Low-Cost, Sustainable Hybrid Aqueous Zinc Metal Batteries Using Ethyl Cellulose as a Binder
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Electrochemical Characterization
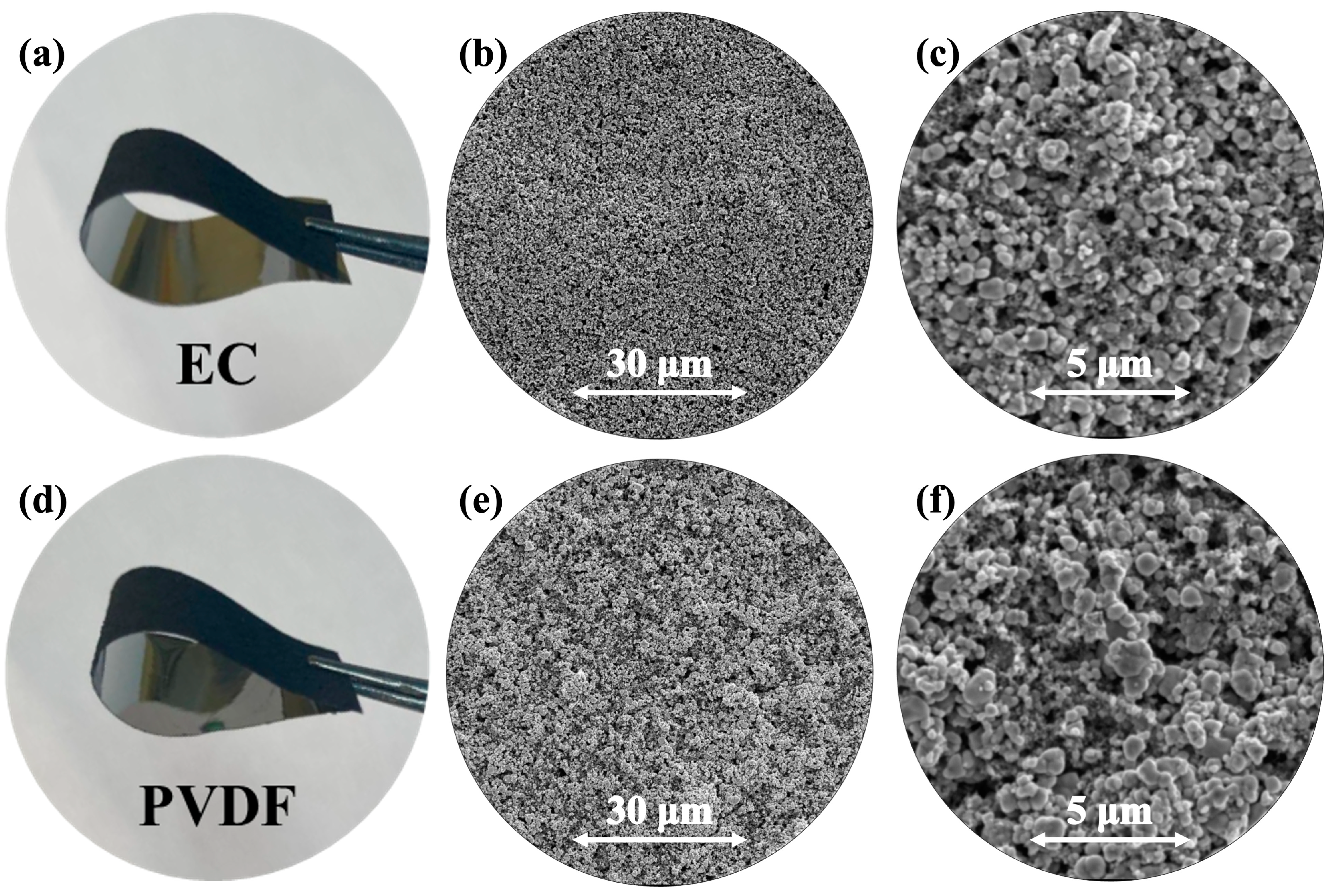
2.2.2. Morphological Characterization
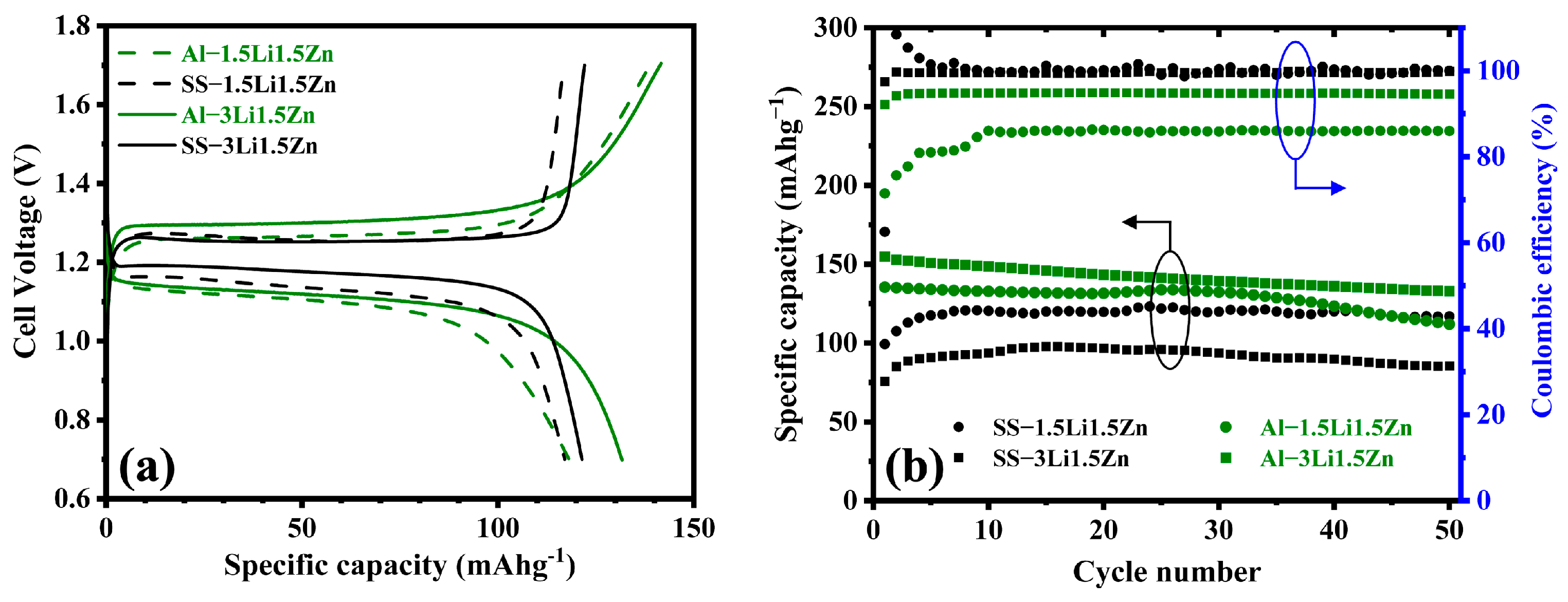
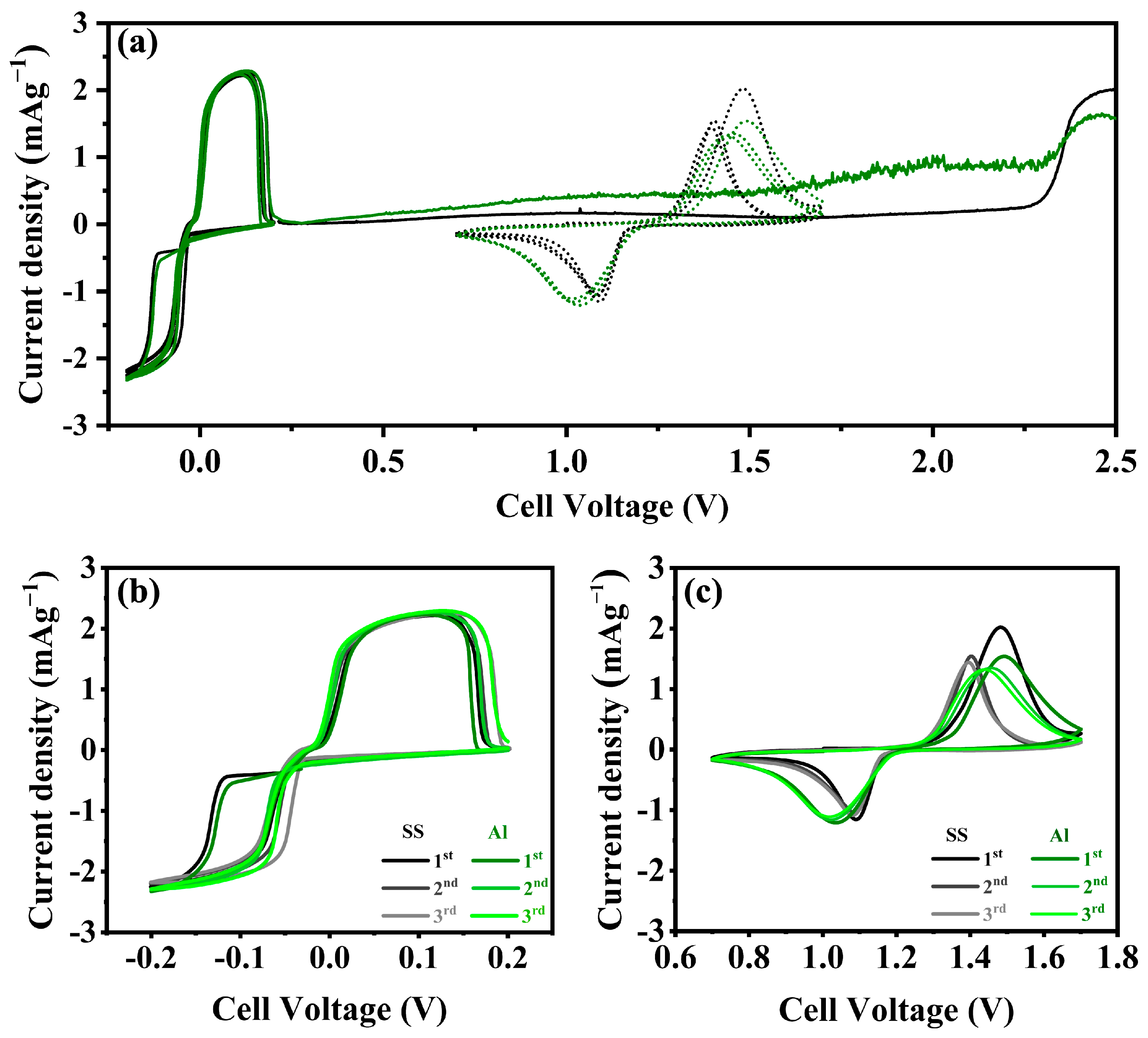
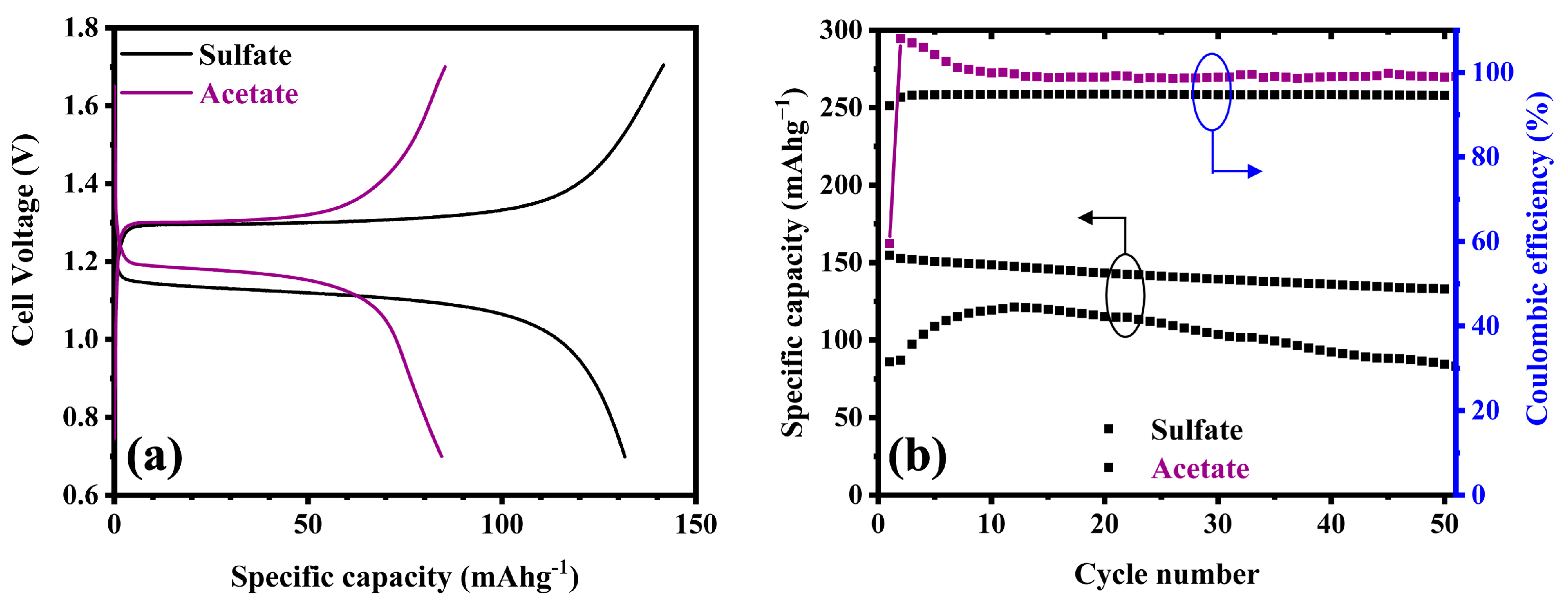
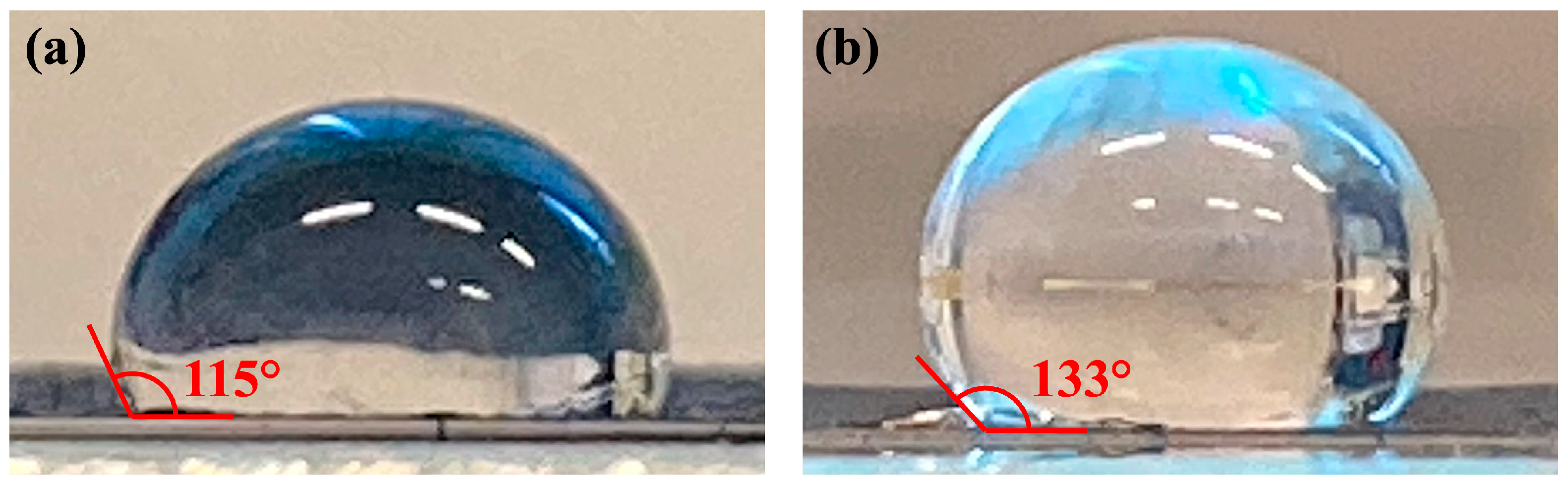
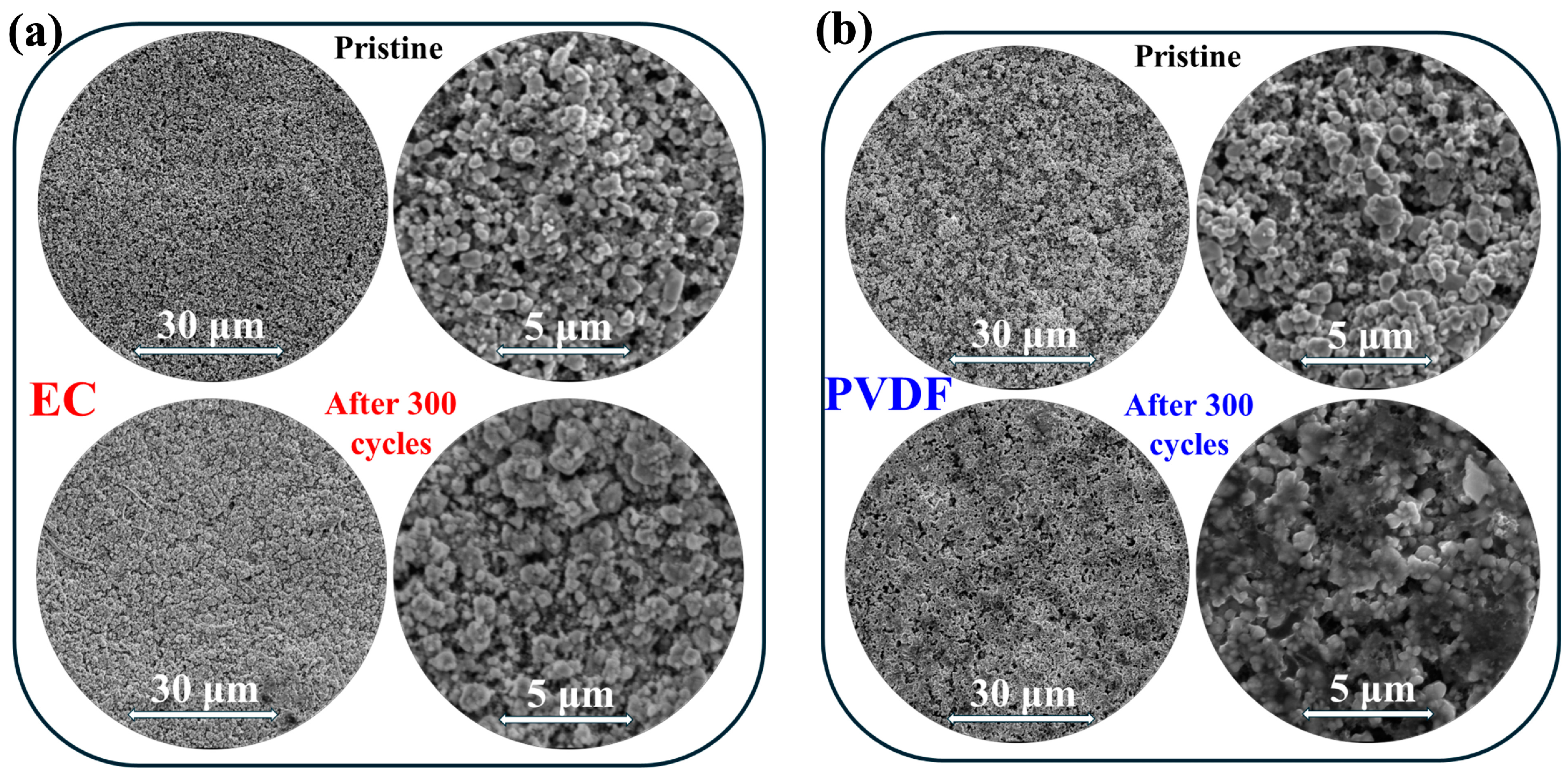
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koholé, Y.W.; Wankouo Ngouleu, C.A.; Fohagui, F.C.V.; Tchuen, G. A Comprehensive Comparison of Battery, Hydrogen, Pumped-Hydro and Thermal Energy Storage Technologies for Hybrid Renewable Energy Systems Integration. J. Energy Storage 2024, 93, 112299. [Google Scholar] [CrossRef]
- Sun, H.; Xiang, X.; Wang, X.; Tsai, H.S.; Feng, W. Advanced Photo-Rechargeable Lithium- and Zinc-Ion Batteries: Progress and Prospect. J. Power Sources 2024, 598, 234204. [Google Scholar] [CrossRef]
- Adeyinka, A.M.; Esan, O.C.; Ijaola, A.O.; Farayibi, P.K. Advancements in Hybrid Energy Storage Systems for Enhancing Renewable Energy-to-Grid Integration. Sustain. Energy Res. 2024, 11, 26. [Google Scholar] [CrossRef]
- Abo-Khalil, A.G.; Alobaid, M. A Guide to the Integration and Utilization of Energy Storage Systems with a Focus on Demand Resource Management and Power Quality Enhancement. Sustainability 2023, 15, 14680. [Google Scholar] [CrossRef]
- Bullich-Massagué, E.; Cifuentes-García, F.-J.; Glenny-Crende, I.; Cheah-Mañé, M.; Aragüés-Peñalba, M.; Díaz-González, F.; Gomis-Bellmunt, O. A Review of Energy Storage Technologies for Large Scale Photovoltaic Power Plants. Appl. Energy 2020, 274, 115213. [Google Scholar] [CrossRef]
- Zhang, Z.; Ding, T.; Zhou, Q.; Sun, Y.; Qu, M.; Zeng, Z.; Ju, Y.; Li, L.; Wang, K.; Chi, F. A Review of Technologies and Applications on Versatile Energy Storage Systems. Renew. Sustain. Energy Rev. 2021, 148, 111263. [Google Scholar] [CrossRef]
- Ho, V.C.; Lim, H.; Kim, M.J.; Mun, J. Improving the Performance of Aqueous Zinc-Ion Batteries by Inhibiting Zinc Dendrite Growth: Recent Progress. Chem. Asian J. 2022, 17, e202200289. [Google Scholar] [CrossRef]
- Han, J.; Euchner, H.; Kuenzel, M.; Hosseini, S.M.; Groß, A.; Varzi, A.; Passerini, S. A Thin and Uniform Fluoride-Based Artificial Interphase for the Zinc Metal Anode Enabling Reversible Zn/MnO2 Batteries. ACS Energy Lett. 2021, 6, 3063–3071. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.G.; Liu, H.; You, Z.; Wei, C.; Kang, F. Electrochemical Activation of Commercial MnO Microsized Particles for High-Performance Aqueous Zinc-Ion Batteries. J. Power Sources 2019, 438, 226951. [Google Scholar] [CrossRef]
- Li, H.; Ma, L.; Han, C.; Wang, Z.; Liu, Z.; Tang, Z.; Zhi, C. Advanced Rechargeable Zinc-Based Batteries: Recent Progress and Future Perspectives. Nano Energy 2019, 62, 550–587. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.G.; You, Z.; Wei, C.; Kang, F.; Wei, B. Rechargeable Aqueous Zinc-Ion Batteries: Mechanism, Design Strategies and Future Perspectives. Mater. Today 2021, 42, 73–98. [Google Scholar] [CrossRef]
- Wu, B.; Li, M.; Mazánek, V.; Liao, Z.; Ying, Y.; Oliveira, F.M.; Dekanovsky, L.; Jan, L.; Hou, G.; Antonatos, N.; et al. In Situ Vanadium-Deficient Engineering of V2C MXene: A Pathway to Enhanced Zinc-Ion Batteries. Small Methods 2024, 8, e2301461. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, H.; Liu, J.H.; Gao, Y.; Cao, X.; Zhan, C.; Wang, Y.; Wang, S.; Chou, S.L.; Dou, S.X.; et al. Vanadium-Based Cathodes for Aqueous Zinc-Ion Batteries: Mechanism, Design Strategies and Challenges. Energy Storage Mater. 2022, 50, 21–46. [Google Scholar] [CrossRef]
- Li, S.; Qin, L.; Li, L.; Cheng, H.; Fang, G.; Liang, S.; Zhu, Q.; Chen, M. Porous Structure ZnV2O4/C-N Composite Activating Vanadium-Based Cathode in Aqueous Zinc-Ion Batteries. Mater. Today Commun. 2021, 27, 102271. [Google Scholar] [CrossRef]
- Li, Q.; Ye, X.; Yu, H.; Du, C.; Sun, W.; Liu, W.; Pan, H.; Rui, X. Pre-Potassiated Hydrated Vanadium Oxide as Cathode for Quasi-Solid-State Zinc-Ion Battery. Chin. Chem. Lett. 2022, 33, 2663–2668. [Google Scholar] [CrossRef]
- Mathew, V.; Sambandam, B.; Kim, S.; Kim, S.; Park, S.; Lee, S.; Alfaruqi, M.H.; Soundharrajan, V.; Islam, S.; Putro, D.Y.; et al. Manganese and Vanadium Oxide Cathodes for Aqueous Rechargeable Zinc-Ion Batteries: A Focused View on Performance, Mechanism, and Developments. ACS Energy Lett. 2020, 5, 2376–2400. [Google Scholar] [CrossRef]
- Han, M.; Qin, L.; Liu, Z.; Zhang, L.; Li, X.; Lu, B.; Huang, J.; Liang, S.; Zhou, J. Reaction Mechanisms and Optimization Strategies of Manganese-Based Materials for Aqueous Zinc Batteries. Mater. Today Energy 2021, 20, 100626. [Google Scholar] [CrossRef]
- Syed, W.A.; Kakarla, A.K.; Bandi, H.; Shanthappa, R.; Yu, J.S. Copper Substituted Manganese Prussian Blue Analogue Composite Nanostructures for Efficient Aqueous Zinc-Ion Batteries. J. Energy Storage 2024, 99, 113325. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, J.; Hu, Q.; Hao, T.; Cao, H.; Huang, X.; Liu, Y.; Zhang, Y.; Lin, D.; Tang, Y.; et al. Prussian Blue Analogs Cathodes for Aqueous Zinc Ion Batteries. Mater. Today Energy 2022, 29, 101095. [Google Scholar] [CrossRef]
- Li, M.; Maisuradze, M.; Sciacca, R.; Hasa, I.; Giorgetti, M. A Structural Perspective on Prussian Blue Analogues for Aqueous Zinc-Ion Batteries. Batter. Supercaps 2023, 6, e202300340. [Google Scholar] [CrossRef]
- Liu, J.; Shen, Z.; Lu, C.Z. Research Progress of Prussian Blue and Its Analogues for Cathodes of Aqueous Zinc Ion Batteries. J. Mater. Chem. A Mater. 2024, 12, 2647–2672. [Google Scholar] [CrossRef]
- Li, C.; Hu, L.; Ren, X.; Lin, L.; Zhan, C.; Weng, Q.; Sun, X.; Yu, X. Asymmetric Charge Distribution of Active Centers in Small Molecule Quinone Cathode Boosts High-Energy and High-Rate Aqueous Zinc-Organic Batteries. Adv. Funct. Mater. 2024, 34, 2313241. [Google Scholar] [CrossRef]
- Dey, G.; Fayaz, A.; Jasmin, R.M.; Dinesan, S.; Sampath, S. A Copolymer of Benzoquinone and Pyrrole as High Rate, Durable Polymer Electrode for Aqueous Zn- and Mg-Ion Based Batteries. Chem. Asian J. 2025, 20, e202401135. [Google Scholar] [CrossRef]
- Song, Z.; Huang, Q.; Lv, Y.; Gan, L.; Liu, M. Multi-N-Heterocycle Donor-Acceptor Conjugated Amphoteric Organic Superstructures for Superior Zinc Batteries. Angew. Chem.—Int. Ed. 2024, 64, e202418237. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Hao, J.; Wang, Z.; Mao, J.; Guo, Z. Recent Progress and Perspectives on Aqueous Zn-Based Rechargeable Batteries with Mild Aqueous Electrolytes. Energy Storage Mater. 2019, 20, 410–437. [Google Scholar] [CrossRef]
- Innocenti, A.; Bresser, D.; Garche, J.; Passerini, S. A Critical Discussion of the Current Availability of Lithium and Zinc for Use in Batteries. Nat. Commun. 2024, 15, 4068. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Mariani, A.; Varzi, A.; Passerini, S. Green and Low-Cost Acetate-Based Electrolytes for the Highly Reversible Zinc Anode. J. Power Sources 2021, 485, 229329. [Google Scholar] [CrossRef]
- Zhong, X.; Wang, F.; Ding, Y.; Duan, L.; Shi, F.; Wang, C. Water-in-Salt Electrolyte Zn/LiFePO4 Batteries. J. Electroanal. Chem. 2020, 867, 114193. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Peng, X.; Dong, S.; Ma, J.; Cui, G.; Chen, L. High-Voltage Zn/LiMn0.8Fe0.2PO4 Aqueous Rechargeable Battery by Virtue of “Water-in-Salt” Electrolyte. Electrochem. Commun. 2016, 69, 6–10. [Google Scholar] [CrossRef]
- Zhang, X.; Ge, X.; Shen, Z.; Ma, H.; Wang, J.; Wang, S.; Liu, L.; Liu, B.; Liu, L.; Zhao, Y. Green Water-Based Binders for LiFePO4/C Cathodes in Li-Ion Batteries: A Comparative Study. New J. Chem. 2021, 45, 9846–9855. [Google Scholar] [CrossRef]
- Prosini, P.P.; Carewska, M.; Cento, C.; Masci, A. Poly Vinyl Acetate Used as a Binder for the Fabrication of a LiFePO4-Based Composite Cathode for Lithium-Ion Batteries. Electrochim. Acta 2014, 150, 129–135. [Google Scholar] [CrossRef]
- Bresser, D.; Buchholz, D.; Moretti, A.; Varzi, A.; Passerini, S. Alternative Binders for Sustainable Electrochemical Energy Storage-the Transition to Aqueous Electrode Processing and Bio-Derived Polymers. Energy Environ. Sci. 2018, 11, 3096–3127. [Google Scholar] [CrossRef]
- Islam, S.; Alfaruqi, M.H.; Mathew, V.; Song, J.; Kim, S.; Kim, S.; Jo, J.; Baboo, J.P.; Pham, D.T.; Putro, D.Y.; et al. Facile Synthesis and the Exploration of the Zinc Storage Mechanism of β-MnO2 Nanorods with Exposed (101) Planes as a Novel Cathode Material for High Performance Eco-Friendly Zinc-Ion Batteries. J. Mater. Chem. A Mater. 2017, 5, 23299–23309. [Google Scholar] [CrossRef]
- Ding, Y.; Zhong, X.; Yuan, C.; Duan, L.; Zhang, L.; Wang, Z.; Wang, C.; Shi, F. Sodium Alginate Binders for Bivalency Aqueous Batteries. ACS Appl. Mater. Interfaces 2021, 13, 20681–20688. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.; Maheshwari, R.; Verma, K.; Sharma, S.; Ghode, P.; Tekade, R.K. Coating Technologies in Pharmaceutical Product Development; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128144879. [Google Scholar]
- Riaz, A.; Abdul-wahhab, A.; Irfan, M. Aqueous Polymeric Coatings: New Opportunities in Drug Delivery Systems; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128212226. [Google Scholar]
- Wasilewska, K.; Winnicka, K. Ethylcellulose-a Pharmaceutical Excipient with Multidirectional Application in Drug Dosage Forms Development. Materials 2019, 12, 3386. [Google Scholar] [CrossRef]
- Zhang, J.; Zhong, H.; Zheng, C.; Xia, Y.; Liang, C.; Huang, H.; Gan, Y.; Tao, X.; Zhang, W. All-solid-state batteries with slurry coated LiNi0.8Co0.1Mn0.1O2 composite cathode and Li6PS5Cl electrolyte: Effect of binder content. J. Power Sources 2018, 391, 73–79. [Google Scholar] [CrossRef]
- Zuo, X.; Ma, X.; Wu, J.; Deng, X.; Xiao, X.; Liu, J.; Nan, J. Self-supporting ethyl cellulose/poly(vinylidene fluoride) blended gel polymer electrolyte for 5 V high-voltage lithium-ion batteries. Electrochim. Acta 2018, 271, 582–590. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Li, H.; Hasa, I.; Passerini, S. Challenges and Strategies for High-Energy Aqueous Electrolyte Rechargeable Batteries. Angew. Chem.—Int. Ed. 2021, 60, 598–616. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado Pico, P.P.; Colonna, S.; Ronci, F. Low-Cost, Sustainable Hybrid Aqueous Zinc Metal Batteries Using Ethyl Cellulose as a Binder. Batteries 2025, 11, 189. https://doi.org/10.3390/batteries11050189
Machado Pico PP, Colonna S, Ronci F. Low-Cost, Sustainable Hybrid Aqueous Zinc Metal Batteries Using Ethyl Cellulose as a Binder. Batteries. 2025; 11(5):189. https://doi.org/10.3390/batteries11050189
Chicago/Turabian StyleMachado Pico, Pedro Pablo, Stefano Colonna, and Fabio Ronci. 2025. "Low-Cost, Sustainable Hybrid Aqueous Zinc Metal Batteries Using Ethyl Cellulose as a Binder" Batteries 11, no. 5: 189. https://doi.org/10.3390/batteries11050189
APA StyleMachado Pico, P. P., Colonna, S., & Ronci, F. (2025). Low-Cost, Sustainable Hybrid Aqueous Zinc Metal Batteries Using Ethyl Cellulose as a Binder. Batteries, 11(5), 189. https://doi.org/10.3390/batteries11050189






