Intergranular Crack of Cathode Materials in Lithium-Ion Batteries Subjected to Rapid Cooling During Transient Thermal Runaway
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
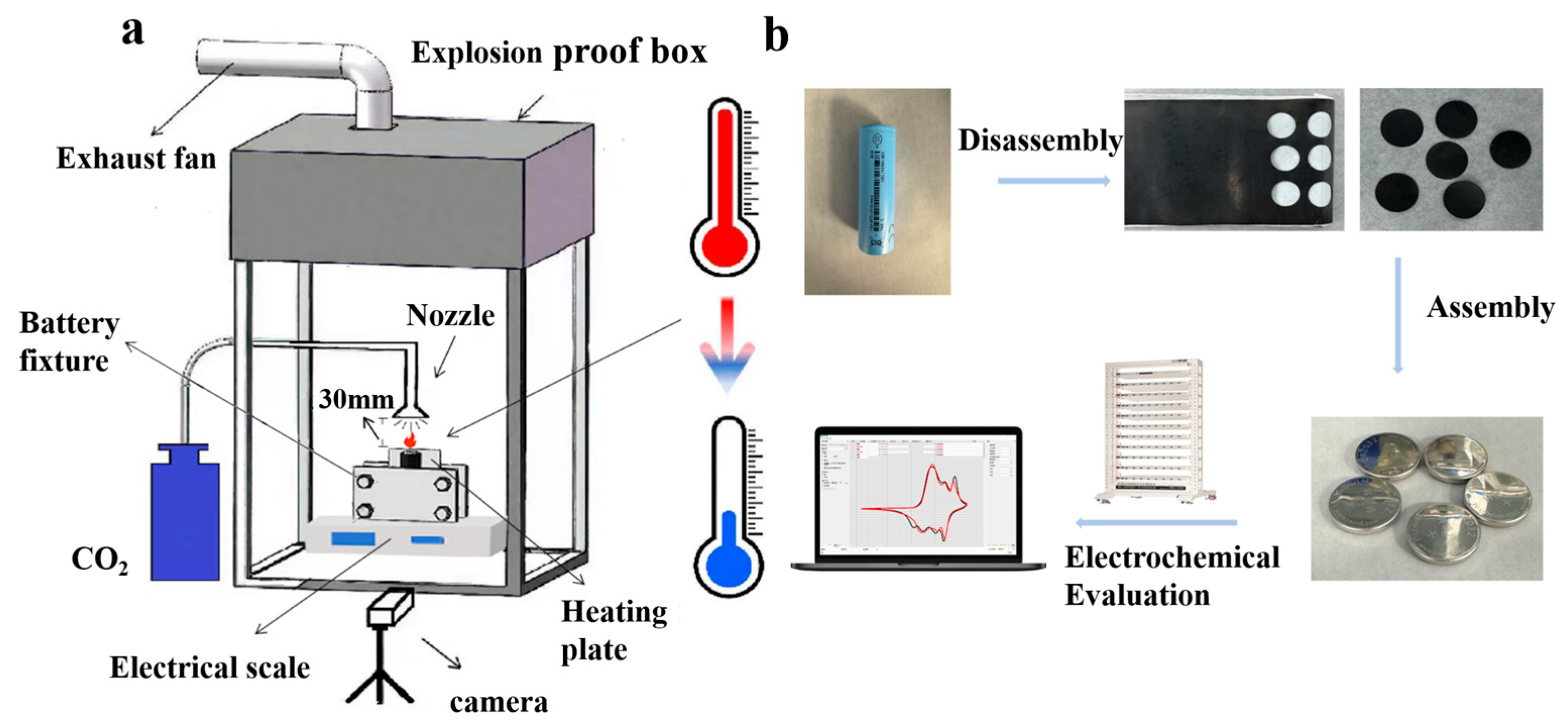
2.2. Experimental Design
2.3. Characterization and Electrochemical Tests
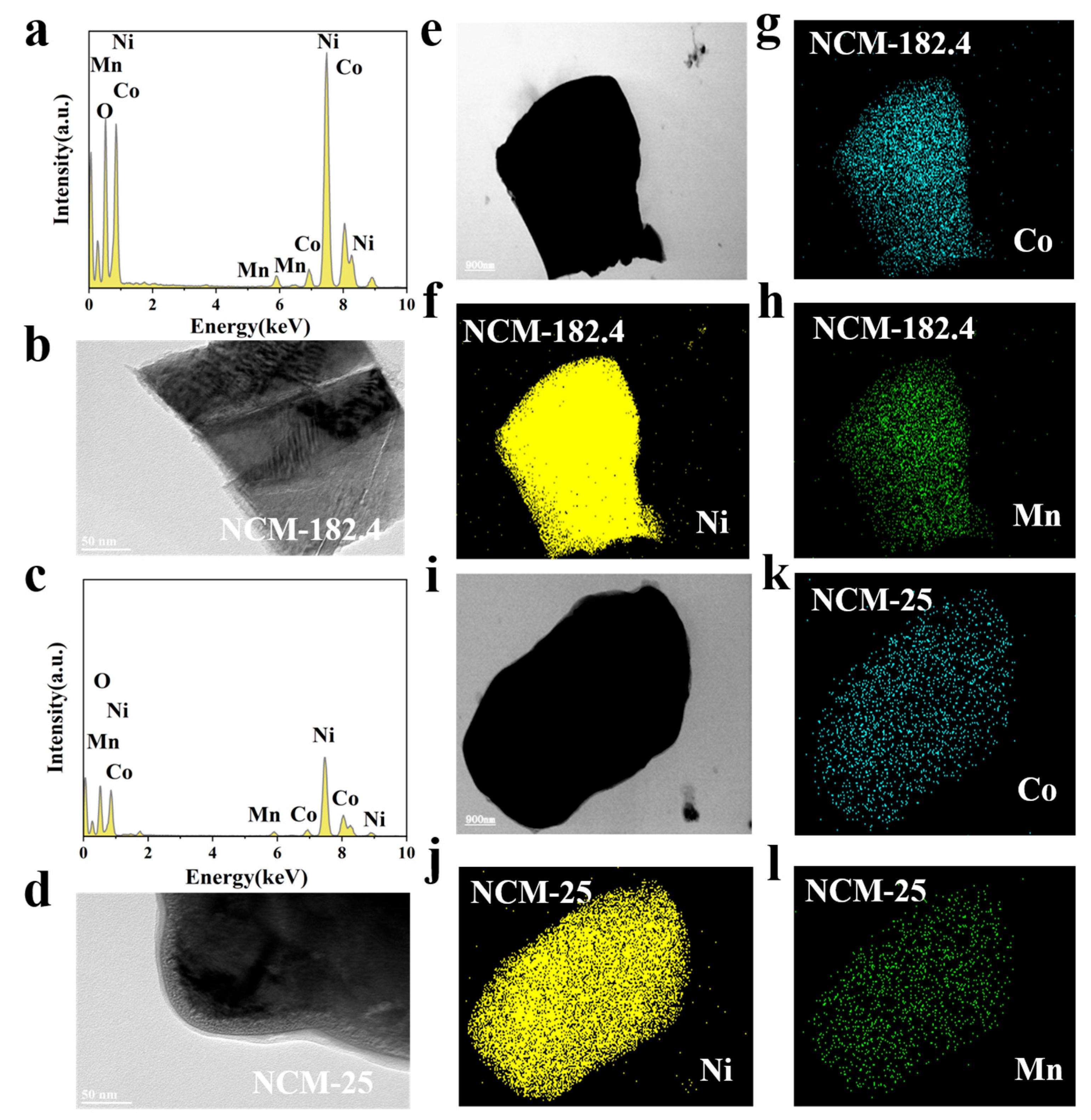
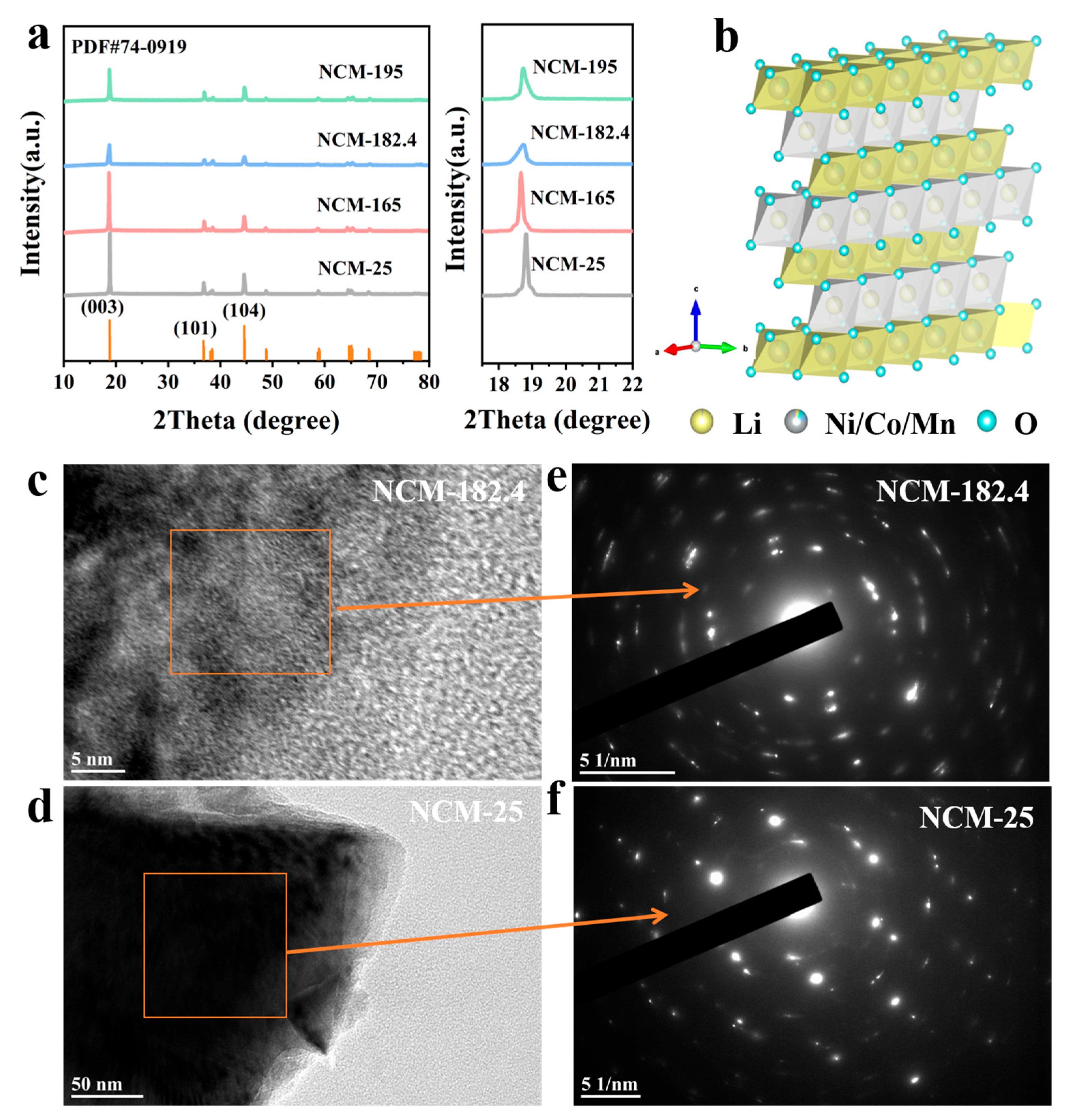
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jenis, P.; Zhang, T.; Ramasubramanian, B.; Lin, S.; Rayavarapu, P.R.; Yu, J.; Ramakrishna, S. Recent progress and hurdles in cathode recycling for Li-ion batteries. Circ. Econ. 2024, 3, 100087. [Google Scholar] [CrossRef]
- Wang, T.; Luo, H.; Bai, Y.; Li, J.; Belharouak, I.; Dai, S. Direct Recycling of Spent NCM Cathodes through Ionothermal Lithiation. Adv. Energy Mater. 2020, 10, 2001204. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Abdullah, M.F.; Dawood, M.K.; Wei, B.; Subramanian, Y.; Azad, A.T.; Nourin, S.; Afroze, S.; Taweekun, J.; Azad, A.K. Innovative lithium-ion battery recycling: Sustainable process for recovery of critical materials from lithium-ion batteries. J. Energy Storage 2023, 67, 107551. [Google Scholar]
- Guo, A.; Xing, Z.; Liu, Y.; Lu, W.; Wang, A.; Wu, J.; Chai, G.; Shi, Y.; Jiang, J.; Ma, Y. The disassembly analysis and thermal runaway characteristics of NCM811 family battery cells. J. Therm. Anal. Calorim. 2024, 150, 141–149. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, J.; Hu, Y.; Zhang, L.; Zhang, W.; Chen, Y.; Zhang, G.; Jiang, L.; Dai, Z.; Wen, Y. Investigations on the essential causes of the degrading properties of ternary lithium-ion batteries with different nickel content during thermal runaway stage. Int. J. Electrochem. Sci. 2024, 19, 100491. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Zhao, Z.; Ma, Y.; Duan, Q.; Li, H.; Sun, J.; Wang, Q. The efficiency of dodecafluoro-2-methylpentan-3-one in suppressing NCM 811 lithium-ion battery fire. Process Saf. Environ. Prot. 2024, 186, 1432–1446. [Google Scholar] [CrossRef]
- Binder, J.O.; Culver, S.P.; Zeier, W.G.; Janek, J. A Rapid and Facile Approach for the Recycling of High-Performance LiNi1−x−y Cox MnyO2 Active Materials. ChemSusChem 2021, 14, 441–448. [Google Scholar] [CrossRef]
- Li, L.; Bian, Y.; Zhang, X.; Xue, Q.; Fan, E.; Wu, F.; Chen, R. Economical recycling process for spent lithium-ion batteries and macro- and micro-scale mechanistic study. J. Power Sources 2018, 377, 70–79. [Google Scholar] [CrossRef]
- Gao, S.; Feng, X.; Lu, L.; Kamyab, N.; Du, J.; Coman, P.; White, R.E.; Ouyang, M. An experimental and analytical study of thermal runaway propagation in a large format lithium ion battery module with NCM pouch-cells in parallel. Int. J. Heat Mass Transf. 2019, 135, 93–103. [Google Scholar] [CrossRef]
- Hu, J.; Tang, X.; Zhu, X.; Liu, T.; Wang, X. Suppression of thermal runaway induced by thermal abuse in large-capacity lithium-ion batteries with water mist. Energy 2024, 286, 129669. [Google Scholar] [CrossRef]
- Zhou, M.; Li, B.; Li, J.; Xu, Z. Pyrometallurgical Technology in the Recycling of a Spent Lithium Ion Battery: Evolution and the Challenge. ACS ES&T Eng. 2021, 1, 1369–1382. [Google Scholar] [CrossRef]
- Yoo, E.; Lee, U.; Kelly, J.C.; Wang, M. Life-cycle analysis of battery metal recycling with lithium recovery from a spent lithium-ion battery. Resour. Conserv. Recycl. 2023, 196, 107040. [Google Scholar] [CrossRef]
- Zhao, L.; Li, W.; Luo, W.; Zheng, M.; Chen, M. Numerical study of critical conditions for thermal runaway of lithium-ion battery pack during storage. J. Energy Storage 2024, 84, 110901. [Google Scholar] [CrossRef]
- Peng, W.; Zhang, Y.; Zhang, S.; Liu, X. Experimental study on the inhibition effect of water mist containing additives on the thermal runaway of lithium battery. Process Saf. Environ. Prot. 2024, 182, 999–1007. [Google Scholar]
- Xiao, X.; Chen, B.; Jin, X.; Zeng, Q.; Tian, Y.; Li, Q. Experimental Study on the Effect of Synergistic Extinguishing Method Based on Liquid Nitrogen on Lithium-Ion Battery Fire After Thermal Runaway. Fire 2024, 7, 479. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Duan, Q.; Chen, M.; Xu, J.; Zhao, C.; Sun, J.; Wang, Q. Experimental study on the synergistic effect of gas extinguishing agents and water mist on suppressing lithium-ion battery fires. J. Energy Storage 2020, 32, 101801. [Google Scholar] [CrossRef]
- Xu, J.; Guo, P.; Duan, Q.; Yu, X.; Zhang, L.; Liu, Y.; Wang, Q. Experimental study of the effectiveness of three kinds of extinguishing agents on suppressing lithium-ion battery fires. Appl. Therm. Eng. 2020, 171, 115076. [Google Scholar] [CrossRef]
- Ye, C.; Liu, J.; Zhang, Q.; Jin, X.; Zhao, Y.; Pan, Z.; Chen, G.; Qiu, Y.; Ye, D.; Gu, L.; et al. Activating Metal Oxides Nanocatalysts for Electrocatalytic Water Oxidation by Quenching-Induced Near-Surface Metal Atom Functionality. J. Am. Chem. Soc. 2021, 143, 14169–14177. [Google Scholar] [CrossRef]
- Luo, D.; Ding, X.; Hao, X.; Xie, H.; Cui, J.; Liu, P.; Yang, X.; Zhang, Z.; Guo, J.; Sun, S.; et al. Ni/Mn and Al Dual Concentration-Gradients To Mitigate Voltage Decay and Capacity Fading of Li-Rich Layered Cathodes. ACS Energy Lett. 2021, 6, 2755–2764. [Google Scholar]
- Wang, Z.; Zhao, Q.; Yin, B.; Zhai, H.; Wang, J.; An, W. Characterization of fire behaviors associated with a thermal runaway in large-scale commercial LiNi0.8Co0.1Mn0.1O2/graphite cells under external ignition. Case Stud. Therm. Eng. 2023, 47, 103126. [Google Scholar] [CrossRef]
- Gong, Z.; Gu, C.; Sun, J.; Wang, H.; Li, Y.; Zhou, X.; Jia, Y.; Han, D. Experimental study on thermal runaway characteristic and residue of Li(Ni0.8Co0.1Mn0.1)O2 lithium-ion batteries induced by overcharge. J. Energy Storage 2023, 68, 107705. [Google Scholar]
- Borchers, A.; Pieler, T. Programming pluripotent precursor cells derived from Xenopus embryos to generate specific tissues and organs. Genes 2010, 1, 413–426. [Google Scholar] [CrossRef]
- Jia, Z.; Qin, P.; Li, Z.; Wei, Z.; Jin, K.; Jiang, L.; Wang, Q. Analysis of gas release during the process of thermal runaway of lithium-ion batteries with three different cathode materials. J. Energy Storage 2022, 50, 104302. [Google Scholar] [CrossRef]
- Li, H.; Lu, J.; Wang, J.; Yang, L. Study on extinguishing performance and suppression mechanism of thermal runaway combustible gas flame of lithium battery by NH4H2PO4 water mist. Case Stud. Therm. Eng. 2025, 71, 106208. [Google Scholar]
- Chang, C.; Wang, R. Experimental investigation of thermal runaway behaviour and inhibition strategies in large-capacity lithium iron phosphate (LiFePO4) batteries for electric vehicles. Int. J. Electrochem. Sci. 2024, 19, 100877. [Google Scholar]
- Pastor, J.V.; García, A.; Monsalve-Serrano, J.; Golke, D. Analysis of the aging effects on the thermal runaway characteristics of Lithium-Ion cells through stepwise reactions. Appl. Therm. Eng. 2023, 230, 120685. [Google Scholar] [CrossRef]
- Li, L.; Bian, Y.; Zhang, X.; Guan, Y.; Fan, E.; Wu, F.; Chen, R. Process for recycling mixed-cathode materials from spent lithium-ion batteries and kinetics of leaching. Waste Manag. 2018, 71, 362–371. [Google Scholar]
- Wang, K.; Ouyang, D.; Yuan, S.; Wu, D.; Zhang, J.; Chang, C.; Yan, K.; Sun, H.; Qian, X. Experimental study on inhibition effect of Novec-1230 on thermal runaway fire of lithium-ion battery packs induced by overcharging. J. Energy Storage 2025, 120, 116451. [Google Scholar] [CrossRef]
- Sloop, S.E.; Crandon, L.; Allen, M.; Lerner, M.M.; Zhang, H.; Sirisaksoontorn, W.; Gaines, L.; Kim, J.; Lee, M. Cathode healing methods for recycling of lithium-ion batteries. Sustain. Mater. Technol. 2019, 22, e00113. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, K.; Sun, J.; Wang, Q. A Review of Fire-Extinguishing Agents and Fire Suppression Strategies for Lithium-Ion Batteries Fire. Fire Technol. 2022, 60, 817–858. [Google Scholar]
- Wang, F.; Sheng, H.; Li, W.; Gerken, J.B.; Jin, S.; Stahl, S.S. Stable Tetrasubstituted Quinone Redox Reservoir for Enhancing Decoupled Hydrogen and Oxygen Evolution. ACS Energy Lett. 2021, 6, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, M.; Kolekar, S.; Ma, Y.; Diaz, H.C.; Kalappattil, V.; Das, R.; Eggers, T.; Gutierrez, H.R.; Phan, M.H.; Batzill, M. Strong room-temperature ferromagnetism in VSe(2) monolayers on van der Waals substrates. Nat. Nanotechnol. 2018, 13, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Winter, L.R.; Chen, J.G. N2 Fixation by Plasma-Activated Processes. Joule 2021, 5, 300–315. [Google Scholar] [CrossRef]
- Huang, Z.; Li, X.; Wang, Q.; Duan, Q.; Li, Y.; Li, L.; Wang, Q. Experimental investigation on thermal runaway propagation of large format lithium ion battery modules with two cathodes. Int. J. Heat Mass Transf. 2021, 172, 121077. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Ye, C.; Jin, M.; Zhong, G.; Liu, S.; Liu, Y.; Tai, Z. Intergranular Crack of Cathode Materials in Lithium-Ion Batteries Subjected to Rapid Cooling During Transient Thermal Runaway. Batteries 2025, 11, 363. https://doi.org/10.3390/batteries11100363
Li S, Ye C, Jin M, Zhong G, Liu S, Liu Y, Tai Z. Intergranular Crack of Cathode Materials in Lithium-Ion Batteries Subjected to Rapid Cooling During Transient Thermal Runaway. Batteries. 2025; 11(10):363. https://doi.org/10.3390/batteries11100363
Chicago/Turabian StyleLi, Siqi, Changchun Ye, Ming Jin, Guobin Zhong, Shi Liu, Yajie Liu, and Zhixin Tai. 2025. "Intergranular Crack of Cathode Materials in Lithium-Ion Batteries Subjected to Rapid Cooling During Transient Thermal Runaway" Batteries 11, no. 10: 363. https://doi.org/10.3390/batteries11100363
APA StyleLi, S., Ye, C., Jin, M., Zhong, G., Liu, S., Liu, Y., & Tai, Z. (2025). Intergranular Crack of Cathode Materials in Lithium-Ion Batteries Subjected to Rapid Cooling During Transient Thermal Runaway. Batteries, 11(10), 363. https://doi.org/10.3390/batteries11100363





