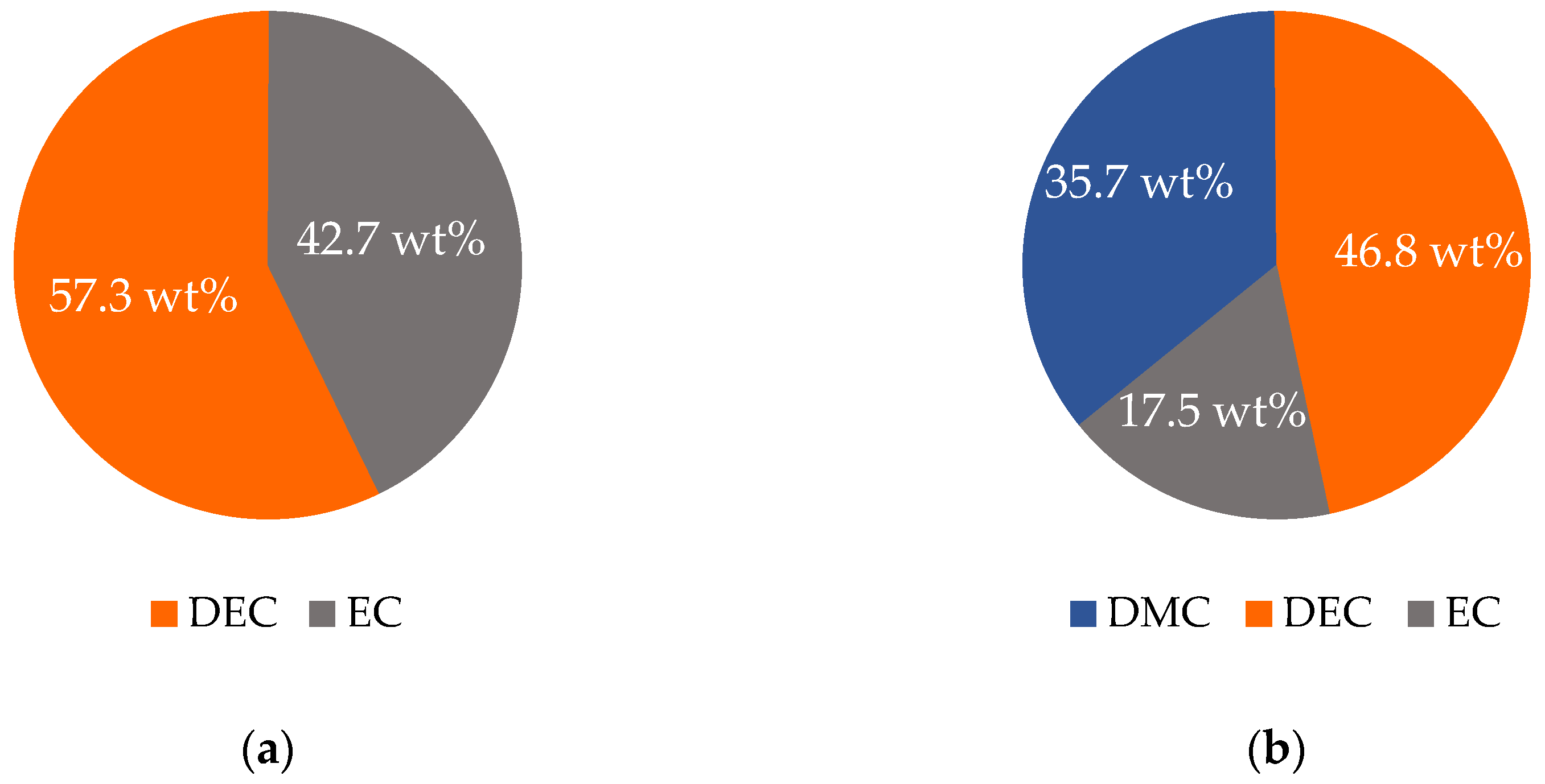1. Introduction
As an essential means of energy storage for a large variety of applications, primarily electromobility and stationary power storage, lithium-ion batteries (LIBs) recently experienced a tremendous rise in production in the international market, which is expected to further accelerate in the next decades [
1,
2]. Considering the criticality and scarcity of some LIB materials, efficient recycling strategies for end-of-life (EoL) batteries are crucial for closed material recirculation, which is required for a sustainable battery market [
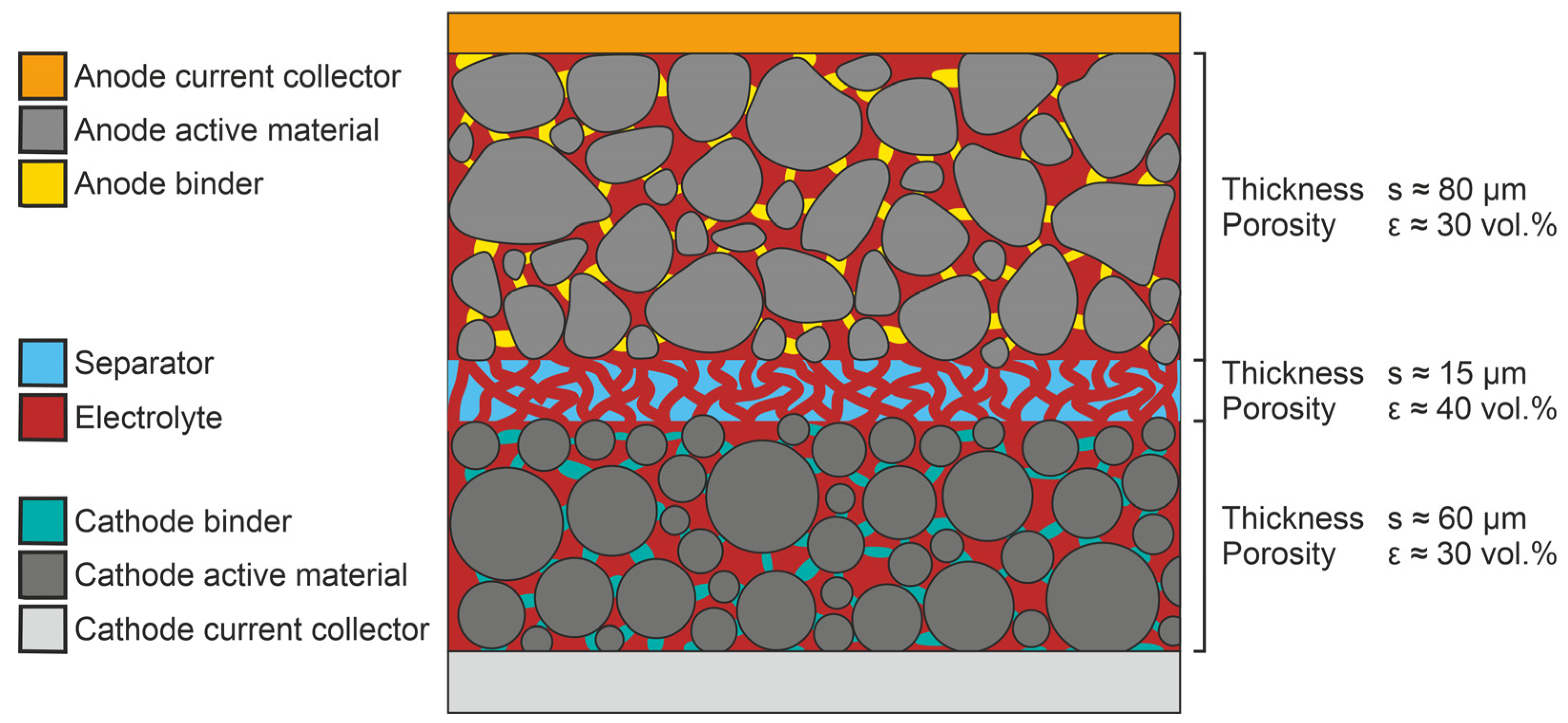
1]. The schematic structure of a LIB cell, with the respective materials of its electrodes and the separator, is visualized in
Figure 1. A LIB cell is an assembly of various functional units, which results in complex waste that requires sophisticated recycling strategies. The aim is to maximize the overall recycling efficiency and to meet material-purity demands on the production side [
1,
3]. Mechanical direct recycling is an approach which pursues a function-conserving recovery of electrode coating materials and, thus, the possibility of their re-use without effortful resynthesis. However, this technology is under development and not yet implemented on a large scale. A currently widespread approach combines mechanical separation steps and hydrometallurgical treatment [
4,
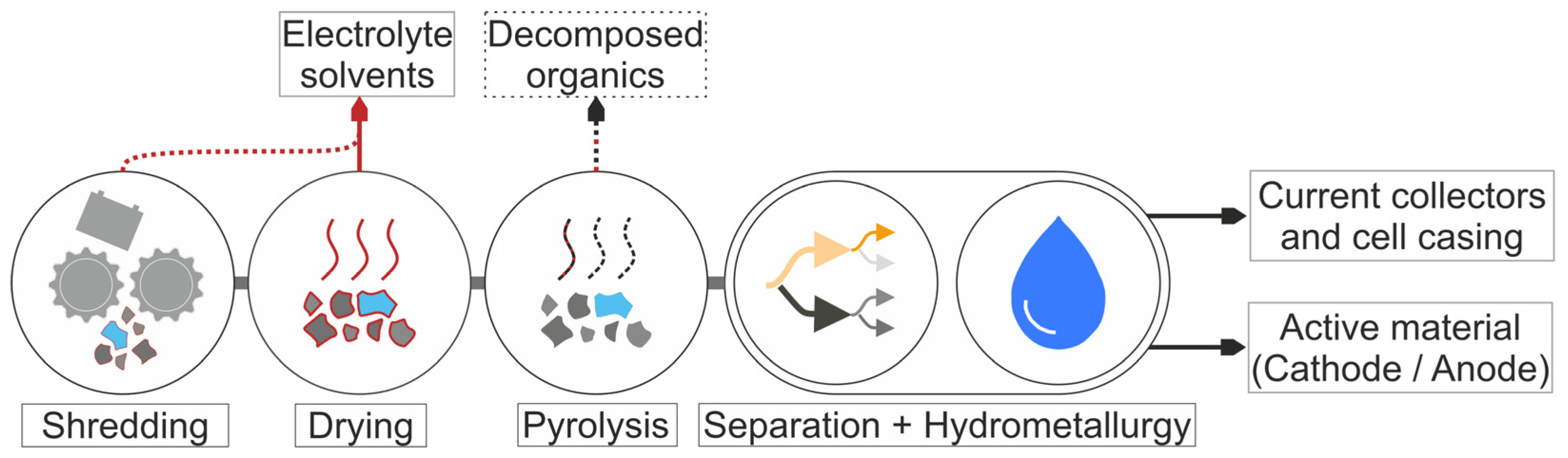
5].
Figure 2 shows a simplified flow diagram for this process with the main material flows. The batteries are discharged and disassembled to at least the module level, followed by shredding, which physically separates the active material, conductor and separator foils into individual streams [
6]. When the cell is crushed, the liquid electrolyte, which accounts for around 10 to 20 wt% on the cell level and consists of conductive salt (≈20 wt% of the liquid phase) and a solvent mixture, is released. In the electrolyte, the conductive salt dissociates into solvated ions and ion pairs through interactions with the solvent molecules. The electrolyte fills the pores of the electrodes and the separator and covers their surfaces to provide an efficient Li-ion transport between the electrodes.
Among the large variety of electrolyte solvents used, the most common solvents are dimethyl carbonate (DMC), diethyl carbonate (DEC) and ethyl methyl carbonate (EMC) as linear carbonates, as well as ethylene carbonate (EC) and propylene carbonate (PC) as cyclic carbonates. Common electrolyte–solvent mixtures consist, for example, of EMC and EC in a mass ratio of 7:3, DMC and EC in a mass ratio of 1:1, or DMC, EMC and DEC in a mass ratio of 1:1:1 [
7,
8,
9,
10,
11]. Some of the electrolyte is consumed during the formation of electrode–electrolyte interphases, namely the solid electrolyte interphase (SEI) on the anode and the cathode electrolyte interphase (CEI) on the cathode. The SEI forms through the reductive decomposition of solvents, salts, and additives, with a preferential reduction of EC, while the CEI forms via oxidative electrolyte reactions at high voltages, where linear carbonates are more susceptible to decomposition. The electrolyte is also partially chemically altered during cycling over the cell lifetime [
12,
13,
14,
15,
16]. However, our own gravimetric and spectrometric measurements during drying suggest that most solvents remain largely unchanged and are still present in the cell. Electrolyte components and their decomposition products can significantly impair the recycling process at multiple stages. Hazardous components that form flammable and explosive vapor–air mixtures pose serious risks to the environment and to worker safety, and their corrosiveness requires resistant materials and increases maintenance costs [
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28]. During sieving, the residues act as unintended binding agents, causing particle agglomeration and reducing separation efficiency. In flotation, the altered wettability of the electrode material’s surface caused by electrolyte solvents hinders separation performance. In hydrometallurgical leaching, electrolyte residues are major contaminants that decrease metal recovery and promote adverse side reactions [
29,
30,
31].
Targeted and channeled removal of the electrolyte components in early-stage thermal treatment prevents safety issues, mitigates the environmental impact, enhances material handling and increases the efficiency of downstream process steps. In a two-step thermal treatment, first, the drying step at a lower temperature aims to remove (and recover) the organic solvents and their decomposition products, while the subsequent pyrolysis step at elevated temperatures aims to decompose binder materials and potential residual solvents. The drying of the comminuted material is performed in inert gas atmosphere or under vacuum [
5,
32,
33,
34]. Low temperatures are set to avoid the decomposition of the conductive salt and the melting of the separator, as well as the formation of adverse compounds [
9,
11,
35].
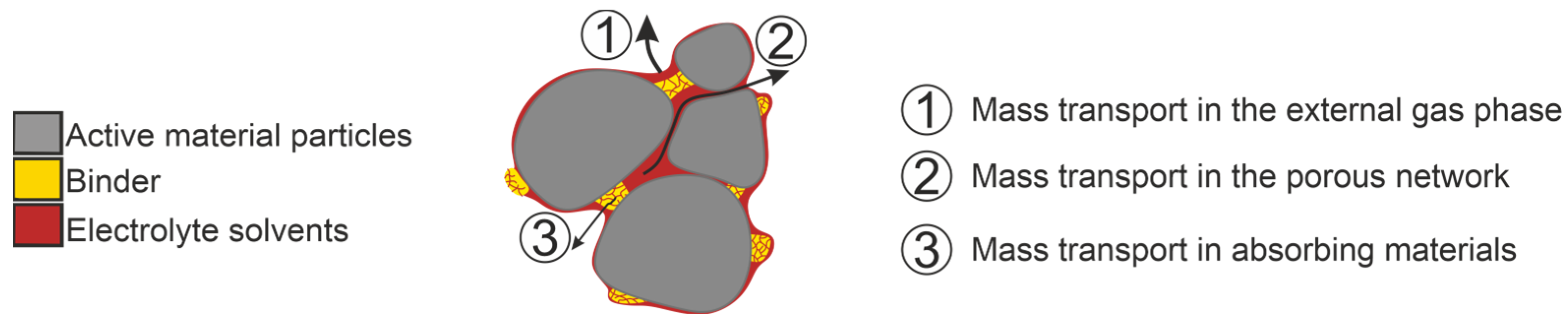
Figure 3 illustrates the individual mass transfer mechanisms taking place in a porous, sorbing material, such as electrodes and separators. If the surface of the material to be dried is covered with solvent, the drying is determined solely by the mass transport in the surrounding external gas phase. If this resistance is not limiting, the mass transport in the porous network becomes dominant as soon as the liquid level recedes to the interior. From this point on, the drying also depends on structural properties of the material, such as porosity and pore-size distribution. Mass transfer in the polymeric materials comes into effect after pore emptying and governs the drying to low residual solvent loadings.
In addition to mass transport kinetics, drying is also governed by the thermodynamic equilibria at phase boundaries. Within the electrolyte, both solvent–solvent and solvent–salt interactions determine the vapor–liquid equilibrium, while solvent–solid interactions are described by the sorption equilibrium.
The removal and recovery of electrolyte components from crushed batteries by means of low-temperature thermal treatment has been the subject of some studies to date.
The vapor–liquid equilibria of common solvent mixtures and their phase transitions during drying have been comprehensively investigated in several studies [
36,
37,
38]. Stehmann et al. [
35,
39] conducted simulations to predict the component-specific decrease of the solvent amount during evaporation of electrolyte–solvent mixtures at different temperatures under low total pressures and carrier gas flow rates. Accompanying pilot-scale drying experiments of shredded cells at 120 °C (reduced pressure, no carrier gas flow) showed an expected insufficient removal of high boiling compounds, such as EC. Due to the assumption of thermodynamic equilibrium in the simulation, only a tendential suitability for reproducing the experimental measurements could be confirmed. Werner et al. [
40] and Zachmann et al. [
41] investigated the electrolyte–solvent removal during the thermal drying of manually opened (different disassembly levels) and comminuted EoL-LIB cells containing DMC, EMC, DEC and EC. The experiments were performed at ambient pressure, over a wide temperature range from room temperature to 150 °C, and in some cases using nitrogen as purge gas. These studies provide valuable insight into the influence of drying conditions on the electrolyte–solvent volatilization for model solvent mixtures and from commercial LIB cells. However, comprehensive investigations regarding optimum drying parameters for efficient removal of the electrolyte solvents are demanded. The governing influences for heat and mass transfer (gas-phase flow-field and gas-phase composition) linked to the applied boundary conditions have not been considered to the required extent.
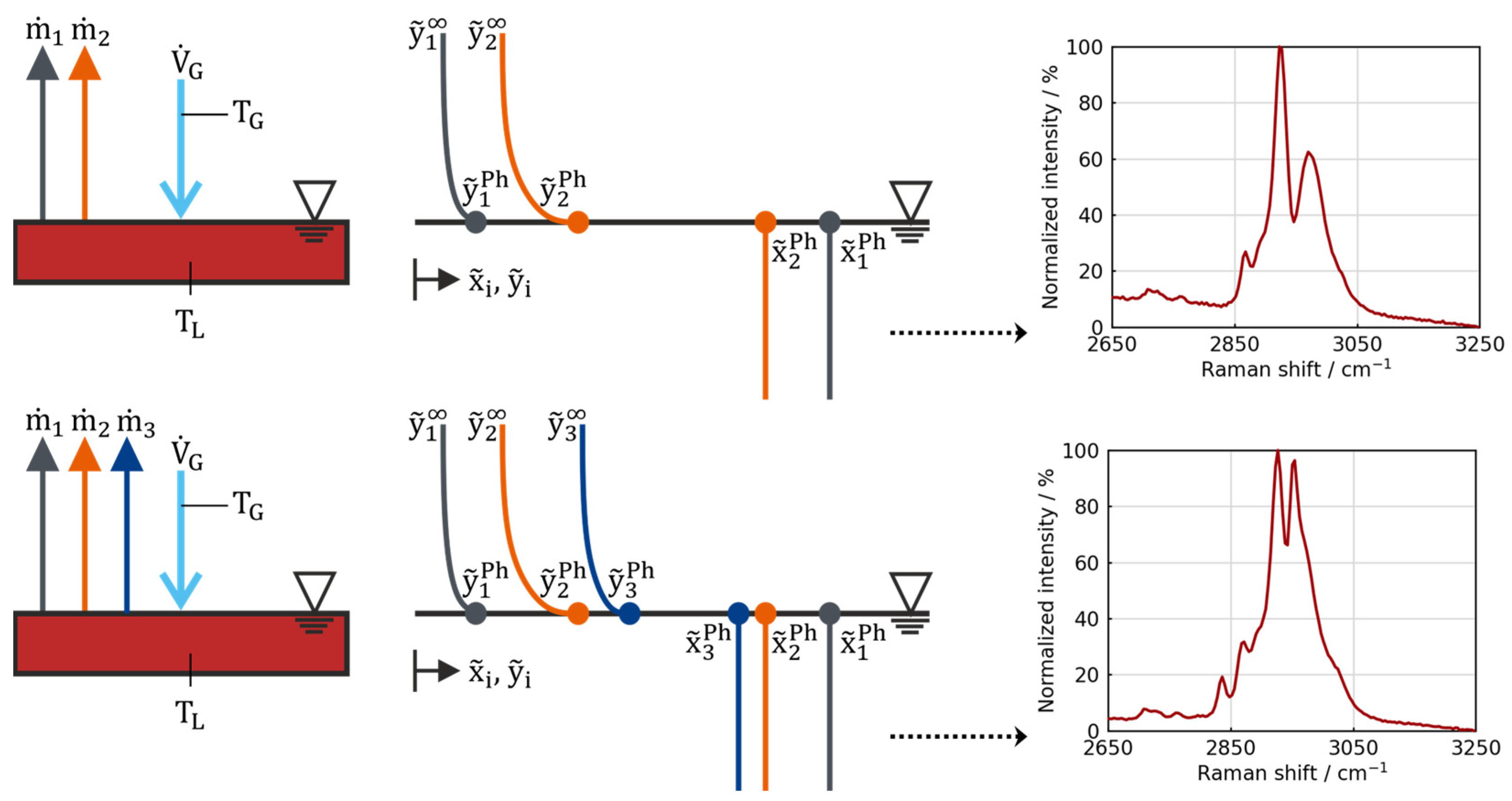
This study focuses on the drying behavior of representative electrolyte–solvent mixtures, with special consideration of the defined process boundary conditions (temperatures, carrier gas flow, geometry). The investigation centers on the initial drying phase, when internal resistances—such as diffusion in the porous network of the electrodes and their electrode–electrolyte interphases and in the polymers—can be neglected (see
Figure 2). Consequently, the drying behavior, characterized by the kinetics and selectivity of evaporation, is solely influenced by the heat and mass transfer in the external gas phase and the thermodynamic phase equilibrium at the vapor–liquid boundary. Due to the low concentration of conductive salt, its influence on the vapor pressure of the solvents is disregarded. The experimental results and findings of this study demonstrate how drying boundary conditions affect the evaporation of individual components. By deliberately simplifying the complex material system, individual influences can be analyzed in isolation. To transfer these results to other dryers, the study presents a simulation model that predicts the evaporation behavior of electrolyte–solvent mixtures based solely on dryer-specific parameters. After validation, the simulation model enables the calculation of minimum required drying times.
3. Mathematical Description
The evaporation (phase transition: liquid ↔ gas) of solvent mixtures can be described by coupling the mass transport kinetics in the gas phase and liquid phase under inclusion of the thermodynamic phase equilibrium at the liquid–gas boundary layer. The solvent evaporation flux from the liquid surface into the bulk gas phase is calculated for the case of unidirectional diffusion using the logarithmic Stefan approach. It is derived from the Maxwell–Stefan equation for mass transport in multi-component systems by formally assuming that all binary diffusion coefficients are equal. The steady-state molar flow
of the evaporating species from the liquid–gas phase boundary into the gas phase (index g) is given by the following:
denotes the boundary surface area for the mass transfer from gas/vapor to the liquid phase, and
is the molar density of the gas phase. The relative molar flux
introduced by E. U. Schlünder represents the composition of the evaporation flow as the ratio of the evaporation flow of a single component
to the total evaporation flow
:
Equation (1) takes into account the additional convective mass flux, which describes the unselective drag flows of one solvent species by the evaporation of another, leading to an increased total evaporation flow [
45]. In contrast, this mathematically simplified solution neglects the influence of the diffusion of one component in another. The binary mass transfer numbers of the solvent species in the carrier gas (
of species i in the gas phase G are directly interconnected via their respective diffusion coefficients
. Their calculation on the basis of characterization experiments with pure DEC is explained in more detail in
Appendix B. The driving gradient for evaporation is the molar fraction of species i between the phase boundary
and in the ambiance
. The Raoult–Dalton law is used to calculate the thermodynamic phase equilibrium at the liquid–gas interface. The activity of component i in the mixture is taken into account with a concentration-dependent activity coefficient determined from vapor–liquid equilibrium data, if available (see
Section 4.1 and
Appendix A).
is then calculated as the ratio of the saturation vapor pressure of component i at the respective temperature (
) and the total pressure.
is the ratio of the evaporation flow of component i and the total gas flow, which comprises the cumulated evaporation flows of all species and the carrier gas flow. The stirred liquid phase is assumed to be ideally mixed. To enable the calculation with temperature-independent material properties, the experiments are conducted at isothermal conditions (constant temperature) [
46].
The evaporation flux of a given species is then used to calculate the amount of this substance in the liquid–solvent mixture over time:
Based on this, the composition of the liquid phase is determined as follows:
Finally, the conversion to masses and mass fractions is carried out using the molar masses of the respective species. The simulation provides the time-resolved solvent amount and the corresponding mass fractions of the individual components. These two quantities are the basis for the display of the results.
4. Results and Discussion
4.1. Analysis of Thermodynamic and Diffusion Properties
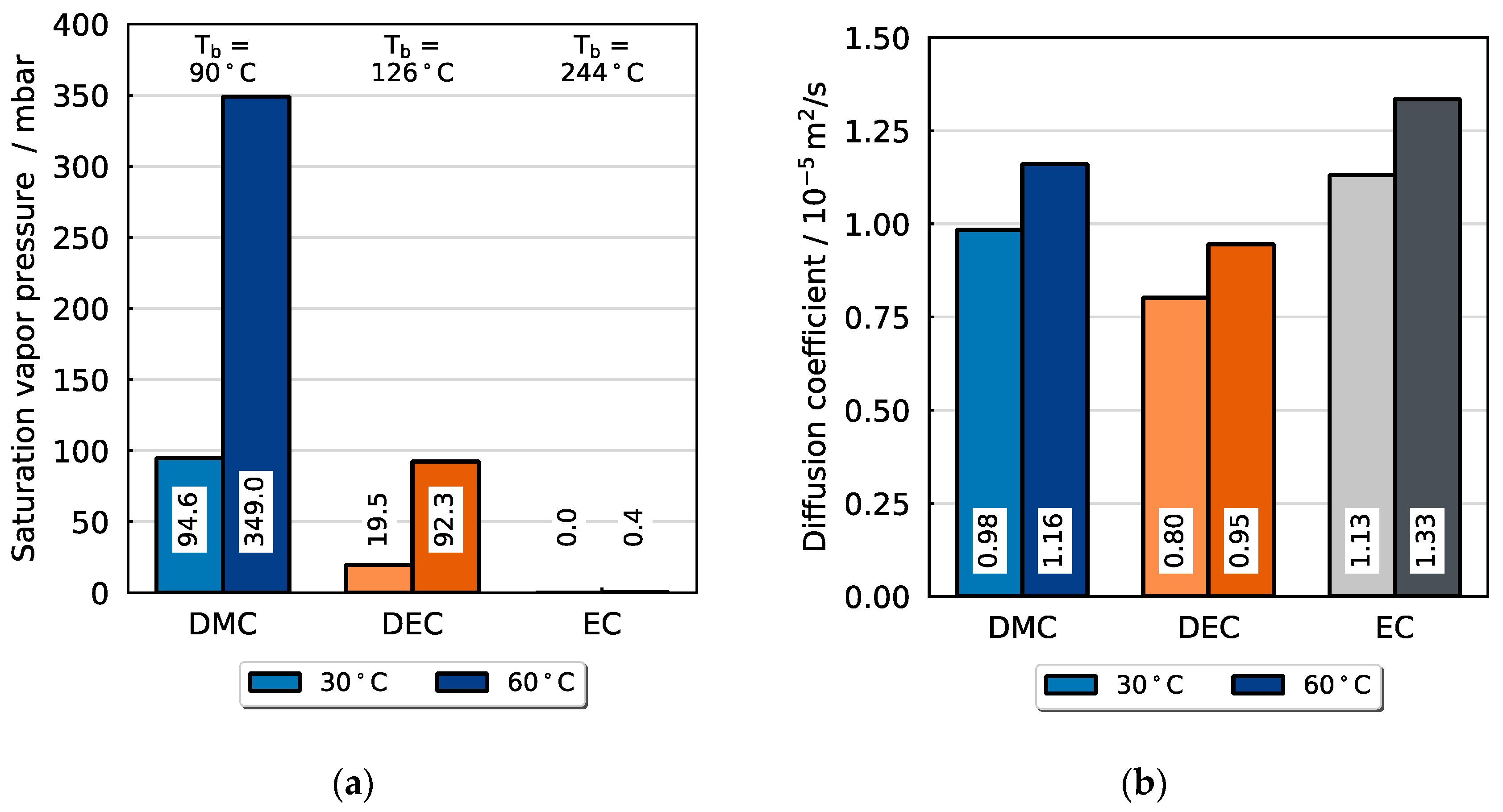
Figure 6 depicts the saturation vapor pressures and the binary diffusion coefficients in nitrogen (carrier gas) for the temperatures applied in the experiments, 30 °C and 60 °C (correlations compare
Section 3). As can be seen in the equations and plots (see
Appendix A), the vapor pressure exhibits a considerably stronger increase with temperature than the diffusion coefficient in the gas phase. The plot displays the exceptionally low vapor pressure of EC for both temperatures. At 30 °C, the ratio of the vapor pressures of DMC and EC is around 2400, then it decreases to around 810 at 60 °C. The ratio of the vapor pressures of DEC and EC is around 100 and 160 at 30 °C and 60 °C, respectively. Therefore, the liquid–gas phase transition of EC is significantly unfavored towards DMC and DEC. While this aspect is apparently crucial for the selectivity of evaporation of the mixture, it should still be noted that EC shows a slight preference in terms of mass transport in the carrier gas flow compared to the other solvents, as can be seen from the diffusion coefficients in the gas phase. The increased mobility of EC is explained by the lower molar weight and molecule size. For both temperatures, 30 and 60 °C, the diffusion coefficient of EC is 1.15 times and 1.41 times that of DMC and DEC, respectively.
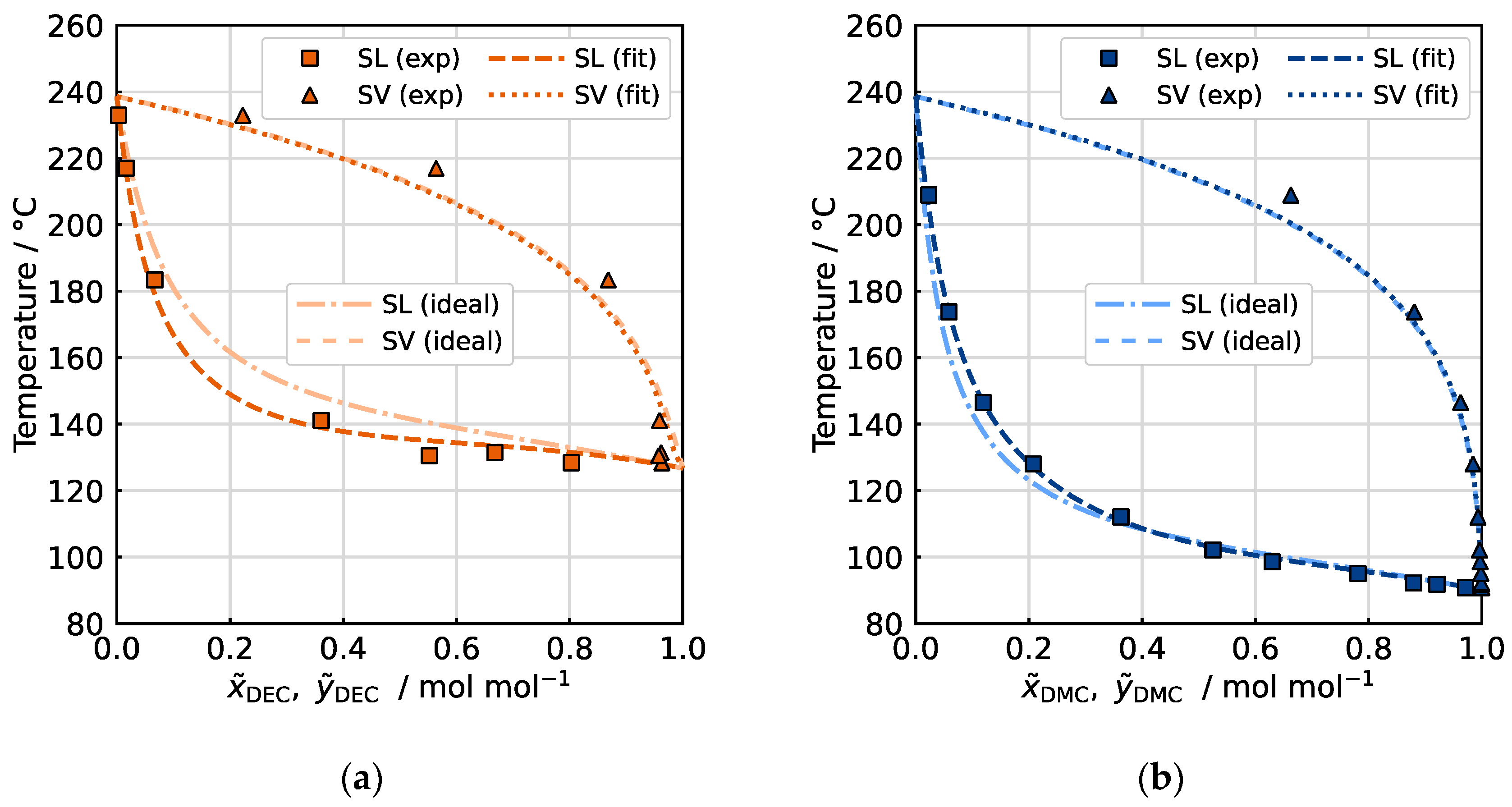
However, the influence of the mutual interactions of the species involved in the mixture on the thermodynamic phase equilibrium can be crucial when describing the vapor–liquid equilibria of such electrolyte–solvent mixtures. According to the literature, binary mixtures of linear carbonates DMC, DEC and EMC exhibit near-ideal behavior due to their similar chemical structures and physical properties. However, stronger molecular interactions occur in mixtures of linear and cyclic carbonates, such as EC or PC [
36]. The vapor–liquid equilibria in
Figure 7 demonstrate that both binary mixtures DMC/EC and DEC/EC exhibit nearly ideal thermodynamic behavior, with minor deviations: DEC is slightly less volatile than expected in an ideal mixture with EC, resulting in a lower proportion in the gas phase relative to its liquid-phase molar fraction. Conversely, DMC shows marginally increased volatility, leading to an elevated gas-phase proportion. In summary, the liquid-to-gas phase transition of the high-boiling EC is affected when mixed with these substances.
In total, the evaporation of EC is substantially disadvantaged regarding the thermodynamic properties, so that its slightly more favorable diffusion properties are presumably insignificant. Against this background, the selectivity of the evaporation of a solvent mixture containing these components is expected to be controlled by the respective vapor pressures of each component. In the next step, it is investigated whether the mathematical approach explained in
Section 3 is suitable for describing the evaporation of the multicomponent mixture in a time- and component-resolved manner under the assumptions made.
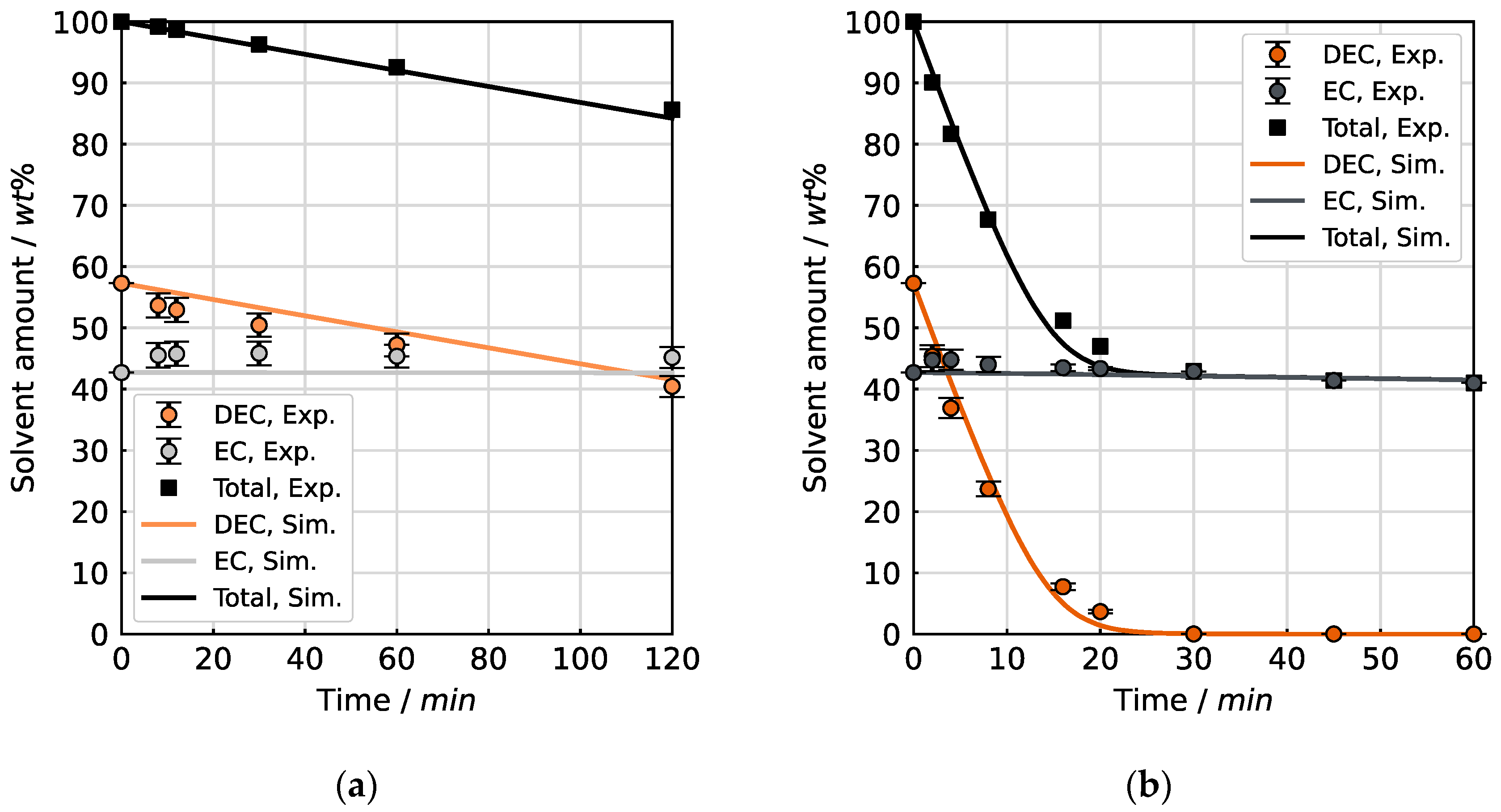
4.2. Evaporation of the Binary Mixture
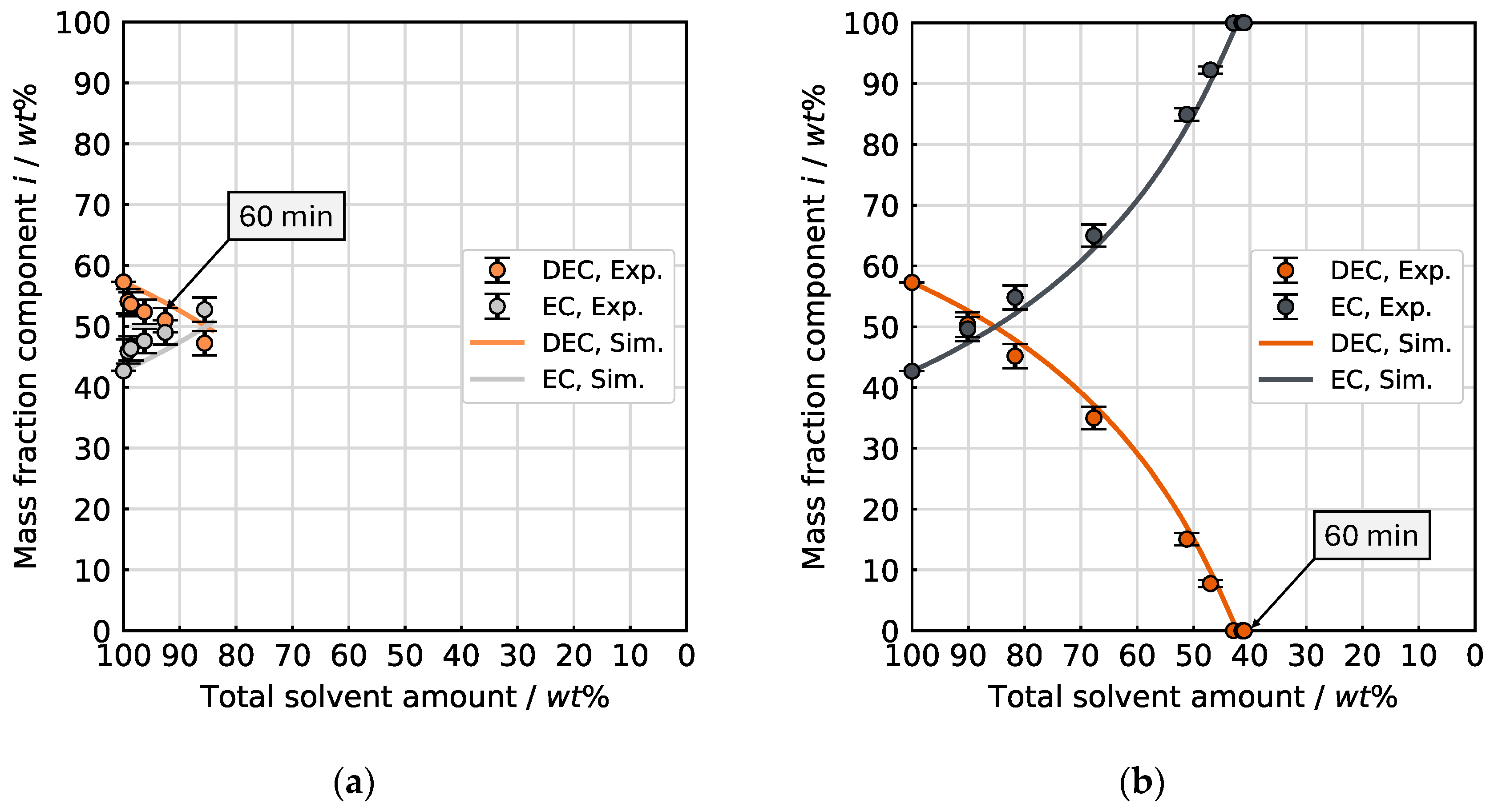
Initially, the binary solvent mixture of 57.3 wt% (50 mol%) DEC and 42.7 wt% (50 mol%) EC was investigated.
Figure 8 shows the experimental data compared with the results of the simulation for the evaporation of the DEC/EC mixture at the LDR and HDR conditions. The change of the mass fractions is plotted as a function of the amount of solvent remaining in the sample vessel relative to the start.
The two experiments show a significant increase in the evaporation rate for the HDR conditions in comparison to the LDR conditions, as indicated by the remaining solvent amount after the same drying time of 60 min. The respective final measurement point corresponds to 60 min drying time. At the HDR conditions, approximately 59 wt% of the solvent mixture has evaporated after 60 min, leading to a remainder of 41 wt%. In contrast, around 92 wt% of the initial solvent amount is left under the LDR conditions after 60 min.
This acceleration for the HDR conditions is explained through intensified mass transfer (, Equation (1)) and increased concentration gradients between the vapor–liquid boundary layer and the surrounding gas phase (, Equation (1)). The higher temperature results in enhanced solvent vapor pressure at the vapor–liquid boundary layer and raised diffusion kinetics of the vapor molecules through the surrounding gas phase. The enlarged mass flow of the carrier gas provides for a thinner concentration boundary layer thickness and greater dilution of the evaporated solvent in the gas phase, thus intensifying the evaporation rate. For both drying conditions, EC steadily enriches (and DEC depletes) at an approximately constant rate with decreasing solvent amount. With regard to evaporation selectivity, there is practically no difference between the two drying curves. This means that the volatilization of DEC is greatly preferred compared to EC, with the selectivity being independent of the drying conditions. The experiments follow the expectations in terms of evaporation kinetics and selectivity. For the present solvent mixture and the selected conditions, the evaporation selectivity is controlled by the vapor pressure as the governing thermodynamic property. The mass transport kinetics of the individual species in the gas phase determine the overall evaporation rate.
In the simulation for the binary mixture, the molecular interactions between the two solvents in the mixture are taken into account by concentration-dependent activity coefficients [
18]. The calculated curve and the measurement points align throughout their entire course. This observation shows that the employed simulation approach with the material system properties used can reproduce the evaporation selectivity for this binary solvent mixture for the adjusted boundary conditions.
An assessment of the predictive accuracy of the simulation regarding evaporation kinetics is conducted in
Figure 9. In these plots, the measured solvent amount per component at certain points in time is visualized along with the calculated solvent amount of both solvents DEC and EC. They show the decreasing solvent amount over time—broken down by components for the LDR and HDR conditions, respectively. Consequently, these figures allow the assessment of simulative reproduction of the evaporation kinetics for different drying conditions.
At the LDR conditions, at the end of the experiment after 120 min, 86 wt% of the initial solvent is left, as opposed to 84 wt% as predicted by the simulation. In the case of the HDR conditions, the calculated share of 42 wt% of the initial solvent remaining after 60 min is according to the experiment. For both experiments, the simulation is in agreement with the measured values, accurately predicting not only the total solvent amount but also the kinetics of both components individually. The slightly larger deviation observed for the LDR conditions can be attributed to the higher uncertainty of spectrometric composition measurements in nearly equimolar mixtures.
The course (ascent/slope) of the curve from the simulation is in accordance with the experimental data points for both experiments. Especially noticeable is the low evaporated amount of EC, which also means that DEC represents almost the total evaporation flow. A conclusion follows that the low-evaporating component EC is indeed the substance within this solvent mixture that limits the initial drying process (evaporation from a free liquid surface) of electrolytes during LIB recycling.
4.3. Evaporation of the Ternary Mixture
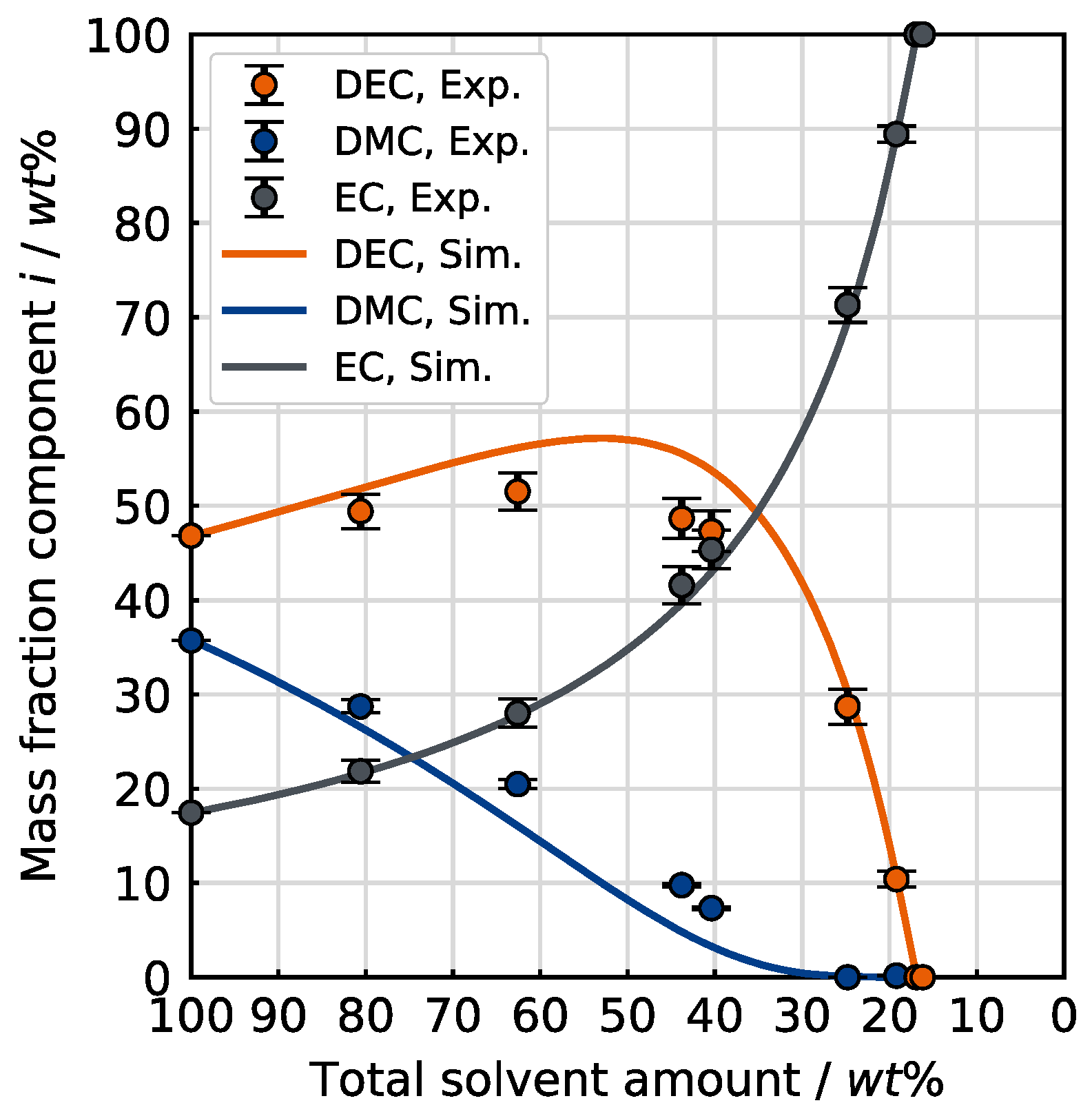
In the next step, the evaporation of a ternary mixture was investigated. The low boiling solvent DMC is included as the third electrolyte solvent with an initial mass fraction in the mixture of 35.7 wt% (40 mol%), while DEC and EC account for 46.8 wt% (40 mol%) and 17.5 wt% (20 mol%), respectively. The experiment was conducted using HDR drying conditions.
Figure 10 illustrates the evaporation of the ternary mixture at the selected conditions, comparing the experimental and simulation results.
The experiment shows a continuous enrichment of EC. When the remaining solvent consists of nearly pure EC, the overall solvent amount is around 16 wt% of the initial amount. From this, it can be deduced that only 8 wt% of the EC initially present has evaporated when both DMC and DEC are volatilized. This observation emphasizes the low volatility of EC and, thus, its key role in thermal drying processes within LIB recycling processes. Even at these drying conditions with extraordinarily high mass transfer numbers compared to technical dryers, the evaporation rate of EC is low. Furthermore, the plot shows a steady depletion of DMC, while the share of DEC in the mixture first increases, then passes through a maximum of 55 wt%, then decreases again. The experimental data are in accordance with the expectations based on the comparison of the partial vapor pressure of the individual solvents, whereupon DMC is volatilized first, closely followed by DEC and eventually EC. As in the case of the investigated binary mixture, the evaporation selectivity of this ternary mixture is thermodynamically controlled by the vapor pressures of the initial solvents. The evaporation behavior of the ternary mixture was not investigated at the LDR conditions.
The calculation is based on the assumption of thermodynamically ideal mixtures due to the unavailability of ternary vapor–liquid equilibrium (VLE) data. As shown in
Figure 10, the change in solvent composition with decreasing solvent amount predicted by the simulation is in agreement with the experimental data. Although the simulation neglects intermolecular interactions, this agreement can be explained by the fact that the mixture predominantly consists of linear carbonates throughout the experiment, which exhibit only weak interactions. As the EC mass fraction increases, the stronger interactions between the linear carbonates and EC gradually become more significant. However, this effect only becomes relevant in the final stage of the experiment, meaning it cannot be captured by the experimental measurement with the required level of detail.
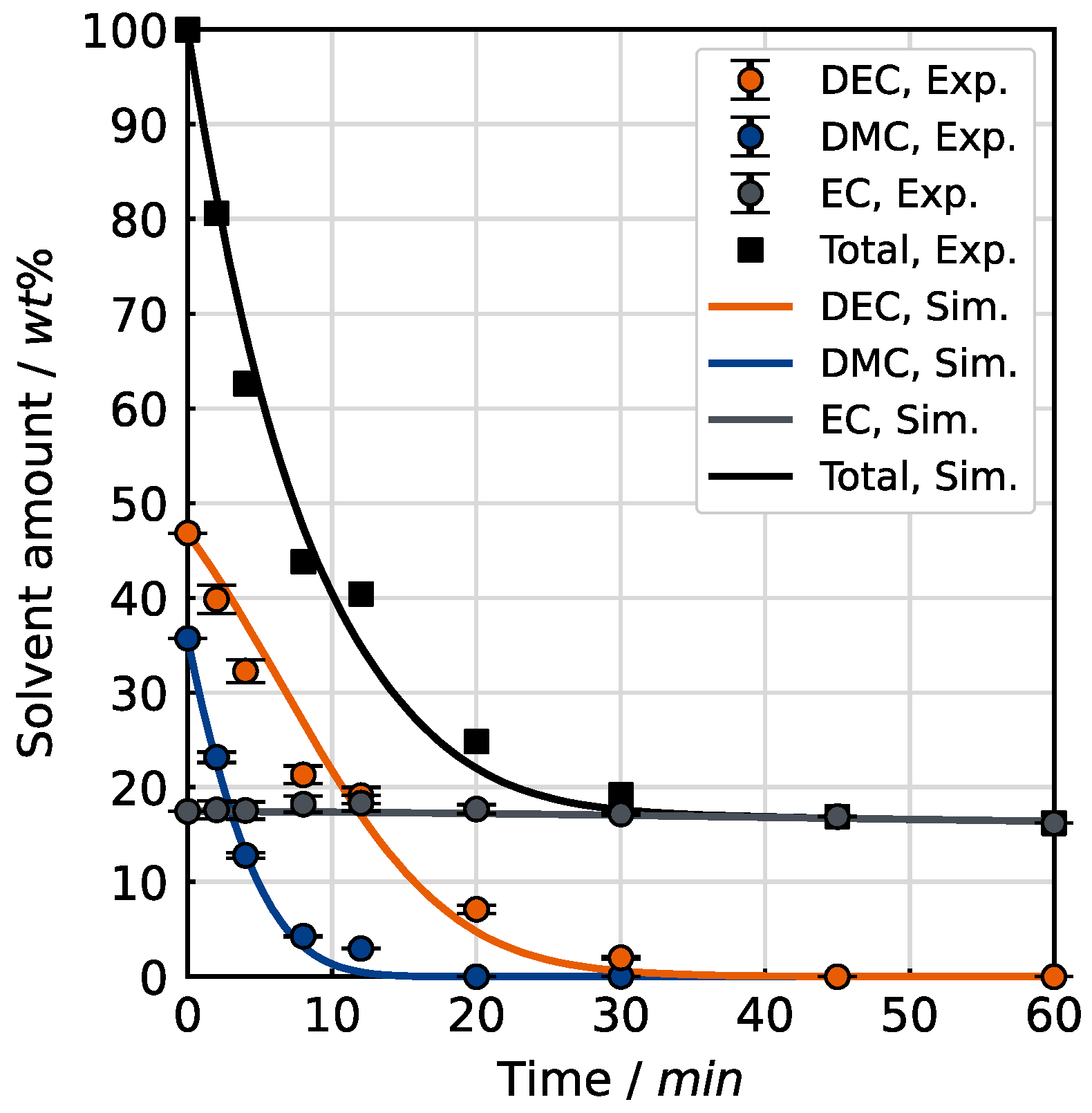
Figure 11 depicts the evaporation kinetics. The measured solvent amount per component is compared to the calculated solvent amount of DMC, DEC and EC taken from the simulation.
The minor deviations for the decrease in solvent amount for each component can be attributed to the neglection of the molecular interactions between the species in the mixture. Overall, the simulation can also reproduce the evaporation behavior of the ternary mixture.
The experiments on the initial drying, which is controlled by the thermodynamics at the free liquid surface and the mass transfer kinetics in the surrounding external gas phase, confirm the expectations that the high-boiling electrolyte solvent EC has significantly lower drying rates than the low boiling DMC and DEC. This means that there is practically a transition from the initial multi-component evaporation to pure-substance evaporation, where EC is clearly the crucial component. The presented simulation model can predict the kinetics and selectivity of the evaporation of multicomponent electrolyte–solvent mixtures from a free surface. Provided that the necessary thermodynamic and gas-kinetic properties of the substances are available, the simulation model can be flexibly applied to predict the drying behavior across a wide range of electrolyte mixtures, as well as other solvent mixtures.
5. Conclusions
This study provides a comprehensive investigation of the initial drying behavior of electrolyte–solvent mixtures under defined process boundary conditions, such as temperature, carrier gas flow and geometry. Focusing on the early drying phase, when the solvent-wetted surface of comminuted battery material is directly exposed to the surrounding gas phase, mass transport resistances within the porous network of electrodes and polymers and sorptive interactions between solvent and solid are negligible. Solvent evaporation is solely governed by gas-phase heat and mass transfer and the thermodynamic phase equilibrium at the vapor–liquid interface.
The experimental results demonstrate that evaporation selectivity is governed by the vapor pressure of the individual solvents, while the drying kinetics are determined by the vapor pressure and the mass transport of the solvent in the gas phase. Low volatility of ethylene carbonate (EC) is the limiting factor in low-temperature thermal treatment. Drying under vacuum or, even further, enhanced carrier gas flow are effective approaches to address the exceedingly low vapor pressures of such solvents, maintaining low temperatures to prevent substantial adverse reactions.
A simulation model for the mathematical description of the solvent-mixture evaporation (at constant temperature) was developed and experimentally validated for the binary and ternary mixtures. It describes the drying behavior (kinetics and selectivity) of the solvent mixtures using the mass transport approach for the gas phase coupled with the thermodynamic phase equilibrium at the vapor–liquid boundary. The simulation reproduces the selectivity and kinetics of the solvent evaporation measured in the experiments in good accordance. Minor deviations in the ternary case are attributed to the assumption of an ideal mixture and are expected to be reduced by incorporating the corresponding non-ideal phase equilibria. The validated simulation model is applicable for calculating minimum required drying times relying only on boundary conditions derived from dryer-specific parameters. Although it is limited to pure solvent evaporation from a free solvent surface, it provides a solid foundation for developing an extended framework that can accurately design and scale real drying processes of EoL batteries with complex material interactions.
To increase the applicability of this simulation model for a reliable prediction of the electrolyte–solvent removal throughout the entire drying process, it needs to be expanded in the following steps. Since the majority of solvent fills the pores of the electrodes and separator, with decreasing solvent content, structural influences are gradually enhanced, and molecular interactions in the electrolyte are altered. As the drying progresses, additional mass transport resistances in the porous and absorbing materials in the battery cell come into play, and the steadily increasing concentration of conductive salt affects the electrolyte solvents’ vapor–liquid equilibrium.
As the simulation model is based exclusively on boundary conditions derived from dryer-specific parameters, it is applicable for determining minimum required drying times across a wide range of dryers and process conditions. Ongoing and future experimental investigations aim to quantify the influences of structural and sorptive mass transport resistance, as well as the solvent/salt interactions in the electrolyte on drying. Significantly reduced drying rates towards low residual solvent loadings were observed in drying experiments with electrodes and separators. Since mass transport within polymers is significantly slower than in the gas phase, the presence of absorbed electrolyte solvent in the polymer components (specifically the binders in the electrodes and the polymer-based commercial separators) is likely. In this case, the release of EC could be strongly favored over DMC and DEC due to its smaller molecule size and, thus, higher mobility. Determining the diffusion coefficients and sorption equilibria of solvents in absorbing materials allows for describing effective drying kinetics.
Overall, this study lays the foundation for a physics-based, holistic simulation framework that can predict electrolyte–solvent evaporation across various dryer configurations and process boundary conditions. Such a model is essential for optimizing thermal drying in the mechanical–hydrometallurgical recycling process of liquid electrolyte batteries.