Optimizing Porous Transport Layers in PEM Water Electrolyzers: A 1D Two-Phase Model
Abstract
1. Introduction
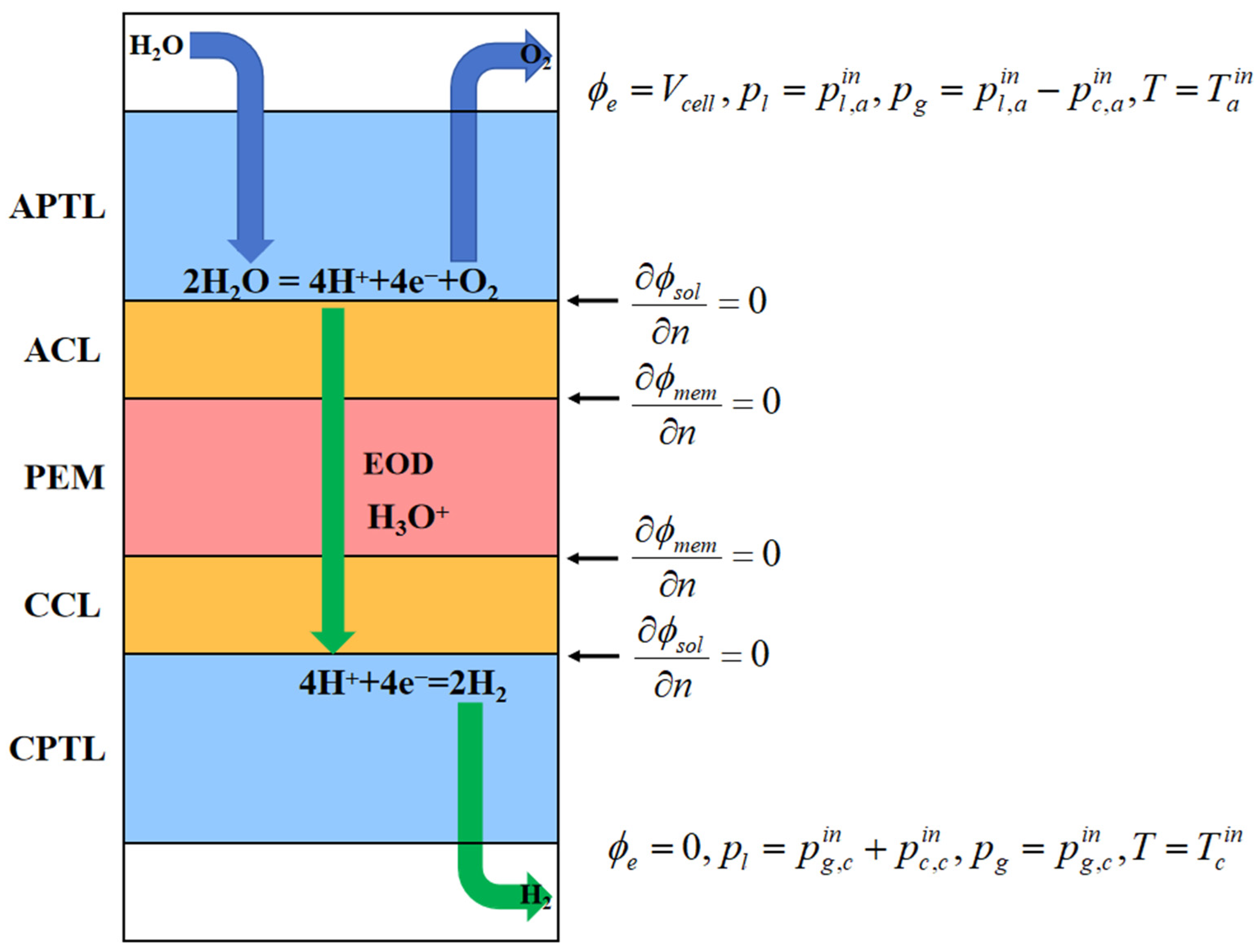
2. Model Description
2.1. Assumptions
- Steady-state operation: the system is modeled under equilibrium conditions with ideal gas behavior for all gaseous species, while the membrane electrode assembly (MEA) is treated as macroscopically homogeneous.
- Unidirectional transport: mass transfer is constrained to the through-plane direction (x-direction), neglecting in-plane gradients.
- Membrane selectivity: the proton exchange membrane is considered impermeable to gas species (O2, H2) while maintaining perfect proton conductivity.
- Simplified physics: Gravitational effects are neglected due to dominant capillary and pressure-driven flows. Interfacial contact resistances between adjacent layers are not considered.
- Electrical insulation: all structural components are assumed to be perfectly insulated, preventing current leakage.
2.2. Analytical Solution
2.2.1. Electrochemistry
2.2.2. Two-Phase Flow
Mass Conservation
- and denote the evaporation and condensation rate coefficients, respectively.
- is the conversion rate of liquid water to dissolved water, which is driven by the difference between the equilibrium water content and the actual water content of the membrane electrode:where
- and are the mass transfer coefficients of adsorption and desorption, respectively.
- denotes the equilibrium water content and is the saturated vapor pressure.
Energy Conservation
2.2.3. Dissolved Water
2.2.4. Overpotential Analysis
2.3. Calculation Implementation
3. Discussion of Results
3.1. Model Validation
3.2. Influence of the Structure of PTL
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PEMWE | Proton exchange membrane water electrolyzer |
| PTL | Porous transport layer |
| MEA | Membrane electrode assembly |
| CL | Catalyst layer |
| EOD | Electro-osmotic drag |
References
- Chi, J.; Yu, H. Water electrolysis based on renewable energy for hydrogen production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Wang, J.; Wen, J.; Wang, J.; Yang, B. Water electrolyzer operation scheduling for green hydrogen production: A review. Renew. Sustain. Energy Rev. 2024, 203, 114779. [Google Scholar] [CrossRef]
- Hughes, J.; Clipsham, J.; Chavushoglu, H.; Rowley-Neale, S.J. Polymer electrolyte electrolysis: A review of the activity and stability of non-precious metal hydrogen evolution reaction and oxygen evolution reaction catalysts. Renew. Sustain. Energy Rev. 2021, 139, 110709. [Google Scholar] [CrossRef]
- Shirvanian, P.; Berkel, F. Novel components in Proton Exchange Membrane Water Electrolyzers (PEMWE): Status, challenges and future needs. A mini review. Electrochem. Commun. 2020, 114, 106704. [Google Scholar] [CrossRef]
- Zhao, C.; Yuan, S.; Cheng, X.; Shen, S.; Zhan, N. Agglomerate Engineering to Boost PEM Water Electrolyzer Performance. Adv. Energy Mater. 2024, 14, 2401588. [Google Scholar] [CrossRef]
- Tao, L.; Lv, F.; Wang, D.; Luo, H.; Lin, F. Mass-efficient catalyst layer of hierarchical sub-nanosheets on nanowire for practical proton exchange membrane electrolyzer. Joule 2024, 8, 450–460. [Google Scholar] [CrossRef]
- Wang, H.; Gu, X.; Zheng, X.; Pan, H.; Zhu, J.; Chen, S. Disentangling the size-dependent geometric and electronic effects of palladium nanocatalysts beyond selectivity. Sci. Adv. 2019, 5, 6413. [Google Scholar] [CrossRef]
- Wu, T.; Han, M.; Xu, Z. Size Effects of Electrocatalysts: More Than a Variation of Surface Area. ACS Nano. 2022, 16, 8531–8539. [Google Scholar] [CrossRef]
- Hao, S.; Sheng, H.; Liu, M.; Huang, J.; Zheng, G.; Zhang, F. Torsion strained iridium oxide for efficient acidic water oxidation in proton exchange membrane electrolyzers. Nat. Nanotechnol. 2021, 16, 1371–1377. [Google Scholar] [CrossRef]
- Wang, K.; Mao, R.; Liu, R.; Zhang, J.; Zhao, H.; Ran, W. Intentional corrosion-induced reconstruction of defective NiFe layered double hydroxide boosts electrocatalytic nitrate reduction to ammonia. Nat. Water 2023, 1, 1068–1078. [Google Scholar] [CrossRef]
- Xie, Z.; Liang, X.; Kang, Z.; Zou, Y.; Wang, X. High-Porosity, Layered Iridium Oxide as an Efficient, Durable Anode Catalyst for Water Splitting. CCS Chem. 2025, 7, 216–228. [Google Scholar] [CrossRef]
- Ge, S.; Xie, R.; Huang, B.; Zhang, Z.; Liu, H.; Kang, X. A robust chromium–iridium oxide catalyst for high-current–density acidic oxygen evolution in proton exchange membrane electrolyzers. Energy Environ. Sci. 2023, 16, 3734–3742. [Google Scholar] [CrossRef]
- Hegge, F.; Lombeck, F.; Ortiz, E.; Bohn, L.; Holst, M.; Kroschel, M. Vierrath. Efficient and Stable Low Iridium Loaded Anodes for PEM Water Electrolysis Made Possible by Nanofiber Interlayers. ACS Appl. Energy Mater. 2020, 3, 8276–8284. [Google Scholar] [CrossRef]
- Maier, M.; Smith, K.; Dodwell, J.; Hinds, G.; Shearing, P. Mass transport in PEM water electrolysers: A review. Int. J. Hydrogen Energy 2022, 47, 30–56. [Google Scholar] [CrossRef]
- Toghyani, S.; Afshari, E.; Baniasadi, E.; Atyabi, S. Thermal and electrochemical analysis of different flow field patterns in a PEM electrolyzer. Electrochim. Acta 2018, 267, 234–245. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Du, S.; Zhao, C. Multiphase flow dynamics in metal foam proton exchange membrane fuel cell. Renew. Energy 2024, 226, 120489. [Google Scholar] [CrossRef]
- Dang, D.; Zhou, B. Numerical analysis of bubble behavior in proton exchange membrane water electrolyzer flow field with serpentine channel. Int. J. Hydrogen Energy 2024, 88, 688–701. [Google Scholar] [CrossRef]
- Bhaskaran, S.; Pandey, D.; Surasani, V.; Tsotsas, E. LBM studies at pore scale for graded anodic porous transport layer (PTL) of PEM water electrolyzer. Int. J. Hydrogen Energy 2022, 47, 31551–31565. [Google Scholar] [CrossRef]
- Omrani, R.; Shabani, B. Gas diffusion layer modifications and treatments for improving the performance of proton exchange membrane fuel cells and electrolysers: A review. Int. J. Hydrogen Energy 2017, 42, 28515–28536. [Google Scholar] [CrossRef]
- Sinyakov, M.; Mensharapov, R.; Ivanov, B. Study of the water electrolyzer with proton exchange membrane performance based on Ti current collectors charged with hydrogen. Electroanalysis 2025, 37, e202400091. [Google Scholar] [CrossRef]
- Pushkarev, A.; Pushkareva, I.; Solovyev, M. On the influence of porous transport layers parameters on the performances of polymer electrolyte membrane water electrolysis cells. Electrochim. Acta 2021, 399, 139436. [Google Scholar] [CrossRef]
- Lin, R.; Huo, J.; Cai, X.; Lan, S.; Hao, Z. Numerical study of the effects of wettability and hierarchical porosity on oxygen transport within the porous transport layer of proton exchange membrane electrolyzers. J. Power Sources 2024, 614, 235030. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, Z.; Jiao, K. Sensitivity analysis of uncertain parameters based on an improved proton exchange membrane fuel cell analytical model. Energy Convers. Manag. 2018, 164, 639–654. [Google Scholar] [CrossRef]
- Vetter, R.; Schumacher, J. Free open reference implementation of a two-phase PEM fuel cell model. Comput. Phys. Commun. 2019, 234, 223–234. [Google Scholar] [CrossRef]
- García-Salaberri, P. 1D two-phase, non-isothermal modeling of a proton exchange membrane water electrolyzer: An optimization perspective. J. Power Sources 2022, 521, 230915. [Google Scholar] [CrossRef]
- Chen, J.; Sun, Y.; Hu, D.; Lv, H. Performance modeling and mechanism study of proton exchange membrane water electrolyzer coupled with water electroosmosis. Energy Convers. Manag. 2024, 315, 118753. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.; Mergel, J. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Kang, Z.; Mo, J.; Yang, G. Performance Modeling and Current Mapping of Proton Exchange Membrane Electrolyzer Cells with Novel Thin/Tunable Liquid/Gas Diffusion Layers. Electrochim. Acta 2017, 255, 405–416. [Google Scholar] [CrossRef]





| Parameter | Symbol | Value |
|---|---|---|
| Thickness of CL | 20 μm | |
| Porosity of CL | 0.4 | |
| Thickness of PEM | 180 μm | |
| Reference pressure | Pref | 1 atm |
| Reference temperature | Tref | 353.15 K |
| Reference anode exchange current density | ja | 5 × 10−3 A cm2 |
| Reference cathode exchange current density | jc | 50 A cm2 |
| Anode transfer coefficient | 0.5 | |
| Cathode transfer coefficient | 0.5 | |
| Coverage exponent | ns | 2 |
| Electronic conductivity of CL | 5000 S/m | |
| Electronic conductivity of PTL | 1250 S/m |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Liu, J.; Du, S. Optimizing Porous Transport Layers in PEM Water Electrolyzers: A 1D Two-Phase Model. Batteries 2025, 11, 222. https://doi.org/10.3390/batteries11060222
Zhang L, Liu J, Du S. Optimizing Porous Transport Layers in PEM Water Electrolyzers: A 1D Two-Phase Model. Batteries. 2025; 11(6):222. https://doi.org/10.3390/batteries11060222
Chicago/Turabian StyleZhang, Lu, Jie Liu, and Shaojie Du. 2025. "Optimizing Porous Transport Layers in PEM Water Electrolyzers: A 1D Two-Phase Model" Batteries 11, no. 6: 222. https://doi.org/10.3390/batteries11060222
APA StyleZhang, L., Liu, J., & Du, S. (2025). Optimizing Porous Transport Layers in PEM Water Electrolyzers: A 1D Two-Phase Model. Batteries, 11(6), 222. https://doi.org/10.3390/batteries11060222







