1. Introduction
Silicon has emerged as a highly promising alternative anode material for Li-ion batteries. It has a theoretical capacity of 3578 mAh/g, which is nearly ten times that of graphite at 372 mAh/g [
1,
2]. Its adoption is hindered by severe volume expansion: fully lithiated silicon (Li
15Si
4) swells by ≈275% at room temperature, compared to just 13% for graphite (LiC
6) [
3]. Such drastic expansion induces particle fracture, electrode pulverization, and unstable solid electrolyte interphase (SEI) formation, leading to rapid capacity fade and poor cycle life.
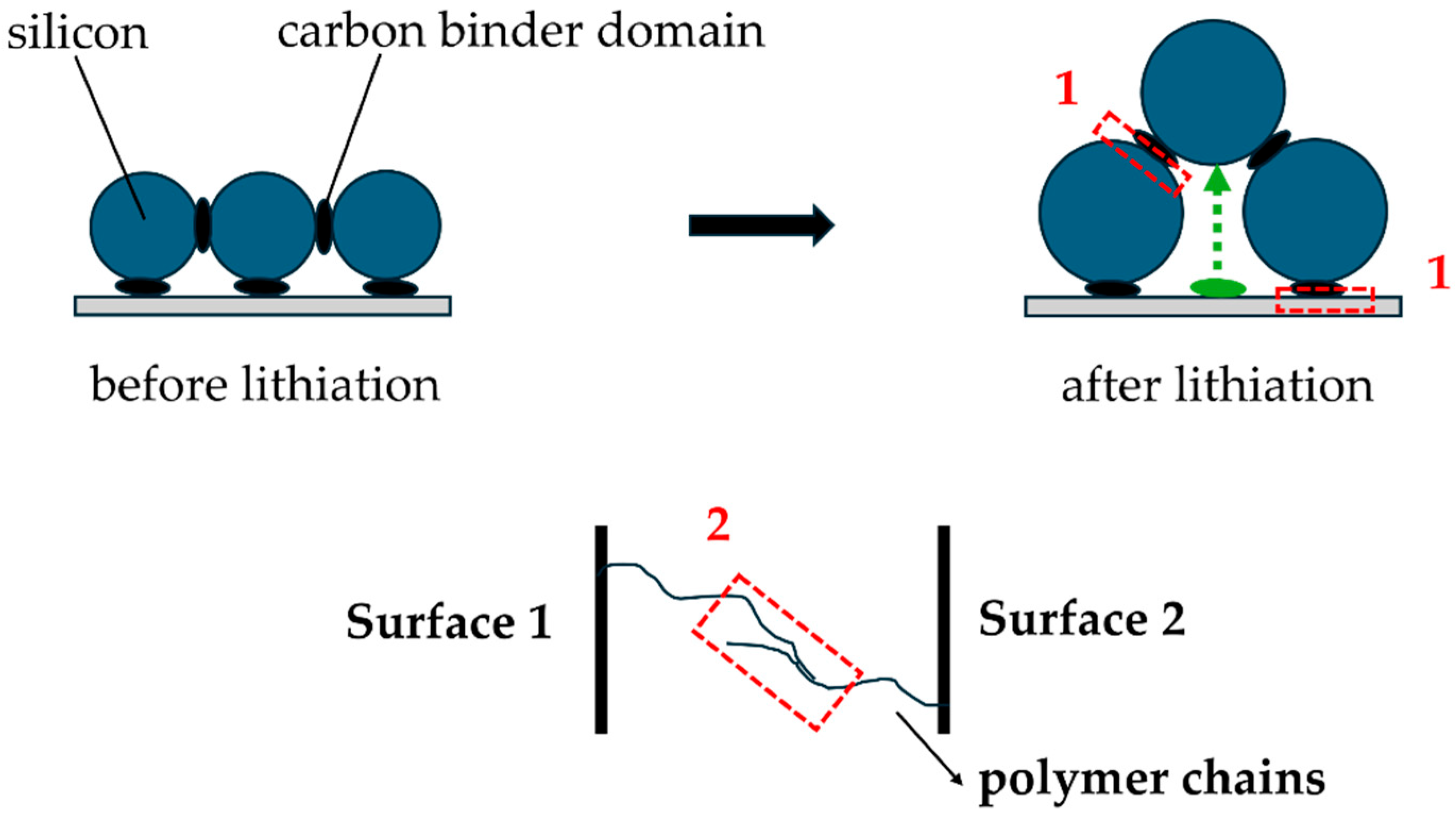
Figure 1 illustrates that when active particles increase in volume, particle rearrangement and translation necessarily happen due to the rigidity of the current collector. The figure further presents two types of connections required for proper binder functionality: adhesive and cohesive. Adhesive connections occur when a binder attaches to active materials or the current collector, a process partially determined by distinct functional groups. Cohesive connections occur when binder molecules connect to other binder molecules through polymer chain interactions. During volume-change cycles, the silicon electrode requires the binder to elongate on the order of particle sizes (green arrow in
Figure 1), and the bonds must be able to repair or successfully reattach themselves in case of bond release or failure. If connections are too rigid, meaning that the large adhesion and cohesion limits do not allow for strain that will dissipate the accumulated stress in the electrode, this could instead lead to fracture and irreversible structural changes.
Enhancing the binder flexibility and self-healing capabilities of polymer binders is essential for improving the mechanical integrity and long-term cycling performance of silicon electrodes. To enhance flexibility, one approach is to select polymers with intrinsically flexible backbones, such as polyacrylic acid (PAA), polyvinylidene fluoride (PVDF), and styrene–butadiene rubber (SBR), which offer both elasticity and processability [
4,
5]. However, PVDF is limited by weak van der Waals interactions; therefore, it cannot maintain Si electrode integrity, leading to a rapid capacity fade to 1000 mAh/g after 10 cycles [
6,
7]. On the other hand, PAA films tend to be brittle when fully dried, limiting their ability to accommodate extreme Si volume fluctuations without cracking [
6]. The PAA binder’s performance is also sensitive to slurry pH [
6]. Sun and Wheeler [
8] found that in the first four cycles at a C/10 rate, the capacity was around 1650 mAh/g, which was improved with a pH of 4.5 (2600 mAh/g).
Another approach is to reduce the polymer chain length or molecular weight. The structure of polymer chains, such as chain length, significantly impacts a binder’s strength, stiffness, and flexibility. Shorter chains exhibit fewer entanglements and greater segmental mobility, enabling facile bond breakage/reformation under stress. For instance, Kasinathan et al. found that the PAA binder with the lowest molecular weight (250 kDa), compared to that with the highest weight (1250 kDa), achieved the best electrochemical and mechanical performance [
9]. Yet, excessive chain shortening can weaken adhesion to the current collector, compromising electrode cohesion [
10]. To address this adhesion challenge, the design of multilayer silicon anodes (MLA) has emerged as a promising structural strategy. By introducing interfacial/substrate layers, such as carbon nanotube (CNT) scaffolds or graphene interlayers, between the silicon and current collector, researchers have demonstrated the improved mechanical accommodation of volume changes and enhanced charge transfer during cycling [
11].
For facilitating self-healing behavior, one approach is to use stronger and reversible interchain interactions, such as hydrogen bonding, electrostatic forces, metal–ligand coordination, disulfide bonds, and boronate ester bonds [
12]. These dynamic bonds enable polymer networks to autonomously reconfigure and recover after mechanical disruption, thereby improving structural resilience and electrode lifespan.
In contrast, while native PVDF is a well-known and often-used solvent-soluble binder, it is known not to work well for silicon electrodes. Some research groups have explored chemical modifications to this binder and the silicon surface to control adhesion. Yoo et al. achieved improved electrode performance through the chemical modification of PVDF with hydroxyl groups, leading to a more homogeneous PVDF distribution [
1]. Comparatively, Huang et al. introduced a functional buffer layer on silicon particles, enhancing solid–electrolyte interface (SEI) layer formation and maintaining a capacity of around 2000 mAh/g after 200 cycles while using a traditional PVDF binder [
2].
Additionally, blending polymers into hybrid or composite networks can induce synergistic effects, enhancing flexibility, interfacial adhesion, and electrochemical performance simultaneously. Such multiphase systems can integrate the mechanical softness of flexible polymers with the robustness or functionality of more rigid components, offering a promising route toward multifunctional binder materials for next-generation batteries [
8].
This study aims to achieve three main objectives related to the expansion of silicon particles. First, we shorten commercial PVDF chains and introduce carboxyl end groups via dehydrofluorination and oxidative cleavage. Second, we evaluate the electrochemical and mechanical performance of low-molecular weight polymer binders to see if they adequately balance flexibility and adhesion. Third, we fabricate and test multilayer silicon anodes by incorporating an engineered adhesion layer to maintain interfacial cohesion while leveraging the benefits of low-molecular weight binder systems.
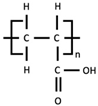
To address the first objective of this work, PVDF is chemically modified. Although controlling the synthesis of PVDF binder from monomeric feedstock is ideal, safely conducting the manufacturing process is challenging due to the high-temperature and high-pressure reaction involving reactive and toxic vinylidene difluoride (VDF) gas precursor. Therefore, we opted to chemically modify off-the-shelf PVDF products. This modification involves two key reactions: dehydrofluorination and oxidative cleavage. These reactions modify PVDF by shortening its polymer chains and incorporating carboxyl functional groups at the chain termini. In the dehydrofluorination step, hydrogen and fluorine atoms are removed from the PVDF polymer backbone, resulting in the formation of carbon–carbon double bonds (C=C). In the subsequent oxidative cleavage reaction achieved through treatment with ozone and hydrogen peroxide, these unsaturated sites undergo bond scission, introducing oxygen-containing functional groups such as carboxyl (-COOH) [
3,
13,
14,
15]. The reactions can be summarized as shown in
Figure 2 [
16,
17,
18].
For the second objective, we explored the use of low-molecular weight polymer binders, including poly(acrylic acid) (PAA) and Jeffamine
TM D-2000 (Huntsman Corporation, The Woodlands, TX, USA), as well as novel binders such as PVDF-HFP, polyacrylonitrile (PAN), and polyimide (PI). These binders differ in structure, molecular weight, melting point, and glass transition temperature, potentially offering enhanced flexibility to accommodate the significant volume expansion of silicon particles. In
Table 1, the structures and properties of those binders are listed. The glass transition point is the temperature at which a polymer transitions from a hard, glassy state to a soft, rubbery state. For example, at temperatures below the glass transition point, the polymer chains are in a rigid state, exhibiting very limited mobility. In this state, the material is rigid and brittle because the molecular chains are locked in place, preventing them from sliding past one another. This lack of mobility contributes to the stiffness and brittleness of the material. Similarly, the melting point of a binder is essential for determining its thermal processing capabilities, stability, and safety under operational conditions.
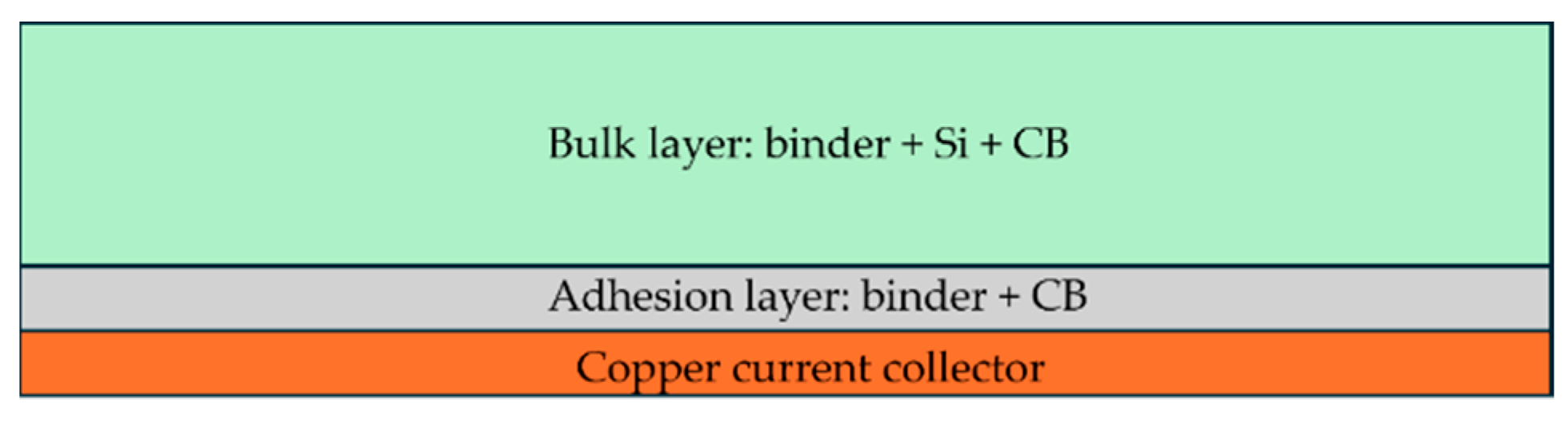
Lastly, for the third objective, we designed an adhesion layer which is introduced between the bulk electrode material and the current collector, as shown in
Figure 3. This adhesion layer is composed of long-chain polymer binder, such as PVDF and PAA, and carbon black, which enhances adhesion while also maintaining electrical conductivity.
2. Materials and Methods
2.1. Chemical Sources
Carbon black (C45) was obtained from Timcal (Lac-des-Îles, Terrebonne, QC, Canada). Silicon nanoparticles (~100 nm) were purchased from Nanostructured & Amorphous Materials, Inc. (Houston, TX, USA). The following were obtained from Sigma-Aldrich (St. Louis, MO, USA): polyacrylic acid (PAA, MW = 450 kDa and 2 kDa), polyvinylidene fluoride (PVDF, MW = 534 kDa), polyacrylonitrile (PAN, MW = 70 kDa), poly(vinylidene fluoride-co-hexafluoropropylene (PVDF-HFP, MW = 400 kDa), JeffamineTM (MW = 2kDa), LiOH, NaOH, NH4OH, NMP, CH2Cl2, and H2O2. Polyimide (PI, MW = 500 kDa) (P84 SG Tecapowder) was sourced from Ensinger GmbH (Lenzing, Austria). Annealed copper sheets were purchased from Nimrod Copper Co. (Springfield, VA, USA). Electrolyte (1.0 M LPF6 in EC and DEC with a volume ratio of 50:50) and fluoroethylene carbonate (FEC) were purchased from Sigma-Aldrich.
2.2. Binder Preparation
The binders were prepared by magnetically stirring the binder powders with deionized water (for PAA) or NMP (for PVDF, PI, PAN, JeffamineTM, PVDF-HFP) at a weight ratio of 1:12 for 6 h at room temperature. Each container was covered with a lid to minimize solvent evaporation.
2.3. Si Electrode Preparation
The fabricated silicon electrodes were composed of 60 wt% silicon, 30 wt% binder, and 10 wt% carbon black (C45). Dry silicon and C45 powders were ground with a mortar and pestle for 20 min. The binder solution and ground particles were then mixed in a sealed jar using a high-speed rotating homogenizer (Algimax GX 300, MONITEX Industrial Co., Ltd., New Taipei City, Taiwan) for 24 s. For the coating process, a 7 cm × 9 cm copper sheet was first cleaned using isopropyl alcohol. The electrode slurry was then coated onto the copper sheet using a coating bar to a thickness of 75 μm. The anode was subsequently dried in an oven at 100 °C for 6 h and calendered to achieve a 50% porosity.
The selected anode composition (60% silicon, 30% binder, and 10% carbon black) was based on prior work and in recognition of the challenge of using only silicon as the active material, as compared to silicon–graphite mixtures. We are aware that commercial electrodes would be preferred at a lower optimized amount of inerts, such as around 5–15% binder. However, a larger fraction allows us to evaluate binder performance in a laboratory setting. Nguyen, Yoon, Seo, Guduru, and Lucht [
6] noted that when binder content is reduced to more realistic levels (~10%), the relative trends in performance remain similar but overall stability decreases, especially for less effective binders like PVDF. This demonstrates that the high-binder formulation is a deliberately exaggerated but useful test to compare binder chemistries under controlled yet challenging conditions.
2.4. Cell Assembly and Electrochemical Characterization
Half cells were assembled using a silicon composite electrode as the working electrode, a lithium metal counter electrode, a microporous polypropylene separator (Celgard 2325, Celgard, LLC, Charlotte, NC, USA), and an electrolyte composed of 1.0 M LiPF6 in a solvent mixture: EC:DEC:FEC 45:45:10 (v/v). All cells were allowed to soak in the electrolyte overnight to ensure thorough penetration of the electrodes. Cycling was performed using a Maccor 4300 battery tester (Maccor, Inc., Tulsa, OK, USA) at room temperature; each cell underwent 3 formation cycles at a C/20 rate, followed by 4 lithiation cycles at rates of C/10, C/5, C/3, 1C, and 2C, coupled with a C/3 delithiation rate. The voltage window spanned from 0.05 to 1.5 V. The sequence of cycles is then repeated a second time, followed by a concluding set of C/10 cycles, leading to a total of 44 cycles after formation. All electrochemical measurements were conducted at room temperature.
2.5. Peeling Test
Peeling tests were conducted using an Instron 3343 stress–strain tester to evaluate the adhesion/cohesion of the binders. The laminate side of the electrode, measuring 30 mm × 54 mm, was attached to a 20 mm-wide strip of aluminum-backed adhesive tape. The electrode portion underneath the adhesive tape was removed from its current collector by pulling the tape at a constant displacement rate of 500 mm/min, as described more fully in ref. [
26]. The applied force was measured, and load/displacement plots were generated to analyze the results.
2.6. Nano-Indentation Test
Nano-indentation was performed using a Leco Micro Hardness Tester to apply a controlled force of 100 g. The force was applied via a sharp indenter tip, resulting in a small, precise impression on the surface of the electrode. The depth of the indentation was accurately measured using a Keyence VHX-7000 microscope ((Keyence Corporation of America, Itasca, IL, USA)).
2.7. FTIR Test
Fourier-transform infrared (FTIR) spectroscopy analyses were performed to characterize the chemical structure of the modified solid powders. The FTIR spectra were recorded using a Thermo Scientific Nicolett iS50 from Thermo Fisher Scientific. Samples were prepared by grinding the powders and pressing them into pellets. The FTIR spectra were collected over a wavenumber range of 400 to 4000 cm−1, with a resolution of 4 cm−1.
2.8. XPS Test
X-ray photoelectron spectroscopy (XPS) analyses were conducted to investigate the elemental composition and chemical states of the elements present in the synthesized solid powders. The measurements were performed using a Thermo Scientific k-Alpha XPS ((Thermo Fisher Scientific Inc., Waltham, MA, USA). Samples were prepared by dispersing the powders on double-sided tape mounted on XPS sample holders. The binding energy scale was calibrated using the C 1s peak at 284.8 eV as a reference. The data analysis included peak fitting using CasaXPS, where peaks were fitted with a combination of Gaussian–Lorentzian functions, and a Shirley background was subtracted to determine the elemental composition and oxidation states.
2.9. DSC Test
Differential scanning calorimetry (DSC) measurements were carried out to investigate the thermal properties of the modified materials, including their melting and crystallization temperatures. The analyses were performed using a DSC Q2000, TA Instruments (TA Instruments, New Castle, DE, USA). Approximately 5–10 mg of each sample was sealed in an aluminum pan and subjected to a controlled temperature program under a nitrogen atmosphere to prevent oxidative degradation. The temperature program typically involved heating the sample from 100 °C to 200 °C at a heating rate of 2 °C/min, followed by a cooling down process at the same rate.
2.10. Dehydrofluorination and Oxidative Cleavage Steps
The dehydrofluorination steps were conducted as follows: A total of 1.6 g of PVDF powder was dissolved in 18.4 g of NMP and stirred at 70 °C for 30 min in an N2 atmosphere. Subsequently, 0.64 g of 5 wt% sodium hydroxide solution was added to the PVDF solution. The mixture was stirred at 180 rpm and maintained at 70 °C for durations of 5, 20, and 60 min. This process resulted in a coagulated emulsion with a brown color. The solid products were then rinsed with ethanol and deionized water and subsequently dried in a vacuum at 70 °C overnight.
The oxidative cleavage steps were performed as follows: At the conclusion of the dehydrofluorination steps, the resulting products were dissolved in CH2Cl2 at a weight ratio of 1:250. The glass reactor vessel was submerged in dry ice. Ozone was bubbled through the enclosed solution for 10 min at a flow rate of 4 standard cubic feet per h (SCFH), followed by stirring for 60 min. Nitrogen was then purged through the reactor to eliminate excess ozone. The dry ice was then removed, allowing the reactor to return to room temperature. Next, 0.5 g of 50 wt% H2O2 solution was added to the reactor, and the reaction was then continued for an additional 60 min. The final solid products were collected by filtering with additional deionized water.
2.11. Post-Mortem Analysis
Post-mortem analysis was performed to evaluate the integrity and degradation of electrode materials, with a specific focus on delamination of the anode surfaces. Cells after cycling were disassembled in an inert atmosphere to prevent additional degradation from exposure to air. The electrodes were then separated from the cell.
2.12. Multilayer Anode Preparation
Two different multilayer anodes were prepared, one made of PVDF and the other made of PAA binders. The binder (dissolved in solvent) was further mixed with carbon black in a mass ratio of 1:0.25, respectively. The mixture was magnetically stirred for 1 h at room temperature. The resulting slurry was then coated to a thickness of 50 μm using a coating bar on the copper sheet, which was previously sanded and treated with 10 wt% oxalic acid in water to remove any potential copper oxide layers. The adhesion layer was then dried at 100 °C for 3 h to eliminate any moisture. Subsequently, a bulk electrode layer comprising a binder, silicon, and carbon black in water was coated on top of the adhesion layer. This electrode was further dried in an oven at 100 °C for 6 h.
4. Conclusions
This work presents an exploration of binder engineering strategies aimed at enhancing the mechanical integrity and electrochemical performance of silicon anodes for lithium-ion batteries, with a particular focus on polymer chain length, chemical functionality, and electrode architecture.
The first strategy involved chemically modifying commercial PVDF binders through dehydrofluorination, followed by oxidative cleavage, to introduce carboxyl (-COOH) functional end groups and shorten the polymer chains. FTIR and XPS confirmed the successful incorporation of -COOH groups, while DSC analysis indicated structural and thermal changes consistent with reduced chain length. While we know the modified PVDF samples, particularly those subjected to extended dehydrofluorination reaction times (up to 60 min), produce shorter chain lengths, we did not have a reliable way to quantify the exact chain lengths. These samples exhibited significantly reduced adhesion to the current collector and inferior mechanical strength, as evidenced by peel tests and nano-indentation. This resulted in poor cycling performance in Si anodes, despite minor gains in flexibility that modestly improved high-rate stability, as shown for the shortest-chain variant (f60). These results suggest that excessive chain shortening leads to binder fragmentation into oligomeric structures, compromising interfacial adhesion. What is the ideal chain length? Undoubtedly, this depends on the polymer and the surfaces to which it is bound, but it should be long enough to ensure adequate adhesion/cohesion to the materials potentially at each end of the chain. Continued work is needed to answer this question.
Incorporating chemical modifications into PVDF binders can enhance the performance of silicon anodes. However, these modifications are likely to increase production costs due to higher material expenses. Therefore, while performance improvements are achievable, they must be carefully weighed against the associated cost implications.
However, the modified PVDF samples, particularly those subjected to extended dehydrofluorination reaction times (up to 60 min, resulting in the shortest chain length, showed markedly reduced adhesion to the current collector and diminished mechanical robustness, as confirmed by peel tests and nanoindentation. These structural weaknesses translated into poor cycling stability in Si anodes. Although the most extensively modified variant (f60) exhibited slightly enhanced flexibility, which provided modest improvements in high-rate performance, this benefit was insufficient to compensate for the overall mechanical and electrochemical degradation.
In the second strategy, low-molecular weight polymer binders were evaluated, namely, PAA(2000) and Jeffamine™. These were also compared to alternative binders, PVDF-HFP, PAN, and PI. Of these, PI emerged as the most promising, exhibiting both strong adhesion and superior capacity retention across a range of C-rates. In contrast, Jeffamine™, PAN, and PVDF-HFP-based electrodes demonstrated relatively poor cycling stability and mechanical performance. These deficiencies were attributed to either insufficient adhesion or inadequate mechanical flexibility to accommodate volume expansion. Moreover, blends of short-chain and long-chain PAA binders were tested, revealing an initially promising electrochemical profile; however, they suffered from capacity fade at higher C-rates, likely due to poor adhesion to the current collector.
To address this issue, a third strategy focused on the design and fabrication of multilayer anodes incorporating an adhesion layer between the copper current collector and the Si electrode. When a PAA-based adhesion layer was used beneath a water-soluble bulk electrode layer of similar composition, solvent blending between the two layers resulted in degraded adhesion and electrochemical performance. However, by using a PVDF adhesion layer, which was processed in NMP and insoluble in the subsequent application of an aqueous PAA layer, this interlayer blending was avoided. The resulting multilayer configuration (PAA(2000) + PAA on PVDF) demonstrated a significantly enhanced average capacity (up to 500 mAh/g higher) and improved adhesion strength compared to its single-layer counterpart or the PAA-on-PAA configuration. This finding confirms that solvent incompatibility can be strategically leveraged to construct mechanically robust multilayer electrodes that preserve layer separation and maintain structural integrity under cycling stress.
Taken together, the results underscore the complex interplay among polymer chain architecture, interfacial adhesion, mechanical compliance, and electrode design in Si-based anodes. While flexibility introduced via chain shortening may aid stress dissipation, sufficient chain entanglement and strong adhesion to the current collector remain essential for cycling stability. Moreover, multilayer electrode designs that decouple adhesion from bulk properties offer a powerful route to optimize both mechanical resilience and electrochemical performance, particularly when solvent compatibility is carefully managed. At this early stage of development, we acknowledge that PVDF-PAA has not yet demonstrated a clear performance advantage over previous neat PAA work. We are not presenting it as a fully developed technology ready to replace existing methods, but rather as a step forward in the ongoing learning and development process.