Stable Manganese-Based High-Entropy Prussian Blue for Enhanced Sodium-Ion Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Synthesis
2.1.1. Synthesis of FeMn-PBA
2.1.2. Synthesis of HE-PBA
2.2. Materials Characterizations
2.3. Electrochemical Measurements
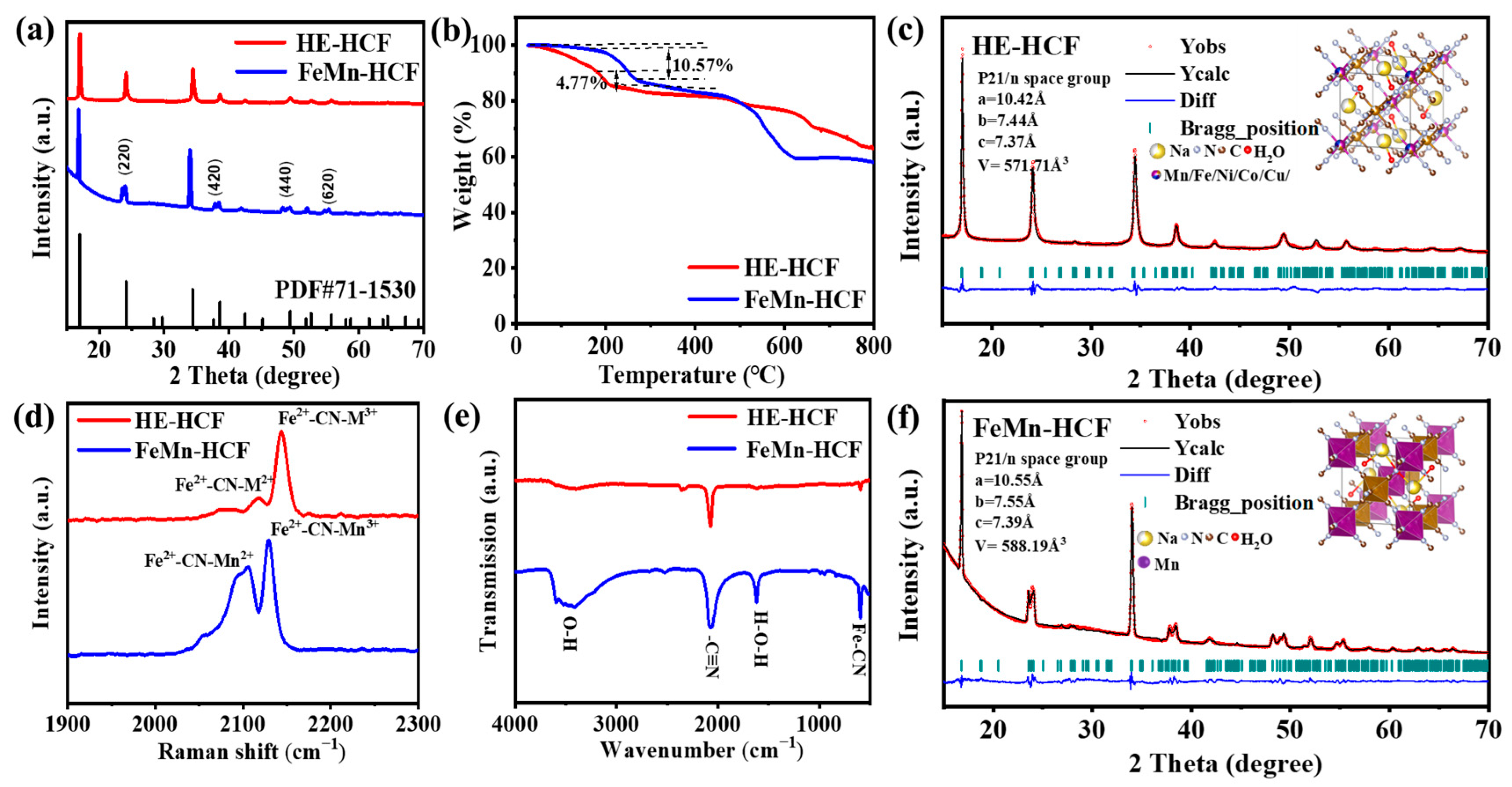
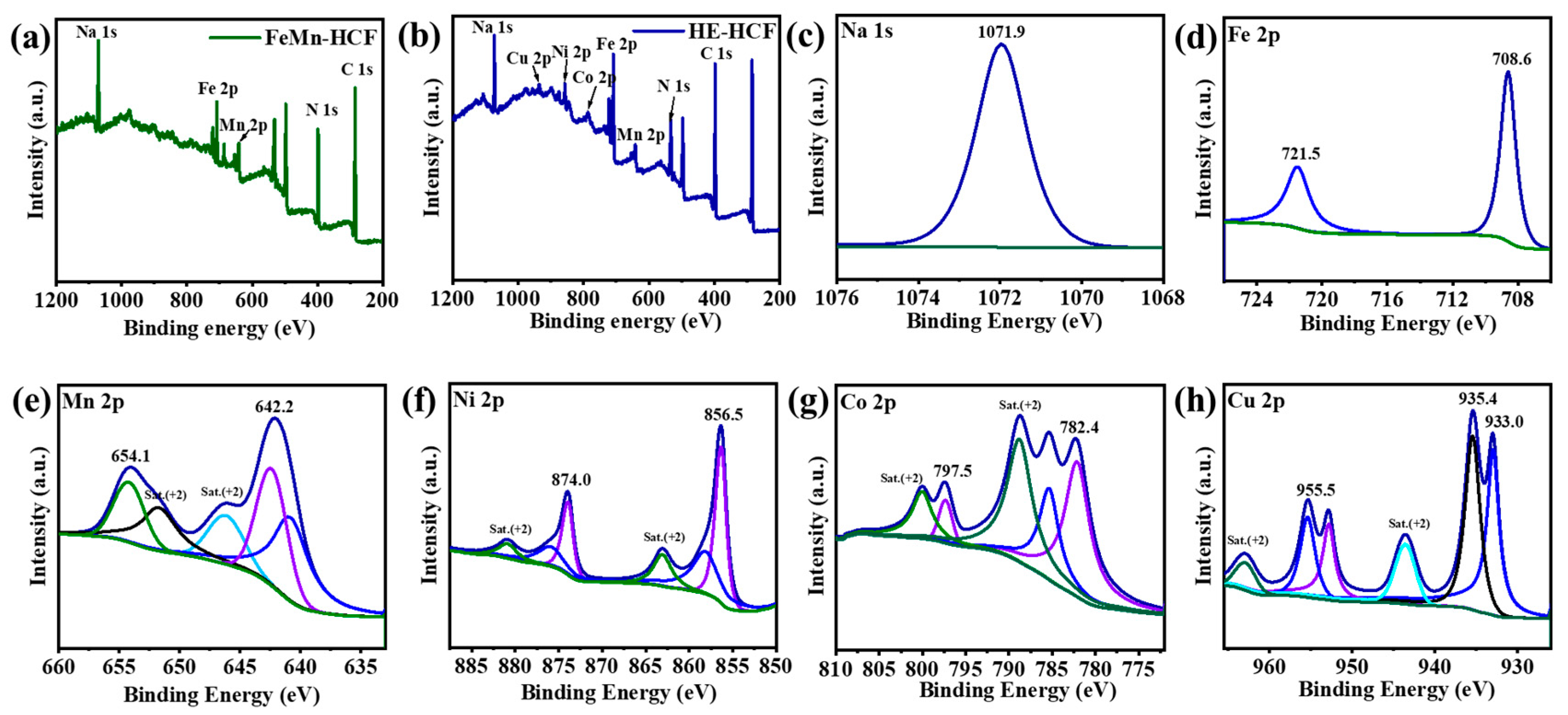
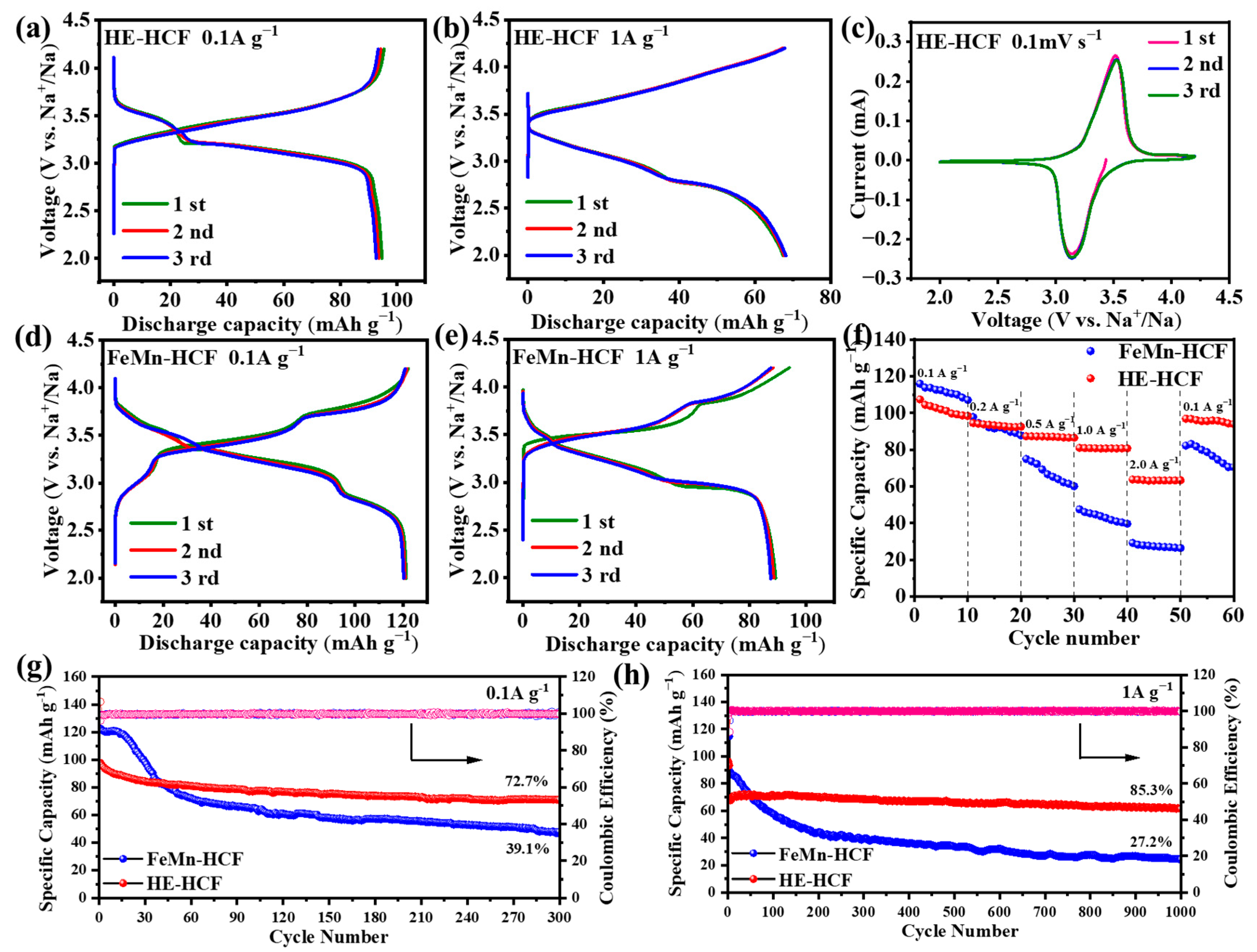
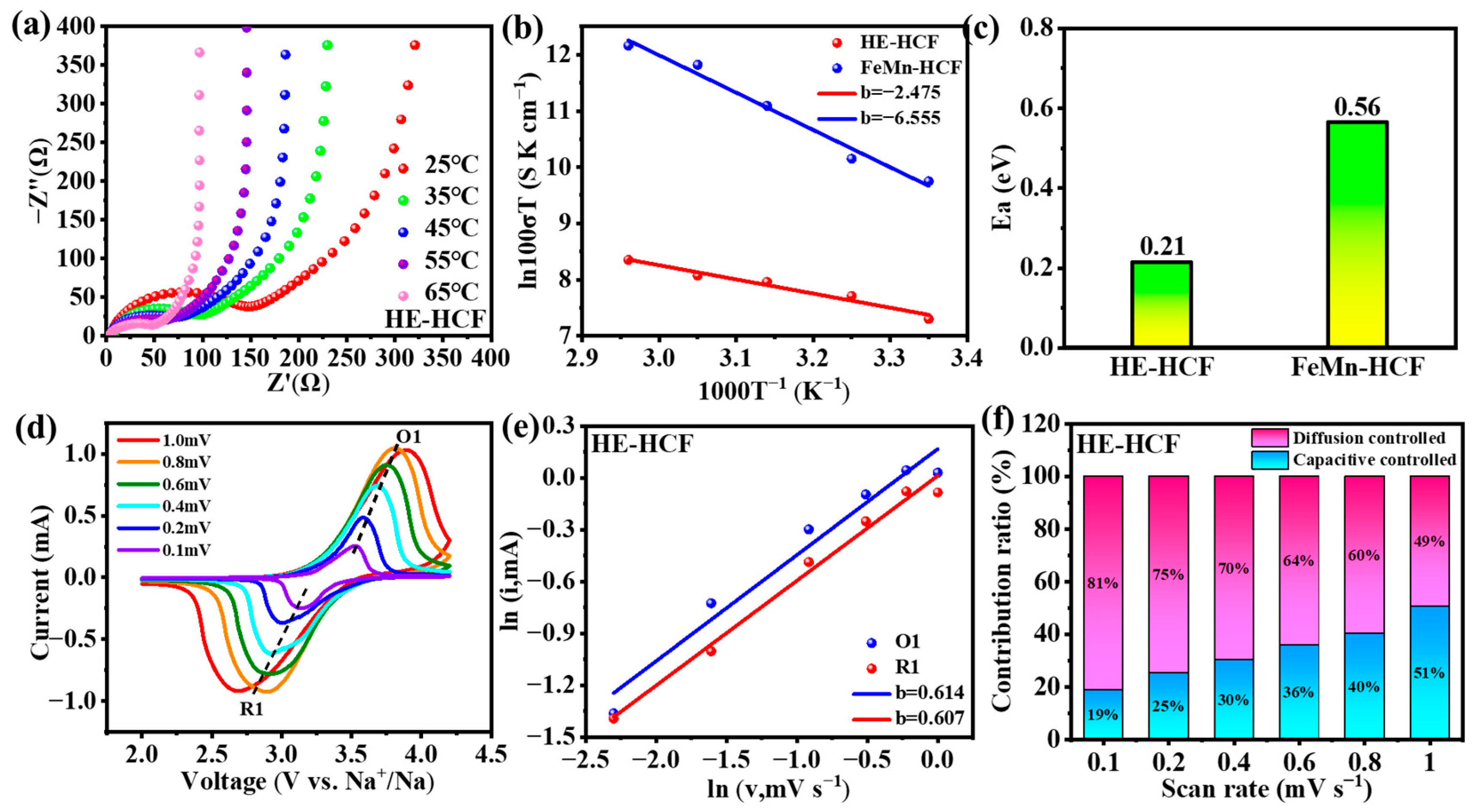
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PB | Prussian blue |
| PBAs | Prussian blue Analogs |
| SIBs | Sodium-ion batteries |
| EIS | Electrochemical impedance spectroscopy |
| GITT | Galvanostatic intermittent titration technique |
| XRD | X-ray diffraction |
| TGA | Thermogravimetric analysis |
| CV | Constant voltage |
References
- Yang, C.; Xin, S.; Mai, L.; You, Y. Materials Design for High-Safety Sodium-Ion Battery. Adv. Energy Mater. 2021, 11, 2000974. [Google Scholar] [CrossRef]
- Qi, S.; Wu, D.; Dong, Y.; Liao, J.; Foster, C.W.; O’Dwyer, C.; Feng, Y.; Liu, C.; Ma, J. Cobalt-Based Electrode Materials for odium-Ion Batteries. Chem. Eng. J. 2019, 370, 185–207. [Google Scholar] [CrossRef]
- Jin, T.; Ji, X.; Wang, P.; Zhu, K.; Zhang, J.; Cao, L.; Chen, L.; Cui, C.; Deng, T.; Liu, S. High-Energy Aqueous Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2021, 60, 11943–11948. [Google Scholar] [CrossRef]
- Jiang, M.; Hou, Z.; Ren, L.; Zhang, Y.; Wang, J.-G. Prussian Blue and Its Analogues for Aqueous Energy Storage: From Fundamentals to Advanced Devices. Energy Storage Mater. 2022, 50, 618–640. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Ji, L.; Wang, L.; Xu, B.B.; Shahzad, M.W.; Tang, Y.; Zhu, Y.; Yan, M.; Sun, G. Boosting the Sodium Storage Performance of Prussian Blue Analogs by Single-Crystal and High-Entropy Approach. Energy Storage Mater. 2023, 58, 1–8. [Google Scholar] [CrossRef]
- Maier, J. Thermodynamic aspects and morphology of nano-structured ion conductors: Aspects of nano-ionics part I. Solid State Ion. 2002, 154–155, 291–301. [Google Scholar] [CrossRef]
- Zhang, G.; Gao, H.; Zhang, D.; Xiao, J.; Sun, L.; Li, J.; Li, C.; Sun, Y.; Yuan, X.; Huang, P. Transformative catalytic carbon conversion enabling superior graphitization and nanopore engineering in hard carbon anodes for sodium-ion batteries. Carbon Energy 2025, 7, e713. [Google Scholar] [CrossRef]
- Joos, M.; Kang, X.; Merkle, R.; Maier, J. Water uptake of solids and its impact on ion transport. Nat. Mater. 2025, 24, 821–834. [Google Scholar] [CrossRef]
- Wu, F.; Wu, S.; Ye, X.; Ren, Y.; Wei, P. Research Progress of High-Entropy Cathode Materials for Sodium-Ion Batteries. Chin. Chem. Lett. 2025, 36, 109851. [Google Scholar] [CrossRef]
- Wu, R.; Ren, B.; Wang, X.; Lin, J.; Li, X.; Zheng, J.; Yang, H.Y.; Shang, Y. Vacancy Remediation in Prussian Blue Analogs for High-Performance Sodium and Potassium Ion Batteries. Adv. Funct. Mater. 2024, 35, 2418018. [Google Scholar] [CrossRef]
- Dai, J.; Tan, S.; Wang, L.; Ling, F.; Duan, F.; Ma, M.; Shao, Y.; Rui, X.; Yao, Y.; Hu, E. High-Voltage Potassium Hexacyanoferrate Cathode via High-Entropy and Potassium Incorporation for Stable Sodium-Ion Batteries. ACS Nano 2023, 17, 20949–20961. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ma, Y.; Dreyer, S.L.; Wang, Q.; Wang, K.; Goonetilleke, D.; Omar, A.; Mikhailova, D.; Hahn, H.; Breitung, B. High-Entropy Metal–Organic Frameworks for Highly Reversible Sodium Storage. Adv. Mater. 2021, 33, 2101342. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, S.; Li, N.; Han, J.; Zhang, S.; Zhuang, Z.; Sun, Z.; Wang, X.; Wu, X.; Chen, Z.; et al. Long-Durable Potassium Ion Batteries Enabled by Medium-Entropy Lattice Engineering on Prussian Blue Analogues Cathodes. Adv. Energy Mater. 2024, 15, 2405007. [Google Scholar] [CrossRef]
- Maier, J. Utility of Simple Rate Equations for Solid State Reactions. Z. Anorg. Allg. Chem. 2005, 631, 433. [Google Scholar] [CrossRef]
- Chen, M.; Li, X.; Yan, Y.; Yang, Y.; Xu, Q.; Liu, H.; Xia, Y. Polypyrrole-Coated K2Mn[Fe(CN)6] Stabilizing Its Interfaces and Inhibiting Irreversible Phase Transition during the Zinc Storage Process in Aqueous Batteries. ACS Appl. Mater. Interfaces J. 2022, 14, 1092–1101. [Google Scholar] [CrossRef]
- Deng, L.; Qu, J.; Niu, X.; Liu, J.; Zhang, J.; Hong, Y.; Feng, M.; Wang, J.; Hu, M.; Zeng, L. Defect-Free Potassium Manganese Hexacyanoferrate Cathode Material for High-Performance Potassium-Ion Batteries. Nat. Commun. 2021, 12, 2167. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, W.; Liu, Q.; Wang, J.; Chou, S.; Liu, H.; Dou, S. Prussian Blue Analogues for Sodium-Ion Batteries: Past, Present, and Future. Adv. Mater. 2022, 34, 2108384. [Google Scholar] [CrossRef]
- Qian, Z.; Luo, R.-J.; Ma, C.; Du, C.-Y.; Zeng, J.; Xu, X.; Mei, Z.; Zhou, Z.-T.; Fu, Z.-W.; Zhou, Y.-N. Six Element High-Entropy Prussian Blue Analogue Cathode Enabling High Cycle Stability for Sodium-Ion Batteries. Chem. Eng. J. 2024, 500, 156767. [Google Scholar] [CrossRef]
- Hong, P.; Xu, C.; Yan, C.; Dong, Y.; Zhao, H.; Lei, Y. Prussian blue and its analogues for commercializing fast-charging sodium/potassium-ion batteries. ACS Energy Lett. 2025, 10, 750–778. [Google Scholar] [CrossRef]
- Du, M.; Geng, P.; Pei, C.; Jiang, X.; Shan, Y.; Hu, W.; Ni, L.; Pang, H. High-Entropy Prussian Blue Analogues and Their Oxide Family as Sulfur Hosts for Lithium-Sulfur Batteries. Angew. Chemie Int. Ed. 2022, 61, e202209350. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, T.; Chen, H.; Suo, X.; Liang, J.; Zhu, W.; Li, H.; Dai, S. Room Temperature Synthesis of High-Entropy Prussian Blue Analogues. Nano Energy 2021, 79, 105464. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, S.; Zhao, L.; Li, C.; Wang, G.; Qiu, J. Entropy Engineering Constrain Phase Transitions Enable Ultralong-life Prussian Blue Analogs Cathodes. Adv. Sci. 2024, 11, 2402340. [Google Scholar] [CrossRef]
- Chou, C.; Tuan, H. Whispers of Entropic Distortion Elevating Voltage and Electrochemical Depth in Potassium Hexacyanoferrate Cathodes. Adv. Funct. Mater. 2024, 35, 2418680. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, B.; Hua, W.; Liang, Y.; Zhang, W.; Du, Y.; Peleckis, G.; Indris, S.; Gu, Q.; Cheng, Z. A Disordered Rubik’s Cube-Inspired Framework for Sodium-Ion Batteries with Ultralong Cycle Lifespan. Angew. Chem. Int. Ed. 2023, 62, e202215865. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Sari, F.N.I.; Saputro, B.W.; Ting, J.-M. Structural and Defect Modulations of Co-Precipitation Synthesized High-Entropy Prussian Blue Analogue Nanocubes via Cu/Zn Co-Doping for Enhanced Electrochemical Performance. J. Mater. Chem. A 2023, 11, 19483–19495. [Google Scholar] [CrossRef]
- Xiao, J.; Gao, H.; Xiao, Y.; Wang, S.; Gong, C.; Huang, Z.; Sun, B.; Dong, C.-L.; Guo, X.; Liu, H. A hydro-stable and phase-transition-free P2-type cathode with superior cycling stability for high-voltage sodium-ion batteries. Chem. Eng. J. 2025, 506, 160010. [Google Scholar] [CrossRef]
- Lu, X.; Zhong, H.-Y.; Huang, P.-W.; Wu, Y.-N.; Xu, S.-Y.; Tan, X.-Y.; Wu, X.-H. Constructing FeSe2 nanorods supported on ketjen black with superior cyclability for potassium-ion batteries. J. Mater. Chem. A 2024, 12, 19995–20005. [Google Scholar] [CrossRef]
- Hurlbutt, K.; Giustino, F.; Volonakis, G.; Pasta, M. Origin of the High Specific Capacity in Sodium Manganese Hexacyanomanganate. Chem. Mater. J. 2022, 34, 4336–4343. [Google Scholar] [CrossRef]
- Ge, L.; Song, Y.; Niu, P.; Li, B.; Zhou, L.; Feng, W.; Ma, C.; Li, X.; Kong, D.; Yan, Z. Elaborating the Crystal Water of Prussian Blue for Outstanding Performance of Sodium Ion Batteries. ACS Nano 2024, 18, 3542–3552. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Gao, Y.; Zhang, H.; Liu, Z.; Zhang, W.; Li, L.; Qiao, Y.; Yang, W.; Wang, J.; Dou, S. Ball Milling Solid-State Synthesis of Highly Crystalline Prussian Blue Analogue Na2−x MnFe(CN)6 Cathodes for All-Climate Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2022, 61, e202205867. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Hu, Y.; Pramudya, Y.; Diemant, T.; Wang, Q.; Goonetilleke, D.; Tang, Y.; Zhou, B.; Hahn, H.; Wenzel, W. Resolving the Role of Configurational Entropy in Improving Cycling Performance of Multicomponent Hexacyanoferrate Cathodes for Sodium-Ion Batteries. Adv. Funct. Mater. 2022, 32, 2202372. [Google Scholar] [CrossRef]
- Huang, Y.; Xie, M.; Zhang, J.; Wang, Z.; Jiang, Y.; Xiao, G.; Li, S.; Li, L.; Wu, F.; Chen, R. A Novel Border-Rich Prussian Blue Synthetized by Inhibitor Control as Cathode for Sodium Ion Batteries. Nano Energy 2017, 39, 273–283. [Google Scholar] [CrossRef]
- Tang, Y.; Li, W.; Feng, P.; Zhou, M.; Wang, K.; Wang, Y.; Zaghib, K.; Jiang, K. High-Performance Manganese Hexacyanoferrate with Cubic Structure as Superior Cathode Material for Sodium-Ion Batteries. Adv. Funct. Mater. 2020, 30, 1908754. [Google Scholar] [CrossRef]
- Bie, X.; Kubota, K.; Hosaka, T.; Chihara, K.; Komaba, S. Synthesis and Electrochemical Properties of Na-Rich Prussian Blue Analogues Containing Mn, Fe, Co, and Fe for Na-Ion Batteries. J. Power Sources 2018, 378, 322–330. [Google Scholar] [CrossRef]
- Liu, Y.; He, D.; Han, R.; Wei, G.; Qiao, Y. Nanostructured Potassium and Sodium Ion Incorporated Prussian Blue Frameworks as Cathode Materials for Sodium-Ion Batteries. Commun. Chem. 2017, 53, 5569–5572. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Wang, S.; Hui, K.S.; Li, H.-F.; Liang, F.; Wu, X.-L.; Zhang, Q.; Zhou, W.; Chen, L.; Chen, F. [Fe(CN)6] Vacancy-Boosting Oxygen Evolution Activity of Co-Based Prussian Blue Analogues for Hybrid Sodium-Air Battery. Mater. Today Energy 2021, 20, 100572. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, M.; Liu, T.; Peng, N.; Yu, H.; Zheng, R.; Zhang, L.; Shui, M.; Shu, J. Copper Hexacyanoferrate as Ultra-High Rate Host for Aqueous Ammonium Ion Storage. Chem. Eng. J. 2021, 421, 127767. [Google Scholar] [CrossRef]
- Wang, Q.; Sarkar, A.; Wang, D.; Velasco, L.; Azmi, R.; Bhattacharya, S.S.; Bergfeldt, T.; Düvel, A.; Heitjans, P.; Brezesinski, T. Multi-Anionic and -Cationic Compounds: New High Entropy Materials for Advanced Li-Ion Batteries. Energy Environ. Sci. 2019, 12, 2433–2442. [Google Scholar] [CrossRef]
- Zhou, Z.; Dong, Y.; Ma, Y.; Zhang, H.; Meng, F.; Ma, Y.; Wu, Y. Innovative high-entropy strategy extending traditional metal substitution for optimizing Prussian blue analogues in rechargeable batteries. Susmat 2025, 5, e265. [Google Scholar] [CrossRef]
- Gu, Y.; Lu, Y.; Dai, P.; Cao, X.; Zhou, Y.; Tang, Y.; Fang, Z.; Wu, P. Self-assembled high-entropy Prussian blue analogue nanosheets enabling efficient sodium storage. J. Colloid Interface Sci. 2025, 677, 307–313. [Google Scholar] [CrossRef]
- Kumar, K.; Kundu, R. Empowering Energy Storage Technology: Recent Breakthroughs and Advancement in Sodium-Ion Batteries. ACS Appl. Energy Mater. J. 2024, 7, 3523–3539. [Google Scholar] [CrossRef]
- Zhou, P.; Liu, X.; Weng, J.; Wang, L.; Wu, X.; Miao, Z.; Zhao, J.; Zhou, J.; Zhuo, S. Synthesis, Structure, and Electrochemical Properties of O′3-Type Monoclinic NaNi0.8Co0.15Al0.05O2 Cathode Materials for Sodium-Ion Batteries. J. Mater. Chem. A 2019, 7, 657–663. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, Q.; Zhao, X.; Zhang, J.; Liu, X.; Wang, T.; Zhang, N.; Jiao, L.; Chen, J.; Fan, L. Hierarchical Engineering of Porous P2-Na2/3Ni1/3Mn2/3O2 Nanofibers Assembled by Nanoparticles Enables Superior Sodium-Ion Storage Cathodes. Adv. Funct. Mater. 2020, 30, 1907837. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Liu, Z.; Li, H.; Huang, Y.; Liu, W.; Ruan, D.; Cai, X.; Yu, X. Dual-Strategy of Cu-Doping and O3 Biphasic Structure Enables Fe/Mn-Based Layered Oxide for High-Performance Sodium-Ion Batteries Cathode. J. Power Sources 2023, 567, 232930. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Xiao, Y.; Zhang, D.; Yuan, X.; Xiao, J.; Zhao, Y.; Gao, H.; Liu, H. Stable Manganese-Based High-Entropy Prussian Blue for Enhanced Sodium-Ion Storage. Batteries 2025, 11, 328. https://doi.org/10.3390/batteries11090328
Li C, Xiao Y, Zhang D, Yuan X, Xiao J, Zhao Y, Gao H, Liu H. Stable Manganese-Based High-Entropy Prussian Blue for Enhanced Sodium-Ion Storage. Batteries. 2025; 11(9):328. https://doi.org/10.3390/batteries11090328
Chicago/Turabian StyleLi, Congcong, Yang Xiao, Dingyi Zhang, Xinyao Yuan, Jun Xiao, Yufei Zhao, Hong Gao, and Hao Liu. 2025. "Stable Manganese-Based High-Entropy Prussian Blue for Enhanced Sodium-Ion Storage" Batteries 11, no. 9: 328. https://doi.org/10.3390/batteries11090328
APA StyleLi, C., Xiao, Y., Zhang, D., Yuan, X., Xiao, J., Zhao, Y., Gao, H., & Liu, H. (2025). Stable Manganese-Based High-Entropy Prussian Blue for Enhanced Sodium-Ion Storage. Batteries, 11(9), 328. https://doi.org/10.3390/batteries11090328






