Abstract
Lithium–sulfur batteries (LSBs) are favorable candidates for advanced energy storage, boasting a remarkable theoretical energy density of 2600 Wh kg−1. Moreover, several challenges hinder their practical implementation, including sulfur’s intrinsic electrical insulation, the shuttle effect of lithium polysulfides (LiPSs), sluggish redox kinetics of Li2S2/Li2S, and the uncontrolled growth of Li dendrites. These issues pose significant obstacles to the commercialization of LSBs. A viable strategy to address these challenges involves using MXene materials, 2D transition metal carbides, and nitrides (TMCs/TMNs) as hosts, functional separators, or interlayers. MXenes offer exceptional electronic conductivity, adjustable structural properties, and abundant polar functional groups, enabling strong interactions with both S cathodes and Li anodes. Despite their advantages, current MXene synthesis methods predominantly rely on acid etching, which is associated with environmental concerns, low production efficiency, and limited structural versatility, restricting their potential in LSBs. This review provides a comprehensive overview of traditional and environmentally sustainable MXene synthesis techniques, emphasizing their applications in developing S cathodes, Li anodes, and functional separators for LSBs. Additionally, it discusses the challenges and outlines future directions for advancing MXene-based solutions in LSBs technology.
1. Introduction
Conventional lithium-ion batteries (LIBs) face significant challenges owing to their limited energy conversion efficiency and high production costs, making them less capable of meeting the increasing demand for energy storage, especially in the electric vehicle sector [1,2]. In contrast, LSBs offer several advantages, such as an impressive energy density of 2600 Wh kg−1, eco-friendliness, and the low cost of sulfur (S). These features make LSBs highly promising for advancing electric vehicles and smart grid technologies [3,4,5]. However, several inherent challenges hinder the commercialization of LSBs [6]. First, S and its discharge products, such as Li2S, exhibit extremely poor electronic conductivity, significantly impeding redox reactions and reducing S utilization. For instance, the conductivities of S and Li2S are as low as ~5 × 10−30 and ~10−13 S cm−1, respectively [7]. Second, LiPSs are highly soluble in the electrolyte during cycling, leading to the shuttle effect. This phenomenon accelerates capacity decay and lowers the battery’s coulombic efficiency (CE). LiPSs can penetrate the separator and reach the Li anode, where they react with Li, causing corrosion. Third, Li’s spontaneous reaction with the liquid electrolyte forms a fragile solid electrolyte interphase (SEI) on the anode, further reducing the CE. Lastly, uneven Li deposition can result in internal short circuits, posing significant safety risks to the battery.
To overcome these challenges, scientists have developed various approaches to enhance the activity of LSBs. For S cathodes, C-based hosts like porous C [8], CNTs [9], and hollow C spheres [10] have been engineered to entrap polysulfides within their nanopores. Nevertheless, the non-polar nature of C composites limits their ability to effectively immobilize polysulfides. To address this, polar host materials with strong chemisorption characteristics for LiPSs have garnered significant interest. These include heteroatom-doped carbons (for instance, B-doped [11] and N-doped [12]), metal oxides and sulfides [13,14], MXene [15], and GO [16], have gained significant attention. For Li anodes, various strategies have been explored to stabilize the Li/electrolyte interface and adjust Li deposition. These include the use of electrolyte additives, 3D current collectors, protective physical layers, and solid electrolytes [17].
MXenes are a versatile family of 2D TMCs and TMNs. The first discovered MXene, Ti3C2Tx (where Tx signifies surface-terminating groups like O, OH, and F), is synthesized using selectively etching of an A-layer from the parent MAX phase. The MAX phase has the common formula Mn+1AX; here, M is the early transition metals (TMs), A is typically an element from groups IIIA or IVA (e.g., Ti, V, Nb, or Mo), X represents C or N, and n varies from 1 to 3. The derived MXenes follow the formula Mn+1XnTx; here, M denotes the TMs site, X refers to the C or N atoms, n varies from 1 to 4, and Tx indicates the functional groups attached to the surface. Structurally, MXenes preserve a hexagonal close-packed (hcp) arrangement like the basal plane of their MAX phase. In this framework, the TM atoms (M) form a densely packed layer, while the X atoms occupy octahedral sites between these M atom layers.
MXene, characterized by outstanding conductivity, remarkable mechanical properties, and adjustable interlayer distance [18,19,20], presents significant promise for advancing LSBs (Figure 1). Its potential is evident in several key respects: (i) MXene’s superior conductivity facilitates efficient reduction of S at the cathode, thereby achieving high S consumption. (ii) The ample polar functional groups on its surface exhibit a robust affinity for LiPSs, efficiently minimizing their dissolution into the electrolyte and enhancing the CE of LSBs. Furthermore, these functional groups act as catalysts, accelerating the conversion of LiPSs into Li2S2 and Li2S, thereby improving reaction kinetics. (iii) The polar functional groups also enable robust chemisorption of Li+, promoting uniform Li nucleation and deposition. (iv) MXene’s exceptional mechanical strength ensures electrode stability during cycling, while its customizable architecture makes it adaptable for various scenarios. Despite these benefits, the practical implementation of MXene in LSBs is constrained by environmentally detrimental preparation processes and suboptimal structural design.
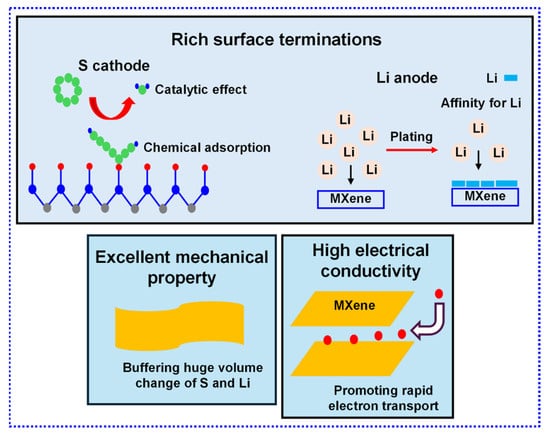
Figure 1.
Distinctive benefits of MXene for LSBs.
In this work, we first explore the preparation approaches of MXene, transitioning from conventional techniques to eco-friendly alternatives. We thoroughly evaluate how these methods impact the cost, energy efficiency, environmental sustainability, and structural characteristics of the resulting MXene. Subsequently, we review recent progress in the application of MXene as a cathode and anode host, as well as a functional interlayer or separator in LSBs (Figure 2), with a focus on the relationship between MXene’s surface chemistry and the energy storage performance of LSBs. The studies highlighted in Figure 2 collectively showcase the evolution of MXenes in LSBs, beginning with the discovery of Ti3C2Tx MXene in 2011. Early work demonstrated MXene’s potential as a conductive S host (2015) and as a separator coating (2016) to suppress polysulfide shuttling. Subsequent research explored MXene as a Li host (2017) and incorporated N-doping (2018) and porous architecture (2019) to enhance active material utilization and ion transport. Hybrid structures such as MXene@CNF (2020) and vertically aligned MXenes (2022) improved Li plating stability and ionic conductivity. Recent innovations include multifunctional composites like TiS2/TiO2@MXene (2023) and 3D TiN-MXene-Co@CNT networks (2024), which synergistically address conductivity, polysulfide retention, and structural stability. These advancements have progressively established MXenes as versatile and high-performance components for next-generation LSBs. Lastly, we propose future directions for MXene synthesis and discuss its potential for real-world applications in LSBs.
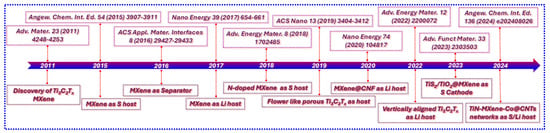
Figure 2.
Key advancements in the MXene for LSBs.
2. Synthesis of MXene
MXene originates from the MAX phase (Mn+1AXn), a ternary layered carbide or nitride, where “A” denotes elements from groups IIIA or IVA, for instance, Al, Si, or Ti. Due to the robust metallic M-A bonds with high bond energy [21], simple mechanical exfoliation cannot separate MXene sheets from the MAX phase. To overcome this, researchers have developed selective etching techniques to eliminate the A-layer from the MAX phase, thereby producing MXene. This etching process generates dangling bonds on the M atomic layer, which accelerates the attachment and coordination of various surface terminations. The synthesis method plays a critical role in determining MXene’s characteristics, including its surface chemistry, sheet size, and structure, as different etching techniques lead to distinct physicochemical characteristics. Traditional etching methods often involve F-containing compounds, which are highly detrimental to the environment and human health. Conversely, green synthesis methods eliminate the use of F, providing a safer and more eco-friendly alternative. This section offers a detailed review of both traditional and green synthesis approaches for MXene. Furthermore, a comparison of the differences between these methods is presented in Table 1.

Table 1.
The differences between traditional and green preparation routes [5].
2.1. Traditional Preparation Routes
2.1.1. HF Etching
In 2011, Gogotsi and colleagues achieved the successful delamination of Ti3AlC2 to synthesize Ti3C2 MXene for the first time using HF etching [22]. This process involves the selective elimination of A-layer from the MAX phase, as outlined below:
Ti3AlC2 + 3HF → AlF3 + 3/2H2 (g)↑ + Ti3C2
Ti3C2 + 2H2O → Ti3C2(OH)2 + H2 (g)↑
Ti3C2 + 2HF → Ti3C2F2 + H2 (g)↑
HF removes Al atoms from Ti3AlC2 through a substitution reaction, releasing H2 as a byproduct. The resulting Ti3C2 reacts further with H2O and HF to yield Ti3C2Tx (Tx: -F, -OH). In 2015, Rosen et al. successfully synthesized Mo2CTx MXene by etching Mo2Ga2C MAX phase, which contains extra A-layer atoms, using HF acid as the etchant [23]. This approach was later applied to produce other MXenes, such as Nb4C3Tx [24] and Hf3C2Tx [25]. Naguib and colleagues immersed the MAX phase in a 50 wt% HF solution for 2 h, followed by washing, etching, and centrifugation, yielding a multi-layered, accordion-type MXene architecture that is difficult to replicate using other etching techniques. This distinctive structure results from the exothermic removal of Al and the release of H2 during the process [26]. Since its introduction, the HF etching route has remained the most widely employed due to its simplicity and rapid effectiveness in etching the MAX phase. Moreover, HF’s extreme corrosiveness poses significant environmental and health hazards and can lead to the over-etching of MXene, adversely affecting its performance. Consequently, there is an urgent need for milder, less toxic, and more eco-friendly alternatives to HF etching technique.
2.1.2. In Situ HF Etching
To address the corrosion challenges associated with HF, Ghidiu and colleagues developed an in situ HF generation etching method. This technique includes the reaction of 6 M HCl with 1.98 g of LiF to produce in situ HF, which specifically removes the Al atomic layer from Ti3AlC2. The process yields a clay-type MXene with exceptional dispersion and hydrophilicity, suitable for direct use as a flexible thin-film electrode using a simple rolling technique [27]. Additionally, this etching process can be modified by substituting LiF and HCl with other fluoride salts (NaF, KF, CsF) and strong acids like H2SO4. Halim’s team suggested that NH4HF2 could serve as an alternative to HF for etching the Al layer from Ti3AlC2 [28]. Feng et al [29]. further elucidated the etching mechanism, presenting it through the following reaction formulae:
Ti3AlC2 + XHF2 → XaAlFb + AlF3 + H2 (g)↑ + Ti3C2
AlF3 + cH2O → AlF3. cH2O
Ti3C2 + XHF2 + H2O → Ti3C2Fx(OH)yXz
In this context, “X” represents cations such as NH4+, K+, and Na+; while a, b, c, x, y, and z denote the numerical values [29]. The equations above highlight that both H+ and F− ions are essential for MXene production, suggesting that this method operates through a reaction process similar to HF etching.
Compared to conventional HF etching, this approach offers several advantages: (i) it involves safer and milder preparation processes; and (ii) cations in the solution, such as Li+ and NH4+, intercalate between MXene layers, increasing interlayer spacing and reducing van der Waals forces, thereby simplifying synthesis, resulting in fewer -F terminations and more -O terminations, which minimizes side reactions. As a result, this method has become a preferred approach among researchers for synthesizing MXene. However, some challenges persist, such as the formation of unetched residual MAX phase biproducts, lower etching efficacy, and the lengthy nature of the process.
2.1.3. Molten Fluorine Salt Etching
Compared to carbide MAX precursors, nitride MAX precursors have stronger bonds between the A and M layer atoms, requiring greater energy to eliminate the A-layer. Furthermore, Tin+1Nn precursors are less stable and can easily dissolve in HF. To overcome these challenges, Urbankowski et al. introduced this etching route in 2016 for synthesizing nitride MXene [30]. In this approach, Ti4AlN3 was etched using a molten fluoride salt mixture (LiF, KF, and NaF in a mass ratio of 29:59:12 wt%) and calcined at 550 °C in an Ar atmosphere to eliminate the Al layer. After etching, fluoride impurities were eliminated with H2SO4, and Ti4N3Tx NSs were obtained through TBAOH intercalation and sonication. This method relies on precise temperature control and the use of an inert atmosphere to selectively etch the Al layer while maintaining the structural integrity of Ti-N bonds in Ti4N3. Nitride MXenes prepared using this method exhibit improved electronic conductivity and a higher DOS. Additionally, non-terminated Ti4N3 displays metallic and magnetic characteristics. Furthermore, Ti4N3Tx MXenes exhibit lower crystallinity compared to those produced by the previously mentioned etching methods, resulting in reduced flexibility.
2.1.4. Water-Free Etching
Acid-etching methods typically rely on water as a solvent, which can limit the potential applications of MXene. For instance, certain polymerization reactions cannot proceed in the presence of water, and quantum dot (0D) materials cannot be integrated with MXene under aqueous conditions. Moreover, even trace amounts of water in MXene materials can significantly degrade battery performance. As a result, MXene must undergo vacuum annealing before cell assembly. To address these limitations, Natu and colleagues introduced an innovative method that utilizes organic polar solvents like DMF, NMP, DMSO, and PC in combination with NH4HF2 to etch MAX precursors in the water-free atmosphere. This process produces Ti3C2Tx with a high-content of -F terminations (Figure 3a) [31]. MXene composite electrodes prepared using this approach demonstrated twice the anode capacity in SIBs compared to those etched in water. Moreover, this method requires significantly more time (196 h compared to 48 h for aqueous etching) and necessitates working in a glove box, adding operational complexity for researchers.
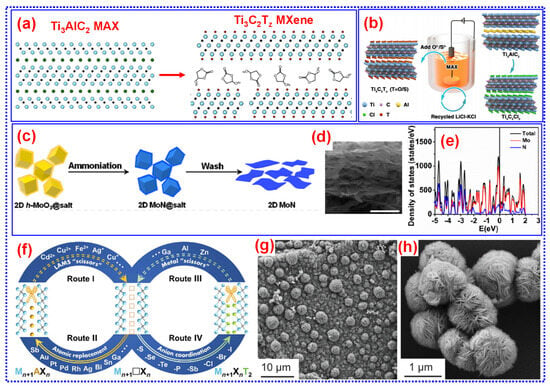
Figure 3.
(a) Pictorial demonstration of water-free etching approach. Adapted from [31], Copyright 2020, Elsevier B.V. (b) Preparation of MXene from the MAX phase by molten salt assisted electrochemical etching and the in situ modification of surface terminations. Adapted from [32], Copyright 2021, Wiley-VCH. (c–e) Pictorial demonstration of preparation and DOS of 2D MoN. Adapted from [33], Copyright 2017, American Chemical Society. (f) Pictorial representation of structural editing of MAX phases and MXenes using chemical scissor-mediated intercalation procedure. Adapted from [34], Copyright 2023, The AAAS. SEM micrograph of (g) microspheres developing on carpets, and (h) individual microspheres. Adapted from [35], Copyright 2023, The AAAS.
2.2. Green Synthesis
Traditional MXene synthesis methods suffer from several limitations, including high energy requirements, the use of toxic HF, and the production of hazardous waste, making them less environmentally sustainable and impractical for industrial-scale applications. As a result, there is an urgent demand for green, energy-efficient, controllable, and environmentally friendly synthesis approaches. Furthermore, conventional methods limit MXene surface terminations to -F and -OH, whereas green preparation approaches can create new terminations with stronger affinities for S and Li, enabling improved structural control of MXene. These methods also allow for electronic modulation by creating defect vacancies and single-atom doping, thereby enhancing MXene’s characteristics [36]. Exploring green preparation strategies is therefore essential for achieving greater structural diversity, advanced surface chemistry, precise synthesis, and broader potential advantages of MXene.
2.2.1. Electrochemical Etching
Etching with F-containing agents creates -F terminations on MXene, which can react with residual water to produce HF, potentially causing electrode corrosion. Hence, the development of F-free etching methods for MXene is crucial. Electrochemical etching offers a viable alternative, utilizing a cathode–anode system submerged in an electrolyte. By applying a voltage difference and carefully adjusting the voltage range (etching potential) within the reaction window between the A and M atomic layers, the A-layer in the MAX phase can be precisely removed [37]. In 2017, Sun et al. pioneered the preparation of MXene using an F-free solution through the electrochemical etching approach [38]. Building on this, Yang et al., in 2018, successfully etched Ti3AlC2 using a two-electrode electrochemical system [39]. In this method, a mixture of NH4Cl and TMAOH was used as an electrolyte, with Ti3AlC2 acting as both the cathode and anode. The chemical reactions involved proceeded as below:
Ti3AlC2 − 3e− + 3Cl− → Ti3C2 + AlCl3
Ti3C2 + 2OH− − 2e− → Ti3C2(OH)2
Ti3C2 + 2H2O → Ti3C2(OH)2 + H2 (g)↑
Shen and colleagues initiated a molten salt-assisted electrochemical etching technique. By subjecting the MAX precursor to a 2 V voltage in a LiCl-KCl molten salt environment, they successfully synthesized Ti3C2Cl2 without introducing any metal impurities. To modify the surface terminations, they substituted -Cl with -O or -S by utilizing different salts, streamlining the process of functional group modification and broadening the variety of achievable functional groups (Figure 3b) [32]. This technique significantly accelerates MXene preparation due to the application of an electric field. Moreover, it offers a safer etching atmosphere, and the resulting MXene film features a large lateral size. However, the harsh etching conditions constrain the MXene yield, presenting challenges for scaling up production.
2.2.2. Salt-Templated Approach
Gogotsi and colleagues introduced a salt-templated technique to enable the large-scale synthesis of nitride MXene [33]. The process involved coating NaCl with MoO3, achieved by calcining a Mo precursor deposited NaCl under an Ar atmosphere. This was followed by ammoniation to convert the coating into MoN. Lastly, the NaCl was dissolved through washing, successfully isolating MoN. By adjusting the precursor and synthesis parameters, it is possible to produce a broader range of nitride MXenes. The resulting product exhibits metallic characteristics and exceptional rate capabilities (Figure 3c–e). This approach offers significant promise for producing high-quality nitride MXenes without relying on etching processes.
2.2.3. Alkali Etching Approach
Aluminum’s amphoteric nature allows it to react with acids and bases, making NaOH a viable etchant for synthesizing Ti3C2Tx [40]. In this process, OH− ions react with Al to form Al(OH)4−, which interacts with Ti3C2 to yield Ti3C2Tx. However, the Ti3C2Tx structure is unstable in alkaline environments and prone to corrosion. To address this, Li and colleagues introduced an alkali-assisted hydrothermal technique, carefully controlling the NaOH concentration (27.5 M) and reaction temperature (270 °C) to prevent framework corrosion [41]. Despite its effectiveness, this method requires high temperatures and concentrated alkali, raising safety concerns and limiting scalability. As a safer alternative, Xuan et al. utilized organic bases such as TBAOH as etching agents to intercalate and delaminate Ti3AlC2, facilitating the formation of Al(OH)4− as the surface termination of Ti3C2 [42]. Although the alkali-assisted hydrothermal method yields MXene with great purity (92 wt%), the resulting material is often multilayered and requires additional delamination to achieve few-layer or monolayer MXene for broader applications. On the other hand, the TBAOH method necessitates HF pretreatment to remove surface oxides from Ti3AlC2, introducing safety risks.
2.2.4. Lewis Acidic Molten Salt Etching
Although many MAX phases contain non-Al atomic layers, studies on synthesizing MXene from the MAX phases without Al layers remain scarce. Li’s group demonstrated a process using high-temperature ZnCl2 molten salts to process MAX phases with Al atomic layers. This approach enabled the formation of novel MAX precursors with Zn as the A-layer, such as Ti3ZnC2, V2ZnC, and Ti2ZnN [43]. Building on this work, Huang’s group introduced this approach. In this strategy, Lewis acid cations with greater redox potentials oxidize and selectively etch the A-layers of MAX phases with lesser redox potentials [44]. Using CuCl2 molten salts at elevated temperatures, this method successfully etched MAX phases with A-layers made of elements like Si, Zn, and Ga, resulting in MXene materials rich in -Cl terminations. The details of the etching process are as follows:
Ti3SiC2 + 2CuCl2 → Ti3C2 + SiCl4 (g)↑ + 2Cu
Ti3C2 + CuCl2 → Ti3C2Cl2 + Cu
Huang and colleagues, in 2023, proposed a “chemical scissor” strategy for structural modification of MAX phases and MXene, paving the way for 3D assembly of 2D materials (Figure 3f) [34]. This innovative technique expands the variety of MAX phases that can be utilized and enables precise tuning of MXene’s surface functional groups and structural characteristics. Despite its potential, the application of this approach is restricted by the intrinsic characteristics of traditional Lewis acidic molten salts, such as their low redox potential and instability at high temperatures. Additionally, the chemical mechanisms underlying the intercalation process remain insufficiently understood.
2.2.5. UV Induced Selective Etching
UV irradiation has been reported to increase the surface reactivity of materials, with Mo2Ga2C standing out for its exceptional UV-absorbing characteristics. Mei and colleagues, in 2020, leveraged a UV-sensitive Mo2Ga2C precursor and employed UV irradiation under mild conditions in the presence of phosphoric acid to etch Ga atoms. This process yielded Mo2C MXene NSs, which, after ultrasonic delamination, demonstrated potential as anodes for rechargeable batteries [45]. This approach allows for the synthesis of MXene within just a few hours, significantly dropping fabrication time. Moreover, the Mo2C exhibits mesoporous architecture, making it highly favorable for electrochemical applications. Nevertheless, the technique may require specialized equipment and precise control of the reaction conditions, potentially necessitating advanced technical expertise. In summary, while this etching offers notable benefits in certain applications, its strengths and limitations should be carefully evaluated based on the intended use.
2.2.6. CVD
CVD is a versatile technique that enables gaseous precursors to react and form solid materials, which are then deposited onto substrates for fabricating thin films, and heterostructures. Xu and colleagues employed the CVD method to directly synthesize ultrathin α-Mo2C 2D crystals with lateral dimensions exceeding 100 μm by employing methane as the C source and Cu/Mo foils as substrates at 1085 °C [46]. Later, Geng and colleagues utilized CVD to grow Mo2C on graphene, achieving lateral sizes till the centimeter dimension [47]. Notably, the Mo2C structures synthesized on graphene exhibit greater uniformity and possess hexagonal structures compared to pure Mo2C crystals. Recently, Talapin and colleagues developed a “carpet-like” Ti2CCl2 structure by depositing a gas blend of CH4 and TiCl4 onto a surface of Ti at 950 °C. This MXene featured a unique spherical architecture formed through bending and unfolding, resulting in a highly accessible surface with exposed catalytically active edges, offering exceptional Li+ storage capacity (Figure 3g,h) [35]. CVD offers several advantages: (i) it enables the direct preparation of MXene, free of surface functional groups, enabling a detailed examination of its internal atomic layer structure; (ii) it produces MXene with greater lateral sizes, great surface quality, less defect density, and precise control; (iii) it eliminates the need for MAX phase precursors, significantly reducing preparation time. Despite these advantages, the range of MXenes that can be synthesized through CVD is currently limited. Furthermore, the great production costs and stringent reaction environments, such as extended durations, specific gas requirements, and elevated temperatures, pose significant challenges for large-scale purposes.
2.2.7. Thermal Reduction
In 2020, Sun and colleagues proposed a novel thermal reduction as a straightforward and eco-friendly approach for preparing MXene. This process involved heating the Ti2SC MAX phase in an Ar and H2 atmosphere at 800 °C to precisely remove S atoms. The process was followed by ultrasound-supported treatment, yielding free-standing 2D Ti2C NSs [48]. This straightforward technique shows promise for large-scale industrial production. However, it is crucial to keep the temperature below 700 °C to prevent the nucleation of TiO2, which can adversely impact MXene yield and quality.
2.2.8. In Situ Hydrothermal Process
The hydrothermal process involves conducting a reaction within a sealed container, utilizing water as the solvent at a controlled atmosphere inside the reactor [49,50,51]. Song and colleagues employed an in situ hydrothermal process to prepare amorphous MoS2 integrated with F-free Mo2CTx. This was achieved using NH4Cl as the etchant, DMSO as the S source, and Mo2Ga2C as the starting material, under conditions of 180 °C for five days [52]. This study offers fresh perspectives on developing novel heterogeneous MXenes beyond traditional types, broadening the scope of MXene research. Moreover, the incorporation of MoS2 imparts a distinctive morphology and heterojunction structure to the heterogeneous Mo2C, enhancing its structural stability. This modification adjusts the Li+ storage activity and facilitates ion movement. Additionally, the hetero-Mo2C sample demonstrated outstanding cycle stability, retaining a capacity of 683.9 mAh g−1 after 1200 cycles (Figure 4a–c).
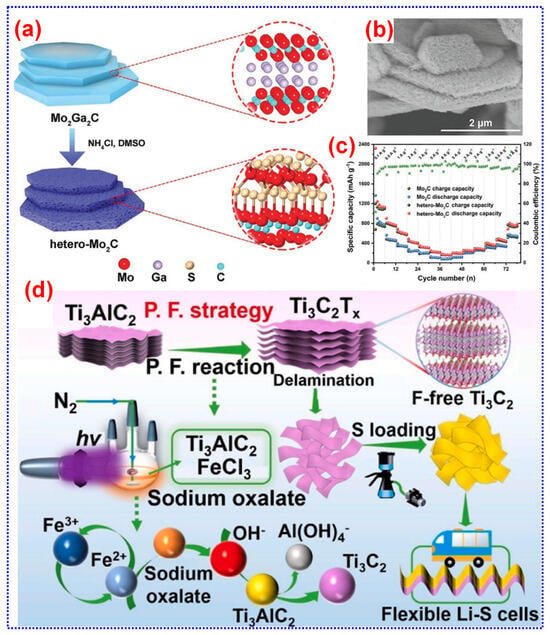
Figure 4.
(a–c) Diagram illustrating the preparation and FE-SEM image of hetero-Mo2C and the cycle performance of hetero-Mo2C and Mo2C samples at 1 A g−1. Adapted from [52], Copyright 2022, Wiley-VCH. (d) Pictorial demonstration of P.F. approach for the preparation of Ff-Ti3C2. Adapted from [53], Copyright 2022, American Chemical Society.
2.2.9. Photo-Fenton (P.F.)
The P.F. is among the widely investigated advanced oxidation processes, recognized as a promising approach for pollutant removal through reactive oxygen groups in both homo- and heterogeneous iron-mediated techniques [54,55,56,57]. Xiao and colleagues introduced a low temperature “soft chemistry” technique depending on the P.F. process. In this process, Ti3AlC2 was immersed in a solution of Fe (III) and Na2C2O4 under continuous stirring. H2O2 was then added, and the mixture was exposed to UV illumination while stirring for 10 h. After centrifugal drying, F-free Ti3C2 (Ff-Ti3C2) with a purity of up to 95% was obtained [53]. During the P.F. reaction, reactive oxygen species (HO* and O2*−) are continuously generated, weakening the Ti-Al bond in the MAX phase and promoting the high concentration of OH− formation, which facilitates the topochemical delamination of Al layer. The resulting Ff-Ti3C2 displays exceptional mechanical stability, enhanced wettability of the electrolyte, and robust electrocatalytic action for the decomposition of Li2S, making it an excellent S cathode host for advanced LSBs (Figure 4d). However, the use of light sources and oxidative processes in the P.F. technique may result in extensive synthesis periods compared to further preparation techniques, potentially enhancing experimental complication and costs.
2.2.10. Physical Vacuum Distillation
Feng and colleagues introduced an acid- and F-free physical vacuum distillation technique for the direct synthesis of various MXenes [58]. This method began with the incorporation of low-boiling-point Zn into the MAX phase using ZnCl2 molten salt. Later, the A-layer is removed through this technique, leading to the successful fabrication of a range of F-free MXenes with different TMs. These included Nb2CTx, Ti3C2Tx, Nb4C3Tx, Ta2CTx, and Ti2NTx, where Tx denotes -Cl and -O functional groups. The activity is governed by the following reactions:
Ti3AlC2 (s) + 1.5ZnCl2 (s) → Ti3C2 (s) + 1.5 Zn (s) + AlCl3 (g)↑
Ti3C2 (s) + ZnCl2 (s) → Ti3C2Cl2 (s) + 1.5 Zn (s)
Zn (s) → Zn (g)↑
The structure of MXene and specific surface area (SSA) can be customized by varying the temperature, making them suitable for diverse applications. This synthesis approach eliminates the need for acids or bases, and the metallic Zn can be reclaimed after evaporation, offering an eco-friendly, cost-efficient, and sustainable approach for producing various MXenes. Additionally, this technique shows great promise for large-scale production owing to its affordability, straightforwardness, and precise control. Furthermore, despite its capability for single-step MXene synthesis, the method operates at high temperatures. Therefore, developing alternative preparation techniques that work at room temperature or lower is crucial for enabling the industrial utilization of MXenes.
Therefore, MXenes can be produced through various synthesis techniques, including acid, and electrochemical etching, salt templating, and physical vacuum distillation. Traditional acid etching typically yields MXenes with surface functional groups like -OH, -O, and -F. In contrast, methods like electrochemical etching, salt templating, and vacuum distillation may present alternative functional groups, like S or Cl. These electronegative elements exhibit strong interactions with S and Li, enhancing electrochemical performance. By selecting and optimizing the preparation approach, the surface chemistry of MXenes may be effectively tailored to enhance the performance of LSBs. Table 2 summarizes the preparation approaches, electrochemical performance, and characteristics of MXenes, while Table 3 outlines design recommendations for their application in S cathodes, separators, and Li anodes in LSBs.

Table 2.
The characteristics, benefits, limitations, electrochemical characteristics, and impact on surface terminations associated with various MXene preparation techniques [5].

Table 3.
Guiding principles for utilizing MXene in various applications for LSBs [5].
3. The Characteristics of MXenes
3.1. Structural Characteristics
Atomic structure of MXenes is key to investigating their unique properties. As earlier discussed, MAX precursors are layered hexagonal materials classified under the P63/mmc space group, where alternating “MX” and “A” layers stack along the c-axis. When the A-layer is removed, MXenes retain the hexagonal close-packed structure of their parent MAX phases. This structural continuity means that the compositional and structural diversity of MAX precursors clearly translate into a wide range of MXenes. Researchers have identified numerous new MAX precursors to prepare unique MXenes. Depending on the choice of “M,” MXenes can be categorized into four distinct varieties [62], as depicted in Figure 5. The first and most common category is mono-TM MXenes. Examples include Ti2C [63,64,65,66], Ti2N [67,68,69,70], Nb2C [71,72], V2C [71,73], V2N [74,75], Cr2C [76,77], Ta2C [78,79], Mo2C [23,80,81], Ti3C2 [82,83,84], Zr3C2 [85], Hf3C2 [25], Ta4C3 [26,86], Nb4C3 [87,88], V4C3 [89,90], and Ti4N3 [30,91], among others. The second category consists of randomly arranged double-TM (double-M) MXenes, such as (Ti,Nb)2C [26,92], (Cr,V)3C2 [26,93], (Nb,V)2C [94], (Nb,Ti)4C3 [95], (Nb,Zr)4C3 [95], and (Mo,V)4C3 [96], among others. The third category includes ordered double-M MXenes, such as Mo2TiC2 [97], Cr2TiC2 [98], Mo2Ti2C3 [97], and Mo2ScC2 [99]. The final category is ordered divacancy MXenes, for example, Mo1.33C [100] and W1.33C [101]. Newly, some high-entropy MXenes have also been investigated, including Ti1.1V0.7CrxNb1.0Ta0.6C3Tz (Tz = -F, -O, -OH) [102], TiVNbMoC3Tx [103], TiVCrMoC3Tx [103], and (Ti1/3V1/6Zr1/6Nb1/6Ta1/6)CxN1-xTy [104].
MXenes can also be prepared from non-MAX precursors. For instance, layered carbides like Zr3Al3C5 [85], Hf3(AlSi)4C5 [25], and Mo2Ga2C [23] have been utilized to produce Zr3C2Tx, Hf3C2Tx, and Mo2CTx, respectively. Additionally, surface terminations are critical to the structural diversity and characteristics of MXenes, with their nature strongly influenced by the preparation process. These surface groups can be precisely tailored through post-treatment processes. For example, F terminations can be eliminated through high-temperature vacuum annealing [105] or alkali behavior [106]. O terminations can be introduced by exposing MXenes to O2 [107], CO2 [108], and Li2O treatment [44], or by transforming OH terminations through vacuum annealing [105]. Conversely, O terminations can be eliminated under a H2 atmosphere [105], while halide terminations can be eliminated using LiH [44]. Additionally, terminations of S, Se, and Te can be incorporated by treating MXenes with NaNH2, Li2S, Li2Se, and Li2Te, respectively [44]. However, conventional etching methods often lead to the oxidation and hydrolysis of resulting MXene moieties. Thus, it is crucial to establish a clear structure–property relationship for MXenes to better understand how to prevent their degradation. This will help improve their oxidative and aqueous-phase stability.
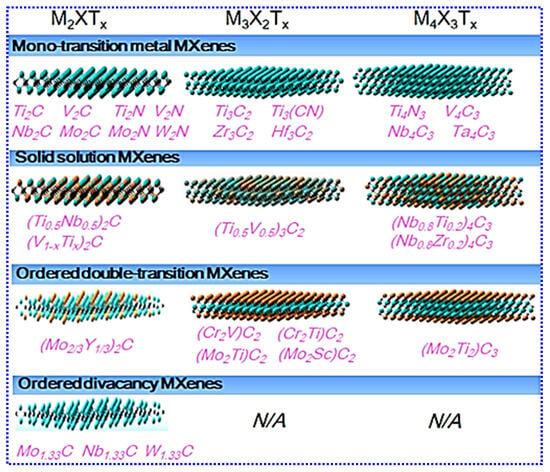

Figure 5.
Structures of synthesized MXenes. Adapted from [109], Copyright 2021, Wiley-VCH.
3.2. Electronic Characteristics
Theoretical studies have revealed that MXenes can display diverse electronic properties, ranging from metallic and semiconductor-like to topological insulating behaviors depending on their M and X atoms, as well as T surface terminations [110]. Interestingly, the electronic characteristics of MXenes are more significantly influenced by their external metal atomic layer than the internal metal layer [111]. While most MXenes exhibit a metallic nature, a subset has been predicted to function as semiconductors. Kawazoe and colleagues demonstrated through simulations that Sc2CT2 (T = F, OH, and O), Ti2CO2, Zr2CO2, and Hf2CO2 are semiconductors with band gaps spanning 0.24 to 1.8 eV [112]. The additional valence electrons contributed by N atoms make Mn+1CnTx generally more favorable as semiconductors, whereas Mn+1NnTx tends to exhibit stronger magnetic properties [112]. Surface terminations are also pivotal in shaping MXenes electronic characteristics. For instance, their work functions range widely from 1.6 to 8.0 eV [113] and can be finely tuned by modifying the surface termination groups [113]. Generally, OH terminations typically result in lower work functions owing to their effective surface dipole moment, while terminations of O and F yield greater work functions due to their great electronegativity [114].
To date, all experimentally synthesized MXenes have exhibited metallic or semi-metallic characteristics, with no semiconducting MXenes realized yet. Studies show that MXene electrical conductivity is significantly affected by factors like the precursor material, its size, etching techniques, and post-treatment processes [115]. For instance, post-annealing treatments can efficiently eliminate water, intercalants, and surface functional groups, resulting in enhanced conductivity (Figure 6a–c) [105]. Recently, Gogotsi and colleagues set a record for MXene conductivity, achieving approximately 20,000 S cm−1 for Ti3C2Tx by optimizing the Ti3AlC2 MAX [115]. Surface terminations of MXenes, typically uncovered by synthesis methods, are often diverse and contribute to their wide-ranging properties [113]. For instance, the commonly studied Ti3C2Tx generally features terminations like -O, -OH, and -F. These surface groups can be modified through vacuum annealing, allowing adjustments in the work function from 4.1 to 4.8 eV (Figure 6d,e) [114]. Additional chemical treatments, for instance, grafting diazonium surface groups [113], or using ammonium hydroxide [116], N2H4, or UV-ozone [117], provide further control over the MXenes work function. Talapin and co-workers in 2020 reported superconducting transitions in Nb2C with terminations such as -Cl2, -S2, -Se, and -NH at temperatures below 10 K [67]. Moreover, the functional groups present on MXenes facilitate their integration with a variety of organic materials and ligands, greatly enhancing their application potential [118].
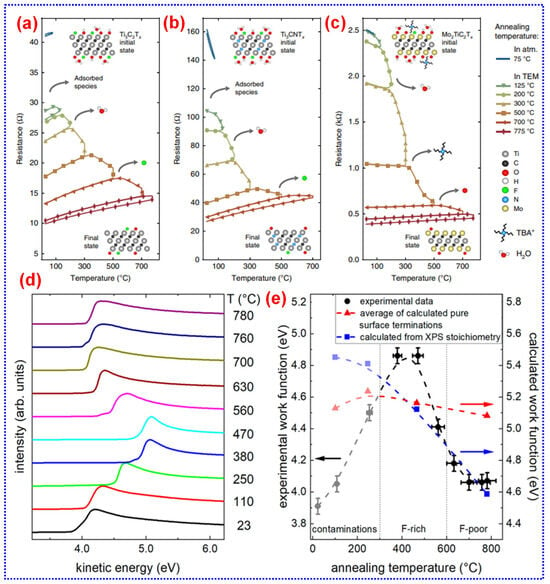
Figure 6.
(a–c) Electrical response of MXene films to heat treatment. Adapted from [105], Copyright 2019, Springer Nature. (d) Secondary electron cutoff (SECO) spectra of Ti3C2Tx after heat treatment at various temperatures. (e) Work function measurements of Ti3C2Tx with respect to temperature. Adapted from [114], Copyright 2019, American Chemical Society.
3.3. Mechanical Characteristics
MXenes significantly enhance the performance of S cathodes through three main advantages: (1) enabling uniform and efficient interactions with numerous S moieties; (2) maintaining electrocatalytic activity to facilitate the conversion of polysulfides; and (3) accommodating the substantial volume changes of S during charging and discharging. These benefits stem from the exceptional mechanical stability of MXenes, which permits them to endure the significant volume expansions of S without structural degradation. This stability ensures consistent bonding with S, thereby enhancing the cathode’s performance. The mechanical characteristics of MXenes are closely linked to their surface terminations, thickness, and structural attributes. First-principles measurements by Sun and colleagues demonstrated that 2D Ti2C can withstand considerable strain, enduring 9.5, 18, and 17% under bi- and uni-axial tension beside the x- and y-axes [119]. Additionally, functionalizing the surface with oxygen was found to prevent the failure of the Ti layer, allowing the material to stretch further to 20, 28, and 26.5% in the corresponding directions. This highlights the critical role of surface termination in enhancing the mechanical strength of MXenes [119]. Balke’s group investigated the mechanical properties of stacked Ti3C2 NSs with various surface terminations (-F, -O, and -OH) using DFT [120]. Their findings revealed that OH-terminated MXene layers had a minimal gap distance of 0.57 Å, whereas O- and F-terminated layers exhibited a gap distance of approximately 2.5 Å. The improved elastic constants of OH-terminated MXenes were attributed to the formation of hydrogen bonds between their hydroxyl groups. However, the presence of H2O molecules in the interlayer spaces significantly weakens the mechanical strength of MXenes. Structurally, bare Ti2C displayed greater stiffness and strength compared to bare Ti3C2 and Ti4C3. The Young’s modulus values for these materials were calculated using DFT and molecular dynamics (MD) simulations, yielding 597, 502, and 534 GPa, respectively [119]. Under high strain, the first crack in Ti4C3 was observed to originate at its center, whereas failure in Ti3C2 and Ti2C initiated at their edges [119]. Experimentally, the Young’s modulus of Ti3C2Tx and Nb4C3Tx was evaluated at 0.33 [121] and 0.39 TPa [122], respectively. Compared to other 2D materials, MXenes displayed exceptional mechanical performance, underscoring their competitiveness in this regard.
Although monolayer MXenes exhibit excellent mechanical properties, widely used MXene films are typically composed of stacked multilayer NSs, resulting in significantly lower mechanical performance. For instance, Gogotsi et al. reported that pure Ti3C2Tx films exhibit a Young’s modulus of 3.52 GPa and a uniaxial tensile strain of 1%, significantly lower than the predicted values for monolayer NSs [123]. This disparity is primarily due to the inherent properties of Ti3C2Tx NSs, like their size, defects, imperfect edges, and weak interlayer forces. Low-quality MXene NSs can further compromise mechanical performance, as the interlayer forces in MXene films are primarily van der Waals interactions, which do not provide sufficient mechanical strength to bulk films. Strengthening the interlayer interactions has proven to be a highly efficient strategy for improving the MXenes performance. One common strategy involves introducing ionic or bonding agents. For example, incorporating polyvinyl alcohol (PVA) into Ti3C2Tx films significantly improved their strength [123]. Gogotsi and colleagues demonstrated that the Ti3C2Tx/PVA composite films achieved a tensile strength of 91 ± 10 MPa, almost four times greater than that of Ti3C2Tx films. Similarly, Kim and colleagues drew inspiration from mussel adhesive proteins to utilize dopamine for enhancing interlayer interactions and structural organization in Ti3C2Tx films [124]. By polymerizing dopamine in situ on the surfaces of Ti3C2Tx flakes, they created a thin adhesive layer, resulting in a greatly aligned and densely packed architecture. This modification improved the tensile strength by sevenfold and significantly enhanced the films elongation. Cheng and colleagues developed a high-performance Ti3C2Tx film using a two-step bridging strategy [125]. This process involved sequentially bridging Ti3C2Tx NSs with sodium alginate through hydrogen bonding and Ca2+ ions via ionic bonding. The resulting modified Ti3C2Tx film demonstrated exceptional mechanical properties, including an in-plane tensile strength of 436 MPa and a Young’s modulus of 14 GPa, representing approximately 6.9 and 2.5-fold improvements, respectively, compared to pure Ti3C2Tx NSs. Building on this work, the same team recently implemented an effective densification technique that combined sequential bridging with hydrogen and covalent bonding. This method utilized borate ions for hydrogen bonding and sodium carboxymethyl cellulose (CMC) for covalent bonding [126]. The resulting mixed Ti3C2Tx film achieved remarkable mechanical performance, with a tensile strength of 583 ± 16 MPa, a young’s modulus of 27.8 ± 2.8 GPa, and an impressive toughness of 15.9 ± 1.0 MJ m−3.
4. Principle of LSBs
As illustrated in Figure 7, LSBs are composed of four main components: the S cathode, metal anode, organic liquid electrolyte, and a porous polymer separator. Unlike conventional LIBs that operate through an “ion intercalation/deintercalation” process, LSBs function based on redox reactions among elemental S and the metal. In LSBs, S acts as the final charge moiety, so the operational cycle typically begins with the discharge activity. During discharge, the metal anode undergoes oxidation, releasing metal cations that migrate toward the cathode in the presence of electric field. At the cathode, these cations react with S to produce metal sulfides. The reverse reactions happen during the charging phase.
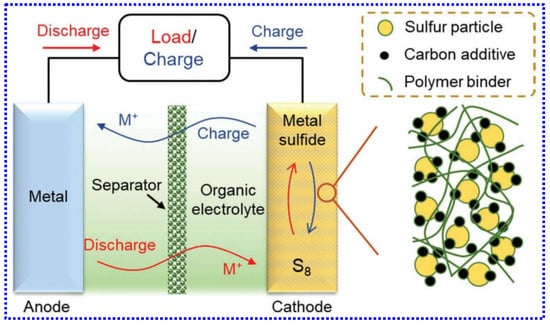
Figure 7.
Pictorial demonstration showing the configuration of LSBs. Adapted from [127], Copyright 2023, Wiley-VCH.
Figure 8 depicts the typical charge and discharge processes of LSBs in ether-related electrolytes. The entire reaction between Li and S is expressed as 2Li + S → Li2S. However, the actual conversion process between S and metal sulfides is more complex. At room temperature, elemental S exists as crown-shaped S8 rings, and the charge/discharge process implies the breaking and reforming of these S8 molecules. During this process, multi-stage LiPSs (Li2Sx, 1 ≤ x ≤ 8) are formed as intermediates [128]. Out of these intermediates, long-chain polysulfides (Li2Sx, 4 ≤ x ≤ 8) are greatly soluble in ether-related electrolytes. This high solubility has both advantages and disadvantages. On the positive side, it enables faster reaction kinetics due to liquid-phase reactions. However, it also leads to the undesirable shuttle effect, where dissolved polysulfides migrate between the electrodes, causing premature battery degradation. In contrast, short-chain polysulfides (Li2Sx, 1 ≤ x < 4) exhibit moderate solubility, reducing dissolution and shuttle-related issues. Nevertheless, their poor conductivity and slower kinetics, resulting from solid-phase reactions, hinder complete charge/discharge cycles. This limitation leads to reduced battery capacity, higher polarization, and diminished performance.
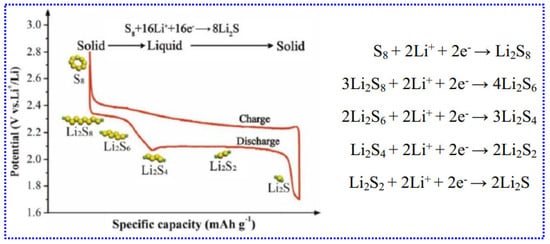
Figure 8.
Typical charge/discharge curves of a LSBs. Adapted from [129], Copyright 2022, Springer Nature.
5. Challenges and Solutions of LSBs
5.1. Challenges of LSBs
The charge storage activity in LSBs is governed by the reversible redox process between S8 and Li2S, expressed as follows:
S8 + 16Li+ + 16e− ↔ 8Li2S
During the discharge activity of LSBs, multiple intermediate reactions occur, forming various LiPSs (Li2Sx, 1 ≤ x ≤ 8), as illustrated in Figure 8. Discharge curve features two distinct voltage plateaus. The first plateau, observed between 2.4 and 2.1 V, corresponds to the decrease in S8 into long-chain LiPSs, providing a theoretical capacity of 418 mAh g−1, which involves the transfer of 0.5 electrons/S atom. The second plateau, from 2.1 to 1.7 V, reflects the conversion of LiPSs into Li2S2/Li2S, with a theoretical capacity of 1254 mAh g−1, representing 1.5 electron transfers/S atom. Due to the great solubility of LiPSs in the electrolyte, the discharge method includes phase transitions: solid (S8) → liquid (LiPSs) → solid (Li2S2/Li2S). During charging, the reverse reaction takes place, where Li2S2/Li2S is oxidized to LiPSs and eventually back to S8 [8]. However, the charge activity is significantly influenced by electric fields and LiPSs concentration gradients. Some LiPSs dissolve in the electrolyte, diffuse across the separator to an anode, and are degraded to short-chain polysulfides Li2Sn (2 ≤ n ≤ 4). These short-chain polysulfides then migrate back to the cathode where they are reoxidized to LiPSs. This repetitive cycle, called as the “shuttle effect,” causes active S loss, anode passivation, and several issues, including rapid capacity decay, reduced cycle life, minimal CE, and severe self-discharge [130]. Another challenge is the extremely low electrical conductivity of S (5 × 10−30 S cm−1 at 25 °C) and Li2S (10−30 S cm−1 at 25 °C), which limits S utilization and reduces capacity. Furthermore, the substantial volume expansion (80%) during the conversion of S8 (2.03 g cm−3) to Li2S (1.66 g cm−3) can also damage the cathode’s structural integrity [131]. For the Li anode, dendrite formation poses a critical safety risk by potentially causing short circuits. Compared to LIBs, the LSBs are more complex due to direct interactions between LiPSs and the Li anode, leading to “dead Li” formation and material loss [132]. Moreover, volume changes during plating and stripping of Li can compromise the anode’s structural stability, eventually causing its failure [133].
5.2. Solutions of LSBs
For the commercial viability of LSBs, it is crucial to enhance their practical capacity and extend cycle life. Achieving these goals primarily relies on mitigating the shuttle effect, improving electrical conductivity, reducing volume changes, and preventing Li dendrite formation. Key approaches involve the strategic design of the cathode, incorporating an interlayer between the cathode and the separator, and optimizing anode structure.
5.2.1. Rational Construction of S Cathode
Since Nazar and colleagues presented the utilization of greatly ordered mesoporous C (CMK-3) for fabricating CMK-3/S composite cathodes in 2009 [134], the design of S cathodes has become a primary strategy for enhancing the electrochemical activity of LSBs. The conductive mesoporous C structure physically traps the S, improves its utilization, and accommodates the volume changes during the charge–discharge cycles, thereby significantly boosting capacity and cycle stability [135]. Since then, extensive research has been conducted on developing composite materials to encapsulate S, leading to notable advancements in the field [136]. Broadly, the materials used to host S in composite cathodes can be classified to three main categories.
The first category includes conductive C materials, including graphene [137], CNTs [138], C nanofibers (CNFs) [139], various porous C structures [140], and their hybrids [141,142]. These C materials form a conductive framework that facilitates rapid electron transport, enabling efficient utilization of S. Additionally, their porous architecture physically confines S and LiPSs, helping to mitigate the shuttle effect and assist significant volume changes during cycling. Moreover, the nonpolar conjugated C planes offer limited ability to strongly bind polar LiPSs, resulting in capacity declining in S/C composite cathodes. To address this issue, incorporating polar sites into the C matrix through heteroatom doping [143,144,145] introduces additional anchoring points that chemically bind LiPSs, further improving the cycle stability of LSBs [146].
The second category consists of polar inorganic compounds [147], including TM oxides (TMOs) (e.g., TiO2 [148] and MnO2 [149]), TM sulfides (e.g., TiS2 [144] and MoS2 [150]), TMNs (e.g., TiN [151] and VN [152]), and TMCs (e.g., Ti3C2Tx [153] and V2CTx [154]). These materials can chemically trap LiPSs within their frameworks, exhibiting significantly stronger binding energies (B.Es) with LiPSs compared to C materials. As a result, they more effectively prevent the shuttle effect and improve the cycling performance of LSBs. However, most inorganic compounds suffer from low conductivity. To address this, they are often combined with C materials to create S hosts, which concurrently suppress the shuttle effect, enhance conductivity, and accommodate the volume variations of LSBs [155]. This confinement can lead to the accumulation of LiPSs, slowing their redox kinetics, particularly in electrodes with great S loading or during prolonged cycling. Therefore, it is essential not only to adsorb LiPSs but also to enhance the reaction kinetics among LiPSs and Li2S. This strategy helps minimize their accumulation, establish a dynamic balance of adsorption and conversion, and further mitigate the shuttle effect while improving S utilization [156]. Consequently, the third major category of S host materials centers on catalytic materials designed to accelerate the conversion between LiPSs and Li2S. These include metals (e.g., Pt [157], Pt@Ni [158]), metal compounds (Fe3O4 [159], CoP [160], MoS2 [161]), metal-free inorganic compounds (black phosphorous [162], C3N4 [163]), and their composites. To improve electrical conductivity further, these catalytic materials are often integrated with conductive C frameworks, including graphene [161] and porous C [158].
5.2.2. Rational Construction of Interlayer Between Cathode and Separator
Adding an interlayer among the cathode and separator offers an additional mechanism to mitigate the shuttle effect of LiPSs by providing physical confinement and/or chemical adsorption. Research on interlayers has primarily focused on the following categories: (i) C materials with porous architecture and high conductivity, enabling them to physically trap LiPSs while also acting as the upper current collector to enable electron transport and improve LiPSs utilization [164]; (ii) polar metal compounds [165] or heteroatom-doped C [166], which chemically bind LiPSs to prevent their shuttle; (iii) negatively charged materials for instance Nafion [167], which prevent LiPSs through electrostatic repulsion; and (iv) catalytic materials, including metal compounds [168] and metals [169], which accelerate redox kinetics by promoting the conversion of adsorbed LiPSs [170]. Interlayer materials can be applied as coatings or vacuum-filtered layers on the separator or exist as independent components. An ideal interlayer should demonstrate strong LiPSs adsorption, high catalytic activity for LiPSs conversion, and possess properties such as being thin, lightweight, and highly conductive to minimize its impact on the overall cell design.
5.2.3. Rational Construction of Li Anode
To enhance the structural stability of Li anodes in LSBs, several approaches have been exploited. These include designing suitable Li hosts [171], applying protective coatings [172], modifying electrolytes to improve the stability of the SEI [173], and transitioning from liquid to solid-state electrolytes [174]. Among these, the advanced preparation of Li hosts has proven particularly effective in suppressing Li dendrite growth and accommodating the volume variations of Li anodes. A novel Li host should possess the following characteristics: (i) light, durable, and porous to effectively accommodate Li; (ii) electrochemical stability during Li plating and stripping processes; (iii) high conductivity and a narrow Li+ diffusion energy barrier to facilitate fast electrochemical kinetics; and (iv) lithophilic properties to ensure efficient interaction with Li and promote uniform Li nucleation [175].
6. Possibility of MXenes for LSBs
Thanks to their outstanding characteristics, 2D MXenes have been widely investigated and utilized in LSBs, playing critical roles in the cathode, as an interlayer between the cathode and separator, and in the Li anode [176]. Their metallic conductivity promotes efficient electron transport, thereby improving S utilization [177]. The 2D structure offers abundant reaction sites for S conversion and can be engineered into 3D porous frameworks to support high S loading and accommodate volume expansion during cycling [178]. Additionally, MXenes exhibit strong B.E with LiPSs through the formation of metal-S bonds [179] and demonstrate catalytic mechanism to speed up the redox reaction kinetics between LiPSs and Li2S [180]. Consequently, MXenes are considered promising candidates for constructing high-performance cathodes and interlayers in LSBs. In addition to their applications in cathodes and interlayers, MXenes also serve as excellent Li hosts. Their metallic conductivity, coupled with a minimal Li+ diffusion energy barrier, enables rapid Li+/electron transfer and supports fast electrochemical kinetics [181]. Additionally, the abundant surface terminations on MXenes promote homogeneous Li nucleation and growth [182], while their 2D structure offers significant opportunities to design advanced MXene/Li composite structures that effectively inhibit Li dendrite formation [183]. Although other 2D materials, such as graphene, TMOs, and layered double hydroxides (LDHs), have also been explored for LSBs, none of them combine all the advantages offered by MXenes. Graphene, for instance, exhibits excellent conductivity, structural stability, and adjustable surface functionality, making it a favorable S host. However, it lacks catalytic activity to accelerate LiPSs conversion, and its actual conductivity is often reduced due to the incomplete reduction of GO [184]. Similarly, some 2D TMOs and LDHs can adsorb LiPSs and catalyze their conversion, but their limited conductivity restricts S employment [185]. Therefore, MXenes stand out as highly promising materials for constructing high-performance LSBs, offering a unique combination of properties that address key challenges in battery design.
6.1. Conductivity
Since the pioneering discovery of MXene by Gogotsi in 2011 [22], researchers have successfully prepared more than 30 distinct stoichiometric phases of MXenes [62]. These materials are renowned for their exceptional electrical conductivity. For instance, a transparent Ti2CTx film, 100 nm thick and produced via the spin-coating method, exhibits a conductivity of 5.25 × 105 S m−1 [186]. Likewise, a Ti3C2Tx film with a thickness of 214 nm, fabricated through blade coating, achieves a remarkable conductivity of 1.51 × 106 S m−1 [187]. The conductivity of MXenes is primarily governed by interfacial interactions between MXene NSs and can be precisely controlled by altering their composition, structural arrangement, surface terminations, stacking sequence, and intercalants [67]. For example, by controlling the terminations of Nb2C MXene to exclusively include -Cl groups, a remarkable superconductive transition is observed in Nb2CCl2 MXene at 6.3 K [67]. This tunability highlights the versatility and potential of MXenes in applications requiring tailored electrical properties. In LSBs, the metallic conductivity of MXenes enables rapid electron transfer, which supports efficient S utilization and contributes to high capacity and excellent rate performance [177]. Nazar’s group was the first to utilize Ti2CTx MXene NSs as a highly effective S host for LSBs [188]. The resulting S/Ti2CTx cathode, containing 70 wt.% S, achieved an impressive capacity of nearly 1200 mAh g−1 at 0.2 C and 1000 mAh g−1 at a 1 C rate. This demonstrated MXene’s ability to enhance electrical conductivity and electrochemical performance. Furthermore, a Ti3C2Tx interlayer, with a thickness of 522 nm, was vacuum filtrated onto a PP separator. Acting as an upper current collector, this interlayer facilitated electron transfer and enhanced LiPSs utilization. This configuration significantly boosted the capacity of the LSBs, highlighting the advantages of MXenes as interlayer materials for enhancing battery performance [189].
6.2. Structural Variety
The 2D structure of MXenes offers extensive surface contact with S. Moreover, Van der Waals forces and hydrogen bonding cause MXene NSs to stack together, which hinders Li-ion diffusion and limits the maximum utilization [190]. To overcome this, structural modifications can be implemented, such as transforming 2D NSs to 0D nanodots (NDs) or 1D nanoribbons (NRs) or assembling them into 3D porous networks. For instance, 0D Ti3C2Tx NDs were generated in situ and distributed within Ti3C2Tx NSs alongside NRs, which acted as reaction initiators to exfoliate the NSs into smaller NDs. This strategy effectively mitigated the restacking of NSs and self-agglomeration of NDs [191]. Similarly, delaminating 2D Ti3C2 MXenes through a shaking process in KOH solution produced MXene NRs, which facilitated high S loading and rapid Li+ diffusion [192]. Additionally, the abundant surface terminations of 2D MXenes allow their assembly into 3D networks, providing numerous active sites for S use and ample space for great S loading. For instance, a 3D flower type porous MXene structure was synthesized through hydrothermal treatment of Ti3C2Tx MXene in an ethylenediamine solution [193]. This structure exhibited an SSA of 132.8 m2 g−1 and a notable pore volume of 0.262 cm3 g−1. When S was loaded at 61.5 wt.%, the resulting MXene/S cathode achieved an aerial capacity of 10.04 mAh cm−2 with an S loading of 10.5 mg cm−2. These versatile MXene architectures present significant opportunities for developing high-performance LSBs with enhanced S loading.
6.3. Adsorption of Soluble Polysulfides Through Chemical Interactions
MXene NSs produced through etching methods are characterized by abundant surface terminations, which play a crucial role in shaping their magnetic [194], electronic [195], and optoelectronic characteristics [196]. These terminations are influenced by the synthesis mechanism [197] or can be customized by post-treatment techniques [67]. Consequently, the characteristics of MXenes may be precisely tuned by modulating their surface terminations. In the context of LSBs, these terminations establish stable chemical interactions with LiPSs, effectively diminishing the shuttle effect and enhancing cycling stability. This functionality makes MXenes highly advantageous as S hosts or as interlayers placed between the cathode and separator [180]. Nazar’s team used XPS analysis to uncover the robust interaction between Ti-based MXenes (for instance Ti2CTx, Ti3C2Tx, and Ti3CNTx) and LiPSs [188]. Their findings suggest that the -OH terminations on MXenes are initially utilized by LiPSs, resulting in the development of thiosulfate and polythionate species. Once the -OH groups are depleted, some Ti atoms with unoccupied orbitals are exposed, allowing these Ti atoms to accept electrons from supplementary LiPSs and form Ti-S bonds through Lewis’s acid–base relations. This dual mechanism, comprising thiosulfate/polythionate transformation and Lewis’s acid–base interactions, enables MXenes to effectively trap LiPSs (Figure 9a). Additionally, DFT measurements validated that Ti-S bond formation is the primary interaction between MXenes and LiPSs [198]. However, the B.E between MXenes and LiPSs must be carefully optimized. Excessively high B.E may cause the breakdown of LiPSs, while too low B.E may fail to securely adsorb them [199]. Achieving an optimal B.E is essential for balancing the binding strength and ensuring the stability of LiPSs. DFT calculations indicate that bare Ti2C free of surface terminations forms excessively strong Ti-S bonds with LiPSs. This interaction disrupts the S-S and Li-S bonds in LiPSs, thereby impeding the reversible reaction among LiPSs and Li2S [198]. As a result, bare Ti2C MXene is not an ideal S host. To address this issue, introducing surface terminations on MXenes is an effective strategy. These terminations facilitate additional reactions with LiPSs and reduce the strength of Ti-S interactions. The B.Es with LiPSs for terminated MXenes follow the order: Ti2CF2 < Ti2CO2 < Ti2C(OH)2 (Figure 9b). On Ti2CO2 and Ti2CF2 surfaces, LiPSs are physically adsorbed, while they bond more strongly to Ti2C(OH)2 surfaces. In this case, the H atoms in Ti2C(OH)2 lower the repulsive forces between negatively charged O and S atoms [198]. Other surface terminations, like -S, -N, and -Cl [200], as well as vacancy defects [201], can also be utilized to adjust MXenes. For instance, S-terminated Ti2C MXene demonstrates a high B.E with LiPSs and a relatively less Li+ diffusion energy barrier, making it an efficient S host with excellent capability to suppress the shuttle effect [200].
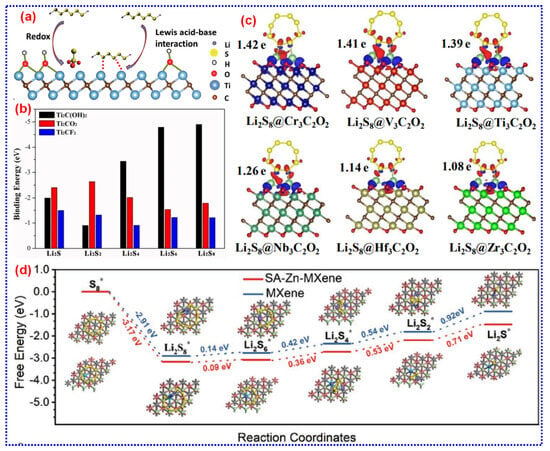
Figure 9.
(a) Chemical trapping of LiPSs using MXenes with -OH termination. Adapted from [179], Copyright 2017, Wiley-VCH. (b) B.Es of LiPSs on the terminated Ti2C free of electrolytes. Adapted from [198], Copyright 2017, American Chemical Society. (c) Charge density between Li2S8 and six O-terminated M3C2O2 (M = Cr, V, Ti, Nb, Hf, and Zr). Adapted from [202], Copyright 2019, Royal Society of Chemistry. (d) Gibbs free energy reports of single atom Zn-implanted MXene and MXene interacted with LiPSs. Adapted from [203], Copyright 2020, Wiley-VCH.
In addition to Ti-based MXenes, the interactions of other MXenes with LiPSs have been investigated theoretically [201]. DFT measurements studied six O-terminated M3C2O2 (M = Cr, V, Ti, Nb, Hf, and Zr) MXenes, all of which demonstrated the ability to trap soluble LiPSs (Figure 9c) [202]. Among these, Cr3C2O2 presented the greatest anchoring effect. Furthermore, the study revealed that the anchoring effect for soluble LiPSs increases as the lattice constant of MXenes decreases, offering valuable insights for selecting and designing MXene-based S hosts. The composition, surface terminations, and vacancy defects of MXenes significantly influence their adsorption capabilities with LiPSs. Unlike theoretical MXene models that often assume a single type of termination, actual MXene NSs typically feature a mixture of terminations, leading to more complex interaction mechanisms with LiPSs. Consequently, the theoretical results utilized to predict LiPS adsorption need further refinement to enhance their accuracy. Guided by these theoretical insights, exploring MXenes with novel compositions and diverse terminations remains an essential area of research.
6.4. Catalytic Role in Accelerating the Conversion of Soluble Polysulfides
MXenes exhibit exceptional properties, including metallic conductivity, a high SSA, a tunable bandgap structure, and remarkable carrier anisotropic mobility, making them highly promising electrocatalysts with excellent activity, selectivity, and prolonged loading lifetimes [204]. As a result, MXenes and MXene-based nanocomposites have been successfully utilized in various electrocatalytic applications, such as the HER, OER, N2 RR, O2 RR, and CO2 RR [205]. In the context of LSBs, MXenes have demonstrated their effectiveness as catalysts in facilitating the transformation of LiPSs to Li2S. By lowering the redox reaction barrier and accelerating redox kinetics, MXenes significantly enhances the rate performance of LSBs [154].
The catalytic performance of MXenes was strongly influenced by surface terminations and vacancies. Using the climbing-image nudged elastic band technique, the decomposition barriers of Li2S on functionalized Ti3C2T2 MXenes were calculated in the following order: Ti3C2S2 < Ti3C2O2 < Ti3C2F2 < Ti3C2N2 < Ti3C2Cl2. Notably, all these values are significantly lower than the natural decomposition barrier of Li2S [180]. Among them, Ti3C2S2 and Ti3C2O2 exhibit the lowest decomposition barriers for Li2S6, highlighting their efficiency in accelerating the transformation between LiPSs and Li2S. These two MXenes also have the shortest Li+ diffusion energy barriers, making them excellent candidates for S host materials. Furthermore, introducing suitable vacancies on the MXene can further boost their catalytic activity. For instance, Ti3C2Cl2, with a 1/16 Cl deficiency, exhibits significantly reduced decomposition barriers for Li2S and Li2S6, at 0.316 and 0.537 eV, respectively, compared to 1.625 and 1.532 eV for the non-deficient surface. This demonstrates a substantial improvement in catalytic performance [180].
Beyond modifying surface terminations, heteroatom doping, such as with N or S, is a powerful strategy to enhance the catalytic activity of MXenes in LSBs. For example, a porous N-doped Ti3C2 with active electrocatalytic properties was synthesized via a melamine-formaldehyde template method [206]. This porous structure, characterized by a high SSA and exceptional conductivity, offered numerous active sites for LiPSs adsorption and facilitated their transformation. The incorporation of N atoms further improved electron transfer and lowered the decomposition barrier of Li2S, thereby accelerating LiPSs conversion and amplifying the catalytic mechanism. Additionally, a single Zn atom implantation into MXenes was explored as an S host, significantly reducing the energy barriers for the conversion of Li2S4 to Li2S2 and Li2S. This improvement is attributed to the great electronegativity of Zn atoms (Figure 9d), enabling the LSBs to achieve outstanding rate performance, delivering a capacity of 640 mAh g−1 at 6 C [203].
6.5. Prevention of Li Dendrite Growth
MXene NSs feature a distinctive 2D architecture, metallic conductivity, rapid Li+ diffusion, and abundant surface terminations, making them highly effective as Li hosts for suppressing dendrite formation and accommodating volume changes of Li during cycling [207]. First, the structural versatility and exceptional mechanical properties of MXenes offer significant opportunities for designing MXene/Li composite architectures [208]. Second, their metallic conductivity combined with a low Li+ diffusion energy barrier ensures efficient electron and Li+ passage, enabling greater electrochemical kinetics [208]. Third, the surface terminations of MXenes exhibit a strong affinity for Li, offering numerous nucleation sites that promote homogeneous Li deposition and inhibit dendrite formation [182]. For instance, a lamellar Ti3C2Tx-Li metal film was developed using a roll-to-roll process. In this structure, Li was evenly distributed between the MXene sheets, and the conductive Ti3C2Tx framework directed controlled Li dendrite growth within nanoscale gaps. This approach effectively suppressed vertical dendrite development, mitigating the risk of separator piercing [209].
MXenes hold significant potential for enhancing the performance of LSBs by serving as high-performance cathode materials and interlayers between the cathode and separator. Their metallic conductivity, diverse structures, robust chemical adsorption of LiPSs, and catalytic activity for the transformation of LiPSs to Li2S make them ideal candidates for this application. Incorporating MXenes as S hosts or interlayer materials in LSBs can boost S consumption and capacity, mitigate the shuttle effect to improve cycle performance, and accelerate redox reaction kinetics, thereby enhancing rate performance. Additionally, MXenes exhibit a strong affinity for Li, promoting uniform Li nucleation and suppressing dendrite growth, which lowers the risk of short circuits. Consequently, MXenes are widely regarded as promising materials for S hosts in cathodes, effective interlayer components, and reliable matrices in Li anodes for LSBs.
7. Theoretical Investigations on MXene-Based LSBs
Recently, extensive research has focused on designing S hosts with robust chemical interactions with LiPSs and investigating the fundamental mechanisms underlying these materials [210]. With their interactive surfaces and excellent conductivity, MXenes have emerged as highly favorable anchoring materials for S cathodes [211]. Gaining a comprehensive understanding of their fundamental properties and potential applications is crucial for advancing both science and technology in this field. This section provides a concise summary of key theoretical insights into LSBs using MXenes with uniform or non-uniform functionalization (Figure 10).
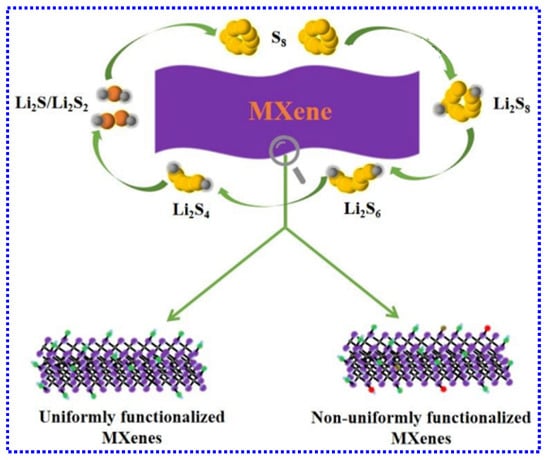
Figure 10.
Representation of theoretical insights into LSBs utilizing uniformly or non-uniformly functionalized MXenes. Adapted from [212], Copyright 2019, Royal Society of Chemistry.
7.1. MXenes with Uniform Functionalization
7.1.1. MXenes with OH-Functionalization
In 2015, Nazar’s group pioneered the use of the Ti2CTx MXene phase as an S host material [188]. They investigated the surface reactivity of Ti3C2 and Ti3CN MXenes upon interaction with LiPSs through XPS and DFT analyses. Their study revealed that before the formation of Ti-S bonds through Lewis’s acid–base interactions, the terminal -OH groups on Ti3C2 and Ti3CN are steadily utilized by trapped LiPSs, leading to the generation of thiosulfate and polythionate arbitrates. Subsequent research by Rao and collaborators highlighted the strong interactions between S in polysulfide chains and hydrogen in -OH groups, which facilitate the binding of LiPSs to OH-terminated MXenes. This interaction improves the retention of S species within the cathode area of LSBs [198]. Moreover, MXenes are typically prepared from the MAX phases by etching Al or Si using aqueous HF or HCl/LiF solutions, a process that introduces -F, -OH, and -O groups onto the MXene surface [213]. Identifying which specific functional groups dominate or whether they act synergistically remains a challenge. Understanding the anchoring mechanisms of these surface terminations toward LiPSs is therefore critical for advancing experimental research on MXenes.
7.1.2. MXenes with O/F-Functionalization
The surface terminations of MXenes are heavily influenced by etching conditions and post-synthetic treatments [213]. While there are discrepancies in quantifying termination group concentrations using characterizations like XPS and NMR, it is generally accepted that -F and -O are the dominant functional groups on MXene surfaces. Recent studies have explored the B.E, bond characteristics, and electron transfer associated with -O and -F uniformly functionalized MXenes [198,201]. Zhao and colleagues provided a detailed analysis of the anchoring mechanisms of O-terminated Ti3C2 and Ti2C MXenes on LiPSs (Figure 11a) [201]. Their findings revealed that interactions between Li+ in LiPSs and O-atoms in Ti2CO2 and Ti3C2O2 monolayers enable O-terminated MXenes to bind LiPSs with control binding strength, stabilizing soluble LiPSs. This behavior demonstrates the amphiphilic nature of MXenes, with lithiophilic properties stemming from Li-O bonding and sulfiphilic characteristics from Ti-S bonding. Fan and collaborators extended this investigation by examining the chemical interactions between LiPSs and six M3C2O2-type MXenes, where M represents Cr, V, Ti, Nb, Hf, and Zr (Figure 11b). Their findings confirmed significant Li-O interactions between these MXenes and LiPSs, akin to the behavior observed with Ti2CO2. Additionally, they identified a correlation between B.Es and lattice constants of M3C2O2 MXenes, where smaller lattice constants resulted in stronger binding with LiPSs [202]. In another work, Wei’s team explored the synergistic effects of LiPSs binding and the catalytic delithiation of Li2S using Ti3C2 MXenes (Figure 11c) [180]. Their results demonstrated that O- and S-terminated Ti3C2 MXenes are highly effective as hosts for S cathodes, corroborating Zhao’s earlier findings [201].
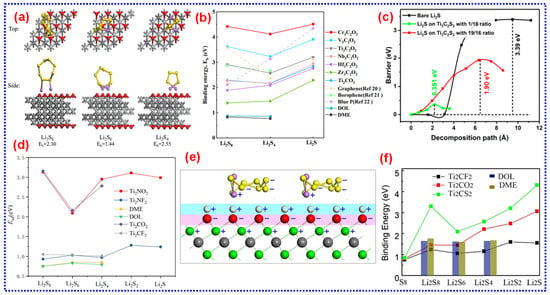
Figure 11.
(a) The optimized configurations of Li2S8, Li2S6, and Li2S4 adsorbed on a Ti3C2O2 monolayer, along with their respective B.Es. Adapted from [201], Copyright 2017, Elsevier B.V. (b) Graphs showing the calculated B.Es of LiPSs with different M3C2O2 MXenes. Adapted from [202], Copyright 2019, Royal Society of Chemistry. (c) Decomposition barriers of bare Li2S (black) compared to Li2S on a Ti3C2S2 supercell, with Li2S to Ti3C2S2 ratios of 1:16 (green) and 19:16 (red). Adapted from [180], Copyright 2019, American Chemical Society. (d) Adsorption energies of Li2Sx species on different substrates. Adapted from [214], Copyright 2018, Elsevier B.V. (e) Diagram of charged atoms in Li2Sx and MXenes, where “+” denotes electropositive and “-” denotes electronegative atoms. Ti is represented by green spheres, S by yellow spheres, Li by purple spheres, O/F by red spheres within a pink area, and H by white spheres within a light blue area. Adapted from [198], Copyright 2017, American Chemical Society. (f) Comparison of B.Es of Li2Sx with Ti2CS2, Ti2CO2, and Ti2CF2. Adapted from [200], Copyright 2018, Elsevier B.V.
Lin and colleagues investigated the properties of O/F-functionalized Ti2N as substrates, demonstrating that Ti2NO2 and Ti2NF2 exhibit average adsorption energies for LiPSs (Figure 11d) [214]. They found that these MXenes strike an optimal balance between strong adsorption and preserving the structural integrity of LiPSs. Additionally, DOS analysis for S8, Li2S8, Li2S6, Li2S4, Li2S2, and Li2S adsorbed on Ti2NO2 validated that the metallic nature of Ti2NO2 and Ti2NF2 remains intact after adsorption. This retention of metallic properties allows trapped LiPSs species to be readily reduced, with their free electrons facilitating the redox reactions of S species. Similar behavior was observed for F/O-functionalized Ti2C (Ti2CF2 and Ti2CO2) [215]. As a result, Ti2NO2, Ti2NF2, Ti2CF2, and Ti2CO2 are promising candidates for LSBs with improved electrochemical characteristics. Moreover, Rao and collaborators noted that an attraction between Ti and S atoms weakens due to the improved repulsive forces exerted by O/F-termination groups on S atoms [198]. This explains the lower B.Es of LiPSs on functionalized MXenes compared to bare MXenes, with B.E following the order: Ti2CF2 < Ti2CO2 < Ti2C(OH)2. Interestingly, introducing H atoms partially mitigates this repulsive force, as the positively charged H atoms enhance attraction to S atoms. This phenomenon is pictorially illustrated in Figure 11e.
Theoretical calculations reveal that after interacting with long-chain LiPSs, the -O groups on MXenes increase while the -OH groups decrease. This occurs because the protruding H atoms on the MXene surface are more readily replaced by Li, as the dissociation energy required for H removal is lower than that for -OH groups. As a result, the conductivity of MXenes remains largely unaffected by LiPSs adsorption, with their band gaps exhibiting minimal adjustment. Additionally, surface-sulfurized Ti2C (Ti2CS2) have been investigated (Figure 11f) [200]. Compared to Ti2CO2 and Ti2CF2, Ti2CS2 demonstrates the robust affinity for LiPSs, with B.Es following the trend: Ti2CF2 < Ti2CO2 < Ti2CS2. This enhanced binding is attributed to Li’s tendency to interact with negatively charged atoms, for instance, O and F, while being repelled by positively charged Ti atoms. S-terminated MXenes strike a balance between attractive (F-Li) and repulsive (F-Ti) forces because of S’s lower electronegativity (S: 2.58; O: 3.44; F: 3.98), consistent with findings reported by Rao and colleagues [198]. Although S-terminated MXenes have not yet been experimentally synthesized, their robust affinity for polysulfides suggests significant potential for mitigating the LiPSs shuttle effect. Additionally, their metallic properties and low-energy barriers for Li diffusion facilitate the electrochemical reactions of S moieties. These features make S-terminated MXenes promising candidates for designing S host materials for high-performance LSBs.
7.2. MXenes with Non-Uniform Functionalization
Theoretical models of MXenes used in DFT measurements typically assume idealized structures with surfaces uniformly terminated by a single type of functional group. However, in reality, MXene surfaces are far from uniform and cannot be exclusively terminated with one specific functional group. Experimental findings consistently demonstrate that MXenes possess complex and diverse surface terminations [213]. Therefore, it is essential to investigate the interactions between non-uniformly functionalized MXene surfaces and LiPSs to enhance their performance as effective anchoring materials for LSBs. Chung and colleagues explored the anchoring behavior of LiPSs on F/O-functionalized Ti2C employing spin-polarized DFT models [216]. Their study examined the effects of substitutional, vacancy, and S-trapped sites on the F/O-functionalized Ti2C surfaces (Figure 12a–i). On the Ti2CO2 surface, F-substitutional sites weaken the interactions between Li and O atoms, thereby preventing the neutralization of S atoms. This suppression effect is influenced by the presence of -F functional groups, even during LiPSs adsorption. In contrast, O-substitutional sites showed robust interactions with Li sites than F sites, making them energetically more favorable for anchoring LiPSs. Additionally, the study revealed that surface vacancy sites, regions partially devoid of -F/-O groups, can stabilize S atoms but pose a risk of losing active material. This is due to the robust interactions between exposed Ti and S atoms, which can break the S-S covalent bonds in S species. Therefore, MXene preparation strategies should prioritize reducing functional group vacancies rather than aiming for uniform functionalization with a single type of group.
Figure 12.
Optimized configurations of Li2S8 anchored at various sites on F/O-functionalized Ti2C surfaces: (a) substitutional, (b) vacancy, and (c) S-trapped sites for F-functionalized Ti2C; and (d) substitutional, (e) vacancy, and (f) S-trapped sites for O-functionalized Ti2C. The spheres represent different atoms: Ti (gray), C (black), F (sky blue), O (red), S (yellow), and Li (green). Electron localization functions of Li2S8 are shown for the vacancy sites of (g) F-functionalized and (h) O-functionalized Ti2C, along with (i) an isolated Li atom on the S-trapped site of F-functionalized Ti2C. The localized electron density values were normalized on a scale from 0 to 1. Adapted from [216], Copyright 2018, Elsevier B.V.
The widespread adoption of LSBs is still limited by the challenge of soluble LiPSs dissolving into the electrolyte. Although theoretical predictions about the anchoring mechanisms of LiPSs on MXenes may be difficult to replicate experimentally due to differences between theoretical models and real-world results, these investigations have offered valuable insights. They contribute to advancing MXene design and provide critical guidance for optimizing MXenes as anchoring materials to achieve high-performance LSBs.
8. Experimental Advances of MXenes-Based LSBs
LSBs have gathered significant interest for their exceptional energy storage capabilities. Moreover, several inherent drawbacks have hindered their commercial viability, with low conductivity, the shuttle effect, slow reaction kinetics at the S cathode, dendrite formation, and volume fluctuations in the Li anode [217]. Thanks to their distinctive characteristics, for instance, excellent metallic conductivity, efficient polysulfide trapping, rich lithiophilic surface groups, and a customizable structure, MXenes have been widely applied in various parts of LSBs, showing great promise for enhancing their electrochemical performance [218]. This section offers a comprehensive review of recent developments in the utilization of MXenes to the cathode, anode, and separator in LSBs.
8.1. MXene-Based S Cathode
First, the S cathode and its discharge moieties have extremely low conductivity (S: 5 × 10−30; Li2S: 10−13 S cm−1), leading to significant overpotentials and reduced energy efficiency during cycling. During discharge, S forms an insulating, insoluble layer on the cathode surface, resulting in poor consumption of the active S material and sluggish kinetics. Second, the density variation between S and Li2S (1.67 vs. 2.03 g cm−3) causes substantial volume change when S is converted to Li2S during discharge, leading to detachment of S from the cathode structure and significant capacity loss. Lastly, the soluble LiPSs generated during discharge diffuse into the electrolyte. Driven by the concentration gradient, long-chain LiPSs migrate to Li anode, where they are diminished to short-chain LiPSs. During charging, these short-chain LiPSs move back to the cathode and are re-oxidized into higher-order LiPSs, creating a shuttle effect. This phenomenon lowers the CE and ultimately causes battery failure [219]. To address these challenges, MXene-based S hosts have been developed. To optimize the performance of LSBs, MXene-based S hosts should possess the following key characteristics: (i) ample surface terminations combined with great conductivity to efficiently capture polysulfides and facilitate fast electron transport; (ii) a well-controlled structure with strong mechanical characteristics and a high SSA to deliver numerous reaction sites for S conversion and accommodate volume fluctuation; and (iii) abundant active sites that reduce redox energy barriers, improving the conversion kinetics of polysulfides.
8.1.1. Direct Use of MXenes as S Host
Electrochemical characteristics of LSBs can be greatly improved by directly incorporating S into MXene NSs. For instance, an accordion-like Ti3C2, fabricated via HF etching, was infused with S using a melt-diffusion approach, resulting in a S/Ti3C2 composite containing 57.6 wt.% S. This composite delivered an initial capacity of 1291 mAh g−1 at 0.2 A g−1 and retained 970 mAh g−1 after 100 cycles [220]. Similarly, a S@MXene composite was prepared through a hydrothermal treatment, where S nanoparticles (NPs) were uniformly distributed within the interlayer space and deposited on the accordion-type Ti3C2Tx surface (Figure 13a,b). Featuring a great areal S loading of 4 mg cm−2 and 67.1 wt.% S content, the S@MXene composite demonstrated an initial capacity of 1477.2 mAh g−1 at 0.2 C, retained 82% of its capacity after 100 cycles (Figure 13c), and achieved a capacity of 860.2 mAh g−1 at a rate of 2 C [221].
Using DMSO intercalation treatment, accordion-like Ti2CTx MXene was delaminated into thinner, layered NSs with increased SSA (Figure 13d). These NSs were then combined with S through a melt-diffusion process to form a uniform Ti2CTx/S composite [188]. When coated onto a C paper current collector, the resulting Ti2CTx/S electrode, containing 70 wt.% S, demonstrated a capacity of 1090 mAh g−1 at 0.5 C and exhibited exceptional cycle performance over 650 cycles, with a low-capacity decay of just 0.05% per cycle (Figure 13e). Further, the behavior of S/MXene composites can be enhanced by adding a protective coating. For example, a S/delaminated Ti3C2Tx composite was encapsulated with a PDA layer, which efficiently mitigated the LiPSs shuttle effect and accommodated S’s volume growth [222]. The S/Ti3C2Tx@PDA electrode, with a S content of 60 wt.%, achieved a capacity of 1044 mAh g−1 after 150 cycles at 0.2 C, a high-rate capacity of 624 mAh g−1 at 6 C, and retained 556 mAh g−1 after 330 cycles at 0.5 C, even with an S loading of 4.4 mg cm−2. Single-layered MXene NSs synthesized using a one-step LiF/HCl etching approach have proven to be excellent conductive hosts for S. Using this method, Ti3C2Tx NSs (Figure 13f) with a wide range of flake sizes were produced and combined with S via a melt-diffusion technique to form a Ti3C2Tx/S composite (Figure 13g). By incorporating a single-walled CNTs (SWCNTs) interlayer between the cathode and separator, the Ti3C2Tx/S cathode with 50 wt.% S achieved a capacity of 1458 mAh g−1 at 0.1 A g−1, retained 608 mAh g−1 at 8.2 A g−1, and demonstrated remarkable stability with a capacity decay of just 0.04% per cycle at 0.8 A g−1 over 1500 cycles. Even with an increased S content of 80 wt.% and an S loading of 3.5 mg cm−2, the cathode exhibited an impressive initial capacity of 675.2 mAh g−1 at 1.5 A g−1 and maintained 92.6% retention in capacity after 600 cycles (Figure 13h) [223].
Thanks to its 2D morphology and exceptional mechanical characteristics, MXenes are well-suited as flexible substrates for S electrodes. The negatively charged surfaces of single-layered MXene NSs, resulting from abundant functional groups, allow them to disperse uniformly in aqueous solutions, forming stable colloidal suspensions. By introducing Na2Sx and HCOOH into a Ti3C2Tx colloidal suspension, S NPs are synthesized in situ, producing a homogeneous Ti3C2Tx/S ink (Figure 13i,j) [224]. A vacuum filtration applied to a separator transforms this ink into a freestanding S@Ti3C2Tx film (Figure 13j,k). This film, containing 70 wt.% S, achieved capacities of 1184 and 1044 mAh g−1 at 0.2 and 2 C, respectively, and retained 724 mAh g−1 after 800 cycles at 0.2 C (Figure 13l). Furthermore, a pouch cell was constructed, employing the S@Ti3C2Tx as the cathode and a Li ribbon as the anode, housed within a commercial plastic casing. This setup delivered an initial capacity of 1263 mAh g−1 at 0.5 C and retained 1119 mAh g−1 after five cycles under bending conditions, outperforming its performance under flat conditions (initial capacity of 1124 mAh g−1 at 0.5 C with 903 mAh g−1 retention after 5 cycles) [224]. Additionally, a flexible Ti3C2Tx/S film was fabricated using a straightforward vapor deposition technique to insert S onto a robust freestanding Ti3C2Tx paper. Containing 30 wt.% S, this Ti3C2Tx/S paper delivered capacities of 1383 and 1075 mAh g−1 at 0.1 and 2 C and exhibited an exceptionally low capacity decay of just 0.014% per cycle over 1500 cycles at 1 C. A prototype full cell, utilizing the Ti3C2Tx/S paper as the cathode and a pre-lithiated germanium anode, achieved an initial capacity of 483 mAh g−1 at 0.34 A g−1 following precycling, maintained 351 mAh g−1 at 1.68 A g−1, and retained 226 mAh g−1 after 186 cycles at 1.68 A g−1 [225]. These findings underscore the remarkable improvements in energy storage performance when MXene NSs are directly utilized as S hosts for LSBs. However, a persistent challenge remains: the restacking of MXene NSs, which hinders Li-ion diffusion and prevents MXenes from reaching their full potential [190]. To overcome this problem, researchers have explored various strategies, including chemical modification, structural control of MXenes, and the development of MXene-based composites.
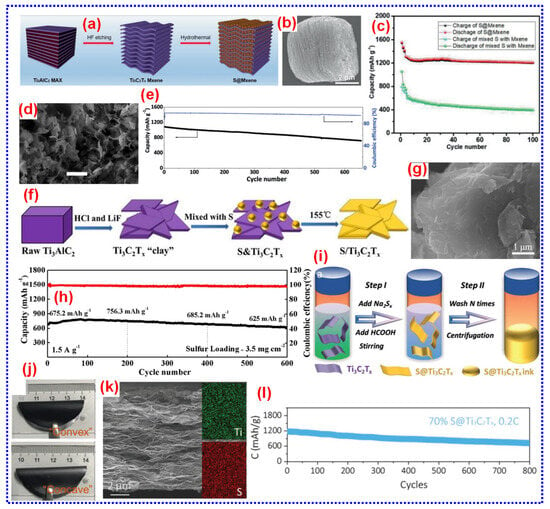

Figure 13.
(a) Pictorial illustration, (b) SEM micrograph, and (c) cycle activity of S@accordion-type Ti3C2Tx composite. Adapted from [221], Copyright 2020, De Gruyter. (d) SEM micrograph and (e) cycling stability of the delaminated Ti2C/S composite. Adapted from [188], Copyright 2015, Wiley-VCH. (f) Schematic, (g) SEM micrograph, and (h) cycling stability of the S/Ti3C2Tx electrode. Adapted from [223], Copyright 2018, Royal Society of Chemistry. (i) Diagram illustrating the preparation of S@Ti3C2Tx ink. (j) Optical image, (k) SEM micrograph, and (l) cycling stability of S@Ti3C2Tx film electrode. Adapted from [224], Copyright 2018, Wiley-VCH.
8.1.2. Chemical Adjustment of MXenes as S Host
Chemical adjustment is a viable approach to enhance the performance of MXenes. Studies have shown that doping heteroatoms like N, S, or metal atoms into conductive hosts can greatly improve their catalytic properties [226]. In LSBs, heteroatom-doped MXenes show improved chemical adsorption of LiPSs and improved catalytic activity [203]. For example, single Zn atom-inserted Ti3C2Tx layers were prepared by etching Al layers from the Ti3AlC2 MAX phase using molten ZnCl2, resulting in Zn atoms being embedded at Ti sites [203]. The Zn inserted MXene revealed robust interactions with LiPSs due to the great electronegativity of Zn, facilitating rapid conversion between LiPSs and Li2S2/Li2S by accelerating the nucleation of Li2S2/Li2S. After S incorporation, the Zn inserted MXene NSs were covered around S balls (Figure 14a,b). The resulting S@Zn-inserted MXene electrode, with an S content of 89 wt.%, achieved a capacity of 1136 mAh g−1 at 0.2 C and 517 mAh g−1 at 6 C, retaining 706 mAh g−1 after 400 cycles at 1 C (Figure 14c), surpassing the performance of S@MXene. Additionally, further adjustment with 1,3-diisopropenylbenzene improved structural stability, increasing the electrode’s capacity to 1210 mAh g−1 at 0.2 C and 640 mAh g−1 at 6 C.
Doping MXenes with heteroatoms like N and S has been shown to significantly improve their performance in LSBs [227]. For example, a 3D crumpled N-doped Ti3C2Tx network was prepared through heat treatment of negatively charged Ti3C2Tx flakes and positively charged melamine (Figure 14d) [228]. N doping improved the electrical conductivity of MXene, strengthened its interaction with LiPSs, and enhanced its catalytic behavior. As a result, the crumpled N-doped MXene/S electrode, containing 73.85 wt.% S and an S loading of 1.5 mg cm−2, delivered a capacity of 1144 mAh g−1 at 0.2 C and demonstrated excellent cycling performance, retaining 610 mAh g−1 at 2 C after 1000 cycles (Figure 14e). Even with a greater S loading of 5.1 mg cm−2, the electrode achieved an initial capacity of 765 mAh g−1 at 0.2 C and retained 588 mAh g−1 after 500 cycles (Figure 14f). Additionally, a porous N-doped Ti3C2 with a SSA of 171.5 m2 g−1 was synthesized using a melamine-formaldehyde template method [206]. The porous architecture, combined with N doping, enhanced the MXene’s ability to adsorb LiPSs and its catalytic activity. The N-doped MXene/S electrode, containing 64 wt.% S, revealed a capacity of 1072 mAh g−1 at 0.5 C, excellent rate capability with 729 mAh g−1 at 3 C, and outstanding cycling performance, showing only 0.033% capacity decay per cycle over 1200 cycles at 2 C. Even at a high S loading of 8.2 mg cm−2, the electrode achieved a remarkable areal capacity of 9 mAh cm−2. When incorporated into a pouch cell with an S loading of 1.5 mg cm−2, it retained 65.3 and 79.1% of its capacity at 0.1 C and 0.2 C after 100 cycles, respectively.
Heteroatom doping has been proven to significantly increase the adsorption capability and catalytic behavior of MXenes toward LiPSs. Consequently, further research is warranted to explore the introduction of various dopant atoms, for instance, S, P, Ni, and Co. Additionally, in LSBs, surface engineering of MXenes remains largely confined to theoretical computational studies, with limited experimental validation. Given the significant impact of MXene terminations on their adsorption B.E and the breakdown barrier of LiPSs, it is essential to study MXenes with well-defined terminations to achieve optimal performance in LSBs.
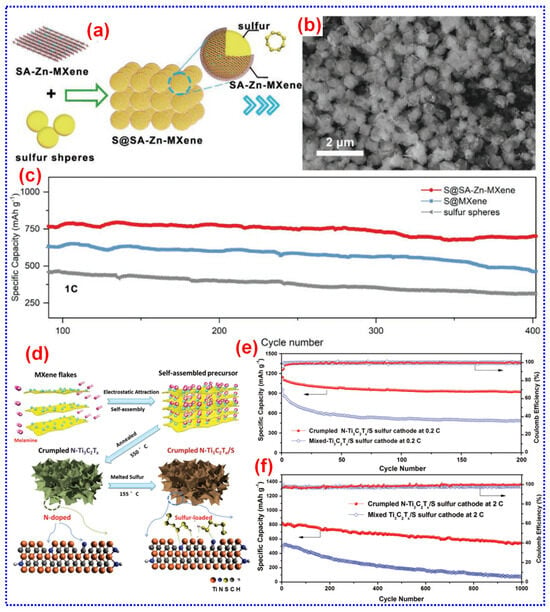

Figure 14.
(a) Diagram illustration the preparation, (b) SEM micrograph, and (c) cycling stability of S electrode coated with single atom Zn inserted MXene (S@SA-Zn-MXene). Adapted from [203], Copyright 2020, Wiley-VCH. (d) Illustration of synthesis. Cycling stability of crumpled N-doped Ti3C2Tx/S electrode at (e) 2 C with an S loading of 1.5 mg cm−2 and (f) 0.2 C with an S loading of 5.1 mg cm−2. Adapted from [228], Copyright 2018, Wiley-VCH.
8.1.3. Structure Engineering of MXenes for S Host
Structural engineering, focused on constructing open configurations for MXenes, is essential for minimizing restacking and maximizing exposed SSA, thereby enhancing their utilization [229]. Different approaches have been devised to achieve this, including expanding the interlayer spacing [230] and creating open structures such as 0D [191], 1D [192], and 3D frameworks [193]. A widely adopted approach to expanding the interlayer spacing of MXenes involves introducing molecular or ionic spacers between the 2D MXene NSs. For instance, Li+ intercalation enhanced the interlayer distance of V2C MXene from 0.736 to 0.939 nm (Figure 15a), facilitating Li+ movement within the S electrode [230]. By employing a rGO/CNT composite as a flexible scaffold, a freestanding S@Li+-intercalated V2C/rGO-CNT electrode with 70 wt% S content was constructed. This electrode demonstrated excellent stability, with minimal capacity decay of 0.053 and 0.051% per cycle over 500 cycles at 1 C and 2 C, respectively (Figure 15b). Another strategy to mitigate restacking and create open pathways for rapid Li+ movement involves transforming 2D MXene NSs into 0D NDs. Using sodium alginate as a reaction initiator, 2D Ti3C2Tx NSs were hydrothermally treated to produce 0D Ti3C2Tx NDs that were interspersed in situ among the Ti3C2Tx NSs [191]. Sodium alginate facilitated the exfoliation process by interacting strongly with Ti sites on Ti3C2Tx, triggering C-Ti bond cleavage and layer separation. This yielded a composite of Ti3C2Tx NDs and NSs that effectively prevented restacking of NSs and aggregation of NDs, enabling spatial immobilization of LiPSs and catalytic transformation of high-loaded S moieties. The resulting cathode, containing 67.6 wt% S and 1.8 mg cm−2 S loading, achieved an impressive capacity of 1609 mAh g−1 at 0.05 C and 882 mAh g−1 at 3 C. Furthermore, with a high S loading of 13.8 mg cm−2, it delivered exceptional areal and volumetric capacities of 13.7 mAh cm−2 and 1957 mAh cm−3, respectively, at 0.05 C.
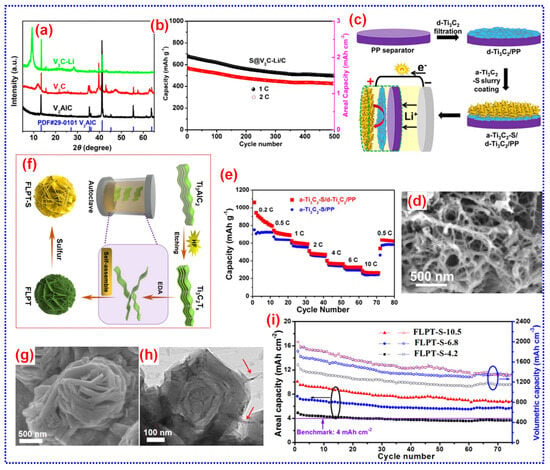
Figure 15.
(a) XRD patterns of V2AlC, V2C, and Li+-intercalated V2C. (b) Cycle study of S@Li+-intercalated V2C. Adapted from [230], Copyright 2020, American Chemical Society. (c) SEM micrograph of a-Ti3C2 NRs. (d) Illustration of fabrication and (e) rate performance of flexible a-Ti3C2-S/delaminated-Ti3C2/PP electrode. Adapted from [192], Copyright 2018, American Chemical Society. (f) Pictorial demonstration of preparation, (g) SEM, and (h) TEM micrographs. (i) Cycle study at 1/30 C of 3D flower-type porous Ti3C2Tx-S composite. Adapted from [193], Copyright 2019, American Chemical Society.
Transforming 2D NSs into 1D NRs offers another efficient approach to preventing MXene NSs from restacking. Wang and colleagues developed Ti3C2 MXene NRs by subjecting multilayer Ti3C2 MXene to a shaking treatment in a KOH solution. This process created numerous open macropores, which facilitated high S loading and rapid Li+ diffusion (Figure 15c) [192]. The delaminated Ti3C2 NSs were vacuum-filtrated onto a PP separator, serving as the interlayer to physically and chemically suppress the shuttle effect. Consequently, a slurry of Ti3C2 NRs/S was deposited onto the modified separator, forming an all-MXene-based integrated electrode (Figure 15d). With an S content of 68 wt%, this electrode demonstrated impressive performance, achieving capacities of 1062 mAh g−1 at 0.2 C and 288 mAh g−1 at 10 C (Figure 15e).
Developing 3D MXene networks with organized porous structures from 2D MXene NSs is a highly effective approach to enhance Li-ion movement [193]. These frameworks provide physical confinement and chemical adsorption of LiPSs, suppress the shuttle effect, support high S loading, and accommodate the volumetric variation of S, thereby significantly improving the behavior of MXene-based LSBs. For instance, a 3D flower type porous Ti3C2Tx network was synthesized using ethane diamine as an aid during a hydrothermal treatment process, which served as an S host (Figure 15f) [193]. Within this network, the conductive Ti3C2Tx NSs were arranged at various angles, facilitating rapid electron and Li+ transport while supporting a great tap density after S loading (Figure 15g,h). This structure achieved an outstanding areal capacity of 10.04 mAh cm−2, even with an S loading of 10.5 mg cm−2 (Figure 15i). Additionally, a 3D Ti3C2Tx foam was fabricated using a hydrazine-induced foaming technique, resulting in a well-arranged porous architecture with a SSA of 182.9 m2 g−1 and a pore volume of 0.461 cm3 g−1 [153]. As an S host, the freestanding Ti3C2Tx foam/S electrode, containing 71.1 wt% S, recorded an initial capacity of 1226.4 mAh g−1 at 0.2 C, a capacity of 711.0 mAh g−1 at 5 C, and an exceptionally low-capacity decay of approximately 0.025% per cycle over 1000 cycles at 1 C.
Strategic structural optimization of MXenes is essential for maximizing the exposed SSA of MXene NSs, enhancing Li-ion diffusion, and preserving their metallic conductivity. Advanced MXene architecture provides numerous active sites for the chemical adsorption of LiPSs and facilitates their transformation to Li2S. Whether employed as S hosts or separator coatings, such structured MXenes significantly boost the electrochemical performance of LSBs. For instance, MXenes with expanded interlayer spacing exhibit rapid Li+ diffusion and improved S utilization [230]. Meanwhile, 0D and 1D MXenes effectively mitigate the restacking challenges associated with 2D NSs [191,192]. Furthermore, 3D porous MXene frameworks demonstrate excellent areal and volumetric capacities, even at high S loadings [193]. Overall, the structural optimization of MXenes opens up new possibilities and presents exciting opportunities for advancing their applications in LSBs.
8.1.4. Development of MXene-Based Composites as S Host
C materials and TM compounds have traditionally been considered effective S hosts [12]. C materials, with their highly porous structures, physically trap LiPSs, provide ample space for increased S loading, and accommodate volume expansion [231]. TM compounds, on the other hand, chemically anchor LiPSs, thereby effectively mitigating the shuttle effect [232]. Leveraging their unique 2D structure and exceptional conductivity, MXenes have emerged as ideal candidates for creating composite materials with C materials or TM compounds. These composites capitalize on the complementary benefits of both components for LSBs. In such systems, MXenes create a continuous conductive framework that supports efficient charge transfer while exhibiting strong LiPSs adsorption and catalytic properties. Simultaneously, the secondary materials contribute their inherent functions while acting as spacers to prevent MXene restacking and facilitate Li+ transport [233]. As a result, MXene-based composites demonstrate excellent electrochemical performance as S hosts, highlighting a promising pathway for MXene applications in LSBs.
MXene/C composites: C materials, known for their high conductivity, adjustable porous structures, and low cost, have been extensively utilized as S hosts in LSBs [234]. Combining MXenes with C materials in composite structures leverages the inherent benefits of both components. MXene NSs improve the electrical conductivity of the composite, enabling efficient electron transfer, while C materials act as spacers, preventing MXene restacking and facilitating rapid ion transport. Moreover, C materials, particularly 1D CNTs and 2D graphene, open up significant possibilities for advanced structural designs when integrated with MXene NSs [235]. This synergistic combination results in MXene/C composite-based S cathodes that deliver excellent electrochemical performance, even under conditions of high S loading.
MXene/CNT composites are renowned for their exceptional conductivity and open structures, enabling high S loading and superior performance [179]. By simply combining 1D CNTs with 2D MXene NSs, 3D CNT/MXene composite frameworks were developed and utilized as S hosts. Combining 1D CNTs with 2D MXene NSs has led to the development of 3D CNT/MXene networks, which have been effectively employed as S hosts. Examples include CNTs/Ti2C (83 wt% S content), CNTs/Ti3C2 (79 wt% S content), and CNTs/Ti3CN (83 wt% S content), all of which delivered initial capacities above 1200 mAh g−1 at 0.05 C and retained ≈ 450 mAh g−1 after 1200 cycles at 0.5 C [179]. Mo2C MXene, known for its strong interaction with LiPSs, efficiently suppresses the shuttle effect. Integrating CNTs with Mo2C MXene produced a Mo2C-CNT composite with an increased SSA (116.8 cm2 g−1) and great conductivity, which was used as an S host [236]. The Mo2C-CNT/S electrode, containing 87.1 wt% S, exhibited an initial capacity of 1235 mAh g−1 with a retention of 74.9% after 250 cycles at 0.1 C and a capacity of 519 mAh g−1 at 5 C. At a great S loading of 5.6 mg cm−2, the electrode achieved an initial capacity of 959 mAh g−1. Additionally, positively charged polyethyleneimine (PEI) modification enabled the electrostatic self-assembly of CNTs with Ti3C2Tx, forming a Ti3C2Tx@PEI-CNT composite (Figure 16a) featuring a highly porous 3D architecture (Figure 16b,c) [237]. This composite is utilized as both an S host and an interlayer. The resulting LSBs provided a capacity of 980 mAh g−1 at 1 C after 500 cycles with an S loading of 2.6 mg cm−2 (Figure 16d). Even at an S loading of 5.8 mg cm−2, the batteries achieved an impressive areal capacity of 7.1 mAh cm−2 at 2.4 mA cm−2 and retained a capacity above 4 mAh cm−2 after 100 cycles at 9.7 mA cm−2.
Characterized by their µm scale diameters and excellent conductivity, 1D C fibers are ideal candidates for integration with MXenes. This combination forms uniform MXene@C fiber composites with well-dispersed MXene NSs (Figure 16e) [238]. Incorporating a C/S slurry into this framework enables the MXene NSs to serve multiple purposes, including suppressing the shuttle effect, facilitating rapid electron transport, and enhancing the flexibility of C fibers to accommodate S’s volume extension. Consequently, the Ti3C2@C fiber-S cathode, featuring a uniform S loading of 4 mg cm−2, achieved an initial capacity of 1058.4 mAh g−1 and maintained 59.1% retention in capacity after 1000 cycles at 1 C (Figure 16f). Incorporating 2D graphene and rGO with MXenes has also proven effective in creating high-performance S cathodes for LSBs [239]. Various 3D MXene/graphene composites have been designed to function as S hosts, offering enhanced ion transport and S loading capabilities [190]. For instance, a 3D porous Ti3C2Tx/rGO hybrid aerogel was synthesized through hydrothermal treatment of Ti3C2Tx and GO, followed by freeze-drying. This aerogel served as a freestanding cathode to accommodate Li2S6 in LSBs (Figure 16g,h) [239]. The 3D interconnected MXene/rGO framework (Figure 16h) facilitated rapid Li-ion diffusion and electron transport, delivering a capacity of 1270 mAh g−1 at 0.1 C. It also demonstrated a low-capacity decay of 0.07% over 500 cycles at 1 C (Figure 16i) and an aerial capacity of 5.27 mAh cm−2 with an S loading of 6 mg cm−2. In another example, a freestanding 3D porous MXene/graphene hybrid aerogel was fabricated using a straightforward liquid infiltration–evaporation process and employed as a host for Li2S [240]. The resulting Li2S@MXene/graphene cathode, with a great Li2S loading till 9 mg cm−2, achieved 545 mAh g−1, retained 88% capacity over 100 cycles at 0.2 C, and delivered an aerial capacity of 3.42 mAh cm−2 at 2.0 C. Furthermore, a 3D layered Ti3C2Tx MXene/rGO hybrid was assembled utilizing a straightforward liquid-phase impregnation technique and subsequently loaded with S. This Ti3C2Tx/rGO/S cathode, with an S content of 70.4 wt%, exhibited a capacity of 1144.2 mAh g−1 at 0.5 C and retained 878.4 mAh g−1 after 300 cycles [190].
Porous C materials deliver additional space for S loading and help address the issue of volume extension during cycling [241], making MXene/porous C composites highly effective as hosts for high S content. One example is the synthesis of a 3D robust Ti3C2Tx@mesoporous C hybrid network by carbonizing an MXene@MOF-5 network [242]. After S loading, the MXene@mesoporous C-S cathode, containing 72.88 wt% S and an S loading of 2 mg cm−2, delivered an impressive initial capacity of 1225.8 mAh g−1 and retained 704.6 mAh g−1 after 300 cycles at 0.5 C. Similarly, hollow porous C spheres were combined with Ti3C2 MXene to create a sandwiched composite [243]. The resulting cathode, with an S content of 76.5 wt%, achieved an initial capacity of 1397.5 mAh g−1 at 0.05 C and sustained 494.7 mAh g−1 after 500 cycles at 1 C.
Additionally, porous C have been adjusted with heteroatom doping before being combined with MXene. This doping enhances polarity, which strengthens the affinity for LiPSs to mitigate shuttle effect. It also delivers high conductivity, ensuring efficient S utilization [244]. For instance, a composite of N-doped porous C and Ti3C2 was created with a 3D sponge-like architecture and plentiful mesopores (417.6 m2 g−1). This composite was formed by carbonizing a composite of ZIF-67 and Ti3C2. After loading with 80 wt% S, the cathode achieved a capacity of 759 mAh g−1 at 1 C and demonstrated exceptional cycling stability over 800 cycles, with a capacity decay of just 0.04% per cycle [245]. Moreover, another composite of MXene and Co/N-co-doped porous C was prepared through the in situ self-assembly of bimetallic ZIFs on Ti3C2Tx NSs, followed by calcination and etching [246]. This composite exhibited a high SSA of 726.6 m2 g−1 (Figure 16j) and a pore volume of 0.71 cm3 g−1 (Figure 16k), with an 81.9 wt% S content as a host. Coupled with an MXene-modified PP separator, the cathode with an S loading of 1.5 mg cm−2 delivered capacities of 1333.9 and 579.2 mAh g−1 at 0.2 and 7 C rates (Figure 16l). Even at a higher S loading of 5.2 mg cm−2, the cathode exhibited the initial capacity of 924.7 mAh g−1 at 1 C and maintained 616.7 mAh g−1 after 1000 cycles [246].
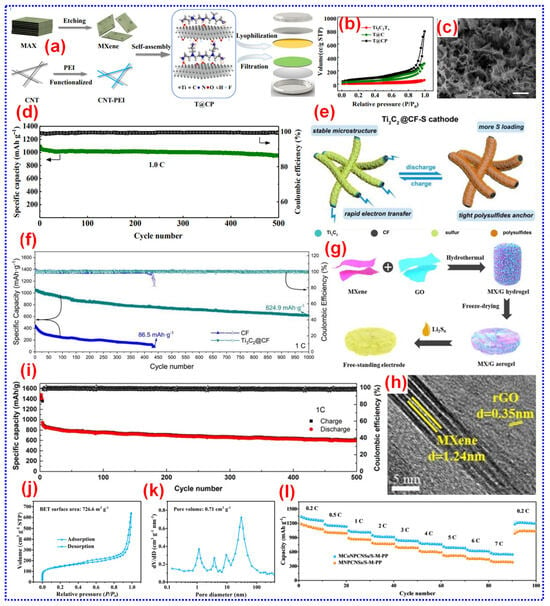

Figure 16.
(a) Illustration of synthesis, (b) N2 adsorption–desorption isotherms, and (c) SEM micrograph of Ti3C2Tx@PEI-CNTs. (d) Cycling performance of LSBs using a Ti3C2Tx@PEI-CNTs-S cathode paired with a Ti3C2Tx@PEI-CNTs interlayer. Adapted from [237], Copyright 2019, Elsevier B.V. (e) Pictorial representation and (f) cycling stability of Ti3C2@C fiber-S cathode. Adapted from [238], Copyright 2020, Royal Society of Chemistry. (g) Diagram illustrating the fabrication, (h) TEM micrograph, and (i) cycling performance of MXene/graphene aerogel electrode. Adapted from [239], Copyright 2019, Royal Society of Chemistry. (j) N2 adsorption–desorption isotherms and (k) pore size distribution of the MXene/Co,N-codoped porous C. (l) Rate capability of LSBs with MXene/Co,N-codoped porous C/S cathode, and MXene-modified PP separator. Adapted from [246], Copyright 2019, American Chemical Society.
MXene/TM compound composites: TM compounds, such as TMOs [247] and TMSs [248], exhibit intense chemical interactions with LiPSs. When combined with MXenes, these compounds help prevent the restacking of MXene NSs and increase the chemical adsorption of LiPSs, effectively reducing the shuttle effect. While some metal compounds have limited electrical conductivity [247], MXenes’ metallic conductivity compensates for this drawback. Consequently, composites of MXenes and TM compounds are considered promising S hosts for LSBs as they efficiently suppress the shuttle effect and provide great conductivity for improved S utilization.
The in situ growth of TM compounds on MXene NSs is a commonly employed approach for producing composites. For instance, a 3D hierarchical porous MXene/1T-2H MoS2-C was created through a hydrothermal technique involving ammonium molybdate and thiourea with MXene and glucose, followed by annealing in a N2 atmosphere and then in ammonia at 600 °C (Figure 17a,b) [249]. The NH3 treatment introduced N-doping into the MoS2 NSs, generated S vacancy defects, and facilitated a phase transition in MoS2 from the 2H to the 1T phase. As an S host in LSBs, the S vacancy defects and the 1T MoS2 phase demonstrated strong LiPSs trapping and accelerated redox kinetics. Additionally, high electrical conductivity of the 1T MoS2, MXene, and N-doped C enabled rapid electron transport. The 3D porous network (153.3 m2 g−1) provided ample space for great S loading and accommodated volume variation. Consequently, the MXene/1T-2H MoS2-C/S cathode, containing 79.6 wt% S, achieved an initial capacity of 1194.7 mAh g−1 at 0.1 C and maintained 799.3 mAh g−1 after 300 cycles at 0.5 C (Figure 17c). A pouch cell using this cathode exhibited an initial capacity of 915.2 mAh g−1 at 0.2 C and maintained stable cycling performance for 40 cycles, showcasing the electrode’s advantages [249]. Similarly, a VO2-V2C hybrid was synthesized through the in situ growth of VO2 rod clusters on 2D V2C NSs via hydrothermal treatment of a VO2 precursor in the presence of V2C [154]. Both the VO2 NRs and V2C MXene demonstrated robust adsorption capabilities and elevated catalytic activity for LiPSs. As a result, the VO2-V2C/S cathode, with 72.7 wt% S content, exhibited a capacity of 1250 mAh g−1 at 0.2 C, 585 mAh g−1 at 4 C, and retained 69.1% of its capacity after 500 cycles at 2 C. Even with an S loading of 10.2 mg cm−2, the cathode achieved an aerial capacity of 9.3 mAh cm−2 at 0.2 C and maintained 6.5 mAh cm−2 after 200 cycles, demonstrating excellent performance.
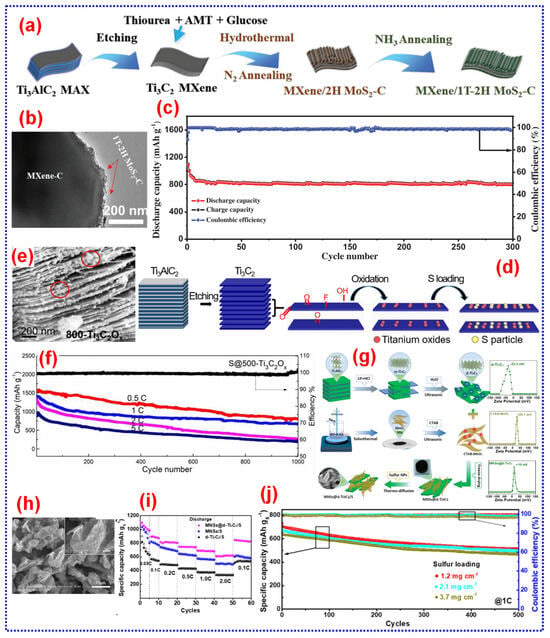
Figure 17.
(a) Illustration of preparation procedure, (b) TEM image, and (c) cycling stability of the MXene/1T-2H MoS2-C-S electrode. Adapted from [249], Copyright 2018, Wiley-VCH. (d) Diagram of in situ synthesis, (e) SEM micrograph, and (f) cycling performance of the S@TiO2-Ti3C2 composite. Adapted from [250], Copyright 2019, Elsevier B.V. (g) Illustration of synthesis, (h) SEM image, (i) rate performance, and (j) cycling performance of MnO2@delaminated MXene/S composite. Adapted from [251], Copyright 2018, American Chemical Society.
Interestingly, TMOs can be directly generated on MXene NSs through partial surface oxidation, leading to the formation of TMOs/MXene composites [3]. Following this approach, an accordion-type TiO2/Ti3C2 heterostructure was fabricated using a simple flash heat treatment of Ti3C2 in air (Figure 17d,e) [250]. The TiO2 layer effectively inhibited the restacking of MXene NSs, improved LiPSs adsorption, and boosted catalytic activity for LiPSs conversion. Simultaneously, MXene component provided excellent conductivity, while the accordion-type architecture allowed for high S loading and accommodated significant volume variations during cycling. Consequently, the TiO2-Ti3C2 heterostructure, with an S content of 80 wt%, achieved an initial capacity of 1567 mAh g−1 at 0.5 C and maintained 803 mAh g−1 after 1000 cycles (Figure 17f).
Ex situ assembly offers an efficient method for fabricating composites, enabling precise control over the ratio of MXene and TM compounds. Using an electrostatic self-assembly process with CTAB-functionalized MnO2 NSs and delaminated Ti3C2 MXene, a 3D mesoporous MnO2@Ti3C2 aerogel framework was developed as an S host (Figure 17g,h) [251]. The polar MnO2 and Ti3C2 MXene exhibited strong LiPSs adsorption capabilities, enabling the MnO2@Ti3C2 aerogel, with an S loading of 70 wt%, to deliver an initial capacity of 1140 mAh g−1 at 0.05 C. The aerogel maintained 91% of its capacity after 500 cycles at 1 C. Even at an S loading of 3.7 mg cm−2, the aerogel retained a capacity of 474 mAh g−1 after 500 cycles at 1 C, which was comparable to the 491 mAh g−1 achieved with an S loading of 2.1 mg cm−2 (Figure 17i,j).
MXene/covalent organic frameworks (COFs) composites: Two-dimensional COFs, characterized by their well-developed porous structures and adjustable pore sizes, offer precisely designed structures and heteroatom compositions for controlled chemical interactions with LiPSs, positioning them as a promising class of S host materials for LSBs. Moreover, COFs generally suffer from poor electrical conductivity [252]. This limitation can be effectively addressed by combining COFs with MXenes. An MXene/COFs composite was recently developed as an S host to harness their synergistic properties [253]. Through the in situ polymerization of 1,4-dicyanobenzene, COFs with an ordered porous structure were anchored onto Ti3C2 NSs. This composite featured hierarchical micro- and meso-porous architecture with an SSA of 318 m2 g−1. The lithophilic N sites within the covalent triazine frameworks and the sulfurophilic Ti sites in MXene provided robust chemical adsorption of LiPSs, efficiently suppressing the shuttle effect. Consequently, the S@covalent triazine frameworks/Ti3C2 cathode, containing 76 wt% S and an S loading of 1.5 mg cm−2, delivered an initial capacity of 1441 mAh g−1 at 0.2 C and exhibited excellent cycling performance, with a capacity decay as low as 0.014% per cycle over 1000 cycles at 1 C. Even at an S loading of 5.6 mg cm−2, the cathode retained a capacity of 816 mAh g−1 after 100 cycles at 0.2 C, corresponding to 94% retention [253].
8.1.5. MXene Employed as a Conductive Binder for S Cathodes
Beyond their role as S hosts, 2D MXene NSs, recognized for their excellent mechanical characteristics, can also function as conductive binders for fabricating flexible and freestanding electrodes [254]. Traditional electrode preparation typically involves blending active materials with a conductive additive and an insulating polymer binder (PTFE or PVDF) to create a slurry, which is then deposited onto a metal current collector. However, using polymer binders often compromises the electrode’s conductivity and rate capability. By incorporating 2D MXenes as conductive binders, a continuous MXene network is formed, offering exceptional conductivity and flexibility. Moreover, MXenes’ inherent ability to chemically adsorb and catalytically interact with LiPSs makes them particularly advantageous as conductive binders for S cathodes in LSBs. This dual functionality enhances both the structural stability and electrochemical behavior of the electrodes.
Using small S molecules (S2-4) as active materials instead of traditional S8 offers a fundamental solution to the shuttle effect by preventing the formation of LiPSs. Xu and collaborators developed ultramicroporous C (UMC) with a pore size of 0.55 nm to encapsulate S2-4, achieving exceptional cycling stability [255]. They further designed a freestanding, flexible electrode by combining MXene as a conductive binder and substrate with S2-4/UMC (Figure 18a,b) [254]. In this design, MXene NSs created a conductive framework that facilitated rapid electron transport, significantly enhancing the rate capability of S2-4/UMC electrode (Figure 18c). Consequently, the MXene-bonded S2-4/UMC electrode achieved capacities of 1029.7 mAh g−1 at 0.1 C and 502.3 mAh g−1 at 2 C (Figure 18d), along with exceptional cycling performance, retaining 91.9% of its capacity after 200 cycles at 0.1 C. These results markedly surpassed that of traditional S2-4/UMC electrodes using a PVDF binder and Al current collector. Additionally, this MXene-based electrode fabrication approach has been successfully applied to SCs [256] and Li/Na/K-ion batteries [257,258], demonstrating its versatility for developing high-performance energy storage devices.
Figure 18.
(a) Illustration of synthesis, (b) digital photograph, (c) SEM micrograph, and (d) rate performance of MXene-bonded S2-4/UMC electrodes. Adapted from [254], Copyright 2019, Royal Society of Chemistry.
MXene NSs can also function as conductive additives to improve the conductivity of S electrodes. For instance, incorporating Ti2C MXene into an S@TiO2 composite resulted in the S@TiO2/Ti2C electrode achieving an initial capacity of 1408.6 mAh g−1 at 0.2 C [259]. Similarly, integrating Ti3C2Tx into polyethyleneimine (PEI)-modified Ketjen black/S NPs, along with a Ketjen black@Ti3C2Tx-deposited PP separator, allowed for an LSB with an aerial S loading of 5.6 mg cm−2 to deliver an aerial capacity of 4.5 mAh cm−2 at 0.2 C while maintaining 74% retention capacity over 100 cycles [260].
As summarized in Table 4, MXenes demonstrate exceptional electrochemical performance when applied in the S cathodes of LSBs. This performance stems from their metallic conductivity, versatile structures, robust chemical interactions with LiPSs, and effective catalytic activity for LiPSs conversion. To address the issue of restacking commonly observed in 2D MXene NSs, advanced MXene architectures have been developed, including structures with expanded interlayer spacing and 3D porous designs. These innovations optimize the benefits of MXenes by fully exposing their surfaces to enhance S utilization, LiPSs adsorption, and catalytic activity. Moreover, the increased structural space supports high S loading and accommodates the volume expansion of S. In addition, the creation of MXene-based composites generates synergistic effects, preventing NSs restacking while improving their chemical adsorption and catalytic capabilities for LiPSs. Such enhancements significantly boost the performance of LSBs, even under high S loading conditions, which is a critical requirement for their practical application.

Table 4.
Electrochemical characteristics of MXene-based S cathode in LSBs [6].
Despite significant progress, the application of MXenes in S cathodes remains in its infancy. Current research has predominantly centered on Ti-based MXenes, particularly Ti3C2Tx, with limited attention given to other MXene types. However, the potential of MXenes beyond Ti3C2Tx can be anticipated based on their unique properties and theoretical predictions of their interactions with LiPSs. For instance, DFT calculations suggest that Cr3C2O2 exhibits the robust interaction with LiPSs among six oxygen-terminated M3C2O2 (M = Cr, V, Ti, Nb, Hf, and Zr) MXenes [202]. Similarly, Nb2C MXenes, depending on their surface terminations, demonstrate intriguing superconducting transitions at low temperatures [67], indicating their potential as promising candidates for LSBs and warranting further experimental exploration. The surface terminations of MXenes significantly influence their chemical adsorption and catalytic properties toward LiPSs, yet experimental investigations into surface engineering of MXenes as S hosts remain unexplored. Strategies such as heteroatom doping and the introduction of vacancies could further optimize their performance and are promising areas for future research. These challenges also present exciting opportunities to advance MXenes for high-performance S cathodes in LSBs. For practical LSB applications, achieving high S loading in MXene-based cathodes is essential, but additional factors such as reducing the E/S ratio and enhancing the tap density of electrode must also be addressed. Investigating pouch-cell-based configurations with optimized MXene utilization, active material loading, and electrolyte management could yield deeper insights, enabling enhanced energy density and prolonged cycling life for LSBs.
8.2. MXene-Based Interlayer Between Cathode and Separator in LSBs
Beyond advanced designs for S cathodes, incorporating an interlayer between the cathode and separator is a highly efficient approach to boost the performance of LSBs. This interlayer, which can either be freestanding or deposited onto the separator, plays a dual role: it acts as a barrier to retain LiPSs within the cathode region and serves as an additional current collector to boost the application of LiPSs (Figure 19a) [189].
8.2.1. Separator Modified with Pristine MXene
A straightforward vacuum-filtration method allows for the efficient assembly of 2D MXene NSs onto the separator in LSBs. This approach creates a conformal Ti3C2Tx layer with a mass loading of just 0.1 mg cm−2 and a thickness of 522 nm, applied to PP membrane, forming a Ti3C2Tx-modified separator free of noticeable pores or gaps (Figure 19a) [189]. Leveraging the elevated conductivity and strong polysulfide adsorption capabilities of Ti3C2Tx layer, LSBs equipped with a sulfur/super P cathode (68% S content) and this modified separator demonstrated a capacity of 550 mAh g−1 after 500 cycles, with a low-capacity decay rate of 0.062% per cycle at 0.5 C (Figure 19b). Thinner Ti3C2Tx coatings allow for rapid Li+ diffusion (Figure 19c), and even a coating as thin as 100 nm, with a mass loading of just 0.016 mg cm−2, effectively limits polysulfide diffusion [261]. To further enhance electrical conductivity and electrolyte wettability, 10 wt% CNTs were incorporated to the 0.016 mg cm−2 Ti3C2Tx coating. The resulting Ti3C2Tx/CNTs-10–functionalized PP separator enabled LSBs with a S/CNT cathode (70% S content) to deliver a capacity of 640 mAh g−1 at 2 C and retain this capacity after 200 cycles at 1 C. Glass fiber membranes, which are known for their high porosity and excellent electrolyte uptake, have also been utilized as separators in LSBs. When modified with a Ti3C2Tx layer via vacuum-filtration, these separators allowed LSBs with an S/super P cathode (70% S content) to achieve capacities of 1366 and 802 mAh g−1 at 0.5 and 2 A g−1 [262]. Additionally, MXenes can be deposited onto separators using binders. For instance, Ti3C2Tx MXene debris, obtained through hydrothermal treatment of MXene, were coated onto a biodegradable eggshell membrane to fabricate a modified separator for LSBs [263]. The MXene debris provided strong chemical confinement of polysulfides, while the eggshell membrane, rich in hydroxyl and amine functional groups, improved electrolyte infiltration and enhanced polysulfide affinity. Consequently, LSBs employing a S/Ketjen black cathode (67% S content) and this MXene/eggshell membrane separator demonstrated excellent rate performance, achieving a capacity of 948 mAh g−1 at 1 C and retaining 74% of the capacity after 250 cycles at 0.5 C.
8.2.2. Separator Enhanced with MXene-Based Composites
MXene-based composites, similar to their role as S hosts, can also modify separators in LSBs to suppress the shuttle effect and improve Li-ion diffusion through synergistic interactions between MXenes and complementary components. C materials are particularly popular for creating such composites. For example, a 3D Ti3C2Tx/CNT network with a mass loading of 0.16 mg cm−2 was fabricated by vacuum-filtrating a mixed solution of PVP-treated CNTs and Ti3C2Tx NSs onto a PP separator. When paired with a S/CNT cathode (70% S content), LSBs using this modified separator delivered an initial capacity of 1415 mAh g−1 at 0.1 C, maintaining a capacity decay as low as 0.06% per cycle over 600 cycles at 1 C [264]. Additionally, an N-doped Ti3C2/C (N-Ti3C2/C) composite was fabricated by calcining ZIF-67-decorated Ti3C2 NSs, followed by blending the product with porous C and a binder to create a slurry. This slurry was then deposited on PP separator, producing a modified separator with a mass loading of 0.6 mg cm−2 [265]. The N doping in Ti3C2 enhanced its chemical affinity for LiPSs and introduced lithiophilic sites to assist efficient Li-ion diffusion. Simultaneously, the porous C provided robust physical adsorption of LiPSs and lowered local current density, which helped delay the formation of Li dendrites. The N-Ti3C2/C interlayer efficiently mitigated the shuttle effect and suppressed dendrite growth in Li anodes (Figure 19d). LSBs using the N-Ti3C2/C@PP separator and a S/CNT cathode (79% S content) delivered excellent performance, achieving capacities of 1332 mAh g−1 at 0.1 C and 675 mAh g−1 at 2 C. The LSBs also exhibited a low-capacity decay rate of 0.07% per cycle over 500 cycles at 0.5 C with an S loading of 3.4 mg cm−2. Even at a high S loading of 10.3 mg cm−2, the LSBs maintained a constant areal capacity of 6.3 mAh cm−2 after 50 cycles at 0.1 C (Figure 19d). In another study, a Ti3C2Tx/GO membrane with excellent mechanical strength was fabricated through vacuum filtration of a mixed solution containing Ti3C2Tx and GO [266]. This freestanding membrane, positioned between the cathode and separator, served as a dual-function barrier, providing both physical and chemical confinement to block LiPSs (Figure 19e). When implemented in LSBs with a S/CNT cathode (70.5% S content), the Ti3C2Tx/GO interlayer significantly boosted battery performance, achieving an initial capacity of 1621.5 mAh g−1 at 0.1 C and retaining 833.2 mAh g−1 over 200 cycles. The batteries also demonstrated an excellent rate capacity of 640 mAh g−1 at 5 C (Figure 19f).
TMOs with a robust chemical affinity for LiPSs represent a promising class of materials to complement Ti3C2Tx. By partially oxidizing Ti3C2Tx via hydrothermal method, TiO2 NPs were synthesized in situ on Ti3C2Tx NSs, resulting in the formation of a TiO2-Ti3C2Tx heterostructure [267]. This heterostructure was then integrated with graphene and applied onto a PP separator using vacuum filtration, creating a compact and thin coating with a mass loading of 0.15 mg cm−2. In this configuration, the TiO2 NPs acted as adsorption centers to capture LiPSs, while the heterointerface facilitated rapid diffusion of LiPSs from TiO2 to Ti3C2Tx. The Ti3C2Tx contributed a great catalytic mechanism for efficient LiPSs conversion, and the graphene formed a conductive structure that physically restricted LiPSs migration (Figure 19g,h). Consequently, LSBs featuring a CMK-3/S cathode (70% S content, 1.2 mg cm−2 S loading) and the TiO2-MXene-graphene-coated separator delivered a capacity of 800 mAh g−1, with a minimal capacity decay of 0.028% per cycle over 1000 cycles at 2 C (Figure 19i). Even at a higher S loading of 5.1 mg cm−2 in the S/CNT cathode (75% S content), the battery retained 93% of its capacity after 200 cycles at 0.5 C [267].
Ti3C2Tx/polymer heterostructures have also been employed to enhance separators in LSBs. A notable example is the creation of a Ti3C2Tx-Nafion interlayer, achieved by vacuum-filtering a solution of Ti3C2Tx and Nafion onto a PP separator [268]. In this composite interlayer, Nafion was evenly distributed among the Ti3C2Tx NSs, promoting efficient Li+ transport while electrostatically repelling LiPSs. Consequently, LSBs with a S/C black cathode (74.1% S content) and the Ti3C2Tx/Nafion adjusted PP separator demonstrated an initial capacity of 1234 mAh g−1 at 0.2 C, a capacity of 794 mAh g−1 at 3 C, and a minimal capacity decay of 0.03% per cycle over 1000 cycles at 1 C.

Figure 19.
(a) Diagram illustrating Li-S cells equipped with PP and PP separators functionalized with Ti3C2Tx. (b) Cycling performance of Li-S cell featuring the Ti3C2Tx-functionalized PP separator. Adapted from [189], Copyright 2016, American Chemical Society. (c) Illustration of Li-ion diffusion through Ti3C2Tx-modified PP separators with varying mass loadings in LSBs. Adapted from [261], Copyright 2019, Elsevier B.V. (d) Diagram showing the preparation of N-doped Ti3C2Tx/C composite and its role in preventing Li dendrites formation when utilized as an interlayer. Additionally, it highlights the cycling stability of Li-S cells with high S loading, featuring N-doped Ti3C2Tx/C-modified PP separator. Adapted from [265], Copyright 2019, Elsevier B.V. (e) Optical image of freestanding Ti3C2Tx/GO membrane. (f) Rate performance comparison of Li-S cells using the Ti3C2Tx/GO@PP and standard PP separators. Adapted from [266], Copyright 2020, American Chemical Society. (g) Illustration of TiO2-Ti3C2Tx heterostructures demonstrating their role in trapping LiPSs and facilitating the conversion mechanism. (h) TEM micrograph of the TiO2-Ti3C2Tx heterostructures, with the corresponding SAED pattern shown in the inset. (i) Cycling stability of Li-S cell equipped with an oxidized Ti3C2Tx/graphene interlayer. Adapted from [267], Copyright 2019, Wiley-VCH.
Inserting an interlayer between the cathode and separator offers a straightforward yet efficient approach to mitigating the shuttle effect in LSBs. Both pristine MXenes and MXene-based composites have been utilized to construct interlayers, leading to substantial improvements in the electrochemical performance of LSBs (Table 5). Research findings suggest that an MXene-based interlayer with a thickness of just 100 nm is sufficient to suppress LiPSs. However, as an additional component in LSBs, it is essential to develop interlayers that are thinner and lighter while maintaining their effectiveness in restricting the shuttle effect. Moreover, exploring other MXene types beyond Ti3C2Tx, particularly those with enhanced LiPSs adsorption capabilities and superior catalytic efficiency, could further advance interlayer technology for LSBs.

Table 5.
Electrochemical characteristics of LSBs with MXene-based interlayer between cathode and separator [6].
8.3. MXene-Based Li Metal Anode
Effectively addressing the issue of dendrite formation in Li anodes can be achieved by constructing an appropriate host for Li metal [269]. MXene materials have shown great potential as Li hosts, offering a promising approach to control Li dendrite formation and growth, thereby enabling stable Li anodes. The surface terminations of MXenes demonstrate a strong affinity for Li, promoting controlled nucleation [182]. Their metallic conductivity and high Li-ion diffusion rates support fast electrochemical kinetics [181]. Furthermore, the structural versatility and exceptional mechanical strength provide numerous possibilities for designing advanced MXene/Li composite architectures [270]. Consequently, MXenes have gained considerable attention for improving the performance of Li anodes, playing a key role in advancing high-performance LSBs.
8.3.1. Lamellar MXene-Li Anode
A lamellar-structured Ti3C2Tx-Li metal film was created using a roll-to-roll mechanical technique, taking advantage of the inherent ductility of Li and the lubricating properties of atomic layers (Figure 20a) [209]. The Ti3C2Tx facilitated controlled nanoscale development of Li dendrites within the interlayer spaces, efficiently suppressing their vertical expansion. This Ti3C2Tx-Li film exhibited stable voltage curves with a minor overpotential of 32 mV at a 1 mA cm−2. LSBs with this Ti3C2Tx-Li anode and an S/C cathode achieved an energy density of 656 Wh kg−1 after 100 cycles at 0.5 mA cm−2. Furthermore, lamellar Ti3C2Tx layers were fabricated via self-assembly process at a water–air interface and applied to the Li anode surface using a rolling technique. This process formed a smooth, dense protective layer on the Li anode [270]. Li preferred to grow horizontally along the aligned NSs of MXene, with nucleation sites forming between the sheets to encourage uniform Li distribution during cycling (Figure 20b). Consequently, the Ti3C2Tx-Li anode maintained a smooth surface after 100 cycles, achieved a cycle life of 900 h at 1 mA cm−2 with a stripping/plating capacity of 0.5 mAh cm−2, and delivered a high stripping/plating capacity of 35 mAh cm−2 (Figure 20c).
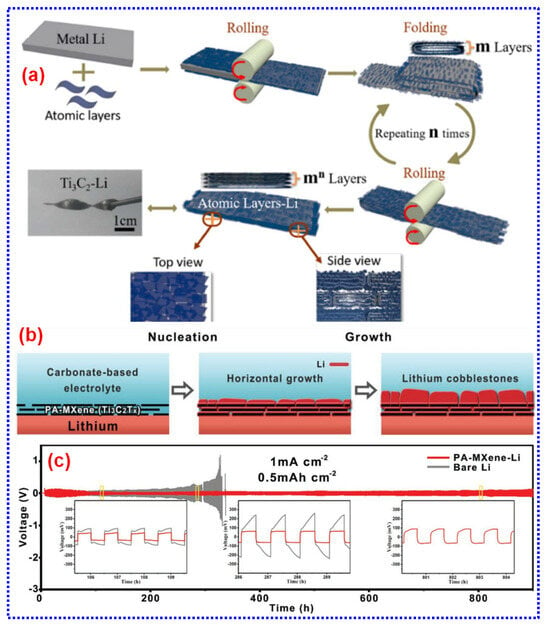
Figure 20.
Lamellar Ti3C2Tx-Li anode. (a) Diagram illustrating the synthesis of lamellar Ti3C2-Li film. Adapted from [209], Copyright 2017, Elsevier B.V. (b) Diagram illustrating the Li plating on the parallel-aligned Ti3C2Tx layers. (c) Cycling stability of the symmetric cell with the parallel aligned Ti3C2Tx-Li and pristine Li anodes at 1 mA cm−2 with 0.5 mAh cm−2 Li deposition. Adapted from [270], Copyright 2019, Wiley-VCH.
A layered rGO/Ti3C2Tx film was prepared through vacuum filtration and then infused with molten Li metal to create a Li-rGO/Ti3C2Tx anode [271]. DFT measurements revealed that the interface between Ti3C2Tx and rGO exhibited robust Li adsorption, acting as preferential sites to guide Li nucleation and growth. Additionally, the rGO/Ti3C2Tx composite demonstrated a short energy barrier for Li-ion diffusion, allowing the Li-rGO/Ti3C2Tx anode to maintain stable cycling for 1000 h at 10 mA cm−2. The composite anode also showed exceptional resistance to corrosion from LiPSs. When coupled with an S/C cathode, LSBs employing the Li-rGO/Ti3C2Tx anode exhibited an initial capacity of 1070 mAh g−1 at 0.2 C and sustained 409 mAh g−1 after 300 cycles at 1 C, resulting in a capacity retention of 64.5%.
8.3.2. Perpendicular MXene-Li Anode
Compared to lamellar Ti3C2Tx, perpendicular Ti3C2Tx structures offer greater advantages in promoting Li-ion transfer. Using a straightforward rolling and cutting technique, perpendicular Ti3C2Tx-Li arrays with dual periodic interspaces were created. These structures significantly enhanced rapid Li+ transport and effectively minimized dendrite formation during Li stripping and plating processes [181]. During deep Li stripping, the perpendicular Ti3C2Tx arrays preserved their periodic interspaces. Upon Li plating, Li preferentially deposited within the interspaces of the MXene arrays, guided by the surface terminations of the MXene (Figure 21a). This mechanism effectively suppressed Li dendrite growth and accommodated the volume changes of Li (Figure 21b). In comparison, perpendicular rGO and Cu arrays were also developed, but Li managed to nucleate and grow on the top of these arrays rather than within the gaps (Figure 21c–e), highlighting the distinctive advantage of Ti3C2Tx arrays in preventing dendrite growth. Consequently, the perpendicular Ti3C2Tx-Li anode achieved an overpotential of 25 mV, a high capacity of 2056 mAh g−1, and stable cycling for 1700 h (Figure 21f).
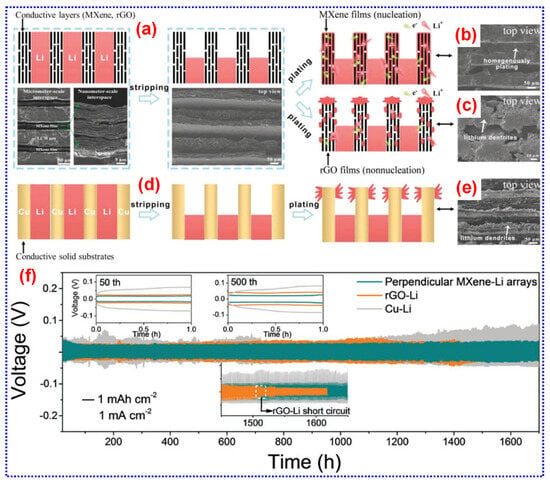
Figure 21.
Perpendicular Ti3C2Tx-Li anode. (a) Diagram showing the stripping/plating states of the perpendicular Ti3C2Tx-Li and rGO-Li arrays, along with SEM micrographs of (b) the perpendicular Ti3C2Tx-Li arrays and (c) the rGO-Li after initial stripping for 20 mAh cm−2 and plating for 1 mAh cm−2. (d) Diagram illustrating the stripping/plating states of the vertically aligned Cu-Li arrays, and (e) SEM micrographs of the Cu-Li arrays after initial stripping for 20 mAh cm−2 and plating for 1 mAh cm−2. (f) Cycling stability of Ti3C2Tx-Li, rGO-Li, and Cu-Li arrays at 1 mA cm−2 with 1 mAh cm−2 Li deposition. Adapted from [181], Copyright 2019, Wiley-VCH.
Another approach involved arranging Ti3C2Tx stacks perpendicularly and randomly dispersing them on a Li anode by pressing the stacks onto Li foils [272]. This configuration created ample interlayer space for Li storage, while the Ti3C2Tx stacks guided Li growth in the perpendicular direction. As a result, the structure maintained a less overpotential of 15 to 20 mV over 1125 cycles at 3 mA cm−2. Additionally, in a Li/Li symmetric cell, the structure exhibited an overpotential of 120 mV after 1050 cycles at 10 mA cm−2 with a capacity of 10 mAh cm−2.
8.3.3. 3D MXene-Li Anode
The development of a 3D MXene network as a Li host effectively suppresses Li dendrite growth and minimizes volume changes during the Li stripping and plating activities. A 3D porous Ti3C2Tx/rGO aerogel (Figure 22a) was created through hydrothermal treatment of a mixed Ti3C2Tx and rGO solution [208]. In this structure, the Ti3C2Tx NSs promoted homogeneous Li nucleation, while rGO contributed to enhanced mechanical stability. The 3D conductive structure enabled rapid electron and Li-ion transport, leading to uniform Li deposition. Furthermore, the interconnected pores provided a stable framework for great Li loading and helped mitigate volume fluctuations. Consequently, the Ti3C2Tx/rGO aerogel exhibited remarkable cycling stability over 350 cycles and maintained a low overpotential of 42 mV at a current density of 10 mA cm−2 (Figure 22b). Additionally, a Ti3C2Tx/graphene network was created by freeze-drying a mixed suspension of Ti3C2Tx and GO, followed by a spark reduction process to transform GO into graphene (Figure 22c). This framework possessed a SSA of 259 m2 g−1 with an interconnected porous architecture (Figure 22d) [273]. After infusion with molten Li (Figure 22c), the 3D Ti3C2Tx/graphene network achieved a great Li mass loading of approximately 92%. The resulting Ti3C2Tx/graphene-Li anode demonstrated exceptional cycling efficiency (~99%), an extended lifespan of 2700 h (Figure 22e), and outstanding stability over 230 cycles, even at a current density of 20 mA cm−2.
Figure 22.
(a) SEM micrograph and digital image of Ti3C2/rGO aerogel. (b) Voltage curves of symmetric cells utilizing Ti3C2/rGO aerogel and rGO scaffold under a condition of 10 mA cm−2@1 mAh cm−2. Adapted from [208], Copyright 2018, Wiley-VCH. (c) Diagram illustrating the preparation and digital images of 3D Ti3C2Tx/graphene-Li anode. (d) SEM micrograph and optical image of 3D Ti3C2Tx/graphene film. (e) CE of Ti3C2Tx/graphene-Li anode over 2700 h under a condition of 0.5 mA cm−2@5 mAh cm−2. Adapted from [273], Copyright 2019, American Chemical Society. (f) SEM micrograph of Ti3C2Tx@cellulose NFs film. (g) Voltage curves of symmetric cells using Ti3C2Tx@cellulose NFs/Li and Cu/Li under the condition of 0.5 mA cm−2@1 mAh cm−2. Adapted from [182], Copyright 2020, Elsevier B.V.
A Ti3C2Tx@cellulose NFs paper was developed as a Li host using spin-steaming technology by assembling Ti3C2Tx dispersions surrounding trace amounts of cellulose NFs [182]. Initially, Ti3C2Tx NSs bonded with cellulose NFs through intermolecular hydrogen bonding, forming layered micelle structures. These micelles then curled layer by layer into spherical shapes via drum granulation, resulting in Ti3C2Tx@cellulose NFs microspheres distributed among the Ti3C2Tx NSs (Figure 22f). In the fabricated Ti3C2Tx@cellulose NFs paper, the Ti3C2Tx NSs enabled uniform Li nucleation, effectively preventing dendrite formation. At the same time, the Ti3C2Tx@cellulose NFs microspheres, with their expanded interspaces, enhanced reaction kinetics, supporting great Li stripping/plating capacities and excellent rate performance. Consequently, the Ti3C2Tx@cellulose-Li anode displayed a stable overpotential of 60 mV for more than 250 cycles at 1 mA cm−2. Additionally, it exhibited exceptional cycling stability, maintaining an overpotential of 47 mV for over 1300 h at 0.5 mA cm−2 (Figure 22g).
8.3.4. Alteration of MXene-Based Li Anode
Efficient Li nucleation is crucial for mitigating Li dendrite formation as it promotes uniform Li growth. Conductive substrates with dispersed metal atoms have been identified as effective nucleation agents [274]. This makes MXenes combined with metal atoms a promising strategy for achieving dendrite-free Li electrodes. For instance, etching the Ti3AlC2 MAX phase with molten ZnCl2 salts immobilized Zn atoms at Ti sites in the resulting Ti3C2Clx MXene (Zn-MXene), which guided Li nucleation and growth [275]. In pure MXene layers, Li typically formed a flat layer across the surface. However, in Zn-MXene layers, the existence of Zn atoms enabled Li nucleation at lower plating levels due to the formation of a Zn-Li alloy phase. This process promoted vertical Li growth along the edges of Zn-MXene layers, resulting in the formation of Li spheres and a bowl-type Li structure devoid of dendrites. The Li-Zn-MXene electrode demonstrated exceptional performance, including a remarkably low overpotential of 11.3 mV, a cycle life of 1200 h at 1 mAh cm−2, and a high stripping/plating capacity of 40 mAh cm−2.
Beyond immobilizing metal atoms within MXenes, directly modifying the MXene surface with metal atoms can also effectively promote uniform Li growth. For instance, coating a Ti3C2Tx substrate with an amorphous liquid metal (GaInSnZn, with a melting point of 3 °C) served as nucleation sites, enabling homogeneous Li deposition [276]. In symmetric cells, the liquid metal-Ti3C2Tx/Li electrode exhibited stable cycling for over 300 h at 0.5 mA cm−2 and a Li deposition capacity of 0.5 mAh cm−2.
A 2D lamellar Ti3C2Tx has proven to be highly effective for constructing dendrite-free Li anodes. Advanced Ti3C2Tx/Li architectures, including lamellar/perpendicular Ti3C2Tx-Li, and 3D/modified Ti3C2Tx-Li configurations, have significantly alleviated the dendrite issue and enhanced electrochemical performance. However, most research has focused on Ti3C2Tx MXene. Future studies should investigate other MXene types, particularly those with great conductivity, low Li-ion diffusion energy barriers, and excellent stability. Additionally, chemical modification of MXenes holds promise for further improving Li nucleation efficiency. Developing MXene-Li superlattice structures, where MXene NSs and Li layers alternate at the atomic level, represents an exciting and valuable avenue for innovation.
9. Summary and Prospects
MXenes are highly promising materials for building high-performance LSBs due to their metallic conductivity, structural diversity, strong chemical adsorption of LiPSs, effective catalytic activity for LiPS conversion, and ability to enable homogeneous Li deposition. The metallic conductivity of MXenes improves S utilization, their diverse structures enable high S loading, and their robust chemical adsorption significantly mitigates the shuttle effect. Additionally, their catalytic activity enhances electrochemical kinetics. By leveraging these properties, MXenes used as S cathodes or as interlayers between the cathode and separator contribute to achieving great capacity, long cycling life, and excellent rate capability in LSBs. Further, the electrochemical behavior of MXenes can be enhanced through chemical modifications, structural engineering, and hybridization with complementary components such as C and TMOs. Furthermore, MXenes with advanced structures and tailored modifications hold promise as effective hosts for Li metal anodes, addressing the critical challenge of dendrite formation. Despite significant progress, the development of MXene-based LSBs is still in its infancy, requiring further research and innovation to fully unlock their potential.
A key challenge in utilizing MXenes for LSBs lies in developing a deeper understanding of their mechanisms for chemically adsorbing LiPSs and catalyzing their conversion. Although some studies have emphasized the critical influence of surface terminations on MXenes, the complexity of the intermediate species formed during the charge/discharge activities in LSBs complicates the full elucidation of their interactions with MXenes. Advancing fundamental research in this area remains both essential and challenging. To address this, a combination of computational and experimental methods is crucial for uncovering these mechanisms in greater detail.
Surface engineering and chemical modifications, such as tailoring terminations, creating vacancies, and introducing doped atoms, significantly impact the characteristics of MXenes, including their conductivity, adsorption capacity, catalytic activity, and Li affinity. These factors, in turn, have a profound impact on the characteristics of MXene-based LSBs. Despite their importance, relatively little attention has been given to optimizing MXenes for use as S hosts, interlayers between the cathode and separator, or Li hosts. Expanding research in this area is critical to fully harness the potential of MXenes. Insights from progress in other fields, particularly advancements in tailoring MXene terminations, vacancies, and doping, can serve as valuable references to accelerate this effort. Recently, significant strides have been made in controlling MXene terminations beyond the conventional -O, -OH, and -F groups. Exploring the effects of these novel terminations on MXene-based LSBs represents a promising and necessary direction for future research.
MXenes represents the largest family of 2D materials, with more than 100 stoichiometric phases theoretically identified. Moreover, only about 30 types have been successfully prepared, and research has mainly focused on Ti-based MXenes, particularly Ti3C2Tx. Since Ti3C2Tx is susceptible to oxidation when exposed to water and air, improving its stability through chemical modifications is essential. Moreover, the extensive variety within the MXene family, along with their customizable surface terminations, offers significant potential for the development of high-performance LSBs. The identification of new MXenes with boosted stability and electrochemical properties remains a hopeful research direction that will require substantial effort. DFT calculations are proving to be an effective tool to guide the design of MXene compositions and surface terminations.
For practical LSBs, it is crucial to address challenges related to both the S cathode and Li anode. In addition to these, factors such as S content, S loading, areal capacity, E/S ratio, and Li excess need to be optimized to accomplish great energy density and longer cycle life. Exploring the application of MXenes in pouch cells is especially valuable as it can offer important insights and speed up their practical adoption in LSBs.
In conclusion, MXenes, with their distinctive advantages and rapidly advancing research, show great potential for LSBs. Key future directions include gaining a deeper understanding of their mechanisms for adsorbing and catalyzing LiPSs, advancing chemical modifications and surface engineering to improve characteristics, and discovering new MXene species with enhanced stability and electrochemical properties. These advancements will unlock the full potential of MXenes and pave the way for the construction of high-performance LSBs with broad practical applications.
Author Contributions
Conceptualization, N.K.; formal analysis, N.K.; investigation, N.K.; resources, H.H. and S.M.; data curation, N.K., H.H. and S.M.; writing—original draft preparation, N.K.; writing—review and editing, N.K., H.H. and S.M.; visualization, N.K.; supervision, N.K., H.H. and S.M.; project administration, H.H. and S.M.; funding acquisition, H.H. and S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Nano and Material Technology Development Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (RS-2024-00449682).
Acknowledgments
N. Kitchamsetti is grateful to the staff of CNST-JNTUH and Nanospan India PVT, LTD, for their valuable assistance during the preparation process.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kitchamsetti, N.; Mannem, C.K.; Chakra, C.S.; de Barros, A.L.F. A review of the exploration of nanostructured electrode materials, electrolytes, and performance in metal-ion (Li, Na, K, and Zn) hybrid capacitors. J. Energy Storage 2025, 106, 114836. [Google Scholar] [CrossRef]
- Kitchamsetti, N.; Kalubarme, R.S.; Chikate, P.R.; Park, C.J.; Ma, Y.R.; Shirage, P.M.; Devan, R.S. An investigation on the effect of Li-ion cycling on the vertically aligned brookite TiO2 nanostructure. ChemistrySelect 2019, 4, 6620–6626. [Google Scholar] [CrossRef]
- Kitchamsetti, N.; Cho, J.S. A roadmap of recent advances in MXene@MOF hybrids, its derived composites: Synthesis, properties, and their utilization as an electrode for supercapacitors, rechargeable batteries and electrocatalysis. J. Energy Storage 2024, 80, 110293. [Google Scholar] [CrossRef]
- Kitchamsetti, N.; Payyavula, S.; Cho, J.S. A review on hollow nanostructures, its derived composites: Preparation, structural control, alterations, and their utilization as an electrode for supercapacitors, rechargeable batteries and electrocatalysis. J. Energy Storage 2024, 98, 113143. [Google Scholar] [CrossRef]
- Li, Y.X.; Feng, Y.S.; Li, L.X.; Yin, X.; Cao, F.F.; Ye, H. Green synthesis and applictions of MXene for lithium-sulfur batteries. Energy Storage Mater. 2024, 67, 103257. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, Q.; Liu, Y.; Xu, B. Status and Prospects of MXene-Based Lithium-Sulfur Batteries. Adv. Funct. Mater. 2021, 31, 2100457. [Google Scholar] [CrossRef]
- Shi, C.; Takeuchi, S.; Alexander, G.V.; Hamann, T.; O’Neill, J.; Dura, J.A.; Wachsman, E.D. High Sulfur Loading and Capacity Retention in Bilayer Garnet Sulfurized-Polyacrylonitrile/Lithium-Metal Batteries with Gel Polymer Electrolytes. Adv. Energy Mater. 2023, 13, 2301656. [Google Scholar] [CrossRef]
- Fu, A.; Wang, C.; Pei, F.; Cui, J.; Fang, X.; Zheng, N. Recent Advances in Hollow Porous Carbon Materials for Lithium-Sulfur Batteries. Small 2019, 15, 1804786. [Google Scholar] [CrossRef]
- Jiang, J.; Fan, Q.; Zheng, Z.; Kaiser, M.R.; Chou, S.; Konstantinov, K.; Liu, H.; Lin, L.; Wang, J. The Dual Functions of Defect-Rich Carbon Nanotubes as Both Conductive Matrix and Efficient Mediator for Li-S Batteries. Small 2021, 17, 2103535. [Google Scholar] [CrossRef]
- Ma, H.; Yu, Z.; Li, H.; Guo, D.; Zhou, Z.; Jin, H.; Wu, L.; Chen, X.; Wang, S. Tandem Carbon Hollow Spheres with Tailored Inner Structure as Sulfur Immobilization for Superior Lithium-Sulfur Batteries. Adv. Funct. Mater. 2024, 34, 2310301. [Google Scholar] [CrossRef]
- Li, J.; Li, G.; Wang, R.; He, Q.; Liu, W.; Hu, C.; Zhang, H.; Hui, J.; Huo, F. Boron-Doped Dinickel Phosphide to Enhance Polysulfide Conversion and Suppress Shuttling in Lithium-Sulfur Batteries. ACS Nano 2024, 18, 17774–17785. [Google Scholar] [CrossRef]
- Zuo, X.; Zhen, M.; Liu, D.; Fu, L.; Qiu, Y.; Liu, H.; Zhang, Y. Hollow and Porous N-Doped Carbon Framework as Lithium-Sulfur Battery Interlayer for Accelerating Polysulfide Redox Kinetics. Adv. Funct. Mater. 2024, 34, 2405486. [Google Scholar] [CrossRef]
- Cheng, R.; Xian, X.; Manasa, P.; Liu, J.; Xia, Y.; Guan, Y.; Wei, S.; Li, Z.; Li, B.; Xu, F.; et al. Carbon Coated Metal-Based Composite Electrode Materials for Lithium Sulfur Batteries: A Review. Chem. Rec. 2022, 22, e202200168. [Google Scholar] [CrossRef]
- Kitchamsetti, N.; Cho, J.S. A roadmap of MOFs derived porous carbon, oxides, chalcogenides, and phosphides of metals: Synthesis, properties, parameter modulation and their utilization as an electrode for Li/Na/K-ion batteries. J. Energy Storage 2024, 84, 110947. [Google Scholar] [CrossRef]
- Zuo, X.; Wang, L.; Zhen, M.; You, T.; Liu, D.; Zhang, Y. Multifunctional TiN-MXene-Co@CNTs Networks as Sulfur/Lithium Host for High-Areal-Capacity Lithium-Sulfur Batteries. Angew. Chem. Int. Ed. 2024, 136, e202408026. [Google Scholar] [CrossRef]
- Li, Z.; Liang, G.; Wang, T.; Liu, J.; Cheng, C.; Ao, G.; Guan, Z.; Tao, T.; Zhu, J. Sulfur nanosheets deposited on reduced graphene oxide enable excellent cycling life for lithium-sulfur battery. Carbon 2024, 229, 119512. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, J.; Hu, X.; Li, Y.; Du, H.; Wang, K.; Du, Z.; Gong, X.; Ai, W.; Huang, W. Lithiophilic sites dependency of lithium deposition in Li metal host anodes. Nano Energy 2022, 94, 106883. [Google Scholar] [CrossRef]
- Kitchamsetti, N.; de Barros, A.L.F. Recent advances in MXenes based composites as photocatalysts: Synthesis, properties and photocatalytic removal of organic contaminants from wastewater. ChemCatChem 2023, 15, e202300690. [Google Scholar] [CrossRef]
- Kitchamsetti, N.; Kim, D. A facile method for synthesizing MOF derived ZnCo2O4 particles on MXene nanosheets as a novel anode material for high performance hybrid supercapacitors. Electrochim. Acta 2023, 441, 141824. [Google Scholar] [CrossRef]
- VahidMohammadi, A.; Rosen, J.; Gogotsi, Y. The world of two-dimensional carbides and nitrides (MXenes). Science 2021, 372, eabf1581. [Google Scholar] [CrossRef]
- Khan, K.; Tareen, A.K.; Iqbal, M.; Ye, Z.; Xie, Z.; Mahmood, A.; Mahmood, N.; Zhang, H. Recent progress in emerging novel MXenes based materials and their fascinating sensing applications. Small 2023, 19, 2206147. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Meshkian, R.; Näslund, L.; Halim, J.; Lu, J.; Barsoum, M.W.; Rosen, J. Synthesis of two-dimensional molybdenum carbide, Mo2C, from the gallium based atomic laminate Mo2Ga2C. Scr. Mater. 2015, 108, 147–150. [Google Scholar] [CrossRef]
- Zhao, S.; Meng, X.; Zhu, K.; Du, F.; Chen, G.; Wei, Y.; Gogotsi, Y.; Gao, Y. Li-ion uptake and increase in interlayer spacing of Nb4C3 MXene. Energy Storage Mater. 2017, 8, 42–48. [Google Scholar] [CrossRef]
- Zhou, J.; Zha, X.; Zhou, X.; Chen, F.; Gao, G.; Wang, S.; Shen, C.; Chen, T.; Zhi, C.; Eklund, P.; et al. Synthesis and Electrochemical Properties of Two-Dimensional Hafnium Carbide. ACS Nano 2017, 11, 3841–3850. [Google Scholar] [CrossRef]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Transition Metal Carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef]
- Halim, J.; Lukatskaya, M.R.; Cook, K.M.; Lu, J.; Smith, C.R.; Näslund, L.; May, S.J.; Hultman, L.; Gogotsi, Y.; Eklund, P.; et al. Transparent Conductive Two-Dimensional Titanium Carbide Epitaxial Thin Films. Chem. Mater. 2014, 26, 2374–2381. [Google Scholar] [CrossRef]
- Feng, A.; Yu, Y.; Wang, Y.; Jiang, F.; Yu, Y.; Mi, L.; Song, L. Two-dimensional MXene Ti3C2 produced by exfoliation of Ti3AlC2. Mater. Des. 2017, 114, 161–166. [Google Scholar] [CrossRef]
- Urbankowski, P.; Anasori, B.; Makaryan, T.; Er, D.; Kota, S.; Walsh, P.L.; Zhao, M.; Shenoy, V.B.; Barsoum, M.W.; Gogotsi, Y. Synthesis of two-dimensional titanium nitride Ti4N3 (MXene). Nanoscale 2016, 8, 11385–11391. [Google Scholar] [CrossRef]
- Natu, V.; Pai, R.; Sokol, M.; Carey, M.; Kalra, V.; Barsoum, M.W. 2D Ti3C2Tz MXene Synthesized by Water-free Etching of Ti3AlC2 in Polar Organic Solvents. Chem 2020, 6, 616–630. [Google Scholar] [CrossRef]
- Shen, M.; Jiang, W.; Liang, K.; Zhao, S.; Tang, R.; Zhang, L.; Wang, J.Q. One-Pot Green Process to Synthesize MXene with Controllable Surface Terminations using Molten Salts. Angew. Chem. Int. Ed. 2021, 133, 27219–27224. [Google Scholar] [CrossRef]
- Xiao, X.; Yu, H.; Jin, H.; Wu, M.; Fang, Y.; Sun, J.; Hu, Z.; Li, T.; Wu, J.; Huang, L.; et al. Salt-Templated Synthesis of 2D Metallic MoN and Other Nitrides. ACS Nano 2017, 11, 2180–2186. [Google Scholar] [CrossRef]
- Ding, H.; Li, Y.; Li, M.; Chen, K.; Liang, K.; Chen, G.; Lu, J.; Palisaitis, J.; Persson, P.; Eklund, P.; et al. Chemical scissor-mediated structural editing of layered transition metal carbides. Science 2023, 379, 1130–1135. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, C.; Filatov, A.S.; Cho, W.; Lagunas, F.; Wang, M.; Vaikuntanathan, S.; Liu, C.; Klie, R.F.; Talapin, D.V. Direct synthesis and chemical vapor deposition of 2D carbide and nitride MXenes. Science 2023, 379, 1242–1247. [Google Scholar] [CrossRef]
- Zhao, M.; Peng, H.J.; Zhang, Z.W.; Li, B.Q.; Chen, X.; Xie, J.; Chen, X.; Wei, J.Y.; Zhang, Q.; Huang, J.Q. Activating Inert Metallic Compounds for High-Rate Lithium-Sulfur Batteries Through In Situ Etching of Extrinsic Metal. Angew. Chem. Int. Ed. 2019, 58, 3779–3783. [Google Scholar] [CrossRef]
- Anasori, B.; Naguib, M.; Editors, G. Two-dimensional MXenes. MRS Bull. 2023, 48, 238–244. [Google Scholar] [CrossRef]
- Sun, W.; Shah, S.A.; Chen, Y.; Tan, Z.; Gao, H.; Habib, T.; Radovic, M.; Green, M.J. Electrochemical etching of Ti2AlC to Ti2CTx (MXene) in low-concentration hydrochloric acid solution. J. Mater. Chem. A 2017, 5, 21663–21668. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, P.; Wang, F.; Ricciardulli, A.G.; Lohe, M.R.; Blom, P.W.M.; Feng, X. Fluoride-Free Synthesis of Two-Dimensional Titanium Carbide (MXene) Using A Binary Aqueous System. Angew. Chem. Int. Ed. 2018, 130, 15717–15721. [Google Scholar] [CrossRef]
- Kumar, S. Fluorine-Free MXenes: Recent Advances, Synthesis Strategies, and Mechanisms. Small 2024, 20, 2308225. [Google Scholar] [CrossRef]
- Li, T.; Yao, L.; Liu, Q.; Gu, J.; Luo, R.; Li, J.; Yan, X.; Wang, W.; Liu, P.; Chen, B.; et al. Fluorine-Free Synthesis of High-Purity Ti3C2Tx (T=OH, O) via Alkali Treatment. Angew.Chem. Int. Ed. 2018, 57, 6115–6119. [Google Scholar] [CrossRef]
- Xuan, J.; Wang, Z.; Chen, Y.; Liang, D.; Cheng, L.; Yang, X.; Liu, Z.; Ma, R.; Sasaki, T.; Geng, F. Organic-Base-Driven Intercalation and Delamination for the Production of Functionalized Titanium Carbide Nanosheets with Superior Photothermal Therapeutic Performance. Angew.Chem. Int. Ed. 2016, 128, 14789–14794. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, Z.; Pan, Y.; Dai, R.; Wu, Y.; Zhuang, Z.; Wang, D.; Peng, Q.; Chen, C.; Li, Y. Functionalization of Hollow Nanomaterials for Catalytic Applications: Nanoreactor Construction. Adv. Mater. 2019, 31, 1800426. [Google Scholar] [CrossRef]
- Li, Y.; Shao, H.; Lin, Z.; Lu, J.; Liu, L.; Duployer, B.; Persson, P.O.Å.; Eklund, P.; Hultman, L.; Li, M.; et al. A general Lewis acidic etching route for preparing MXenes with enhanced electrochemical performance in non-aqueous electrolyte. Nat. Mater. 2020, 19, 894–899. [Google Scholar] [CrossRef]
- Mei, J.; Ayoko, G.A.; Hu, C.; Bell, J.M.; Sun, Z. Two-dimensional fluorine-free mesoporous Mo2C MXene via UV-induced selective etching of Mo2Ga2C for energy storage. Sustain. Mater. Technol. 2020, 25, e00156. [Google Scholar] [CrossRef]
- Xu, C.; Wang, L.; Liu, Z.; Chen, L.; Guo, J.; Kang, N.; Ma, X.L.; Cheng, H.M.; Ren, W. Large-area high-quality 2D ultrathin Mo2C superconducting crystals. Nat. Mater. 2015, 14, 1135–1141. [Google Scholar] [CrossRef]
- Geng, D.; Zhao, X.; Chen, Z.; Sun, W.; Fu, W.; Chen, J.; Liu, W.; Zhou, W.; Loh, K.P. Direct Synthesis of Large-Area 2D Mo2C on In Situ Grown Graphene. Adv. Mater. 2017, 29, 1700072. [Google Scholar] [CrossRef]
- Mei, J.; Ayoko, G.A.; Hu, C.; Sun, Z. Thermal reduction of sulfur-containing MAX phase for MXene production. Chem. Eng. J. 2020, 395, 125111. [Google Scholar] [CrossRef]
- Kitchamsetti, N.; Kim, D. High performance hybrid supercapacitor based on hierarchical MOF derived CoFe2O4 and NiMn2O4 composite for efficient energy storage. J. Alloys Compd. 2023, 959, 170483. [Google Scholar] [CrossRef]
- Kitchamsetti, N.; Samtham, M.; Singh, D.; Choudhary, E.; Rondiya, S.R.; Ma, Y.R.; Cross, R.W.; Dzade, N.Y.; Devan, R.S. Hierarchical 2D MnO2@1D mesoporous NiTiO3 core-shell hybrid structures for high-performance supercapattery electrodes: Theoretical and experimental investigations. J. Electroanal. Chem. 2023, 936, 117359. [Google Scholar] [CrossRef]
- Park, J.; Jo, S.; Kitchamsetti, N.; Zaman, S.; Kim, D. The development of NiCo2O4/PVP/PANI heterogeneous nanocomposites as an advanced battery-type electrode material for high-performing supercapacitor application. J. Alloys Compd. 2022, 926, 166815. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, C.; Shou, H.; Zhang, P.; Wan, P.; Guo, X.; Yu, Z.; Wang, W.; Chen, S.; Chu, W.; et al. In Situ Architecting Endogenous Heterojunction of MoS2 Coupling with Mo2CTx MXenes for Optimized Li+ Storage. Adv. Mater. 2022, 34, 2108809. [Google Scholar] [CrossRef]
- Liang, L.; Niu, L.; Wu, T.; Zhou, D.; Xiao, Z. Fluorine-Free Fabrication of MXene via Photo-Fenton Approach for Advanced Lithium-Sulfur Batteries. ACS Nano 2022, 16, 7971–7981. [Google Scholar] [CrossRef]
- Lin, J.; Tian, W.; Guan, Z.; Zhang, H.; Duan, X.; Wang, H.; Sun, H.; Fang, Y.; Huang, Y.; Wang, S. Functional Carbon Nitride Materials in Photo-Fenton-Like Catalysis for Environmental Remediation. Adv. Funct. Mater. 2022, 32, 2201743. [Google Scholar] [CrossRef]
- Kitchamsetti, N.; Chakra, C.S.; de Barros, A.L.F.; Kim, D. Development of MOF Based Recyclable Photocatalyst for the Removal of Different Organic Dye Pollutants. Nanomaterials 2023, 13, 336. [Google Scholar] [CrossRef]
- Chavan, R.; Bhat, N.; Parit, S.; Narasimharao, K.; Devan, R.S.; Patil, R.B.; Karade, V.C.; Pawar, N.V.; Kim, J.H.; Jadhav, J.P.; et al. Development of magnetically recyclable nanocatalyst for enhanced fenton and photo-Fenton degradation of MB and Cr (VI) photo-reduction. Mater. Chem. Phys. 2023, 293, 126964. [Google Scholar] [CrossRef]
- Kitchamsetti, N.; Narsimulu, D.; Chinthakuntla, A.; Chakra, C.S.; de Barros, A.L.F. Bimetallic MOF derived ZnCo2O4 nanocages as a novel class of high performance photocatalyst for the removal of organic pollutants. Inorg. Chem. Commun. 2022, 144, 109946. [Google Scholar] [CrossRef]
- An, Y.; Tian, Y.; Man, Q.; Shen, H.; Liu, C.; Xiong, S.; Feng, J. Fluorine-and acid-free strategy toward scalable fabrication of two-dimensional MXenes for sodium-ion batteries. Nano Lett. 2023, 23, 5217–5226. [Google Scholar] [CrossRef]
- Liu, Y.H.; Li, L.X.; Wen, A.Y.; Cao, F.F.; Ye, H. A Janus MXene/MOF separator for the all-in-one enhancement of lithium-sulfur batteries. Energy Storage Mater. 2023, 55, 652–659. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, P.; Soomro, R.A.; Zhu, Q.; Xu, B. Advances in the synthesis of 2D MXenes. Adv. Mater. 2021, 33, 2103148. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Xu, R. New materials in hydrothermal synthesis. Acc. Chem. Res. 2001, 34, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Gogotsi, Y.; Anasori, B. The Rise of MXenes. ACS Nano 2019, 13, 8491–8494. [Google Scholar] [CrossRef]
- Ma, G.; Shao, H.; Xu, J.; Liu, Y.; Huang, Q.; Taberna, P.L.; Simon, P.; Lin, Z. Li-ion storage properties of two-dimensional titanium-carbide synthesized via fast one-pot method in air atmosphere. Nat. Commun. 2021, 12, 5085. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Qin, G.; Luo, K.; Lu, J.; Li, Y.; Liang, G.; Huang, Z.; Zhou, J.; Hultman, L.; et al. Halogenated Ti3C2 MXenes with electrochemically active terminals for high-performance zinc ion batteries. ACS Nano 2021, 15, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Baek, K.W.; Kitchamsetti, N.; Kim, H.W.; Cho, J.S. Prussian blue analogue-derived porous nanocages with hollow (Co,Fe)O nanoparticles as anodes for lithium ion batteries. J. Mater. Sci. Technol. 2025, 223, 76–90. [Google Scholar] [CrossRef]
- Yun, J.; Park, J.; Ryoo, M.; Kitchamsetti, N.; Goh, T.S.; Kim, D. Piezo-triboelectric hybridized nanogenerator embedding MXene based bifunctional conductive filler in polymer matrix for boosting electrical power. Nano Energy 2023, 105, 108018. [Google Scholar] [CrossRef]
- Kamysbayev, V.; Filatov, A.S.; Hu, H.; Rui, X.; Lagunas, F.; Wang, D.; Klie, R.F.; Talapin, D.V. Covalent surface modifications and superconductivity of two-dimensional metal carbide MXenes. Science 2020, 369, 979–983. [Google Scholar] [CrossRef]
- Soundiraraju, B.; George, B.K. Two-dimensional titanium nitride (Ti2N) MXene: Synthesis, characterization, and potential application as surface-enhanced Raman scattering substrate. ACS Nano 2017, 11, 8892–8900. [Google Scholar] [CrossRef]
- Kitchamsetti, N.; Cho, J.S.; Chakra, C.S. Prussian blue analogue derived porous hollow nanocages comprising polydopamine-derived N-doped C coated CoSe2/FeSe2 nanoparticles composited with N-doped graphitic C as an anode for high-rate Na-ion batteries. Chem. Eng. J. 2024, 495, 153353. [Google Scholar] [CrossRef]
- Lee, J.S.; Kitchamsetti, N.; Cho, J.S. Hierarchical porous nanofibers comprising N-doped graphitic C and ZIF-8 derived hollow N-doped C nanocages for long-life K-ion battery anodes. Chem. Eng. J. 2024, 487, 150465. [Google Scholar] [CrossRef]
- Naguib, M.; Halim, J.; Lu, J.; Cook, K.M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. New two-dimensional niobium and vanadium carbides as promising materials for Li-ion batteries. J. Am. Chem. Soc. 2013, 135, 15966–15969. [Google Scholar] [CrossRef]
- Kitchamsetti, N. A review on recent advances in Prussian blue, its analogues, and their derived materials as electrodes for high performance supercapacitors. J. Energy Storage 2023, 73, 108958. [Google Scholar] [CrossRef]
- Kim, I.; Cho, H.; Kitchamsetti, N.; Yun, J.; Lee, J.; Park, W.; Kim, D. A Robust Triboelectric Impact Sensor with Carbon Dioxide Precursor-Based Calcium Carbonate Layer for Slap Match Application. Micromachines 2023, 14, 1778. [Google Scholar] [CrossRef]
- Venkateshalu, S.; Cherusseri, J.; Karnan, M.; Kumar, K.S.; Kollu, P.; Sathish, M.; Thomas, J.; Jeong, S.K.; Grace, A.N. New method for the synthesis of 2D vanadium nitride (MXene) and its application as a supercapacitor electrode. ACS Omega 2020, 5, 17983–17992. [Google Scholar] [CrossRef]
- Park, J.; Kim, Y.; Kitchamsetti, N.; Jo, S.; Lee, S.; Song, J.; Park, W.; Kim, D. FeV LDH coated on sandpaper as an electrode material for high-performance flexible energy storage devices. Polymers 2023, 15, 1136. [Google Scholar] [CrossRef]
- Tran, M.H.; Malik, A.M.; Dürrschnabel, M.; Regoutz, A.; Thakur, P.; Lee, T.L.; Perera, D.; Luna, L.M.; Albe, K.; Rohrer, J.; et al. Experimental and theoretical investigation of the chemical exfoliation of Cr-based MAX phase particles. Dalton Trans. 2020, 49, 12215–12221. [Google Scholar] [CrossRef]
- Kitchamsetti, N.; Kim, D. Facile synthesis of hierarchical core–shell heterostructured ZnO/SnO2@NiCo2O4 nanorod sheet arrays on carbon cloth for high performance quasi-solid-state asymmetric supercapacitors. J. Mater. Res. Technol. 2022, 21, 590–603. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Ma, Y.; Liu, J.; Wang, P.; Luo, J.; Rui, Y.; Wu, Y. Ta4C3 Nanosheets as a Novel Therapeutic Platform for Photothermal-Driven ROS Scavenging and Immune Activation against Antibiotic-Resistant Infections in Diabetic Wounds. Small 2024, 20, 2400741. [Google Scholar] [CrossRef]
- Zaw, N.Y.W.; Jo, S.; Park, J.; Kitchamsetti, N.; Jayababu, N.; Kim, D. Clay-assisted hierarchical growth of metal-telluride nanostructures as an anode material for hybrid supercapacitors. Appl. Clay Sci. 2022, 225, 106539. [Google Scholar] [CrossRef]
- Halim, J.; Kota, S.; Lukatskaya, M.R.; Naguib, M.; Zhao, M.Q.; Moon, E.J.; Pitock, J.; Nanda, J.; May, S.J.; Gogotsi, Y.; et al. Synthesis and characterization of 2D molybdenum carbide (MXene). Adv. Funct. Mater. 2016, 26, 3118–3127. [Google Scholar] [CrossRef]
- Kitchamsetti, N.; Samtham, M.; Didwal, P.N.; Kumar, D.; Singh, D.; Bimli, S.; Chikate, P.R.; Basha, D.A.; Kumar, S.; Park, C.J.; et al. Theory abide experimental investigations on morphology driven enhancement of electrochemical energy storage performance for manganese titanate perovskites electrodes. J. Power Sources 2022, 538, 231525. [Google Scholar] [CrossRef]
- Peng, C.; Wei, P.; Chen, X.; Zhang, Y.; Zhu, F.; Cao, Y.; Wang, H.; Yu, H.; Peng, F. A hydrothermal etching route to synthesis of 2D MXene (Ti3C2, Nb2C): Enhanced exfoliation and improved adsorption performance. Ceram. Int. 2018, 44, 18886. [Google Scholar] [CrossRef]
- Kitchamsetti, N.; Didwal, P.N.; Mulani, S.R.; Patil, M.S.; Devan, R.S. Photocatalytic activity of MnTiO3 perovskite nanodiscs for the removal of organic pollutants. Heliyon 2021, 7, e07297. [Google Scholar] [CrossRef]
- Patil, M.S.; Kitchamsetti, N.; Mulani, S.R.; Rondiya, S.R.; Deshpande, N.G.; Patil, R.A.; Cross, R.W.; Dzade, N.Y.; Sharma, K.K.; Patil, P.S.; et al. Photocatalytic behavior of Ba (Sb/Ta)2O6 perovskite for reduction of organic pollutants: Experimental and DFT correlation. J. Taiwan Inst. Chem. Eng. 2021, 122, 201–209. [Google Scholar] [CrossRef]
- Zhou, J.; Zha, X.; Chen, F.Y.; Ye, Q.; Eklund, P.; Du, S.; Huang, Q. A two-dimensional zirconium carbide by selective etching of Al3C3 from nanolaminated Zr3Al3C5. Angew. Chem. Int. Ed. 2016, 55, 5008–5013. [Google Scholar] [CrossRef]
- Kitchamsetti, N.; Ma, Y.R.; Shirage, P.M.; Devan, R.S. Mesoporous perovskite of interlocked nickel titanate nanoparticles for efficient electrochemical supercapacitor electrode. J. Alloys Compd. 2020, 833, 155134. [Google Scholar] [CrossRef]
- Ghidiu, M.; Naguib, M.; Shi, C.; Mashtalir, O.; Pan, L.M.; Zhang, B.; Yang, J.; Gogotsi, Y.; Billinge, S.J.L.; Barsoum, M.W. Synthesis and characterization of two-dimensional Nb4C3 (MXene). Chem. Commun. 2014, 50, 9517–9520. [Google Scholar] [CrossRef]
- Kitchamsetti, N.; Choudhary, R.J.; Phase, D.M.; Devan, R.S. Structural correlation of a nanoparticle-embedded mesoporous CoTiO3 perovskite for an efficient electrochemical supercapacitor. RSC Adv. 2020, 10, 23446–23456. [Google Scholar] [CrossRef]
- Tran, M.H.; Schäfer, T.; Shahraei, A.; Dürrschnabel, M.; Luna, L.M.; Kramm, U.I.; Birkel, C.S. Adding a new member to the MXene family: Synthesis, structure, and electrocatalytic activity for the hydrogen evolution reaction of V4C3Tx. ACS Appl. Energy Mater. 2018, 1, 3908–3914. [Google Scholar] [CrossRef]
- Kitchamsetti, N.; Chikate, P.R.; Patil, R.A.; Ma, Y.R.; Shirage, P.M.; Devan, R.S. Perforated mesoporous NiO nanostructures for an enhanced pseudocapacitive performance with ultra-high rate capability and high energy density. CrystEngComm 2019, 21, 7130–7140. [Google Scholar] [CrossRef]
- Narasimharao, K.; Ramana, G.V.; Sreedhar, D.; Vasudevarao, V. Synthesis of graphene oxide by modified hummers method and hydrothermal synthesis of graphene-NiO nano composite for supercapacitor application. J. Mater. Sci. Eng. 2016, 5, 6. [Google Scholar] [CrossRef]
- Jo, S.; Kitchamsetti, N.; Cho, H.; Kim, D. Microwave-assisted hierarchically grown flake-like NiCo layered double hydroxide nanosheets on transitioned polystyrene towards triboelectricity-driven self-charging hybrid supercapacitors. Polymers 2023, 15, 454. [Google Scholar] [CrossRef]
- Kitchamsetti, N.; Ramteke, M.S.; Rondiya, S.R.; Mulani, S.R.; Patil, M.S.; Cross, R.W.; Dzade, N.Y.; Devan, R.S. DFT and experimental investigations on the photocatalytic activities of NiO nanobelts for removal of organic pollutants. J. Alloys Compd. 2021, 855, 157337. [Google Scholar] [CrossRef]
- Han, M.; Shuck, C.E.; Rakhmanov, R.; Parchment, D.; Anasori, B.; Koo, C.M.; Friedman, G.; Gogotsi, Y. Beyond Ti3C2Tx: MXenes for electromagnetic interference shielding. ACS Nano 2020, 14, 5008–5016. [Google Scholar] [CrossRef]
- Yang, J.; Naguib, M.; Ghidiu, M.; Pan, L.M.; Gu, J.; Nanda, J.; Halim, J.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional Nb-based M4C3 solid solutions (MXenes). J. Am. Ceram. Soc. 2016, 99, 660–666. [Google Scholar] [CrossRef]
- Pinto, D.; Anasori, B.; Avireddy, H.; Shuck, C.E.; Hantanasirisakul, K.; Deysher, G.; Morante, J.R.; Porzio, W.; Alshareef, H.N.; Gogotsi, Y. Synthesis and electrochemical properties of 2D molybdenum vanadium carbides-solid solution MXenes. J. Mater. Chem. A 2020, 8, 8957–8968. [Google Scholar] [CrossRef]
- Anasori, B.; Xie, Y.; Beidaghi, M.; Lu, J.; Hosler, B.C.; Hultman, L.; Kent, P.R.C.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional, ordered, double transition metals carbides (MXenes). ACS Nano 2015, 9, 9507–9516. [Google Scholar] [CrossRef]
- Anasori, B.; Shi, C.; Moon, E.J.; Xie, Y.; Voigt, C.A.; Kent, P.R.C.; May, S.J.; Billinge, S.J.L.; Barsoum, M.W.; Gogotsi, Y. Control of electronic properties of 2D carbides (MXenes) by manipulating their transition metal layers. Nanoscale Horiz. 2016, 1, 227–234. [Google Scholar] [CrossRef]
- Meshkian, R.; Tao, Q.; Dahlqvist, M.; Lu, J.; Hultman, L.; Rosen, J. Theoretical stability and materials synthesis of a chemically ordered MAX phase, Mo2ScAlC2, and its two-dimensional derivate Mo2ScC2 MXene. Acta Mater. 2017, 125, 476–480. [Google Scholar] [CrossRef]
- Srimuk, P.; Halim, J.; Lee, J.; Tao, Q.; Rosen, J.; Presser, V. Two-dimensional molybdenum carbide (MXene) with divacancy ordering for brackish and seawater desalination via cation and anion intercalation. ACS Sustain. Chem. Eng. 2018, 6, 3739–3747. [Google Scholar] [CrossRef]
- Meshkian, R.; Lind, H.; Halim, J.; Ghazaly, A.E.; Thörnberg, J.; Tao, Q.; Dahlqvist, M.; Palisaitis, J.; Persson, P.O.Å.; Rosen, J. Theoretical Analysis, Synthesis, and Characterization of 2D W1.33C (MXene) with Ordered Vacancies. ACS Appl. Nano Mater. 2019, 2, 6209–6219. [Google Scholar] [CrossRef]
- Zhou, J.; Tao, Q.; Ahmed, B.; Palisaitis, J.; Persson, I.; Halim, J.; Barsoum, M.W.; Persson, P.O.Å.; Rosen, J. High-entropy laminate metal carbide (MAX phase) and its two-dimensional derivative MXene. Chem. Mater. 2022, 34, 2098–2106. [Google Scholar] [CrossRef]
- Nemani, S.K.; Zhang, B.; Wyatt, B.C.; Hood, Z.D.; Manna, S.; Khaledialidusti, R.; Hong, W.; Sternberg, M.G.; Sankaranarayanan, S.K.R.S.; Anasori, B. High-entropy 2D carbide mxenes: TiVNbMoC3 and TiVCrMoC3. ACS Nano 2021, 15, 12815–12825. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Wu, C.; Chen, Y.; Zhu, Q.; Cui, Y.; Wang, H.; Zhang, Y.; Chen, X.; Shang, J.; Li, B.; et al. High-entropy carbonitride MAX phases and their derivative MXenes. Adv. Energy Mater. 2022, 12, 2103228. [Google Scholar] [CrossRef]
- Hart, J.L.; Hantanasirisakul, K.; Lang, A.C.; Anasori, B.; Pinto, D.; Pivak, Y.; van Omme, J.T.; May, S.J.; Gogotsi, Y.; Taheri, M.L. Control of MXenes’ electronic properties through termination and intercalation. Nat. Commun. 2019, 10, 522. [Google Scholar] [CrossRef]
- Dall’Agnese, Y.; Lukatskaya, M.R.; Cook, K.M.; Taberna, P.L.; Gogotsi, Y.; Simon, P. High capacitance of surface-modified 2D titanium carbide in acidic electrolyte. Electrochem. Commun. 2014, 48, 118–122. [Google Scholar] [CrossRef]
- Persson, I.; Halim, J.; Hansen, T.W.; Wagner, J.B.; Darakchieva, V.; Palisaitis, J.; Rosen, J.; Persson, P.O.Å. How much oxygen can a MXene surface take before it breaks? Adv. Funct. Mater. 2020, 30, 1909005. [Google Scholar] [CrossRef]
- Zhang, C.; Ye, W.B.; Zhou, K.; Chen, H.Y.; Yang, J.Q.; Ding, G.; Chen, X.; Zhou, Y.; Zhou, L.; Li, F.; et al. Bioinspired artificial sensory nerve based on nafion memristor. Adv. Funct. Mater. 2019, 29, 1808783. [Google Scholar] [CrossRef]
- Ding, M.; Han, C.; Yuan, Y.; Xu, J.; Yang, X. Advances and promises of 2D MXenes as cocatalysts for artificial photosynthesis. Sol. RRL 2021, 5, 2100603. [Google Scholar] [CrossRef]
- Kim, H.; Alshareef, H.N. MXetronics: MXene-enabled electronic and photonic devices. ACS Mater. Lett. 2019, 2, 55–70. [Google Scholar] [CrossRef]
- Huang, K.; Li, Z.; Lin, J.; Han, G.; Huang, P. Two-dimensional transition metal carbides and nitrides (MXenes) for biomedical applications. Chem. Soc. Rev. 2018, 47, 5109–5124. [Google Scholar] [CrossRef] [PubMed]
- Hantanasirisakul, K.; Gogotsi, Y. Electronic and optical properties of 2D transition metal carbides and nitrides (MXenes). Adv. Mater. 2018, 30, 1804779. [Google Scholar] [CrossRef]
- Jing, H.; Yeo, H.; Lyu, B.; Ryou, J.; Choi, S.; Park, J.H.; Lee, B.H.; Kim, Y.H.; Lee, S. Modulation of the electronic properties of MXene (Ti3C2Tx) via surface-covalent functionalization with diazonium. ACS Nano 2021, 15, 1388–1396. [Google Scholar] [CrossRef]
- Schultz, T.; Frey, N.C.; Hantanasirisakul, K.; Park, S.; May, S.J.; Shenoy, V.B.; Gogotsi, Y.; Koch, N. Surface termination dependent work function and electronic properties of Ti3C2Tx MXene. Chem. Mater. 2019, 31, 6590–6597. [Google Scholar] [CrossRef]
- Mathis, T.S.; Maleski, K.; Goad, A.; Sarycheva, A.; Anayee, M.; Foucher, A.C.; Hantanasirisakul, K.; Shuck, C.E.; Stach, E.A.; Gogotsi, Y. Modified MAX phase synthesis for environmentally stable and highly conductive Ti3C2 MXene. ACS Nano 2021, 15, 6420–6429. [Google Scholar] [CrossRef]
- Lyu, B.; Kim, M.; Jing, H.; Kang, J.; Qian, C.; Lee, S.; Cho, J.H. Large-area MXene electrode array for flexible electronics. ACS Nano 2019, 13, 11392–11400. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Feng, W.; Lu, W.; Li, B.; Yao, H.; Zeng, K.; Ouyang, J. MXenes with tunable work functions and their application as electron-and hole-transport materials in non-fullerene organic solar cells. J. Mater. Chem. A 2019, 7, 11160–11169. [Google Scholar] [CrossRef]
- Wu, H.; Almalki, M.; Xu, X.; Lei, Y.; Ming, F.; Mallick, A.; Roddatis, V.; Lopatin, S.; Shekhah, O.; Eddaoudi, M.; et al. MXene derived metal-organic frameworks. J. Am. Chem. Soc. 2019, 141, 20037–20042. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhou, J.; Si, C.; Sun, Z. Flexible two-dimensional Tin+1Cn (n = 1, 2 and 3) and their functionalized MXenes predicted by density functional theories. Phys. Chem. Chem. Phys. 2015, 17, 15348–15354. [Google Scholar] [CrossRef]
- Come, J.; Xie, Y.; Naguib, M.; Jesse, S.; Kalinin, S.V.; Gogotsi, Y.; Kent, P.R.C.; Balke, N. Nanoscale elastic changes in 2D Ti3C2Tx (MXene) pseudocapacitive electrodes. Adv. Energy Mater. 2016, 6, 1502290. [Google Scholar] [CrossRef]
- Lipatov, A.; Lu, H.; Alhabeb, M.; Anasori, B.; Gruverman, A.; Gogotsi, Y.; Sinitskii, A. Elastic properties of 2D Ti3C2Tx MXene monolayers and bilayers. Sci. Adv. 2018, 4, eaat0491. [Google Scholar] [CrossRef] [PubMed]
- Lipatov, A.; Alhabeb, M.; Lu, H.; Zhao, S.; Loes, M.J.; Vorobeva, N.S.; Dall’Agnese, Y.; Gao, Y.; Gruverman, A.; Gogotsi, Y.; et al. Electrical and elastic properties of individual single-layer Nb4C3Tx MXene flakes. Adv. Electron. Mater. 2020, 6, 1901382. [Google Scholar] [CrossRef]
- Ling, Z.; Ren, C.E.; Zhao, M.Q.; Yang, J.; Giammarco, J.M.; Qiu, J.; Barsoum, M.W.; Gogotsi, Y. Flexible and conductive MXene films and nanocomposites with high capacitance. Proc. Natl. Acad. Sci. USA 2014, 111, 16676–16681. [Google Scholar] [CrossRef]
- Lee, G.S.; Yun, T.; Kim, H.; Kim, I.H.; Choi, J.; Lee, S.H.; Lee, H.J.; Hwang, H.S.; Kim, J.G.; Kim, D.W.; et al. Mussel inspired highly aligned Ti3C2Tx MXene film with synergistic enhancement of mechanical strength and ambient stability. ACS Nano 2020, 14, 11722–11732. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Li, X.; Wang, Y.; Chen, Y.; Xie, X.; Yang, R.; Tomsia, A.P.; Jiang, L.; Cheng, Q. Strong sequentially bridged MXene sheets. Proc. Natl. Acad. Sci. USA 2020, 117, 27154–27161. [Google Scholar] [CrossRef]
- Wan, S.; Li, X.; Chen, Y.; Liu, N.; Du, Y.; Dou, S.; Jiang, L.; Cheng, Q. High-strength scalable MXene films through bridging-induced densification. Science 2021, 374, 96–99. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, T.C.; Alhajji, E.; Tian, Z.; Shi, Z.X.; Zhang, Y.Z.; Alshareef, H.N. MXenes for Sulfur-Based Batteries. Adv. Energy Mater. 2023, 13, 2202860. [Google Scholar] [CrossRef]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.M. Li-O2 and Li-S batteries with high energy storage. Nat. Mater. 2012, 11, 19–29. [Google Scholar] [CrossRef]
- Wang, J.; Du, R.; Yu, C.; Xu, C.; Shi, Z. Application of transition metal compounds in cathode materials for lithium-sulfur battery. Ionics 2022, 28, 5275–5288. [Google Scholar] [CrossRef]
- Fang, R.; Zhao, S.; Sun, Z.; Wang, D.W.; Cheng, H.M.; Li, F. More reliable lithium-sulfur batteries: Status, solutions and prospects. Adv. Mater. 2017, 29, 1606823. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, Q.; An, Y.; Chen, R.; Sun, N.; Wu, F.; Xu, B. A 3D conductive carbon interlayer with ultrahigh adsorption capability for lithium-sulfur batteries. Appl. Surf. Sci. 2018, 440, 770–777. [Google Scholar] [CrossRef]
- Xu, X.; Wang, S.; Wang, H.; Xu, B.; Hu, C.; Jin, Y.; Liu, J.B.; Yan, H. The suppression of lithium dendrite growth in lithium sulfur batteries: A review. J. Energy Storage 2017, 13, 387–400. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, W.; Lei, T.; Jiao, Y.; Huang, J.; Hu, A.; Gong, C.; Yan, C.; Wang, X.; Xiong, J. Strategies toward high-loading lithium-sulfur battery. Adv. Energy Mater. 2020, 10, 2000082. [Google Scholar] [CrossRef]
- Ji, X.; Lee, K.T.; Nazar, L.F. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, X.; Zhang, S.; Hou, Y. Sulfur hosts against the shuttle effect. Small Methods 2018, 2, 1700345. [Google Scholar] [CrossRef]
- Li, C.; Xi, Z.; Guo, D.; Chen, X.; Yin, L. Chemical immobilization effect on lithium polysulfides for lithium-sulfur batteries. Small 2018, 14, 1701986. [Google Scholar] [CrossRef]
- Fang, R.; Zhao, S.; Pei, S.; Qian, X.; Hou, P.X.; Cheng, H.M.; Liu, C.; Li, F. Toward more reliable lithium-sulfur batteries: An all-graphene cathode structure. ACS Nano 2016, 10, 8676–8682. [Google Scholar] [CrossRef]
- Li, M.; Carter, R.; Douglas, A.; Oakes, L.; Pint, C.L. Sulfur vapor-infiltrated 3D carbon nanotube foam for binder-free high areal capacity lithium-sulfur battery composite cathodes. ACS Nano 2017, 11, 4877–4884. [Google Scholar] [CrossRef]
- Zhao, X.; Kim, M.; Liu, Y.; Ahn, H.J.; Kim, K.W.; Cho, K.K.; Ahn, J.H. Root-like porous carbon nanofibers with high sulfur loading enabling superior areal capacity of lithium sulfur batteries. Carbon 2018, 128, 138–146. [Google Scholar] [CrossRef]
- Wang, M.; Xia, X.; Zhong, Y.; Wu, J.; Xu, R.; Yao, Z.; Wang, D.; Tang, W.; Wang, X.; Tu, J. Porous carbon hosts for lithium-sulfur batteries. Chem. Eur. J. 2019, 25, 3710–3725. [Google Scholar] [CrossRef]
- Li, Z.; Li, C.; Ge, X.; Ma, J.; Zhang, Z.; Li, Q.; Wang, C.X.; Yin, L.W. Reduced graphene oxide wrapped MOFs-derived cobalt-doped porous carbon polyhedrons as sulfur immobilizers as cathodes for high performance lithium sulfur batteries. Nano Energy 2016, 23, 15–26. [Google Scholar] [CrossRef]
- Zhang, Z.; Kong, L.L.; Liu, S.; Li, G.R.; Gao, X.P. A high-efficiency sulfur/carbon composite based on 3D graphene nanosheet@carbon nanotube matrix as cathode for lithium-sulfur battery. Adv. Energy Mater. 2017, 7, 1602543. [Google Scholar] [CrossRef]
- Yu, Q.; Lu, Y.; Luo, R.; Liu, X.M.; Huo, K.; Kim, J.K.; He, J.; Luo, Y.S. In Situ Formation of Copper-Based Hosts Embedded within 3D N-Doped Hierarchically Porous Carbon Networks for Ultralong Cycle Lithium-Sulfur Batteries. Adv. Funct. Mater. 2018, 28, 1804520. [Google Scholar] [CrossRef]
- Huang, X.; Tang, J.; Luo, B.; Knibbe, R.; Lin, T.; Hu, H.; Rana, M.; Hu, Y.X.; Zhu, X.; Gu, Q.; et al. Sandwich-like ultrathin TiS2 nanosheets confined within N,S codoped porous carbon as an effective polysulfide promoter in lithium-sulfur batteries. Adv. Energy Mater. 2019, 9, 1901872. [Google Scholar] [CrossRef]
- Qiu, W.; Xiao, H.B.; Li, Y.; Lu, X.H.; Tong, Y.X. Nitrogen and phosphorus codoped vertical graphene/carbon cloth as a binder-free anode for flexible advanced potassium ion full batteries. Small 2019, 15, 1901285. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Hou, Y. Chemical confinement and utility of lithium polysulfides in lithium sulfur batteries. Small Methods 2020, 4, 1900001. [Google Scholar] [CrossRef]
- Balach, J.; Linnemann, J.; Jaumann, T.; Giebeler, L. Metal-based nanostructured materials for advanced lithium-sulfur batteries. J. Mater. Chem. A 2018, 6, 23127–23168. [Google Scholar] [CrossRef]
- Lei, T.; Xie, Y.; Wang, X.; Miao, S.; Xiong, J.; Yan, C. TiO2 feather duster as effective polysulfides restrictor for enhanced electrochemical kinetics in lithium-sulfur batteries. Small 2017, 13, 1701013. [Google Scholar] [CrossRef]
- Ahad, S.A.; Ragupathy, P.; Ryu, S.; Lee, H.W.; Kim, D.K. Unveiling the synergistic effect of polysulfide additive and MnO2 hollow spheres in evolving a stable cyclic performance in Li-S batteries. Chem. Commun. 2017, 53, 8782–8785. [Google Scholar] [CrossRef]
- Wang, H.E.; Li, X.; Qin, N.; Zhao, X.; Cheng, H.; Cao, G.; Zhang, W. Sulfur-deficient MoS2 grown inside hollow mesoporous carbon as a functional polysulfide mediator. J. Mater. Chem. A 2019, 7, 12068–12074. [Google Scholar] [CrossRef]
- Tang, Q.; Li, H.; Pan, Y.Y.; Zhang, J.; Lin, Z.; Chen, Y.; Shu, X.; Qi, W. TiN synergetic with micro-/mesoporous carbon for enhanced performance lithium-sulfur batteries. Ionics 2018, 24, 2983–2993. [Google Scholar] [CrossRef]
- Li, X.X.; Ding, K.; Gao, B.; Li, Q.W.; Li, Y.Y.; Fu, J.; Zhang, X.M.; Chu, P.K.; Huo, K. Freestanding carbon encapsulated mesoporous vanadium nitride nanowires enable highly stable sulfur cathodes for lithium-sulfur batteries. Nano Energy 2017, 40, 655–662. [Google Scholar] [CrossRef]
- Zhao, T.; Zhai, P.F.; Yang, Z.; Wang, J.X.; Qu, L.; Du, F.G.; Wang, J.T. Self-supporting Ti3C2Tx foam/S cathodes with high sulfur loading for high-energy-density lithium-sulfur batteries. Nanoscale 2018, 10, 22954–22962. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, K.; Yu, F.; Qi, R.; Ren, J.; Zhu, Z.Q. VO2 (p)-V2C (MXene) grid structure as a lithium polysulfide catalytic host for high-performance Li-S battery. ACS Appl. Mater. Interfaces 2019, 11, 44282–44292. [Google Scholar] [CrossRef]
- Yu, M.P.; Ma, J.; Song, H.Q.; Wang, A.; Tian, F.; Wang, Y.; Qiu, H.; Wang, R.M. Atomic layer deposited TiO2 on a nitrogen-doped graphene/sulfur electrode for high performance lithium-sulfur batteries. Energy Environ. Sci. 2016, 9, 1495–1503. [Google Scholar] [CrossRef]
- Lim, W.G.; Kim, S.; Jo, C.; Lee, J. A comprehensive review of materials with catalytic effects in Li-S batteries: Enhanced redox kinetics. Angew. Chem. Int. Ed. 2019, 58, 18746–18757. [Google Scholar] [CrossRef]
- Xie, Z.Z.; Chen, G.H.; Liu, Z.; Zhu, X.X.; Liu, D.; Li, R.; Qu, D.; Li, J.S. Electrocatalytic Polysulfide Traps and their Conversion to long-chain Polysulfides using rGO-Pt composite as electrocatalyst to Improve the Performance of Li-S Battery. Int. J. Electrochem. Sci. 2019, 14, 11225–11236. [Google Scholar] [CrossRef]
- Liu, Y.; Kou, W.; Li, X.; Huang, C.; Shui, R.B.; He, G.H. Constructing patch-Ni-shelled Pt@Ni nanoparticles within confined nanoreactors for catalytic oxidation of insoluble polysulfides in Li-S batteries. Small 2019, 15, 1902431. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, R.; Zheng, S.; Liao, K.X.; Shi, P.H.; Fan, J.C.; Xu, Q.J.; Min, Y.L. Long-life Li-S batteries based on enabling the immobilization and catalytic conversion of polysulfides. J. Mater. Chem. A 2019, 7, 21747–21758. [Google Scholar] [CrossRef]
- Ye, Z.Q.; Jiang, Y.; Qian, J.; Li, W.; Feng, T.; Li, L.; Wu, F.; Chen, R. Exceptional adsorption and catalysis effects of hollow polyhedra/carbon nanotube confined CoP nanoparticles superstructures for enhanced lithium-sulfur batteries. Nano Energy 2019, 64, 103965. [Google Scholar] [CrossRef]
- Lin, H.; Yang, L.Q.; Jiang, X.; Li, G.C.; Zhang, T.; Yao, Q.F.; Zheng, G.Y.W.; Lee, J.Y. Electrocatalysis of polysulfide conversion by sulfur-deficient MoS2 nanoflakes for lithium-sulfur batteries. Energy Environ. Sci. 2017, 10, 1476–1486. [Google Scholar] [CrossRef]
- Xu, Z.L.; Lin, S.H.; Onofrio, N.; Zhou, L.; Shi, F.; Lu, W.; Kang, K.; Zhang, Q.; Lau, S.P. Exceptional catalytic effects of black phosphorus quantum dots in shuttling-free lithium sulfur batteries. Nat. Commun. 2018, 9, 4164. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liang, Q.H.; Han, J.W.; Tao, Y.; Liu, D.H.; Zhang, C.; Lv, W.; Yang, Q.H. Catalyzing polysulfide conversion by gC3N4 in a graphene network for long-life lithium-sulfur batteries. Nano Res. 2018, 11, 3480–3489. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.N.; Shao, A.H.; Xiong, D.G.; Liu, J.W.; Lao, C.Y.; Xi, K.; Lu, S.Y.; Jiang, Q.; Yu, J.; et al. Recyclable cobalt-molybdenum bimetallic carbide modified separator boosts the polysulfide adsorption-catalysis of lithium sulfur battery. Sci. China Mater. 2020, 63, 2443–2455. [Google Scholar] [CrossRef]
- Wang, H.Q.; Zhang, W.C.; Xu, J.Z.; Guo, Z. Advances in polar materials for lithium-sulfur batteries. Adv. Funct. Mater. 2018, 28, 1707520. [Google Scholar] [CrossRef]
- Jiang, S.; Chen, M.; Wang, X.; Zhang, Y.; Huang, C.; Zhang, Y.; Wang, Y. Honeycomb-like nitrogen and sulfur dual-doped hierarchical porous biomass carbon bifunctional interlayer for advanced lithium-sulfur batteries. Chem. Eng. J. 2019, 355, 478–486. [Google Scholar] [CrossRef]
- Liu, X.Y.; Shan, Z.Q.; Zhu, K.; Du, J.Y.; Tang, Q.; Tian, J. Sulfur electrode modified by bifunctional nafion/γ-Al2O3 membrane for high performance lithium-sulfur batteries. J. Power Sources 2015, 274, 85–93. [Google Scholar] [CrossRef]
- Mo, Y.X.; Lin, J.X.; Wu, Y.J.; Yin, Z.W.; Lu, Y.Q.; Li, J.T.; Zhou, Y.; Sheng, T.; Huang, L.; Sun, S.G. Core-shell structured S@Co(OH)2 with a carbon-nanofiber interlayer: A conductive cathode with suppressed shuttling effect for high-performance lithium-sulfur batteries. ACS Appl. Mater. Interfaces 2019, 11, 4065–4073. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, Z.X.; Ning, R.; Xi, S.; Tang, W.; Du, Y.H.; Liu, C.; Ren, Z.Y.; Chi, X.; Bai, M.H.; et al. Single-atom coated separator for robust lithium-sulfur batteries. ACS Appl. Mater. Interfaces 2019, 11, 25147–25154. [Google Scholar] [CrossRef]
- Li, S.; Zhang, W.F.; Zheng, J.; Lv, M.Y.; Song, H.; Du, L. Inhibition of polysulfide shuttles in Li-S batteries: Modified separators and solid-state electrolytes. Adv. Energy Mater. 2021, 11, 2000779. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Lin, D.C.; Liang, Z.; Zhao, J.; Yan, K.; Cui, Y. Lithium-coated polymeric matrix as a minimum volume-change and dendrite-free lithium metal anode. Nat. Commun. 2016, 7, 10992. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.Y.; Lee, S.W.; Liang, Z.; Lee, H.W.; Yan, K.; Yao, H.; Wang, H.T.; Li, W.Y.; Chu, S.; Cui, Y. Interconnected hollow carbon nanospheres for stable lithium metal anodes. Nat. Nanotechnol. 2014, 9, 618–623. [Google Scholar] [CrossRef]
- Li, N.W.; Yin, Y.X.; Yang, C.P.; Guo, Y.G. An artificial solid electrolyte interphase layer for stable lithium metal anodes. Adv. Mater. 2016, 28, 1853–1858. [Google Scholar] [CrossRef]
- Yang, C.P.; Fu, K.; Zhang, Y.; Hitz, E.; Hu, L.B. Protected lithium-metal anodes in batteries: From liquid to solid. Adv. Mater. 2017, 29, 1701169. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.L.; Tao, Y.; An, Y.L.; Tian, Y.; Zhang, Y.; Feng, J.; Qian, Y. Recent advances of emerging 2D MXene for stable and dendrite-free metal anodes. Adv. Funct. Mater. 2020, 30, 2004613. [Google Scholar] [CrossRef]
- Nan, J.X.; Guo, X.; Xiao, J.; Li, X.; Chen, W.H.; Wu, W.J.; Liu, H.; Wang, Y.; Wu, M.H.; Wang, G.X. Nanoengineering of 2D MXene-based materials for energy storage applications. Small 2021, 17, 1902085. [Google Scholar] [CrossRef]
- Zhang, C.F.; Cui, L.F.; Abdolhosseinzadeh, S.; Heier, J. Two-dimensional MXenes for lithium-sulfur batteries. InfoMat 2020, 2, 613–638. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, Q.Z.; Soomro, R.A.; He, S.; Sun, N.; Qiao, N.; Xu, B. In situ ice template approach to fabricate 3D flexible MXene film-based electrode for high performance supercapacitors. Adv. Funct. Mater. 2020, 30, 2000922. [Google Scholar] [CrossRef]
- Liang, X.; Rangom, Y.; Kwok, C.Y.; Pang, Q.; Nazar, L.F. Interwoven MXene Nanosheet/Carbon-Nanotube Composites as Li-S Cathode Hosts. Adv. Mater. 2016, 29, 1603040. [Google Scholar] [CrossRef]
- Wang, D.; Li, F.; Lian, R.Q.; Xu, J.; Kan, D.X.; Liu, Y.H.; Chen, G.; Gogotsi, Y.; Wei, Y.J. A general atomic surface modification strategy for improving anchoring and electrocatalysis behavior of Ti3C2T2 MXene in lithium-sulfur batteries. ACS Nano 2019, 13, 11078–11086. [Google Scholar] [CrossRef]
- Cao, Z.J.; Zhu, Q.; Wang, S.; Zhang, D.; Chen, H.; Du, Z.; Li, B.; Yang, S. Perpendicular MXene arrays with periodic interspaces toward dendrite-free lithium metal anodes with high-rate capabilities. Adv. Funct. Mater. 2020, 30, 1908075. [Google Scholar] [CrossRef]
- Wang, C.Y.; Zheng, Z.J.; Feng, Y.Q.; Ye, H.; Cao, F.F.; Guo, Z.P. Topological design of ultrastrong MXene paper hosted Li enables ultrathin and fully flexible lithium metal batteries. Nano Energy 2020, 74, 104817. [Google Scholar] [CrossRef]
- Ren, H.; Yu, R.; Qi, J.; Zhang, L.; Jin, Q.; Wang, D. Hollow Multishelled Heterostructured Anatase/TiO2(B) with Superior Rate Capability and Cycling Performance. Adv. Mater. 2019, 31, 1805754. [Google Scholar] [CrossRef]
- Fang, R.; Chen, K.; Yin, L.C.; Sun, Z.; Li, F.; Cheng, H.M. The regulating role of carbon nanotubes and graphene in lithium-ion and lithium-sulfur batteries. Adv. Mater. 2019, 31, 1800863. [Google Scholar] [CrossRef]
- Park, S.K.; Nakhanivej, P.; Park, H.S. Two-dimensional nanomaterials as emerging pseudocapacitive materials. Korean J. Chem. Eng. 2019, 36, 1557–1564. [Google Scholar] [CrossRef]
- Ying, G.B.; Dillon, A.D.; Fafarman, A.T.; Barsoum, M.W. Transparent, conductive solution processed spincast 2D Ti2CTx (MXene) films. Mater. Res. Lett. 2017, 5, 391–398. [Google Scholar] [CrossRef]
- Jiang, X.; Kuklin, A.V.; Baev, A.; Ge, Y.; Ågren, H.; Zhang, H.; Prasad, P.N. Two-dimensional MXenes: From morphological to optical, electric, and magnetic properties and applications. Phys. Rep. 2020, 848, 1–58. [Google Scholar] [CrossRef]
- Liang, X.; Garsuch, A.; Nazar, L.F. Sulfur cathodes based on conductive MXene nanosheets for high-performance lithium-sulfur batteries. Angew. Chem. Int. Ed. 2015, 127, 3979–3983. [Google Scholar] [CrossRef]
- Song, J.; Su, D.; Xie, X.Q.; Guo, X.; Bao, W.Z.; Shao, G.; Wang, G.X. Immobilizing polysulfides with MXene-functionalized separators for stable lithium-sulfur batteries. ACS Appl. Mater. Interfaces 2016, 8, 29427–29433. [Google Scholar] [CrossRef]
- Bao, W.Z.; Xie, X.Q.; Xu, J.; Guo, X.; Song, J.; Wu, W.J.; Su, D.; Wang, G.X. Confined sulfur in 3D MXene/reduced graphene oxide hybrid nanosheets for lithium-sulfur battery. Chem. Eur. J. 2017, 23, 12613–12619. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, Z.; Li, P.; Meng, X.; Wang, R. Ultrafine Ti3C2 MXene nanodots-interspersed nanosheet for high-energy-density lithium-sulfur batteries. ACS Nano 2019, 13, 3608–3617. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zheng, S.H.; Qin, J.Q.; Zhao, X.; Shi, H.; Wang, X.; Chen, J.; Wu, Z.S. All-MXene-based integrated electrode constructed by Ti3C2 nanoribbon framework host and nanosheet interlayer for high-energy-density Li-S batteries. ACS Nano 2018, 12, 2381–2388. [Google Scholar] [CrossRef]
- Xiao, Z.; Yang, Z.; Li, Z.; Li, P.; Wang, R. Synchronous gains of areal and volumetric capacities in lithium-sulfur batteries promised by flower-like porous Ti3C2Tx matrix. ACS Nano 2019, 13, 3404–3412. [Google Scholar] [CrossRef]
- Khazaei, M.; Arai, M.; Sasaki, T.; Chung, C.Y.; Venkataramanan, N.S.; Estili, M.; Sakka, Y.; Kawazoe, Y. Novel electronic and magnetic properties of two-dimensional transition metal carbides and nitrides. Adv. Funct. Mater. 2013, 23, 2185–2192. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, H.; Goddard, W.A., III. Schottky-barrier-free contacts with two-dimensional semiconductors by surface-engineered MXenes. J. Am. Chem. Soc. 2016, 138, 15853–15856. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.J.; Zhang, Y.; Zhuo, Z.; Neukirch, A.J.; Tretiak, S. Interlayer-decoupled Sc-based MXene with high carrier mobility and strong light-harvesting ability. J. Phys. Chem. Lett. 2018, 9, 6915–6920. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Luo, K.; Li, Y.; Chang, K.; Chen, K.; Zhou, J.; Rosen, J.; Hultman, L.; Eklund, P.; et al. Element replacement approach by reaction with Lewis acidic molten salts to synthesize nanolaminated MAX phases and MXenes. J. Am. Chem. Soc. 2019, 141, 4730–4737. [Google Scholar] [CrossRef]
- Rao, D.; Zhang, L.; Wang, Y.; Meng, Z.S.; Qian, X.; Liu, J.; Shen, X.Q.; Qiao, G.; Lu, R. Mechanism on the improved performance of lithium sulfur batteries with MXene-based additives. J. Phys. Chem. C 2017, 121, 11047–11054. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; She, Z.W.; Fu, Z.H.; Zhang, R.F.; Cui, Y. Understanding the anchoring effect of two-dimensional layered materials for lithium-sulfur batteries. Nano Lett. 2015, 15, 3780–3786. [Google Scholar] [CrossRef]
- Liu, X.B.; Shao, X.F.; Li, F.; Zhao, M.W. Anchoring effects of S-terminated Ti2C MXene for lithium-sulfur batteries: A first-principles study. Appl. Surf. Sci. 2018, 455, 522–526. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, J. Functional group-dependent anchoring effect of titanium carbide-based MXenes for lithium-sulfur batteries: A computational study. Appl. Surf. Sci. 2017, 412, 591–598. [Google Scholar] [CrossRef]
- Li, N.; Meng, Q.Q.; Zhu, X.H.; Li, Z.; Ma, J.; Huang, C.X.; Song, J.; Fan, J. Lattice constant-dependent anchoring effect of MXenes for lithium-sulfur (Li-S) batteries: A DFT study. Nanoscale 2019, 11, 8485–8493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, S.; Hu, R.; Gu, J.; Cui, Y.; Li, B.; Chen, W.; Liu, C.; Shang, J.; Yang, S. Catalytic conversion of polysulfides on single atom zinc implanted MXene toward high-rate lithium-sulfur batteries. Adv. Funct. Mater. 2020, 30, 2002471. [Google Scholar] [CrossRef]
- Wang, H.; Lee, J.M. Recent advances in structural engineering of MXene electrocatalysts. J. Mater. Chem. A 2020, 8, 10604–10624. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, T.; Xie, X.; Jiang, W.; Li, J.; Tian, B.B.; Su, C.L. Oxygen-functionalized ultrathin Ti3C2Tx MXene for enhanced electrocatalytic hydrogen evolution. ChemSusChem 2019, 12, 1368–1373. [Google Scholar] [CrossRef]
- Song, Y.; Sun, Z.; Fan, Z.; Cai, W.; Shao, Y.L.; Sheng, G.; Wang, M.; Song, L.X.; Liu, Z.F.; Zhang, Q.; et al. Rational design of porous nitrogen-doped Ti3C2 MXene as a multifunctional electrocatalyst for Li-S chemistry. Nano Energy 2020, 70, 104555. [Google Scholar] [CrossRef]
- Pang, J.; Mendes, R.G.; Bachmatiuk, A.; Zhao, L.; Ta, H.Q.; Gemming, T.; Liu, H.; Liu, Z.; Rummeli, M.H. Applications of 2D MXenes in energy conversion and storage systems. Chem. Soc. Rev. 2019, 48, 72–133. [Google Scholar] [CrossRef]
- Zhang, X.; Lv, R.; Wang, A.; Guo, W.; Liu, X.J.; Luo, J. MXene aerogel scaffolds for high-rate lithium metal anodes. Angew. Chem. Int. Ed. 2018, 57, 15028–15033. [Google Scholar] [CrossRef]
- Li, B.; Zhang, D.; Liu, Y.; Yu, Y.; Li, S.; Yang, S. Flexible Ti3C2 MXene-lithium film with lamellar structure for ultrastable metallic lithium anodes. Nano Energy 2017, 39, 654–661. [Google Scholar] [CrossRef]
- Pang, Q.; Liang, X.; Kwok, C.Y.; Nazar, L.F. The importance of chemical interactions between sulfur host materials and lithium polysulfides for advanced lithium-sulfur batteries. J. Electrochem. Soc. 2015, 162, A2567. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Peng, H.J.; Zhao, M.; Huang, J.Q. Heterogeneous/homogeneous mediators for high-energy-density lithium–sulfur batteries: Progress and prospects. Adv. Funct. Mater. 2018, 28, 1707536. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, Z.L.; Meng, X.P.; Wang, R. MXene-engineered lithium-sulfur batteries. J. Mater. Chem. A 2019, 7, 22730–22743. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Lin, H.; Yang, D.D.; Lou, N.; Zhu, S.G.; Li, H.Z. Functionalized titanium nitride-based MXenes as promising host materials for lithium-sulfur batteries: A first principles study. Ceram. Int. 2019, 45, 1588–1594. [Google Scholar] [CrossRef]
- Sim, E.S.; Yi, G.S.; Je, M.Y.; Lee, Y.B.; Chung, Y.C. Understanding the anchoring behavior of titanium carbide-based MXenes depending on the functional group in LiS batteries: A density functional theory study. J. Power Sources 2017, 342, 64–69. [Google Scholar] [CrossRef]
- Sim, E.S.; Chung, Y.C. Non-uniformly functionalized titanium carbide-based MXenes as an anchoring material for Li-S batteries: A first-principles calculation. Appl. Surf. Sci. 2018, 435, 210–215. [Google Scholar] [CrossRef]
- Liu, Y.H.; Wang, C.Y.; Yang, S.L.; Cao, F.F.; Ye, H. 3D MXene architectures as sulfur hosts for high-performance lithium-sulfur batteries. J. Energy Chem. 2022, 66, 429–439. [Google Scholar] [CrossRef]
- Li, X.; Guan, Q.H.; Zhuang, Z.; Zhang, Y.Z.; Lin, Y.; Wang, J.; Shen, C.Y.; Lin, H.Z.; Wang, Y.; Zhan, L.; et al. Ordered mesoporous carbon grafted MXene catalytic heterostructure as Li-ion kinetic pump toward high-efficient sulfur/sulfide conversions for Li-S battery. ACS Nano 2023, 17, 1653–1662. [Google Scholar] [CrossRef]
- Yao, W.; Tian, C.X.; Yang, C.; Xu, J.; Meng, Y.F.; Manke, I.; Chen, N.; Wu, Z.; Zhan, L.; Wang, Y.; et al. P-doped NiTe2 with Te-vacancies in lithium-sulfur batteries prevents shuttling and promotes polysulfide conversion. Adv. Mater. 2022, 34, 2106370. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Liu, M.; Chen, Y.; Hou, B.; Zhang, N.; Chen, B.B.; Yang, N.; Chen, K.; Li, J.; An, L. Fabrication of layered Ti3C2 with an accordion-like structure as a potential cathode material for high performance lithium-sulfur batteries. J. Mater. Chem. A 2015, 3, 7870–7876. [Google Scholar] [CrossRef]
- Zhang, F.; Zhou, Y.; Zhang, Y.; Li, D.C.; Huang, Z.C. Facile synthesis of sulfur@titanium carbide Mxene as high performance cathode for lithium-sulfur batteries. Nanophotonics 2020, 9, 2025–2032. [Google Scholar] [CrossRef]
- Yao, Y.; Feng, W.; Chen, M.L.; Zhong, X.W.; Wu, X.J.; Zhang, H.; Yu, Y. Boosting the electrochemical performance of Li-S batteries with a dual polysulfides confinement strategy. Small 2018, 14, 1802516. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Zhang, N.; Zhu, C.C.; Gao, H.; Zhang, X.T. Rationally designing S/Ti3C2Tx as a cathode material with an interlayer for high-rate and long-cycle lithium-sulfur batteries. Nanoscale 2018, 10, 16935–16942. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Li, W.L.; Pan, L.M.; Cullen, C.P.; Liu, Y.; Pakdel, A.; Long, D.H.; Yang, J.; McEvoy, N.; Duesberg, G.S.; et al. In situ formed protective barrier enabled by sulfur@titanium carbide (MXene) ink for achieving high-capacity, long lifetime Li-S batteries. Adv. Sci. 2018, 5, 1800502. [Google Scholar] [CrossRef]
- Tang, H.; Li, W.L.; Pan, L.M.; Tu, K.; Du, F.; Qiu, T.; Yang, J.; Cullen, C.P.; McEvoy, N.; Zhang, C.F. A robust, freestanding MXene-sulfur conductive paper for long-lifetime Li-S batteries. Adv. Funct. Mater. 2019, 29, 1901907. [Google Scholar] [CrossRef]
- Du, Z.Z.; Chen, X.J.; Hu, W.; Chuang, C.H.; Xie, S.; Hu, A.; Yan, W.S.; Kong, X.H.; Wu, X.; Ji, H.X.; et al. Cobalt in nitrogen-doped graphene as single-atom catalyst for high-sulfur content lithium-sulfur batteries. J. Am. Chem. Soc. 2019, 141, 3977–3985. [Google Scholar] [CrossRef]
- Feng, J.; Liu, W.D.; Shi, C.; Zhang, C.Y.; Zhao, X.X.; Wang, T.; Chen, S.Q.; Li, Q.; Song, J. Enabling fast diffusion/conversion kinetics by thiourea-induced wrinkled N,S co-doped functional MXene for lithium-sulfur battery. Energy Storage Mater. 2024, 67, 103328. [Google Scholar] [CrossRef]
- Bao, W.Z.; Liu, L.; Wang, C.Y.; Choi, S.; Wang, D.; Wang, G.X. Facile synthesis of crumpled nitrogen-doped mxene nanosheets as a new sulfur host for lithium-sulfur batteries. Adv. Energy Mater. 2018, 8, 1702485. [Google Scholar] [CrossRef]
- Nguyen, V.P.; Qureshi, Y.; Shim, H.C.; Yuk, J.M.; Kim, J.H.; Lee, S.M. Intercalation-Conversion Hybrid Cathode Enabled by MXene-Driven TiO2/TiS2 Heterostructure for High-Energy-Density Li-S Battery. Small Struct. 2024, 5, 2400196. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, X.B.; Qiao, X.; Zhang, N.; Zhang, C.F.; Ma, Z.; Wang, H.Q. Lithium-ion-engineered interlayers of V2C MXene as advanced host for flexible sulfur cathode with enhanced rate performance. J. Phys. Chem. Lett. 2020, 11, 885–890. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhang, L.; Ma, X.; Lu, H.Q.; Li, L.; Zhang, X.T.; Wu, L. Conversion of polysulfides on core-shell CoP@C nanostructures for lithium-sulfur batteries. Chem. Eng. J. 2023, 454, 140460. [Google Scholar] [CrossRef]
- Liang, Q.; Wang, S.; Yao, Y.; Dong, P.; Song, H. Transition Metal Compounds Family for Li-S Batteries: The DFT-Guide for Suppressing Polysulfides Shuttle. Adv. Funct. Mater. 2023, 33, 2300825. [Google Scholar] [CrossRef]
- Wen, C.Y.; Zheng, X.; Li, X.; Yuan, M.W.; Li, H.F.; Sun, G. Rational design of 3D hierarchical MXene@AlF3/Ni(OH)2 nanohybrid for high-performance lithium-sulfur batteries. Chem. Eng. J. 2021, 409, 128102. [Google Scholar] [CrossRef]
- Guan, H.; Feng, D.; Xu, X.Z.; Chen, Q.; Mei, Y.; Zeng, T.B.; Xie, D. Rational carbon design and confinement of leaf-like Tin trisulfide@Carbon/MXene Ti3C2Tx for enhanced conductivity and lithium storage kinetics. Adv. Compos. Hybrid Mater. 2024, 7, 112. [Google Scholar] [CrossRef]
- Li, Y.Y.; Yang, R.; Xie, J.W.; Li, J.; Huang, H.; Liang, X.Q.; Huang, D.; Lan, Z.Q.; Liu, H.Z.; Li, G.X.; et al. Potassium Ion-Assisted Self-Assembled MXene-K-CNT Composite as High-Quality Sulfur-Loaded Hosts for Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2024, 16, 39771–39783. [Google Scholar] [CrossRef]
- Lv, L.P.; Guo, C.F.; Sun, W.W.; Wang, Y. Strong Surface-Bound Sulfur in Carbon Nanotube Bridged Hierarchical Mo2C-Based MXene Nanosheets for Lithium-Sulfur Batteries. Small 2019, 15, 1804338. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Ming, F.W.; Su, H.; Wu, Y.Q.; Wahyudi, W.; Li, M.L.; Hedhili, M.N.; Sheng, G.; Li, L.J.; Alshareef, H.N.; et al. MXene based self-assembled cathode and antifouling separator for high-rate and dendrite-inhibited Li-S battery. Nano Energy 2019, 61, 478–485. [Google Scholar] [CrossRef]
- Gan, R.; Yang, N.; Dong, Q.; Fu, N.; Wu, R.; Li, C.; Liao, Q.; Li, J.; Wei, Z. Enveloping ultrathin Ti3C2 nanosheets on carbon fibers: A high-density sulfur loaded lithium-sulfur battery cathode with remarkable cycling stability. J. Mater. Chem. A 2020, 8, 7253–7260. [Google Scholar] [CrossRef]
- Song, J.J.; Guo, X.; Zhang, J.Q.; Chen, Y.; Zhang, C.Y.; Luo, L.; Wang, F.Y.; Wang, G.X. Rational design of free-standing 3D porous MXene/rGO hybrid aerogels as polysulfide reservoirs for high-energy lithium-sulfur batteries. J. Mater. Chem. A 2019, 7, 6507–6513. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, N.; Yu, M.L.; Liu, J.S.; Wang, S.; Qiu, J.S. Boosting redox activity on MXene-induced multifunctional collaborative interface in high Li2S loading cathode for high-energy Li-S and metallic Li-free rechargeable batteries. J. Energy Chem. 2019, 37, 183–191. [Google Scholar] [CrossRef]
- Wang, M.Y.; Bai, Z.C.; Yang, T.; Nie, C.; Xu, X.; Wang, Y.X.; Yang, J.; Dou, S.X.; Wang, N. Advances in high sulfur loading cathodes for practical lithium-sulfur batteries. Adv. Energy Mater. 2022, 12, 2201585. [Google Scholar] [CrossRef]
- Bao, W.Z.; Su, D.; Zhang, W.X.; Guo, X.; Wang, G.X. 3D metal carbide@mesoporous carbon hybrid architecture as a new polysulfide reservoir for lithium-sulfur batteries. Adv. Funct. Mater. 2016, 26, 8746–8756. [Google Scholar] [CrossRef]
- Qi, Q.; Zhang, H.; Zhang, P.; Bao, Z.H.; Zheng, W.; Tian, W.B.; Zhang, W.; Zhou, M.; Sun, Z.M. Self-assembled sandwich hollow porous carbon sphere@MXene composites as superior LiS battery cathode hosts. 2D Mater. 2020, 7, 025049. [Google Scholar] [CrossRef]
- Li, T.T.; Liang, L.P.; Chen, Z.; Zhu, J.L.; Shen, P.K. Hollow Ti3C2Tx MXene@CoSe2/N-doped carbon heterostructured composites for multiphase electrocatalysis process in lithium-sulfur batteries. Chem. Eng. J. 2023, 474, 145970. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Tang, W.W.; Zhan, R.M.; Liu, H.; Chen, H.; Yang, J.; Xu, M.W. An N-doped porous carbon/MXene composite as a sulfur host for lithium-sulfur batteries. Inorg. Chem. Front. 2019, 6, 2894–2899. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, T.; Yang, Z.; Chen, Y.; Liu, Y.; Wang, J.X.; Zhai, P.F.; Wu, W. MXene-based Co,N-codoped porous carbon nanosheets regulating polysulfides for high-performance lithium-sulfur batteries. ACS Appl. Mater. Interfaces 2019, 11, 38654–38662. [Google Scholar] [CrossRef]
- Qiu, S.Y.; Wang, C.; Jiang, Z.X.; Zhang, L.S.; Gu, L.L.; Wang, K.X.; Gao, J.; Zhu, X.D.; Wu, G. Rational design of MXene@TiO2 nanoarray enabling dual lithium polysulfide chemisorption towards high-performance lithium-sulfur batteries. Nanoscale 2020, 12, 16678–16684. [Google Scholar] [CrossRef]
- Wang, Q.; Qiao, S.M.; Huang, C.H.; Wang, X.; Cai, C.; He, G.H.; Zhang, F.X. Multi-heterostructured MXene/NiS2/Co3S4 with S-Vacancies to Promote Polysulfide Conversion in Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2024, 16, 24502–24513. [Google Scholar] [CrossRef]
- Zhang, Y.; Mu, Z.; Yang, C.; Xu, Z.; Zhang, S.; Zhang, X.; Li, Y.; Lai, J.P.; Sun, Z.H.; Yang, Y.; et al. Rational design of MXene/1T-2H MoS2-C nanohybrids for high-performance lithium-sulfur batteries. Adv. Funct. Mater. 2018, 28, 1707578. [Google Scholar] [CrossRef]
- Pan, H.; Huang, X.X.; Zhang, R.; Wang, D.; Chen, Y.; Duan, X.M.; Wen, G. Titanium oxide-Ti3C2 hybrids as sulfur hosts in lithium-sulfur battery: Fast oxidation treatment and enhanced polysulfide adsorption ability. Chem. Eng. J. 2019, 358, 1253–1261. [Google Scholar] [CrossRef]
- Zhang, H.; Qi, Q.; Zhang, P.; Zheng, W.; Chen, J.; Zhou, A.; Tian, W.; Zhang, W.; Sun, Z.M. Self-assembled 3D MnO2 nanosheets@ delaminated-Ti3C2 aerogel as sulfur host for lithium-sulfur battery cathodes. ACS Appl. Energy Mater. 2018, 2, 705–714. [Google Scholar] [CrossRef]
- Han, D.D.; Sun, L.H.; Li, Z.; Qin, W.L.; Zhai, L.P.; Sun, Y.Y.; Tang, S.; Fu, Y. Supramolecular channels via crown ether functionalized covalent organic frameworks for boosting polysulfides conversion in Li-S batteries. Energy Storage Mater. 2024, 65, 103143. [Google Scholar] [CrossRef]
- Meng, R.; Deng, Q.; Peng, C.X.; Chen, B.J.; Liao, K.X.; Li, L.; Yang, Z.; Yang, D.L.; Zheng, L.; Zhang, C.; et al. Two-dimensional organic-inorganic heterostructures of in situ-grown layered COF on Ti3C2 MXene nanosheets for lithium-sulfur batteries. Nano Today 2020, 35, 100991. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, Q.; Miao, J.W.; Zhang, P.; Xu, B. 2D MXene nanosheets enable small-sulfur electrodes to be flexible for lithium-sulfur batteries. Nanoscale 2019, 11, 8442–8448. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhao, Q.; An, Y.; Anasori, B.; Wang, H.; Xu, B. Ultra-microporous carbons encapsulate small sulfur molecules for high performance lithium-sulfur battery. Nano Energy 2017, 33, 402–409. [Google Scholar] [CrossRef]
- Yu, L.Y.; Hu, L.F.; Anasori, B.; Liu, Y.T.; Zhu, Q.; Zhang, P.; Gogotsi, Y.; Xu, B. MXene-bonded activated carbon as a flexible electrode for high-performance supercapacitors. ACS Energy Lett. 2018, 3, 1597–1603. [Google Scholar] [CrossRef]
- Sun, N.; Zhu, Q.; Anasori, B.; Zhang, P.; Liu, H.; Gogotsi, Y.; Xu, B. MXene-bonded flexible hard carbon film as anode for stable Na/K-ion storage. Adv. Funct. Mater. 2019, 29, 1906282. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, Q.; Guan, Z.; Zhao, Q.; Sun, N.; Xu, B. A flexible Si@C electrode with excellent stability employing an MXene as a multifunctional binder for lithium-ion batteries. ChemSusChem 2020, 13, 1621–1628. [Google Scholar] [CrossRef]
- Du, C.; Wu, J.; Yang, P.; Li, S.Y.; Xu, J.M.; Song, K.X. Embedding S@TiO2 nanospheres into MXene layers as high rate cyclability cathodes for lithium-sulfur batteries. Electrochim. Acta 2019, 295, 1067–1074. [Google Scholar] [CrossRef]
- Zhang, S.Z.; Zhong, N.; Zhou, X.; Zhang, M.J.; Huang, X.P.; Yang, X.; Meng, R.; Liang, X. Comprehensive design of the high-sulfur-loading Li-S battery based on MXene nanosheets. Nano-Micro Lett. 2020, 12, 112. [Google Scholar] [CrossRef]
- Li, N.; Xie, Y.; Peng, S.; Xiong, X.; Han, K. Ultra-lightweight Ti3C2Tx MXene modified separator for Li-S batteries: Thickness regulation enabled polysulfide inhibition and lithium ion transportation. J. Energy Chem. 2020, 42, 116–125. [Google Scholar] [CrossRef]
- Lin, C.; Zhang, W.K.; Wang, L.; Wang, Z.G.; Zhao, W.; Duan, W.; Zhao, Z.; Liu, B.; Jin, J. A few-layered Ti3C2 nanosheet/glass fiber composite separator as a lithium polysulphide reservoir for high-performance lithium-sulfur batteries. J. Mater. Chem. A 2016, 4, 5993–5998. [Google Scholar] [CrossRef]
- Yin, L.X.; Xu, G.; Nie, P.; Dou, H.; Zhang, X.G. MXene debris modified eggshell membrane as separator for high-performance lithium-sulfur batteries. Chem. Eng. J. 2018, 352, 695–703. [Google Scholar] [CrossRef]
- Li, N.; Cao, W.; Liu, Y.; Ye, H.; Han, K. Impeding polysulfide shuttling with a three-dimensional conductive carbon nanotubes/MXene framework modified separator for highly efficient lithium-sulfur batteries. Colloids Surf. A Physicochem. Eng. Asp. 2019, 573, 128–136. [Google Scholar] [CrossRef]
- Jiang, G.; Zheng, N.; Chen, X.; Ding, G.; Li, Y.; Sun, F.; Li, Y.S. In-situ decoration of MOF-derived carbon on nitrogen-doped ultrathin MXene nanosheets to multifunctionalize separators for stable Li-S batteries. Chem. Eng. J. 2019, 373, 1309–1318. [Google Scholar] [CrossRef]
- Liu, P.; Qu, L.; Tian, X.; Yi, Y.; Xia, J.X.; Wang, T.; Nan, J.; Yang, P.; Wang, T.; Fang, B.; et al. Ti3C2Tx/Graphene Oxide Free-Standing Membranes as Modified Separators for Lithium-Sulfur Batteries with Enhanced Rate Performance. ACS Appl. Energy Mater. 2020, 3, 2708–2718. [Google Scholar] [CrossRef]
- Jiao, L.; Zhang, C.; Geng, C.; Wu, S.; Li, H.; Lv, W.; Tao, Y.; Chen, Z.; Zhou, G.M.; Li, J.; et al. Capture and catalytic conversion of polysulfides by in situ built TiO2-MXene heterostructures for lithium-sulfur batteries. Adv. Energy Mater. 2019, 9, 1900219. [Google Scholar] [CrossRef]
- Wang, J.T.; Zhai, P.F.; Zhao, T.K.; Li, M.J.; Yang, Z.; Zhang, H.; Huang, J. Laminar MXene-Nafion-modified separator with highly inhibited shuttle effect for long-life lithium-sulfur batteries. Electrochim. Acta 2019, 320, 134558. [Google Scholar] [CrossRef]
- Zhan, Y.X.; Shi, P.; Zhang, X.Q.; Ding, F.; Huang, J.Q.; Jin, Z.; Xiang, R.; Liu, X.J.; Zhang, Q. The insights of lithium metal plating/stripping in porous hosts: Progress and perspectives. Energy Technol. 2021, 9, 2000700. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, S.; Li, B.; Gong, Y.; Yang, S. Horizontal growth of lithium on parallelly aligned MXene layers towards dendrite-free metallic lithium anodes. Adv. Mater. 2019, 31, 1901820. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Li, H.; Chen, Z.; Shang, T.X.; Wu, Z.; Zhang, C.; Li, J.; Lv, W.; Tao, Y.; et al. Layered MXene Protected Lithium Metal Anode as an Efficient Polysulfide Blocker for Lithium-Sulfur Batteries. Batter. Supercaps 2020, 3, 892–899. [Google Scholar] [CrossRef]
- Chen, X.; Shang, M.; Niu, J. Inter-layer-calated thin Li metal electrode with improved battery capacity retention and dendrite suppression. Nano Lett. 2020, 20, 2639–2646. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.D.; Zhang, C.F.J.; Lu, P.; Dong, Y.; Wen, P.C.; Wu, Z.S. Conducting and lithiophilic MXene/graphene framework for high-capacity, dendrite-free lithium-metal anodes. ACS Nano 2019, 13, 14308–14318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, H.; Hu, R.; Huang, C.Y.; Zhong, W.J.; Pan, L.M.; Feng, Y.B.; Qiu, T.; Zhang, C.F.; Yang, J. Novel solvothermal preparation and enhanced microwave absorption properties of Ti3C2Tx MXene modified by in situ coated Fe3O4 nanoparticles. Appl. Surf. Sci. 2019, 484, 383–391. [Google Scholar] [CrossRef]
- Gu, J.; Zhu, Q.; Shi, Y.Z.; Chen, H.; Zhang, D.; Du, Z.; Yang, S. Single zinc atoms immobilized on MXene (Ti3C2Clx) layers toward dendrite-free lithium metal anodes. ACS Nano 2020, 14, 891–898. [Google Scholar] [CrossRef]
- Wei, C.L.; Fei, H.F.; Tian, Y.; An, Y.L.; Guo, H.H.; Feng, J.K.; Qian, Y. Isotropic Li nucleation and growth achieved by an amorphous liquid metal nucleation seed on MXene framework for dendrite-free Li metal anode. Energy Storage Mater. 2020, 26, 223–233. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).