Characterization of Industrial Black Mass from End-of-Life LiFePO4-Graphite Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
3. Results
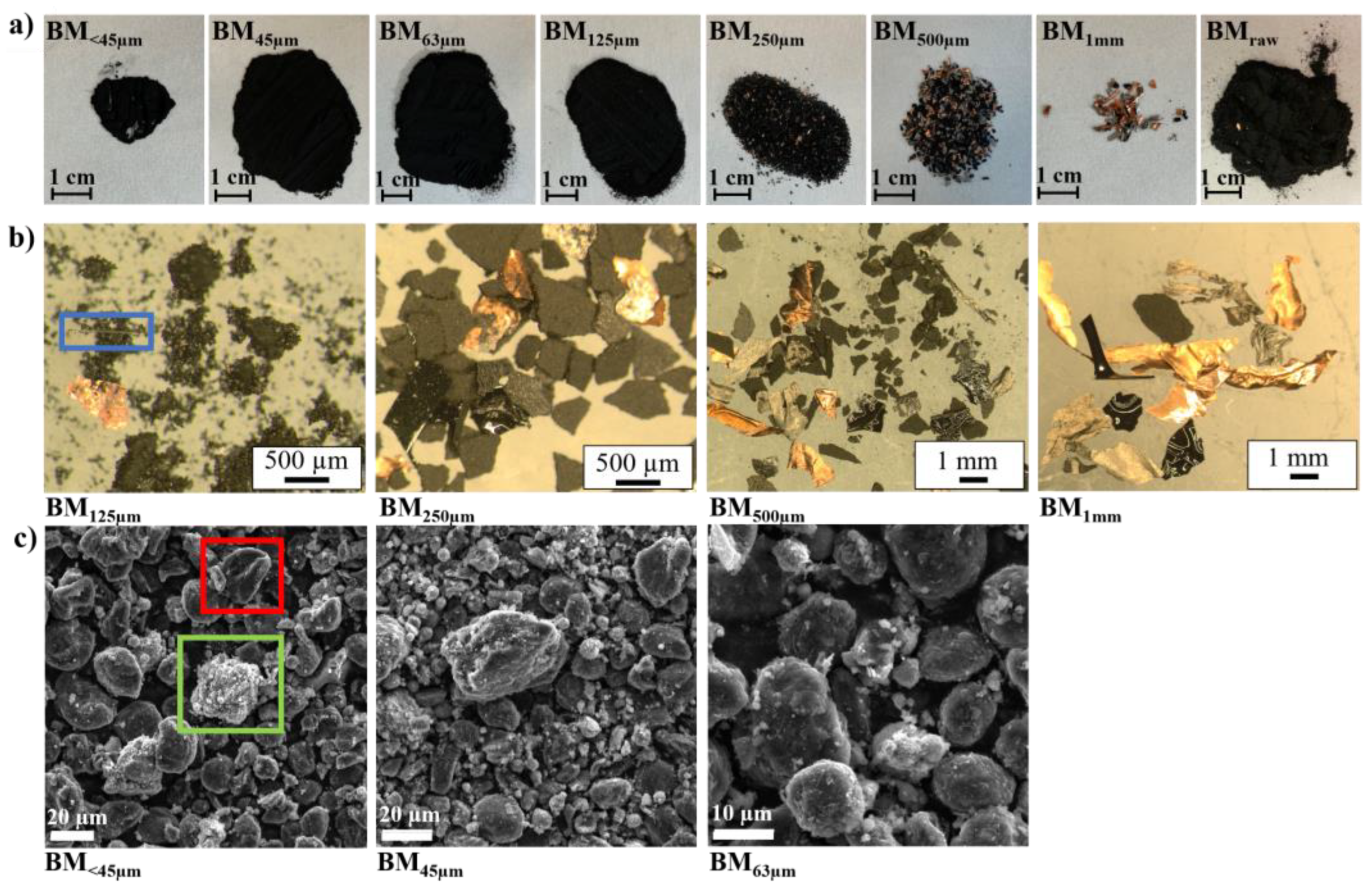
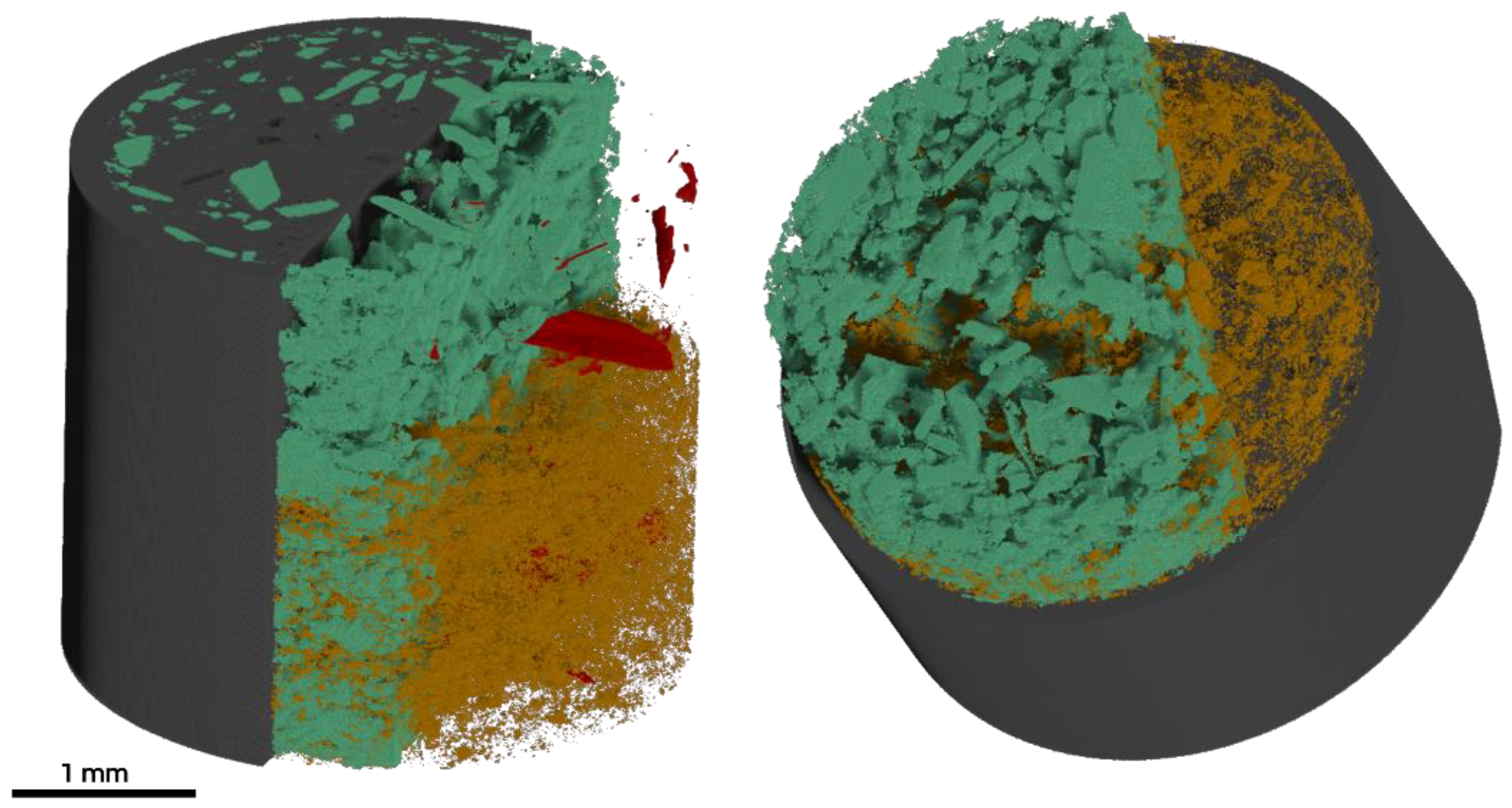
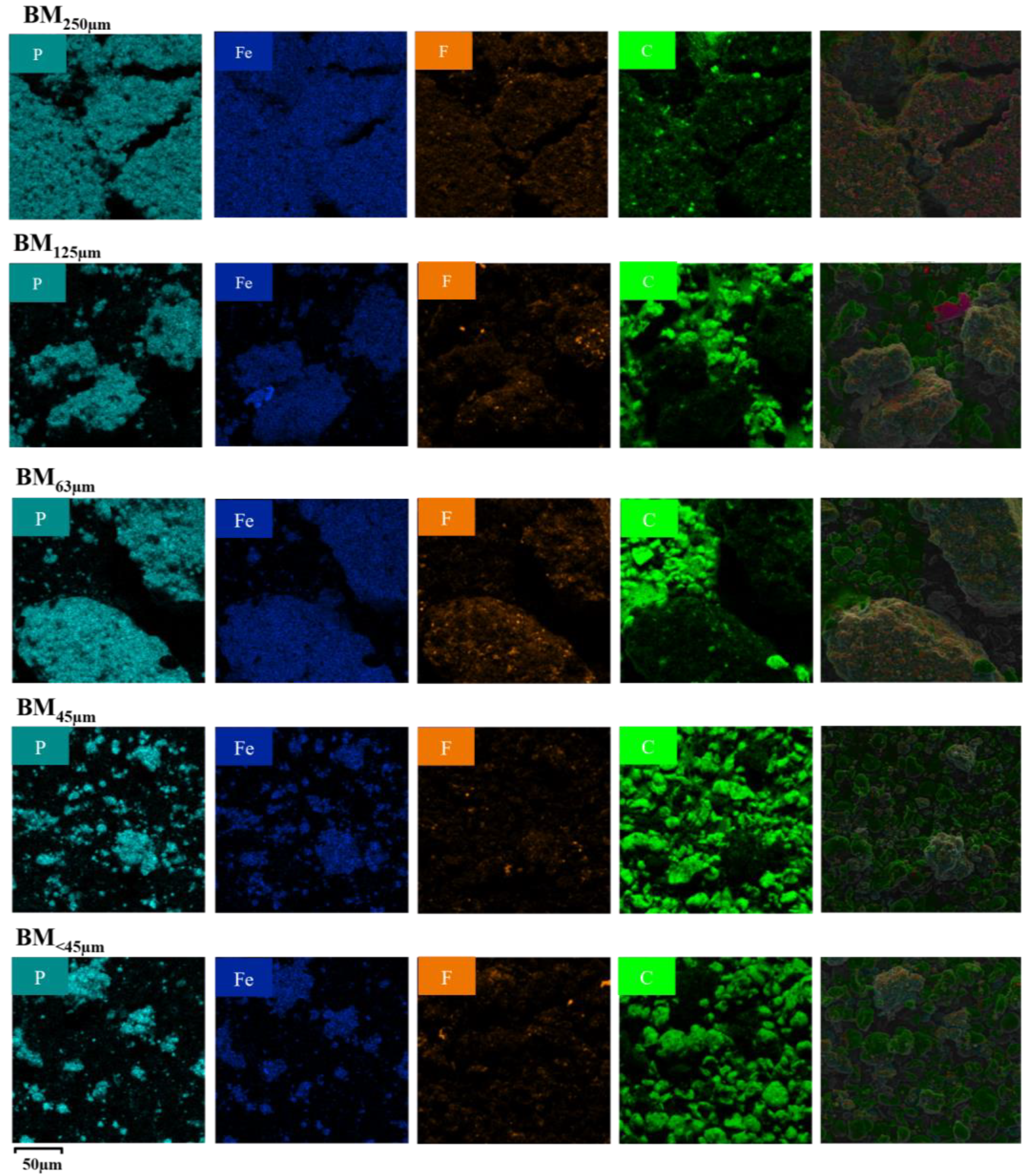
3.1. Visual and Morphological Characteristics
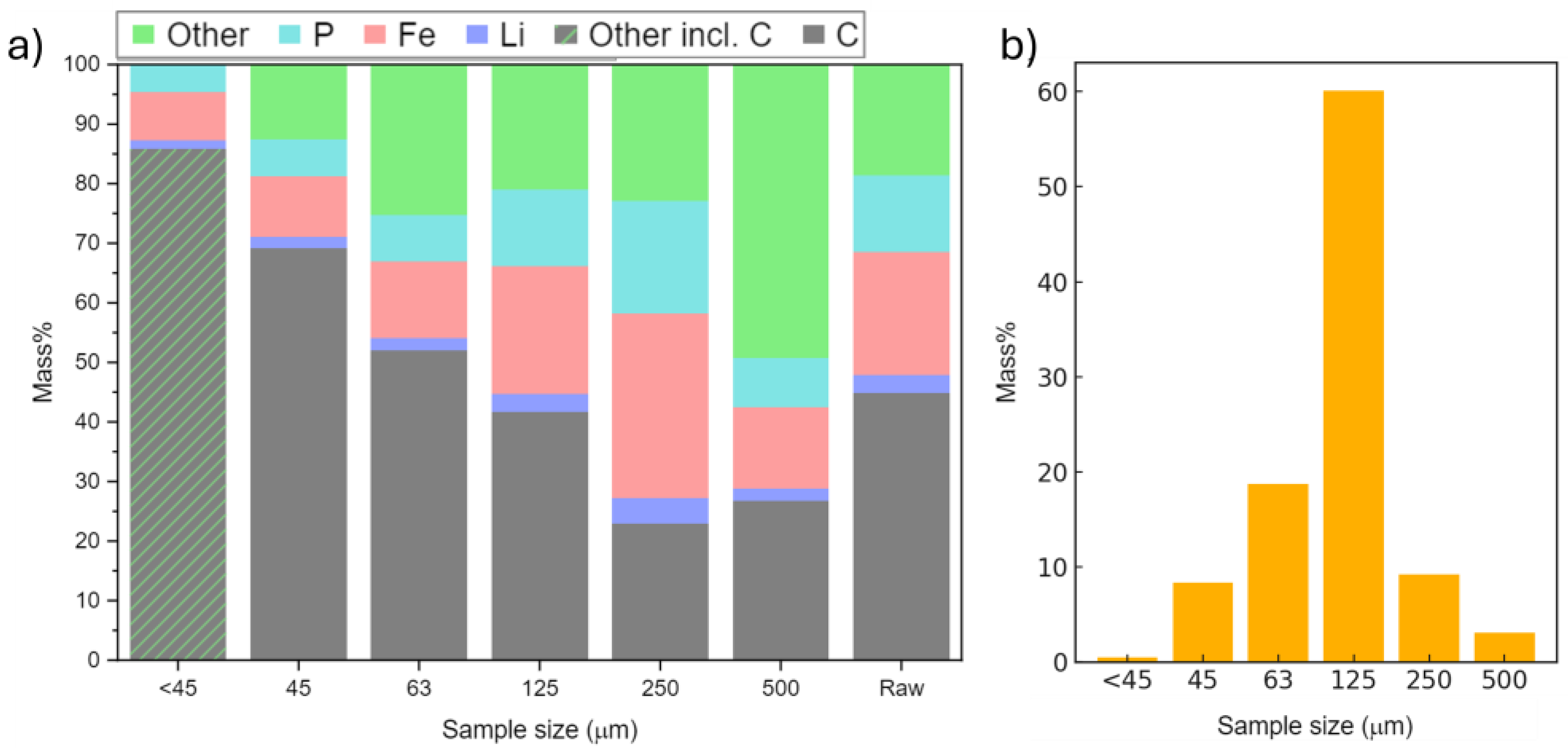
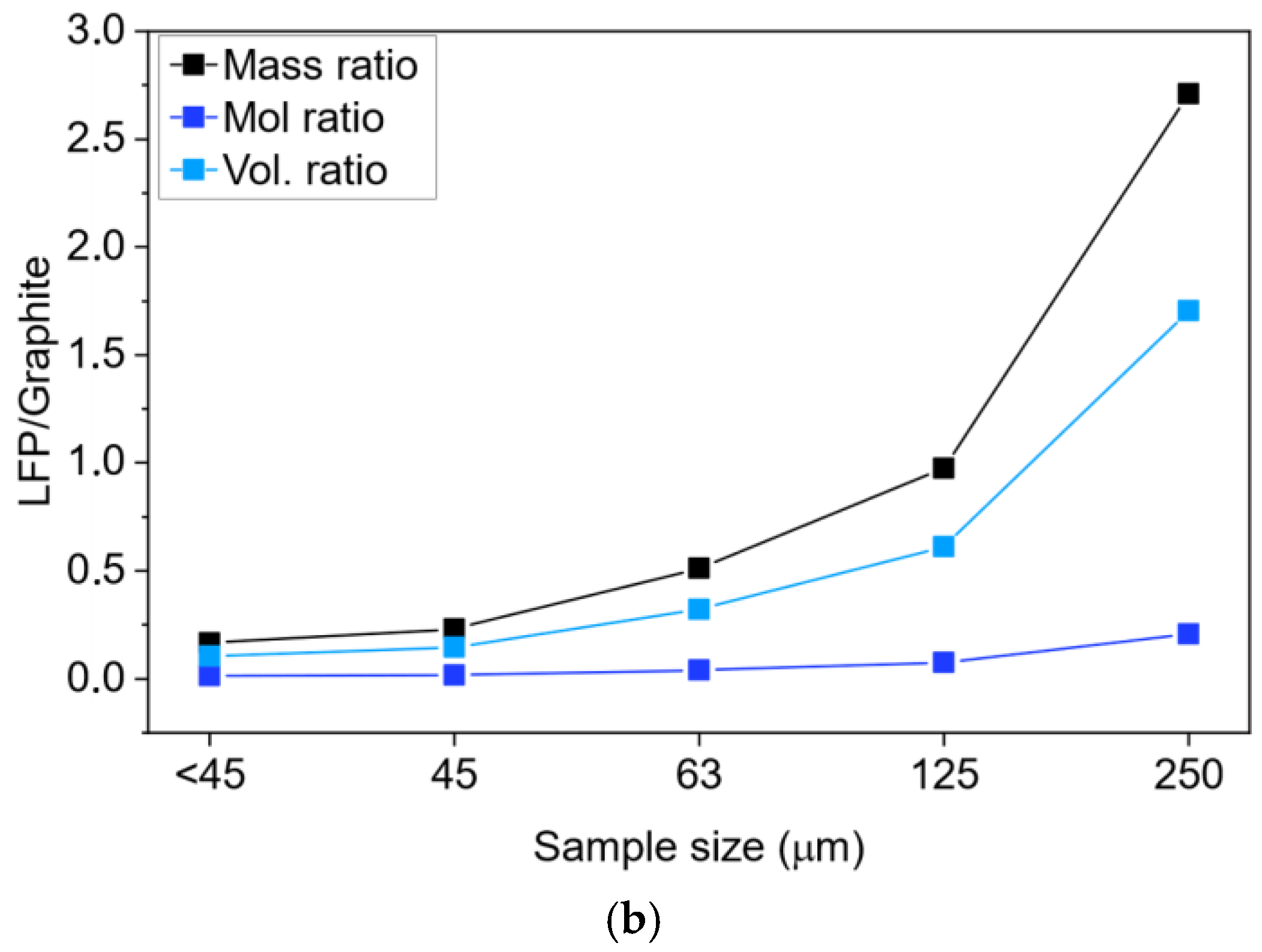
3.2. Compositional Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fleischmann, J.M.H.; Horetsky, E.; Ibrahim, D.; Jautelat, S.; Linder, M.; Schaufuss, P.; Torscht, L.; van de Rijt, A. Battery 2030: Resilient, Sustainable, and Circular. 2023. Available online: https://www.mckinsey.com/industries/automotive-and-assembly/our-insights/battery-2030-resilient-sustainable-and-circular#/ (accessed on 25 March 2025).
- Stone, M. As Electric Vehicles Take Off, We’ll Need to Recycle Their Batteries National Geographics, 2021. Available online: https://www.nationalgeographic.com/environment/article/electric-vehicles-take-off-recycling-ev-batteries (accessed on 25 March 2025).
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Galande, C.; Miranda, A.; Mathkar, A.; Gao, W.; Reddy, A.L.M.; Vlad, A.; Ajayan, P.M. Paintable Battery. Sci. Rep. 2012, 2, 481. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jiang, B.; Xiong, W.; Sun, H.; Lin, Z.; Hu, L.; Tu, J.; Hou, J.; Zhu, H.; Jiao, S. A new cathode material for super-valent battery based on aluminium ion intercalation and deintercalation. Sci. Rep. 2013, 3, 3383. [Google Scholar] [CrossRef]
- Circular Economy: New Law on More Sustainable, Circular and Safe Batteries Enters Into Force. 2023. Available online: https://environment.ec.europa.eu/news/new-law-more-sustainable-circular-and-safe-batteries-enters-force-2023-08-17_en (accessed on 25 March 2025).
- Office, V.T. Battery Policies and Incentives Search 2023. Available online: https://www.energy.gov/eere/vehicles/battery-policies-and-incentives-search#/ (accessed on 25 March 2025).
- Nemko. Compulsory Chinese Certification of Li-ion Batteries & Related Product. 2023. Available online: https://www.nemko.com/blog/compulsory-chinese-certification-of-lithium-ion-batteries-and-related-products (accessed on 25 March 2025).
- Government Notifies Battery Waste Management Rules. 2022. Available online: https://pib.gov.in/PressReleasePage.aspx?PRID=1854433 (accessed on 25 March 2025).
- Law No. 12305 Brazilian National Policy on Solid Waste (Batteries). Iea. Available online: https://www.iea.org/policies/15805-law-no-12305-brazilian-national-policy-on-solid-waste-batteries (accessed on 25 March 2025).
- Wang, X.-T.; Gu, Z.-Y.; Ang, E.H.; Zhao, X.-X.; Wu, X.-L.; Liu, Y. Prospects for managing end-of-life lithium-ion batteries: Present and future. Interdiscip. Mater. 2022, 1, 417–433. [Google Scholar] [CrossRef]
- Hazotte, C.; Leclerc, N.; Diliberto, S.; Meux, E.; Lapicque, F. End-of-life nickel-cadmium accumulators: Characterization of electrode materials and industrial Black Mass. Environ. Technol. 2015, 36, 796–805. [Google Scholar] [CrossRef]
- Thompson, D.; Hyde, C.; Hartley, J.M.; Abbott, A.P.; Anderson, P.A.; Harper, G.D.J. To shred or not to shred: A comparative techno-economic assessment of lithium ion battery hydrometallurgical recycling retaining value and improving circularity in LIB supply chains. Resour. Conserv. Recycl. 2021, 175, 105741. [Google Scholar] [CrossRef]
- Gaines, L. Lithium-ion battery recycling processes: Research towards a sustainable course. Sustain. Mater. Technol. 2018, 17, e00068. [Google Scholar] [CrossRef]
- Zhao, Y.; Pohl, O.; Bhatt, A.I.; Collis, G.E.; Mahon, P.J.; Rüther, T.; Hollenkamp, A.F.A. A Review on Battery Market Trends, Second-Life Reuse, and Recycling. Sustain. Chem. 2021, 2, 167–205. [Google Scholar] [CrossRef]
- Romo, L.A.; López-Fernández, A.; García-Díaz, I.; Fernández, P.; Urbieta, A.; López, F.A. From spent alkaline batteries to ZnxMn3−xO4 by a hydrometallurgical route: Synthesis and characterization. RSC Adv. 2018, 8, 33496–33505. [Google Scholar] [CrossRef]
- Meskers, C.; Caffarey, M.; Van Camp, M. Circular Cities, E-Mobility and the Metals Industry—A World in Transition. In REWAS 2019; Gaustad, G., Fleuriault, C., Gökelma, M., Howarter, J.A., Kirchain, R., Ma, K., Meskers, C., Neelameggham, N.R., Olivetti, E., Powell, A.C., et al., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 313–318. [Google Scholar]
- Yadav, P.; Jie, C.J.; Tan, S.; Srinivasan, M.J. Recycling of cathode from spent lithium iron phosphate batteries. Hazard. Mater. 2020, 399, 123068. [Google Scholar] [CrossRef]
- Li, H.; Xing, S.; Liu, Y.; Li, F.; Guo, H.; Kuang, G. Recovery of Lithium, Iron, and Phosphorus from Spent LiFePO4 Batteries Using Stoichiometric Sulfuric Acid Leaching System. ACS Sustain. Chem. Eng. 2017, 5, 8017–8024. [Google Scholar] [CrossRef]
- Du, M.; Guo, J.-Z.; Zheng, S.-H.; Liu, Y.; Yang, J.-L.; Zhang, K.-Y.; Gu, Z.-Y.; Wang, X.-T.; Wu, X.-L. Direct reuse of LiFePO4 cathode materials from spent lithium-ion batteries: Extracting Li from brine. Chin. Chem. Lett. 2023, 34, 107706. [Google Scholar] [CrossRef]
- Ding, Y.; Fu, J.; Zhang, S.; He, X.; Zhao, B.; Ren, J.; Zhong, J.; Liu, Z. Advances in recycling LiFePO4 from spent lithium batteries: A critical review. Sep. Purif. Technol. 2024, 338, 126551. [Google Scholar] [CrossRef]
- Dolotko, O.; Gehrke, N.; Malliaridou, T.; Sieweck, R.; Herrmann, L.; Hunzinger, B.; Knapp, M.; Ehrenberg, H. Universal and efficient extraction of lithium for lithium-ion battery recycling using mechanochemistry. Commun. Chem. 2023, 6, 49. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, J.; Liu, Y.; Jing, Q.; Yang, C.; Chen, Y.; Wang, C. Synergistic Recovery of Valuable Metals from Spent Nickel–Metal Hydride Batteries and Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2019, 7, 5626–5631. [Google Scholar] [CrossRef]
- Liu, K.; Tan, Q.; Liu, L.; Li, J. Acid-Free and Selective Extraction of Lithium from Spent Lithium Iron Phosphate Batteries via a Mechanochemically Induced Isomorphic Substitution. Environ. Sci. Technol. 2019, 53, 9781–9788. [Google Scholar] [CrossRef]
- Xu, P.; Dai, Q.; Gao, H.; Liu, H.; Zhang, M.; Li, M.; Chen, Y.; An, K.; Meng, Y.S.; Liu, P.; et al. Efficient Direct Recycling of Lithium-Ion Battery Cathodes by Targeted Healing. Joule 2020, 4, 2609–2626. [Google Scholar] [CrossRef]
- Fan, M.; Meng, Q.; Chang, X.; Gu, C.-F.; Meng, X.-H.; Yin, Y.-X.; Li, H.; Wan, L.-J.; Guo, Y.-G. In Situ Electrochemical Regeneration of Degraded LiFePO4 Electrode with Functionalized Prelithiation Separator. Adv. Energy Mater. 2022, 12, 2103630. [Google Scholar] [CrossRef]
- Jing, Q.; Zhang, J.; Liu, Y.; Zhang, W.; Chen, Y.; Wang, C. Direct Regeneration of Spent LiFePO4 Cathode Material by a Green and Efficient One-Step Hydrothermal Method. ACS Sustain. Chem. Eng. 2020, 8, 17622–17628. [Google Scholar] [CrossRef]
- Feng, C.; Cao, Y.; Song, L.; Zhao, B.; Yang, Q.; Zhang, Y.; Wei, X.; Zhou, G.; Song, Y. Direct Regeneration of Industrial LiFePO4 Black Mass Through A Glycerol-Enabled Granule Reconstruction Strategy. Angew. Chem. Int. Ed. 2025, 64, e202418198. [Google Scholar] [CrossRef]
- Wang, Y.; An, N.; Wen, L.; Wang, L.; Jiang, X.; Hou, F.; Yin, Y.; Liang, J.J. Recent progress on the recycling technology of Li-ion batteries. Energy Chem. 2021, 55, 391–419. [Google Scholar] [CrossRef]
- Mousa, E.; Hu, X.; Ånnhagen, L.; Ye, G.; Cornelio, A.; Fahimi, A.; Bontempi, E.; Frontera, P.; Badenhorst, C.; Santos, A.C.; et al. Characterization and Thermal Treatment of the Black Mass from Spent Lithium-Ion Batteries. Sustainability 2023, 15, 15. [Google Scholar] [CrossRef]
- Babanejad, S.; Ahmed, H.; Andersson, C.; Samuelsson, C.; Lennartsson, A.; Hall, B.; Arnerlöf, L.J. High-Temperature Behavior of Spent Li-Ion Battery Black Mass in Inert Atmosphere. Sustain. Metall. 2022, 8, 566–581. [Google Scholar] [CrossRef]
- Donnelly, L.; Pirrie, D.; Power, M.; Corfe, I.; Kuva, J.; Lukkari, S.; Lahaye, Y.; Liu, X.; Dehaine, Q.; Jolis, E.M.; et al. The Recycling of End-of-Life Lithium-Ion Batteries and the Phase Characterisation of Black Mass. Recycling 2023, 8, 59. [Google Scholar] [CrossRef]
- Peschel, C.; van Wickeren, S.; Preibisch, Y.; Naber, V.; Werner, D.; Frankenstein, L.; Horsthemke, F.; Peuker, U.; Winter, M.; Nowak, S. Comprehensive Characterization of Shredded Lithium-Ion Battery Recycling Material. Chem. Eur. J. 2022, 28, e202200485. [Google Scholar] [CrossRef]
- Wilke, C.; Werner, D.M.; Kaas, A.; Peuker, U.A. Influence of the Crusher Settings and a Thermal Pre-Treatment on the Properties of the Fine Fraction (Black Mass) from Me-chanical Lithium-Ion Battery Recycling. Batteries 2023, 9, 514. [Google Scholar] [CrossRef]
- GmbH, A.R. Accurec Battery Recycling. Available online: https://accurec.de/?lang=en (accessed on 17 May 2025).
- Dragonfly; Comet Technologies Canada Inc., Montreal, Canada: Software. Available online: https://www.theobjects.com/dragonfly (accessed on 20 August 2024).
- Dippel, A.-C.; Liermann, H.-P.; Delitz, J.T.; Walter, P.; Schulte-Schrepping, H.; Seeck, O.H.; Franz, H.J. Beamline P02.1 at PETRA III for high-resolution and high-energy powder diffraction. Synchrotron Radiat. 2015, 22, 675–687. [Google Scholar] [CrossRef]
- Juan Rodriguez-Carvajal, T.R. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Condens. Matter. 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Withers, P.J.; Bouman, C.; Carmignato, S.; Cnudde, V.; Grimaldi, D.; Hagen, C.K.; Maire, E.; Manley, M.; Du Plessis, A.; Stock, S.R. X-ray computed tomography. Nat. Rev. Methods Primers 2021, 1, 18. [Google Scholar] [CrossRef]
- Vanderbruggen, A.; Gugala, E.; Blannin, R.; Bachmann, K.; Serna-Guerrero, R.; Rudolph, M. Automated mineralogy as a novel approach for the compositional and textural characterization of spent lithium-ion batteries. Miner. Eng. 2021, 169, 106924. [Google Scholar] [CrossRef]
- Trucano, P.; Chen, R. Structure of Graphite by Neutron Diffraction. Nature 1975, 258, 136–137. [Google Scholar] [CrossRef]
- Janssen, Y.; Santhanagopalan, D.; Qian, D.; Chi, M.; Wang, X.; Hoffmann, C.; Meng, Y.S.; Khalifah, P.G. Reciprocal Salt Flux Growth of LiFePO4 Single Crystals with Controlled Defect Concentrations. Chem. Mater. 2013, 25, 4574–4584. [Google Scholar] [CrossRef]
- Andersson, A.S.; Kalska, B.; Häggström, L.; Thomas, J.O. Lithium extraction/insertion in LiFePO4: An X-ray diffraction and Mössbauer spectroscopy study. Solid State Ion. 2000, 130, 41–52. [Google Scholar] [CrossRef]
- Zou, F.; Manthiram, A. A Review of the Design of Advanced Binders for High-Performance Batteries. Adv. Energy Mater. 2020, 10, 2002508. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bjerre-Christensen, N.; Eriksen, C.B.; Sylvester-Hvid, K.O.; Ravnsbæk, D.B. Characterization of Industrial Black Mass from End-of-Life LiFePO4-Graphite Batteries. Batteries 2025, 11, 210. https://doi.org/10.3390/batteries11060210
Bjerre-Christensen N, Eriksen CB, Sylvester-Hvid KO, Ravnsbæk DB. Characterization of Industrial Black Mass from End-of-Life LiFePO4-Graphite Batteries. Batteries. 2025; 11(6):210. https://doi.org/10.3390/batteries11060210
Chicago/Turabian StyleBjerre-Christensen, Nanna, Caroline Birksø Eriksen, Kristian Oluf Sylvester-Hvid, and Dorthe Bomholdt Ravnsbæk. 2025. "Characterization of Industrial Black Mass from End-of-Life LiFePO4-Graphite Batteries" Batteries 11, no. 6: 210. https://doi.org/10.3390/batteries11060210
APA StyleBjerre-Christensen, N., Eriksen, C. B., Sylvester-Hvid, K. O., & Ravnsbæk, D. B. (2025). Characterization of Industrial Black Mass from End-of-Life LiFePO4-Graphite Batteries. Batteries, 11(6), 210. https://doi.org/10.3390/batteries11060210







