Thermally Stable Carbon Materials from Polybenzoxazines: Structure, Properties, and Supercapacitor Potential
Abstract
1. Introduction
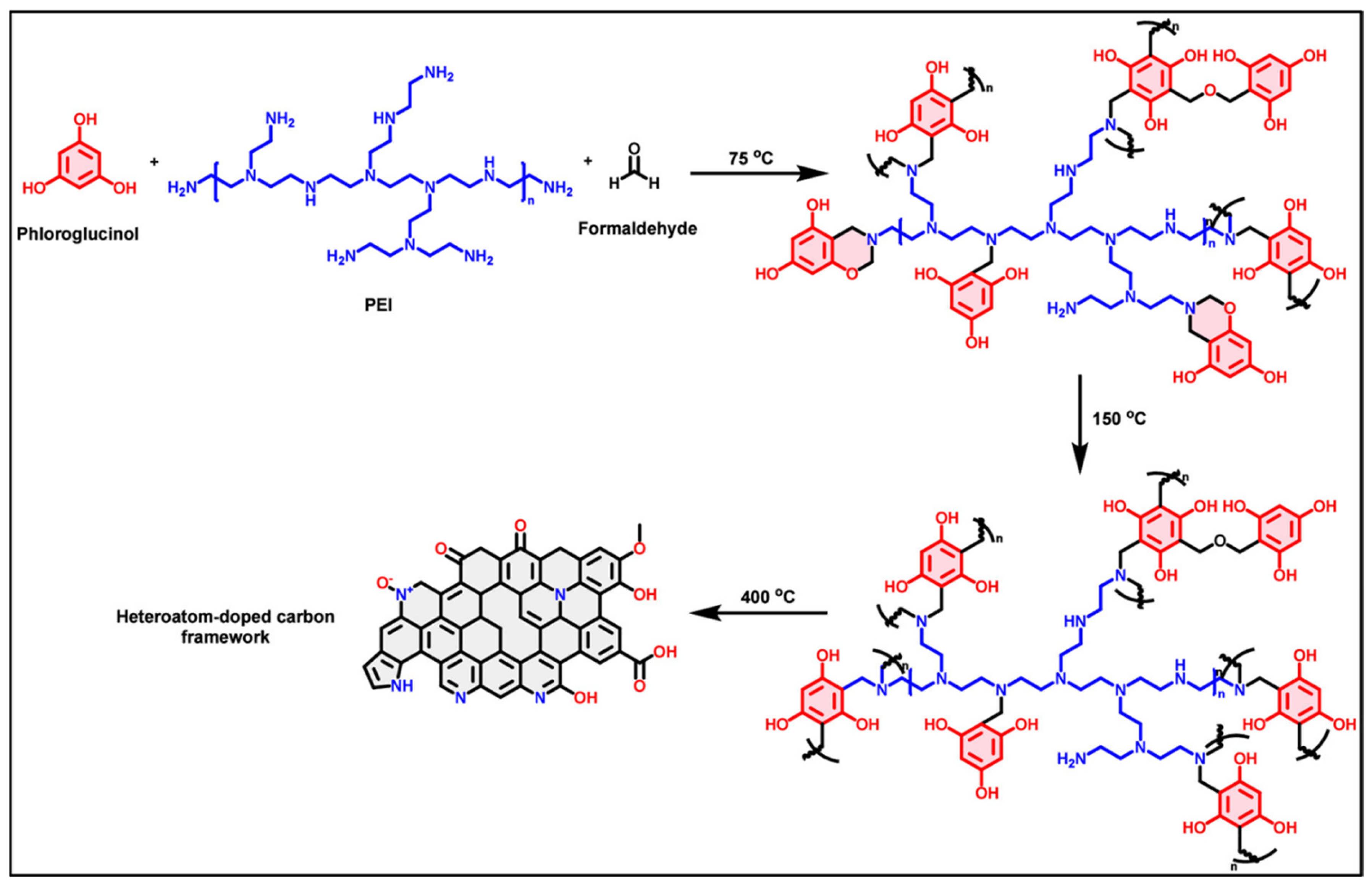
2. Preparation of Carbon-Materials from Polybenzoxazines: Key Methods
2.1. Formation of Solid Structures and Composites
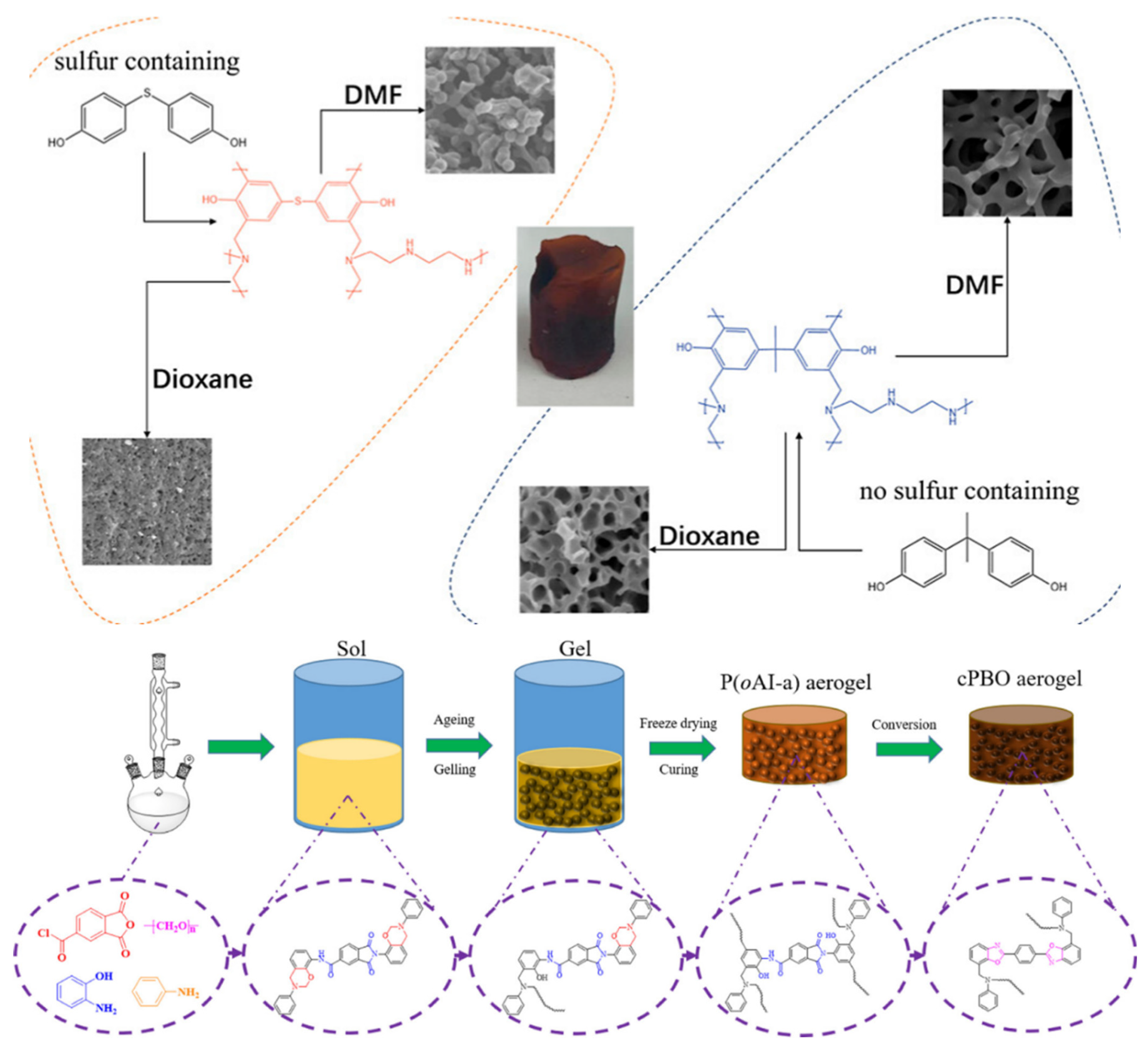
2.2. Development of Carbon Aerogels
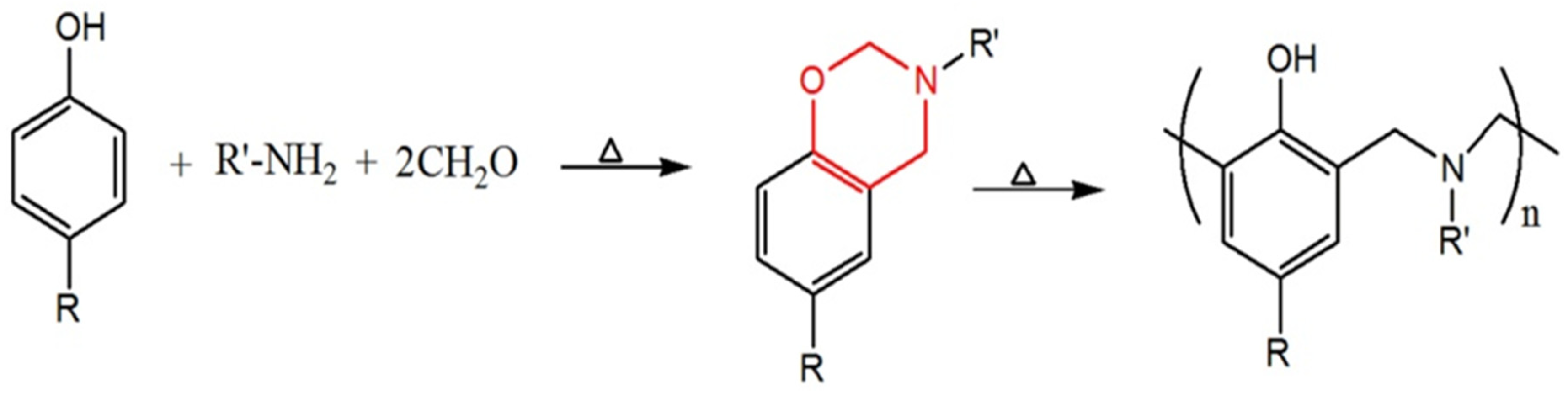
3. Key Characteristics of Polybenzoxazines
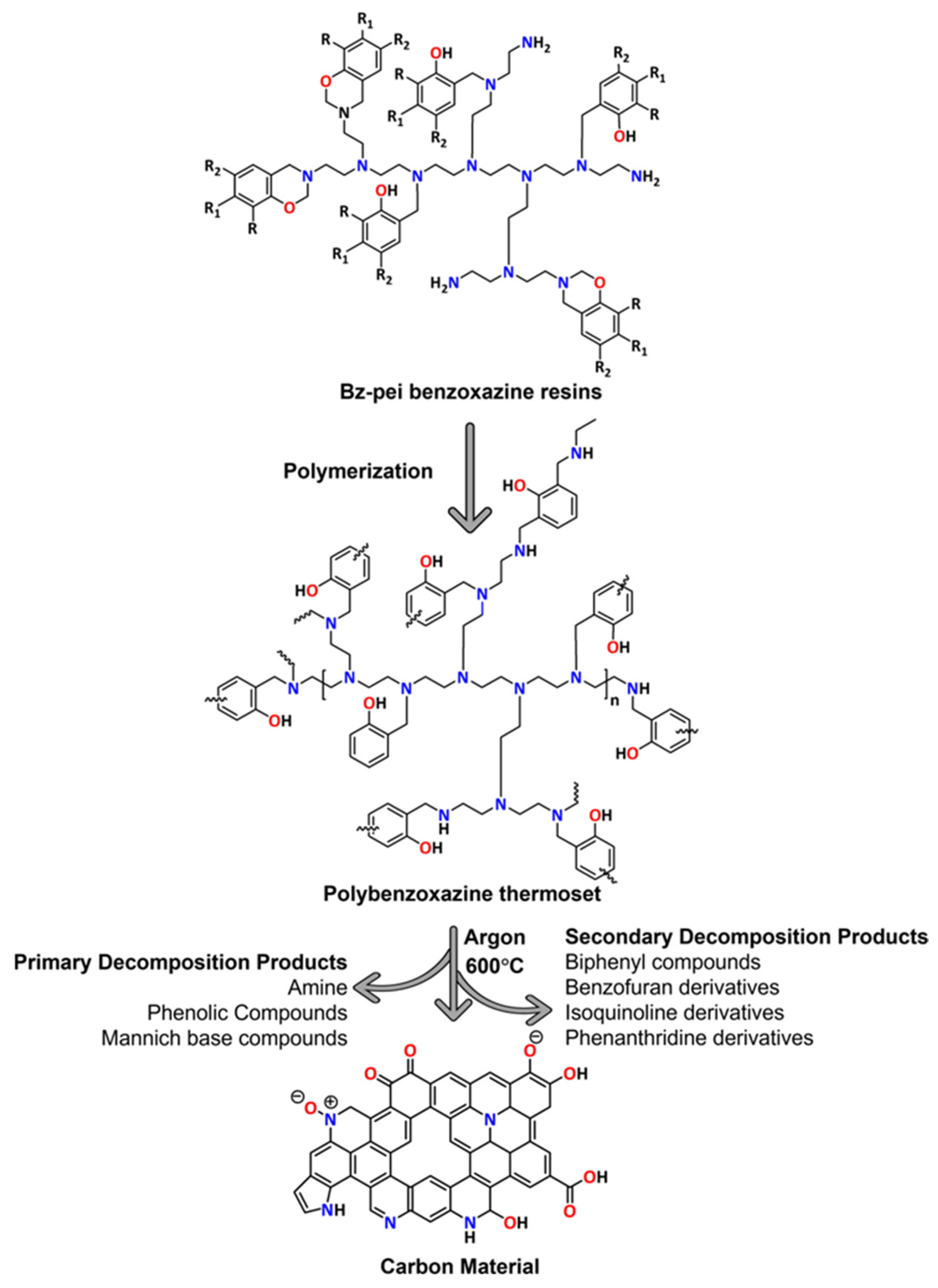
3.1. Thermal Decomposition and Carbon Residue Formation
3.2. Cross-Linking and Char Formation
4. Polybenzoxazine-Based Carbons and Their Electrochemical Performance
4.1. Autocatalysis Synthesis of Poly(benzoxazine-co-resol)-Based Polymer and Carbon Spheres
4.2. Template-Free, Self-Doped Approach to Porous Carbon Spheres for High-Performance Supercapacitors
4.3. Supermolecule Self-Assembly Promotes Porous N, P Co-Doped Reduced Graphene Oxide for High-Energy Supercapacitors
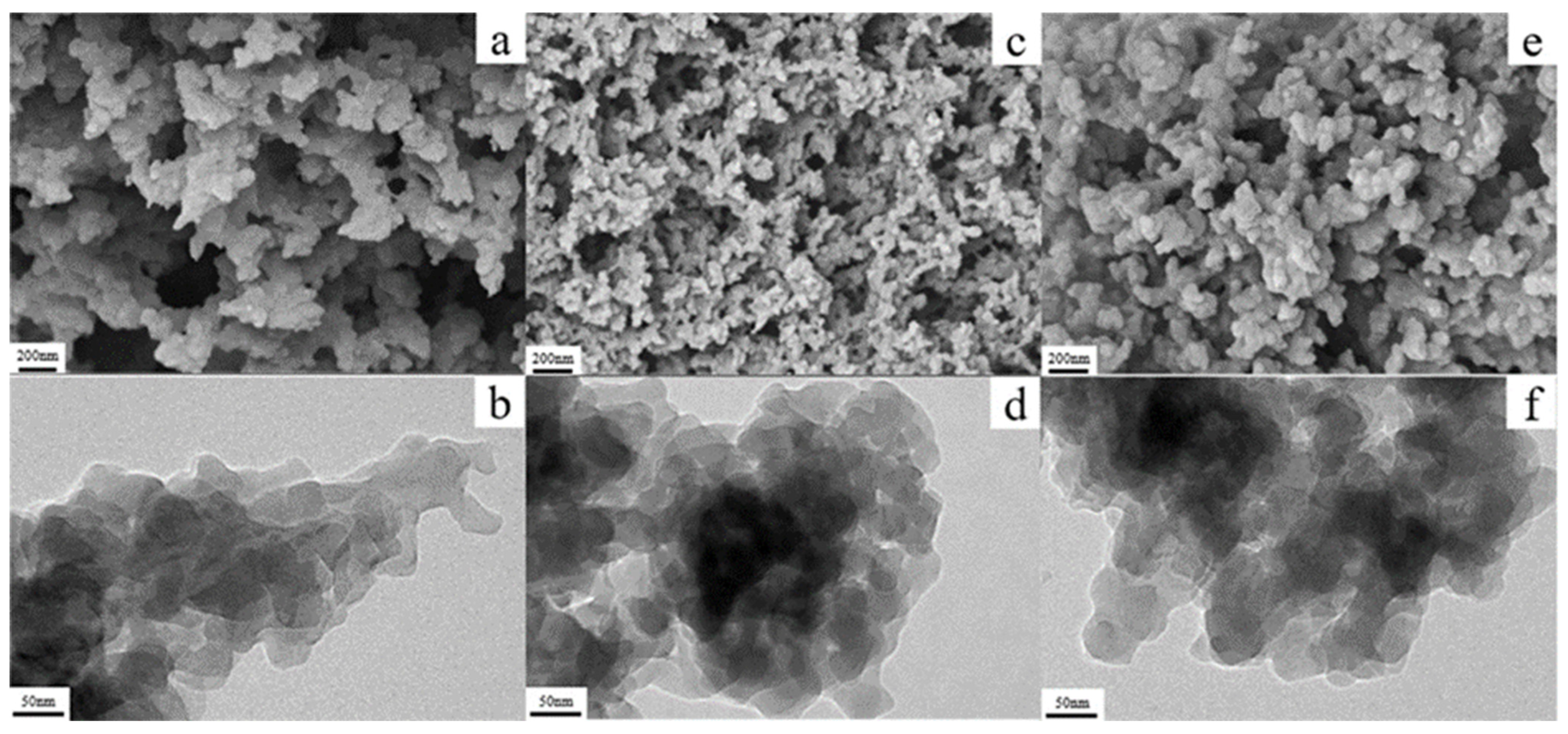
4.4. Electrochemical Aspects of Cross-Linked Polybenzoxazole Aerogels
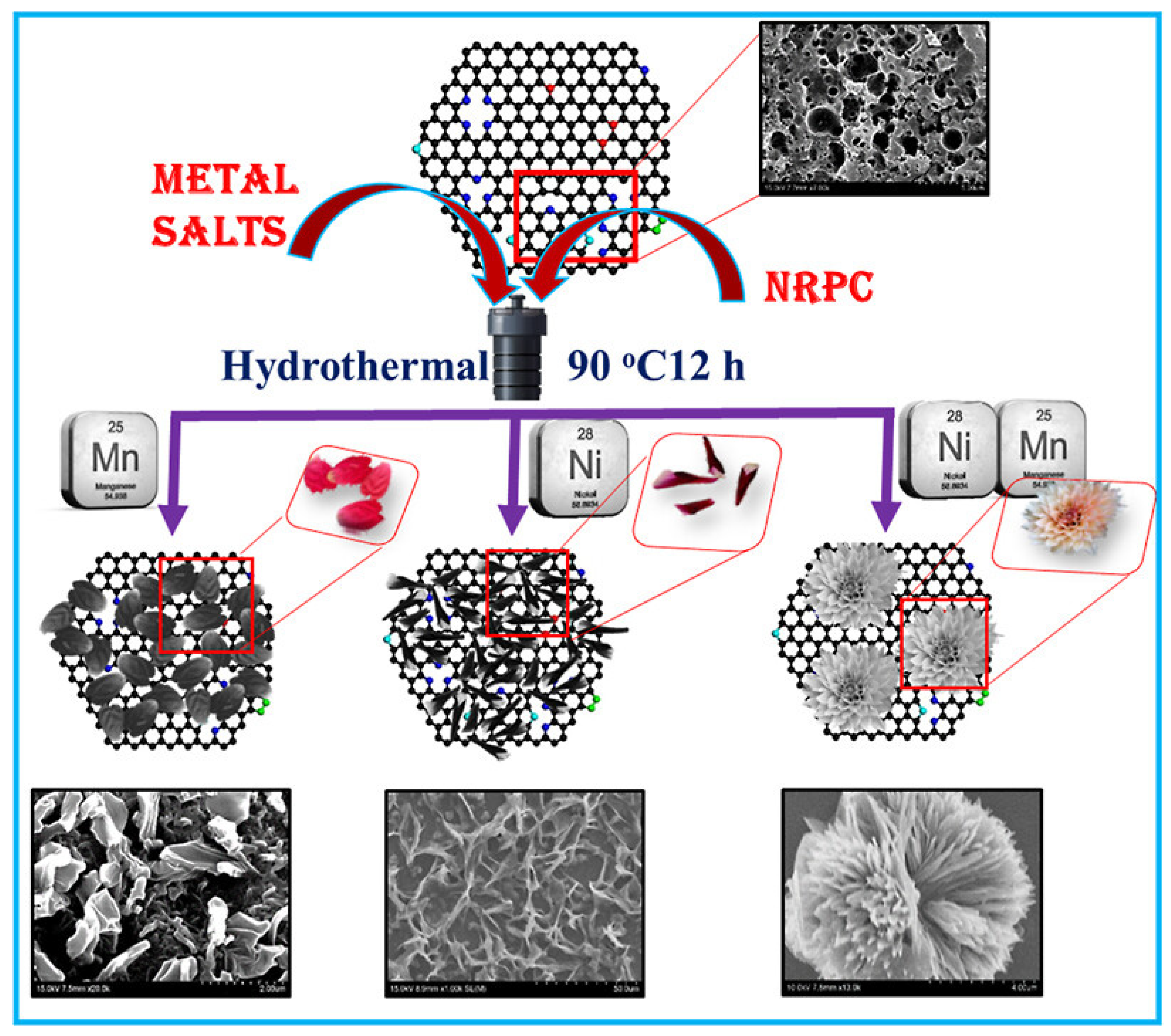
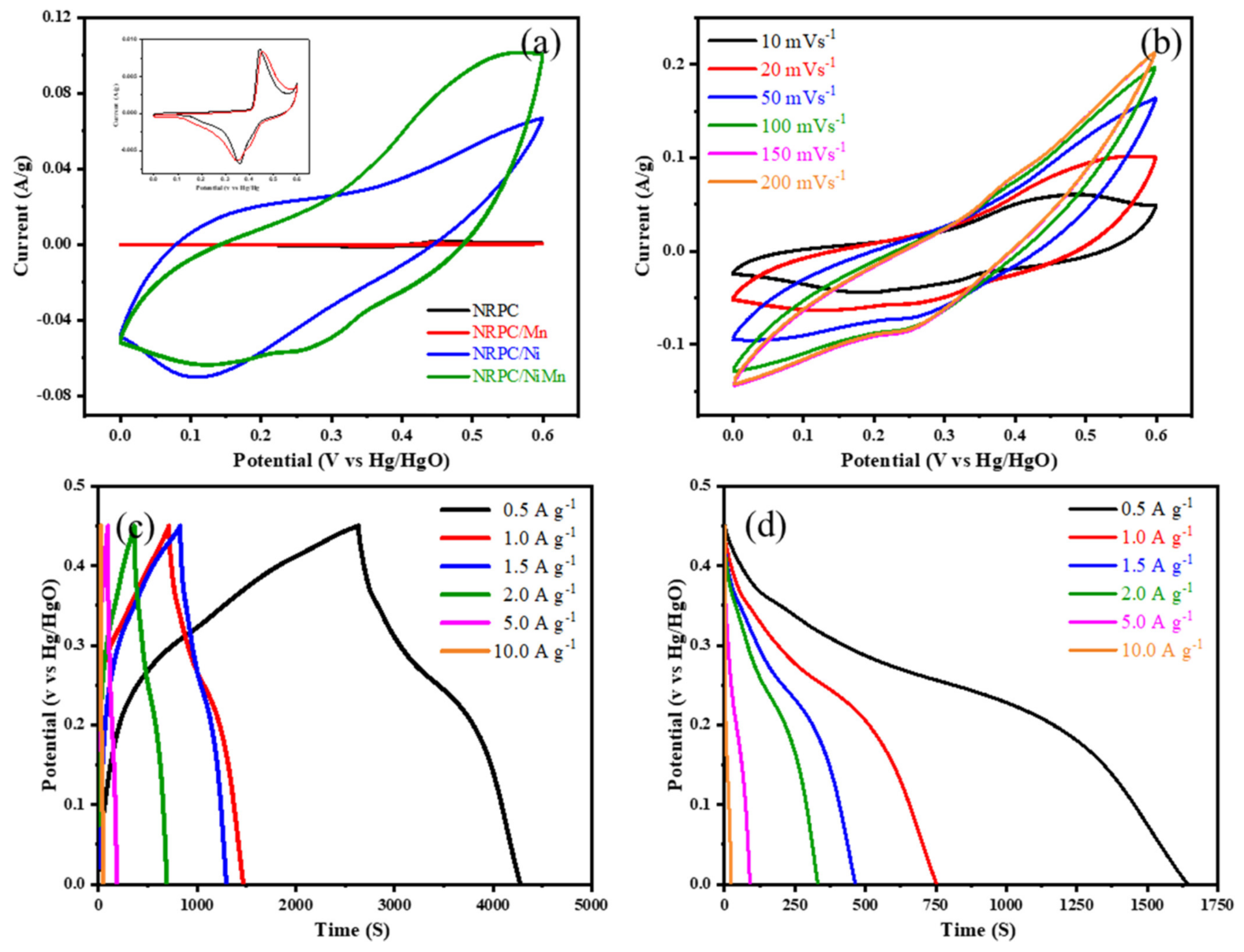
4.5. Electrochemical Aspects of Nitrogen-Rich Porous Carbon/NiMn Hybrids for Supercapacitors
4.6. Electrochemical Aspects Upcycling Polybenzoxazine Thermosets into N-Doped Carbon for Supercapacitors
4.7. Electrochemical Aspects of Enhanced Electrochemical Performance of HC/NiCo@800C Using Redox-Active Electrolytes
4.8. Electrochemical Aspects of Controllable Synthesis of Bifunctional Porous Carbon for Supercapacitors
4.9. Electrochemical Aspects of Development of Aryl Ether-Free Cross-Linked Polymer Membranes for Supercapacitors
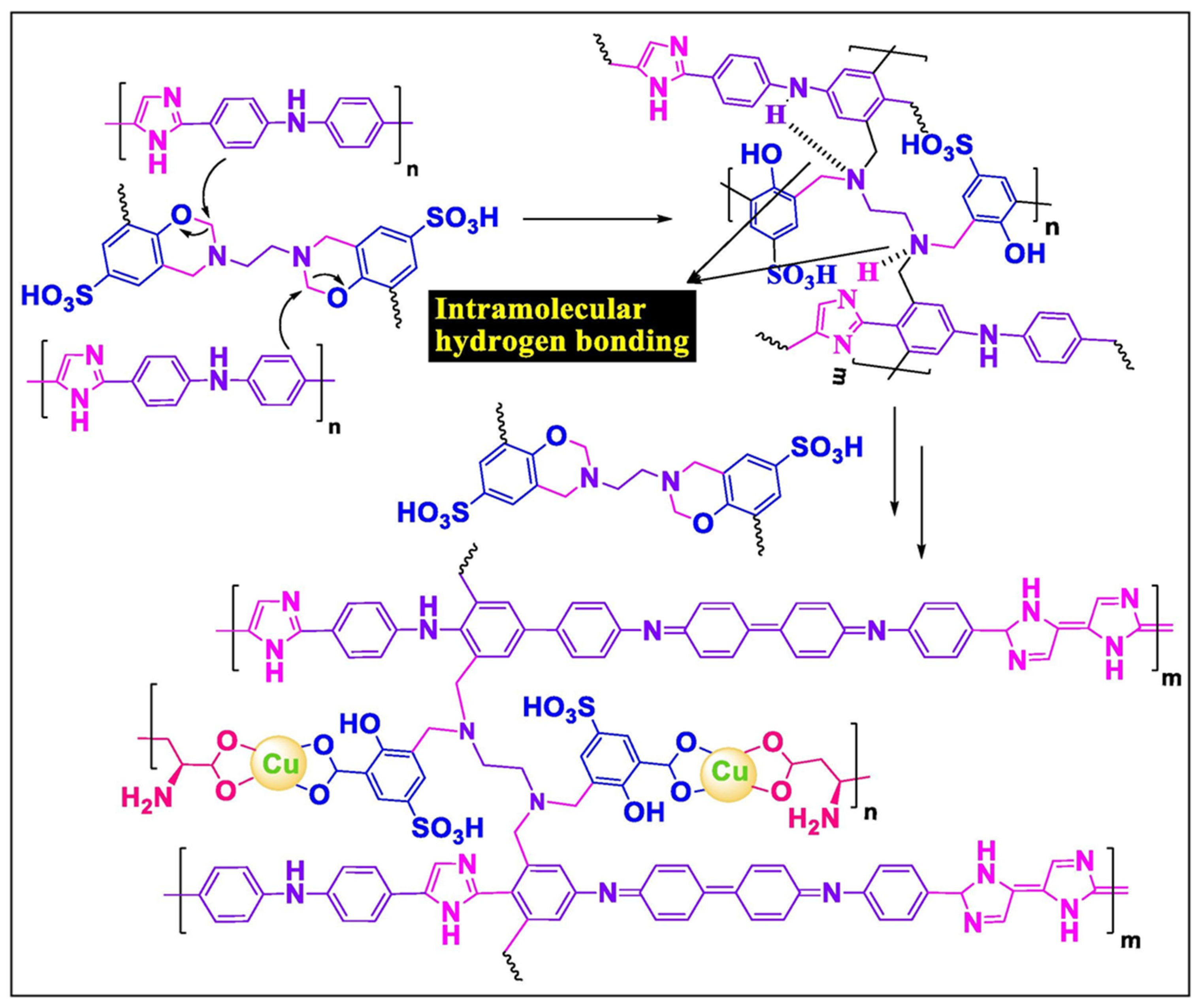
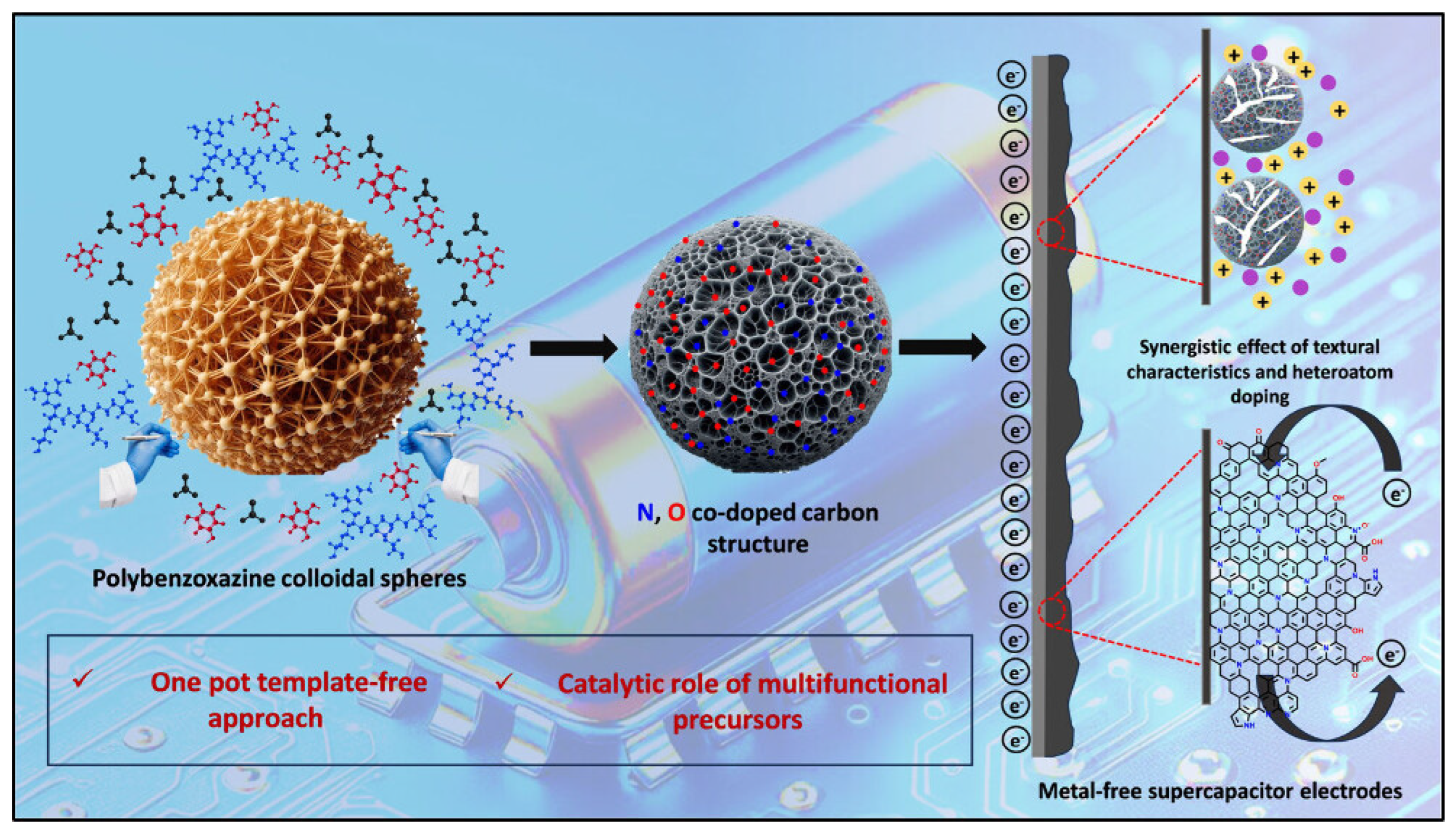
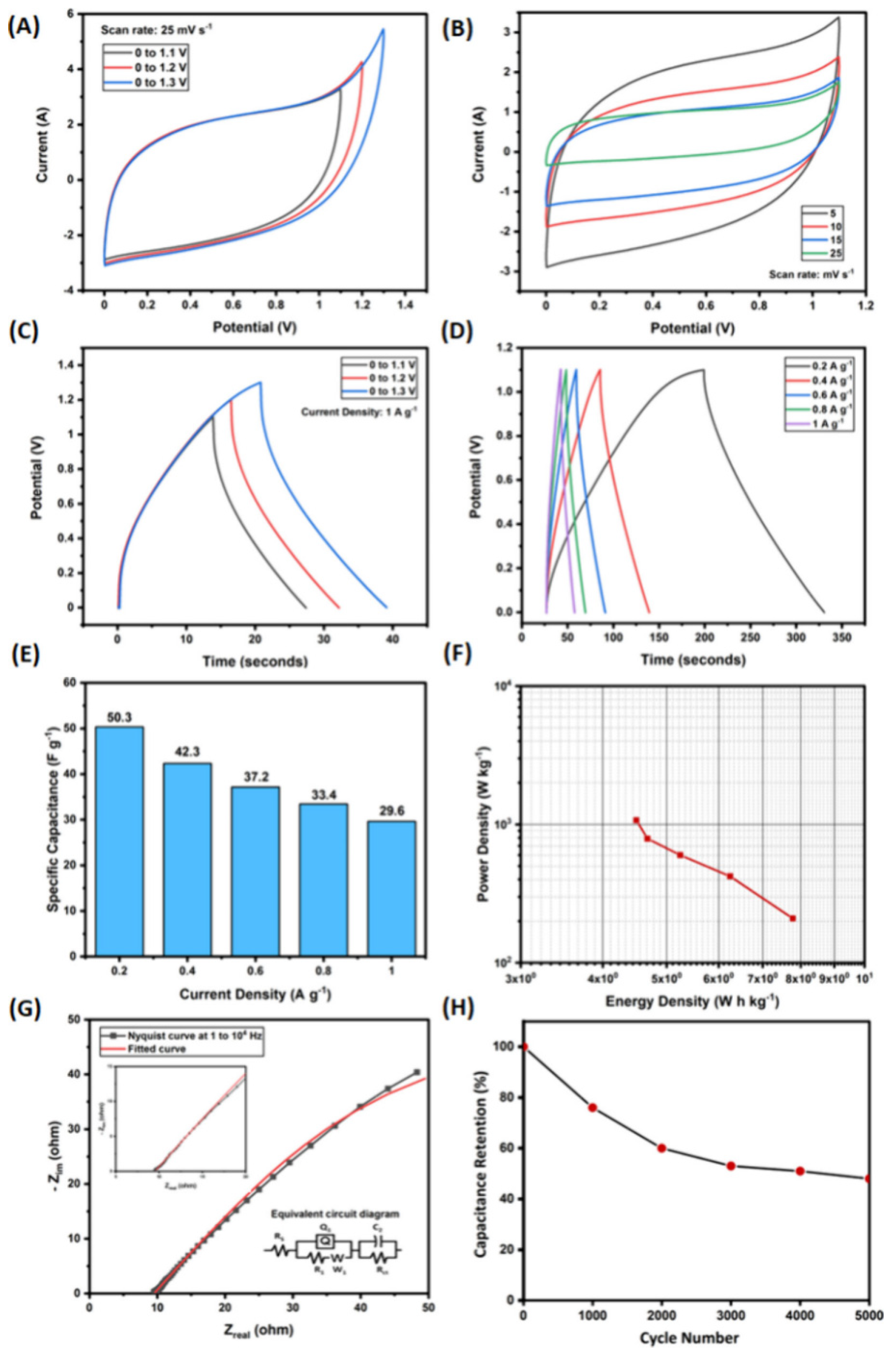
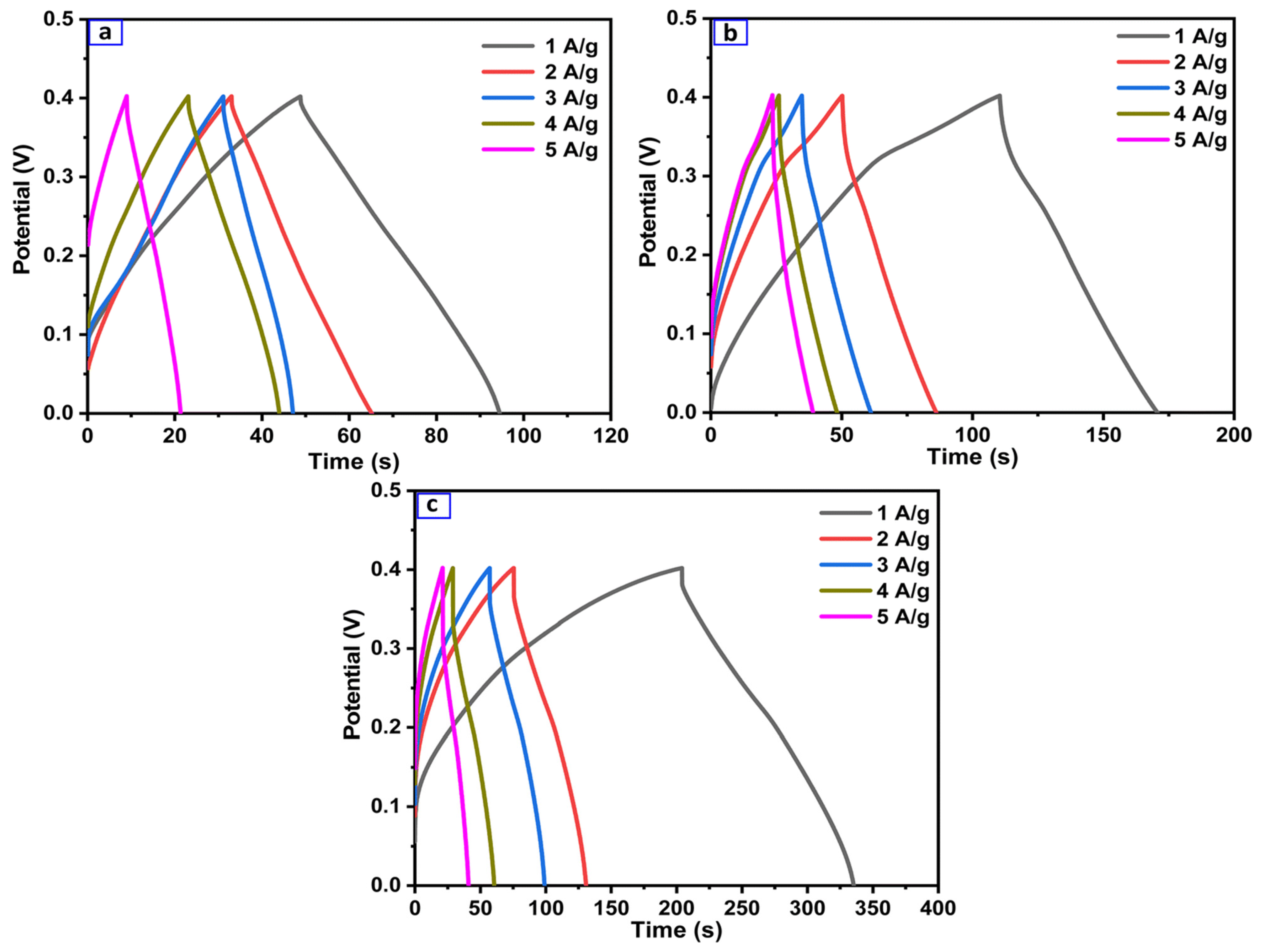
4.10. Electrochemical Aspects of Heteroatom-Enriched Carbon Particles for Supercapacitors
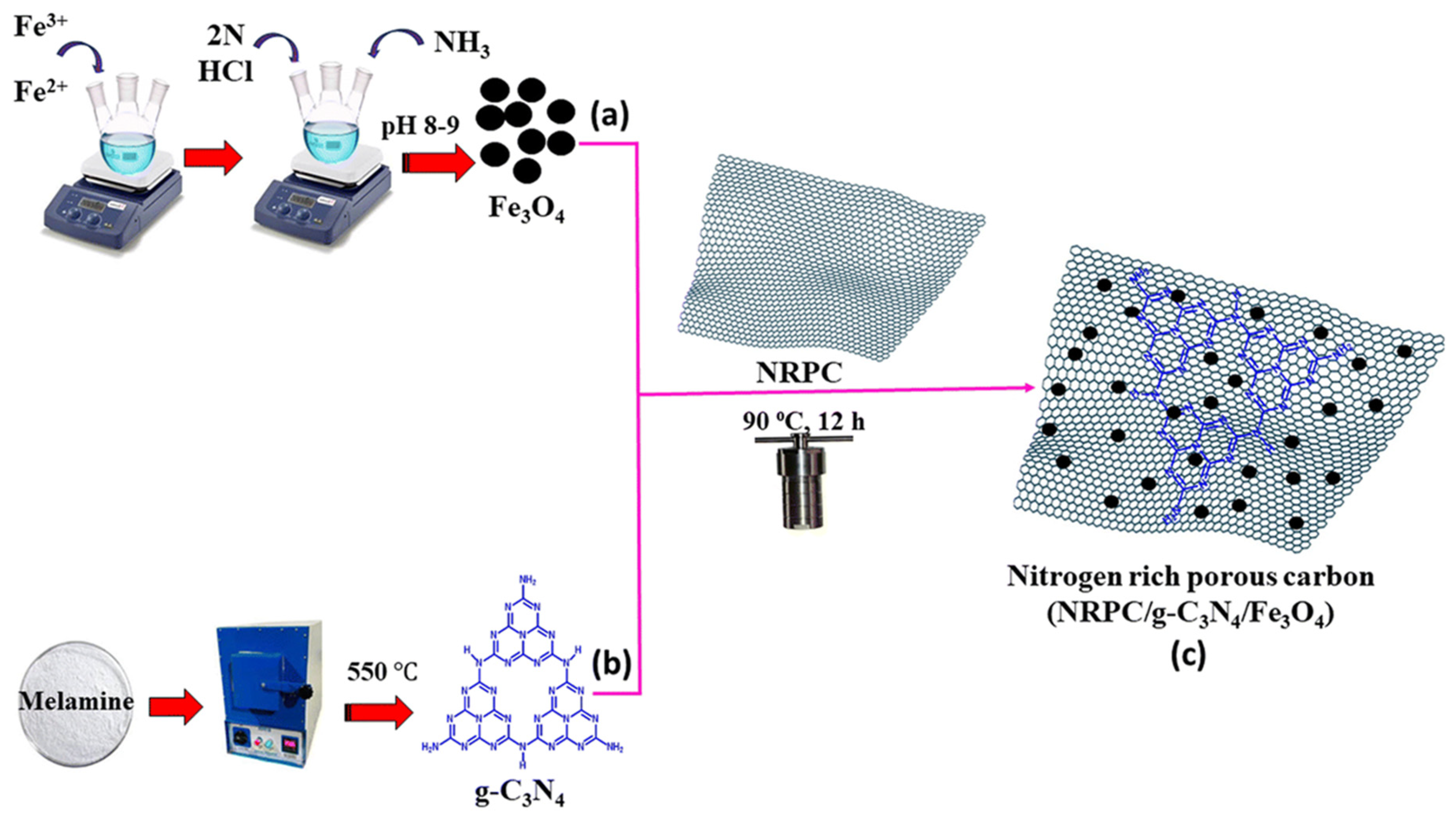
4.11. Electrochemical Aspects of NRPC-Supported G-C3N4/Fe3O4 for Supercapacitors
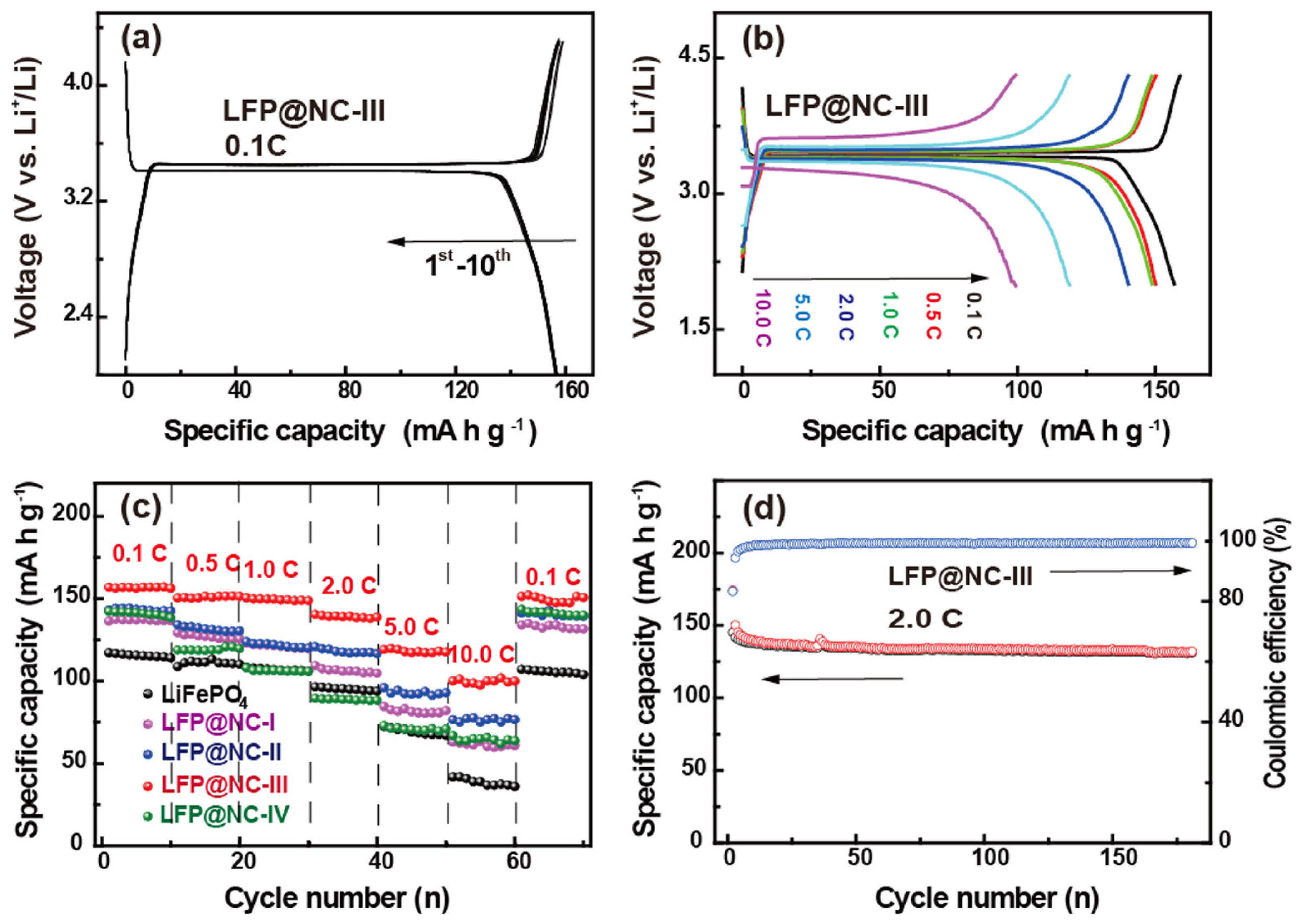
4.12. Electrochemical Aspects of LiFePO4@N-Doped Carbon for Lithium-Ion Batteries
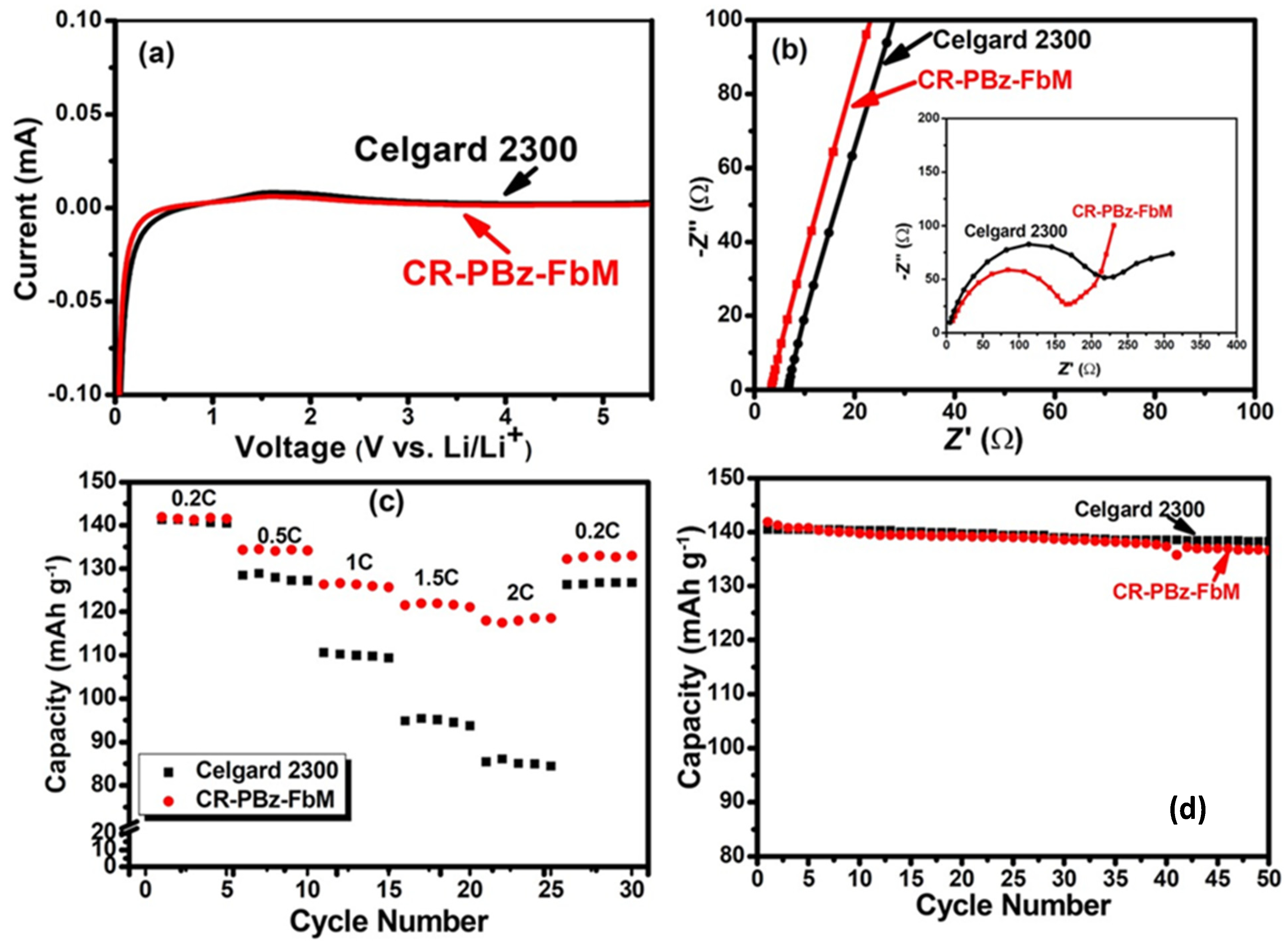
4.13. High-Ion-Conductivity Separator for Li-Ion Batteries Using Cross-Linked Polybenzoxazine
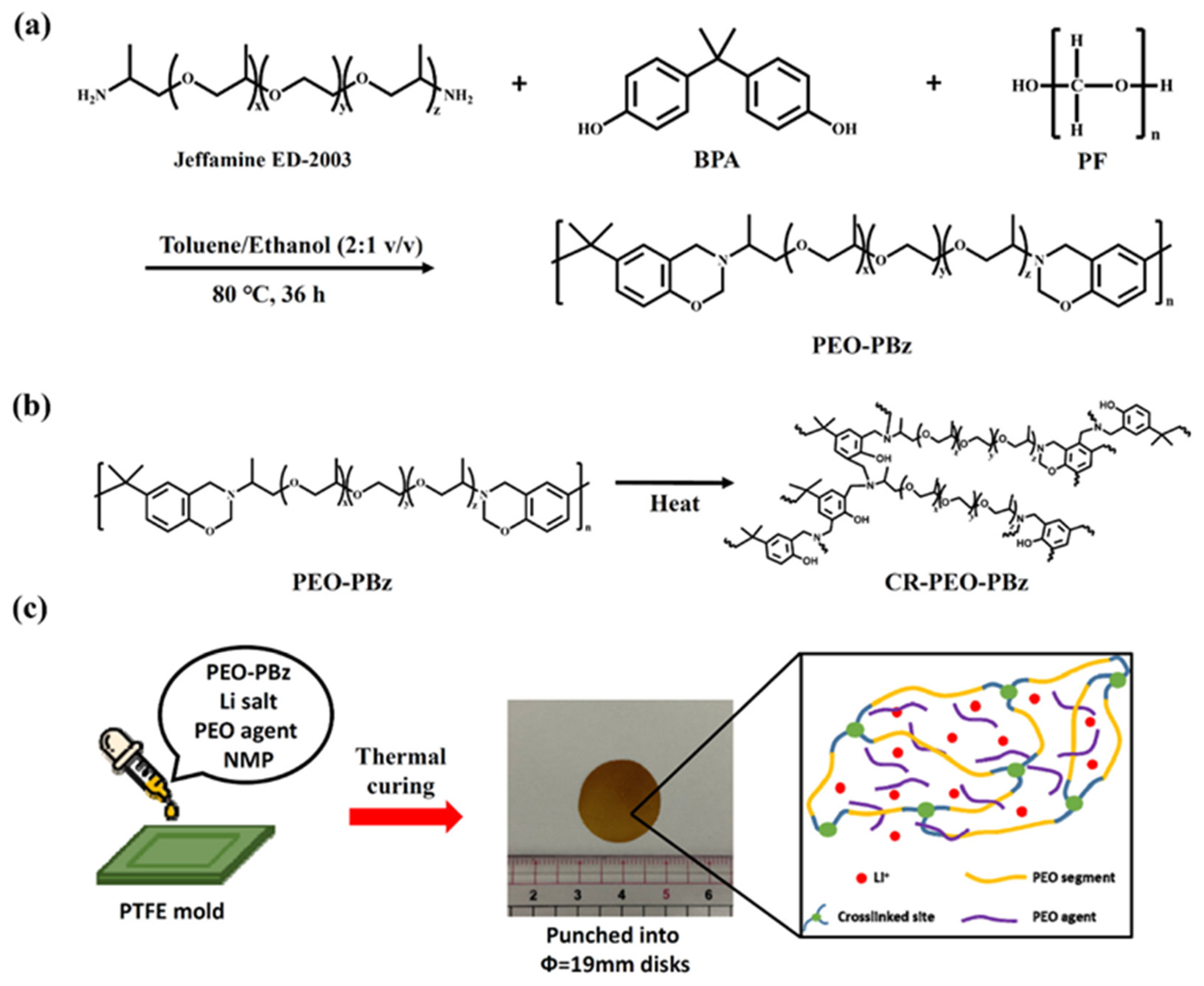
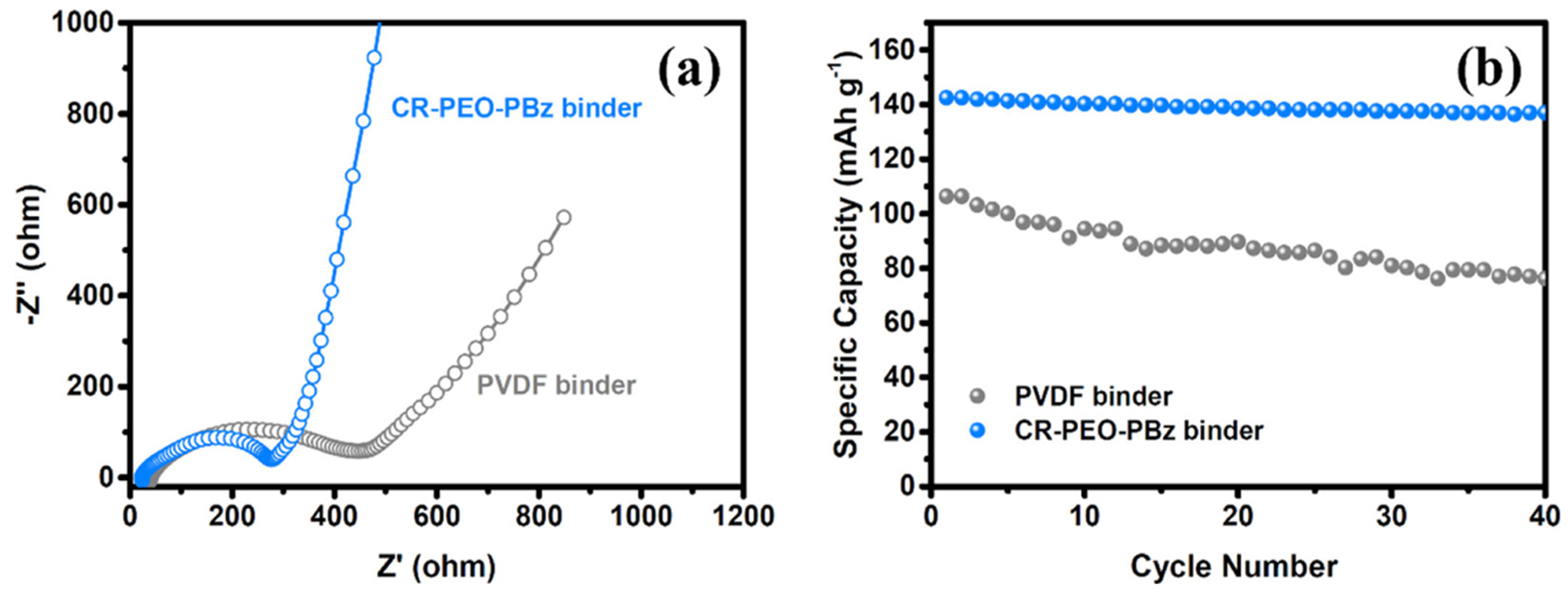
4.14. Cross-Linked Polybenzoxazine Electrolytes for Enhanced Lithium Battery Cycling
5. Conclusions and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Geim, A.K.; Novoselov, K. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [PubMed]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nat. Cell Biol. 1985, 318, 162–163. [Google Scholar]
- Osawa, E.; Kroto, H.W.; Fowler, P.W.; Wasserman, E. The evolution of the football structure for the C60 molecule: A retrospective. Philos. Trans. R. Soc. London Ser. A Phys. Eng. Sci. 1993, 343, 8. [Google Scholar]
- Zhou, Y.; Chen, G.; Zhang, J. A review of advanced metal-free carbon catalysts for oxygen reduction reactions towards the selective generation of hydrogen peroxide. J. Mater. Chem. A 2020, 8, 20849–20869. [Google Scholar]
- Tiwari, A. Advanced Carbon Materials and Technology; Wiley-Scrivener: Beverly, MA, USA, 2013. [Google Scholar]
- Paul, R.; Dai, L. Interfacial aspects of carbon composites. Compos. Interfaces 2018, 25, 539–605. [Google Scholar]
- McCreery, R.L. Advanced Carbon Electrode Materials for Molecular Electrochemistry. Chem. Rev. 2008, 108, 2646–2687. [Google Scholar]
- Feng, L.; Zhang, W.X. The structure and magnetism of graphone. AIP Adv. 2012, 2, 042138. [Google Scholar]
- Gaddam, R.R.; Kumar, N.A.; Narayan, R.; Raju, K.; Zhao, X. Advanced carbon materials for electrochemical energy storage. Nanomater. Synth. 2019, 2019, 385–418. [Google Scholar]
- Ghosh, N.; Kiskan, B.; Yagci, Y. Polybenzoxazines—New high performance thermosetting resins: Synthesis and properties. Prog. Polym. Sci. 2007, 32, 1344–1391. [Google Scholar] [CrossRef]
- Ishida, H. Overview and historical background of polybenzoxazine research. In Handbook of Benzoxazine Resins; Ishida, H., Agag, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 3–81. [Google Scholar]
- Ishida, H.; Froimowicz, P. (Eds.) Advanced and Emerging Polybenzoxazine Science and Technology; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Khan, J.; Momin, S.A.; Mariatti, M. A review on advanced carbon-based thermal interface materials for electronic devices. Carbon 2020, 168, 65–112. [Google Scholar]
- Kiskan, B. Adapting benzoxazine chemistry for unconventional applications. React. Funct. Polym. 2018, 129, 76–88. [Google Scholar] [CrossRef]
- Lyu, Y.; Ishida, H. Natural-sourced benzoxazine resins, homopolymers, blends and composites: A review of their synthesis, manufacturing and applications. Prog. Polym. Sci. 2019, 99, 101168. [Google Scholar]
- Antil, B.; Elkasabi, Y.; Strahan, G.D.; Wal, R.L.V. Development of Graphitic and Non-Graphitic Carbons Using Different Grade Biopitch Sources. Carbon 2025, 232, 119770. [Google Scholar] [CrossRef]
- Asrafali, S.P.; Periyasamy, T.; Lee, J. High-Performance Supercapacitors Using Compact Carbon Hydrogels Derived from Polybenzoxazine. Gels 2024, 10, 509. [Google Scholar] [CrossRef]
- Shah, S.S.; Aziz, M.A.; Marzooqi, M.A.; Khan, A.Z.; Yamani, Z.H. Enhanced Light-Responsive Supercapacitor Utilizing BiVO4 and Date Leaves-Derived Carbon: A Leap towards Sustainable Energy Harvesting and Storage. J. Power Sources 2024, 602, 234334. [Google Scholar] [CrossRef]
- Zhen, H.; Yang, H.; Wang, M.; Lu, G.; Liu, Y.; Run, M. Cyclo-oligomerization of hydroxyl-containing mono-functional benzox azines: A mechanism for oligomer formation. Polym. Chem. 2020, 11, 2325–2331. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Ishida, H. Polymerization of an AB-type benzoxazine monomer toward different polybenzoxazine networks: When diels–alder reaction meets benzoxazine chemistry in a single-component resin. Macromolecules 2019, 52, 7386–7395. [Google Scholar] [CrossRef]
- Wang, Y.; Gawryla, M.D.; Schiraldi, D.A. Effects of freezing conditions on the morphology and mechanical properties of clay and polymer/clay aerogels. J. Appl. Polym. Sci. 2013, 129, 1637–1641. [Google Scholar] [CrossRef]
- Trybuła, D.; Marszałek-Harych, A.; Gazińska, M.; Berski, S.; Jedrzkiewicz, D.; Ejfler, J. N-Activated 1,3-Benzoxazine Monomer as a KeyAgent in Polybenzoxazine Synthesis. Macromolecules 2020, 53, 8202–8215. [Google Scholar] [CrossRef] [PubMed]
- Sini, N.K.; Endo, T. Toward Elucidating the Role of Number of Oxazine Rings and Intermediates in the Benzoxazine Backbone on Their Thermal Characteristics. Macromolecules 2016, 49, 8466–8478. [Google Scholar]
- Ohashi, S.; Ishida, H. Various synthetic methods of benzoxazine monomers. In Advanced and Emerging Polybenzoxazine Science and Technology; Ishida, H., Froimowicz, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3–8. [Google Scholar]
- Kistler, S.S. Coherent Expanded Aerogels and Jellies. Nat. Cell Biol. 1931, 127, 741. [Google Scholar]
- Han, L.; Salum, M.L.; Zhang, K.; Froimowicz, P.; Ishida, H. Intrinsic self-initiating thermal ring-opening polymerization of 1,3-benzoxazines without the influence of impurities using very high purity crystals. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3434–3445. [Google Scholar]
- Fung, A.; Wang, Z.; Lu, K.; Dresselhaus, M.; Pekala, R. Characterization of carbon aerogels by transport measurements. J. Mater. Res. 1993, 8, 1875–1885. [Google Scholar]
- Cui, S.; Arza, C.R.; Froimowicz, P.; Ishida, H. Developing further versatility in benzoxazine synthesis via hydrolytic ring-opening. Polymers 2020, 12, 694. [Google Scholar] [CrossRef]
- Cheng, Z.; DeGracia, K.; Schiraldi, D.A. Sustainable, low flammability, mechanically-strong poly(vinyl alcohol) aerogels. Polymers 2018, 10, 1102. [Google Scholar] [CrossRef]
- Lorjai, P.; Chaisuwan, T.; Wongkasemjit, S. Porous structure of polybenzoxazine-based organic aerogel prepared by sol–gel process and their carbon aerogels. J. Sol-Gel Sci. Technol. 2009, 52, 56–64. [Google Scholar]
- Mahadik-Khanolkar, S.; Donthula, S.; Sotiriou-Leventis, C.; Leventis, N. Polybenzoxazine aerogels. 1. High-yield room temperature acid-catalyzed synthesis of robust monoliths, oxidative aromatization, and conversion to microporous carbons. Chem. Mater. 2014, 26, 1303–1317. [Google Scholar]
- Sun, M.; Sun, H.; Wang, Y.; Sanchez-Soto, M.; Schiraldi, D.A. The relation between the rheological properties of gels and the mechanical properties of their corresponding aerogels. Gels 2018, 4, 33. [Google Scholar] [CrossRef]
- Thubsuang, U.; Ishida, H.; Wongkasemjit, S.; Chaisuwan, T. Self-formation of 3D interconnected macroporous carbon xerogels derived from polybenzoxazine by selective solvent during the sol–gel process. J. Mater. Sci. 2014, 49, 4946–4961. [Google Scholar] [CrossRef]
- Haozhe, X.; Haibo, F.; Ya, L. Preparation and Characterization of Carbon Aerogel Based on MainChain Benzoxazine. ACS Appl. Eng. Mater. 2024, 2, 116–125. [Google Scholar]
- Tiwari, I.; Tanwar, V.; Ingole, P.P.; Nebhani, L. Heteroatom-Enriched Carbon Particles Derived from Multifunctional Polybenzoxazine Particles for High-Performance Supercapacitors. ACS Appl. Energy Mater. 2024, 7, 7185–7204. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, H.; Zhan, G.; Liu, X.; Yang, Y.; Zhuang, Q.; Qian, J. Preparing Multifunctional High-Performance Cross-Linked Polybenzoxazole Aerogels from Polybenzoxazine. ACS Appl. Polym. Mater. 2021, 3, 2352–2362. [Google Scholar] [CrossRef]
- Si, Y.; Ren, T.; Li, Y.; Ding, B.; Yu, J. Fabrication of magnetic polybenzoxazine-based carbon nanofibers with Fe3O4 inclusions with a hierarchical porous structure for water treatment. Carbon 2012, 50, 5176–5185. [Google Scholar] [CrossRef]
- Thubsuang, U.; Ishida, H.; Wongkasemjit, S.; Chaisuwan, T. Advanced and economical ambient drying method for controlled mesopore polybenzoxazine-based carbon xerogels: Effects of non-ionic and cationic surfactant on porous structure. J. Colloid Interface Sci. 2015, 459, 241–249. [Google Scholar] [CrossRef]
- Zhu, Y.; Jiang, Y.; Lin, R.; Yu, S. Research on thermal degradation process of p-nitrophenol-based polybenzoxazine. Polym. Degrad. Stab. 2017, 141, 1–10. [Google Scholar] [CrossRef]
- Zhu, Y.; Gu, Y. Effect of interaction between transition metal oxides and nitrogen atoms on thermal stability of polybenzoxazine. J. Macromol. Sci. Part B 2011, 50, 1130–1143. [Google Scholar]
- Speight, J.G. Industrial Organic Chemistry. In Environmental Organic Chemistry for Engineers; Elsevier: Amsterdam, The Netherlands, 2017; pp. 87–151. [Google Scholar]
- Low, H.Y.; Ishida, H. Structural effects of phenols on the thermal and thermo-oxidative degradation of polybenzoxazines. Polymer 1999, 40, 4365–4376. [Google Scholar]
- Low, H.Y.; Ishida, H. Improved thermal stability of polybenzoxazines by transition metals. Polym. Degrad. Stab. 2006, 91, 805–815. [Google Scholar]
- Low, H.Y.; Ishida, H. An investigation of the thermal and thermo-oxidative degradation of polybenzoxazines with a reactive functional group. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 647–659. [Google Scholar]
- Liu, Y.; Zhang, H.; Wang, M.; Liao, C.; Zhang, J. Thermal degradation behavior and mechanism of polybenzoxazine based on bisphenol-S and methylamine. J. Therm. Anal. Calorim. 2013, 112, 1213–1219. [Google Scholar]
- Liu, Y.; Yue, Z.; Li, Z.; Liu, Z. Thermal degradation behavior and kinetics of polybenzoxazine based on bisphenol-S and aniline. Thermochim. Acta 2011, 523, 170–175. [Google Scholar]
- Huang, S.; Gu, J.; Ye, J.; Fang, B.; Wan, S.; Wang, C.; Ashraf, U.; Li, Q.; Wang, X.; Shao, L.; et al. Benzoxazine monomer derived carbon dots as a broad-spectrum agent to block viral infectivity. J. Colloid Interface Sci. 2019, 542, 198–206. [Google Scholar]
- Hemvichian, K.; Laobuthee, A.; Chirachanchai, S.; Ishida, H. Thermal decomposition processes in polybenzoxazine model dimers investigated by TGA–FTIR and GC–MS. Polym. Degrad. Stab. 2002, 76, 1–15. [Google Scholar]
- Hemvichian, K.; Ishida, H. Thermal decomposition processes in aromatic amine-based polybenzoxazines investigated by TGA and GC–MS. Polymer 2002, 43, 4391–4402. [Google Scholar]
- Hamerton, I.; Thompson, S.; Howlin, B.J.; Stone, C.A. New method to predict the thermal degradation behavior of polybenzox azines from empirical data using structure property relationships. Macromolecules 2013, 46, 7605–7615. [Google Scholar]
- Fang, B.; Lu, X.; Hu, J.; Zhang, G.; Zheng, X.; He, L.; Cao, J.; Gu, J.; Cao, F. pH controlled green luminescent carbon dots derived from benzoxazine monomers for the fluorescence turn-on and turn-off detection. J. Colloid Interface Sci. 2019, 536, 516–525. [Google Scholar]
- Bagherifam, S.; Uyar, T.; Ishida, H.; Hacaloglu, J. The use of pyrolysis mass spectrometry to investigate polymerization and degradation processes of methyl amine-based benzoxazine. Polym. Test. 2010, 29, 520–526. [Google Scholar]
- Zhao, J.; Gilani, M.R.H.S.; Lai, J.; Nsabimana, A.; Liu, Z.; Luque, R.; Xu, G. Autocatalysis Synthesis of Poly(Benzoxazine- Co-Resol)-Based Polymer and Carbon Spheres. Macromolecules 2018, 51, 5494–5500. [Google Scholar]
- Zhang, R.; Wang, L.; Zhao, J.; Guo, S. Effects of Sodium Alginate on the Composition, Morphology, and Electrochemical Properties of Electrospun Carbon Nanofibers as Electrodes for Supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 632–640. [Google Scholar]
- Zhao, J.; Niu, W.; Zhang, L.; Cai, H.; Han, M.; Yuan, Y.; Majeed, S.; Anjum, S.; Xu, G. A Template-Free and Surfactant-Free Method for High-Yield Synthesis of Highly Monodisperse 3- Aminophenol-Formaldehyde Resin and Carbon Nano/Microspheres. Macromolecules 2013, 46, 140–145. [Google Scholar]
- Xue, D.; Zhu, D.; Xiong, W.; Cao, T.; Wang, Z.; Lv, Y.; Li, L.; Liu, M.; Gan, L. Template-Free, Self-Doped Approach to Porous Carbon Spheres with High N/O Contents for High-Performance Supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 7024–7034. [Google Scholar]
- Cheng, H.; Yi, F.; Gao, A.; Liang, H.; Shu, D.; Zhou, X.; He, C.; Zhu, Z. Supermolecule Self-Assembly Promoted Porous N, P Co-Doped Reduced Graphene Oxide for High Energy Density Supercapacitors. ACS Appl. Energy Mater. 2019, 2, 4084–4091. [Google Scholar]
- Chen, Z.; Chen, Y.; Wang, Q.; Yang, T.; Luo, Q.; Gu, K.; Yang, W. Molecularly-Regulating Oxygen-Containing Functional Groups of Ramie Activated Carbon for High-Performance Supercapacitors. J. Colloid Interface Sci. 2024, 665, 772–779. [Google Scholar]
- Si, Y.; Ren, T.; Ding, B.; Yu, J.; Sun, G. Synthesis of mesoporous magnetic Fe3O4@carbon nanofibers utilizing in situ polymerized polybenzoxazine for water purification. J. Mater. Chem. 2012, 22, 4619–4622. [Google Scholar]
- Huang, Z.; Pan, Q.; Smith, D.M.; Li, C.Y. Plasticized Hybrid Network Solid Polymer Electrolytes for Lithium-Metal Batteries. Adv. Mater. Interfaces 2019, 6, 1801445. [Google Scholar]
- Zhang, J.; Li, J.-Y.; Wang, W.-P.; Zhang, X.-H.; Tan, X.-H.; Chu, W.-G.; Guo, Y.-G. Microemulsion Assisted Assembly of 3D Porous S Graphene G-C3N4 Hybrid Sponge as. Adv. Energy Mater. 2018, 8, 1702839. [Google Scholar]
- Periyasamy, T.; Asrafali, S.P.; Kim, S.C. Nitrogen-Rich Porous Carbon/NiMn Hybrids as Electrode Materials for High-Performance Supercapacitors. ACS Appl. Energy Mater. 2022, 5, 15605–15614. [Google Scholar]
- Shaer, C.; Oppenheimer, L.; Lin, A.; Ishida, H. Advanced Carbon Materials Derived from Polybenzoxazines: A Review. Polymers 2021, 13, 3775. [Google Scholar] [CrossRef]
- Ma, X.; Ning, G.; Qi, C.; Xu, C.; Gao, J. Phosphorus and Nitrogen Dual-Doped Few-Layered Porous Graphene: A High-Performance Anode Material for Lithium-Ion. Batteries 2014, 6, 14415–14422. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Tanwar, V.; Tiwari, I.; Ingole, P.P.; Nebhani, L. Sustainable Upcycling of Nitrogen-Enriched Polybenzoxazine Thermosets into Nitrogen-Doped Carbon Materials for Contriving High-Performance Supercapacitors. Energy Fuels 2023, 37, 7445–7467. [Google Scholar] [CrossRef]
- Liu, J.; Ishida, H. Anomalous Isomeric Effect on the Properties of Bisphenol F-Based Benzoxazines: Toward the Molecular Design for Higher Performance. Macromolecules 2014, 47, 5682–5690. [Google Scholar] [CrossRef]
- Yu, Z.; Yu, W.; Jiang, Y.; Wang, Z.; Zhao, W.; Liu, X. Upcycling of Polybenzoxazine to Magnetic Metal Nanoparticle-Doped Laser-Induced Graphene for Electromagnetic Interference Shielding. ACS Appl. Nano Mater. 2022, 5, 13158–13170. [Google Scholar] [CrossRef]
- Asrafali, S.P.; Periyasamy, T.; Kim, S.C. Enhanced Electrochemical Performance of HC/NiCo@ 800 C//HC Using Redox-Active Electrolytes Showing Increased Energy Density. J. Alloys Compd. 2024, 972, 172753. [Google Scholar] [CrossRef]
- Zhuang, X.; Zhang, F.; Wu, D.; Zhuang, X.F. Graphene Coupled Schiff-base Porous Polymers Towards Nitrogen-enriched Porous Carbon. Adv. Mater. 2014, 26, 3081–3086. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, P.; Liu, L.; Zhang, Y.; Yang, J.; Zeng, Z.; Deng, S. Controllable Synthesis of Bifunctional Porous Carbon for Efficient Gas-Mixture Separation and High-Performance Supercapacitor. Chem. Eng. J. 2018, 348, 57–66. [Google Scholar]
- Cao, J.; Han, Y.; Zheng, X.; Wang, Q. Preparation and Electrochemical Performance of Modified Ti3C2Tx/Polypyrrole Composites. J. Appl. Polym. Sci. 2019, 136, 47003. [Google Scholar] [CrossRef]
- Murugan, E.; Munusamy, K.; Vallipparambil Babu, A. Development of Aryl Ether-Free Cross-Linked Polymer Membranes for Sustainable Electrochemical Energy Conversion and Storage Applications. Chem. Eng. J. 2024, 501, 157473. [Google Scholar]
- Devadas, B.; Imae, T. Effect of Carbon Dots on Conducting Polymers for Energy Storage Applications. ACS Sustain. Chem. Eng. 2018, 6, 127–134. [Google Scholar]
- Yao, Z.; Erhong, S.; Wei, C.; Carlo, U.S.; Zhou, J.; Yung-Chang, L.; Zhu, C.; Ma, R.; Liu, P.; Chu, S.; et al. Dual-Metal Interbonding as the Chemical Facilitator for Single-Atom Dispersions. Adv. Mater. 2020, 32, 2003484. [Google Scholar]
- Ertas, Y.; Uyar, T. Main-chain polybenzoxazine nanofibers via electrospinning. Polymer 2014, 55, 556–564. [Google Scholar]
- Ting, F.Y.; Lingna, S.X.H.; Fanfan, W.Y.Z.; Ying, X. Approaching High-Performance Lithium Storage Materials by Constructing. Energy Environ. Mater. 2021, 4, 586–595. [Google Scholar]
- Selvaraj, K.; Yu, B.; Spontón, M.E.; Kumar, P.; Veerasamy, U.S.; Arulraj, A.; Mangalaraja, R.V.; Almarhoon, Z.M.; Sayed, S.R.M.; Kannaiyan, D. Nonylphenol Polybenzoxazines-Derived Nitrogen-Rich Porous Carbon (NRPC)-Supported g-C3N4/Fe3O4 Nanocomposite for Efficient High-Performance Supercapacitor Application. Soft Matter 2024, 20, 7957–7969. [Google Scholar]
- Bheema, R.K.; Verma, A.; Bhaskaran, K.; Etika, K.C. A Review on Recent Progress in Polymer Composites for Effective Electromagnetic Interference Shielding Properties- Structures, Process, Sustainability Approaches. Nanoscale Adv. 2024, 6, 5773–5802. [Google Scholar]
- Zu, G.; Wang, X.; Kanamori, K.; Nakanishi, K. Superhydrophobic Highly Flexible Doubly Cross-Linked Aerogel/Carbon Nanotube Composites as Strain/Pressure Sensors. J. Mater. Chem. B 2020, 8, 4883–4889. [Google Scholar]
- Ananthanarayanan, A.; Wang, Y.; Routh, P.; Sk, M.A.; Than, A.; Lin, M.; Zhang, J.; Chen, J.; Sun, H.; Chen, P. Nitrogen and Phosphorus Co-Doped Graphene Quantum Dots: Synthesis from Adenosine Triphosphate, Optical Properties, and Cellular Imaging. Nanoscale 2015, 7, 8159–8165. [Google Scholar]
- Wang, P.; Zhang, G.; Li, Z.; Sheng, W.; Zhang, Y.; Gu, J.; Zheng, X.; Cao, F. Improved Electrochemical Performance of LiFePO4@N-Doped Carbon Nanocomposites Using Polybenzoxazine as Nitrogen and Carbon Sources. ACS Appl. Mater. Interfaces 2016, 8, 26908–26915. [Google Scholar]
- El-Shinawi, H.; Cussen, E.J.; Corr, S.A. Morphology-Directed Synthesis of LiFePO4 and LiCoPO4 from Nanostructured Li1+2XPO3+X. Inorg. Chem. 2019, 58, 6946–6949. [Google Scholar]
- Li, H.-Y.; LI, G.-A.; Lee, Y.-Y.; Tuan, H.-Y.; Liu, Y.-L. A Thermally Stable Combustion-Resistant and Highly Ion-Conductive Separator for Lithium-Ion. Energy Technol. 2016, 4, 551–557. [Google Scholar]
- Wu, Z.-S.; Winter, A.; Chen, L.; Sun, Y.; Turchanin, A.; Feng, X.; Müllen, K. Three-Dimensional Nitrogen and Boron Co-doped Graphene for High-Performance. Adv. Mater. 2012, 24, 5130–5135. [Google Scholar] [PubMed]
- Raul, D.; Shchadenko, R.S.; Murastov, G.; Lipovka, A.; Fatkullin, M.; Petrov, I.; Tran, T.-H.; Khalelov, A.; Saqib, M.; Villa, N.E.; et al. Ultra-Robust Flexible Electronics by Laser-Driven Polymer-Nanomaterials. Adv. Funct. Mater. 2021, 31, 2008818. [Google Scholar]
- Wang, T.C.; Tsai, C.Y.; Liu, Y.L. Solid Polymer Electrolytes Based on Cross-Linked Polybenzoxazine Possessing Poly(Ethylene Oxide) Segments Enhancing Cycling Performance of Lithium Metal Batteries. ACS Sustain. Chem. Eng. 2021, 9, 6274–6283. [Google Scholar]
- Krüner, B.; Schreiber, A.; Tolosa, A.; Quade, A.; Badaczewski, F.; Pfaff, T.; Smarsly, B.M.; Presser, V. Nitrogen-Containing Novolac-Derived Carbon Beads as Electrode Material for Supercapacitors. Carbon 2018, 132, 220–231. [Google Scholar]
- Zhao, N.; Wu, S.; He, C.; Shi, C.; Liu, E.; Du, X.; Li, J. Hierarchical Porous Carbon with Graphitic Structure Synthesized by a Water Soluble Template Method. Mater. Lett. 2012, 87, 77–79. [Google Scholar]
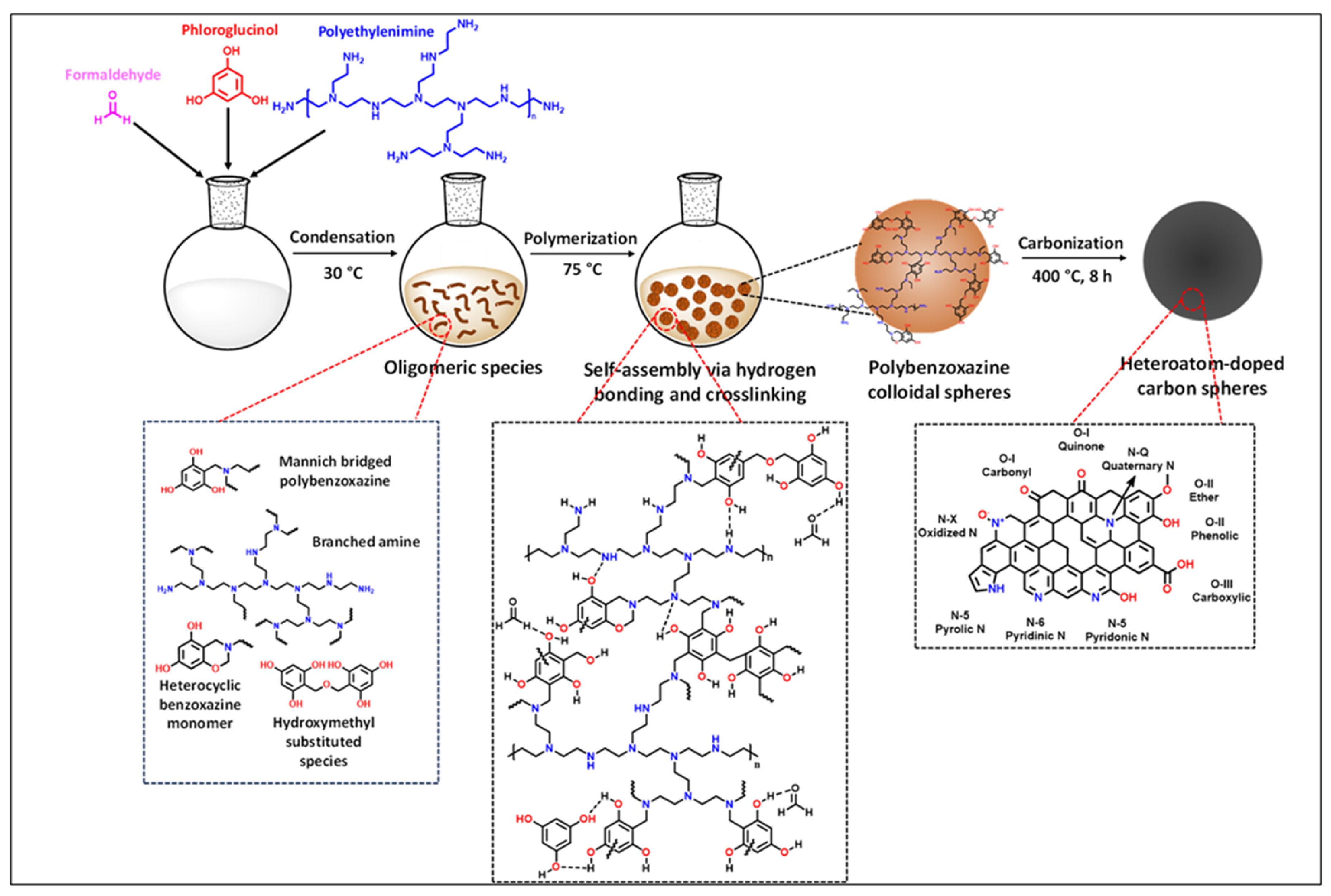
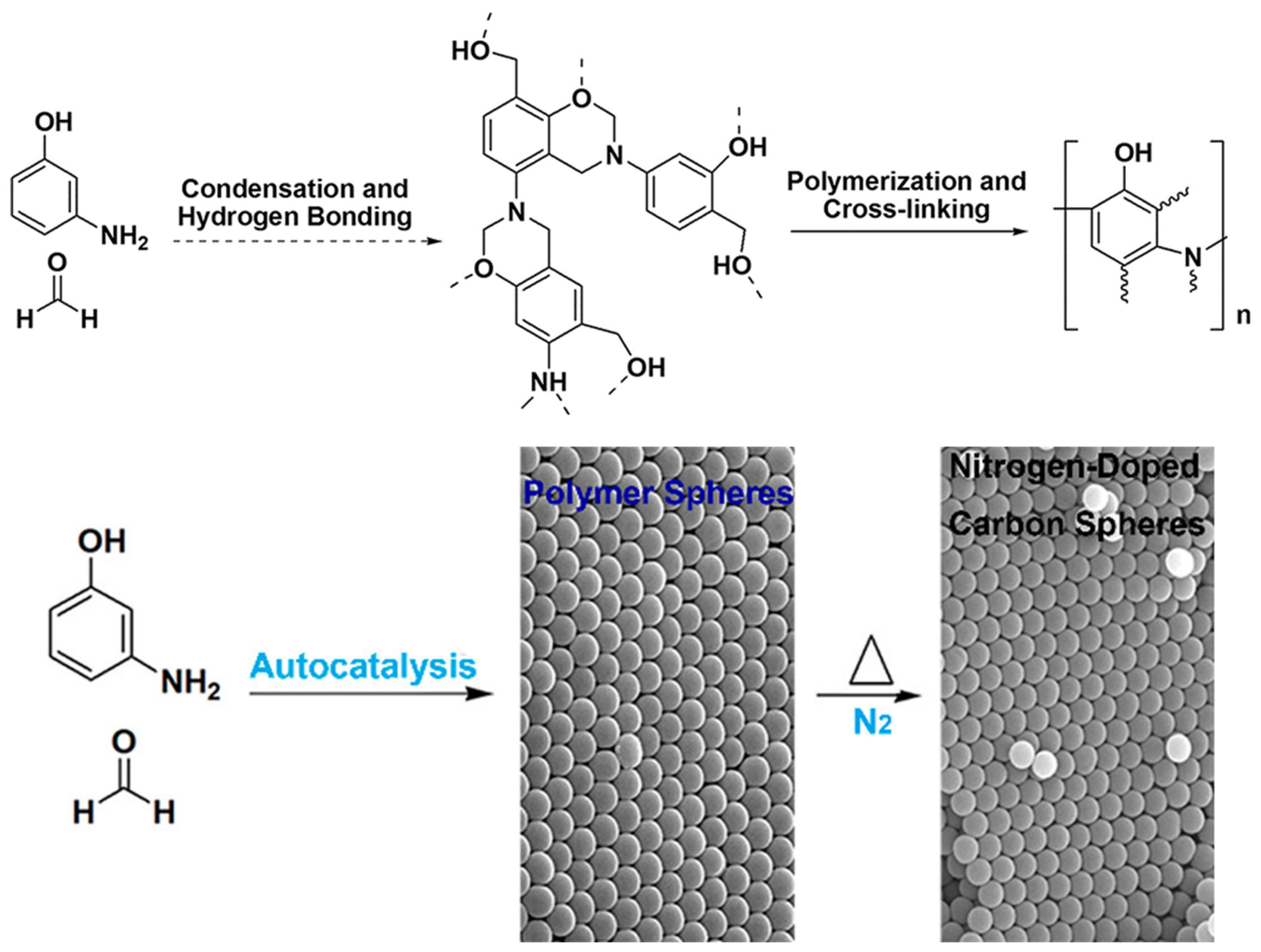

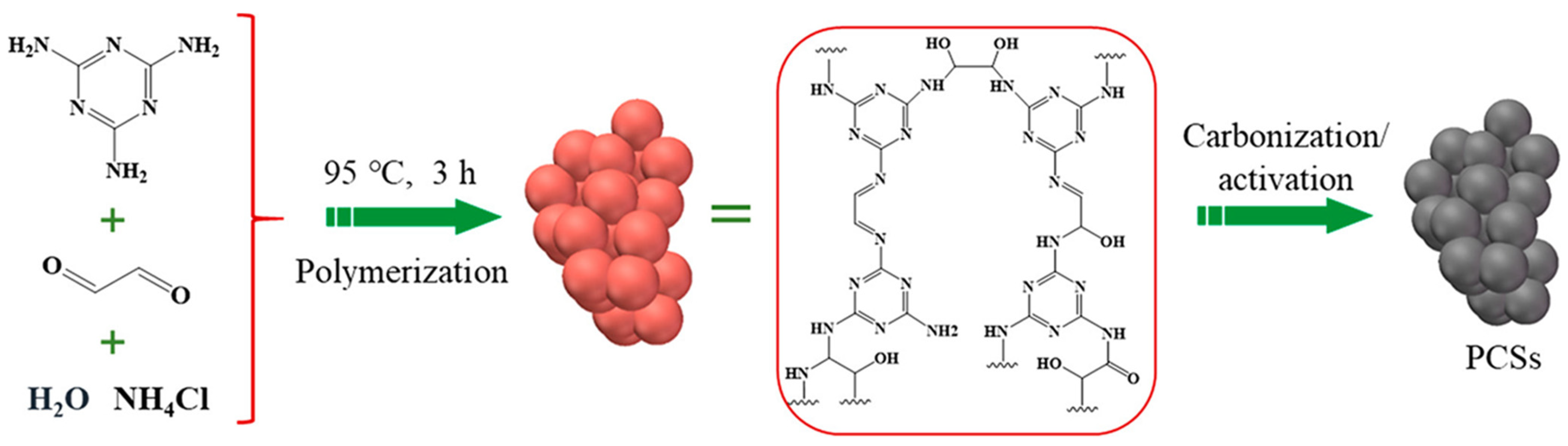

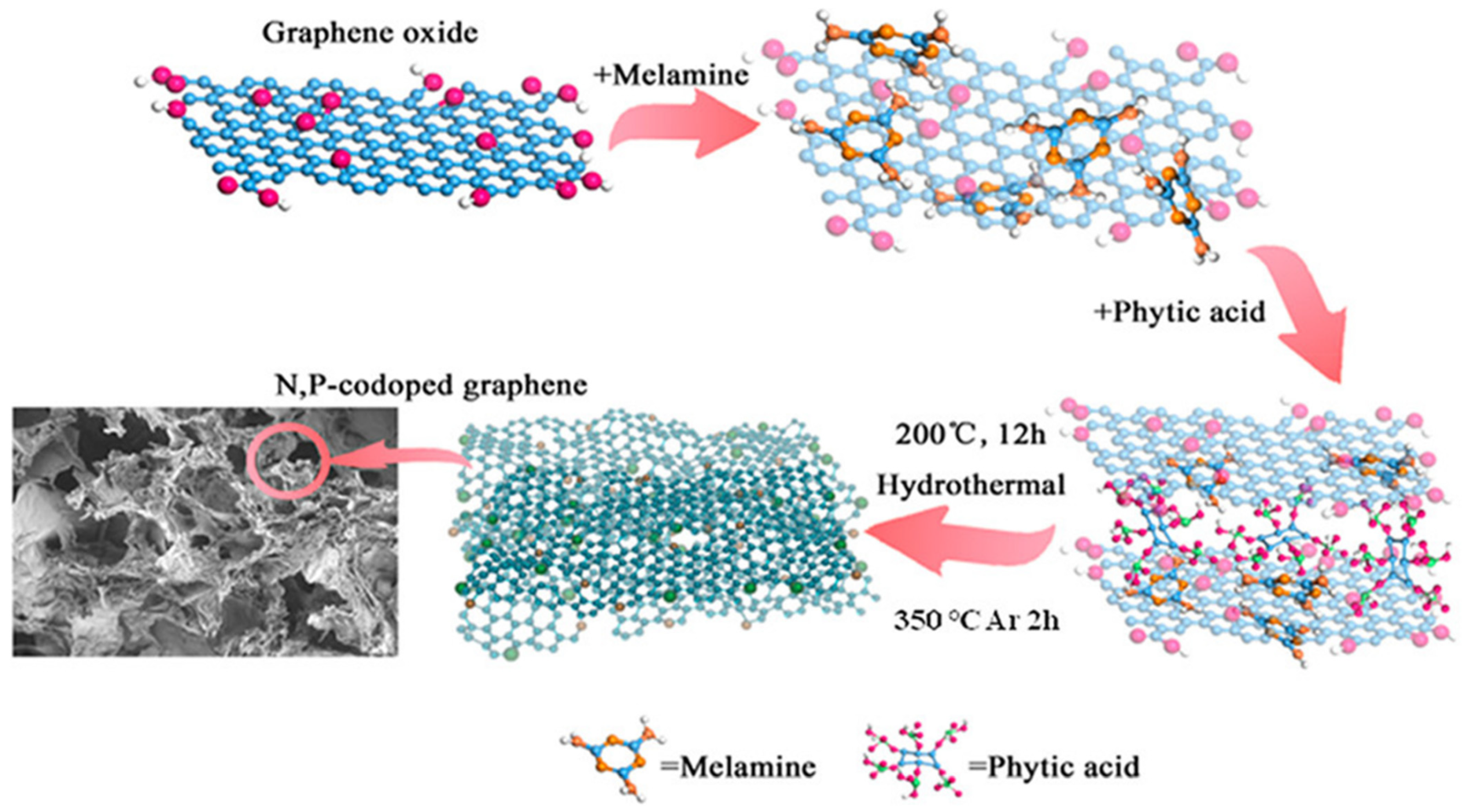



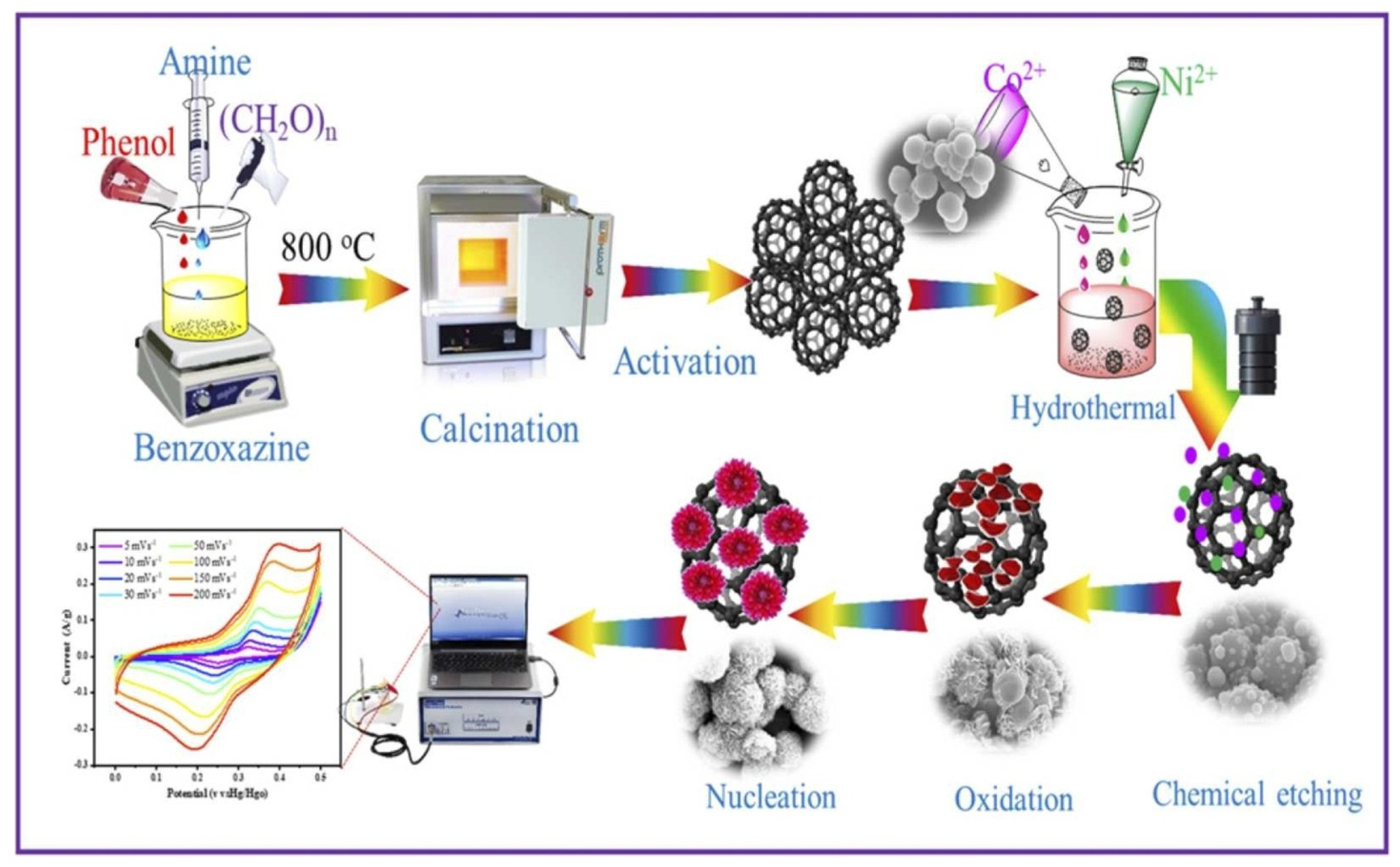
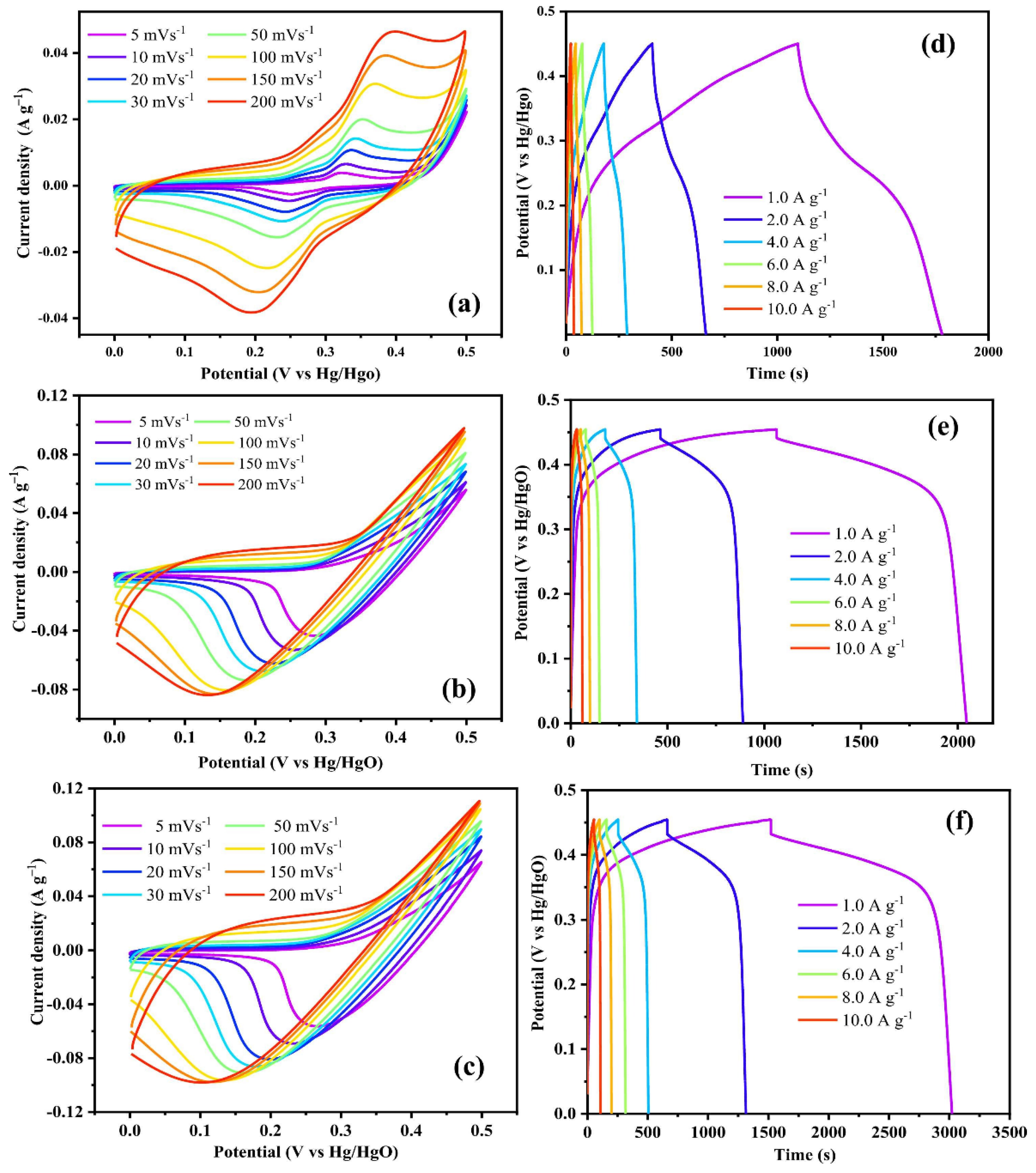
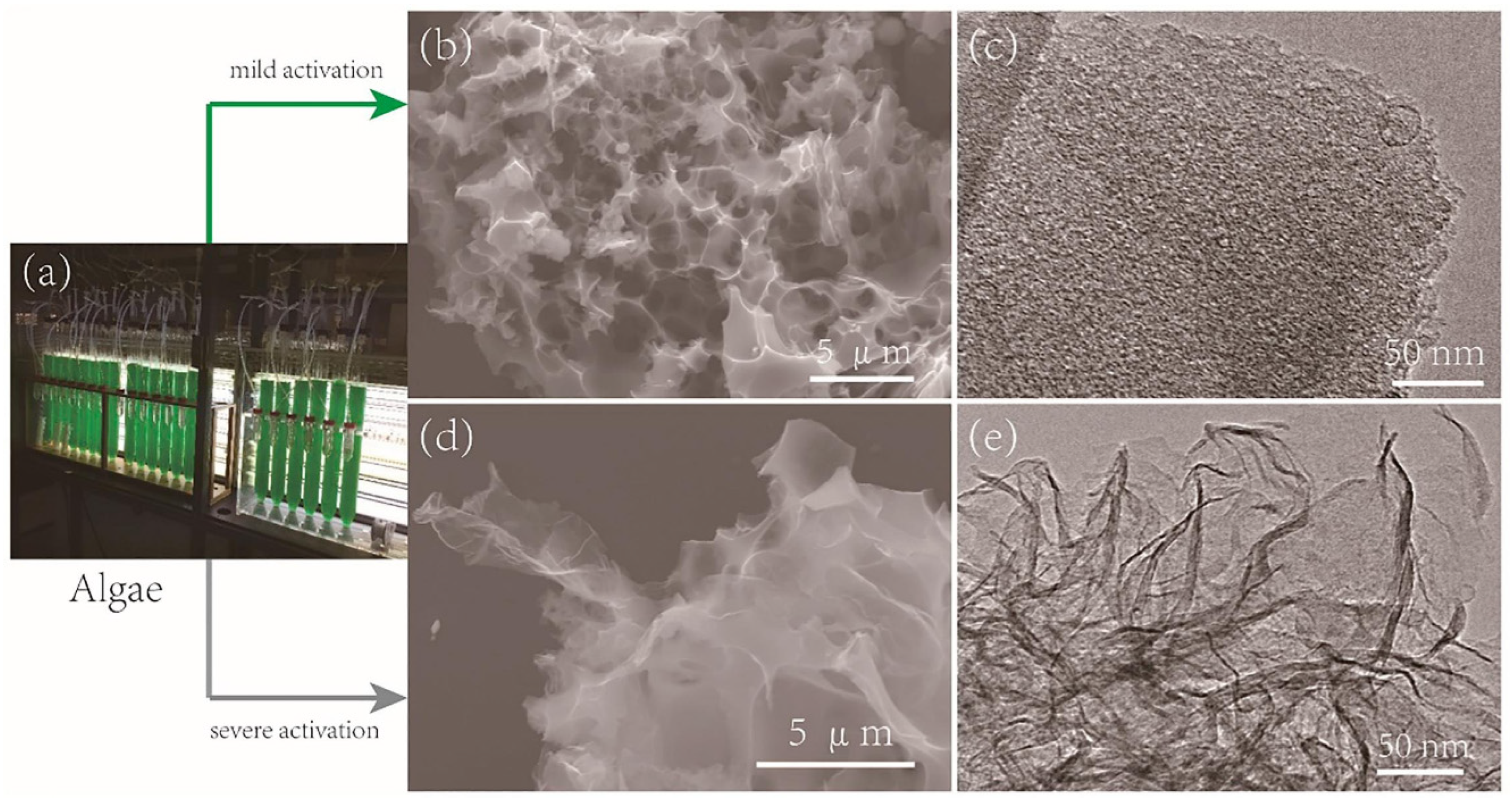
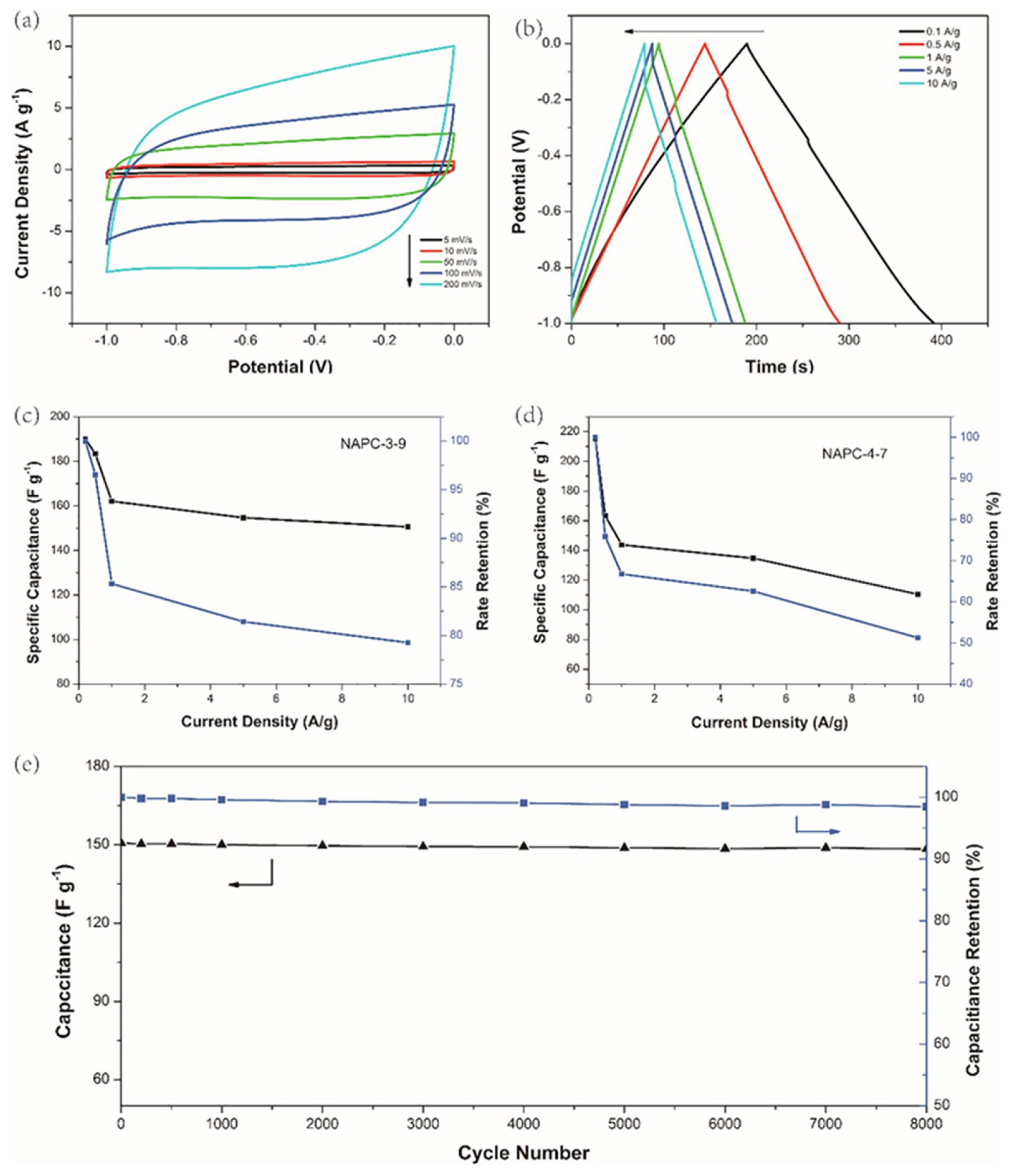




| Synthesis Method | Performance | Scalability | Cost | Environmental Impact |
|---|---|---|---|---|
| Freeze-Drying | High porosity and surface area, beneficial for catalysis and adsorption. | Large-scale processing is possible, but energy-intensive. | High, due to freezing and sublimation energy costs. | High energy consumption but no hazardous solvents. |
| Surfactant-Assisted Synthesis | Precise pore control enhances electrochemical performance. | Requires additional surfactant removal steps, limiting scalability. | Moderate, depends on surfactant type and processing. | Potential waste disposal concerns from surfactants. |
| Template-Free Synthesis | Self-doped carbon materials exhibit large surface area and high electrochemical performance. | Simplified process, making it highly scalable. | Low, as it avoids the use of templates or surfactants. | More sustainable, avoids hazardous chemicals and additional processing steps. |
| Material | Capacitance | Energy Density | Power Density | Cycle Stability |
|---|---|---|---|---|
| HC/NiCo@800 °C//HC [70] | 232 F/g (using RbI electrolyte) | 96.57 Wh/kg (using RbI electrolyte) | - | - |
| Porous carbon [72] | 287.7 F/g (three-electrode), 190.0 F/g (two-electrode) | - | 79.3% at 10 A/g | exceptional |
| PABz-co-Cu MOFs-graft-PIDPA [74] | 387 F/g at 1 A/g | - | - | - |
| NP-rGO [59] | 416 F/g | 22.3 Wh/kg (at 500 W/kg) | - | 94.63% capacitance maintained after 10,000 cycles |
| NRPC/g-C3N4/Fe3O4 [79] | 385 F/g at 1 A/g | - | - | 94.3% after 2000 cycles |
| LFP@NC | - | - | favorable | stable |
| NRPC/NiMn [64] | 1825 F/g | - | - | 78% after 2500 cycles |
| Nitrogen-doped carbon [67] | 700 F/g at 10 A/g | 48 Wh/kg at 8400 W/kg | 8400 W/kg | - |
| N, O-co-doped carbon [37] | 728 F/g at 10 A/g | 56 Wh/kg at 14,246 W/kg | 14246 W/kg | 86% capacitance retention after 2500 cycles |
| Porous carbon spheres [58] | 344 F/g at 1 A/g | 33.37 Wh/kg at 9000 W/kg | 9000 W/kg | 92% capacitance maintained after 5000 cycles |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Periyasamy, T.; Asrafali, S.P.; Lee, J. Thermally Stable Carbon Materials from Polybenzoxazines: Structure, Properties, and Supercapacitor Potential. Batteries 2025, 11, 140. https://doi.org/10.3390/batteries11040140
Periyasamy T, Asrafali SP, Lee J. Thermally Stable Carbon Materials from Polybenzoxazines: Structure, Properties, and Supercapacitor Potential. Batteries. 2025; 11(4):140. https://doi.org/10.3390/batteries11040140
Chicago/Turabian StylePeriyasamy, Thirukumaran, Shakila Parveen Asrafali, and Jaewoong Lee. 2025. "Thermally Stable Carbon Materials from Polybenzoxazines: Structure, Properties, and Supercapacitor Potential" Batteries 11, no. 4: 140. https://doi.org/10.3390/batteries11040140
APA StylePeriyasamy, T., Asrafali, S. P., & Lee, J. (2025). Thermally Stable Carbon Materials from Polybenzoxazines: Structure, Properties, and Supercapacitor Potential. Batteries, 11(4), 140. https://doi.org/10.3390/batteries11040140








