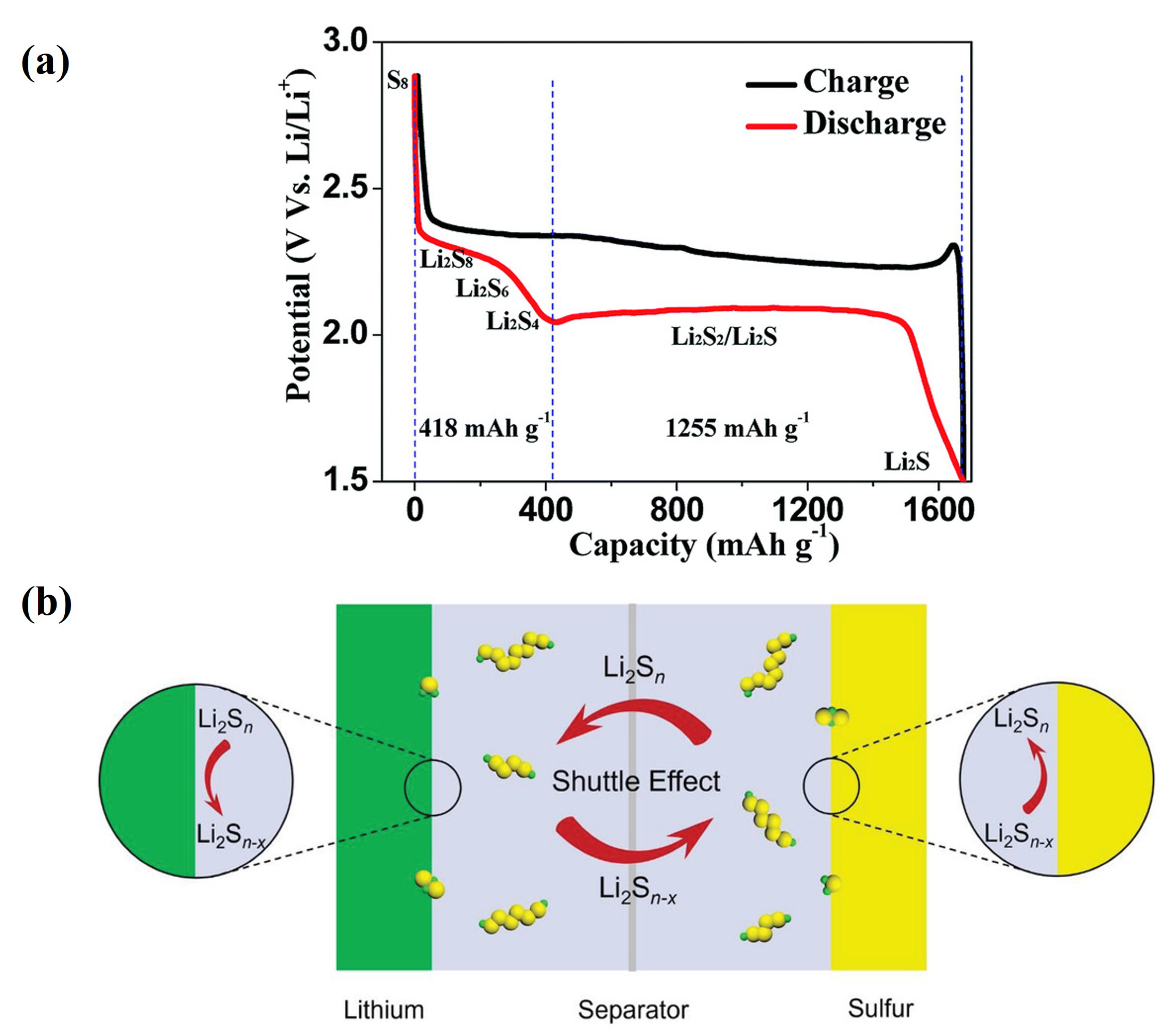
Strategies to Suppress Polysulfide Dissolution and Its Effects on Lithium–Sulfur Batteries
Abstract
1. Introduction
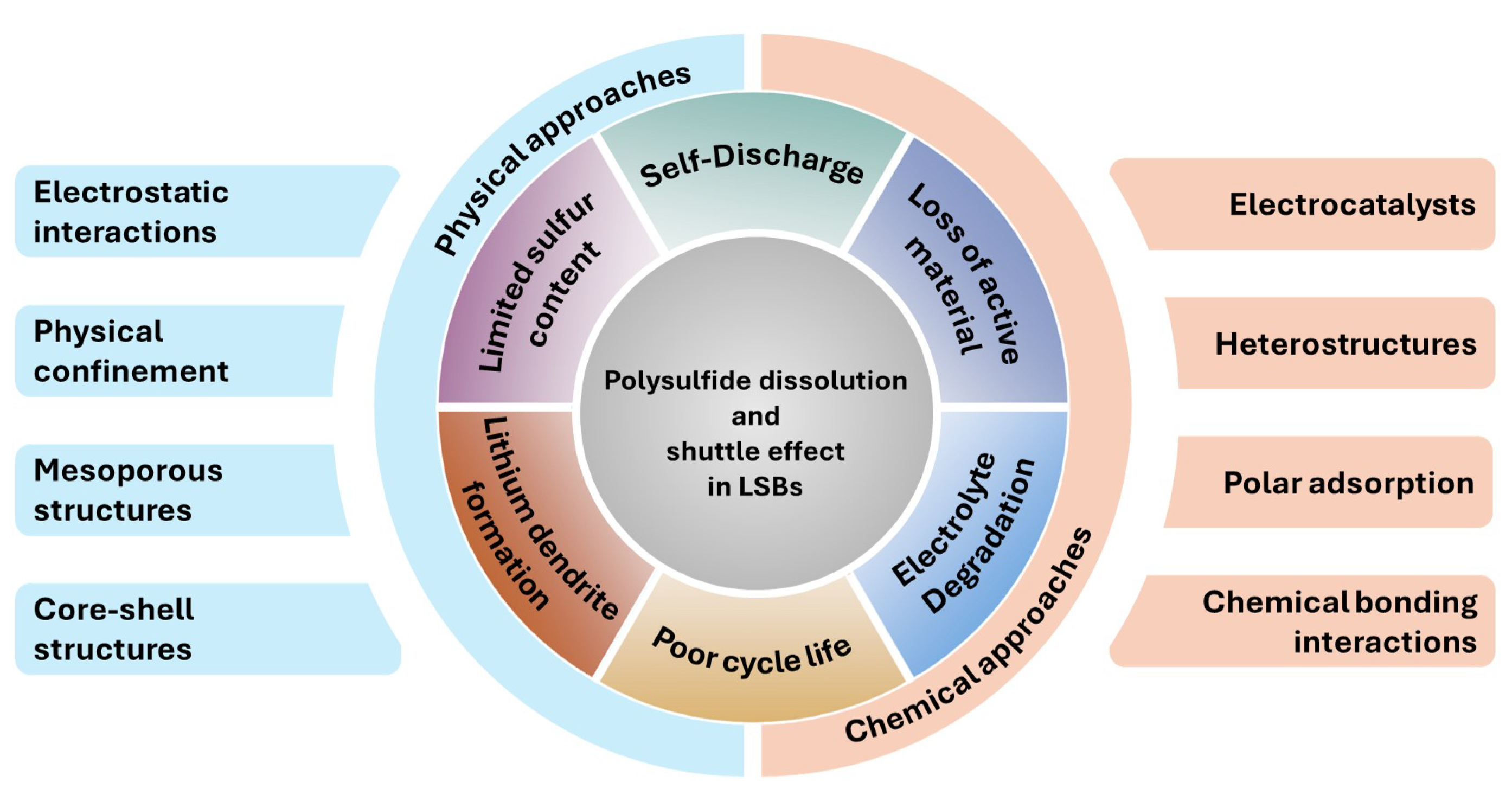
2. Overview and Reaction Mechanism of Polysulfide Dissolution and the Problems Associated
3. Strategies for Polysulfide Trapping and Polysulfide Shuttle Suppression
3.1. Physical Approaches
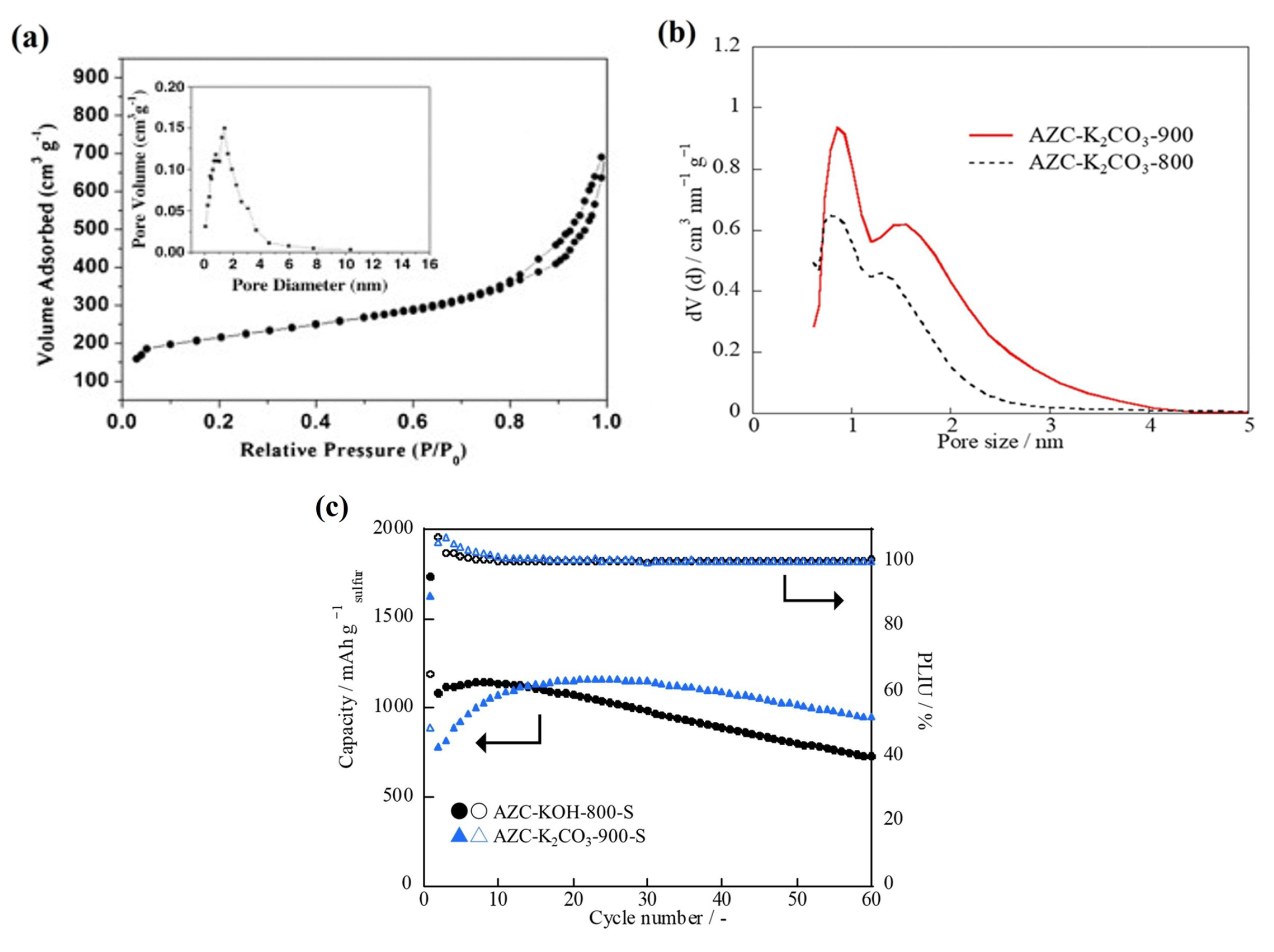
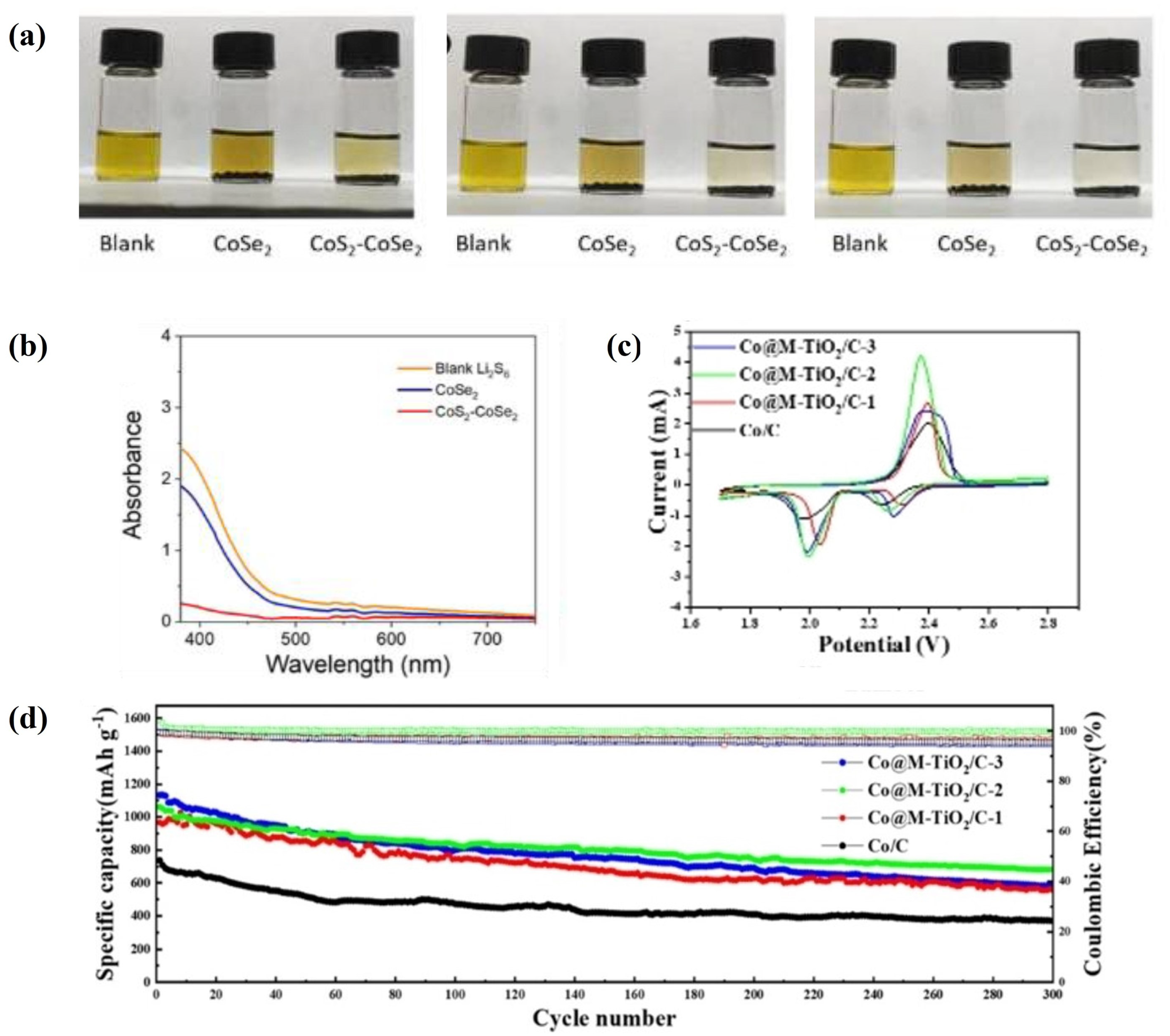
3.2. Chemical Approaches
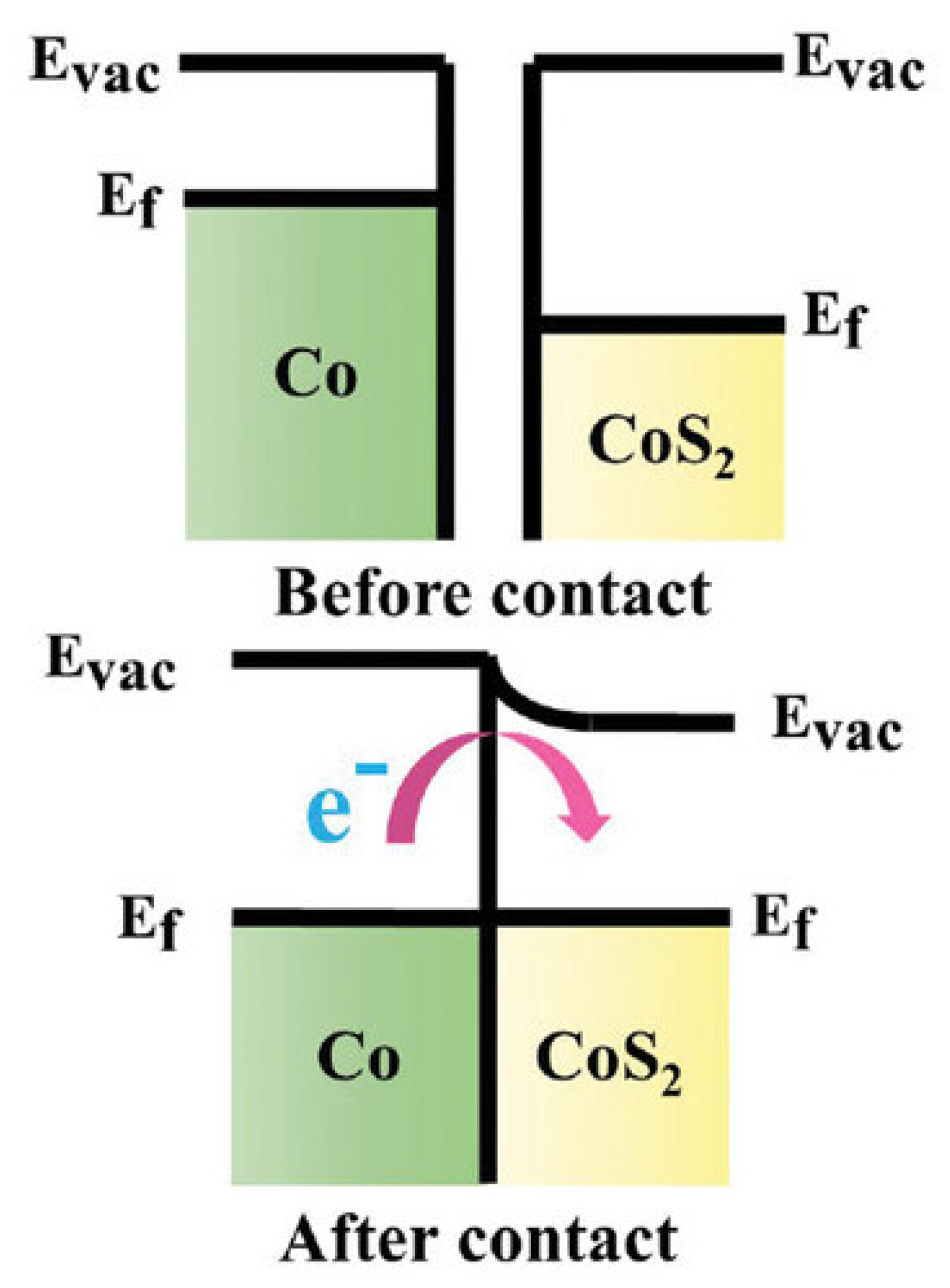
3.2.1. Catalytic Material for Polysulfide Conversion
3.2.2. Separators
3.2.3. Polymer-Based Binders
3.2.4. Comparison of Different Strategies for Mitigation of Polysulfide Dissolution and Shuttle Effects
4. Conclusions and Future Directions
- (1)
- The optimisation and compatibility of materials. The materials must be carefully studied to ensure that their stability and mechanical qualities are sustained during battery operation. The thermal properties, mechanical properties, and structural stability need careful consideration when selecting materials.
- (2)
- Mechanism behind synergistic effects in heterostructures. A deeper understanding of the mechanism underlying the adsorption–catalytic process is crucial in selecting materials with both enhanced catalytic and adsorption activity. This understanding is essential in improving the overall effectiveness of heterostructures in impeding the shuttle effect, as well as the performance of LSBs.
- (3)
- The scalability of synthesis for mass production. While porous structures show promising effects, creating uniform and stable porous structures at nanoscales can be challenging and costly. The complex fabrication processes for heterostructures further add to the costs and complexity, limiting the commercial viability of LSBs. Exploring cost-effective materials and synthesis methods is critical for the commercialisation of LSBs.
- (4)
- Exploring alternatives materials outside of cobalt-based materials. While cobalt-based materials offer high stability and strong adsorption capabilities regarding polysulfides, the usage of cobalt raises concerns associated with environmental impacts, ethical issues, toxicity, and cost-effectiveness, limiting the production of LSBs.
Author Contributions
Funding
Conflicts of Interest
References
- Mitali, J.; Dhinakaran, S.; Mohamad, A.A. Energy Storage Systems: A Review. Energy Storage Sav. 2022, 1, 166–216. [Google Scholar] [CrossRef]
- Huang, C.; Wilson, M.D.; Cline, B.; Sivarajah, A.; Stolp, W.; Boone, M.; Connolley, T.; Leung, C.L.A. Li+ concentration and morphological changes at the anode and cathode interphases inside solid-state lithium metal batteries. J. Phys. Energy 2025, 7, 025009. [Google Scholar]
- Blomgren, G.E. The Development and Future of Lithium Ion Batteries. J. Electrochem. Soc. 2016, 164, A5019–A5025. [Google Scholar] [CrossRef]
- Min, G. 11—Power Supply Sources for Smart Textiles. In Smart Clothes and Wearable Technology; McCann, J., Bryson, D., Eds.; Woodhead Publishing Series in Textiles; Woodhead Publishing: Sawston, UK, 2009; pp. 214–231. [Google Scholar] [CrossRef]
- Gong, Z.; Liu, Z.; Gao, X.W.; Chen, N.; Song, Y.; Wu, X.; Hu, A. Constructing cyclic hydrogen bonding to suppress side reactions and dendrite formation on zinc anodes. Chem.–Eur. J. 2024, 30, e202402558. [Google Scholar]
- Liu, Z.; Song, Y.; Fu, S.; An, P.; Dong, M.; Wang, S.; Lai, Q.; Gao, X.W.; Luo, W.B. Multiphase manganese-based layered oxide for sodium-ion batteries: Structural change and phase transition. Microstructures 2024, 4, 2024036. [Google Scholar]
- Huang, L.; Barker, K.; Liu, X.; Jian, Y.; Skinner, S.J.; Ryan, M.P.; Huang, C. A mixed-anion strategy for constructing rapid ion-conducting Na solid-state electrolyte. Chem. Inorg. Mater. 2025, 6, 100102. [Google Scholar]
- Liu, Z.; Li, S.; Mu, J.; Zhao, L.K.; Gao, X.W.; Gu, Q.; Wang, X.C.; Chen, H.; Luo, W.B. Element-tailored quenching methods: Phase-defective K0. 5Mn1-xCrxO2 cathode materials for potassium ion batteries. Mater. Today Chem. 2024, 40, 102251. [Google Scholar]
- Liu, Z.; Gong, Z.; He, K.; Qiu, P.; Wang, X.C.; Zhao, L.K.; Gu, Q.F.; Gao, X.W.; Luo, W.B. Developments and prospects of carbon anode materials in potassium-ion batteries. Sci. China Mater. 2024, 1–16. [Google Scholar]
- Liu, Z.M.; Wang, D.; Li, S.Z.; Lai, Q.S.; Yang, D.R.; Zhao, L.K.; Mu, J.J.; Wang, X.C.; Gao, X.W.; Luo, W.B. An ultrafast rechargeable hybrid potassium dual-ion capacitor based on carbon quantum dot@ ultrathin carbon film cathode. Rare Met. 2024, 43, 5070–5081. [Google Scholar]
- Manthiram, A.; Fu, Y.; Chung, S.H.; Zu, C.; Su, Y.S. Rechargeable Lithium–Sulfur Batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef]
- Li, M.; Jafta, C.J.; Belharouak, I. 5—Progress of Nanotechnology for Lithium-Sulfur Batteries. In Frontiers of Nanoscience; Raccichini, R., Ulissi, U., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Nanomaterials for Electrochemical Energy Storage; Volume 19, pp. 137–164. [Google Scholar] [CrossRef]
- Mori, R. Cathode Materials for Lithium-Sulfur Battery: A Review. J. Solid State Electrochem. 2023, 27, 813–839. [Google Scholar] [CrossRef]
- Haridas, A.K.; Huang, C. Advances and Challenges in Tuning the Reversibility & Cyclability of Room Temperature Sodium–Sulfur and Potassium–Sulfur Batteries with Catalytic Materials. Mater. Today Energy 2023, 32, 101228. [Google Scholar] [CrossRef]
- Shojaei, M.J.; Sivarajah, A.; Safdar, T.; Magdysyuke, O.V.; Leung, C.L.A.; Huang, C. Advanced battery cathode microstructure analysis through operando synchrotron X-ray tomography and super-resolution deep learning. Solid State Ionics 2025, 422, 116818. [Google Scholar] [CrossRef]
- Liu, Y.; Elias, Y.; Meng, J.; Aurbach, D.; Zou, R.; Xia, D.; Pang, Q. Electrolyte Solutions Design for Lithium-Sulfur Batteries. Joule 2021, 5, 2323–2364. [Google Scholar] [CrossRef]
- Huang, C.; Wilson, M.D.; Cline, B.; Sivarajah, A.; Stolp, W.; Boone, M.N.; Connolley, T.; Leung, C.L.A. Correlating Lithium-Ion Transport and Interfacial Lithium Microstructure Evolution in Solid-State Batteries during the First Cycle. Cell Rep. Phys. Sci. 2024, 5, 101995. [Google Scholar] [CrossRef]
- Leung, C.L.A.; Wilson, M.D.; Connolley, T.; Huang, C. Mapping of Lithium Ion Concentrations in 3D Structures through Development of in Situ Correlative Imaging of X-ray Compton Scattering-Computed Tomography. Synchrotron Radiat. 2024, 31, 888–895. [Google Scholar] [CrossRef]
- Huang, C.; Leung, C.L.A.; Leung, P.; Grant, P.S. A Solid-State Battery Cathode with a Polymer Composite Electrolyte and Low Tortuosity Microstructure by Directional Freezing and Polymerization. Adv. Energy Mater. 2021, 11, 2002387. [Google Scholar] [CrossRef]
- Haridas, A.K.; Huang, C. Advances in Strategic Inhibition of Polysulfide Shuttle in Room-Temperature Sodium-Sulfur Batteries via Electrode and Interface Engineering. Batteries 2023, 9, 223. [Google Scholar] [CrossRef]
- Han, X.; Cai, J.; Wang, X.; Liu, Y.; Zhou, H.; Meng, X. Understanding Effects of Conductive Additives in Lithium-Sulfur Batteries. Mater. Today Commun. 2021, 26, 101934. [Google Scholar] [CrossRef]
- Dong, L.; Liu, J.; Chen, D.; Han, Y.; Liang, Y.; Yang, M.; Yang, C.; He, W. Suppression of Polysulfide Dissolution and Shuttling with Glutamate Electrolyte for Lithium Sulfur Batteries. ACS Nano 2019, 13, 14172–14181. [Google Scholar] [CrossRef]
- Wang, Z.; Ji, H.; Zhou, L.; Shen, X.; Gao, L.; Liu, J.; Yang, T.; Qian, T.; Yan, C. All-Liquid-Phase Reaction Mechanism Enabling Cryogenic Li–S Batteries. ACS Nano 2021, 15, 13847–13856. [Google Scholar] [CrossRef]
- Yu, L.; Ong, S.J.H.; Liu, X.; Mandler, D.; Xu, Z.J. The Importance of the Dissolution of Polysulfides in Lithium-Sulfur Batteries and a Perspective on High-Energy Electrolyte/Cathode Design. Electrochim. Acta 2021, 392, 139013. [Google Scholar] [CrossRef]
- Ren, W.; Ma, W.; Zhang, S.; Tang, B. Recent Advances in Shuttle Effect Inhibition for Lithium Sulfur Batteries. Energy Storage Mater. 2019, 23, 707–732. [Google Scholar] [CrossRef]
- Chung, S.H.; Manthiram, A. Lithium–Sulfur Batteries with the Lowest Self-Discharge and the Longest Shelf Life. ACS Energy Lett. 2017, 2, 1056–1061. [Google Scholar] [CrossRef]
- Chien, Y.C.; Lacey, M.J.; Steinke, N.J.; Brandell, D.; Rennie, A.R. Correlations between Precipitation Reactions and Electrochemical Performance of Lithium-Sulfur Batteries Probed by Operando Scattering Techniques. Chem 2022, 8, 1476–1492. [Google Scholar] [CrossRef]
- Guo, J.; Liu, J. A Binder-Free Electrode Architecture Design for Lithium–Sulfur Batteries: A Review. Nanoscale Adv. 2019, 1, 2104–2122. [Google Scholar] [CrossRef]
- Zhou, L.; Danilov, D.L.; Qiao, F.; Wang, J.; Li, H.; Eichel, R.A.; Notten, P.H.L. Sulfur Reduction Reaction in Lithium–Sulfur Batteries: Mechanisms, Catalysts, and Characterization. Adv. Energy Mater. 2022, 12, 2202094. [Google Scholar] [CrossRef]
- Wu, Y.; Jin, T.; Momma, T.; Yokoshima, T.; Nara, H.; Osaka, T. Potentiostatic Way to Fabricate Li2Sx Cathode with Suppressed Polysulfide Formation. J. Power Sources 2018, 399, 287–293. [Google Scholar] [CrossRef]
- Safdar, T.; Huang, C. Sulfur/Carbon Cathode Material Chemistry and Morphology Optimisation for Lithium–Sulfur Batteries. RSC Adv. 2024, 14, 30743–30755. [Google Scholar] [CrossRef]
- Fahad, S.; Wei, Z.; Kushima, A. In-situ TEM observation of fast and stable reaction of lithium polysulfide infiltrated carbon composite and its application as a lithium sulfur battery electrode for improved cycle lifetime. J. Power Sources 2021, 506, 230175. [Google Scholar]
- Pozio, A.; Di Carli, M.; Aurora, A.; Falconieri, M.; Della Seta, L.; Prosini, P.P. Hard Carbons for Use as Electrodes in Li-S and Li-ion Batteries. Nanomaterials 2022, 12, 1349. [Google Scholar] [CrossRef]
- Wang, M.; Xia, X.; Zhong, Y.; Wu, J.; Xu, R.; Yao, Z.; Wang, D.; Tang, W.; Wang, X.; Tu, J. Porous Carbon Hosts for Lithium–Sulfur Batteries. Chem. Eur. J. 2019, 25, 3710–3725. [Google Scholar] [CrossRef]
- Rao, M.; Li, W.; Cairns, E.J. Porous Carbon-Sulfur Composite Cathode for Lithium/Sulfur Cells. Electrochem. Commun. 2012, 17, 1–5. [Google Scholar] [CrossRef]
- Takahashi, T.; Yamagata, M.; Ishikawa, M. A Sulfur–Microporous Carbon Composite Positive Electrode for Lithium/Sulfur and Silicon/Sulfur Rechargeble Batteries. Prog. Nat. Sci. Mater. Int. 2015, 25, 612–621. [Google Scholar] [CrossRef]
- Tonoya, T.; Hinago, H.; Ishikawa, M. Development of Highly Sulfur-Loadable Microporous Activated Carbon from Azulmic Acid for Lithium-Sulfur Battery. In Electrochemical Society Meeting Abstracts prime2020; The Electrochemical Society, Inc.: Pennington, NJ, USA, 2020; MA2020-02; p. 3454. [Google Scholar] [CrossRef]
- Tonoya, T.; Matsui, Y.; Hinago, H.; Ishikawa, M. Microporous Activated Carbon Derived from Azulmic Acid Precursor with High Sulfur Loading and Its Application to Lithium-Sulfur Battery Cathode. Electrochem. Commun. 2022, 140, 107333. [Google Scholar] [CrossRef]
- Wang, T.; He, J.; Cheng, X.B.; Zhu, J.; Lu, B.; Wu, Y. Strategies toward High-Loading Lithium–Sulfur Batteries. ACS Energy Lett. 2022, 8, 116–150. [Google Scholar] [CrossRef]
- Pan, H.; Huang, X.; Zhang, R.; Zhang, T.; Chen, Y.; Hoang, T.K.A.; Wen, G. Reduced Graphene Oxide-Encapsulated Mesoporous Silica as Sulfur Host for Lithium–Sulfur Battery. J. Solid State Electrochem. 2018, 22, 3557–3568. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, S. 20—Impedance Humidity Sensors Based on Metal Oxide Semiconductors: Characteristics and Mechanism. In Modeling, Characterization, and Production of Nanomaterials, 2nd ed.; Tewary, V.K., Zhang, Y., Eds.; Woodhead Publishing Series in Electronic and Optical Materials; Woodhead Publishing: Sawston, UK, 2023; pp. 549–580. [Google Scholar] [CrossRef]
- Zhao, J. Capillary Force and Surface Wettability. In Encyclopedia of Tribology; Wang, Q.J., Chung, Y.W., Eds.; Springer: Boston, MA, USA, 2013; pp. 295–298. [Google Scholar] [CrossRef]
- Lin, H.; Yang, L.; Jiang, X.; Li, G.; Zhang, T.; Yao, Q.; Zheng, G.W.; Lee, J.Y. Electrocatalysis of Polysulfide Conversion by Sulfur-Deficient MoS2 Nanoflakes for Lithium–Sulfur Batteries. Energy Environ. Sci. 2017, 10, 1476–1486. [Google Scholar] [CrossRef]
- Zhao, W.M.; Shen, J.D.; Xu, X.J.; He, W.X.; Liu, L.; Chen, Z.H.; Liu, J. Functional Catalysts for Polysulfide Conversion in Li–S Batteries: From Micro/Nanoscale to Single Atom. Rare Met. 2022, 41, 1080–1100. [Google Scholar] [CrossRef]
- Wu, L.; Hu, J.; Yang, X.; Liang, Z.; Chen, S.; Liu, L.; Hou, H.; Yang, J. Synergistic Effect of Adsorption and Electrocatalysis of CoO/NiO Heterostructure Nanosheet Assembled Nanocages for High-Performance Lithium–Sulfur Batteries. J. Mater. Chem. A 2022, 10, 23811–23822. [Google Scholar] [CrossRef]
- Azam, S.; Wei, Z.; Wang, R. Cerium Oxide Nanorods Anchored on Carbon Nanofibers Derived from Cellulose Paper as Effective Interlayer for Lithium Sulfur Battery. J. Colloid Interface Sci. 2022, 615, 417–431. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Tian, L.; Pang, L.; Liu, X.; Li, J. Synergic Adsorption and Catalytic Conversion of Polysulfides Based on Polar Mo2C and Pyridinic N for the Enhanced Electrochemical Performance of Li–S Batteries. Mater. Today Sustain. 2022, 18, 100155. [Google Scholar] [CrossRef]
- Gao, X.T.; Zhu, X.D.; Gu, L.L.; Wang, C.; Sun, K.N.; Hou, Y.L. Efficient Polysulfides Anchoring for Li-S Batteries: Combined Physical Adsorption and Chemical Conversion in V2O5 Hollow Spheres Wrapped in Nitrogen-Doped Graphene Network. Chem. Eng. J. 2019, 378, 122189. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, F.; Pan, H.; Wang, L.; Wang, D.; Jiang, Y.; Wang, L.; Qian, Y. Study on the Effect of Transition Metal Sulfide in Lithium–Sulfur Battery. Inorg. Chem. Front. 2019, 6, 477–481. [Google Scholar] [CrossRef]
- Park, Y.Y.; Moon, S.H.; Park, D.H.; Shin, J.H.; Kim, J.H.; Jang, J.S.; Kim, S.B.; Lee, S.N.; Park, K.W. Vanadium Nitride/Reduced Graphene Oxide Composite Interlayer with Dual Lithium-Polysulfide Adsorption Effect for Lithium-Sulfur Batteries. J. Alloys Compd. 2023, 960, 170812. [Google Scholar] [CrossRef]
- Wei, Z.; Sarwar, S.; Azam, S.; Ahasan, M.R.; Voyda, M.; Zhang, X.; Wang, R. Ultrafast Microwave Synthesis of MoTe2@graphene Composites Accelerating Polysulfide Conversion and Promoting Li2S Nucleation for High-Performance Li-S Batteries. J. Colloid Interface Sci. 2023, 635, 391–405. [Google Scholar] [CrossRef]
- Yuan, B.; Zhao, J.; Liu, W.; Liu, H.; Chen, P.; Sun, L.; Guo, X.; Wang, X.; Zhang, W.; Zhang, R.; et al. Preparation and Application of NiS2/NiSe2 Heterostructure as a Sulfur Host in Lithium-Sulfur Batteries. J. Alloys Compd. 2024, 987, 174231. [Google Scholar] [CrossRef]
- Li, Y.; Hao, Y.; Ali, U.; Zhang, Q.; Jin, Z.; Sun, H.; Li, L.; Zhang, L.; Wang, C.; Liu, B. Bimetallic Sulfides Heterostructure Efficient Adsorption and Catalytic of Polysulfides for High-Performance Lithium-Sulfur Batteries. Chem. Eng. J. 2023, 474, 145961. [Google Scholar] [CrossRef]
- Gao, D.; Li, Y.; Guo, Z.; Liu, Z.; Guo, K.; Fang, Y.; Xue, Y.; Huang, Y.; Tang, C. Sc2CO-MXene/h-BN Heterostructure with Synergetic Effect as an Anchoring and Catalytic Material for Lithium-Sulfur Battery. J. Alloys Compd. 2021, 887, 161273. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, L.; Guo, D.; Wang, X.; Fang, G.; Chen, X.; Wang, S. Flower-Like NiS2/WS2 Heterojunction as Polysulfide/Sulfide Bidirectional Catalytic Layer for High-Performance Lithium-Sulfur Batteries. Small 2023, 19, 2206926. [Google Scholar] [CrossRef]
- Liu, X.; Huang, J.Q.; Zhang, Q.; Mai, L. Nanostructured Metal Oxides and Sulfides for Lithium–Sulfur Batteries. Adv. Mater. 2017, 29, 1601759. [Google Scholar] [CrossRef]
- Zhang, B.; Ma, J.; Cui, M.; Zhao, Y.; Wei, S. A Rational Design of a CoS2-CoSe2 Heterostructure for the Catalytic Conversion of Polysulfides in Lithium-Sulfur Batteries. Materials 2023, 16, 3992. [Google Scholar] [CrossRef]
- Chang, Z.; Liu, W.; Feng, J.; Lin, Z.; Shi, C.; Wang, T.; Lei, Y.; Zhao, X.; Song, J.; Wang, G. Cobalt/MXene-derived TiO2 Heterostructure as a Functional Separator Coating to Trap Polysulfide and Accelerate Redox Kinetics for Reliable Lithium-sulfur Battery. Batter. Supercaps 2024, 7, e202300516. [Google Scholar] [CrossRef]
- Zhao, Z.; Yi, Z.; Duan, Y.; Pathak, R.; Cheng, X.; Wang, Y.; Elam, J.W.; Wang, X. Regulating the D-p Band Center of FeP/Fe2P Heterostructure Host with Built-in Electric Field Enabled Efficient Bidirectional Electrocatalyst toward Advanced Lithium-Sulfur Batteries. Chem. Eng. J. 2023, 463, 142397. [Google Scholar] [CrossRef]
- Zheng, M.; Zhao, J.; Wu, W.; Chen, R.; Chen, S.; Cheng, N. Co/CoS2 Heterojunction Embedded in N, S-Doped Hollow Nanocage for Enhanced Polysulfides Conversion in High-Performance Lithium–Sulfur Batteries. Small 2024, 20, 2303192. [Google Scholar] [CrossRef]
- Zhu, C.; Zhou, W.; Chen, M.; Yuan, K.; Chen, N.; Wang, A.; Zhao, D.; Li, L.; Liang, X.; An, M. N Doped Carbon Supported Cobalt/Tungsten Nitride Mott-Schottky Heterojunction as an Efficient Electrocatalyst to Enhance Adsorption and Conversion of Lithium Polysulfides for High-Performance Lithium–Sulfur Batteries. Electrochim. Acta 2024, 489, 144184. [Google Scholar] [CrossRef]
- Peng, L.; Wei, Z.; Wan, C.; Li, J.; Chen, Z.; Zhu, D.; Baumann, D.; Liu, H.; Allen, C.S.; Xu, X.; et al. A fundamental look at electrocatalytic sulfur reduction reaction. Nat. Catal. 2020, 3, 762–770. [Google Scholar]
- Zhang, W.; Li, Y.; Lv, H.; Xie, S.; Zhu, J.; Xu, J.; Jin, H.; Kong, X.; Jin, S.; Wang, H.; et al. A comparison study of the electrocatalytic sulfur reduction activity on heteroatom-doped graphene for Li–S battery. Small Struct. 2023, 4, 2200244. [Google Scholar]
- Cui, Y.; Fang, W.; Zhang, J.; Li, J.; Wu, H.; Sun, Z.; Cai, Y.; Zhang, H.; Zhang, S. Controllable sulfur redox multi-pathway reactions regulated by metal-free electrocatalysts anchored with LiS3* radicals. Nano Energy 2024, 122, 109343. [Google Scholar] [CrossRef]
- Shen, J.; Liang, Z.; Gu, T.; Sun, Z.; Wu, Y.; Liu, X.; Liu, J.; Zhang, X.; Liu, J.; Shen, L.; et al. Revisiting the unified principle for single-atom electrocatalysts in the sulfur reduction reaction: From liquid to solid-state electrolytes. Energy Environ. Sci. 2024, 17, 6034–6045. [Google Scholar]
- Cai, G.; Lv, H.; Zhang, G.; Liu, D.; Zhang, J.; Zhu, J.; Xu, J.; Kong, X.; Jin, S.; Wu, X.; et al. A Volcano Correlation between Catalytic Activity for Sulfur Reduction Reaction and Fe Atom Count in Metal Center. J. Am. Chem. Soc. 2024, 146, 13055–13065. [Google Scholar]
- Shou, H.; Zhou, Q.; Wei, S.; Liu, H.; Lv, H.; Wu, X.; Song, L. High-Throughput Screening of Sulfur Reduction Reaction Catalysts Utilizing Electronic Fingerprint Similarity. JACS Au 2024, 4, 930–939. [Google Scholar] [PubMed]
- Rakhimol, K.R.; Kalarikkal, N.; Thomas, S. Different Types of Separators for Lithium Sulfur Battery. In Nanostructured Materials for Lithium/Sulfur Batteries; Gueye, A.B., Thomas, S., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2024; pp. 431–444. [Google Scholar] [CrossRef]
- Ouellette, R.J.; Rawn, J.D. 1—Structure of Organic Compounds. In Principles of Organic Chemistry; Ouellette, R.J., Rawn, J.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–32. [Google Scholar] [CrossRef]
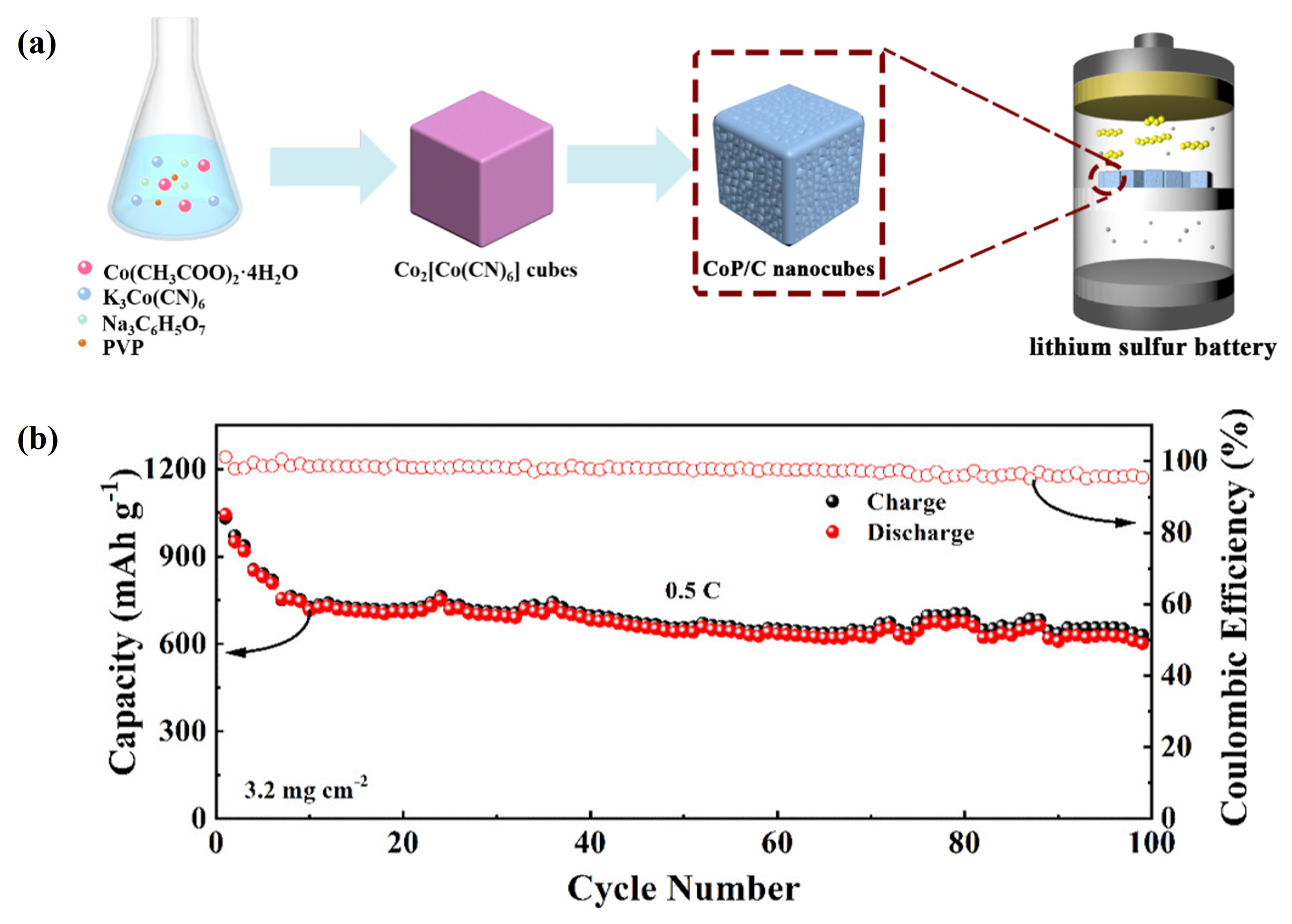
- Lin, J.; Zhang, K.; Zhu, Z.; Zhang, R.; Li, N.; Zhao, C. CoP/C Nanocubes-Modified Separator Suppressing Polysulfide Dissolution for High-Rate and Stable Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2019, 12, 2497–2504. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kang, J.; Kim, S.W.; Halim, W.; Frey, M.W.; Joo, Y.L. Effective Suppression of the Polysulfide Shuttle Effect in Lithium–Sulfur Batteries by Implementing rGO–PEDOT:PSS-Coated Separators via Air-Controlled Electrospray. ACS Omega 2018, 3, 16465–16471. [Google Scholar] [CrossRef]
- Tang, Q.; Li, H.; Pan, Y.; Zhang, J.; Lin, Z.; Chen, Y.; Shu, X.; Qi, W. TiN Synergetic with Micro-/Mesoporous Carbon for Enhanced Performance Lithium–Sulfur Batteries. Ionics 2018, 24, 2983–2993. [Google Scholar] [CrossRef]
- Hong, X.; Jin, J.; Wen, Z.; Zhang, S.; Wang, Q.; Shen, C.; Rui, K. On the Dispersion of Lithium-Sulfur Battery Cathode Materials Effected by Electrostatic and Stereo-Chemical Factors of Binders. J. Power Sources 2016, 324, 455–461. [Google Scholar] [CrossRef]
- Niu, C.; Liu, J.; Qian, T.; Shen, X.; Zhou, J.; Yan, C. Single Lithium-Ion Channel Polymer Binder for Stabilizing Sulfur Cathodes. Natl. Sci. Rev. 2020, 7, 315–323. [Google Scholar] [CrossRef]
- Huang, Y.; Shaibani, M.; Gamot, T.D.; Wang, M.; Jovanović, P.; Dilusha Cooray, M.C.; Mirshekarloo, M.S.; Mulder, R.J.; Medhekar, N.V.; Hill, M.R.; et al. A Saccharide-Based Binder for Efficient Polysulfide Regulations in Li-S Batteries. Nature Commun. 2021, 12, 5375. [Google Scholar] [CrossRef]
- Liao, J.; Liu, Z.; Wang, J.; Ye, Z. Cost-Effective Water-Soluble Poly(Vinyl Alcohol) as a Functional Binder for High-Sulfur-Loading Cathodes in Lithium–Sulfur Batteries. ACS Omega 2020, 5, 8272–8282. [Google Scholar] [CrossRef]
- Qin, T.; Yang, H.; Li, Q.; Yu, X.; Li, H. Design of Functional Binders for High-Specific-Energy Lithium-Ion Batteries: From Molecular Structure to Electrode Properties. Ind. Chem. Mater. 2024, 2, 191–225. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, K.; Fan, Y.; Yuan, M.; Liu, B.; Liu, W.; Shi, F.; Liu, Y.; Chen, W.; Lopez, J.; et al. An Aqueous Inorganic Polymer Binder for High Performance Lithium–Sulfur Batteries with Flame-Retardant Properties. ACS Cent. Sci. 2018, 4, 260–267. [Google Scholar] [CrossRef]
- Han, P.; Chung, S.H.; Chang, C.H.; Manthiram, A. Bifunctional Binder with Nucleophilic Lithium Polysulfide Immobilization Ability for High-Loading, High-Thickness Cathodes in Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2019, 11, 17393–17399. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Ling, M.; Ye, Y.; Li, Z.; Guo, J.; Yao, Y.; Zhu, J.; Lin, Z.; Zhang, S. Acacia Senegal–Inspired Bifunctional Binder for Longevity of Lithium–Sulfur Batteries. Adv. Energy Mater. 2015, 5, 1500878. [Google Scholar] [CrossRef]
- Sun, Q.; He, B.; Zhang, X.Q.; Lu, A.H. Engineering of Hollow Core–Shell Interlinked Carbon Spheres for Highly Stable Lithium–Sulfur Batteries. ACS Nano 2015, 9, 8504–8513. [Google Scholar] [CrossRef]
- Bai, Y.; Nguyen, T.T.; Chu, R.; Kim, N.H.; Lee, J.H. Core-Shell Hollow Nanostructures as Highly Efficient Polysulfide Conversion and Adsorption Cathode for Shuttle-Free Lithium-Sulfur Batteries. Chem. Eng. J. 2023, 454, 140338. [Google Scholar] [CrossRef]
- Li, S.; Fan, Z. Encapsulation Methods of Sulfur Particles for Lithium-Sulfur Batteries: A Review. Energy Storage Mater. 2023, 34, 107–127. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheung, G.; Huang, C. Strategies to Suppress Polysulfide Dissolution and Its Effects on Lithium–Sulfur Batteries. Batteries 2025, 11, 139. https://doi.org/10.3390/batteries11040139
Cheung G, Huang C. Strategies to Suppress Polysulfide Dissolution and Its Effects on Lithium–Sulfur Batteries. Batteries. 2025; 11(4):139. https://doi.org/10.3390/batteries11040139
Chicago/Turabian StyleCheung, Grace, and Chun Huang. 2025. "Strategies to Suppress Polysulfide Dissolution and Its Effects on Lithium–Sulfur Batteries" Batteries 11, no. 4: 139. https://doi.org/10.3390/batteries11040139
APA StyleCheung, G., & Huang, C. (2025). Strategies to Suppress Polysulfide Dissolution and Its Effects on Lithium–Sulfur Batteries. Batteries, 11(4), 139. https://doi.org/10.3390/batteries11040139