Investigations of Dongyue Series Perfluorosulfonic Acid Membranes for Applications in Proton Exchange Membrane Fuel Cells (PEMFCs)
Abstract
1. Introduction
2. Materials and Methods
2.1. Membrane Preparation
2.2. Membrane Characterization
2.2.1. Scanning Electron Microscopy (SEM)
2.2.2. Fourier Transform Infrared (FT-IR) Microspectroscopy
2.2.3. Water Uptake (WU) and Swelling Ratio (SR)
2.2.4. Ion Exchange Capacity (IEC)
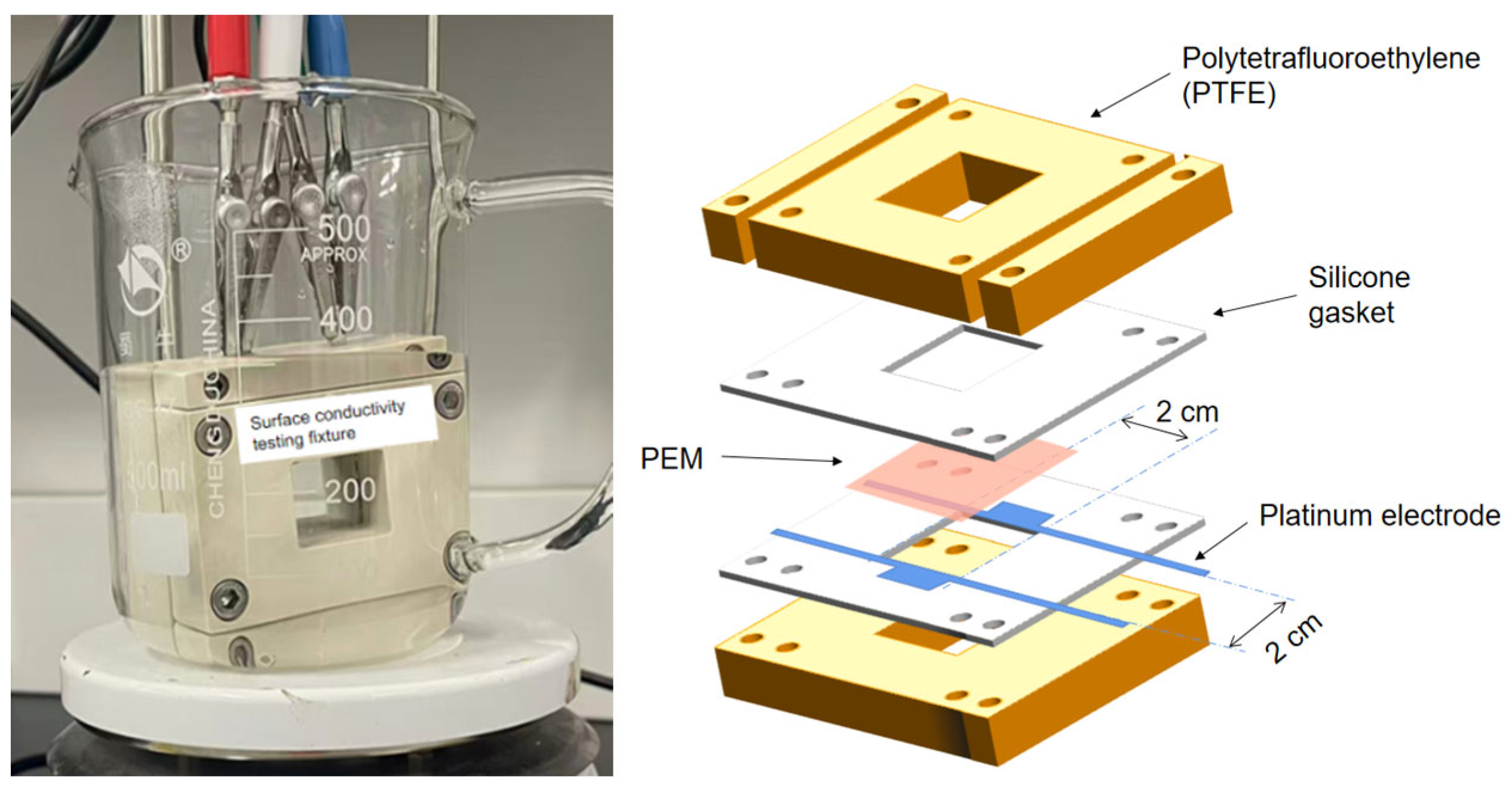
2.2.5. Proton Conductivity
2.2.6. Mechanical Properties
2.2.7. Thermogravimetric Analysis (TGA)
2.2.8. Chemical Stability
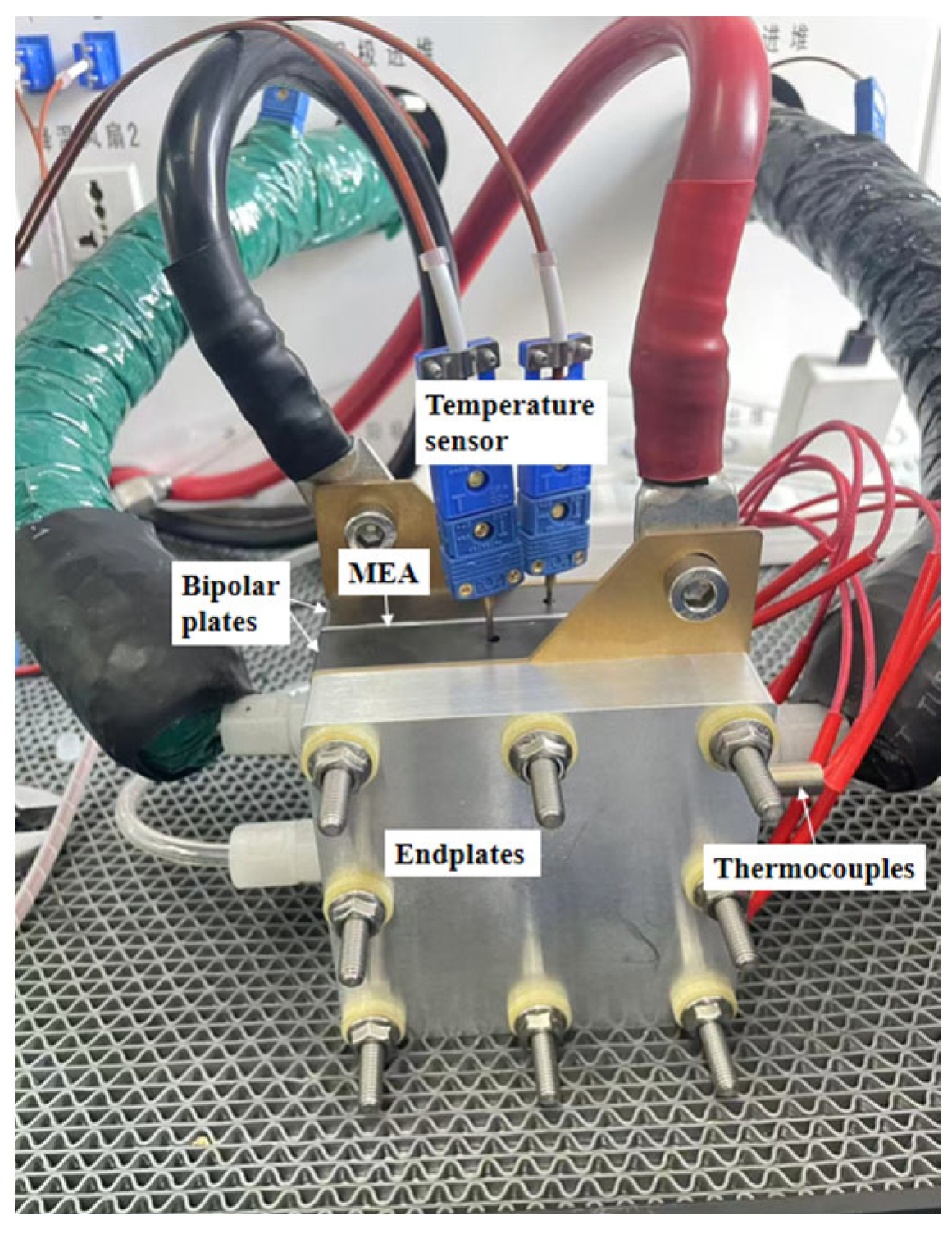
2.3. PEMFC Single-Cell Performance
3. Results and Discussion
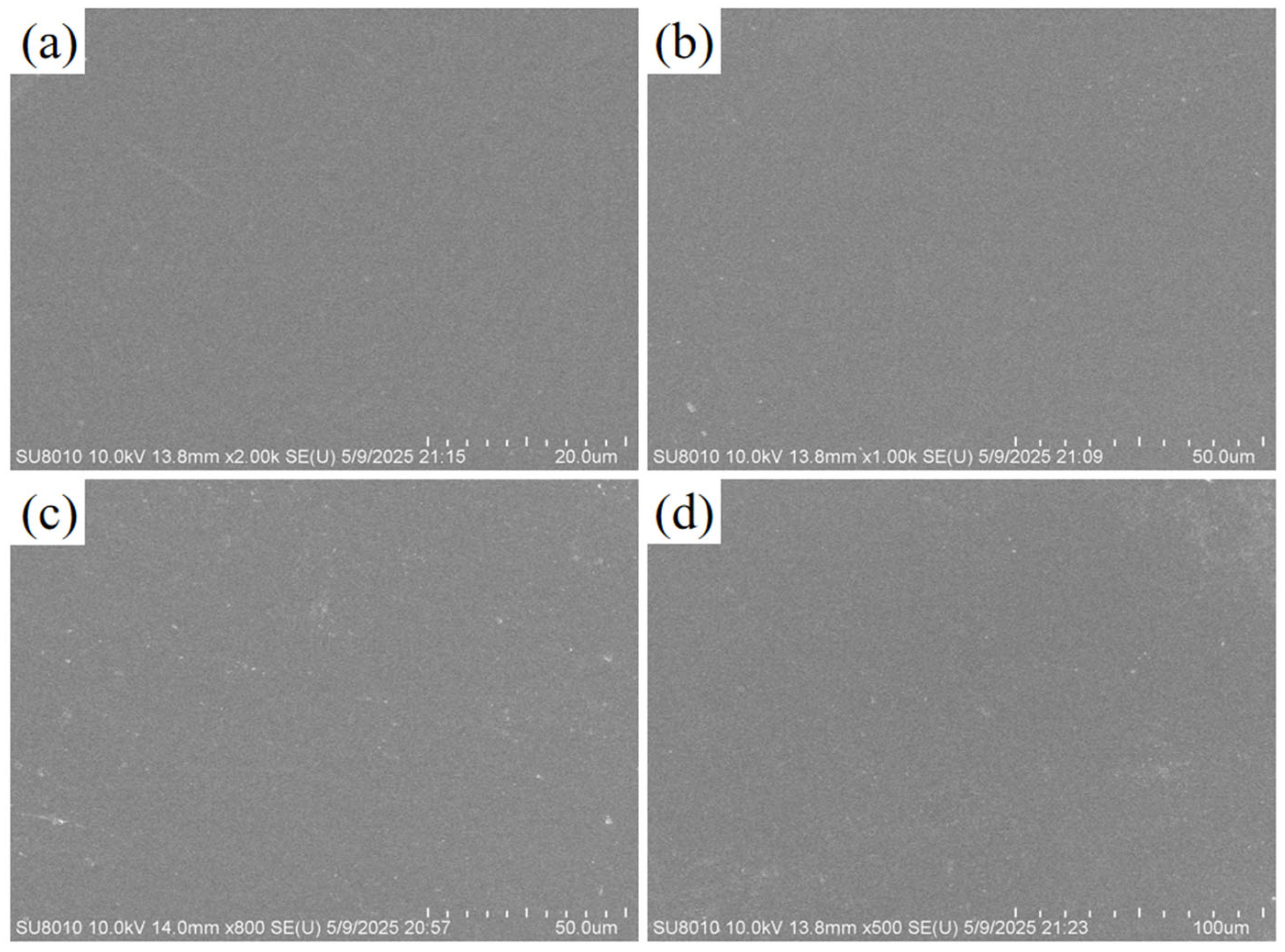
3.1. Morphology of PFSA Membranes
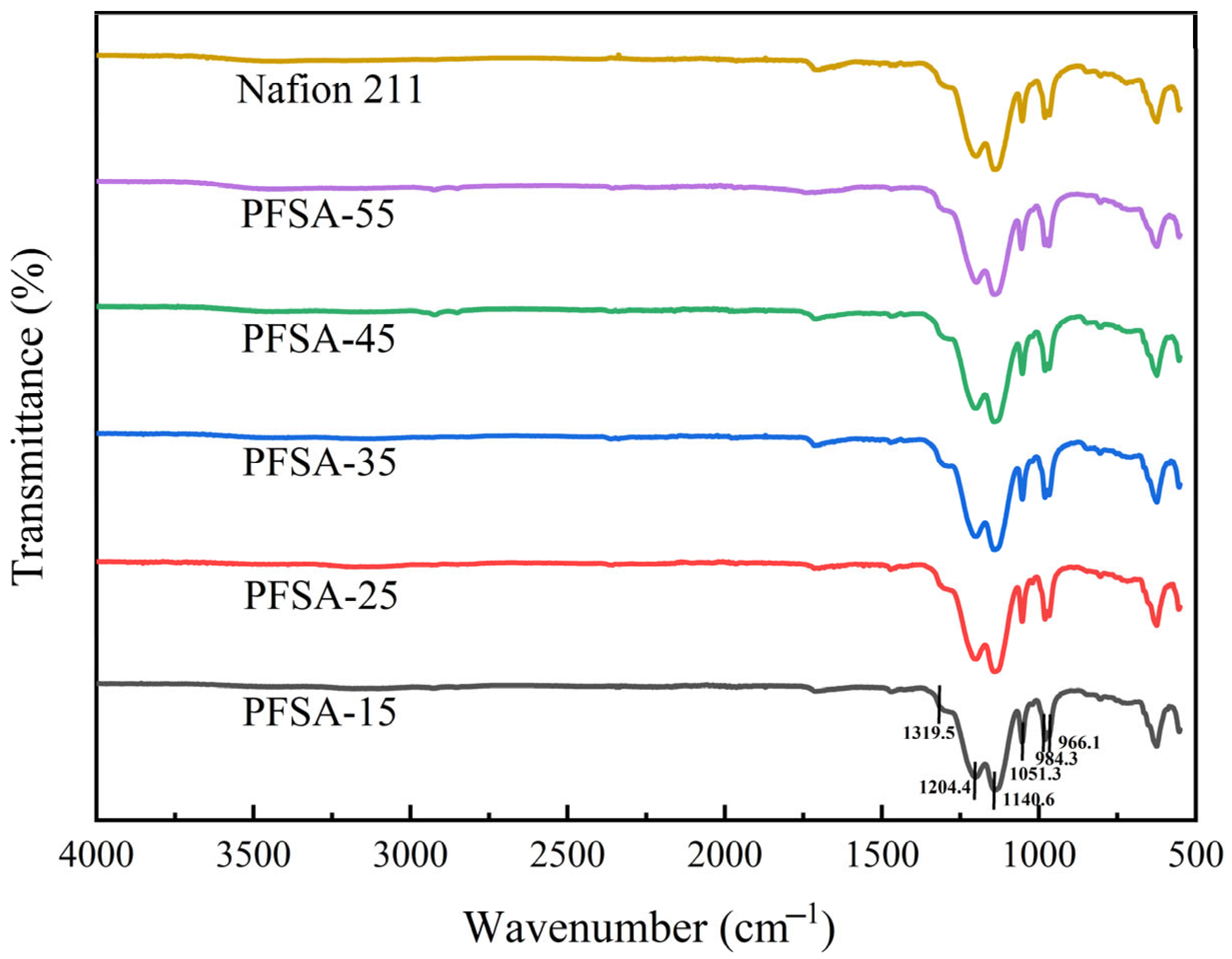
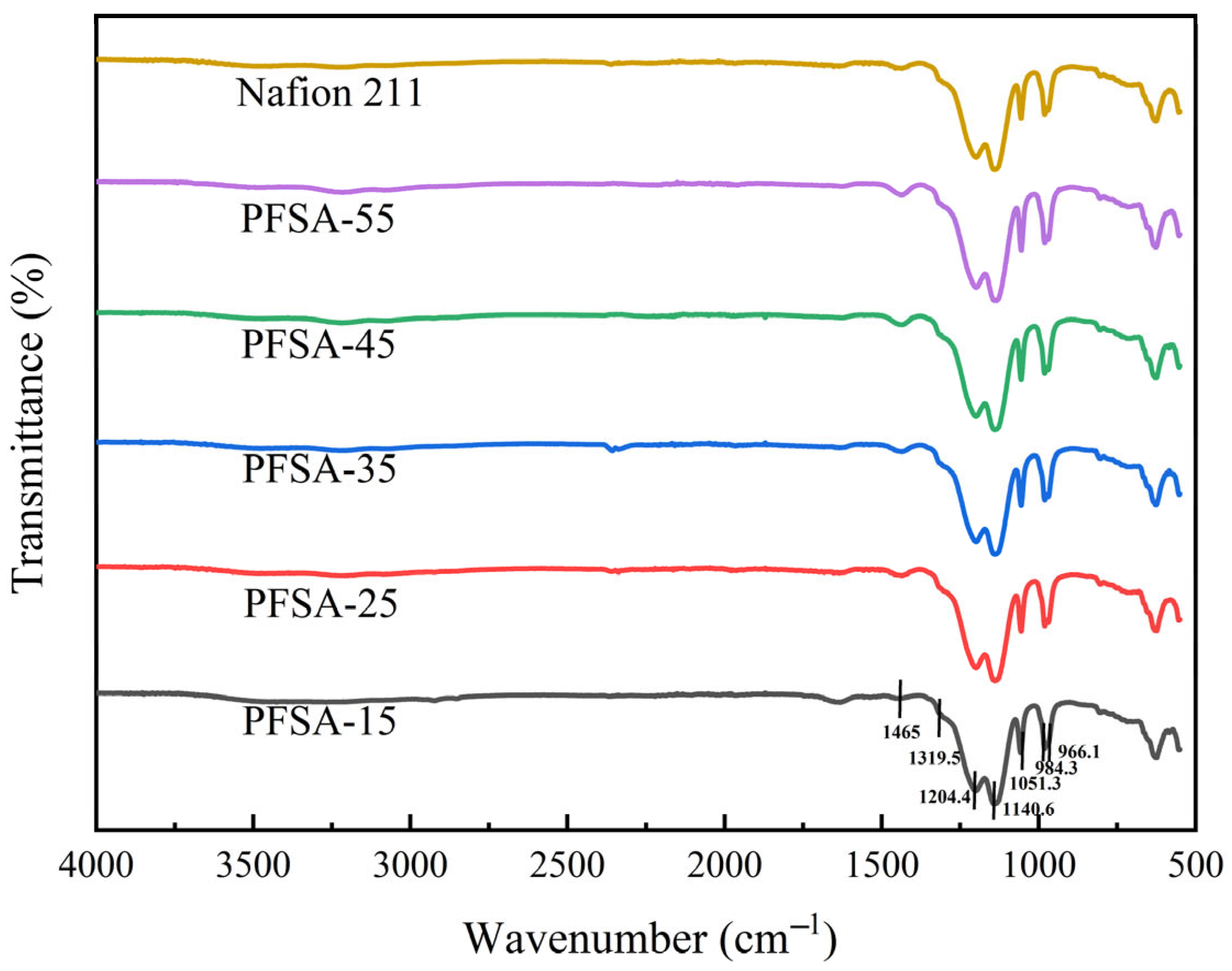
3.2. FT-IR Spectra
3.3. Physicochemical Properties
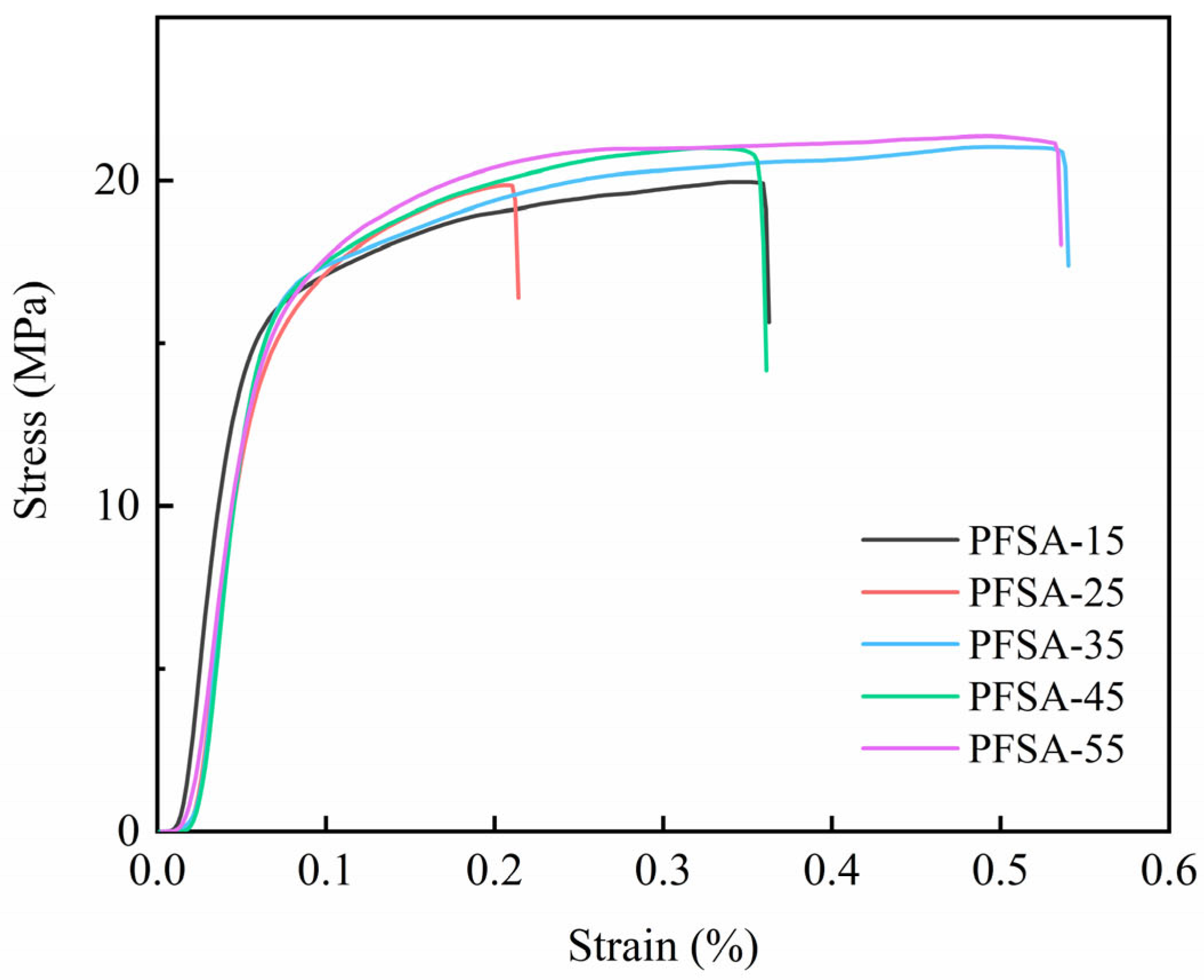
3.4. Mechanical Properties Test Results
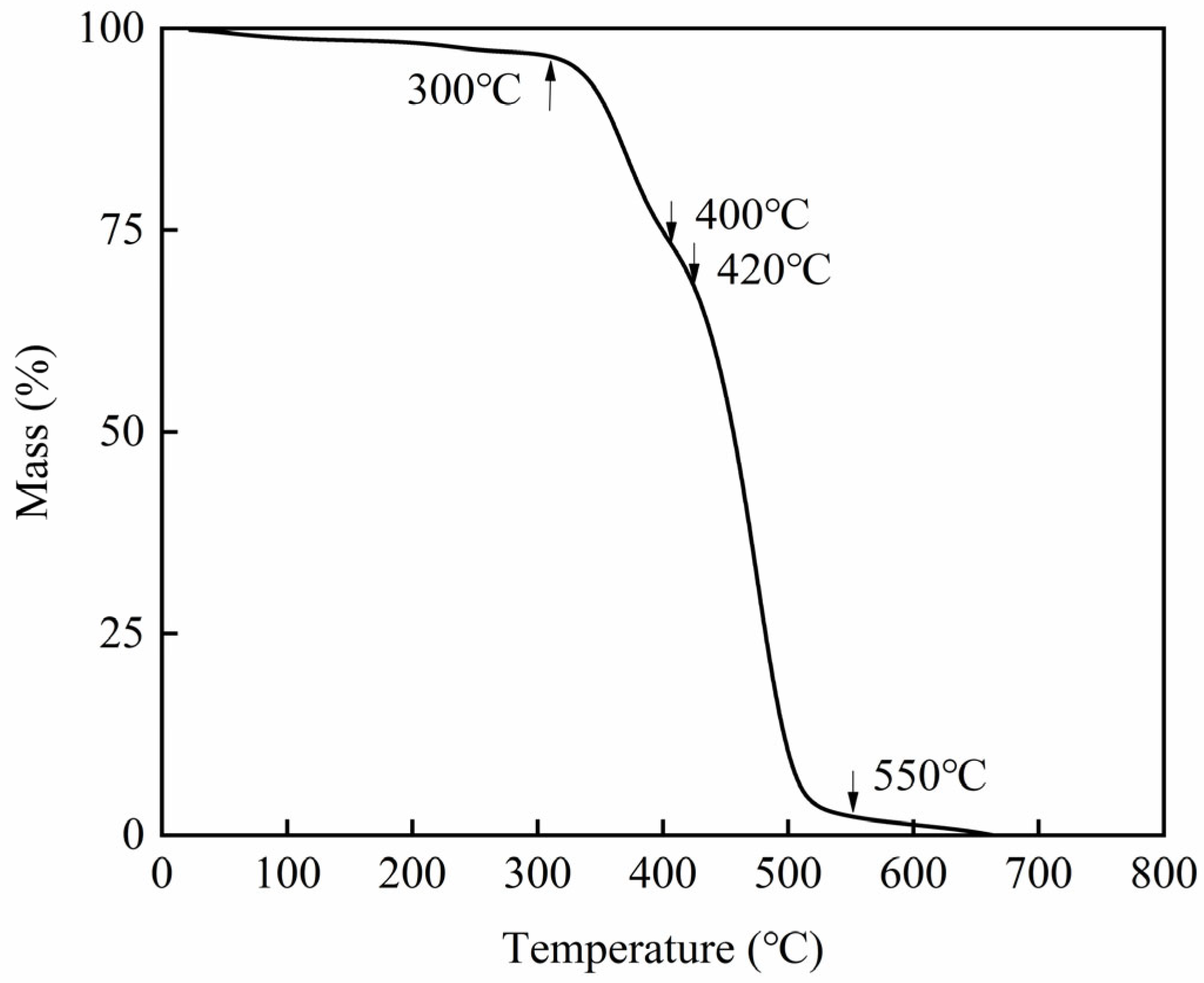
3.5. Thermogravimetric Analysis (TGA) Test Results
3.6. Proton Conductivity Test Results
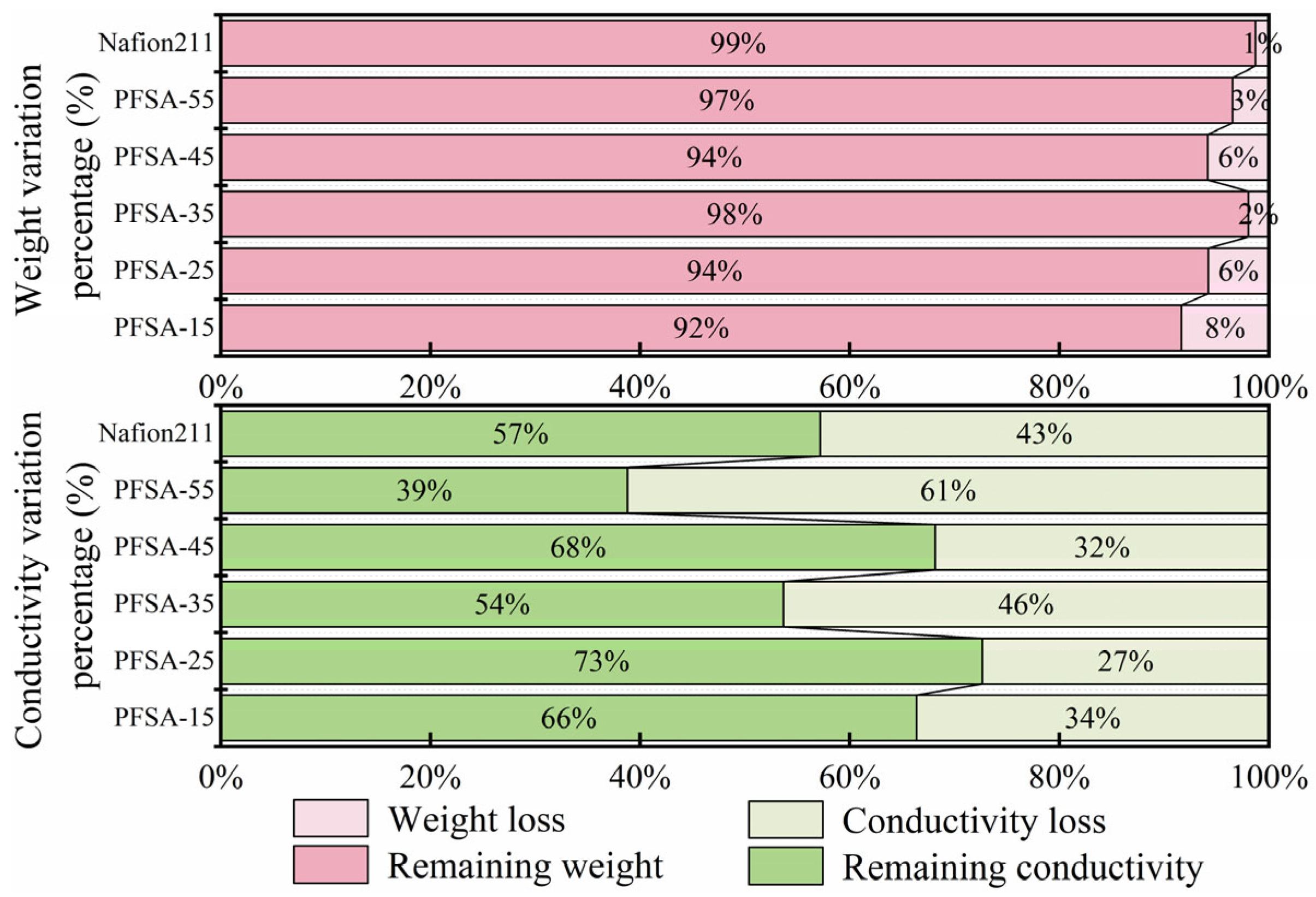
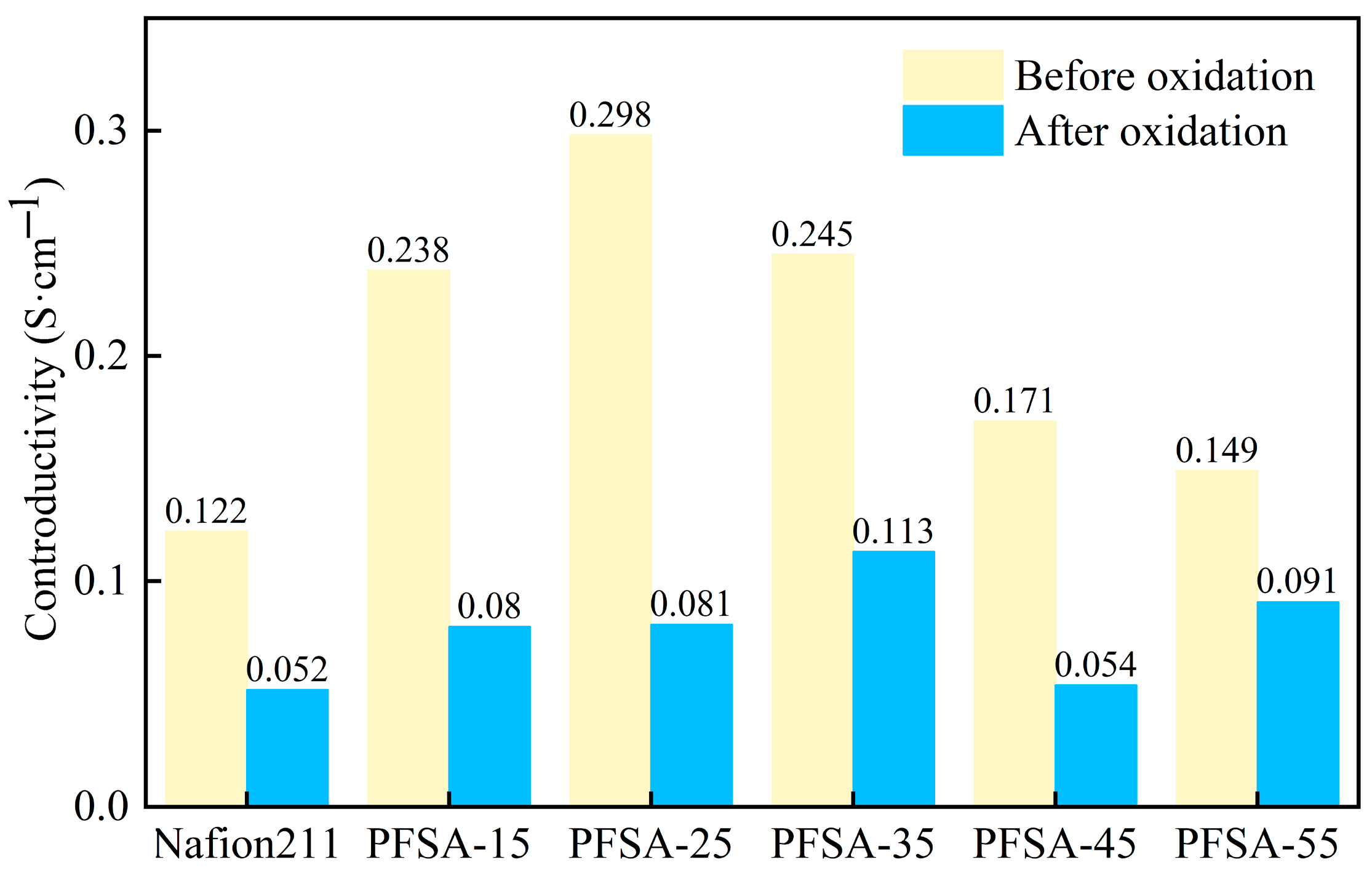
3.7. Oxidation Stability
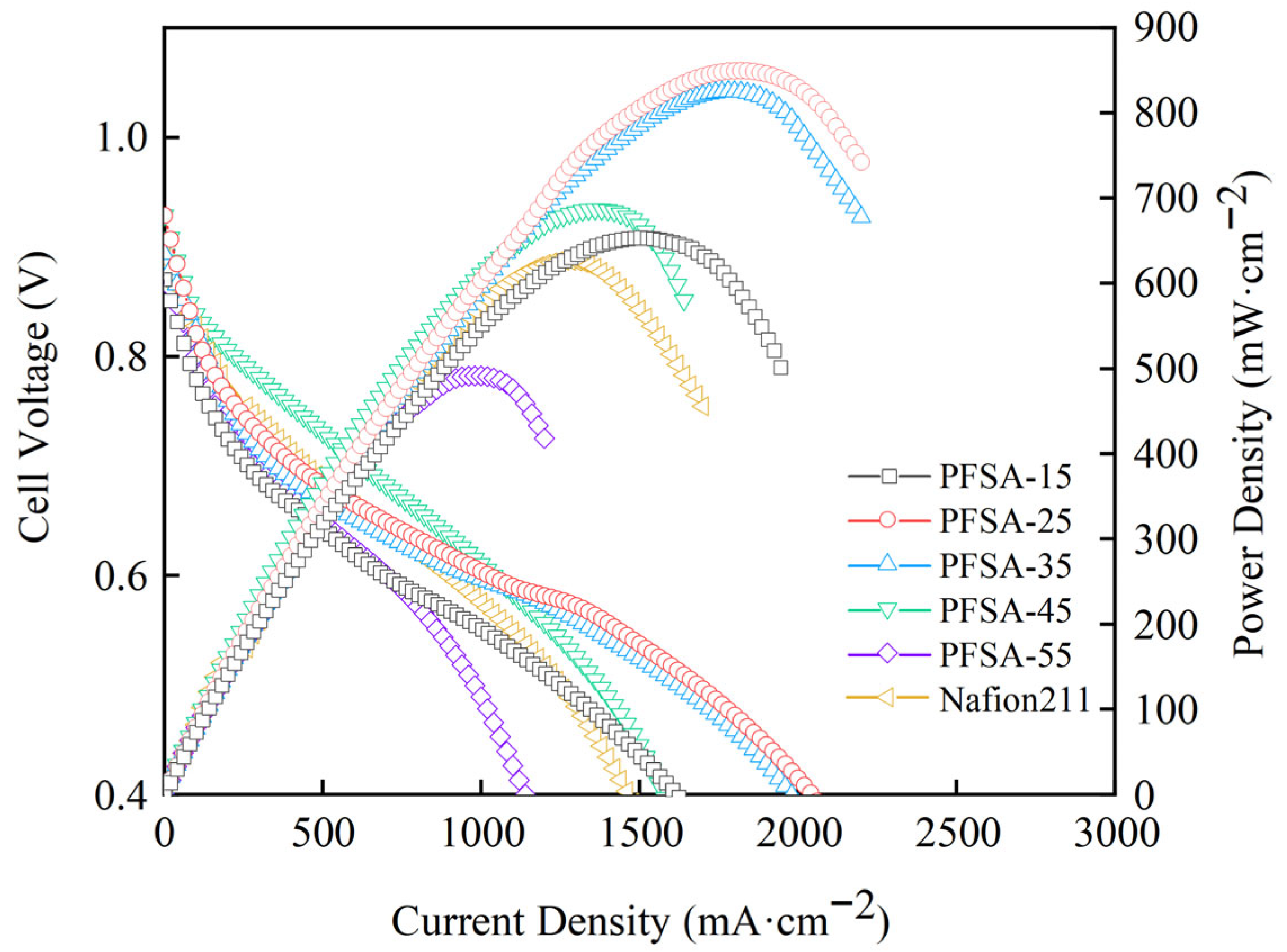
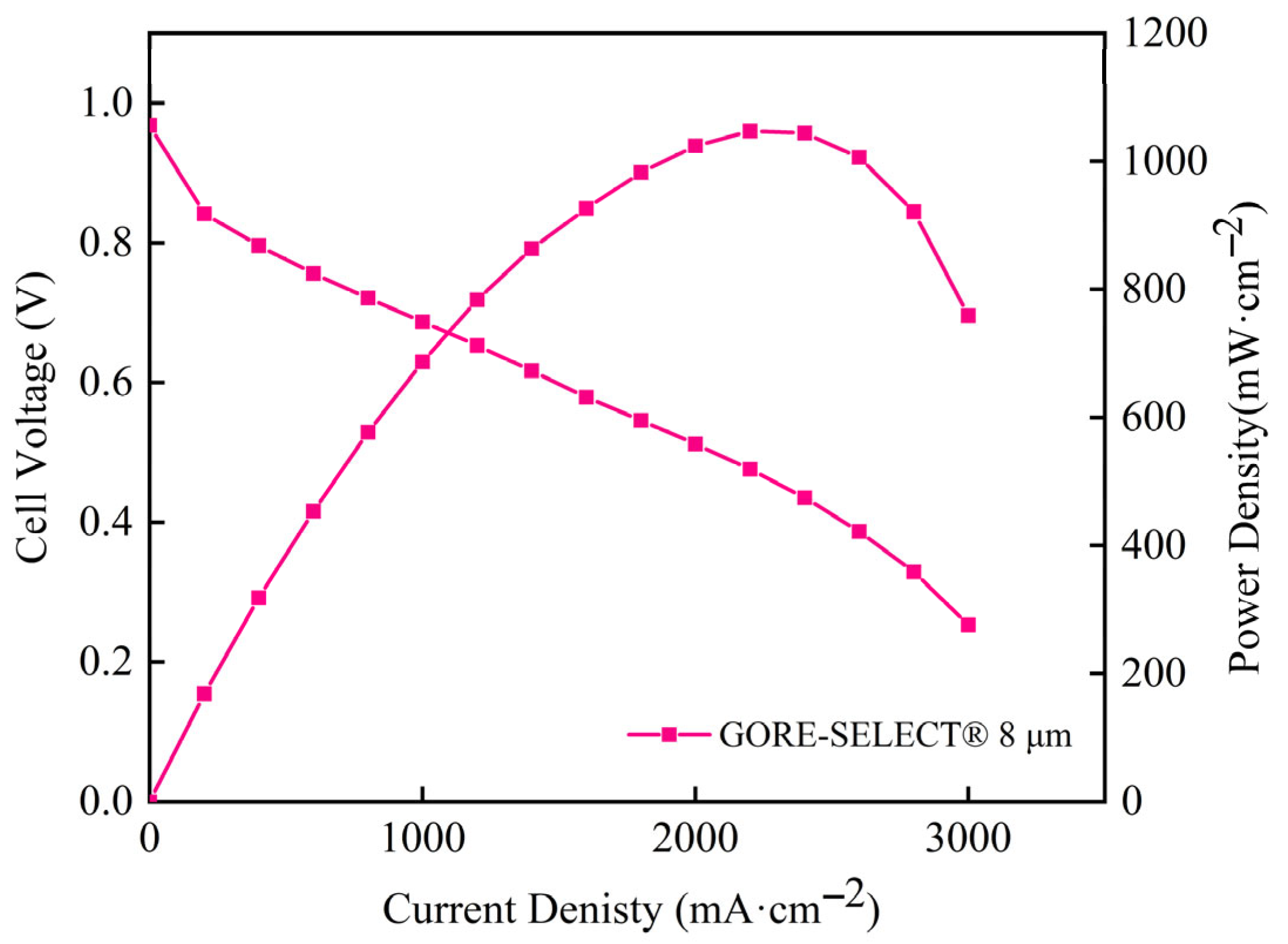
3.8. PEMFC SINGLE Cell Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PFSA | Perfluorosulfonic acid |
| PEMFC | Proton exchange membrane full cell |
| PEM | Proton exchange membrane |
| IEC | Ion exchange capacity |
| DMF | N, N-Dimethylformamide |
| SEM | Scanning electron microscopy |
| FT-IR | Fourier transform infrared |
| WU | Water uptake |
| SR | Swelling ration |
| MEA | Membrane electrode assembly |
| EIS | Electrochemical impedance spectroscopy |
| TGA | Thermogravimetric analysis |
| GDL | Gas diffusion layer |
| GDE | Gas diffusion electrode |
References
- Shafiee, S.; Topal, E. When will fossil fuel reserves be diminished? Energy Policy 2009, 37, 181–189. [Google Scholar] [CrossRef]
- Bach, W. Fossil fuel resources and their impacts on environment and climate. Int. J. Hydrogen Energy 1981, 6, 185–201. [Google Scholar] [CrossRef]
- Li, X.; Ye, T.; Meng, X.; He, D.; Li, L.; Song, K.; Jiang, J.; Sun, C. Advances in the Application of Sulfonated Poly(Ether Ether Ketone) (SPEEK) and Its Organic Composite Membranes for Proton Exchange Membrane Fuel Cells (PEMFCs). Polymers 2024, 16, 2840. [Google Scholar] [CrossRef] [PubMed]
- Peighambardoust, S.J.; Rowshanzamir, R.; Amjadi, R. Review of the proton exchange membranes for fuel cell applications. Int. J. Hydrogen Energy 2010, 35, 9349–9384. [Google Scholar] [CrossRef]
- Wu, C.; Burke, T. Intelligent computer aided optimization on specific power of an OTEC Rankine power plant. Appl. Therm. Eng. 1998, 18, 295–300. [Google Scholar] [CrossRef]
- Ajanovic, A.; Haas, R. Economic prospects and policy framework for hydrogen as fuel in the transport sector. Energy Policy 2018, 123, 280–288. [Google Scholar] [CrossRef]
- Hames, Y.; Kaya, K.; Baltacioglu, E.; Turksoy, A. Analysis of the control strategies for fuel saving in the hydrogen fuel cell vehicles. Int. J. Hydrogen Energy 2018, 43, 10810–10821. [Google Scholar] [CrossRef]
- Meng, X.; Mei, J.; Tang, X.; Jiang, J.; Sun, C.; Song, K. The degradation prediction of proton exchange membrane fuel cell performance based on a transformer model. Energies 2024, 17, 3050. [Google Scholar] [CrossRef]
- Li, H.Y.; Liu, Y.L. Nafion-functionalized electrospun poly(vinylidene fluoride) (PVDF) nanofibers for high performance proton exchange membranes in fuel cells. J. Mater. Chem. A 2014, 2, 3783–3793. [Google Scholar] [CrossRef]
- Devanathan, R. Recent developments in proton exchange membranes for fuel cells. Energy Environ. Sci. 2008, 1, 101–119. [Google Scholar] [CrossRef]
- Park, J.; Oh, H.; Ha, T.; Lee, Y.I.; Min, K. A review of the gas diffusion layer in proton exchange membrane fuel cells: Durability and degradation. Appl. Energy 2015, 155, 866–880. [Google Scholar] [CrossRef]
- Farooqui, U.R.; Ahmad, A.L.; Hamid, N.A. Graphene oxide: A promising membrane material for fuel cells. Renew. Sustain. Energy Rev. 2018, 82 Pt 1, 714–733. [Google Scholar] [CrossRef]
- Peron, J.; Mani, A.; Zhao, X.; Edwards, D.; Adachi, M.; Soboleva, T.; Shi, Z.; Xie, Z.; Navessin, T. Steven Holdcroft Properties of Nafion NR-211 membranes for PEMFCs. J. Membr. Sci. 2010, 356, 44–51. [Google Scholar] [CrossRef]
- Şengül, E.; Erdener, H.; Akay, R.G.; Yücel, H.; Baç, N.; Eroğlu, İ. Effects of sulfonated polyether-etherketone (SPEEK) and composite membranes on the proton exchange membrane fuel cell (PEMFC) performance. Int. J. Hydrogen Energy 2009, 34, 4645–4652. [Google Scholar] [CrossRef]
- Teixeira, F.C.; Teixeira, A.P.; Rangel, C.M. New Modified SPEEK-Based Proton Exchange Membranes. Polymers 2025, 17, 1646. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Negro, E.; Vezzù, K.; Pagot, G.; Cavinato, G.; Nale, A.; Bang, Y.H.; Di Noto, V. Hybrid inorganic-organic proton-conducting membranes based on SPEEK doped with WO3 nanoparticles for application in vanadium redox flow batteries. Electrochim. Acta 2019, 309, 311–325. [Google Scholar] [CrossRef]
- Yu, J.-r.; Yi, B.-l.; Han, M. Influence of the Nation membrane thickness on the performance of proton exchange membrane fuel cells. Chin. J. Power Sources 2001, 25, 384–386. [Google Scholar]
- Dimitrova, P.; Friedrich, K.A.; Vogt, B.; Stimming, U. Transport properties of ionomer composite membranes for direct methanol fuel cells. J. Electroanal. Chem. 2002, 532, 75–83. [Google Scholar] [CrossRef]
- Silva, R.; De Francesco, M.; Pozio, A. Tangential and normal conductivities of Nafion® membranes used in polymer electrolyte fuel cells. J. Power Sources 2004, 134, 18–26. [Google Scholar] [CrossRef]
- Tsampas, M.N.; Pikos, A.; Brosda, S.; Katsaounis, A.; Vayenas, C.G. The effect of membrane thickness on the conductivity of Nafion. Electrochim. Acta 2006, 51, 2743–2755. [Google Scholar] [CrossRef]
- Büchi, F.N.; Scherer, G.G. Investigation of the transversal water profile in Nafion membranes in polymer electrolyte fuel cells. J. Electrochem. Soc. 2001, 148, A183. [Google Scholar] [CrossRef]
- Karimi, M.B.; Mohammadi, F.; Hooshyari, K. Recent approaches to improve Nafion performance for fuel cell applications: A review. Int. J. Hydrogen Energy 2019, 44, 28919–28938. [Google Scholar] [CrossRef]
- Yin, C.; Li, J.; Zhou, Y.; Zhang, H.; Fang, P.; He, C. Phase separation and development of proton transport pathways in metal oxide nanoparticle/nafion composite membranes during water uptake. J. Phys. Chem. C 2018, 122, 9710–9717. [Google Scholar] [CrossRef]
- Di Noto, V.; Boaretto, N.; Negro, E.; Stallworth, P.E.; Lavina, S.; Giffin, G.A.; Greenbaum, S.G. Inorganic–organic membranes based on Nafion, [(ZrO2)·(HfO2)0.25] and [(SiO2)·(HfO2)0.28] nanoparticles. Part II: Relaxations and conductivity mechanism. Int. J. Hydrogen Energy 2012, 37, 6215–6227. [Google Scholar] [CrossRef]
- Vittadello, M.; Negro, E.; Lavina, S.; Pace, G.; Safari, A.; Noto, V.D. Vibrational studies and properties of hybrid inorganic− organic proton conducting membranes based on Nafion and hafnium oxide nanoparticles. J. Phys. Chem. B 2008, 112, 16590–16600. [Google Scholar] [CrossRef] [PubMed]
- Vinothkannan, M.; Ramakrishnan, S.; Kim, A.R.; Lee, H.K.; Yoo, D.J. Ceria Stabilized by Titanium Carbide as a Sustainable Filler in the Nafion Matrix Improves the Mechanical Integrity, Electrochemical Durability, and Hydrogen Impermeability of Proton-Exchange Membrane Fuel Cells: Effects of the Filler Content. ACS Appl. Mater. Interfaces 2020, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Negro, E.; Nale, A.; Pagot, G.; Vezzù, K.; Zawodzinski, T.A.; Meda, L.; Gambaro, C.; Di Noto, V. An efficient barrier toward vanadium crossover in redox flow batteries: The bilayer [Nafion/(WO3)x] hybrid inorganic-organic membrane. Electrochim. Acta 2021, 378, 138133. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Guo, G.-B.; An, S.-L.; Hao, Y.; Zhang, D.; Yan, K.-B. Synthesis and properties of proton exchange membranes via single-step grafting PSBMA onto PVDF modified by TMAH. Acta Phys.-Chim. Sin. 2015, 31, 1905–1913. [Google Scholar] [CrossRef]
- Dai, W.; Shen, Y.; Li, Z.; Yu, L.; Xi, J.; Qiu, X. SPEEK/Graphene oxide nanocomposite membranes with superior cyclability for highly efficient vanadium redox flow battery. J. Mater. Chem. A 2014, 2, 12423–12432. [Google Scholar] [CrossRef]
- Meng, X.; Sun, C.; Mei, J.; Tang, X.; Hasanien, H.M.; Jiang, J.; Fan, F.; Song, K. Fuel cell life prediction considering the recovery phenomenon of reversible voltage loss. J. Power Sources 2025, 625, 235634. [Google Scholar] [CrossRef]
- Sigwadi, R.; Dhlamini, M.; Mokrani, T.; Ṋemavhola, F.; Nonjola, P.; Msomi, P. The proton conductivity and mechanical properties of Nafion®/ZrP nanocomposite membrane. Heliyon 2019, 5, e02240. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.; Han, W.; Liu, B.; Zhai, M.; Li, N.; Wang, Z.; Zhao, J. Multifunctional Laser-Induced Graphene-Based Microfluidic Chip for High-Performance Oocyte Cryopreservation with Low Concentration of Cryoprotectants. Adv. Healthc. Mater. 2024, 13, 2400981. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.N.; Lee, D.C.; Park, S.H.; Kim, W.J. Preparation of Nafion/various Pt-containing SiO2 composite membranes sulfonated via different sources of sulfonic group and their application in self-humidifying PEMFC—ScienceDirect. J. Membr. Sci. 2013, 443, 210–218. [Google Scholar] [CrossRef]
- Arico’, A.S.; Baglio, V.; Blasi, A.D.; Antonucci, V. FTIR spectroscopic investigation of inorganic fillers for composite DMFC membranes. Electrochem. Commun. 2003, 5, 862–866. [Google Scholar] [CrossRef]
- Di Noto, V.; Gliubizzi, R.; Negro, E.; Pace, G. Effect of SiO2 on relaxation phenomena and mechanism of ion conductivity of [Nafion/(SiO2)x] composite membranes. J. Phys. Chem. B 2006, 110, 24972–24986. [Google Scholar] [CrossRef] [PubMed]
- Laporta, M.; Pegoraro, M.; Zanderighi, L. Perfluorosulfonated membrane (Nafion): FT-IR study of the state of water with increasing humidity. Phys. Chem. Chem. Phys. 1999, 1, 4619–4628. [Google Scholar] [CrossRef]
- Kinumoto, T.; Inaba, M.; Nakayama, Y.; Ogata, K.; Umebayashi, R.; Tasaka, A.; Iriyama, Y.; Abe, T.; Ogumi, Z. Durability of perfluorinated ionomer membrane against hydrogen peroxide. J. Power Sources 2006, 158, 1222–1228. [Google Scholar] [CrossRef]
- Gruger, A.; Régis, A.; Schmatko, T.; Colomban, P. Nanostructure of Nafion® membranes at different states of hydration: An IR and Raman study. Vib. Spectrosc. 2001, 26, 215–225. [Google Scholar] [CrossRef]
- Shin, S.-H.; Nur, P.J.; Kodir, A.; Kwak, D.-H.; Lee, H.; Shin, D.; Bae, B. Improving the mechanical durability of short-side-chain perfluorinated polymer electrolyte membranes by annealing and physical reinforcement. ACS Omega 2019, 4, 19153–19163. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Yang, M.; Sun, C.; Xu, S. Liquid water characteristics in the compressed gradient porosity gas diffusion layer of proton exchange membrane fuel cells using the Lattice Boltzmann Method. Energies 2023, 16, 6010. [Google Scholar] [CrossRef]
- Wang, G.; Kang, J.; Yang, S.; Lu, M.; Wei, H. Influence of structure construction on water uptake, swelling, and oxidation stability of proton exchange membranes. Int. J. Hydrogen Energy 2024, 50, 279–311. [Google Scholar] [CrossRef]
- Safronova, E.Y.; Voropaeva, D.Y.; Lysova, A.A.; Korchagin, O.V.; Bogdanovskaya, V.A.; Yaroslavtsev, A.B. On the properties of Nafion membranes recast from dispersion in N-Methyl-2-Pyrrolidone. Polymers 2022, 14, 5275. [Google Scholar] [CrossRef] [PubMed]
- Yazili, D.; Marini, E.; Saatkamp, T.; Münchinger, A.; de Wild, T.; Gubler, L.; Titvinidze, G.; Schuster, M.; Schare, C.; Jörissen, L. Sulfonated Poly (Phenylene sulfone) blend membranes finding their way into proton exchange membrane fuel cells. J. Power Sources 2023, 563, 232791. [Google Scholar] [CrossRef]




| Samples | Thickness (μm) | WU (%) | Length SR (%) | Thickness SR (%) | IEC (mmol g−1) | Proton Conductivity at 80 °C (S cm−1) |
|---|---|---|---|---|---|---|
| PFSA-15 | 15 ± 2 | 28.57 ± 0.12 | 14.08 ± 0.21 | 23.53 ± 2.95 | 1.016 ± 0.005 | 0.326 |
| PFSA-25 | 25 ± 2 | 29.03 ± 0.13 | 18.55 ± 0.08 | 27.59 ± 1.73 | 1.006 ± 0.003 | 0.371 |
| PFSA-35 | 35 ± 2 | 29.17 ± 0.10 | 13.65 ± 0.07 | 30.56 ± 1.40 | 1.021 ± 0.003 | 0.311 |
| PFSA-45 | 45 ± 2 | 30.00 ± 0.08 | 11.69 ± 0.17 | 38.78 ± 1.05 | 1.022 ± 0.001 | 0.204 |
| PFSA-55 | 55 ± 2 | 32.03 ± 0.11 | 16.36 ± 0.09 | 40.35 ± 1.25 | 1.013 ± 0.004 | 0.190 |
| Nafion 211 | 25 | 11.19 ± 0.09 | 3.35 ± 0.25 | 16.00 ± 2.00 | 0.813 ± 0.007 | 0.155 |
| Samples | Tensile Strength (MPa) | Elongation Percentage (%) | Maximum Force (N) | Breaking Force (N) |
|---|---|---|---|---|
| PFSA-15 | 19.96 ± 0.19 | 36.26 ± 0.80 | 7.19 ± 0.05 | 5.64 ± 0.13 |
| PFSA-25 | 19.69 ± 0.17 | 21.40 ± 0.85 | 9.78 ± 0.01 | 8.08 ± 0.10 |
| PFSA-35 | 21.03 ± 0.14 | 54.02 ± 0.88 | 14.72 ± 0.02 | 12.16 ± 0.07 |
| PFSA-45 | 21.00 ± 0.15 | 36.11 ± 0.90 | 18.90 ± 0.04 | 12.75 ± 0.12 |
| PFSA-55 | 21.36 ± 0.16 | 53.59 ± 1.05 | 23.50 ± 0.03 | 19.83 ± 0.11 |
| Recast Nafion 50 μm | 9.6 ± 0.2 [42] | 41 ± 1 [42] | -- | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, G.; Li, X.; Liu, M.; Grigoriev, S.A.; Tolj, I.; Shen, J.; Yue, C.; Sun, C. Investigations of Dongyue Series Perfluorosulfonic Acid Membranes for Applications in Proton Exchange Membrane Fuel Cells (PEMFCs). Batteries 2025, 11, 277. https://doi.org/10.3390/batteries11070277
Meng G, Li X, Liu M, Grigoriev SA, Tolj I, Shen J, Yue C, Sun C. Investigations of Dongyue Series Perfluorosulfonic Acid Membranes for Applications in Proton Exchange Membrane Fuel Cells (PEMFCs). Batteries. 2025; 11(7):277. https://doi.org/10.3390/batteries11070277
Chicago/Turabian StyleMeng, Ge, Xiang Li, Mengjie Liu, Sergey A. Grigoriev, Ivan Tolj, Jiaqi Shen, Chaonan Yue, and Chuanyu Sun. 2025. "Investigations of Dongyue Series Perfluorosulfonic Acid Membranes for Applications in Proton Exchange Membrane Fuel Cells (PEMFCs)" Batteries 11, no. 7: 277. https://doi.org/10.3390/batteries11070277
APA StyleMeng, G., Li, X., Liu, M., Grigoriev, S. A., Tolj, I., Shen, J., Yue, C., & Sun, C. (2025). Investigations of Dongyue Series Perfluorosulfonic Acid Membranes for Applications in Proton Exchange Membrane Fuel Cells (PEMFCs). Batteries, 11(7), 277. https://doi.org/10.3390/batteries11070277











