Synergistic Cationic–Anionic Regulation in Ni-Doped FeSe@C Anodes with Se Vacancies for High-Efficiency Sodium Storage
Abstract
1. Introduction
2. Experimental Section
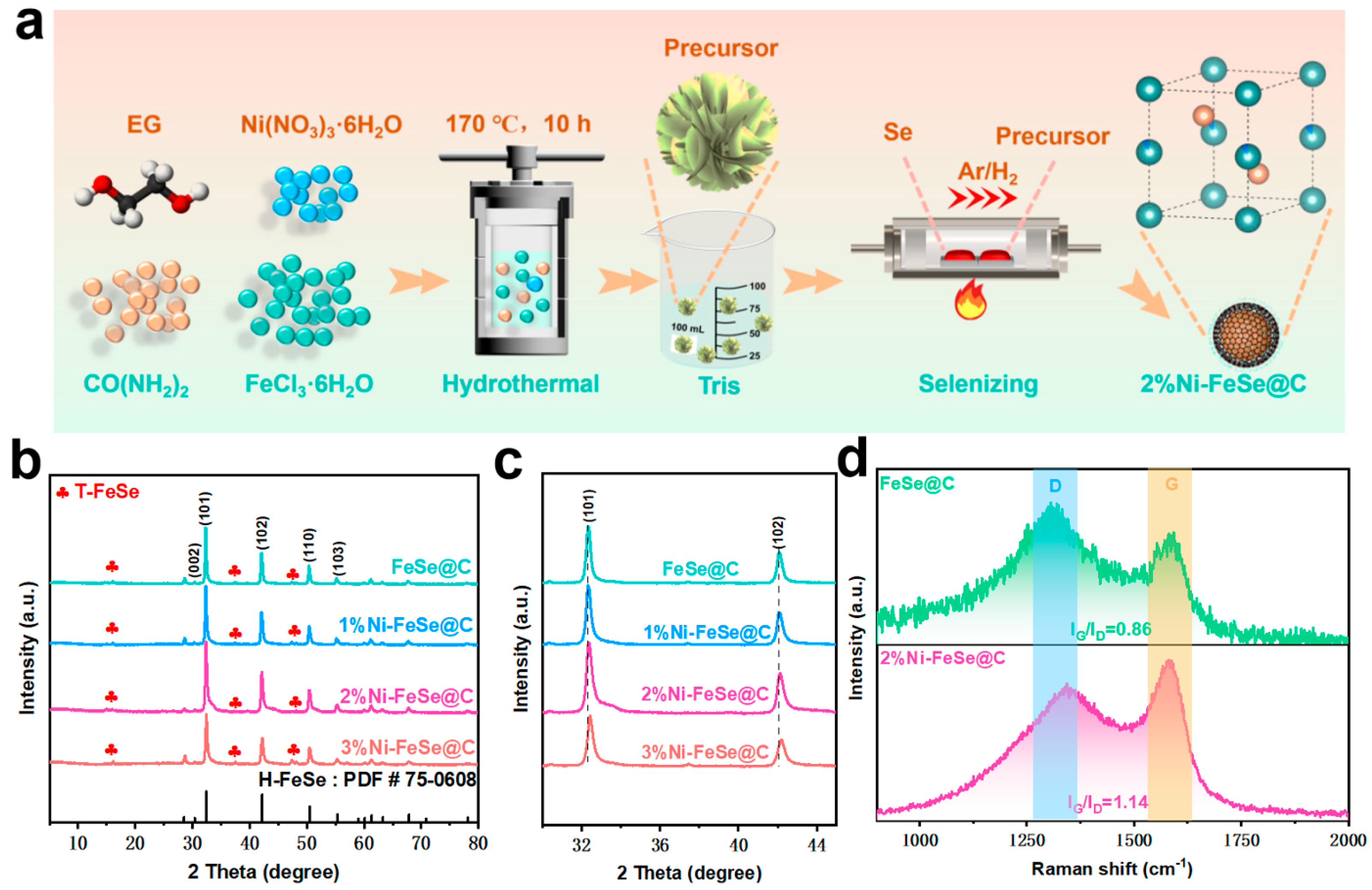
2.1. Synthesis of 2%Ni-FeOOH@C Nanomaterial
2.2. Synthesis of 2%Ni-FeSe@C Nanomaterial
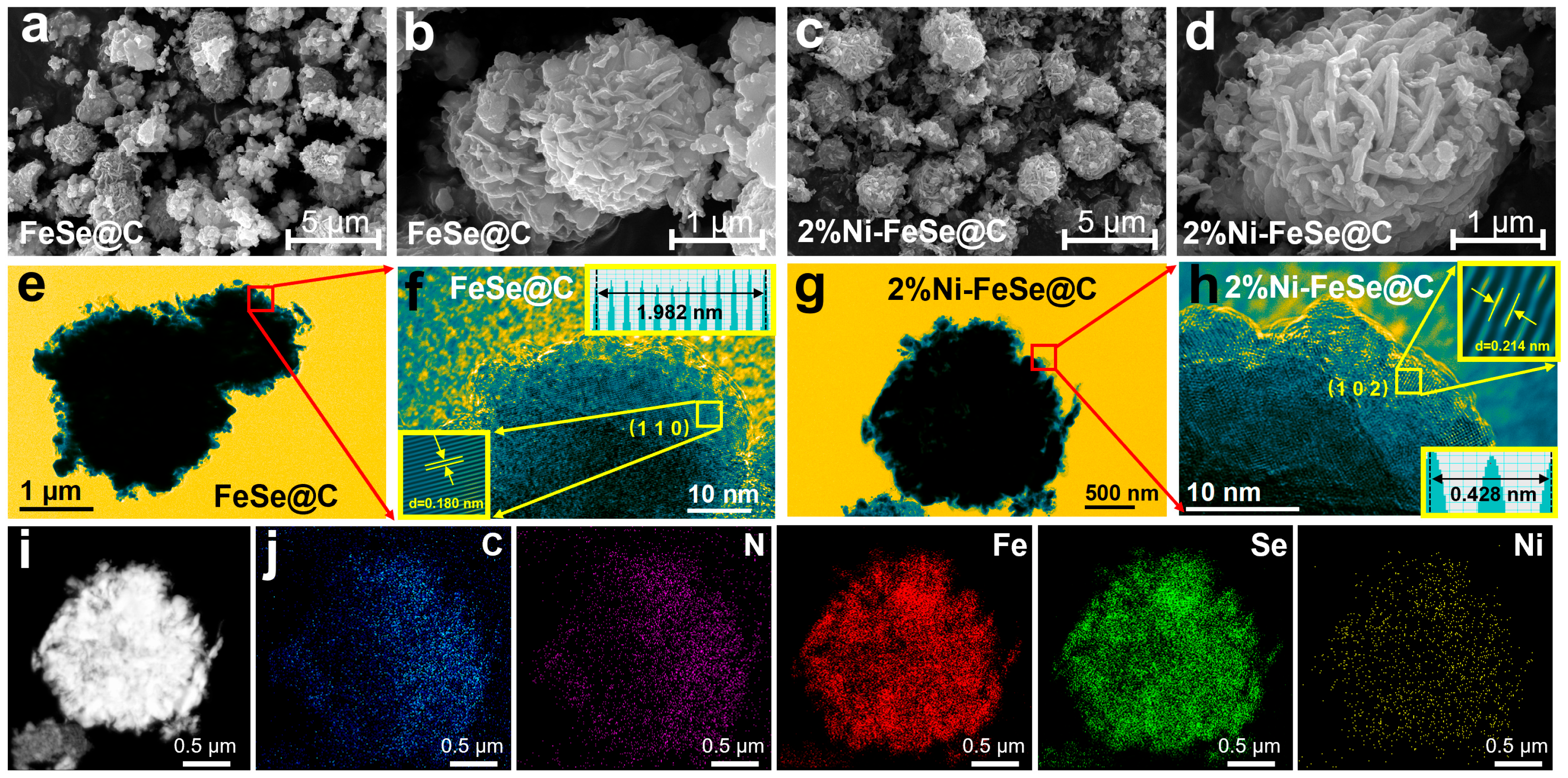
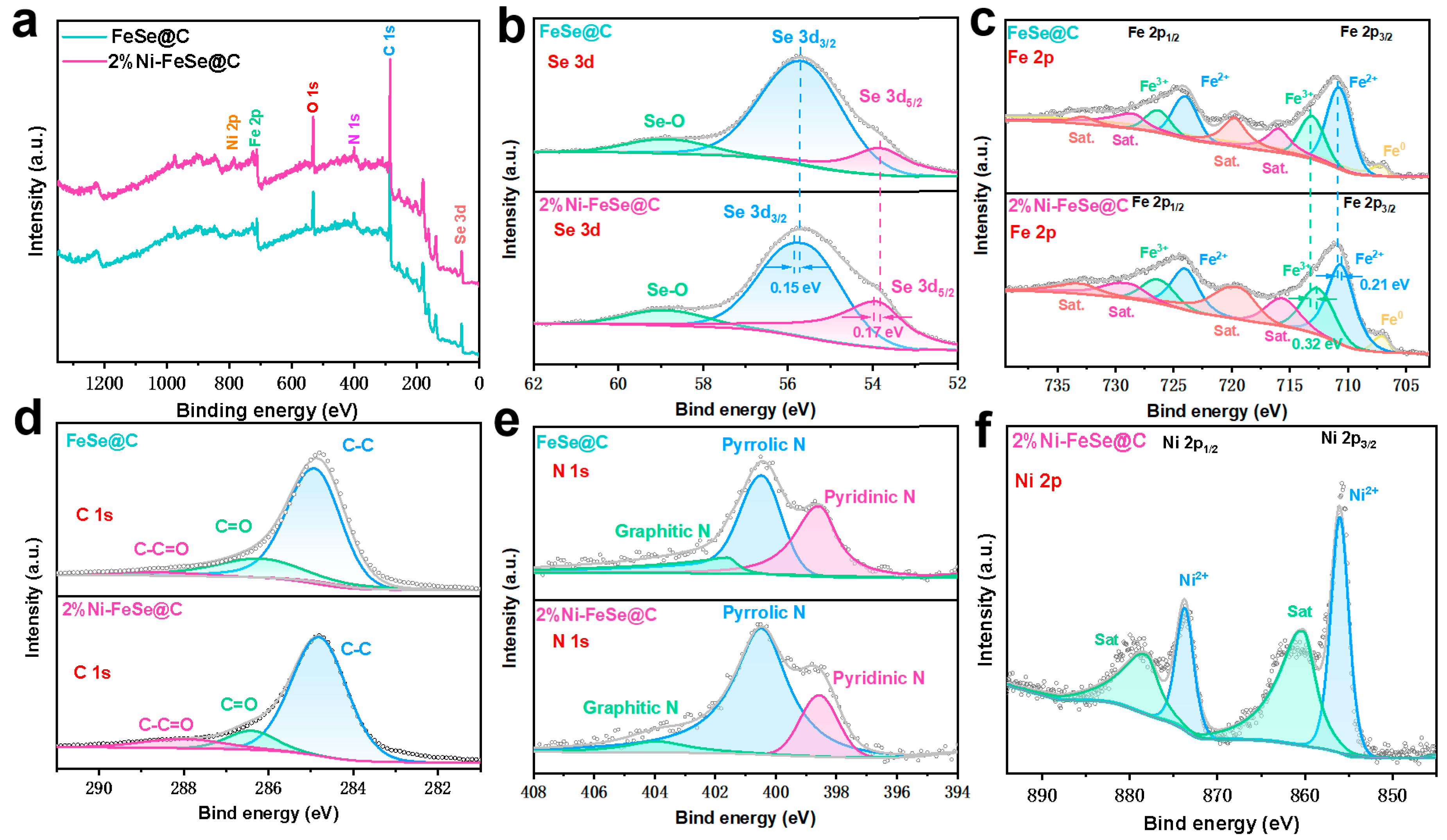
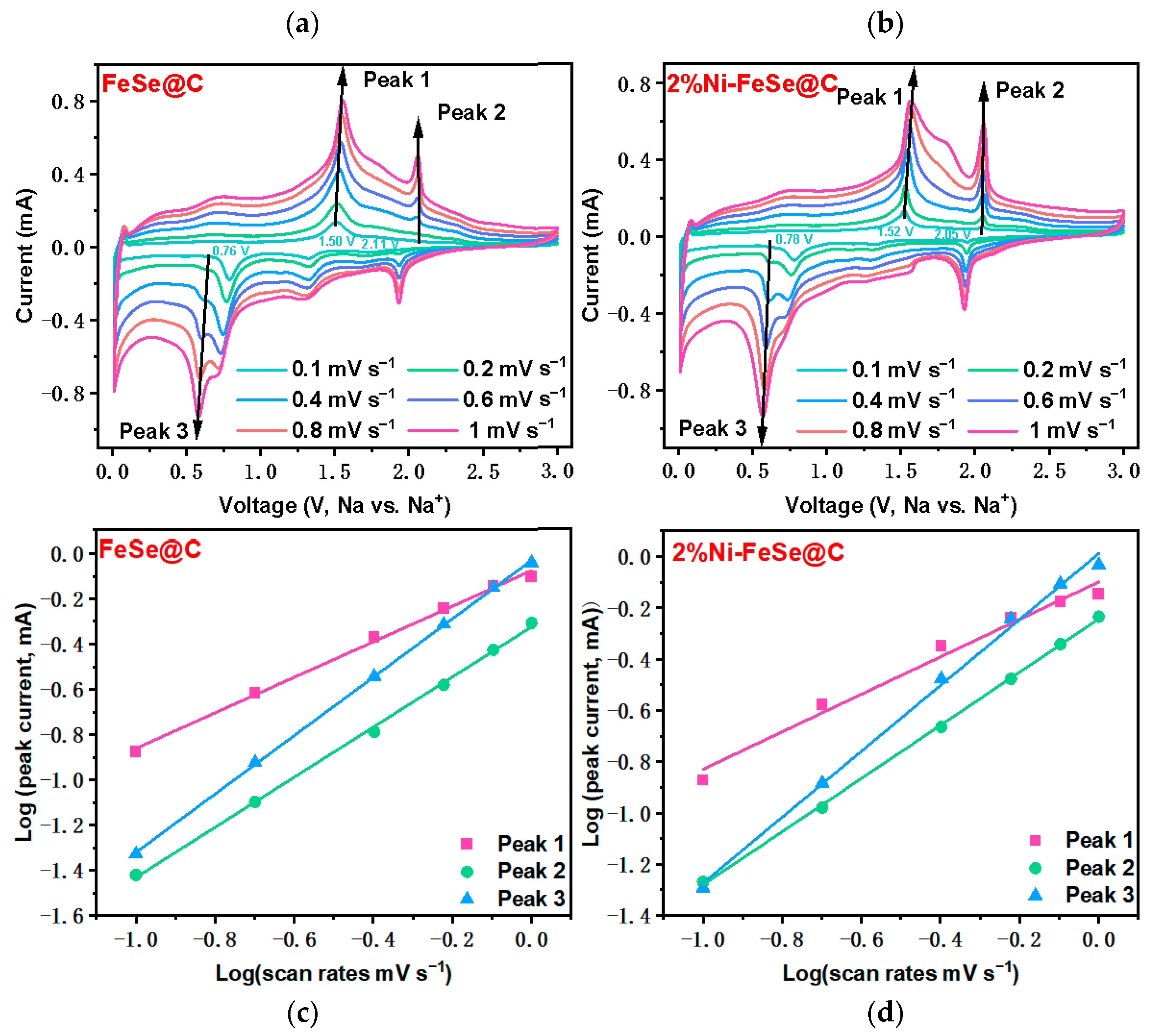
3. Results and Discussion

4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, B.; Qian, K.; Jiao, X.; Yuan, G.; Bai, J.; Wang, H.; Wang, G. Metal Doping and Vacancy Synergistic Induced Electron/Ion Engineering to Optimize the Redox Kinetics of Sodium Storage: A Case Study Mo1-xWxSe2. Energy Storage Mater. 2023, 63, 102998. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Ji, C.; Ma, Y.; Wang, R.; Zhang, S.; Hao, J.; Zhou, T.; Zhang, L.; Zhang, C.; et al. Molybdenum-Based Materials for Alkali Metal-Ion Batteries: Recent Advances and Perspectives. Coord. Chem. Rev. 2024, 506, 215725. [Google Scholar] [CrossRef]
- Wang, C.; Wan, J.; Li, J.; Zhang, L.; Wang, R.; Liu, Y.; Ma, Y.; Qin, Q.; Qian, M.; Li, H.; et al. Enhancing Sodium Storage through Tri-phase Heterointerfaces in Co–Fe–MoSe2@N-doped Carbon Nanocubes with Self-generated Rich Phase Boundaries. J. Alloy. Compd. 2024, 987, 174177. [Google Scholar] [CrossRef]
- Li, J.; Hu, Y.; Guo, Y.; Wang, C.; Huang, Z.; Luo, S.-H.; Yan, S.; Qian, M.; Cheng, Y.; Ma, Y. Rational Design of Nanocubic Fe3O4/FeP@C with Heterostructure as Advanced Anode Material towards Enhanced Lithium Storage. J. Energy Storage 2024, 89, 111642. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, Z.; Jiang, X.; Zhao, Y.; Luo, Y.; Wang, Y.; Wang, Z.; Zhang, Y.; Liu, X.; Fang, B. A Comprehensive Review on Iron-Based Sulfate Cathodes for Sodium-Ion Batteries. Nanomaterials 2024, 14, 1915. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-J.; Ri, S.-B.; Choe, S.-H.; Ri, G.-C.; Kye, Y.-H.; Kim, S.-C. Ab Initio Study of Sodium Cointercalation with Diglyme Molecule into Graphite. Electrochim. Acta 2017, 253, 589–598. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Wang, R.; Zhang, C.; Li, G.; Davey, K.; Zhang, S.; Guo, Z. Progress and Perspectives on Iron-Based Electrode materials for Alkali Metal-Ion Batteries: A Critical Review. Chem. Soc. Rev. 2024, 53, 4154–4229. [Google Scholar] [CrossRef]
- Kong, X.; Luo, S.; Wan, Z.; Li, S. Constructing Hierarchical Carbon Network Wrapped Fe3Se4 Nanoparticles for Sodium Ion Storage and Hydrogen Evolution Reaction. Electrochim. Acta 2021, 392, 138997. [Google Scholar] [CrossRef]
- Ren, M.; Zang, H.; Cao, S.; Liu, W.; Li, M.; Yao, J.; Cai, F.; Cui, J.; Wang, Y. Fe3Se4 Decorating Carbon Nanotubes with Superior Sodium Storage Performance for Sodium-Ion Batteries. J. Energy Storage 2024, 81, 110486. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, Z.; Yao, S.; Gao, Y.; Jin, X.; Chen, G.; Shen, Z.; Du, F. Polymorph Engineering for Boosted Volumetric Na-Ion and Li-Ion Storage. Adv. Mater. 2021, 33, 2100210. [Google Scholar] [CrossRef]
- Chu, K.; Luo, Y.; Shen, P.; Li, X.; Li, Q.; Guo, Y. Unveiling the Synergy of O-vacancy and Heterostructure over MoO3-x/MXene for N2 Electroreduction to NH3. Adv. Energy Mater. 2022, 12, 2103022. [Google Scholar] [CrossRef]
- Yan, Z.; Sun, Z.; Zhao, L.; Liu, H.; Guo, Z.; Qiu, Y.; Wang, P.; Qian, L. In-Situ Induced Sulfur Vacancy from Phosphorus Doping in Fes2 Microflowers for High-Efficiency Lithium Storage. Mater. Today Nano 2022, 20, 100261. [Google Scholar] [CrossRef]
- Ma, Z.; Wen, Z.; Song, Y.; Yang, T.; Tian, X.; Wu, J.; Liu, Y.; Liu, Z.; Wang, H. Effect of Sulfur Modification on Structural and Electrochemical Performance of Pitch-Based Carbon Materials for Lithium/Sodium Ion Batteries. Nanomaterials 2024, 14, 1410. [Google Scholar] [CrossRef]
- Fan, H.-N.; Wang, X.-Y.; Yu, H.-B.; Gu, Q.-F.; Chen, S.-L.; Liu, Z.; Chen, X.-H.; Luo, W.-B.; Liu, H.-K. Enhanced Potassium Ion Battery by Inducing Interlayer Anionic Ligands in MoS1.5Se0.5 Nanosheets with Exploration of the Mechanism. Energy Mater. 2020, 10, 1904162. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Y.; Sun, H.; Zhou, J.; Liu, Z.; Qiu, Z.; Wang, D.; Yang, C.; Zeng, Q.; Peng, Z.; et al. Orthorhombic Cobalt Ditelluride with Te Vacancy Defects Anchoring on Elastic MXene Enables Efficient Potassium-Ion Storage. Adv. Mater. 2021, 33, 2100272. [Google Scholar] [CrossRef]
- He, T.; Chen, L.; Su, Y.; Lu, Y.; Bao, L.; Chen, G.; Zhang, Q.; Chen, S.; Wu, F. The Effects of Alkali Metal Ions with Different Ionic Radii Substituting in Li Sites on the Electrochemical Properties of Ni-Rich Cathode Materials. J. Power Sources 2019, 441, 227195. [Google Scholar] [CrossRef]
- Huang, X.; Xu, C.; Ma, J.; Chen, F. Ionothermal Synthesis of Cu-Doped Fe3O4 Magnetic Nanoparticles with Enhanced Peroxidase-Like Activity for Organic Wastewater Treatment. Adv. Powder Technol. 2018, 29, 796–803. [Google Scholar] [CrossRef]
- Lv, C.; Liu, H.; Li, D.; Chen, S.; Zhang, H.; She, X.; Guo, X.; Yang, D. Ultrafine FeSe Nanoparticles Embedded into 3D Carbon Nanofiber Aerogels with FeSe/Carbon Interface for Efficient and Long-Life Sodium Storage. Carbon 2019, 143, 106–115. [Google Scholar] [CrossRef]
- Wang, S.; Cui, T.; Shao, L.; Yang, S.; Yu, L.; Guan, J.; Shi, X.; Cai, J.; Sun, Z. In-Situ Fabrication of Active Interfaces towards FeSe as Advanced Performance Anode for Sodium-Ion Batteries. J. Colloid Interface Sci. 2022, 627, 922–930. [Google Scholar] [CrossRef]
- Pei, Y.R.; Zhou, H.Y.; Zhao, M.; Li, J.C.; Ge, X.; Zhang, W.; Yang, C.C.; Jiang, Q. High-Efficiency Sodium Storage of Co0.85Se/WSe2 Encapsulated in N-Doped Carbon Polyhedron via Vacancy and Heterojunction Engineering. Carbon Energy 2024, 6, e374. [Google Scholar] [CrossRef]
- Xiong, Z.; Sun, D.; Jia, X.; Zhou, J. Core/Shell FeSe/Carbon Nanosheet-Assembled Microflowers with Ultrahigh Coulombic-Efficiency and Rate Performance as Nonpresodiate Anode for Sodium-Ion Battery. Carbon 2020, 166, 339–349. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Z.; Wang, X.; Hu, H.; Wu, M. MXene/Carbon Composites for Electrochemical Energy Storage and Conversion. Mater. Today Sustain. 2023, 22, 100350. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, S.; Li, X.; Li, Z.; Li, X.; Zhang, W.; Xiang, Y.; Niu, X.; Chen, J.S. Interface Engineering of Fe3Se4/FeSe Heterostructure Encapsulated in Electrospun Carbon Nanofibers for Fast and Robust Sodium Storage. Chem. Eng. J. 2021, 417, 129279. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, X.L.; Tan, P.; Bao, S.; Zhang, X.; Xu, M. Ultrafast Kinetics and High Capacity for Stable Sodium Storage Enabled by Fe3Se4/ZnSe Heterostructure Engineering. Compos. Part B Eng. 2021, 224, 109166. [Google Scholar] [CrossRef]
- Wan, M.; Zeng, R.; Chen, K.; Liu, G.; Chen, W.; Wang, L.; Zhang, N.; Xue, L.; Zhang, W.; Huang, Y. Fe7Se8 Nanoparticles Encapsulated by Nitrogen-Doped Carbon with High Sodium Storage Performance and Evolving Redox Reactions. Energy Storage Mater. 2018, 10, 114–121. [Google Scholar] [CrossRef]
- Song, H.; Xie, Z.; Liao, Y.; Wang, Y.; Tan, C.-K. Effect of Selenium Doping on the Electronic Properties of β-Ga2O3 by First-Principles Calculations. J. Electron. Mater. 2024, 53, 6282–6289. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, X.; Mao, X.; Wen, S.; Dai, P.; Li, L.; Liu, D.; Zhao, X. Boosting Fast and Stable Potassium Storage of Iron Selenide/Carbon Nanocomposites by Electrolyte Salt and Solvent Chemistry. J. Power Sources 2021, 486, 229373. [Google Scholar] [CrossRef]
- Živković, A.; Somers, M.; Camprubi, E.; King, H.E.; Wolthers, M.; de Leeuw, N.H. Changes in CO2 Adsorption Affinity Related to Ni Doping in FeS Surfaces: A DFT-D3 Study. Catalysts 2021, 11, 486. [Google Scholar] [CrossRef]
- Xia, J.; Yuan, Y.; Yan, H.; Liu, J.; Zhang, Y.; Liu, L.; Zhang, S.; Li, W.; Yang, X.; Shu, H. Electrospun SnSe/C Nanofibers as Binder-Free Anode for Lithium–Ion and Sodium-Ion Batteries. J. Power Sources 2020, 449, 227559. [Google Scholar] [CrossRef]
- Shen, W.; Wang, C.; Xu, Q.; Liu, H.; Wang, Y. Nitrogen-Doping-Induced Defects of A Carbon Coating Layer Facilitate Na-Storage in Electrode Materials. Adv. Energy Mater. 2015, 5, 1400982. [Google Scholar] [CrossRef]
- Ma, Q.; Zhuang, Q.; Song, H.; Zhao, C.; Zhang, Z.; Liu, J.; Peng, H.; Mao, C.; Li, G. Large-Scale Synthesis of Fe3Se4/C Composites Assembled by Aligned Nanorods as Advanced Anode Material for Lithium Storage. Mater. Lett. 2018, 228, 235–238. [Google Scholar] [CrossRef]
- Li, S.; Luo, S.; Xi, Z.; Wang, L.; Liu, Y.; Chen, X.; Rong, L.; Wan, Z. Fe3Se4 Rice Grains Anchored on Cotton-Derived Porous Carbon Network for Enhanced Sodium Ion Storage and Hydrogen Evolution Reactions. Appl. Surf. Sci. 2023, 610, 155529. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Liu, H.; Song, Y.; Sun, S.; Li, Q.; Xing, X.; Chen, J. Urchin-Like Fe3Se4 Hierarchitectures: A Novel Pseudocapacitive Sodium-Ion Storage Anode with Prominent Rate and Cycling Properties. Small 2020, 16, 2000504. [Google Scholar] [CrossRef]
- Ye, J.; Zheng, Z.; Xia, G.; Hu, C. Fe3Se4 Nanoparticles Confined in Hollow Carbon Nanocages for High-Performance Sodium-Ion Batteries. Appl. Surf. Sci. 2020, 519, 146260. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X. Reduced Graphene Oxides Decorated NiSe Nanoparticles as High Performance Electrodes for Na/Li Storage. Materials 2019, 12, 3709. [Google Scholar] [CrossRef]
- Bi, R.; Zeng, C.; Huang, H.; Wang, X.; Zhang, L. Metal–Organic Frameworks Derived Hollow NiS2 Spheres Encased in Graphene Layers for Enhanced Sodium-Ion Storage. J. Mater. Chem. A 2018, 6, 14077–14082. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, R.; Mu, D.; Tan, G.; Gao, H.; Li, N.; Chen, R.; Wu, F. Progress in Electrolyte and Interface of Hard Carbon and Graphite Anode for Sodium-Ion Battery. Carbon Energy 2022, 4, 458–479. [Google Scholar] [CrossRef]
- Xi, J.; Yuan, Y.; Zhu, M.; Yin, S.; Chen, Y.; Guo, S.; Du, P. Achieving Long-Life and Efficient Sodium Storage through Encapsulating Fe3Se4 Nanoparticles within Hollow Mesoporous Carbon Nanospheres. J. Energy Storage 2024, 81, 110499. [Google Scholar] [CrossRef]
- Zhaom, W.; Ma, X.; Yue, L.; Zhang, L.; Luo, Y.; Ren, Y.; Zhao, X.-E.; Li, N.; Tang, B.; Liu, Q. A Gradient Hexagonal-Prism Fe3Se4@SiO2@C Configuration as A Highly Reversible Sodium Conversion Anode. J. Mater. Chem. A 2022, 10, 4087–4099. [Google Scholar]
- Ji, P.-G.; Liu, Y.; Han, S.-B.; Yan, Y.-F.; Tolochko, O.V.; Strativnov, E.; Kurbanov, M.S.; Wang, H.; Zhang, C.-W.; Wang, G.-K. Synergistical Heterointerface Engineering of Fe-Se Nanocomposite for High-Performance Sodium-Ion Hybrid Capacitors. Rare Met. 2022, 41, 2470–2480. [Google Scholar] [CrossRef]
- Zhang, K.; Park, M.; Zhou, L.; Lee, G.H.; Shin, J.; Hu, Z.; Chou, S.L.; Chen, J.; Kang, Y.M. Cobalt-Doped FeS2 Nanospheres with Complete Solid Solubility as A High-Performance Anode Material for Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2016, 55, 12822–12826. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Song, H.; Chen, X.; Huo, J. Diffusion of Metal in a Confined Nanospace of Carbon Nanotubes Induced by Air Oxidation. J. Am. Chem. Soc. 2010, 132, 11402–11405. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Liu, S.; Song, H.; Zhou, J. Carbon Nanonion-Assembled Microspheres for Excellent Gravimetric and Volumetric Na-Ion Storage. Carbon 2019, 153, 298–307. [Google Scholar] [CrossRef]
- Li, Y.-H.; Babu, S.K.; Gregory, D.H.; Kheawhom, S.; Chang, J.-K.; Liu, W.-R. Silicon/Hard Carbon Composites Synthesized from Phenolic Resin as Anode Materials for Lithium-Ion Batteries. Nanomaterials 2025, 15, 455. [Google Scholar] [CrossRef] [PubMed]
- Weppner, W.; Huggins, R.A. Determination of the Kinetic Parameters of Mixed-Conducting Electrodes and Application to the System Li3Sb. J. Electro. Chem. Soc. 1977, 124, 1569. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, X.; Liu, Y.; Fang, Y.; Zhou, X.; Bao, J. Rice Husk-Derived Hard Carbons as High-Performance Anode Materials for Sodium-Ion Batteries. Carbon 2018, 127, 658–666. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, Z.; Hao, X.; Wei, Y.; Zhang, H.; Dong, Y.; Wang, Y.; Pan, X.; Hou, Y.; Wang, X. Cellular Carbon-Wrapped FeSe2 Nanocavities with Ultrathin Walls and Multiple Rooms for Ion Diffusion-Confined Ultrafast Sodium Storage. J. Mater. Chem. A 2019, 7, 4469–4479. [Google Scholar] [CrossRef]
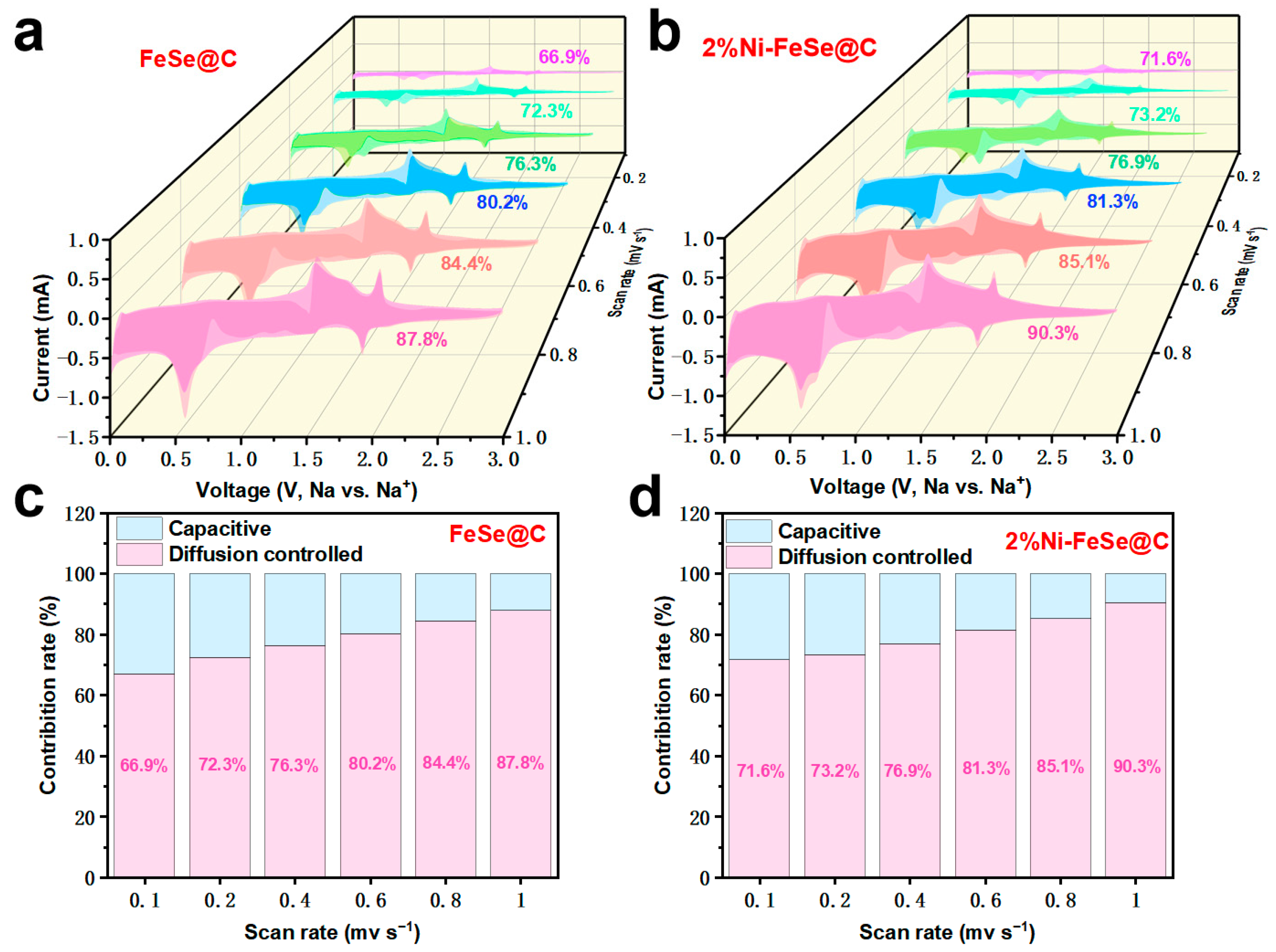
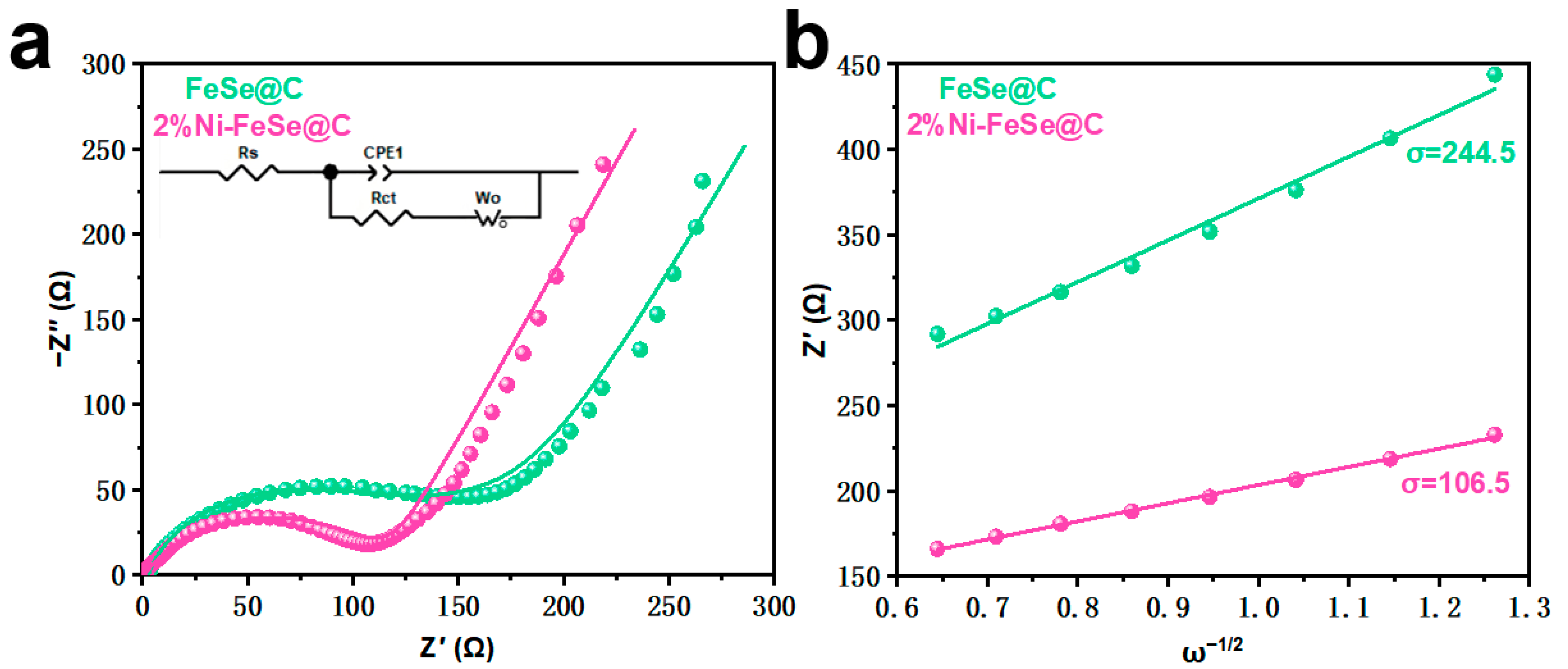
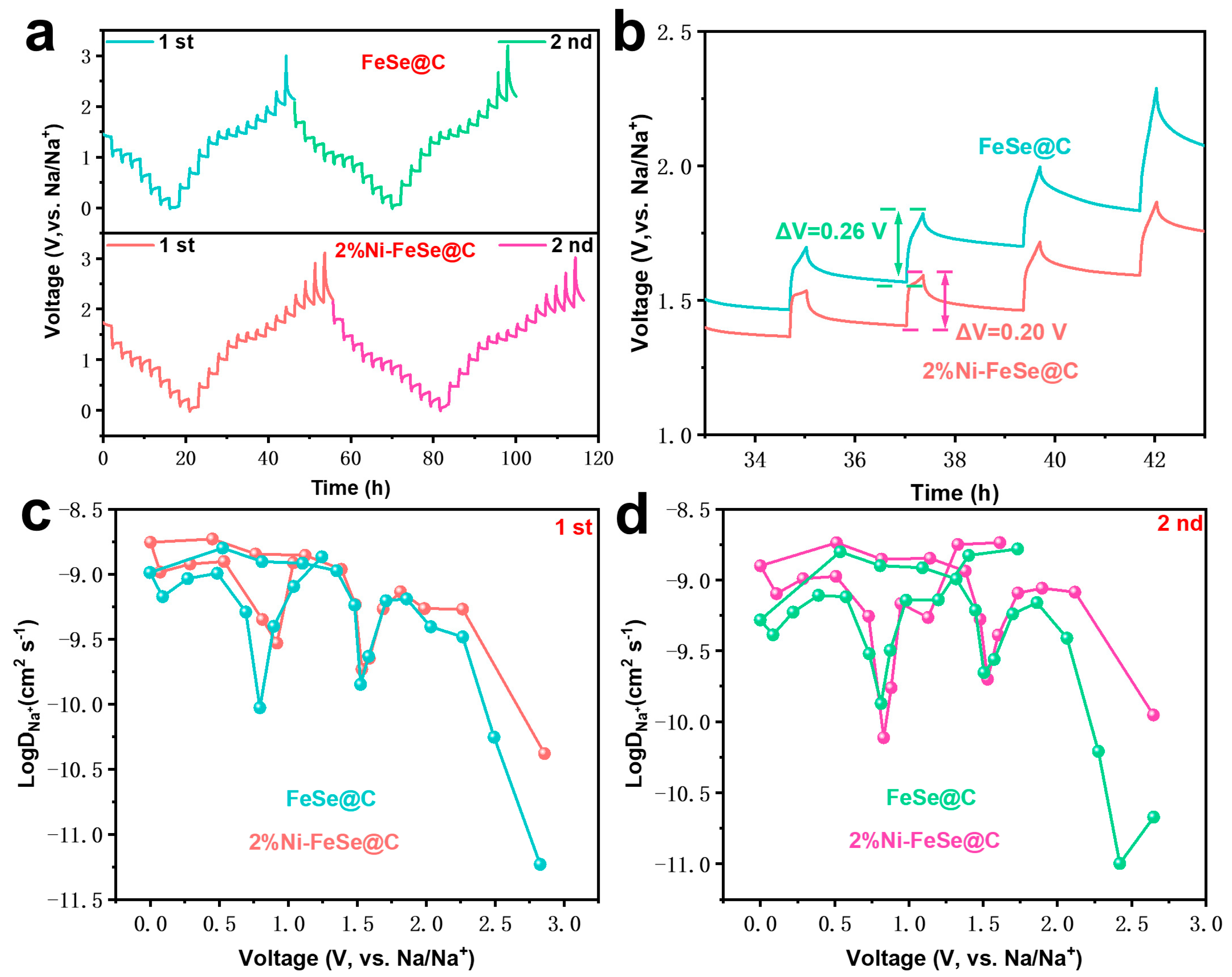
| Samples | Rs (Ω) | Rct (Ω) | σ (Ω S−0.5) | D (cm2 S−1) | I0 (mA cm−2) |
|---|---|---|---|---|---|
| FeSe@C | 3.7 | 117.5 | 244.5 | 5.93 × 10−9 | 22.18 × 10−4 |
| 2%Ni-FeSe@C | 2.9 | 101.0 | 106.5 | 3.13 × 10−8 | 2.54 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Cai, S.; Wang, D.; Wang, X.; Cheng, Y. Synergistic Cationic–Anionic Regulation in Ni-Doped FeSe@C Anodes with Se Vacancies for High-Efficiency Sodium Storage. Batteries 2025, 11, 205. https://doi.org/10.3390/batteries11060205
Wang L, Cai S, Wang D, Wang X, Cheng Y. Synergistic Cationic–Anionic Regulation in Ni-Doped FeSe@C Anodes with Se Vacancies for High-Efficiency Sodium Storage. Batteries. 2025; 11(6):205. https://doi.org/10.3390/batteries11060205
Chicago/Turabian StyleWang, Liang, Shutong Cai, Dingwen Wang, Xiangyi Wang, and Yang Cheng. 2025. "Synergistic Cationic–Anionic Regulation in Ni-Doped FeSe@C Anodes with Se Vacancies for High-Efficiency Sodium Storage" Batteries 11, no. 6: 205. https://doi.org/10.3390/batteries11060205
APA StyleWang, L., Cai, S., Wang, D., Wang, X., & Cheng, Y. (2025). Synergistic Cationic–Anionic Regulation in Ni-Doped FeSe@C Anodes with Se Vacancies for High-Efficiency Sodium Storage. Batteries, 11(6), 205. https://doi.org/10.3390/batteries11060205







