Recent Advances in the Application of MOFs in Supercapacitors
Abstract
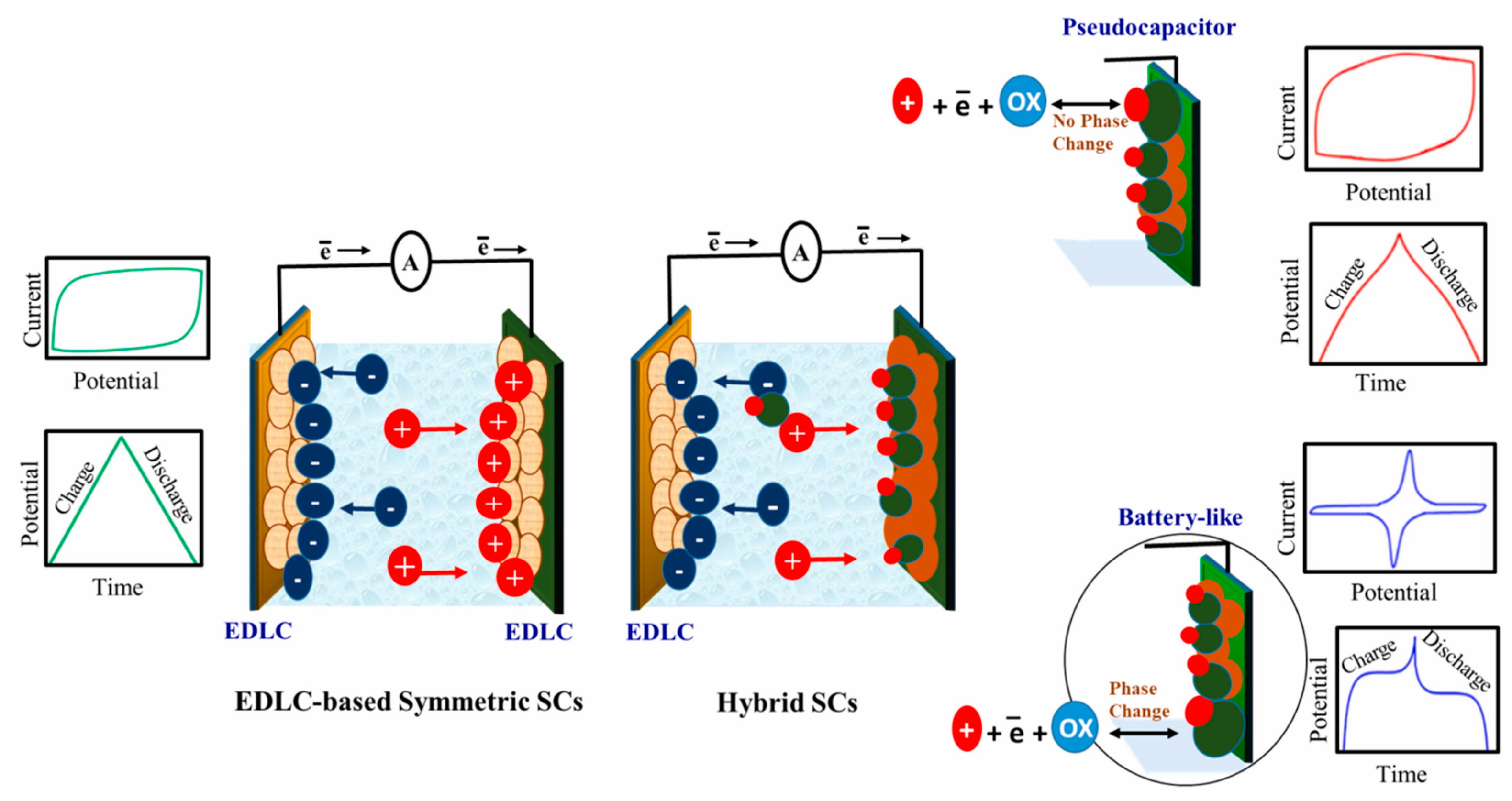
1. Introduction
2. MOF-Based Materials for Supercapacitors
2.1. MOFs and Metal Oxides
2.2. MOFs@Sulfides
2.3. MOF-Based Composites
2.4. MOFs@Oxides/Sulfides
2.5. MOFs@Oxides/Phosphides
2.6. Other Composite Combinations
2.6.1. MOFs/Graphene
2.6.2. MOFs/Phosphides
2.6.3. MOFs-Sulfides
2.6.4. MOFs–Selenides
2.6.5. MOFs–Sulfoselenides
3. Limitations and Outlook
4. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- DNV As, Norway, Energy Transition Outlook 2024—A Global and Regional Forecast to 2050. Available online: www.dnv.com (accessed on 22 April 2025).
- Wang, Y.; Wang, H.; Qu, G. Molybdenum-Based Electrode Materials Applied in High-Performance Supercapacitors. Batteries 2023, 9, 479. [Google Scholar] [CrossRef]
- Qi, P.; Wang, H.; Lu, Y.; Chen, M.; Liu, G.; Li, W.; Huang, C.; Tang, Y. Ammonia-induced N-doped NiCoO2 nanosheet array on Ni foam as a cathode of supercapacitor with excellent rate performance. J. Alloys Compd. 2022, 895, 162535. [Google Scholar] [CrossRef]
- El-Shamy, O.A.A.; Siddiqui, M.R.; Mele, G.; Mohsen, Q.; Alhajeri, M.M.; Deyab, M.A. Synthesis, characterization and application of CNTs@Co-MOF and FCNTs@Co-MOF as superior supercapacitor: Experimental and theoretical studies. J. Mol. Struct. 2025, 1321, 140161. [Google Scholar] [CrossRef]
- Kulkarni, O.; Bhosale, R.; Narale, D.; Pise, S.; Shaikh, T.; Kolekar, S. Dual redox center-based copper-cobalt metal–organic framework as pseudocapacitive electrode material for supercapacitor. Inorg. Chem. Commun. 2025, 172, 113711. [Google Scholar] [CrossRef]
- Bojarajan, A.K.; Gunasekaran, S.S.; Kalluri, S.; Al Omari, S.A.B.; Bakenov, Z.; Sangaraju, S. Tuning capacitance of bimetallic ZnCo2O4 using anionic, cationic and non-ionic surfactants by hydrothermal synthesis for high-performance asymmetric supercapacitor. Inorg. Chem. Commun. 2024, 169, 113035. [Google Scholar] [CrossRef]
- Minakshi, M.; Samayamanthry, A.; Whale, J.; Aughterson, R.; Shinde, P.A.; Ariga, K.; Shrestha, L.K. Phosphorous—Containing Activated Carbon Derived from Natural Honeydew Peel Powers Aqueous Supercapacitors. Chem. Asian J. 2024, 19, e202400622. [Google Scholar] [CrossRef] [PubMed]
- Minakshi, M.; Mujeeb, A.; Whale, J.; Evans, R.; Aughterson, R.; Shinde, P.A.; Ariga, K.; Shrestha Kumar, L. Synthesis of Porous Carbon Honeycomb Structures Derived from Hemp for Hybrid Supercapacitors with Improved Electrochemistry. ChemPlusChem 2024, 89, e202400408. [Google Scholar] [CrossRef]
- Minakshi, M.; Wickramaarachchi, K. Electrochemical aspects of supercapacitors in perspective: From electrochemical configurations to electrode materials processing. Prog. Solid State Chem. 2023, 69, 100390. [Google Scholar] [CrossRef]
- Alam, S.; Urooj, A.; Rehman, S.; Iqbal, M.Z.; Hegazy, H.H. Investigation of metal organic frameworks and their derivatives as electrode materials for hybrid energy storage devices. Mater. Chem. Phys. 2023, 304, 127877. [Google Scholar] [CrossRef]
- Fleischmann, S.; Mitchell, J.B.; Wang, R.; Zhan, C.; Jiang, D.; Presser, V.; Augustyn, V. Pseudocapacitance: From fundamental understanding to high power energy storage materials. Chem. Rev. 2020, 120, 6738–6782. [Google Scholar] [CrossRef]
- Brousse, T.; Belangér, D.; Long, J.W. To Be or Not To Be Pseudocapacitive? J. Electrochem. Soc. 2015, 162, A5185–A5189. [Google Scholar] [CrossRef]
- Costentin, C.; Porter, T.R.; Savéant, J.-M. How do pseudocapacitors store energy? Theoretical analysis and experimental illustration. ACS Appl. Mater. Interfaces 2017, 9, 8649–8658. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S.; Kitaura, R.; Noro, S. Functional Porous Coordination Polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef]
- Li, J.X.; Bao, T.; Zhang, C.; Song, H.; Zou, Y.Y.; Yuan, I.; Xi, Y.; Yu, C.Z.; Liu, C. A general strategy for direct growth of yolk-shell MOF-on-MOF hybrids. Chem. Eng. J. 2023, 472, 144926. [Google Scholar] [CrossRef]
- Arbulu, R.C.; Jiang, Y.B.; Peterson, E.J.; Qin, Y. Metal-organic framework (MOF) nanorods, nanotubes, and nanowires. Angew. Chem. Int. Ed. 2018, 57, 5813–5817. [Google Scholar] [CrossRef]
- Mohammadi, T.; Hosseini, M.G.; Rezvani Jalal, N.; Alavipour, E.; Pastor, E. Binder-free supercapacitors based on nanosheets of Bi-ligand Co metal-organic frameworks: Density functional theory validation. J. Power Sources 2025, 632, 236385. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 974. [Google Scholar] [CrossRef]
- Lei, L.; Cheng, Y.; Chen, C.; Kosari, M.; Jiang, Z.; He, C. Taming structure and modulating carbon dioxide (CO2) adsorption isosteric heat of nickel-based metal organic framework (MOF-74(Ni)) for remarkable CO2 capture. J. Colloid Interface Sci. 2022, 612, 132–145. [Google Scholar] [CrossRef]
- Zhang, W.; Bojdys, M.J.; Pinna, N. A universal synthesis strategy for tunable metal-organic framework nanohybrids. Angew. Chem. Int. Ed. 2023, 62, e202301021. [Google Scholar] [CrossRef]
- Della Roca, J.; Liu, D.; Lin, W. Nanoscale Metal–Organic Frameworks for Biomedical Imaging and Drug Delivery. Acc. Chem. Res. 2011, 44, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Chia, G.H.; Gao, Z. Metal–organic frameworks in fuel cell technologies. Nano Today 2013, 8, 577–597. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, X.; Yang, L.; Hu, Y.; Yuan, M.; Wang, C.; Liu, Q.; Yue, F.; Zhou, D.; Xia, Q. Efficient air epoxidation of cycloalkenes over bimetal-organic framework ZnCoMOF materials. Mol. Catal. 2021, 499, 111300. [Google Scholar] [CrossRef]
- Liang, Y.N.; Yao, W.G.; Duan, J.X.; Chu, M.; Sun, S.Z.; Li, X. Nickel cobalt bimetallic metal-organic frameworks with a layer-and-channel structure for high performance supercapacitors. J. Energy Storage 2021, 33, 102149. [Google Scholar] [CrossRef]
- Lim, G.J.H.; Liu, X.M.; Guan, C.; Wang, J. Co/Zn bimetallic oxides derived from metal organic frameworks for high performance electrochemical energy storage. Electrochim. Acta 2018, 291, 177–187. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, W.; Li, M.; Meng, Z.; Ullah, I.; Nawaz, M.Z.; Wang, J.; Wang, C.; Chu, P.K. (Fe, Co) oxide nanowires on gold nanoparticles modified MOF-derived carbon nanoflakes for high-efficiency sodium-ion batteries and supercapacitors across electrolytes. J. Power Sources 2025, 626, 235793. [Google Scholar] [CrossRef]
- Otun, K.O.; Diop, N.F.; Fasakin, O.; Adam, R.A.M.; Rutavi, G.; Manyala, N. Engineering the structures of ZnCo-MOFs via a ligand effect for enhanced supercapacitor performance. RSC Adv. 2025, 15, 4120–4136. [Google Scholar] [CrossRef]
- Li, H.I.; Eddaoudi, M.; Keeffe, M.O.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Hao, X.; Song, W.; Wang, Y.; Qin, J.; Jiang, J. Recent Advancements in Electrochemical Sensors Based on MOFs and Their Derivatives. Small 2025, 21, 2408624. [Google Scholar] [CrossRef]
- Xie, C.; Li, H.; Niu, B.; Guo, H.; Lin, X. Comparison of ultrasonic vs mechanochemistry methods for fabrication of mixed-ligand Zn-based MOFs for electrochemical determination of luteolin. J. Alloys Compd. 2024, 989, 174363. [Google Scholar] [CrossRef]
- Kumar, R.S.; Kumar, S.S.; Kulandainathan, M.A. Efficient electrosynthesis of highly active Cu3(BTC)2-MOF and its catalytic application to chemical reduction. Microporous Mesoporous Mater. 2013, 168, 57–64. [Google Scholar] [CrossRef]
- Kaur, G.; Kandwal Komal, P.; Sud, D. Sonochemically synthesized Zn (II) and Cd (II) based metal-organic frameworks as fluoroprobes for sensing of 2,6-dichlorophenol. J. Solid State Chem. 2023, 319, 123833. [Google Scholar] [CrossRef]
- Glowniak, S.; Szczesniak, B.; Choma, J.; Jaroniec, M. Recent developments in sonochemical synthesis of nanoporous materials. Molecules 2023, 28, 2639. [Google Scholar] [CrossRef]
- Mendes, R.F.; Rocha, J.; Almeida Paz, F.A. Chapter 8—Microwave Synthesis of Metal-Organic Frameworks. In Metal-Organic Frameworks for Biomedical Applications; Mozafari, M.B.T., Ed.; Woodhead Publishing: Cambridge, UK, 2020; pp. 159–176. [Google Scholar] [CrossRef]
- Kumari, P.; Kareem, A.; Jhariat, P.; Kumar, S.S.; Panda, T. Phase purity regulated by mechano-chemical synthesis of metal-organic frameworks for the electrocatalytic oxygen evolution reaction. Inorg. Chem. 2023, 62, 3457–3463. [Google Scholar] [CrossRef]
- Wang, W.P.; Chai, M.; Zulkifli, M.Y.B.; Xu, K.J.; Chen, Y.L.; Wang, L.Z.; Chen, V.; Hou, J.W. Metal-organic framework composites from a mechanochemical process. Mol. Syst. Des. Eng. 2023, 8, 560–579. [Google Scholar] [CrossRef]
- Vaitsis, C.; Kanellou, E.; Pandis, P.K.; Papamichael, I.; Sourkouni, G.; Zorpas, A.A.; Argirusis, C. Sonochemical synthesis of zinc adipate Metal-Organic Framework (MOF) for the electrochemical reduction of CO2: MOF and circular economy potential. Sustain. Chem. Pharm. 2022, 29, 100786. [Google Scholar] [CrossRef]
- Shakeel, N.; Khan, J.; Al-Kahtani, A.A. Morphology-driven electrochemical attributes of Cu-MOF: A high-performance anodic material for battery supercapacitor hybrids. RSC Adv. 2024, 14, 33941–33951. [Google Scholar] [CrossRef]
- Vaitsis, C.; Sourkouni, G.; Argirusis, C. Metal Organic Frameworks (MOFs) and ultrasound: A review. Ultrason. Sonochemistry 2019, 52C, 106–119. [Google Scholar] [CrossRef]
- Muzaffar, N.; Afzal, A.M.; Hegazy, H.H.; Iqbal, M.W. Recent advances in two-dimensional metal-organic frameworks as an exotic candidate for the evaluation of redox-active sites in energy storage devices. J. Energy Storage 2023, 64, 107142. [Google Scholar] [CrossRef]
- Zhang, G.; Jin, L.; Zhang, R.; Bai, Y.; Zhu, R.; Pang, P. Recent advances in the development of electronically and ionically conductive metal-organic frameworks. Coord. Chem. Rev. 2021, 439, 213915. [Google Scholar] [CrossRef]
- Lee, S.J.; Telfer, S.G. Multicomponent metal-organic frameworks. Angew. Chem. Int. Ed. 2023, 135, e202306341. [Google Scholar] [CrossRef]
- Lin, Z.X.; Richardson, J.J.; Zhou, J.J.; Caruso, F. Direct synthesis of amorphous coordination polymers and metal-organic frameworks. Nat. Rev. Chem. 2023, 7, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.N.; Meng, X.; Dong, L.Z.; Chen, Y.; Li, S.L.; Lan, Y.Q. Coordination polymer-based conductive materials: Ionic conductivity vs. electronic conductivity. J. Mater. Chem. A 2019, 7, 24059–24091. [Google Scholar] [CrossRef]
- Kumar, P.; Vellingiri, K.; Kim, K.-H.; Brown, R.J.C.; Manos, M.J. Modern progress in metal-organic frameworks and their composites for diverse applications. Microporous Mesoporous Mater. 2017, 253, 251–265. [Google Scholar] [CrossRef]
- Bernales, V.; Ortuño, M.A.; Truhlar, D.G.; Cramer, C.J.; Gagliardi, L. Computational Design of Functionalized Metal−Organic Framework Nodes for Catalysis. ACS Cent. Sci. 2018, 4, 5–19. [Google Scholar] [CrossRef]
- Coudert, F.X.; Fuchs, A.H. Computational characterization and prediction of metal–organic framework properties. Coord. Chem. Rev. 2016, 307, 211–236. [Google Scholar] [CrossRef]
- McCarver, G.A.; Rajeshkumar, T.; Vogiatzis, K.D. Computational catalysis for metal-organic frameworks: An overview. Coord. Chem. Rev. 2021, 436, 213777. [Google Scholar] [CrossRef]
- Hai, G.; Gao, H.; Huang, X.; Tan, L.; Xue, X.; Feng, S.; Wang, G. An efficient factor for fast screening of high-performance two-dimensional metal-organic frameworks towards catalyzing the oxygen evolution reaction. Chem. Sci. 2022, 13, 4397–4405. [Google Scholar] [CrossRef]
- Chatterjee, P.; Sengul, M.Y.; Kumar, A.; MacKerell, A.D., Jr. Harnessing deep learning for optimization of Lennard-Jones parameters for the polarizable classical Drude oscillator force field. J. Chem. Theory Comput. 2022, 18, 2388–2407. [Google Scholar] [CrossRef]
- Hai, G.; Wang, H. Theoretical studies of metal-organic frameworks: Calculation methods and applications in catalysis, gas separation, and energy storage. Coord. Chem. Rev. 2022, 469, 214670. [Google Scholar] [CrossRef]
- Demir, H.; Daglar, H.; Gulbalkan, H.C.; Aksu, G.O.; Keskin, S. Recent advances in computational modeling of MOFs: From molecular simulations to machine learning. Coord. Chem. Rev. 2023, 484, 215112. [Google Scholar] [CrossRef]
- Zhao, J.; Burke, A.F. Electrochemical capacitors: Materials, technologies and performance. Energy Storage Mater. 2021, 36, 31–55. [Google Scholar] [CrossRef]
- Song, J.L.; Chai, L.; Kumar, A.; Zhao, M.; Sun, Y.Z.; Liu, X.G.; Pan, J.Q. Precise tuning of hollow and pore size of bimetallic MOFs derivate to construct high-performance nanoscale materials for supercapacitors and sodium-ion batteries. Small 2023, 20, 2306272. [Google Scholar] [CrossRef]
- Kitchamsetti, N. A review on recent advances in Prussian blue, its analogues, and their derived materials as electrodes for high performance supercapacitors. J. Energy Storage 2023, 73, 108958. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, H.; Mei, H.; Sun, D. Recent progress in metal-organic framework-based supercapacitor electrode materials. Coord. Chem. Rev. 2020, 420, 213438. [Google Scholar] [CrossRef]
- Yin, Z.; Wan, S.; Yang, J.; Kurmoo, M.; Zeng, M.-H. Recent advances in post-synthetic modification of metaleorganic frameworks: New types and tandem reactions. Coord. Chem. Rev. 2019, 378, 500–512. [Google Scholar] [CrossRef]
- Li, C.; Sun, X.; Yao, Y.; Hong, G. Recent advances of electrically conductive metal-organic frameworks in electrochemical applications. Mater. Today Nano 2021, 13, 100105. [Google Scholar] [CrossRef]
- Sundriyal, S.; Kaur, H.; Bhardwaj, S.K.; Mishra, S.; Kim, K.-H.; Deep, A. Metal-organic frameworks and their composites as efficient electrodes for supercapacitor applications. Coord. Chem. Rev. 2018, 369, 15–38. [Google Scholar] [CrossRef]
- Chen, D.; Wei, L.; Li, J.; Wu, Q. Nanoporous materials derived from metal-organic framework for super-capacitor application. J. Energy Storage 2020, 30, 101525. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Shao, Z.; Jiang, S.P. Metal-organic frameworks derived porous carbon, metal oxides and metal sulfides-based compounds for supercapacitors application. Energy Storage Mater. 2020, 26, 1–22. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Zhang, B.; Tian, S.; Yang, X.; Ye, H.; Xia, Z.; Zheng, G. Application of MOFs-derived mixed metal oxides in energy storage. J. Electroanal. Chem. 2020, 878, 114576. [Google Scholar] [CrossRef]
- Li, S.; Luo, J.; Wang, J.; Zhu, Y.; Feng, J.; Fu, N.; Wang, H.; Guo, Y.; Tian, D.; Zheng, Y.; et al. Hybrid supercapacitors using metal-organic framework derived nickel-sulfur compounds. J. Colloid Interface Sci. 2024, 669, 265–274. [Google Scholar] [CrossRef]
- Hao, Y.; Guo, H.; Yang, F.; Zhang, J.; Wu, N.; Wang, M.; Li, C.; Yang, W. Hydrothermal synthesis of MWCNT/Ni-Mn-S composite derived from bimetallic MOF for high-performance electrochemical energy storage. J. Alloy. Compd. 2022, 911, 164726. [Google Scholar] [CrossRef]
- Qu, Y.; Sun, L.; Xie, F.; Hu, J.; Tan, H.; Zhang, Y. Metal-organic framework derived r-Ni3S2 nanoparticles with enriched sulfur vacancies for supercapacitor application. Appl. Surf. Sci. 2023, 623, 157037. [Google Scholar] [CrossRef]
- Lee, P.Y.; Lin, L.Y.; Yougbaré, S. Sulfurization of nickel-cobalt fluoride decorating ammonia ions as efficient active material of supercapacitor. J. Solid State Chem. 2022, 313, 123345. [Google Scholar] [CrossRef]
- Naseer, M.; Siyal, S.H.; Najam, T.; Afzal, S.; Iqbl, R.; Ismail, M.A.; Rauf, A.; Shah, S.S.A.; Nazir, M.A. Engineering of metal oxide integrated metal organic frameworks (MO@ MOF) composites for energy and environment sector. Mater. Sci. Eng. B 2025, 313, 117909. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, J.; Zhang, W.; Tan, Y.; Zhao, J.; Tang, B. The electrochemical performance of SnO2 quantum dots@zeolitic imidazolate frameworks-8 (ZIF-8) composite material for supercapacitors. Mater. Lett. 2014, 128, 208–211. [Google Scholar] [CrossRef]
- Cui, H.; Liu, Y.; Ren, W.; Wang, M.; Zhao, Y. Large scale synthesis of highly crystallized SnO2 quantum dots at room temperature and their high electrochemical performance. Nanotechnology 2013, 24, 345602. [Google Scholar] [CrossRef]
- Yu, G.; Xie, X.; Pan, L.; Bao, Z.; Cui, Y. Hybrid nanostructured materials for high-performance electrochemical capacitors. Nano Energy 2013, 2, 213–234. [Google Scholar] [CrossRef]
- Gowdhaman, A.; Kumar, S.A.; Elumalai, D.; Balaji, C.; Sabarinathan, M.; Ramesh, R.; Navaneethan, M. Ni-MOF derived NiO/Ni/r-GO nanocomposite as a novel electrode material for high-performance asymmetric supercapacitor. J. Energy Storage 2023, 60, 106769. [Google Scholar] [CrossRef]
- Das, A.K.; Bera, R.; Maitra, A.; Karan, S.K.; Paria, S.; Halder, L.; Si, S.K.; Bera, A.; Khatua, B.B. Fabrication of an advanced asymmetric supercapacitor based on a microcubical PB@MnO2 hybrid and PANI/GNP composite with excellent electrochemical behaviour. J. Mater. Chem. A 2017, 5, 22242–22254. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Cheng, T.; Wang, Y.; Lai, W.Y.; Pang, H.; Huang, W. A simple approach to boost capacitance: Flexible supercapacitors based on manganese Oxides@MOFs via chemically induced in situ self-transformation. Adv. Mater. 2016, 28, 5242–5248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Y.; Huang, S.; Yuan, Y.; Li, H.; Jin, Z.; Wu, J.; Liao, Q.; Hu, L.; Lu, J.; et al. Co3O4/Ni-based MOFs on carbon cloth for flexible alkaline battery-supercapacitor hybrid devices and near-infrared photocatalytic hydrogen evolution. Electrochim. Acta 2018, 281, 189–197. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, J.; Song, J.; You, Y.; Jin, X.; Ma, J. Anchoring Ni-MOF nanosheet on carbon cloth by zeolite imidazole framework derived ribbonlike Co3O4 as integrated composite cathodes for advanced hybrid supercapacitors. Ceram. Int. 2021, 47, 14001–14008. [Google Scholar] [CrossRef]
- Zheng, J.H.; Zhang, R.M.; Yu, P.F.; Wang, X.G. Binary transition metal oxides (BTMO) (Co-Zn, Co-Cu) synthesis and high supercapacitor performance. J. Alloys Compd. 2019, 772, 359–365. [Google Scholar] [CrossRef]
- Vaitsis, C.; Mechili, M.; Argirusis, N.; Kanellou, E.; Pandis, P.K.; Sourkouni, G.; Zorpas, A.; Argirusis, C. Ultrasound-Assisted preparation methods of nanoparticles for energy-related applications. In Nanotechnology and the Environment; Sen, M., Ed.; IntechOpen: London, UK, 2020; pp. 77–103. [Google Scholar] [CrossRef]
- Shen, L.; Che, Q.; Li, H.; Zhang, X. Mesoporous NiCO2O4 nanowire arrays grown on carbon textiles as binder-free flexible electrodes for energy storage. Adv. Funct. Mater. 2014, 24, 2630–2637. [Google Scholar] [CrossRef]
- Wu, P.; Cheng, S.; Yao, M.; Yang, L.; Zhu, Y.; Liu, P.; Xing, O.; Zhou, J.; Wang, M.; Luo, H.; et al. A low-cost, self-standing NiCO2O4@CNT/CNT multilayer electrode for flexible asymmetric solid-state supercapacitors. Adv. Funct. Mater. 2017, 27, 1702160. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, J.; Yao, H. Ultrathin Ni-MOF nanosheet coated NiCO2O4 nanowire arrays as a high-performance binder-free electrode for flexible hybrid supercapacitors. Ceram. Int. 2019, 45, 24279–24287. [Google Scholar] [CrossRef]
- Xiong, S.; Jiang, S.; Wang, J.; Lin, H.; Lin, M.; Weng, S.; Liu, S.; Jiao, Y.; Xu, Y.; Chen, J. A high-performance hybrid supercapacitor with NiO derived NiO@Ni-MOF composite electrodes. Electrochim. Acta 2020, 340, 135956. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, Q.; Xiong, Y.; Cheng, D.; Zeng, Y.; Bu, Y. Fabrication of 3D Co-doped Ni-based MOF hierarchical micro-flowers as a high-performance electrode material for supercapacitors. Appl. Surf. Sci. 2019, 483, 1158–1165. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Q.; Xu, B.; Gao, F.; Gao, F.; Zhao, C. Flower-shaped multiwalled carbon nanotubes@nickel-trimesic acid MOF composite as a high-performance cathode material for energy storage. Electrochim. Acta 2018, 281, 69–77. [Google Scholar] [CrossRef]
- Li, N.; Li, Y.; Li, Q.; Zhao, Y.; Liu, C.S.; Pang, H. NiO nanoparticles decorated hexagonal Nickel-based metal-organic framework: Self-template synthesis and its application in electrochemical energy storage. J. Colloid Interface Sci. 2021, 581 Pt B, 709–718. [Google Scholar] [CrossRef]
- Chen, J.; Xu, J.; Zhou, S.; Zhao, N.; Wong, C.-P. Template-grown graphene/porous Fe2O3 nanocomposite: A high-performance anode material for pseudocapacitors. Nano Energy 2015, 15, 719–728. [Google Scholar] [CrossRef]
- Chameh, B.; Moradi, M.; Hessari, F.A. Decoration of metal organic frameworks with FeO3 for enhancing electrochemical performance of ZIF-(67 and 8) in energy storage application. Synth. Met. 2020, 269, 116540. [Google Scholar] [CrossRef]
- Chameh, B.; Moradi, M.; Hajati, S.; Hessari, F.A. Design and construction of ZIF(8 and 67) supported Fe3O4 composite as advanced materials of high performance supercapacitor. Phys. E Low-Dimens. Syst. Nanostruct. 2021, 126, 114442. [Google Scholar] [CrossRef]
- Shabani Shayeh, J.; Salari, H. Dendritic fibrous nano metal organic framework: A magnetic core-shell structure as high performance material for electrochemical capacitors. J. Energy Storage 2020, 32, 101734. [Google Scholar] [CrossRef]
- Albiter, E.; Merlano, A.S.; Rojas, E.; Barrera-Andrade, J.M.; Salazar, Á.; Valenzuela, M.A. Synthesis, characterization, and photocatalytic performance of ZnO-graphene nanocomposites: A review. J. Compos. Sci. 2020, 5, 4. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- Sulciute, A.; Nishimura, K.; Gilshtein, E.; Cesano, F.; Viscardi, G.; Nasibulin, A.G.; Ohno, Y.; Rackauskas, S. ZnO nanostructures application in electrochemistry: Influence of morphology. J. Phys. Chem. C 2021, 125, 1472–1482. [Google Scholar] [CrossRef]
- Luo, W.; Zhang, Q.; Zhang, J.; Moioli, E.; Zhao, K.; Züttel, A. Electrochemical reconstruction of ZnO for selective reduction of CO2 to CO. Appl. Catal. B Environ. 2020, 273, 119060. [Google Scholar] [CrossRef]
- Saranya, M.; Ramachandran, R.; Wang, F. Graphene-zinc oxide (G-ZnO) nanocomposite for electrochemical supercapacitor applications. J. Sci. Adv. Mater. Dev. 2016, 1, 454–460. [Google Scholar] [CrossRef]
- Liu, Y.N.; Jin, L.N.; Wang, H.T.; Kang, X.H.; Bian, S.W. Fabrication of three-dimensional composite textile electrodes by metal-organic framework, zinc oxide, graphene and polyaniline for all-solid-state supercapacitors. J. Colloid Interface Sci. 2018, 530, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; He, Y.; Liu, Y.; Kazantseva, N.; Saha, P.; Cheng, Q. ZnO@MOF@PANI core-shell nanoarrays on carbon cloth for high-performance supercapacitor electrodes. J. Energy Chem. 2019, 35, 124–131. [Google Scholar] [CrossRef]
- Zuhri, F.U.; Diantoro, M.; Suryanti, L.; Suprayogi, T.; Nasikhudin, S.; Meevasana, W. ZnO-FC-NiCo MOF for prospective supercapacitor materials. Mater. Today Proc. 2021, 44, 3385–3389. [Google Scholar] [CrossRef]
- Reghunath, S.; Pinheiro, D.; Kr, S.D. A review of hierarchical nanostructures of TiO2: Advances and applications. Appl. Surf. Sci. Adv. 2021, 3, 100063. [Google Scholar] [CrossRef]
- Ramasubbu, V.; Omar, F.S.; Ramesh, K.; Ramesh, S.; Shajan, X.S. Three-dimensional hierarchical nano-structured porous TiO2 aerogel/Cobalt based metal-organic framework (MOF) composite as an electrode material for supercapattery. J. Energy Storage 2020, 32, 101750. [Google Scholar] [CrossRef]
- Feng, J.; Feng, J.; Zhang, C. Thermal conductivity of low density carbon aerogels. J. Porous Mater. 2011, 19, 551–556. [Google Scholar] [CrossRef]
- Keshavarz, L.; Ghaani, M.R.; MacElroy, J.M.D.; English, N.J. A comprehensive review on the application of aerogels in CO2-adsorption: Materials and characterization. Chem. Eng. J. 2021, 412, 128604. [Google Scholar] [CrossRef]
- Kitchamsetti, N.; Samtham, M.; Singh, D.; Choudhary, E.; Rondiya, S.R.; Ma, Y.R.; Cross, R.W.; Dzade, N.Y.; Devan, R.S. Hierarchical 2D MnO2@1D mesoporous NiTiO3 core-shell hybrid structures for high-performance supercapattery electrodes: Theoretical and experimental investigations. J. Electroanal. Chem. 2023, 936, 117359. [Google Scholar] [CrossRef]
- Khan, M.W.; Khan, A.S.; Rohma; Pai, S.H.S.; Mohapatra, G.; Kumar, A.; Ali, R.B.; Sial, Q.A.; Seo, H. Electrodeposition of redox active nickel-manganese metal organic framework and manganese sulfide composite for supercapacitors. Electrochim. Acta 2025, 513, 145554. [Google Scholar] [CrossRef]
- Feng, G.; Zhao, T.; Zhou, L.; Wang, X.; Ding, H.; Jiang, F.; Li, H.; Liu, Y.; Yu, Q.; Cao, H.; et al. Nanocubic transition metal sulfides (FeMn)S2@NC synthesized by MOFs facily for supercapacitors. Vacuum 2025, 231, 113819. [Google Scholar] [CrossRef]
- Kim, M.K.; Kim, W.J.; Kim, M.L.; Ahn, G.H.; Hong, J.P.; Ryu, G.H.; Hong, J. Hierarchically structured transition metal (Cu, Ni) sulfide core–shell electrode for high-performance supercapacitor. J. Alloys Compd. 2025, 1014, 178717. [Google Scholar] [CrossRef]
- Lu, P.; Jiang, X.; Guo, W.; Wang, L.; Zhang, T.; Boyjoo, Y.; Si, W.; Hou, F.; Liu, J.; Dou, S.X.; et al. A Ni–Co sulfide nanosheet/carbon nanotube hybrid film for high-energy and high-power flexible supercapacitors. Carbon 2021, 178, 355–362. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, D.; Cheng, M.; Liu, Z.; Lai, C.; Zhang, C.; Zhou, C.; Xiong, W.; Qin, L.; Shao, B.; et al. Metal sulfide/MOF-based composites as visible-light-driven photocatalysts for enhanced hydrogen production from water splitting. Coord. Chem. Rev. 2020, 409, 213220. [Google Scholar] [CrossRef]
- Song, X.Z.; Zhang, N.; Wang, X.F.; Tan, Z. Recent advances of metal-organic frameworks and their composites toward oxygen evolution electrocatalysis. Mater. Today Energy 2021, 19, 100597. [Google Scholar] [CrossRef]
- Lee, C.S.; Lim, J.M.; Park, J.T.; Kim, J.H. Direct growth of highly organized, 2D ultra-thin nano-accordion Ni-MOF@NiS2@C core-shell for high performance energy storage device. Chem. Eng. J. 2021, 406, 126810. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Han, M.; Xia, Q.; Chen, Q.; Chen, M. Constructing ultra-thin Ni-MOF@NiS2 nanosheets arrays derived from metal organic frameworks for advanced all-solid-state asymmetric supercapacitor. Mater. Res. Bull. 2021, 137, 111186. [Google Scholar] [CrossRef]
- Yue, L.; Wang, X.; Sun, T.; Liu, H.; Li, Q.; Wu, N.; Guo, H.; Yang, W. Ni-MOF coating MoS2 structures by hydrothermal intercalation as high-performance electrodes for asymmetric supercapacitors. Chem. Eng. J. 2019, 375, 121959. [Google Scholar] [CrossRef]
- Wei, J.; Hu, F.; Shen, X.; Chen, B.; Chen, L.; Wang, Z.; Lv, C.; Ouyang, Q. Defective core–shell NiCO2S4/MnO2 nanocomposites for high performance solid-state hybrid supercapacitors. J. Colloid Interface Sci. 2023, 649, 665–674. [Google Scholar] [CrossRef]
- Ali, M.; Afzal, A.M.; Iqbal, M.W.; Mumtaz, S.; Imran, M.; Ashraf, F.; Rehman, A.U.; Muhammad, F. 2D-TMDs based electrode material for supercapacitor applications. Int. J. Energy Res. 2022, 46, 22336–22364. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Zhu, Y.; Zhai, S.; Liu, C.; Fu, N.; Hou, S.; Niu, Y.; Luo, J.; Mu, S.; et al. Metal-organic frameworks-derived NiSe@RGO composites for high-performance hybrid supercapacitors. J. Electroanal. Chem. 2022, 919, 116548. [Google Scholar] [CrossRef]
- Zhang, N.; Meng, Q.; Wu, H.; Hu, X.; Zhang, M.; Zhou, A.; Li, Y.; Huang, Y.; Li, L.; Wu, F.; et al. Co-MOF as Stress-Buffered Architecture: An engineering for improving the performance of NiS/SnO2 heterojunction in lithium storage. Adv. Energy Mater. 2023, 13, 2300413. [Google Scholar] [CrossRef]
- Hu, M.-L.; Masoomi, M.Y.; Morsali, A. Template strategies with MOFs. Coord. Chem. Rev. 2019, 387, 415–435. [Google Scholar] [CrossRef]
- Mei, J.; Liao, T.; Ayoko, G.A.; Bell, J.; Sun, Z. Cobalt oxide-based nanoarchitectures for electrochemical energy applications. Prog. Mater. Sci. 2019, 103, 596–677. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Lin, L.-Y.; Geng, D.-S.; Lee, P.-Y. Systematic synthesis of ZIF-67 derived Co3O4 and N-doped carbon composite for supercapacitors via successive oxidation and carbonization. Electrochim. Acta 2021, 376, 137986. [Google Scholar] [CrossRef]
- Xu, J.; Liu, S.; Liu, Y. Co3O4/ZnO nanoheterostructure derived from core-shell ZIF-8@ZIF-67 for supercapacitors. RSC Adv. 2016, 6, 52137–52142. [Google Scholar] [CrossRef]
- Kitchamsetti, N.; Kim, D. High performance hybrid supercapacitor based on hierarchical MOF derived CoFe2O4 and NiMn2O4 composite for efficient energy storage. J. Alloys Compd. 2023, 959, 170483. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Zakir, A.; Shoukat, W.; Badi, N.; Afzal, A.M.; Fouda, A.M.; Hegazy, H.H. Enhancing the electrochemical efficiency of metal-organic frameworks through integration of chromium nitride interfacial layer for efficient hybrid supercapacitors. J. Energy Storage 2025, 105, 114606. [Google Scholar] [CrossRef]
- Wang, B.; Tan, W.; Fu, R.; Mao, H.; Kong, Y.; Qin, Y.; Tao, Y. Hierarchical mesoporous Co3O4/C@MoS2 core-shell structured materials for electrochemical energy storage with high supercapacitive performance. Synth. Met. 2017, 233, 101–110. [Google Scholar] [CrossRef]
- Hou, S.; Lian, Y.; Bai, Y.; Zhou, Q.; Ban, C.; Wang, Z.; Zhao, J.; Zhang, H. Hollow dodecahedral Co3S4@NiO derived from ZIF-67 for supercapacitor. Electrochim. Acta 2020, 341, 136053. [Google Scholar] [CrossRef]
- Zhao, J.; Li, H.; Li, C.; Zhang, Q.; Sun, J.; Wang, X.; Guo, J.; Xie, L.; Xie, J.; He, B.; et al. MOF for template-directed growth of well-oriented nanowire hybrid arrays on carbon nanotube fibers for wearable electronics integrated with triboelectric nanogenerators. Nano Energy 2018, 45, 420–431. [Google Scholar] [CrossRef]
- Prasad Ojha, G.; Muthurasu, A.; Prasad Tiwari, A.; Pant, B.; Chhetri, K.; Mukhiya, T.; Dahal, B.; Lee, M.; Park, M.; Kim, H.-Y. Vapor solid phase grown hierarchical CuxO NWs integrated MOFs-derived CoS2 electrode for high-performance asymmetric supercapacitors and the oxygen evolution reaction. Chem. Eng. J. 2020, 399, 125532. [Google Scholar] [CrossRef]
- Govindan, R.; Hong, X.-J.; Sathishkumar, P.; Cai, Y.-P.; Gu, F.L. Construction of metal-organic framework-derived CeO2/C integrated MoS2 hybrid for high-performance asymmetric supercapacitor. Electrochim. Acta 2020, 353, 136502. [Google Scholar] [CrossRef]
- Gayathri, S.; Arunkumar, P.; Saha, D.; Han, J.H. Composition engineering of ZIF-derived cobalt phosphide/cobalt monoxide heterostructures for high-performance asymmetric supercapacitors. J. Colloid Interface Sci. 2021, 588, 557–570. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, S.; Sun, J.; Yu, L.; Qian, X.; Hu, R.; Wang, Y.; Zhao, H.; Zhu, J. 2D Fe-containing cobalt phosphide/cobalt oxide lateral heterostructure with enhanced activity for oxygen evolution reaction. Nano Energy 2019, 56, 109–117. [Google Scholar] [CrossRef]
- Wang, J.; Gao, R.; Zheng, L.; Chen, Z.; Wu, Z.; Sun, L.; Hu, Z.; Liu, X. CoO/CoP heterostructured nanosheets with an O-P interpenetrated Interface as a bifunctional electrocatalyst for Na-O2 battery. ACS Catal. 2018, 8, 8953–8960. [Google Scholar] [CrossRef]
- Guo, J.W.; Zhao, H.B.; Yang, Z.W.; Wang, Y.W.; Liu, X.L.; Wang, L.F.; Zhao, Z.H.; Wang, A.Z.; Ding, L.H.; Liu, H.; et al. Hierarchical porous 3D Ni3N-CoN/NC heterojunction nanosheets with nitrogen vacancies for high-performance flexible supercapacitor. Nano Energy 2023, 116, 108763. [Google Scholar] [CrossRef]
- Zhao, J.J.; Liu, N.; Sun, Y.Z.; Xu, Q.H.; Pan, J.Q. Nitrogen-modified spherical porous carbon derived from aluminum-based metal-organic frameworks as activation-free materials for supercapacitors. J. Energy Storage 2023, 73, 109070. [Google Scholar] [CrossRef]
- Zhang, S.; Dai, P.; Liu, H.; Yan, L.; Song, H.; Liu, D.; Zhao, X. Metal-organic framework derived porous flakes of cobalt chalcogenides (CoX, X = O, S, Se and Te) rooted in carbon fibers as flexible electrode materials for pseudocapacitive energy storage. Electrochim. Acta 2021, 369, 137681. [Google Scholar] [CrossRef]
- Zhang, A.; Zhang, Q.; Fu, H.C.; Zong, H.W.; Guo, H.W. Metal-organic frameworks and their derivatives based nanostructure with different dimensionalities for supercapacitors. Small 2023, 19, 2303911. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, V.; Bulla, M.; Devi, R.; Dahiya, R.; Sisodiya, A.K.; Singh, R.B.; Mishra, A.K. Hydrothermally reduced graphene oxide based electrodes for high-performance symmetric supercapacitor. Mater. Lett. 2024, 364, 136364. [Google Scholar] [CrossRef]
- Feng, W.; Liu, C.; Liu, Z.; Pang, H. In-situ growth of N-doped graphene-like carbon/MOF nanocomposites for high-performance supercapacitor. Chin. Chem. Lett. 2024, 35, 109552. [Google Scholar] [CrossRef]
- Kausar, A.; Ahmad, I. Graphene-MOF hybrids in high-tech energy devices—Present and future advances. Hybrid Adv. 2024, 5, 100150. [Google Scholar] [CrossRef]
- Saxena, N.; Bondarde, M.P.; Lokhande, K.D.; Bhakare, M.A.; Dhumal, P.S.; Some, S. One-pot synthesis of rGO/Ppy/Zn-MOF, ternary composite for High-Performance supercapacitor application. Chem. Phys. Lett. 2024, 856, 141605. [Google Scholar] [CrossRef]
- Lian, P.C.; Liu, H.H.; Li, H.; Zhang, Y.; Mei, Y. A facile and mild route to synthesize ultralight and flexible 3D functionalized graphene. J. Porous. Mater. 2018, 25, 905–911. [Google Scholar] [CrossRef]
- Tang, Z.; Lei, Y.; Deng, Y.; Chen, J.; Liu, K.; Zhang, C.; Wang, T.; Lei, Y. MOF-derived MoS2/nitrogen-doped graphene aerogel for supercapacitor electrodes. Diam. Relat. Mater. 2025, 153, 112098. [Google Scholar] [CrossRef]
- Siddiqui, R.; Rani, M.; Shah, A.A.; Razaq, A.; Iqbal, R.; Neffati, R.; Arshad, M. Fabrication of tricarboxylate-neodymium metal organic frameworks and its nanocomposite with graphene oxide by hydrothermal synthesis for a symmetric supercapacitor electrode material. Mater. Sci. Eng. B 2023, 295, 116530. [Google Scholar] [CrossRef]
- Ashraf, M.; Umar, E.; Sunny, M.A.; Iqbal, M.W.; Hassan, H.; Alrobei, H.; Ismayilova, N.A.; Alawaideh, Y.M.; Al-Buriahi, M.S.; Alomairy, S. Reduced graphene oxides embedded zeolitic imidazolate framework-8 and copper sulfide as electrodes for asymmetric supercapacitors and hydrogen evolution reactions. J. Energy Storage 2024, 104, 114479. [Google Scholar] [CrossRef]
- Kumar, R.V.; Vickraman, P.; Raja, T.A.; Bharathi, M.S.R. Exploratory mass dispersoid rGO in-situ influence on nickel phosphate-trimesic acid metal-organic framework for higher energy density hybrid supercapacitors. J. Energy Storage 2024, 102, 114140. [Google Scholar] [CrossRef]
- Hassan, H.; Shoaib, M.; Khan, K.; Ghanem, M.A.; Osman, M. Graphene quantum dots decorated on chromium oxide and zirconium metal-organic framework composite (GQDs@Zr-MOF/Cr2O3) for asymmetric supercapacitors and hydrogen production. Mater. Chem. Phys. 2025, 332, 130225. [Google Scholar] [CrossRef]
- Xiong, C.; Zhang, Y.; Zheng, C.; Yin, Y.; Xiong, Q.; Zhao, M.; Wang, B. Fabrication of metal-organic framework@cellulose nanofibers/reduced graphene oxide-Vitrimer composite electrode materials with shape memory for supercapacitors. Electrochim. Acta 2024, 493, 144373. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Zhao, P.; Zhang, X.; Lu, X.; Qiu, Z.; Qi, B.; Yao, R.; Huang, Y.; Wang, L.; et al. Metal-organic framework-mediated construction of confined ultrafine nickel phosphide immobilized in reduced graphene oxide with excellent cycle stability for asymmetric supercapacitors. J. Colloid Interface Sci. 2023, 649, 616–625. [Google Scholar] [CrossRef]
- Tang, X.R.; Li, N.; Pang, H. Metal-organic frameworks-derived metal phosphides for electrochemistry application. Green Energy Environ. 2022, 7, 636–661. [Google Scholar] [CrossRef]
- Zhou, Q.; Gong, Y.; Tao, K. Calcination/phosphorization of dual Ni/Co-MOF into NiCoP/C nanohybrid with enhanced electrochemical property for high energy density asymmetric supercapacitor. Electrochim. Acta 2019, 320, 134582. [Google Scholar] [CrossRef]
- Liu, S.; Xu, W.; Feng, K.; Shi, X.; Xu, Z.; Teng, R.; Fan, X.; Wang, C. Three-dimensional heterostructured nickel phosphide @ nickel cobalt phosphide nanocomposites with highly porous surface for high-performance supercapacitor. Colloids Surf. A Physicochem. Eng. Asp. 2024, 686, 133342. [Google Scholar] [CrossRef]
- Yi, M.; Lu, B.; Zhang, X.; Tan, Y.; Zhu, Z.; Pan, Z.; Zhang, J. Ionic liquid-assisted synthesis of nickel cobalt phosphide embedded in N, P codoped-carbon with hollow and folded structures for efficient hydrogen evolution reaction and supercapacitor. Appl. Catal. B Environ. 2021, 283, 119635. [Google Scholar] [CrossRef]
- Zhu, Y.; Lao, J.; Xu, F.; Sun, L.; Shao, Q.; Luo, Y.; Fang, S.; Chen, Y.; Yu, C.; Zou, Y. A structurally controllable flower-shaped phosphide derived from metal-organic frameworks for high-performance supercapacitors. J. Electroanal. Chem. 2024, 966, 118376. [Google Scholar] [CrossRef]
- Liu, S.; Xu, W.; Feng, K.; Shi, X.; Wang, C. Bimetallic MOF derived NiMn phosphide for high-performance supercapacitor electrode material. J. Energy Storage 2024, 96, 112684. [Google Scholar] [CrossRef]
- Zhou, H.; Nasser, R.; Zhang, L.; Li, Z.; Li, F.; Song, J.-M. Copper/Cobalt based metal phosphide composites as positive material for supercapacitors. Chem. Eng. J. 2024, 496, 154102. [Google Scholar] [CrossRef]
- Lao, J.; Zhu, Y.; Xu, F.; Sun, L.; Fang, S.; Shao, Q.; Liao, L.; Guan, Y.; Zou, Y. A novel 1D MXene fibers-loaded metal-organic framework derived bimetallic sulfide for high-performance supercapacitors. Fuel 2024, 362, 130904. [Google Scholar] [CrossRef]
- Ramesh, S.; Rabani, I.; Sivasamy, A.; Kakani, V.; Haldorai, Y.; Seo, Y.-S.; Kim, J.-H.; Kim, H.S. Fabrication of nickel-copper sulfide nanoparticles decorated on metal-organic framework composite for supercapacitor application by hydrothermal process. J. Alloys Compd. 2024, 977, 173375. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, H.; Wu, N.; Pan, Z.; Li, C.L.; Chen, Y.; Cao, Y.J.; Yang, W. Trimesic acid-modified 2D NiCo-MOF for high-capacity supercapacitors. J. Alloys Compd. 2023, 934, 167779. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Kang, H.; Jin, T.; Jiao, L. Design, synthesis, and energy-related applications of metal sulfides. Mater. Horiz. 2016, 3, 402–421. [Google Scholar] [CrossRef]
- Ouyang, Y.; Zhang, B.; Wang, C.; Xia, X.; Lei, W.; Hao, Q. Bimetallic metal-organic framework derived porous NiCo2S4 nanosheets arrays as binder-free electrode for hybrid supercapacitor. Appl. Surf. Sci. 2021, 542, 148621. [Google Scholar] [CrossRef]
- Wang, D.; Tian, L.; Huang, J.; Li, D.; Liu, J.; Xu, Y.; Ke, H.; Wei, Q. “One for two” strategy to prepare MOF- derived NiCo2S4 nanorods grown on carbon cloth for high-performance asymmetric supercapacitors and efficient oxygen evolution reaction. Electrochim. Acta 2020, 334, 135636. [Google Scholar] [CrossRef]
- Cai, P.; Liu, T.; Zhang, L.; Cheng, B.; Yu, J. ZIF-67 derived nickel cobalt sulfide hollow cages for high-performance supercapacitors. Appl. Surf. Sci. 2020, 504, 144501. [Google Scholar] [CrossRef]
- Li, G.-C.; Liu, M.; Wu, M.-K.; Liu, P.-F.; Zhou, Z.; Zhu, S.-R.; Liu, R.; Han, L. MOF-derived self-sacrificing route to hollow NiS2/ZnS nanospheres for high performance supercapacitors. RSC Adv. 2016, 6, 103517–103522. [Google Scholar] [CrossRef]
- Cai, F.; Sun, R.; Kang, Y.; Chen, H.; Chen, M.; Li, Q. One-step strategy to a three-dimensional NiS-reduced graphene oxide hybrid nanostructure for high performance supercapacitors. RSC Adv. 2015, 5, 23073–23079. [Google Scholar] [CrossRef]
- Ruan, Y.; Jiang, J.; Wan, H.; Ji, X.; Miao, L.; Peng, L.; Zhang, B.; Lv, L.; Liu, J. Rapid self-assembly of porous square rod-like nickel persulfide via a facile solution method for high-performance supercapacitors. J. Power Sources 2016, 301, 122–130. [Google Scholar] [CrossRef]
- Shrivastav, V.; Sundriyal, S.; Goel, P.; Shrivastav, V.; Tiwari, U.K.; Deep, A. ZIF-67 derived Co3S4 hollow microspheres and WS2 nanorods as a hybrid electrode material for flexible 2V solid-state supercapacitor. Electrochim. Acta 2020, 345, 136194. [Google Scholar] [CrossRef]
- Zhao, W.; Yan, G.; Zheng, Y.; Liu, B.; Jia, D.; Liu, T.; Cui, L.; Zheng, R.; Wei, D.; Liu, J. Bimetal-organic framework derived Cu(NiCo)2S4/Ni3S4 electrode material with hierarchical hollow heterostructure for high performance energy storage. J. Colloid Interface Sci. 2020, 565, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Li, M.D.; Jiang, X.Y.; Liu, J.J.; Liu, Q.; Lv, N.J.; Qi, N.; Chen, Z.Q. A flower-like Co/Ni bimetallic metal-organic framework based electrode material with superior performance in supercapacitors. J. Alloys Compd. 2023, 930, 167354. [Google Scholar] [CrossRef]
- Xiong, D.; Gu, M.; Chen, C.; Lu, C.; Yi, F.-Y.; Ma, X. Rational design of bimetallic metal-organic framework composites and their derived sulfides with superior electrochemical performance to remarkably boost oxygen evolution and supercapacitors. Chem. Eng. J. 2021, 404, 127111. [Google Scholar] [CrossRef]
- Nataraj, N.; Dash, P.; Sakthivel, R.; Lin, Y.-C.; Fang, H.-W.; Chung, R.-J. Simultaneous electrochemical and colorimetric detection of tri-heavy metal ions in environmental water samples employing 3D-MOF/nickel selenide as a synergistic catalyst. Chem. Eng. J. 2024, 485, 149965. [Google Scholar] [CrossRef]
- Al-Shaibah, F.N.; Ibrahim, M.A.A.; Abu Hatab, A.S.; Abotaleb, A.; Sinopoli, A.; Zekri, A.; Ahmad, Y.H.; Al-Qaradawi, S.Y. Rational synthesis of MOF-Derived Cobalt-Based binary selenides nanocrystals for electrochemical oxygen evolution reaction. Appl. Surf. Sci. 2025, 688, 162479. [Google Scholar] [CrossRef]
- Sheikhi, S.; Jalali, F. Copper selenide—Porous carbon derived from metal-organic frameworks as an efficient electrocatalyst for methanol oxidation. Int. J. Hydrogen Energy 2024, 55, 864–874. [Google Scholar] [CrossRef]
- Zhai, Z.; Yan, W.; Dong, L.; Wang, J.; Chen, C.; Lian, J.; Wang, X.; Xia, D.; Zhang, J. Multi-dimensional materials with layered structures for supercapacitors: Advanced synthesis, supercapacitor performance and functional mechanism. Nano Energy 2020, 78, 105193. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, A.; Wang, Y.; Cao, X.; Zhou, Z.; Zhu, T.; Liang, S.; Cao, G. Self-templated synthesis of N-doped CoSe2/C double-shelled dodecahedra for high-performance supercapacitors. Energy Storage Mater. 2017, 8, 28–34. [Google Scholar] [CrossRef]
- Mei, H.; Zhang, L.; Zhang, K.; Gao, J.; Zhang, H.; Huang, Z.; Xu, B.; Sun, D. Conversion of MOF into carbon-coated NiSe2 yolk-shell microspheres as advanced battery-type electrodes. Electrochim. Acta 2020, 357, 136866. [Google Scholar] [CrossRef]
- Wang, Q.; Ran, X.; Shao, W.; Miao, M.; Zhang, D. High performance flexible supercapacitor based on metal-organic-framework derived CoSe2 nanosheets on carbon nanotube film. J. Power Sources 2021, 490, 229517. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, J.; Huang, J.; Wang, L.; Wang, P.; Cai, C.; Lu, M.; Yao, Z.; Yang, Y. Bimetallic MOF-derived (CuCo)Se nanoparticles embedded in nitrogen-doped carbon framework with boosted electrochemical performance for hybrid supercapacitor. Mater. Res. Bull. 2021, 137, 111196. [Google Scholar] [CrossRef]
- Li, Z.; Tian, M.; Chen, Y.; Liu, Y.; Cai, Y.; Wei, W. MOFs derived (Ni0.75Co0.25)Se2 nanoparticles embedded in N-doped nanocarbon for hybrid supercapacitors. Ceram. Int. 2021, 47, 12623–12630. [Google Scholar] [CrossRef]
- Acharya, J.; Ojha, G.P.; Pant, B.; Park, M. Construction of self-supported bimetallic MOF-mediated hollow and porous tri-metallic selenide nanosheet arrays as battery-type electrodes for high-performance asymmetric supercapacitors. J. Mater. Chem. A 2021, 9, 23977–23993. [Google Scholar] [CrossRef]
- Dong, W.; Li, X.; Ye, F.; Xu, X.; Zhang, X.; Yang, F.; Shen, D.; Hong, X.; Yang, S. Synergistically enhanced multimetallic selenide electrode materials derived from ZIF-67 templates for high-performance supercapacitors. J. Energy Storage 2025, 114, 115870. [Google Scholar] [CrossRef]
- Charis Caroline, S.; Das, B.; Pramana, S.S.; Batabyal, S.K. Nickel sulfide-nickel sulfoselenide nanosheets as a potential electrode material for high performance supercapacitor with extended shelf life. J. Energy Storage 2023, 68, 107812. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, S.; Ye, F.; Liu, W.; Lian, J.; Li, G.; Wang, H.; Hu, L.; Wu, L. Hierarchical mesoporous selenium@bimetallic selenide quadrilateral nanosheet arrays for advanced flexible asymmetric supercapacitors. J. Mater. Chem. A 2022, 10, 16212–16223. [Google Scholar] [CrossRef]
- Yi, M.; Wu, A.; Chen, Q.; Cai, D.; Zhan, H. In situ confined conductive nickel cobalt sulfoselenide with tailored composition in graphitic carbon hollow structure for energy storage. Chem. Eng. J. 2018, 351, 678–687. [Google Scholar] [CrossRef]
- Lu, X.; Chen, Q.; Wu, L.; Cui, S.; Li, G.; Zhao, W.; Han, L. A multi-interface bimetallic sulfoselenide–selenite heterojunction as a battery-type cathode for high-performance supercapacitors. New J. Chem. 2024, 48, 15227–15239. [Google Scholar] [CrossRef]
- Huang, S.; Shi, X.-R.; Sun, C.; Duan, Z.; Ma, P.; Xu, S. The Application of Metal–Organic Frameworks and Their Derivatives for Supercapacitors. Nanomaterials 2020, 10, 2268. [Google Scholar] [CrossRef]
- Lan, M.; Wang, X.; Zhao, R.; Dong, M.; Fang, L.; Wang, L. Metal-organic framework-derived porous MnNi2O4 microflower as an advanced electrode material for high-performance supercapacitors. J. Alloys Compd. 2020, 821, 153546. [Google Scholar] [CrossRef]
- Javed, M.S.; Aslam, M.K.; Asim, S.; Batool, S.; Idrees, M.; Hussain, S.; Shah, S.S.A.; Saleem, M.; Mai, W.; Hu, C. High-performance flexible hybrid-supercapacitor enabled by pairing binder-free ultrathin Ni–Co–O nanosheets and metal-organic framework derived N-doped carbon nanosheets. Electrochim. Acta 2020, 349, 136384. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Bi, R.; Mao, F.; Wang, K.; Wu, H.; Wang, X. A polythreaded MnII-MOF and its super-performances for dye adsorption and supercapacitors. Inorg. Chem. Front. 2020, 7, 718–730. [Google Scholar] [CrossRef]
- Deng, T.; Shi, X.; Zhang, W.; Wang, Z.; Zheng, W. In-plane Assembly of Distinctive 2D MOFs with Optimum Supercapacitive Performance. iScience 2020, 23, 101220. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Yu, J.; Liu, Q.; Shao, L.; Zhang, Y.; Sun, Z.; Huang, H. Metal-Organic-Framework-Derived N-, P-, and O-Codoped Nickel/Carbon Composites Homogeneously Decorated on Reduced Graphene Oxide for Energy Storage. ACS Appl. Nano Mater. 2020, 3, 5625–5636. [Google Scholar] [CrossRef]
- Maliha, Z.; Rani, M.; Neffati, R.; Mahmood, A.; Iqbal, M.Z.; Shah, A. Investigation of copper/cobalt MOFs nanocomposite as an electrode material in supercapacitors. Int. J. Energy Res. 2022, 46, 17404–17415. [Google Scholar] [CrossRef]
- Singh, M.K.; Krishnan, S.; Singh, K.; Rai, D.K. CNT Interwoven Cu-MOF: A Synergistic Electrochemical Approach for Solid-State Supercapacitor and Hydrogen Evolution Reaction. Energy Fuels 2024, 38, 12098–12110. [Google Scholar] [CrossRef]
- Anwer, A.H.; Ansari, M.Z.; Mashkoor, F.; Zhu, S.; Shoeb, M.; Jeong, C. Synergistic effect of carbon nanotube and tri-metallic MOF nanoarchitecture for electrochemical high-performance asymmetric supercapacitor applications and their charge storage mechanism. J. Alloys Compd. 2023, 955, 170038. [Google Scholar] [CrossRef]
- Zaka, A.; Iqbal, M.W.; Afzal, A.M.; Hassan, H.; Alharthi, S.; Amin, M.A.; Saeedi, A.M.; Albargi, H.B.; Alhadrami, A.; Alqarni, N.D.; et al. Synergistic innovations in energy Storage: Cu-MOF infused with CNT for supercapattery devices and hydrogen evolution reaction. Inorg. Chem. Commun. 2024, 159, 111739. [Google Scholar] [CrossRef]
- Ji, Y.; Li, W.; You, Y.; Xu, G. In situ synthesis of M (Fe, Cu, Co and Ni)-MOF@MXene composites for enhanced specific capacitance and cyclic stability in supercapacitor electrodes. Chem. Eng. J. 2024, 496, 154009. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Aziz, U.; Aftab, S.; Wabaidur, S.M.; Siddique, S.; Iqbal, M.J. A Hydrothermally Prepared Lithium and Copper MOF Composite as Anode Material for Hybrid Supercapacitor Applications. Angew. Chem. 2023, 8, e202204554. [Google Scholar] [CrossRef]
- Suganthi, S.; Ahmad, K.; Oh, T.H. Progress in MOFs and MOFs-Integrated MXenes as Electrode Modifiers for Energy Storage and Electrochemical Sensing Applications. Molecules 2024, 29, 5373. [Google Scholar] [CrossRef] [PubMed]
- Do, H.H.; Cho, J.H.; Han, S.M.; Ahn, S.H.; Kim, S.Y. Metal–Organic-Framework- and MXene-Based Taste Sensors and Glucose Detection. Sensors 2021, 21, 7423. [Google Scholar] [CrossRef]
- Su, H.; Jin, C.; Zhang, X.; Yu, Z.; Zeng, X. Recent progress in the synthesis and electrocatalytic application of MXene-based metal phosphide composites. Carbon Neutralization 2024, 3, 1009–1035. [Google Scholar] [CrossRef]
- Basha, S.I.; Shah, S.S.; Helal, A.; Aziz, M.A.; Yoo, D.-Y. Unveiling the limitless potential: Exploring metal–organic frameworks (MOFs)/MXene based construction materials. Case Stud. Constr. Mater. 2024, 21, e03586. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, G.-X.; Zhou, H.-J.; Sun, Y.-Y.; Pang, H. Metal–Organic Frameworks Meet MXene: New Opportunities for Electrochemical Application. Energy Mater. Adv. 2023, 4, 0033. [Google Scholar] [CrossRef]
- Tan, H.; Zhou, Y.; Qiao, S.-Z.; Fan, H.J. Metal organic framework (MOF) in aqueous energy devices. Mater. Today 2021, 48, 270–284. [Google Scholar] [CrossRef]
- Ding, M.; Jiang, H.-L. Improving Water Stability of Metal–Organic Frameworks by a General Surface Hydrophobic Polymerization. CCS Chem. 2021, 3, 2740–2748. [Google Scholar] [CrossRef]
- Woodliffe, J.L.; Molinar-Díaz, J.; Clowes, R.; Hussein, O.H.; Lester, E.; Ferrari, R.; Ahmed, I.; Laybourn, A. Continuous flow synthesis of MOF UTSA-16(Zn), mixed-metal and magnetic composites for CO2 capture—Toward scalable manufacture. J. Environ. Chem. Eng. 2024, 12, 114167. [Google Scholar] [CrossRef]
- Woodliffe, J.L.; Ferrari, R.S.; Ahmed, I.; Laybourn, A. Evaluating the purification and activation of metal-organic frameworks from a technical and circular economy perspective. Coord. Chem. Rev. 2021, 428, 213578. [Google Scholar] [CrossRef]
- Laybourn, A.; Robertson, K.; Slater, A.G. Quid pro flow. J. Am. Chem. Soc. 2023, 145, 4355–4365. [Google Scholar] [CrossRef]
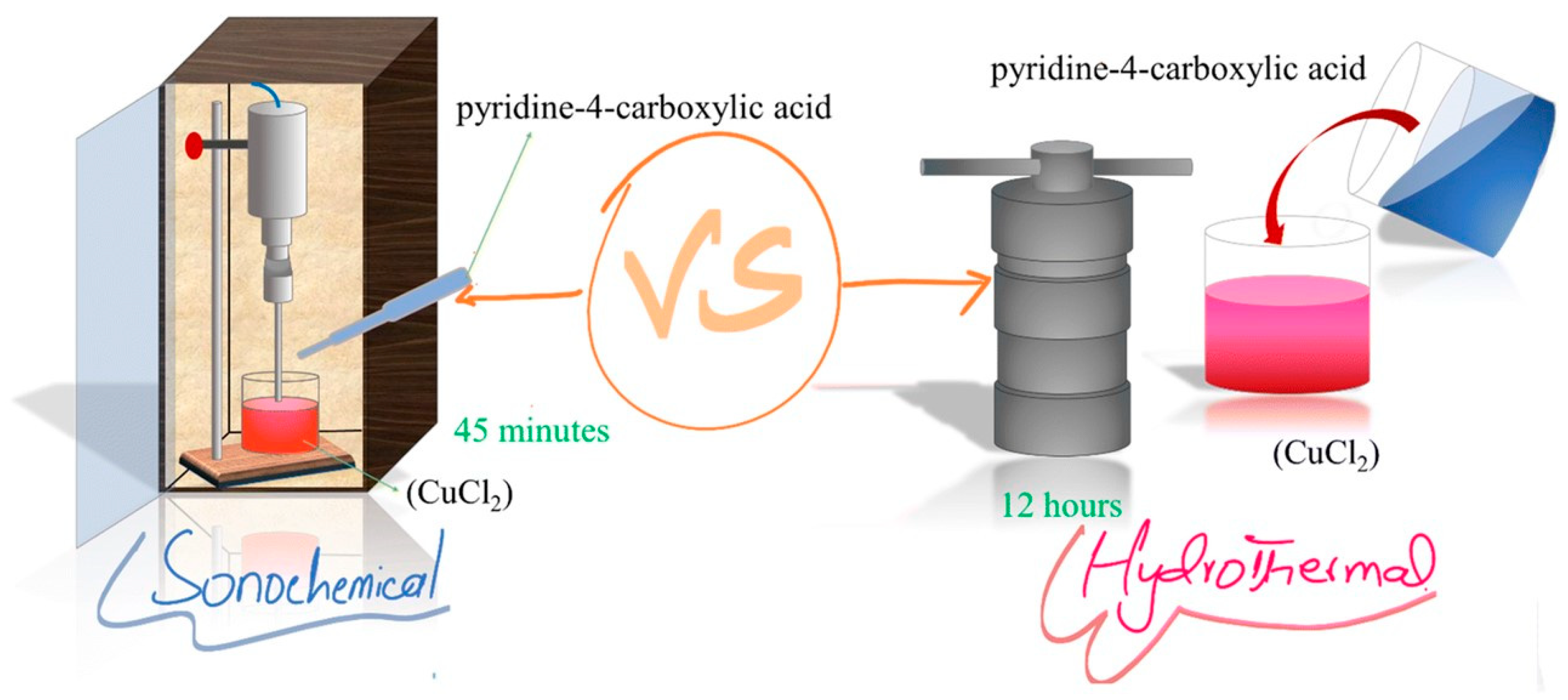
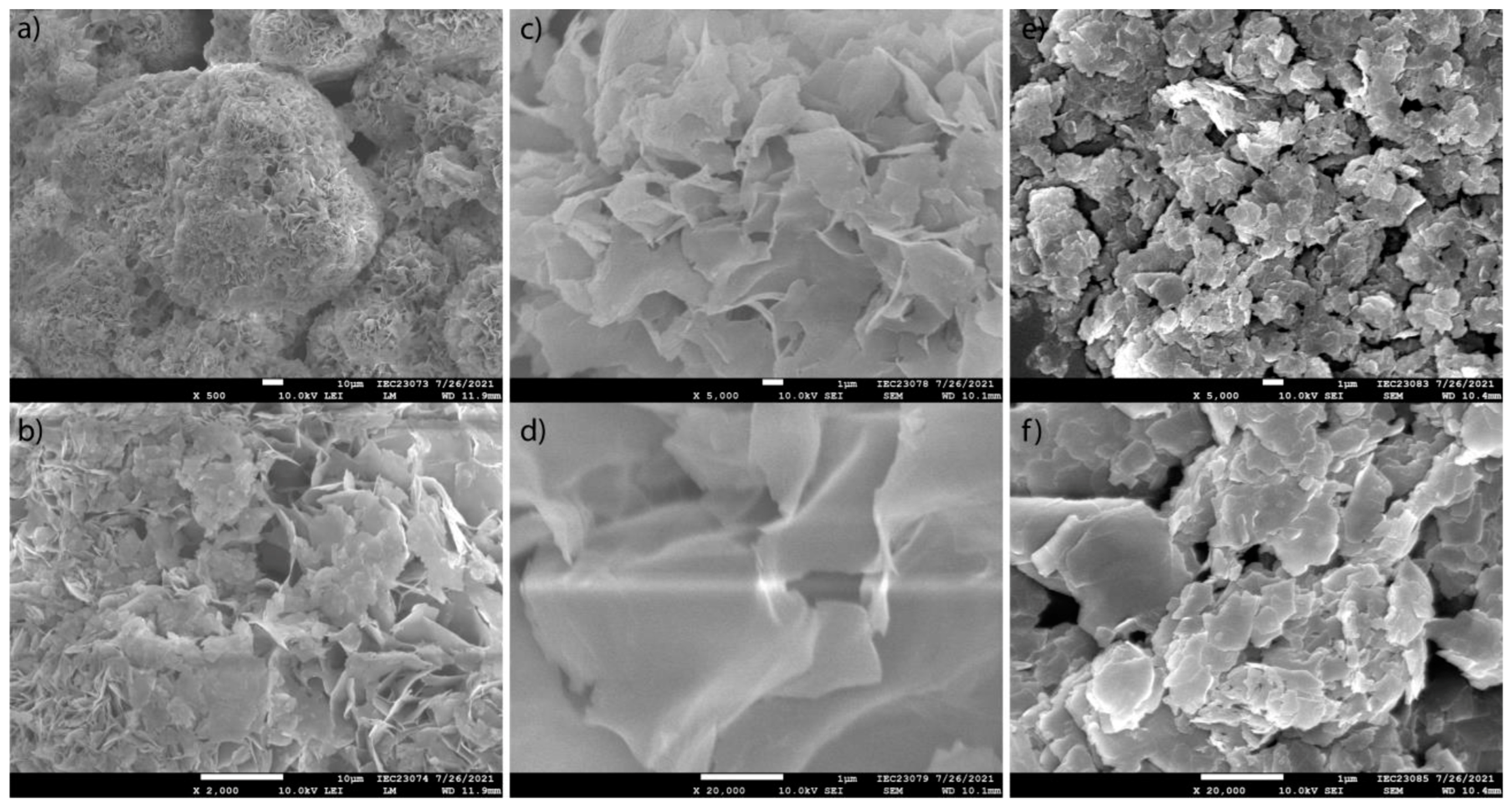


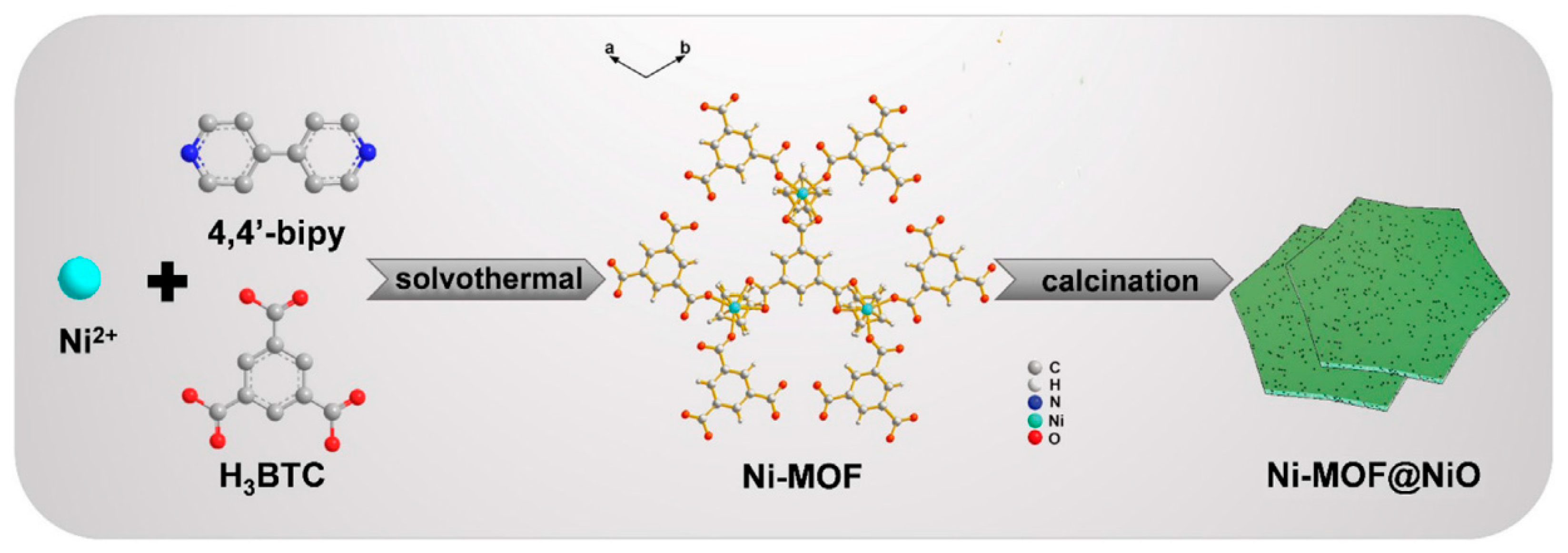
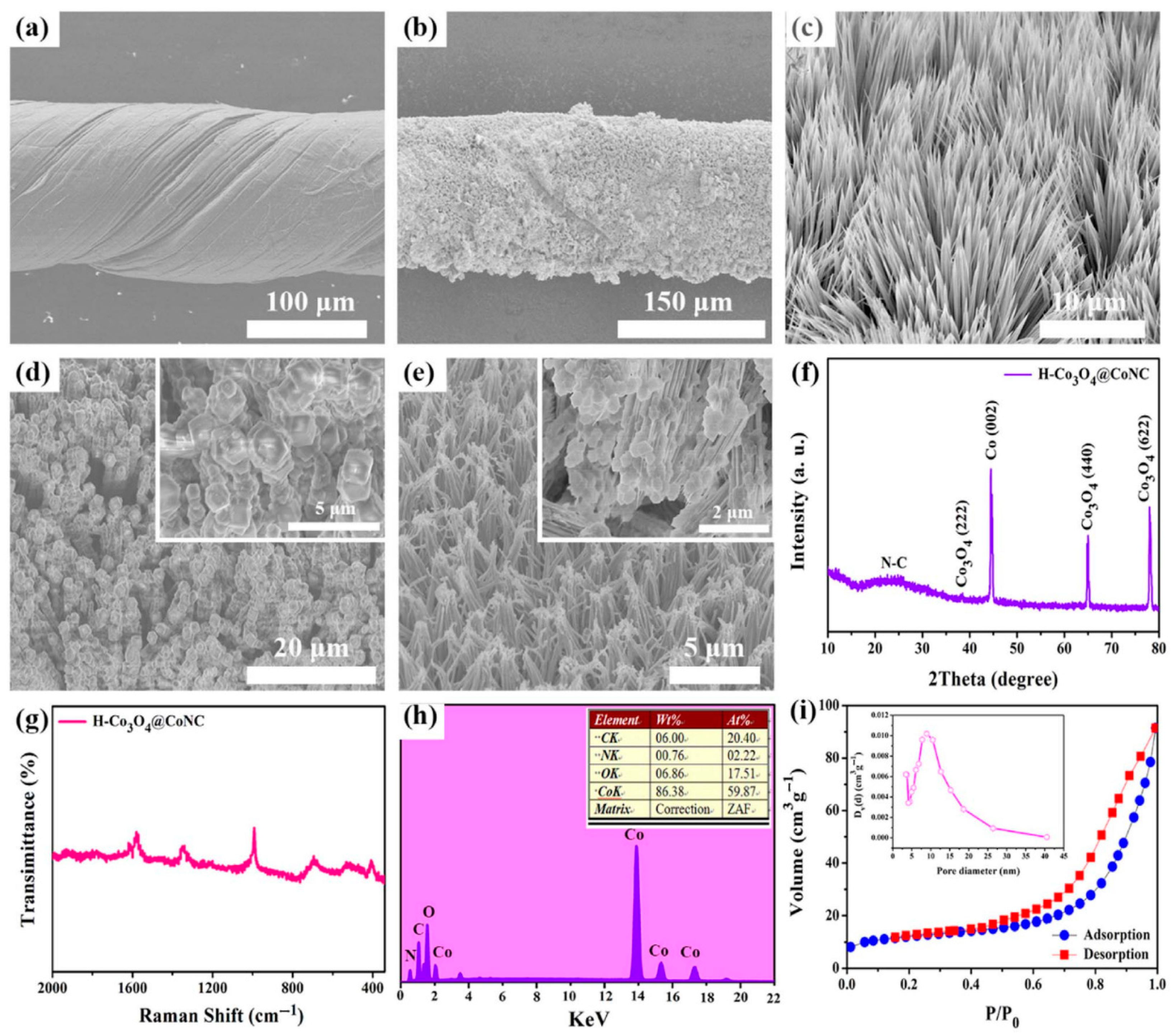
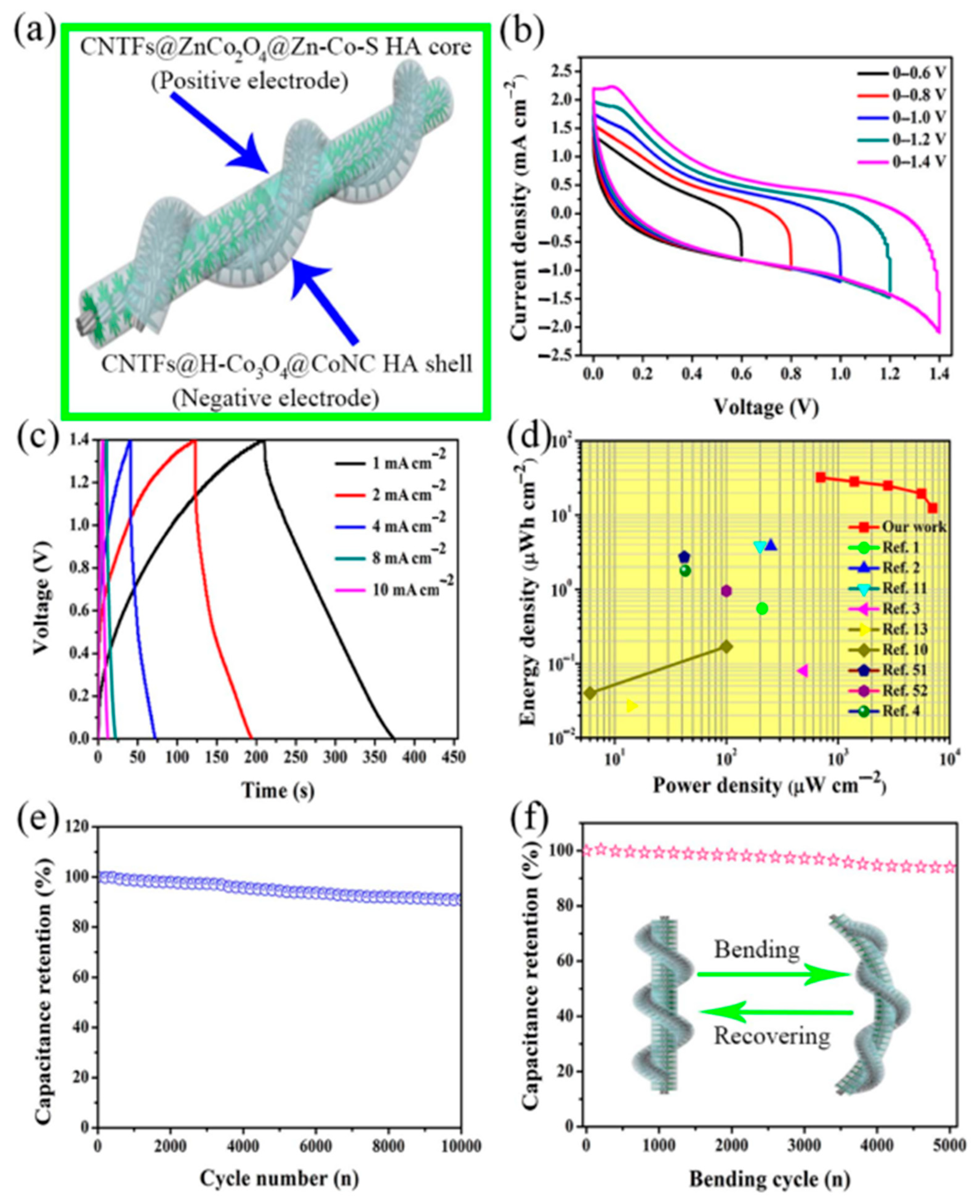

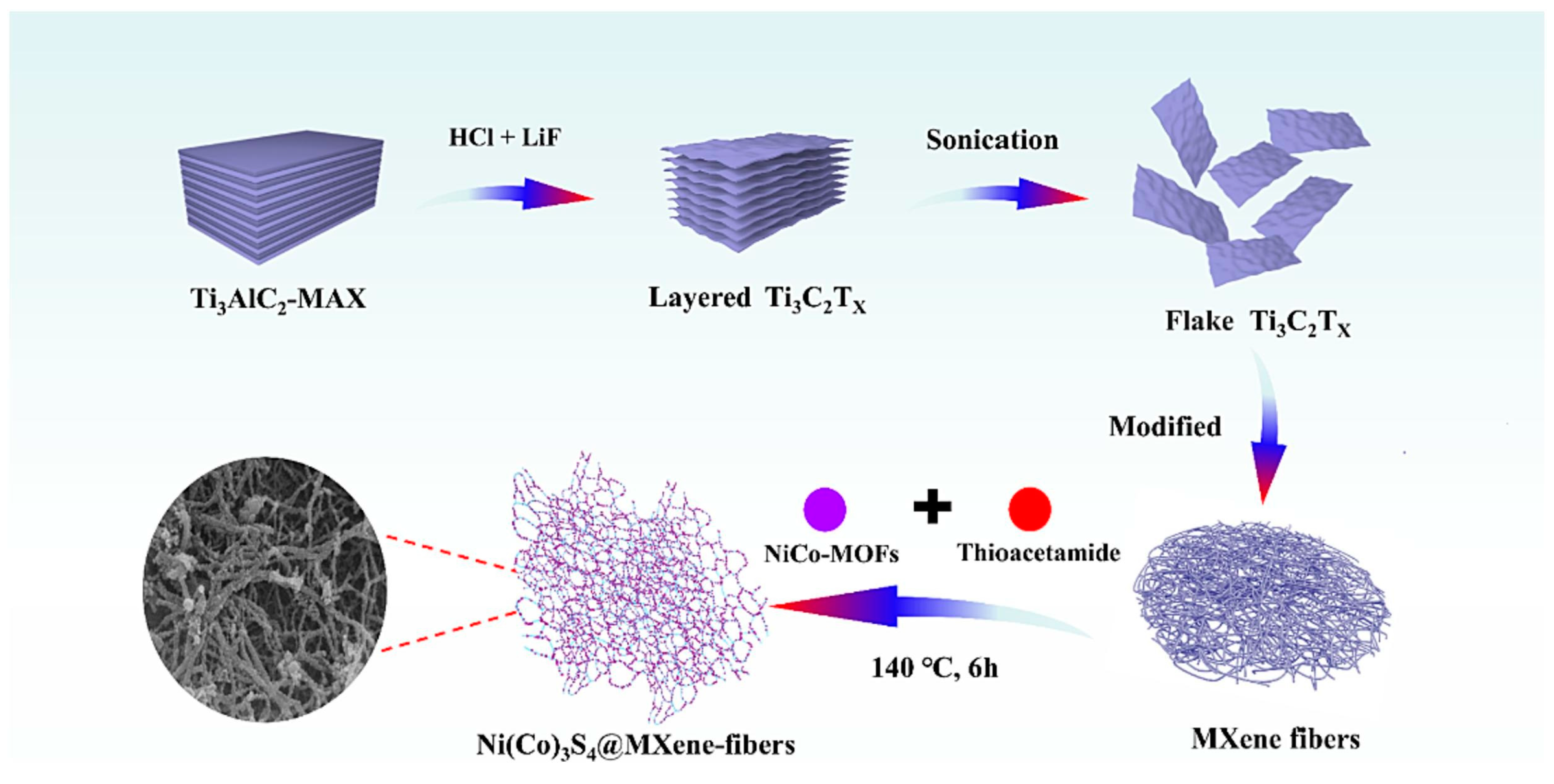

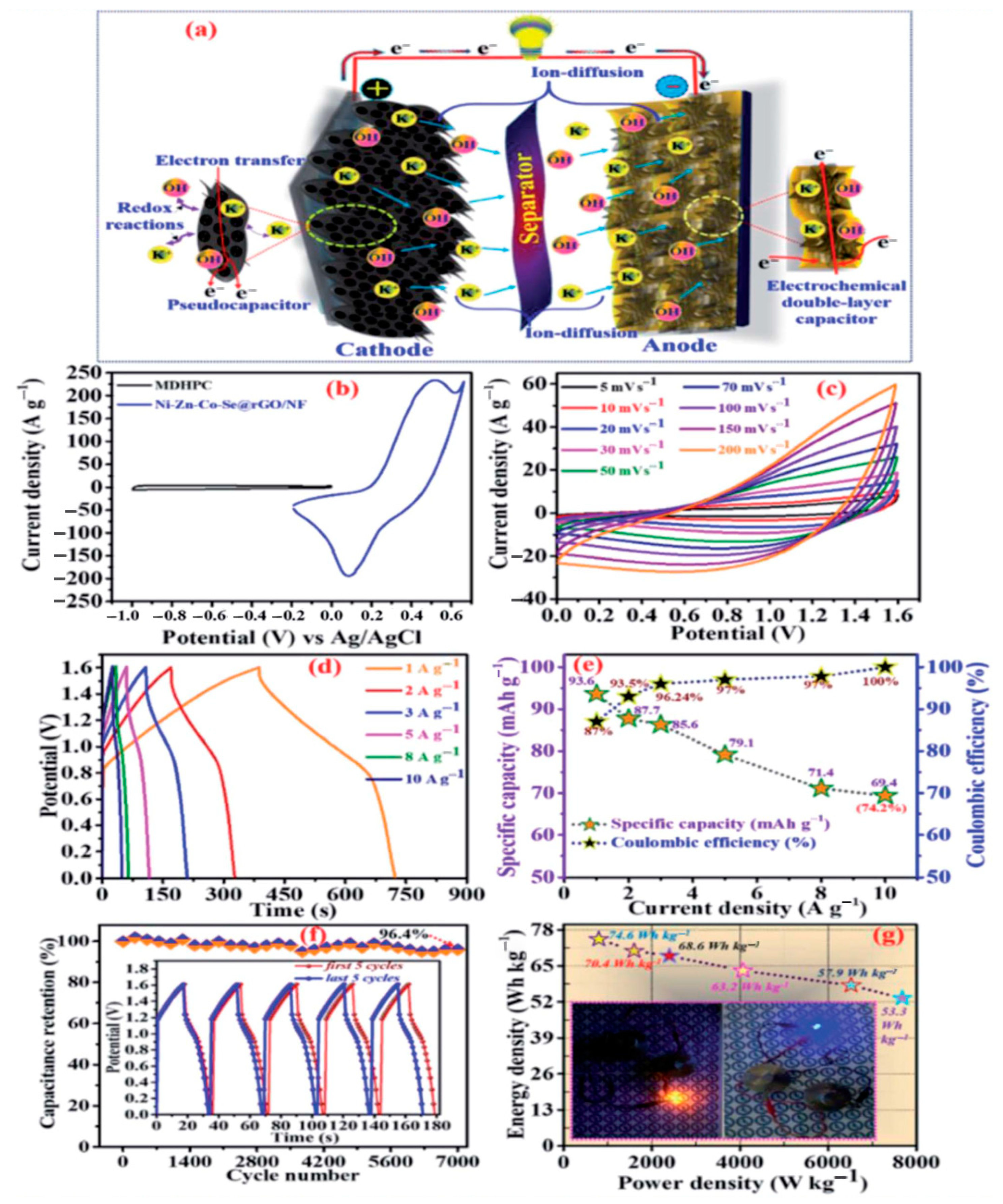
| Material(s)/Devices | Specific Capacitance or Capacity * [F/g] or [C/g] * | Energy Density [Wh/kg] | Power Density [W/kg] | Cycling Stability [% @ Cycles] | Ref. |
|---|---|---|---|---|---|
| Cu-MOF (sonochemically) | 594.2 | 34.4 | 13,765 | 97.95 @ - | [39] |
| Ni-MOF derived NiO/Ni/r-GO//r-GO | 172.2 * | 39.6 | 41,360 | 80 @ 10,000 | [72] |
| NiCo-PTA@PNTs | 1109 | 41.2 | 375 | 79.1 @ 10,000 | [182] |
| NiO@Ni-BTC/NF-12 | 1853 * | 29.1 | 7000 | 94 @ 3000 | [82] |
| Ni/Mn-PTA | 2848 | 142.8 | 800 | 93.25 @ 5000 | [183] |
| ZIF-8/Fe2O3//AC | 1160 | 28.5 | 2398 | 97 @ 1500 | [87] |
| Fe3O4@ZIF-67//AC | 1334.0 | 27.9 | 5488 | 87 @ 3000 | [88] |
| ZIF-8/ZIF-67: 50/50 | 201 | 69 | 840 | 72 @ 5000 | [184] |
| MnO2@NTO | 1054.7 | 36.2 | 11,879.9 | 85.3 @ 5000 | [102] |
| Mn-PTA/NF | 10.25 | 66 | 441 | 81.2 @ 10,000 | [185] |
| CoFe2O4@NiMn2O4/NF//AC | 312.8 | 90.3 | 12,900 | 88.4 @ 10,000 | [120] |
| rGO/Ppy/Zn-MOF | 175.0 | 19.7 | 1792 | 82 @ 7000 | [137] |
| NiCoP/C nanohybrids//AC | 775.7 | 47.6 | 798.9 | 78.1 @ 10,000 | [147] |
| (Ni0.93Mn0.07)2P-18/CC | 851.1 * | 59.8 | 750 | 84.87 @ 5000 | [151] |
| NiCo2(S0.78Se0.22)5/GC//Bi2O2.33/rGO | 476.2 | 47.2 | 93.7 @ 100 | [161] | |
| Ni-MOF-24/Cu3 (HITP)2/CFP | 1424 | 57 | 1500 | 94.3 @ 7000 | [186] |
| Ni-CTP-COOH/GO | 1258.7 | 79.7 | 1275 | 110 @ 5000 | [187] |
| Cu-Co-MOF/rGO | 935.8 | 45.2 | 2495.5 | - | [188] |
| Cu-MOF/CNT Composite | 348.56 | 27.7 | 1640 | 90.15 @ 10,000 | [189] |
| Cu-MOF/CNT | 166.4 | 23.6 | 501.5 | 79.2 @ 10,000 | [190] |
| Cu-MOF/CNT//AC | 1875 * | 47 | 920 | 98.3 @ 15,000 | [191] |
| NiMOF@MX 2//AC (ASC) | 1160.5 | 48.2 | 15,000 | 94 @ 10,000 | [192] |
| Li-Cu-MOF//AC | 171.1 | 36.1 | 5100 | 82.1 @ 1000 | [193] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argirusis, C.; Katsanou, M.-E.; Alizadeh, N.; Argirusis, N.; Sourkouni, G. Recent Advances in the Application of MOFs in Supercapacitors. Batteries 2025, 11, 181. https://doi.org/10.3390/batteries11050181
Argirusis C, Katsanou M-E, Alizadeh N, Argirusis N, Sourkouni G. Recent Advances in the Application of MOFs in Supercapacitors. Batteries. 2025; 11(5):181. https://doi.org/10.3390/batteries11050181
Chicago/Turabian StyleArgirusis, Christos, Maria-Eleni Katsanou, Niyaz Alizadeh, Nikolaos Argirusis, and Georgia Sourkouni. 2025. "Recent Advances in the Application of MOFs in Supercapacitors" Batteries 11, no. 5: 181. https://doi.org/10.3390/batteries11050181
APA StyleArgirusis, C., Katsanou, M.-E., Alizadeh, N., Argirusis, N., & Sourkouni, G. (2025). Recent Advances in the Application of MOFs in Supercapacitors. Batteries, 11(5), 181. https://doi.org/10.3390/batteries11050181










