Protein-Based Strategies for Non-Alkali Metal-Ion Batteries
Abstract
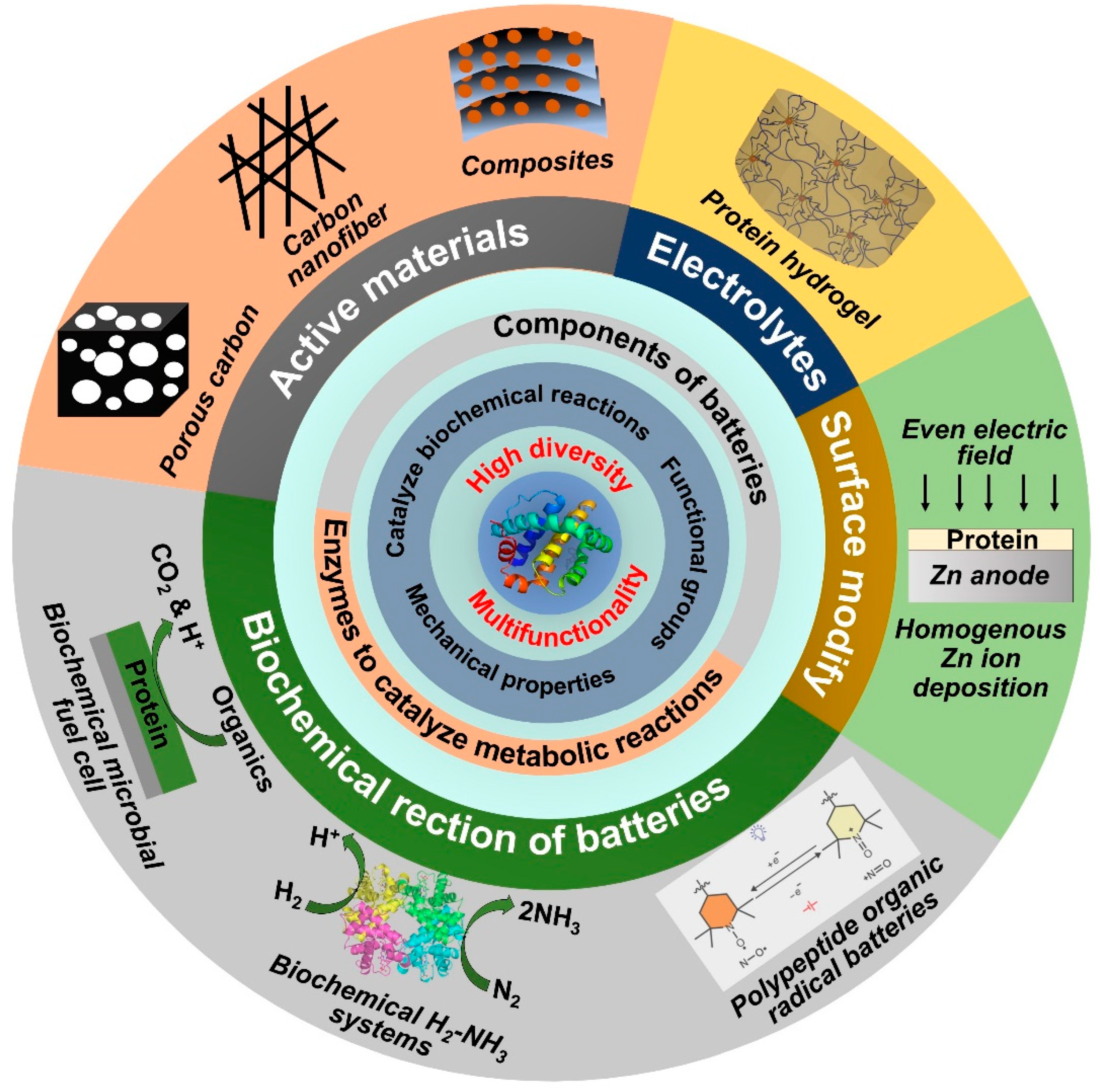
1. Introduction
2. Protein-Derived Materials for Electrodes
2.1. Protein-Derived Carbon
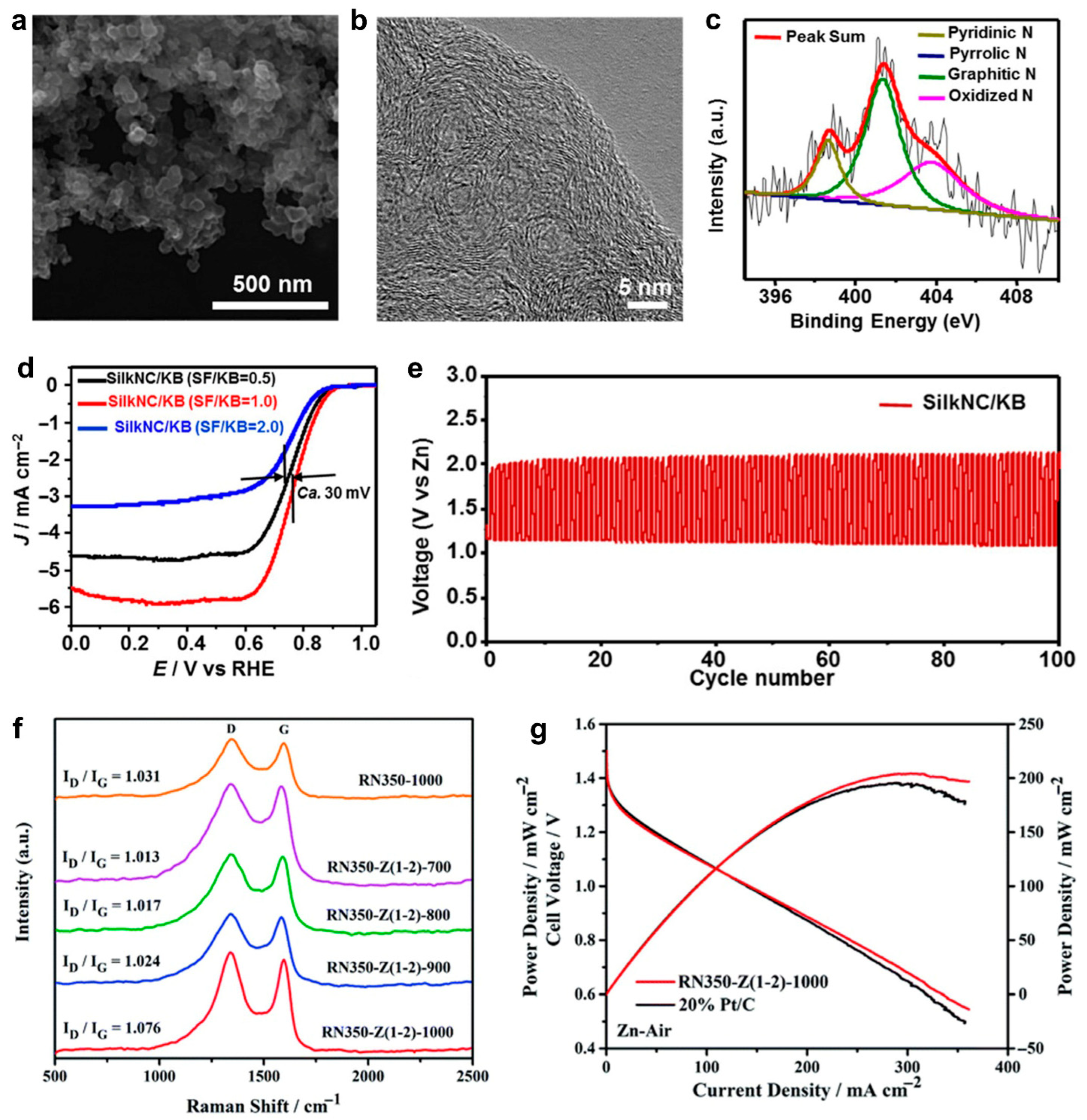
2.1.1. Protein-Derived Carbon for Electrodes of Zn–Air Batteries
2.1.2. Protein-Derived Carbon for Electrodes of Vanadium Redox Flow Batteries
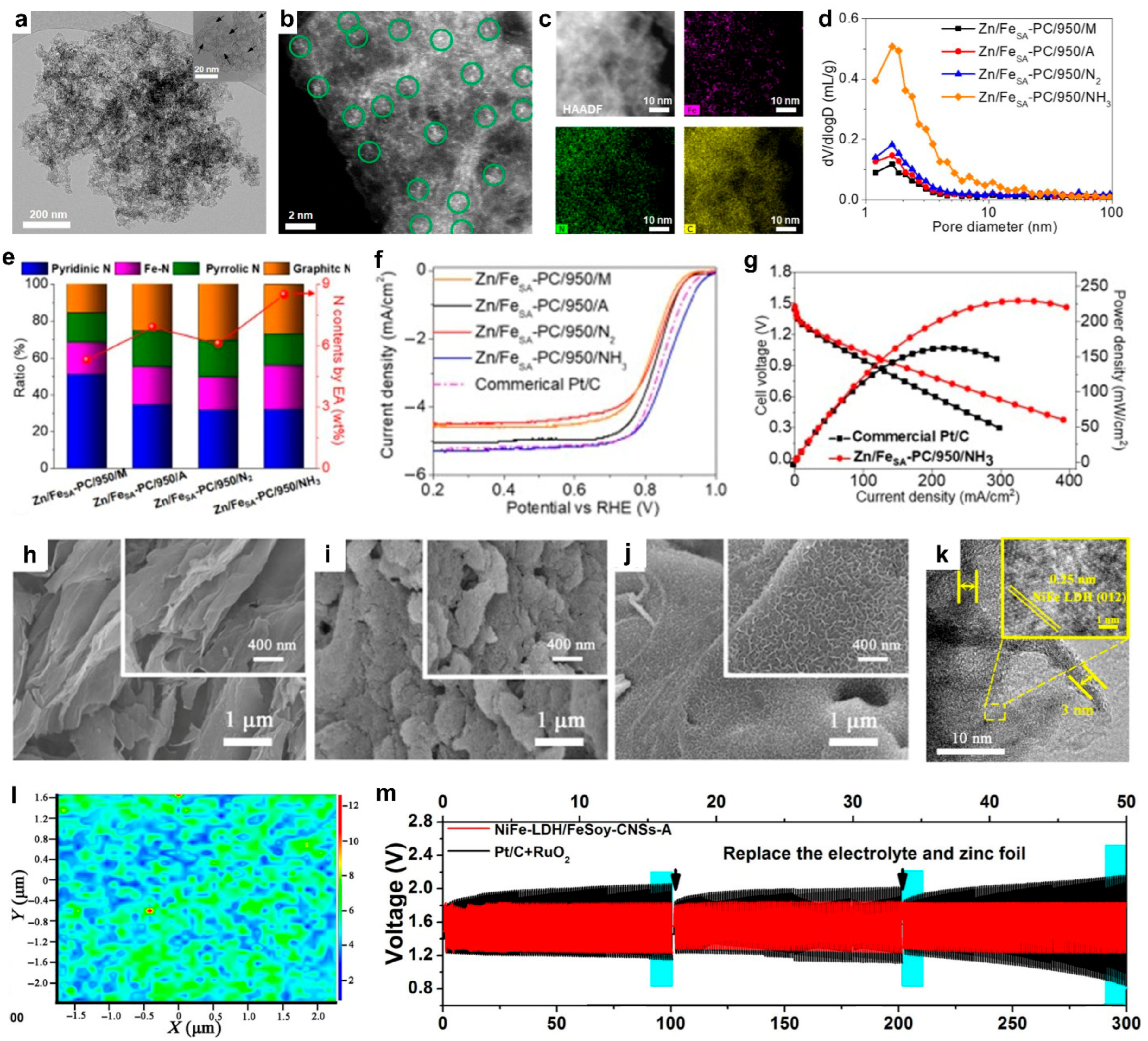
2.2. Protein-Modified Active Materials
3. Protective Films for Zn Metal Anodes
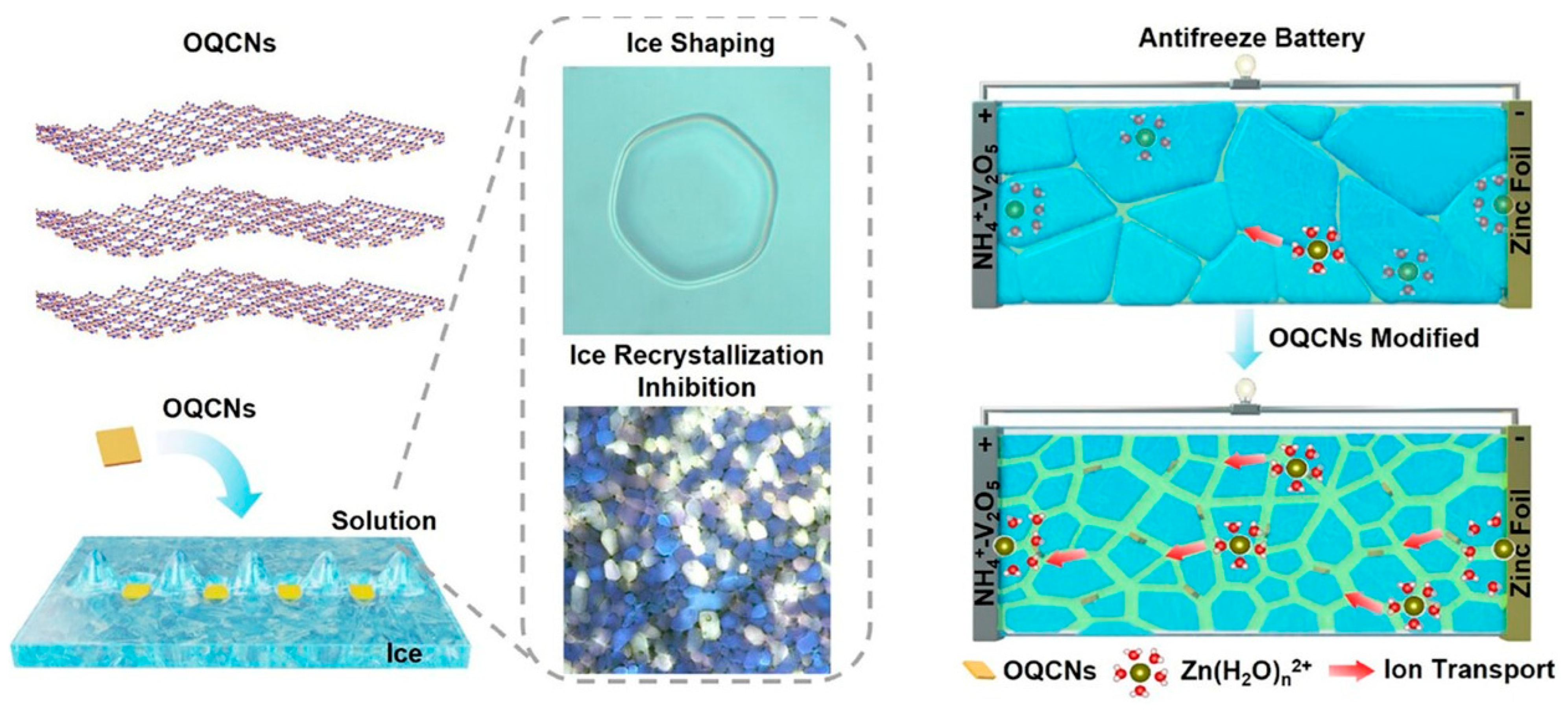
4. Protein-Based Electrolytes for Zn-Based Batteries
4.1. Liquid Electrolytes
4.2. Gel State Electrolytes
4.3. Solid-State Electrolytes
5. Protein-Derived Active Electrodes for Bio-Nanobatteries
6. Organic Radical Batteries
7. Conclusions and Perspectives
- (1)
- Protein-derived biomass carbon materials have attracted attention for their high surface area, porous architecture, and tunable surface chemistry. The biomass carbon derived from proteins such as silk, root nodule protein and zein, has demonstrated excellent performance as electrodes in Zn–air batteries and vanadium redox flow batteries (VRFBs). In Zn–air batteries, nitrogen-doped carbon from silk and protein-rich root nodules have enabled effective oxygen reduction and evolution reactions, offering good catalytic activity and long cycling stability. In VRFBs, carbonized silk and zein-based materials exhibited improved redox kinetics and high electrochemical reversibility, which were attributed to their high nitrogen/oxygen content and interconnected conductive networks.
- (2)
- Protein-modified active materials have improved battery performance by integrating single-atom catalysts or nanocomposites within protein-derived carbon matrices. Fe-N-C structures derived from pig blood or soybean showed enhanced ORR activity and durability in Zn–air batteries. Additionally, bovine serum albumin (BSA) was used as an exfoliation agent to synthesize MoS2/graphene hybrids, improving their dispersion and electrocatalytic performance. The non-alkali metal ion battery systems with the protein-derived active materials demonstrated improved power densities, voltage outputs, and cycling lifespans.
- (3)
- Proteins were able to regulate Zn metal anodes to suppress dendrite formation and side reactions with water in aqueous Zn-ion batteries. Collagen hydrolysate (CH) and silk fibroin (SF) have been successfully applied to construct protective films on the Zn metal anode, leading to smoother Zn deposition that improved cycling stability even under high-temperature conditions. These protein molecules contributed to stable solvation structures and ion transport regulation, demonstrating potential scalability from coin cells to large-format batteries.
- (4)
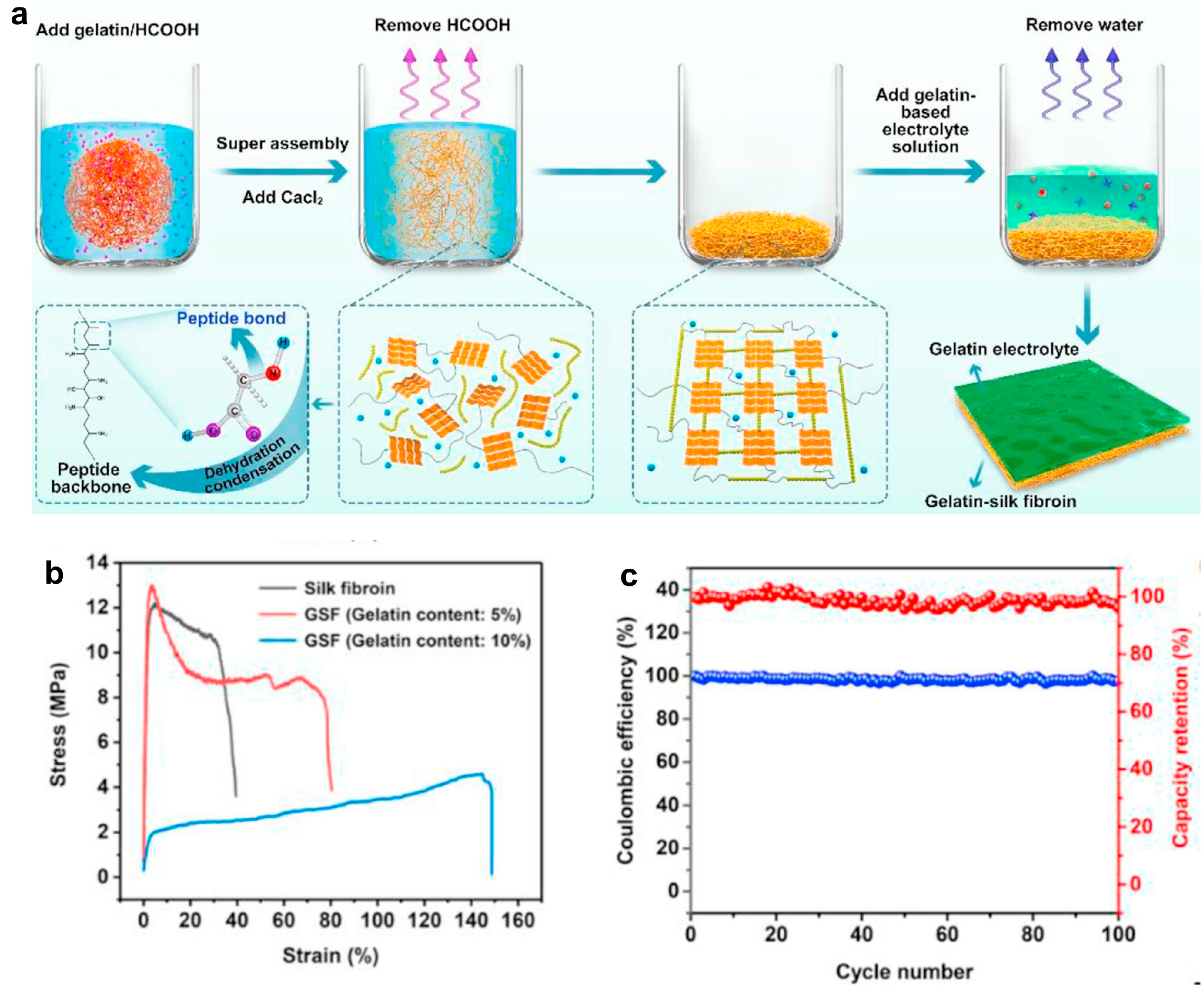
- Proteins also served as promising candidates in gel electrolytes for biodegradable and flexible Zn-ion batteries. A gelatin-silk protein composite formed a plasticized gel matrix with good ionic conductivity and mechanical robustness. The gel electrolyte delivered consistent voltage output and degraded in enzymatic environments, aligning with growing interest in transient and eco-friendly energy storage technologies.
- (5)
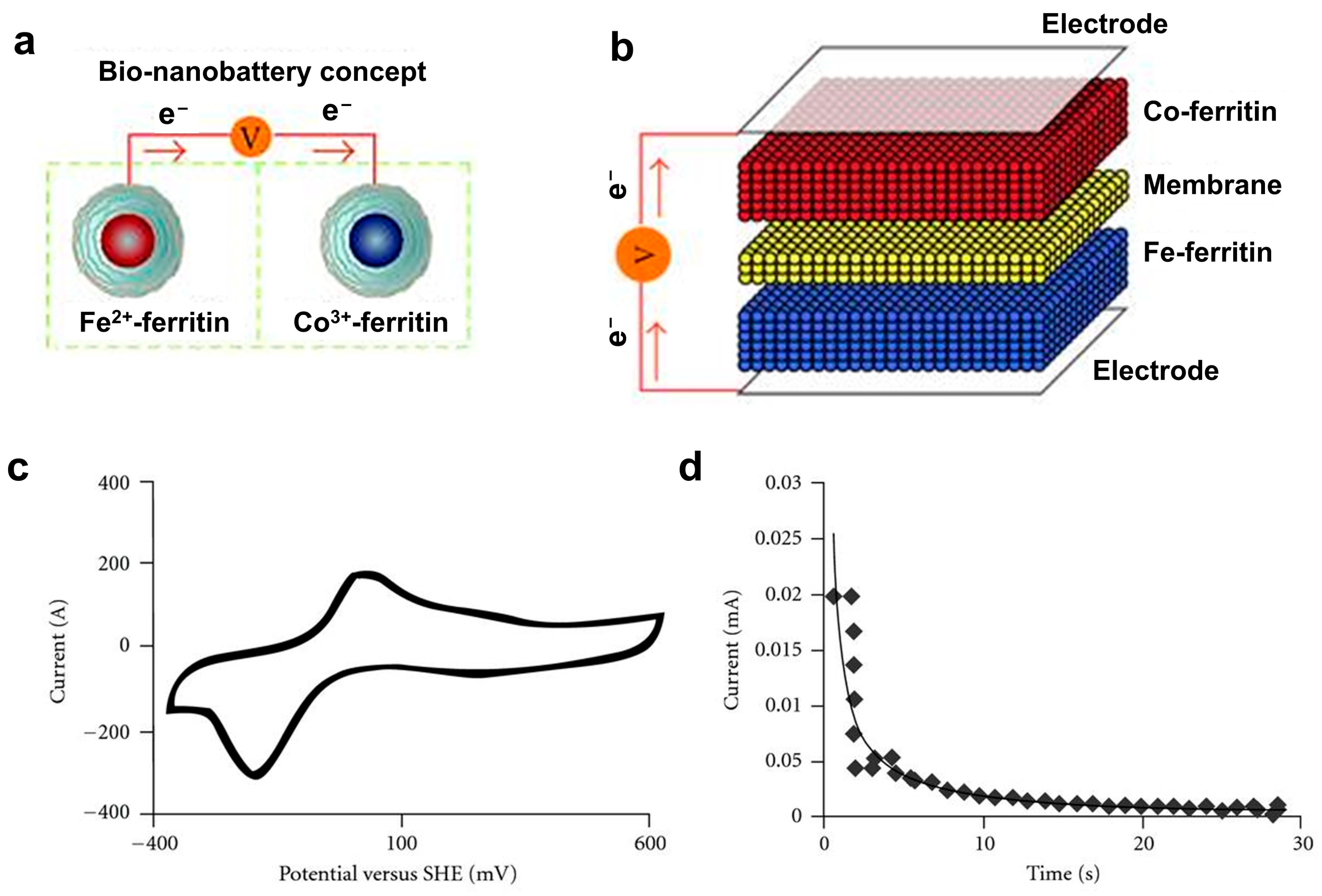
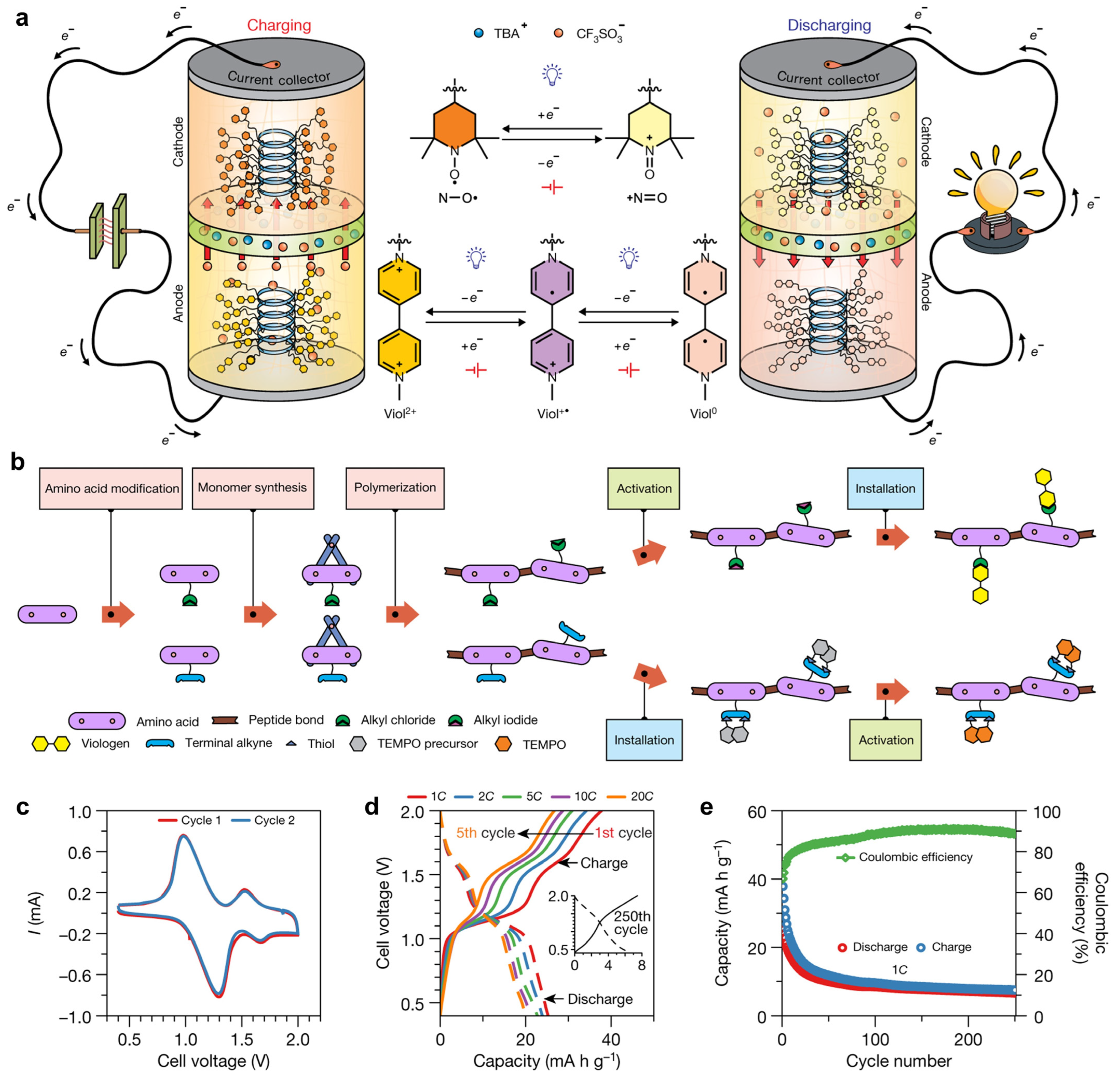
- Ferritin-based bio-nanobatteries have been developed by employing ferritins loaded with Fe(OH)2 and Co(OH)3 as anode and cathode materials, respectively. These nanoscale batteries generated stable voltage and were fully regenerable. In addition, redox-active polypeptides synthesized from amino acids have been applied as the active materials of anode and cathode of batteries, which offered competitive energy storage capacity and cycling stability while being fully degradable under mild acidic conditions.
Author Contributions
Funding
Conflicts of Interest
References
- Hassan, Q.; Viktor, P.; Al-Musawi, T.J.; Mahmood Ali, B.; Algburi, S.; Alzoubi, H.M.; Khudhair Al-Jiboory, A.; Zuhair Sameen, A.; Salman, H.M.; Jaszczur, M. The Renewable Energy Role in the Global Energy Transformations. Renew. Energy Focus 2024, 48, 100545. [Google Scholar] [CrossRef]
- Elalfy, D.A.; Gouda, E.; Kotb, M.F.; Bureš, V.; Sedhom, B.E. Comprehensive Review of Energy Storage Systems Technologies, Objectives, Challenges, and Future Trends. Energy Strateg. Rev. 2024, 54, 101482. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef]
- Zampardi, G.; La Mantia, F. Open Challenges and Good Experimental Practices in the Research Field of Aqueous Zn-Ion Batteries. Nat. Commun. 2022, 13, 687. [Google Scholar] [CrossRef]
- Leong, K.W.; Wang, Y.; Ni, M.; Pan, W.; Luo, S.; Leung, D.Y.C. Rechargeable Zn-Air Batteries: Recent Trends and Future Perspectives. Renew. Sustain. Energy Rev. 2022, 154, 111771. [Google Scholar] [CrossRef]
- Yao, Y.; Lei, J.; Shi, Y.; Ai, F.; Lu, Y.C. Assessment Methods and Performance Metrics for Redox Flow Batteries. Nat. Energy 2021, 6, 582–588. [Google Scholar] [CrossRef]
- Liu, L.; Solin, N.; Inganäs, O. Bio Based Batteries. Adv. Energy Mater. 2021, 11, 2003713. [Google Scholar] [CrossRef]
- Tang, L.; Peng, H.; Kang, J.; Chen, H.; Zhang, M.; Liu, Y.; Kim, D.H.; Liu, Y.; Lin, Z. Zn-Based Batteries for Sustainable Energy Storage: Strategies and Mechanisms. Chem. Soc. Rev. 2024, 53, 4877–4925. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Díez, E.; Ventosa, E.; Guarnieri, M.; Trovò, A.; Flox, C.; Marcilla, R.; Soavi, F.; Mazur, P.; Aranzabe, E.; Ferret, R. Redox Flow Batteries: Status and Perspective towards Sustainable Stationary Energy Storage. J. Power Sources 2021, 481, 228804. [Google Scholar] [CrossRef]
- Feng, X.; Bai, Y.; Liu, M.; Li, Y.; Yang, H.; Wang, X.; Wu, C. Untangling the Respective Effects of Heteroatom-Doped Carbon Materials in Batteries, Supercapacitors and the ORR to Design High Performance Materials. Energy Environ. Sci. 2021, 14, 2036–2089. [Google Scholar] [CrossRef]
- Wang, C.; Yang, X.; Zheng, M.; Xu, Y. Synthesis of β-FeOOH Nanorods Adhered to Pine-Biomass Carbon as a Low-Cost Anode Material for Li-Ion Batteries. J. Alloys Compd. 2019, 794, 569–575. [Google Scholar] [CrossRef]
- Yuan, C.; Bin Wu, H.; Xie, Y.; Lou, X.W. Mixed Transition-Metal Oxides: Design, Synthesis, and Energy-Related Applications. Angew. Chem. Int. Ed. 2014, 53, 1488–1504. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, M.; Xu, Y.; Wang, D.; Liu, D. Cu Ion Induced Morphology Change of Hematite Microspheres as Lithium Ion Battery Anode Material by Solvothermal Synthesis. Ceram. Int. 2019, 45, 2940–2947. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, Y.; Li, H.; Wang, C.; Qin, B.; Li, Z.; Chen, Y.; Jiang, K.; Zhang, H. Incorporating SnO2 Nanodots into Wood Flour-Derived Hierarchically Porous Carbon as Low-Cost Anodes for Superior Lithium Storage. J. Electroanal. Chem. 2020, 856, 113654. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Zhou, J.; Wang, C.; Zhang, W.; Li, Z.; Guo, F.; Chen, H.; Zhang, H. Highly Dispersed SnO2 Nanoparticles Confined on Xylem Fiber-Derived Carbon Frameworks as Anodes for Lithium-Ion Batteries. J. Electroanal. Chem. 2020, 879, 114753. [Google Scholar] [CrossRef]
- Geng, P.; Zheng, S.; Tang, H.; Zhu, R.; Zhang, L.; Cao, S.; Xue, H.; Pang, H. Transition Metal Sulfides Based on Graphene for Electrochemical Energy Storage. Adv. Energy Mater. 2018, 8, 1703259. [Google Scholar] [CrossRef]
- Zheng, M.; Wang, C.; Xu, Y.; Li, K.; Liu, D. A Water-Soluble Binary Conductive Binder for Si Anode Lithium Ion Battery. Electrochim. Acta 2019, 305, 555–562. [Google Scholar] [CrossRef]
- Shi, Y.; Peng, L.; Ding, Y.; Zhao, Y.; Yu, G. Nanostructured Conductive Polymers for Advanced Energy Storage. Chem. Soc. Rev. 2015, 44, 6684–6696. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, Z.; Zou, R.; Xu, Q. Metal-Organic Frameworks for Batteries. Joule 2018, 2, 2235–2259. [Google Scholar] [CrossRef]
- Mehtab, T.; Yasin, G.; Arif, M.; Shakeel, M.; Korai, R.M.; Nadeem, M.; Muhammad, N.; Lu, X. Metal-Organic Frameworks for Energy Storage Devices: Batteries and Supercapacitors. J. Energy Storage 2019, 21, 632–646. [Google Scholar] [CrossRef]
- Wang, C.; Fu, X.; Ying, C.; Liu, J.; Zhong, W. Natural Protein as Novel Additive of a Commercial Electrolyte for Long-Cycling Lithium Metal Batteries. Chem. Eng. J. 2022, 437 Pt 1, 135283. [Google Scholar] [CrossRef]
- Wang, C.; Fu, X.; Lin, S.; Liu, J.; Zhong, W.H. A Protein-Enabled Protective Film with Functions of Self-Adapting and Anion-Anchoring for Stabilizing Lithium-Metal Batteries. J. Energy Chem. 2021, 64, 485–495. [Google Scholar] [CrossRef]
- Wang, C.; Odstrcil, R.; Liu, J.; Zhong, W.H. Protein-Modified SEI Formation and Evolution in Li Metal Batteries. J. Energy Chem. 2022, 73, 248–258. [Google Scholar] [CrossRef]
- Wang, Y.; Katyal, P.; Montclare, J.K. Protein-Engineered Functional Materials. Adv. Healthc. Mater. 2019, 8, e1801374. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ren, L.; Ying, C.; Liu, J.; Zhong, W.H. An Amino Acid-Enabled Separator for Effective Stabilization of Li Anodes. ACS Appl. Mater. Interfaces 2024, 16, 15632–15639. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Fu, X.; Luo, M.; Wang, C.; Zhong, W.H. Interface-Tailored Forces Fluffing Protein Fiber Membranes for High-Performance Filtration. Sep. Purif. Technol. 2022, 278, 119570. [Google Scholar] [CrossRef]
- Fu, X.; Scudiero, L.; Zhong, W.H. A Robust and Ion-Conductive Protein-Based Binder Enabling Strong Polysulfide Anchoring for High-Energy Lithium-Sulfur Batteries. J. Mater. Chem. A 2019, 7, 1835–1848. [Google Scholar] [CrossRef]
- Ying, C.; Wang, C.; Zhong, W.H.; Liu, J. Dual Anchoring Mechanism of Protein-Based Binder for Lithium-Sulfur Batteries. J. Phys. Chem. C 2024, 128, 14522–14528. [Google Scholar] [CrossRef]
- Li, Y.; Ding, P.; Cai, L.; Shi, L.; Zhao, Y.; Liu, H.; Yuan, H.; Yu, D.; Guo, C.; Gao, Q.; et al. Eco-Friendly Soy Protein-Based Solid-State Electrolyte Exhibiting Stable High-Rate Cyclic Performances by Molecular Regulation Design. Adv. Energy Mater. 2025, 15, 2501056. [Google Scholar] [CrossRef]
- Ying, C.; Wang, C.; Zhong, W.H.; Liu, J. Effects of Anions and Protein Structures on Protein-Based Solid Electrolytes. Adv. Mater. Technol. 2023, 8, 2201875. [Google Scholar] [CrossRef]
- Ju, Z.; Lu, G.; Sheng, O.; Yuan, H.; Zhou, S.; Liu, T.; Liu, Y.; Wang, Y.; Nai, J.; Zhang, W.; et al. Soybean Protein Fiber Enabled Controllable Li Deposition and a LiF-Nanocrystal-Enriched Interface for Stable Li Metal Batteries. Nano Lett. 2022, 22, 1374–1381. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ren, L.; Ying, C.; Shang, J.; Mccloy, J.; Liu, J. Soy Protein as a Dual-Functional Bridge Enabling High Performance Solid Electrolyte for Li Metal Batteries. J. Power Sources 2024, 620, 235260. [Google Scholar] [CrossRef]
- Miao, J.; Fang, Y.; Wang, H.; Lyu, L.; Bai, W.; Li, B.; Kong, D.; Xu, T.; Li, X.; Xu, Z.L.; et al. Dendrite Suppression Enabled Longevous Sodium Metal Batteries by Sodiophilic Zein/MXene Nanofiber Modulated Polypropylene Separator. Energy Storage Mater. 2024, 71, 103591. [Google Scholar] [CrossRef]
- Ren, L.; Ying, C.; Wang, C.; Guo, Y.; Liu, J.; Zhong, W.H. Zein Protein-Functionalized Separator through a Viable Method for Trapping Polysulfides and Regulating Ion Transport in Li-S Batteries. J. Energy Storage 2024, 100 Pt A, 113547. [Google Scholar] [CrossRef]
- Ding, C.; Huang, L.; Guo, Y.; Lan, J.-L.; Yu, Y.; Fu, X.; Zhong, W.-H.; Yang, X. Let It Catch: A Short-Branched Protein for Efficiently Capturing Polysulfides in Lithium–Sulfur Batteries. Adv. Energy Mater. 2020, 10, 1903642. [Google Scholar] [CrossRef]
- Ding, C.; Huang, L.; Guo, Y.; Lan, J.-L.; Yu, Y.; Fu, X.; Zhong, W.H.; Yang, X. An Ultra-Durable Gel Electrolyte Stabilizing Ion Deposition and Trapping Polysulfides for Lithium-Sulfur Batteries. Energy Storage Mater. 2020, 27, 25–34. [Google Scholar] [CrossRef]
- Guo, Y.; Sireesha, P.; Wang, C.; Ren, L.; Ying, C.; Liu, J.; Zhong, W. An Interpenetrated Protein-Polar Polymer Interlayer for Suppressing Shuttle Effect in Li-S Batteries. J. Power Sources 2025, 630, 236145. [Google Scholar] [CrossRef]
- Zhang, Y.; Yue, K.; Zhao, H.; Wu, Y.; Duan, L.; Wang, K.L. Bovine Serum Albumin Assisted Synthesis of Fe3O4@C@Mn3O4 Multilayer Core-Shell Porous Spheres as Anodes for Lithium Ion Battery. Chem. Eng. J. 2016, 291, 238–243. [Google Scholar] [CrossRef]
- Wang, C.; Ying, C.; Shang, J.; Karcher, S.E.; McCloy, J.; Liu, J.; Zhong, W.H. A Bioinspired Coating for Stabilizing Li Metal Batteries. ACS Appl. Mater. Interfaces 2022, 14, 43886–43896. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.F.P.; Brito-Pereira, R.; Gonçalves, R.; Silva, M.P.; Costa, C.M.; Silva, M.M.; De Zea Bermudez, V.; Lanceros-Méndez, S. Silk Fibroin Separators: A Step Toward Lithium-Ion Batteries with Enhanced Sustainability. ACS Appl. Mater. Interfaces 2018, 10, 5385–5394. [Google Scholar] [CrossRef]
- Chen, W.; Li, Z.; Yang, K.; Zhang, D.; Luo, M.; Ling, Y.; Liu, C.; Zhou, X. Low-Temperature Carbonized MXene/Protein-Based Eggshell Membrane Composite as Free-Standing Electrode for Flexible Supercapacitors. Int. J. Biol. Macromol. 2023, 226, 588–596. [Google Scholar] [CrossRef]
- Minakshi, M.; Higley, S.; Baur, C.; Mitchell, D.R.G.; Jones, R.T.; Fichtner, M. Calcined Chicken Eggshell Electrode for Battery and Supercapacitor Applications. RSC Adv. 2019, 9, 26981–26995. [Google Scholar] [CrossRef]
- Wang, C.; Zhong, W.-H. Natural Protein-Based Strategies for Batteries; World Scientific: Singapore, 2023. [Google Scholar] [CrossRef]
- Wu, Y.; Li, H.; Liu, T.; Xu, M. Versatile Protein and Its Subunit Biomolecules for Advanced Rechargeable Batteries. Adv. Mater. 2023, 35, e2305063. [Google Scholar] [CrossRef]
- Wang, C.; Zhong, W. Promising Sustainable Technology for Energy Storage Devices: Natural Protein-Derived Active Materials. Electrochim. Acta 2023, 441, 141860. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, X.L.; Yang, Y.L.; Suo, G.; Zhang, L.; Lu, S.; Chen, Z.G. Biomass-Derived Carbon for High-Performance Batteries: From Structure to Properties. Adv. Funct. Mater. 2022, 32, 2201584. [Google Scholar] [CrossRef]
- Wang, C.; Xie, N.H.; Zhang, Y.; Huang, Z.; Xia, K.; Wang, H.; Guo, S.; Xu, B.Q.; Zhang, Y. Silk-Derived Highly Active Oxygen Electrocatalysts for Flexible and Rechargeable Zn-Air Batteries. Chem. Mater. 2019, 31, 1023–1029. [Google Scholar] [CrossRef]
- Hao, M.; Dun, R.; Su, Y.; He, L.; Ning, F.; Zhou, X.; Li, W. In Situ Self-Doped Biomass-Derived Porous Carbon as an Excellent Oxygen Reduction Electrocatalyst for Fuel Cells and Metal-Air Batteries. J. Mater. Chem. A 2021, 9, 14331–14343. [Google Scholar] [CrossRef]
- Lee, M.E.; Jang, D.; Lee, S.; Yoo, J.; Choi, J.; Jin, H.J.; Lee, S.; Cho, S.Y. Silk Protein-Derived Carbon Fabric as an Electrode with High Electro-Catalytic Activity for All-Vanadium Redox Flow Batteries. Appl. Surf. Sci. 2021, 567, 150810. [Google Scholar] [CrossRef]
- Wang, R.; Li, Y. Twin-Cocoon-Derived Self-Standing Nitrogen-Oxygen-Rich Monolithic Carbon Material as the Cost-Effective Electrode for Redox Flow Batteries. J. Power Sources 2019, 421, 139–146. [Google Scholar] [CrossRef]
- Lee, M.E.; Jin, H.J.; Yun, Y.S. Synergistic Catalytic Effects of Oxygen and Nitrogen Functional Groups on Active Carbon Electrodes for All-Vanadium Redox Flow Batteries. RSC Adv. 2017, 7, 43227–43232. [Google Scholar] [CrossRef]
- Park, M.; Ryu, J.; Kim, Y.; Cho, J. Corn Protein-Derived Nitrogen-Doped Carbon Materials with Oxygen-Rich Functional Groups: A Highly Efficient Electrocatalyst for All-Vanadium Redox Flow Batteries. Energy Environ. Sci. 2014, 7, 3727–3735. [Google Scholar] [CrossRef]
- Ye, L.; Qi, S.; Cheng, T.; Jiang, Y.; Feng, Z.; Wang, M.; Liu, Y.; Dai, L.; Wang, L.; He, Z. Vanadium Redox Flow Battery: Review and Perspective of 3D Electrodes. ACS Nano 2024, 18, 18852–18869. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, J.; Jang, J.H.; Jin, H.; Paidi, V.K.; Lee, S.H.; Lee, K.S.; Kim, P.; Yoo, S.J. Waste Pig Blood-Derived 2D Fe Single-Atom Porous Carbon as an Efficient Electrocatalyst for Zinc–Air Batteries and AEMFCs. Appl. Surf. Sci. 2021, 563, 150208. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, J.; Ran, S.; Qiu, L.; Sun, W.; Yu, Y.; Chen, J.; Zhu, Z. A Robust Bifunctional Catalyst for Rechargeable Zn-Air Batteries: Ultrathin NiFe-LDH Nanowalls Vertically Anchored on Soybean-Derived Fe-N-C Matrix. Nano Res. 2021, 14, 1175–1186. [Google Scholar] [CrossRef]
- Puglia, M.K.; Malhotra, M.; Chivukula, A.; Kumar, C.V. “simple-Stir” Heterolayered MoS2/Graphene Nanosheets for Zn-Air Batteries. ACS Appl. Nano Mater. 2021, 4, 10389–10398. [Google Scholar] [CrossRef]
- Zhi, J.; Li, S.; Han, M.; Chen, P. Biomolecule-Guided Cation Regulation for Dendrite-Free Metal Anodes. Sci. Adv. 2020, 6, eabb1342. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lv, W.; Yang, W.; Jin, Y.; Jin, Q.; Sun, B.; Zhang, Z.; Wang, T.; Zheng, L.; Shi, X.; et al. In Situ Construction of Protective Films on Zn Metal Anodes via Natural Protein Additives Enabling High-Performance Zinc Ion Batteries. ACS Nano 2022, 16, 11392–11404. [Google Scholar] [CrossRef]
- Zhu, M.; Ran, Q.; Huang, H.; Xie, Y.; Zhong, M.; Lu, G.; Bai, F.-Q.; Lang, X.-Y.; Jia, X.; Chao, D. Interface Reversible Electric Field Regulated by Amphoteric Charged Protein-Based Coating Toward High-Rate and Robust Zn Anode. Nano-Micro Lett. 2022, 14, 219. [Google Scholar] [CrossRef]
- Zhou, B.; Miao, B.; Gao, Y.; Yu, A.; Shao, Z. Self-Assembled Protein Nanofilm Regulating Uniform Zn Nucleation and Deposition Enabling Long-Life Zn Anodes. Small 2023, 19, e2300895. [Google Scholar] [CrossRef]
- Zhang, S.J.; Hao, J.; Wu, H.; Chen, Q.; Ye, C.; Qiao, S.Z. Protein Interfacial Gelation toward Shuttle-Free and Dendrite-Free Zn–Iodine Batteries. Adv. Mater. 2024, 36, e2404011. [Google Scholar] [CrossRef]
- Guo, W.; Xu, L.; Su, Y.; Tian, Z.; Qiao, C.; Zou, Y.; Chen, Z.; Yang, X.; Cheng, T.; Sun, J. Tailoring Localized Electrolyte via a Dual-Functional Protein Membrane toward Stable Zn Anodes. ACS Nano 2024, 18, 10642–10652. [Google Scholar] [CrossRef]
- Zhai, S.; Song, W.; Jiang, K.; Tan, X.; Zhang, W.; Yang, Y.; Chen, W.; Chen, N.; Zeng, H.; Li, H.; et al. Mimicking Ion and Water Management in Poultry Breeding for Highly Reversible Zinc Ion Batteries. Energy Environ. Sci. 2023, 16, 5479–5489. [Google Scholar] [CrossRef]
- Xu, X.; Li, S.; An, J.; Luo, Z.; Du, J.; Zhong, J.; Yu, M.; Yang, S.; Li, B. A Dynamically Assembled Bionic Ion Pump Interface towards High-Rate and Stable-Cycling Zinc Metal Batteries. Energy Environ. Sci. 2024, 18, 689–701. [Google Scholar] [CrossRef]
- Zhang, X.; Liao, T.; Long, T.; Cao, Y.K.; Zeng, X.X.; Deng, Q.; Liu, B.; Wu, X.W.; Wu, Y.P. In Situ Buildup of Zinc Anode Protection Films with Natural Protein Additives for High-Performance Zinc Battery Cycling. ACS Appl. Mater. Interfaces 2023, 15, 32496–32505. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Qu, Q.; Jiang, B.; Zou, T.; Zhen, S.; Wang, P.; Li, K.; Zhang, J.; Guo, H.; Zhang, T. Zein Improved GF Separator for Dendrite-Free Aqueous Zinc-Ion Batteries. J. Mater. Chem. A 2024, 12, 31185–31194. [Google Scholar] [CrossRef]
- Zhu, J.; Tie, Z.; Bi, S.; Niu, Z. Towards More Sustainable Aqueous Zinc-Ion Batteries. Angew. Chem. 2024, 136, e202403712. [Google Scholar] [CrossRef]
- Zhu, Z.; Ma, H.; Du, H.; Zhang, L.; Wu, J.; Gao, C.; Li, W.; Chen, X.; Su, Y.; Wang, D.; et al. Antifreeze Protein Mimics Realizing Stable Low-Temperature-Resistant Aqueous Zn-Ion Batteries with High Water Content. Angew. Chem. Int. Ed. 2025, 64, e202505325. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Y.; Xie, L.; Xu, R.; Zhang, R.; Gao, M.; Tian, W.; Li, D.; Qiao, L.; Wang, T.; et al. Humidity-Sensitive, Shape-Controllable, and Transient Zinc-Ion Batteries Based on Plasticizing Gelatin-Silk Protein Electrolytes. Mater. Today Energy 2021, 21, 100712. [Google Scholar] [CrossRef]
- Shen, P.; Xu, S.; Li, D.; Lin, Y.; Zhang, Y.; Huang, J.; Wang, X.; Xu, J.; Teng, W.; Shen, Y.; et al. A Bioinspired Flexible Hydrogel Electrolyte with β-Sheet–Directed Interphase for Dendrite-Free Zn Metal Batteries. Energy Storage Mater. 2025, 81, 104464. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Ge, R.; Moretti, M.; Yin, J.; Zhao, Z.; Valle-Pérez, A.U.; Liu, H.; Tian, Z.; Guo, T.; et al. Peptide Gel Electrolytes for Stabilized Zn Metal Anodes. ACS Nano 2024, 18, 164–177. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, S.; Xu, Z.; Chen, L.; Li, G. Biodegradable Pea Protein Fibril Hydrogel-Based Quasi-Solid-State Zn-Ion Battery. ACS Appl. Mater. Interfaces 2023, 15, 49060–49070. [Google Scholar] [CrossRef]
- Xu, X.; Li, S.; Yang, S.; Li, B. Superelastic Hydrogel Electrolyte Incorporating Helical Protein Molecules as Zinc Ion Transport Pathways to Enhance the Cycling Stability of Zinc Metal Batteries. Energy Environ. Sci. 2024, 17, 7919–7931. [Google Scholar] [CrossRef]
- Li, M.; Xu, T.; Huang, L.; Hu, Z.; Zhou, C.; Li, D.; Zhang, J.; Hu, E.; Chen, Z. Biodegradable Electrolyte toward Green Flexible Zinc-Air Batteries. ACS Sustain. Chem. Eng. 2024, 12, 17147–17157. [Google Scholar] [CrossRef]
- De Cachinho Cordeiro, I.M.; Li, A.; Lin, B.; Ma, D.X.; Xu, L.; Eh, A.L.S.; Wang, W. Solid Polymer Electrolytes for Zinc-Ion Batteries. Batteries 2023, 9, 343. [Google Scholar] [CrossRef]
- Watt, G.D.; Kim, J.W.; Zhang, B.; Miller, T.; Harb, J.N.; Davis, R.C.; Choi, S.H. A Protein-Based Ferritin Bio-Nanobattery. J. Nanotechnol. 2012, 2012, 516309. [Google Scholar] [CrossRef]
- Janoschka, T.; Hager, M.D.; Schubert, U.S. Powering up the Future: Radical Polymers for Battery Applications. Adv. Mater. 2012, 24, 6397–6409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Monteiro, M.J.; Jia, Z. Stable Organic Radical Polymers: Synthesis and Applications. Polym. Chem. 2016, 7, 5589–5614. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Easley, A.D.; Kang, N.; Khan, S.; Lim, S.M.; Rezenom, Y.H.; Wang, S.; Tran, D.K.; Fan, J.; Letteri, R.A.; et al. Polypeptide Organic Radical Batteries. Nature 2021, 593, 61–66. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Z.; Zhang, P.; Wei, J.; Li, J.; Wang, H.; Wen, S.; Liang, J.; Chen, Y.; Dai, T.; et al. Artificial α-Amino Acid Based on Cysteine Grafted Natural Aloe-Emodin for Aqueous Organic Redox Flow Batteries. Nat. Commun. 2025, 16, 2965. [Google Scholar] [CrossRef]



| Protein Type | Carbon Type | System Type | Electrochemical Performance | Electrolyte | Counter Electrode | Reference |
|---|---|---|---|---|---|---|
| Silk | Defect-rich and N-doped carbon | Zn–air | Voltage gap = 1.39 V, voltaic efficiency = 41.3% after 100 cycles | 6.0 M KOH + 0.2 M ZnAC, PVA aqueous gel | Zn | [47] |
| Root nodule | Fe, Mo, S, N self-doped porous carbon | Zn–air | Half-wave potentials are 0.723 V (vs. RHE) in 0.1 M HClO4 and 0.868 V and 0.1 M KOH solution | 6 M KOH + 0.2 M Zn(CH3CO2)2 | Zn | [48] |
| Fuel cell | Flow rate of H2 was 10 mL min−1, 17.6 mW cm−2 with an open-circuit voltage of 0.966 V | H2 | air | Graphite | |||
| Silk | Carbon fabrics | All-vanadium redox flow batteries | Energy efficiency = 86.8% | 1.6 M VOSO4 in 4 M H2SO4 solution | Symmetrical | [49] |
| Twin cocoon | Self-standing monolithic carbon | All-vanadium redox flow batteries | 50% redox potential decrease and 192% diffusion slope increase | 1.0 M VOSO4 + 3.0 M H2SO4 | Pt, Ag/AgCl | [50] |
| Pyroprotein | Carbon felts@ pyroprotein | All-vanadium redox flow batteries | ΔEp = 0.17 V, energy efficiency = 90% at 40 mA cm−2 | 0.1 M VOSO4 in 2 M H2SO4 | Three electrodes | [51] |
| Zein | Zein-coated carbon black | All-vanadium redox flow batteries | Energy efficiency = 85.2% after 100th cycles | Positive: 2 M VOSO4 in 3 M H2SO4 Negative: 2 M VOSO4 in 3 M H2SO4 | Symmetrical | [52] |
| Protein Type | Electrode | Systems | Electrochemical Performance | Separator | Electrolyte | Counter Electrode | Reference |
|---|---|---|---|---|---|---|---|
| Pig blood | 2D Zn-Fe single-atom porous carbon catalyst | Zn–air | 220 mW cm−2 | / | 6.0 M KOH | Zn | [54] |
| AEMFCs | 352 mW cm−2 | FAA-3-20 (coating toward cathode) | H2/O2 | Symmetrical gas diffusion layer | |||
| Bovine serum albumin | Protein-coated MoS2/Gr nanosheet | Zn–air | 130 W h kg−1, OCV = 1.4 V | Whatman filter paper | 4 M KOH | Zn | [56] |
| Soybean | NiFe-LDH nanowalls anchored on Fe-N-C matrix | Zn–air | OER (Ej=10 = 1.53 V vs. RHE) and ORR (E1/2 = 0.91 V vs. RHE) | / | 6.0 M KOH + 0.2 M ZnAC | Zn | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Wang, C.; Zhong, W.-H. Protein-Based Strategies for Non-Alkali Metal-Ion Batteries. Batteries 2025, 11, 318. https://doi.org/10.3390/batteries11090318
Wang Q, Wang C, Zhong W-H. Protein-Based Strategies for Non-Alkali Metal-Ion Batteries. Batteries. 2025; 11(9):318. https://doi.org/10.3390/batteries11090318
Chicago/Turabian StyleWang, Qian, Chenxu Wang, and Wei-Hong Zhong. 2025. "Protein-Based Strategies for Non-Alkali Metal-Ion Batteries" Batteries 11, no. 9: 318. https://doi.org/10.3390/batteries11090318
APA StyleWang, Q., Wang, C., & Zhong, W.-H. (2025). Protein-Based Strategies for Non-Alkali Metal-Ion Batteries. Batteries, 11(9), 318. https://doi.org/10.3390/batteries11090318







