Effects of Crystalline Diamond Nanoparticles on Silicon Thin Films as an Anode for a Lithium-Ion Battery
Abstract
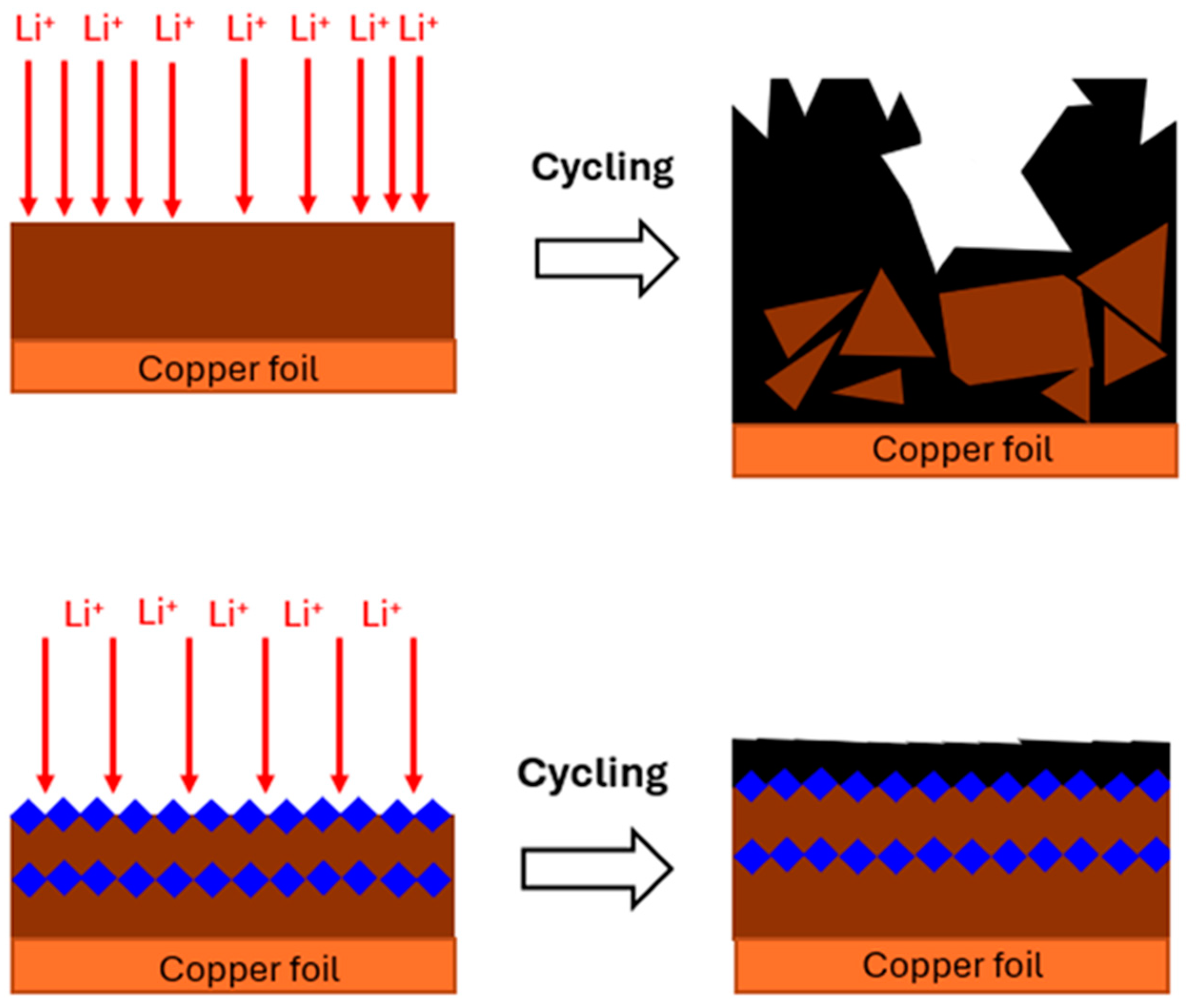
1. Introduction
2. Materials and Methods
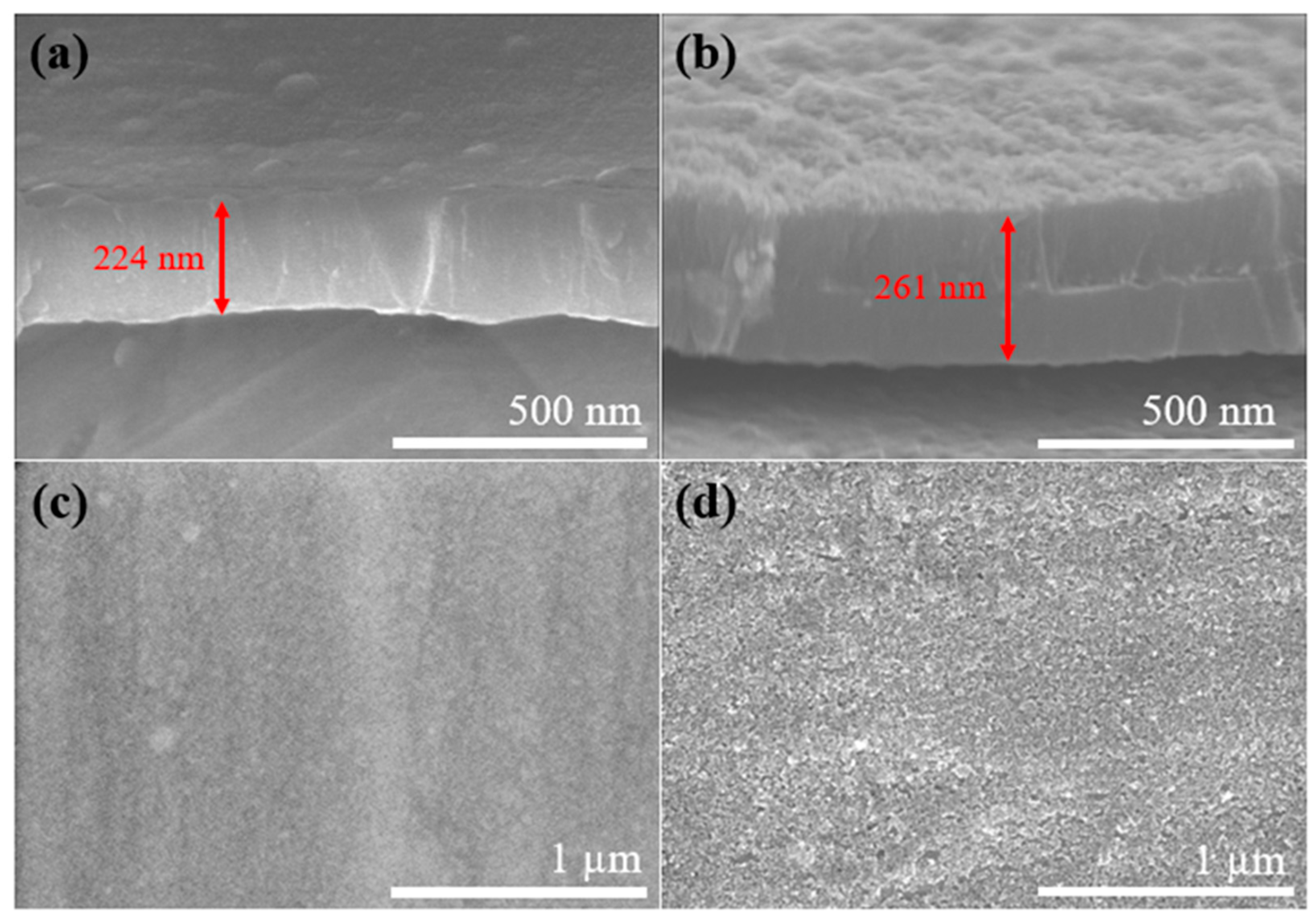
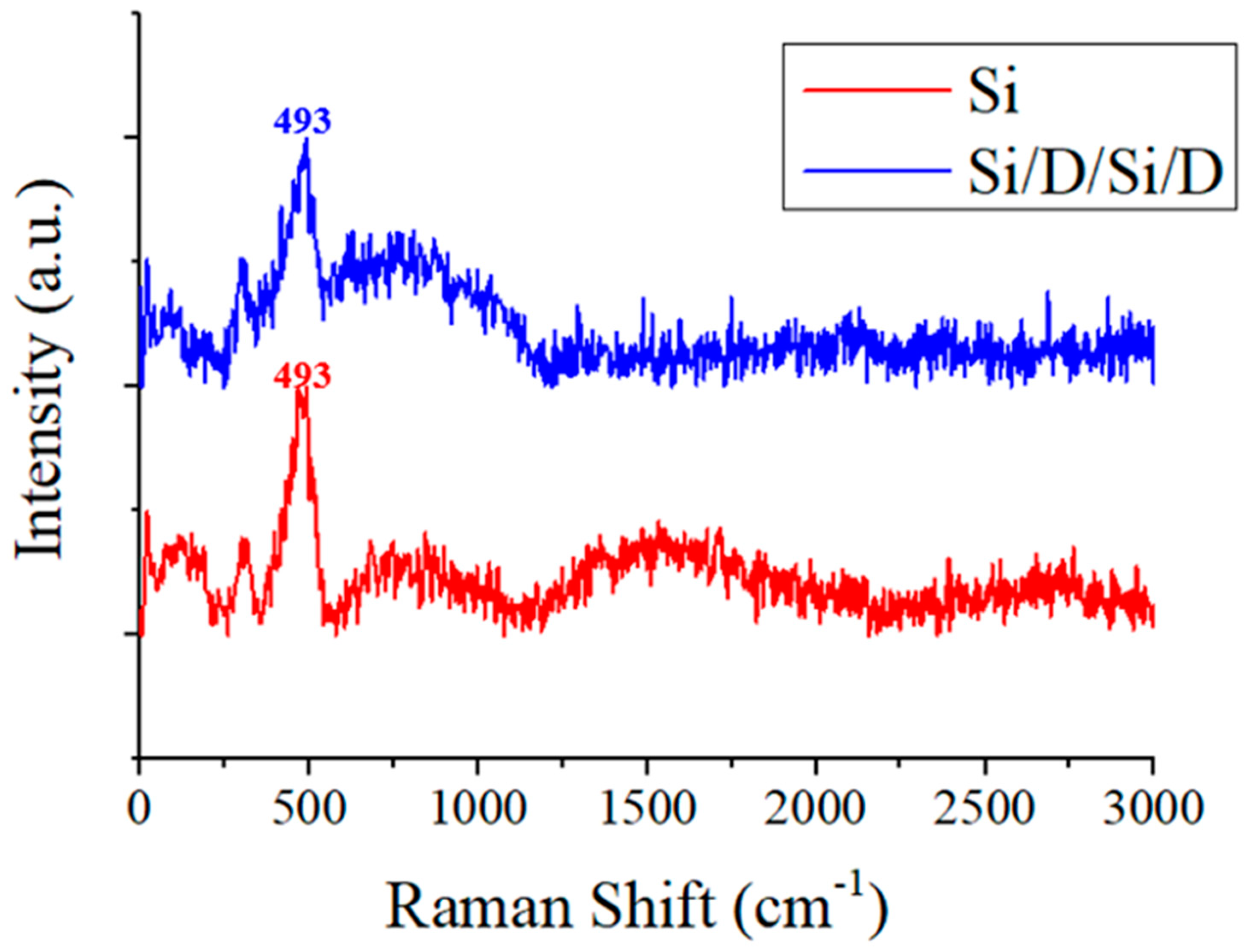
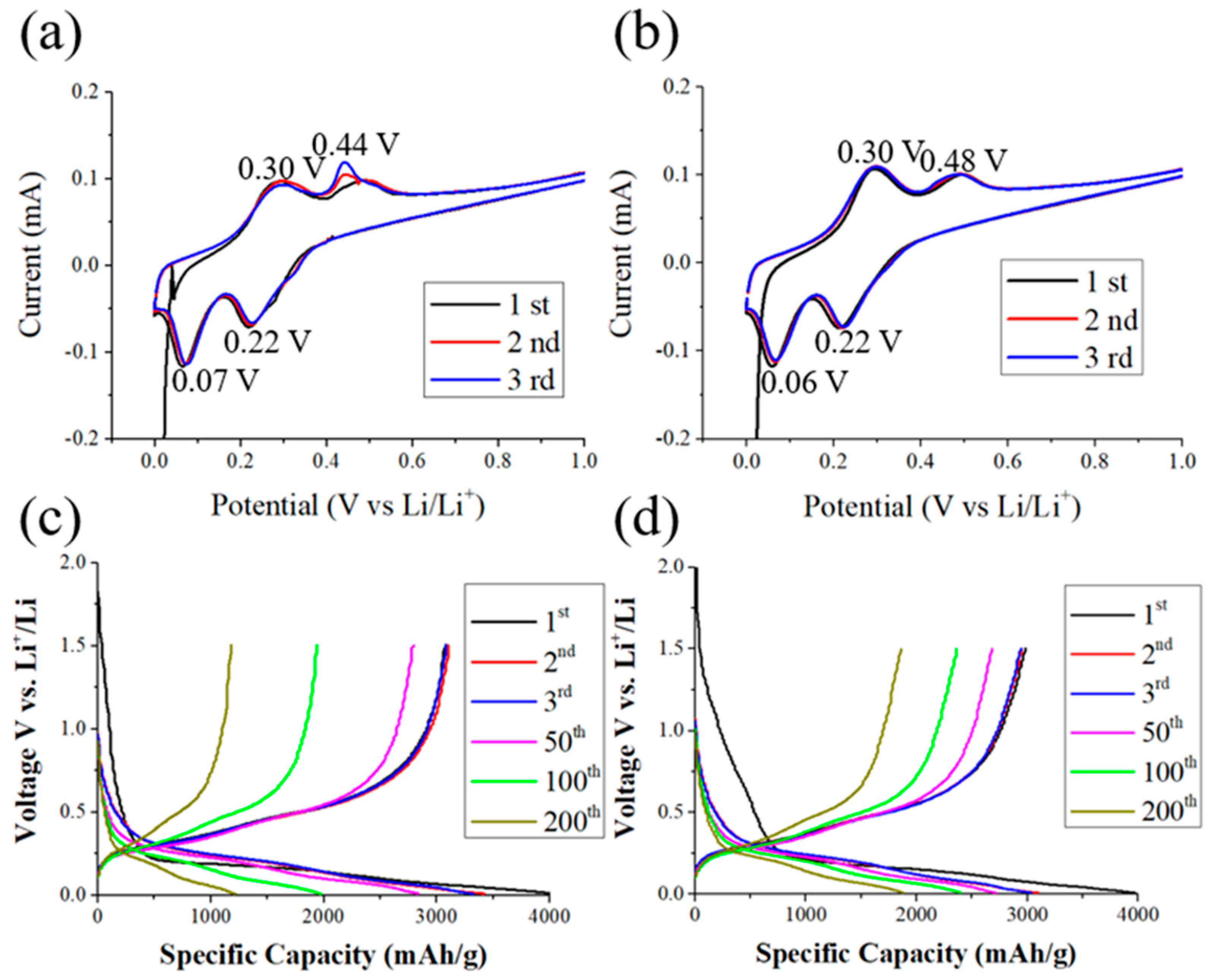
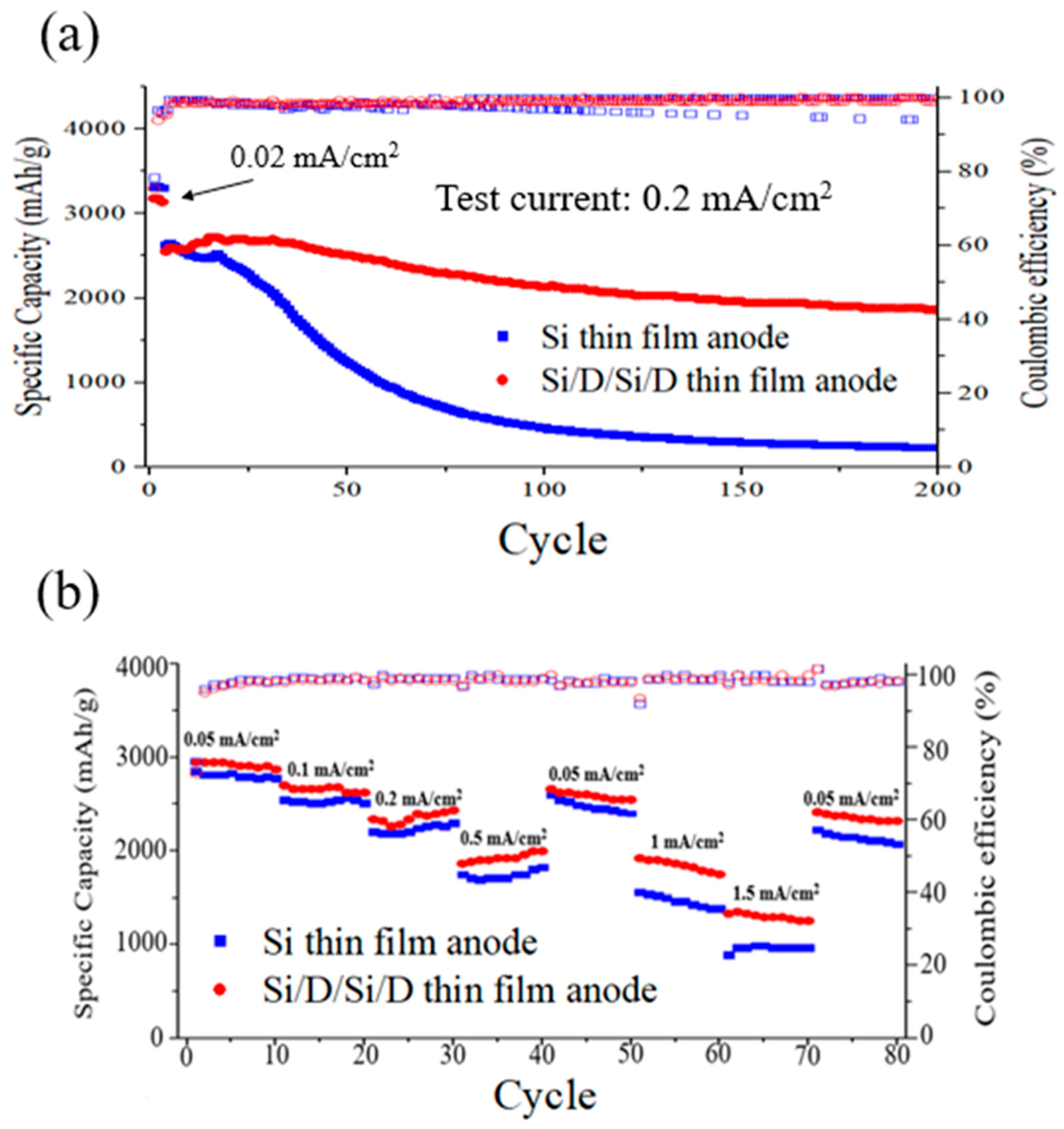
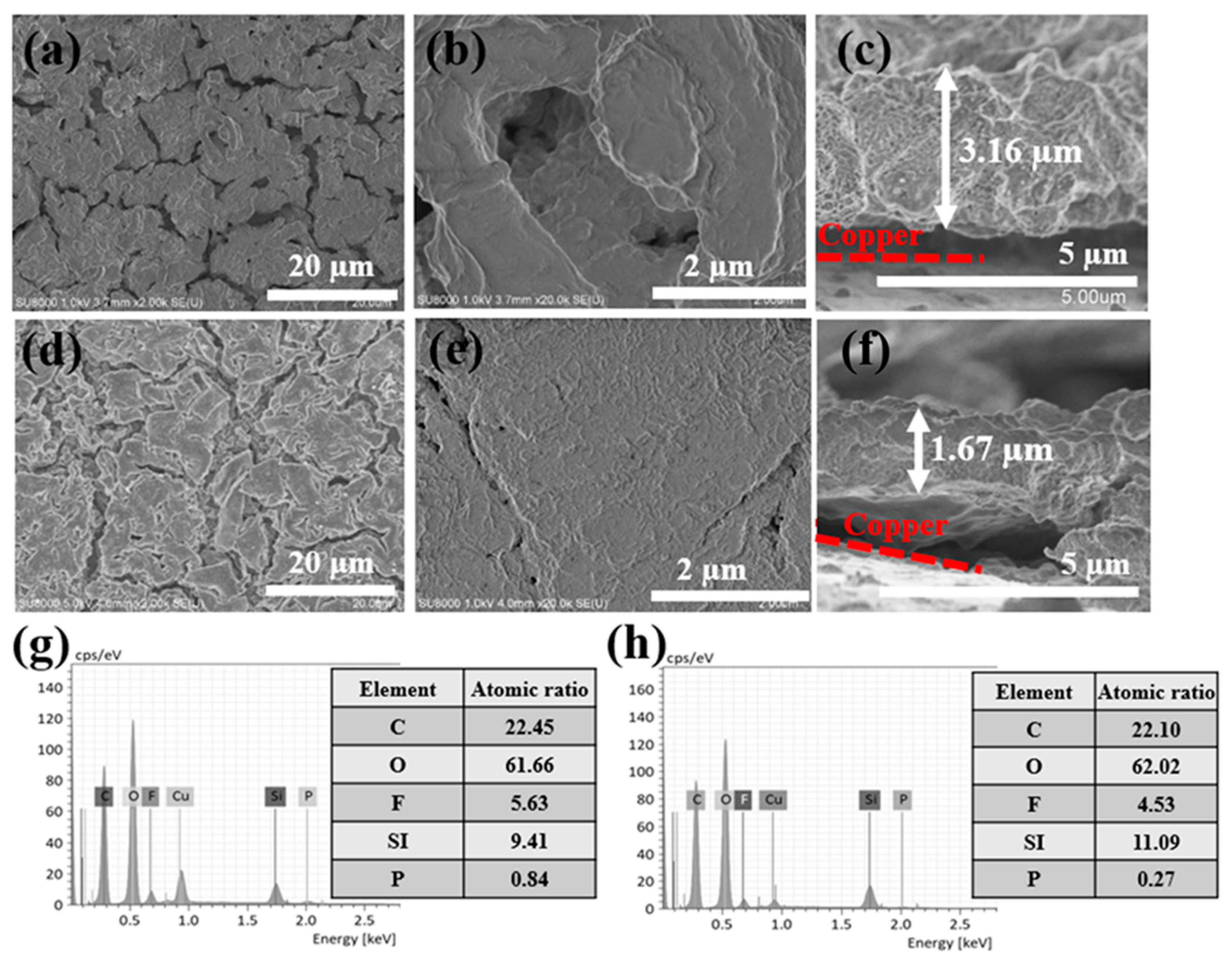
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, L.; Wu, Z.; Yuan, S.; Zhang, X.B. Advances and challenges for flexible energy storage and conversion devices and systems. Energy Environ. Sci. 2014, 7, 2101–2122. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Wolverton, C.; Isaacs, E.D. Electrical energy storage for transportation—Approaching the limits of, and going beyond, lithium-ion batteries. Energy Environ. Sci. 2012, 5, 7854–7863. [Google Scholar] [CrossRef]
- Bai, S.; Chen, S.; Shen, X.; Zhua, G.; Wang, G. Nanocomposites of hematite (α-Fe2O3) nanospindles with crumpled reduced graphene oxide nanosheets as high-performance anode material for lithium-ion batteries. RSC Adv. 2012, 2, 10977–10984. [Google Scholar] [CrossRef]
- Ge, M.; Cao, C.; Biesold, G.M.; Sewell, C.D.; Hao, S.M.; Huang, J.; Lin, Z. Recent advances in silicon-based electrodes: From fundamental research toward practical applications. Adv. Mater. 2021, 33, 2004577. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Deng, Y.; Hu, K.; Xu, B.; Wang, N.; Bai, Z.; Yang, J. Nanostructure designing and hybridizing of high-capacity silicon-based anode for lithium-ion batteries. Prog. Nat. Sci. Mater. Int. 2023, 33, 16–36. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Yan, Z.; Cheng, F.; Chen, J. Structure design and mechanism analysis of silicon anode for lithium-ion batteries. Sci. China Mater. 2019, 62, 1515–1536. [Google Scholar] [CrossRef]
- Kim, H.; Seo, M.; Park, M.H.; Cho, J. A critical size of silicon nano-anodes for lithium rechargeable batteries. Angew. Chem. Int. Ed. 2010, 49, 2146–2149. [Google Scholar] [CrossRef]
- Wu, H.; Cui, Y. Designing nanostructured Si anodes for high energy lithium ion batteries. Nano Today 2012, 7, 414–429. [Google Scholar] [CrossRef]
- Liu, L.; Lyu, J.; Li, T.; Zhao, T. Well-constructed silicon-based materials as high-performance lithium-ion battery anodes. Nanoscale 2016, 8, 701–722. [Google Scholar] [CrossRef]
- Jiang, S.; Hu, B.; Sahore, R.; Zhang, L.; Liu, H.; Zhang, L.; Zhang, Z. Surface-functionalized silicon nanoparticles as anode material for lithium-ion battery. ACS Appl. Mater. Interfaces 2018, 10, 44924–44931. [Google Scholar] [CrossRef]
- Zamfir, M.R.; Nguyen, H.T.; Moyen, E.; Lee, Y.H.; Pribat, D. Silicon nanowires for Li-based battery anodes: A review. J. Mater. Chem. A 2013, 1, 9566–9586. [Google Scholar] [CrossRef]
- Wen, Z.; Lu, G.; Mao, S.; Kim, H.; Cui, S.; Yu, K.; Chen, J. Silicon nanotube anode for lithium-ion batteries. Electrochem. Commun. 2013, 29, 67–70. [Google Scholar] [CrossRef]
- Salah, M.; Murphy, P.; Hall, C.; Francis, C.; Kerr, R.; Fabretto, M. Pure silicon thin-film anodes for lithium-ion batteries: A review. J. Power Sources 2019, 414, 48–67. [Google Scholar] [CrossRef]
- Tong, L.; Wang, P.; Chen, A.; Qiu, F.; Fang, W.; Yang, J.; Wang, C.; Yang, Y. Improved Electrochemical Performance of Binder-Free Multi-Layered Silicon/Carbon Thin Film Electrode for Lithium-Ion Batteries. Carbon 2019, 153, 592–601. [Google Scholar] [CrossRef]
- Chen, Y.X.; Liao, H.C.; Cheng, Y.W.; Huang, J.H.; Liu, C.P. Scalable Interlayer Nanostructure Design for High-Rate (10C) Submicron Silicon-Film Electrode by Incorporating Silver Nanoparticle. ACS Appl. Mater. Interfaces 2023, 15, 18845–18856. [Google Scholar] [CrossRef] [PubMed]
- Jhan, C.-Y.; Sung, S.-H.; Tzeng, Y. Silicon–Nanodiamond-Based Anode for a Lithium-Ion Battery. Nanomaterials 2024, 14, 43. [Google Scholar] [CrossRef]
- Jhan, C.-Y.; Wang, P.-S.; Sung, S.-H.; Tzeng, Y. Effects of volume-confinement on lithium-ion battery with silicon-based anode. Mater. Today Commun. 2024, 39, 108578. [Google Scholar] [CrossRef]
- Tzeng, Y.; Jhan, C.Y.; Wu, Y.H. Effects of pyrolysis on high-capacity Si-based anode of lithium ion battery with high coulombic efficiency and long cycling life. Nanomaterials 2022, 12, 469. [Google Scholar] [CrossRef]
- Tzeng, Y.; Jhan, C.Y.; Chiu, K.M.; Wu, Y.C.; Chen, G.Y.; Wang, P.S. Si–Ni-alloy-assisted very high-areal-capacity silicon-based anode on Ni foam for lithium ion battery. Mater. Today Chem. 2023, 30, 101570. [Google Scholar] [CrossRef]
- Tzeng, Y.; Jhan, C.Y.; Wu, Y.C.; Chen, G.Y.; Chiu, K.M.; Guu SY, E. High-ICE and high-capacity retention silicon-based anode for lithium-ion battery. Nanomaterials 2022, 12, 1387. [Google Scholar] [CrossRef]
- Marcins, G.; Butikova, J.; Tale, I.; Polyakov, B.; Kalendarjov, R.; Muhin, A. Crystallization processes of amorphous Si by thermal annealing and pulsed laser processing. IOP Conf. Ser. Mater. Sci. Eng. 2011, 23, 012035. [Google Scholar] [CrossRef]
- Becker, C.; Dogan, P.; Ruske, F.; Gorka, B.; Sánchez-Vicens, A.; Conrad, E.; Hüpkes, J. Solid phase crystallized silicon thin-film solar cells on temperature-stable ZnO: Al contact layers. In Proceedings of the 23rd European Photovoltaic Solar Energy Conference, Valencia, Spain, 1–5 September 2008; pp. 2045–2048. [Google Scholar]
- Lee, S.H.; Choi, M.; Jung, Y.I.; Sim, S.J.; Moon, J.K.; Choi, J.; Kim, S. Deposition and characterization of silicon thin film on stainless steel by electron beam evaporation. Thin Solid Films 2022, 756, 139380. [Google Scholar] [CrossRef]
- Wang, J.W.; He, Y.; Fan, F.; Liu, X.H.; Xia, S.; Liu, Y.; Zhu, T. Two-phase electrochemical lithiation in amorphous silicon. Nano Lett. 2013, 13, 709–715. [Google Scholar] [CrossRef]
- Adhitama, E.; Van Wickeren, S.; Neuhaus, K.; Frankenstein, L.; Demelash, F.; Javed, A.; Placke, T. Revealing the role, mechanism, and impact of AlF3 coatings on the interphase of silicon thin film anodes. Adv. Energy Mater. 2022, 12, 2201859. [Google Scholar] [CrossRef]
- Tong, L.; Wang, P.; Fang, W.; Guo, X.; Bao, W.; Yang, Y.; Qiu, F. Interface engineering of silicon/carbon thin-film anodes for high-rate lithium-ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 29242–29252. [Google Scholar] [CrossRef] [PubMed]
- Tamirat, A.G.; Lui, Y.; Dong, X.; Wang, C.; Wang, Y.; Xia, Y. Ultrathin Silicon Nanolayer Implanted NixSi/Ni Nanoparticles as Superlong-Cycle Lithium-Ion Anode Material. Small Struct. 2021, 2, 2000126. [Google Scholar] [CrossRef]
- Mukanova, A.; Jetybayeva, A.; Myung, S.T.; Kim, S.S.; Bakenov, Z. A mini-review on the development of Si-based thin film anodes for Li-ion batteries. Mater. Today Energy 2018, 9, 49–66. [Google Scholar] [CrossRef]
- Jaumann, T.; Balach, J.; Klose, M.; Oswald, S.; Langklotz, U.; Michaelis, A.; Giebeler, L. SEI-component formation on sub 5 nm sized silicon nanoparticles in Li-ion batteries: The role of electrode preparation, FEC addition and binders. Phys. Chem. Chem. Phys. 2015, 17, 24956–24967. [Google Scholar] [CrossRef]
- Aryanfar, A.; Brooks, D.J.; Colussi, A.J.; Hoffmann, M.R. Quantifying the dependence of dead lithium losses on the cycling period in lithium metal batteries. Phys. Chem. Chem. Phys. 2014, 16, 24965–24970. [Google Scholar] [CrossRef]
- Philippe, B.; Dedryvère, R.; Allouche, J.; Lindgren, F.; Gorgoi, M.; Rensmo, H.; Edström, K. Nanosilicon electrodes for lithium-ion batteries: Interfacial mechanisms studied by hard and soft X-ray photoelectron spectroscopy. Chem. Mater. 2012, 24, 1107–1115. [Google Scholar] [CrossRef]
- Philippe, B.; Dedryvère, R.; Gorgoi, M.; Rensmo, H.; Gonbeau, D.; Edström, K. Role of the LiPF6 salt for the long-term stability of silicon electrodes in Li-ion batteries–A photoelectron spectroscopy study. Chem. Mater. 2013, 25, 394–404. [Google Scholar] [CrossRef]
- Elazari, R.; Salitra, G.; Gershinsky, G.; Garsuch, A.; Panchenko, A.; Aurbach, D. Li ion cells comprising lithiated columnar silicon film anodes, TiS2 cathodes and fluoroethyene carbonate (FEC) as a critically important component. J. Electrochem. Soc. 2012, 159, A1440. [Google Scholar] [CrossRef]
- Dalavi, S.; Guduru, P.; Lucht, B.L. Performance enhancing electrolyte additives for lithium ion batteries with silicon anodes. J. Electrochem. Soc. 2012, 159, A642. [Google Scholar] [CrossRef]
- Etacheri, V.; Haik, O.; Goffer, Y.; Roberts, G.A.; Stefan, I.C.; Fasching, R.; Aurbach, D. Effect of fluoroethylene carbonate (FEC) on the performance and surface chemistry of Si-nanowire Li-ion battery anodes. Langmuir 2012, 28, 965–976. [Google Scholar] [CrossRef]
- Sun, X.; Wang, C.; Zhang, X.; Zhai, X.; Feng, J.; Li, H. Nanodiamond-modified separators and optimized graphene anodes for highly enhanced performance of lithium-ion batteries. J. Alloys Compd. 2022, 929, 167204. [Google Scholar] [CrossRef]
- Wu, J.J.; Bennett, W.R. Fundamental investigation of Si anode in Li-ion cells. In Proceedings of the 2012 IEEE Energytech, Cleveland, OH, USA, 29–31 May 2012; pp. 1–5. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzeng, Y.; Jhan, C.-Y.; Sung, S.-H.; Chiou, Y.-Y. Effects of Crystalline Diamond Nanoparticles on Silicon Thin Films as an Anode for a Lithium-Ion Battery. Batteries 2024, 10, 321. https://doi.org/10.3390/batteries10090321
Tzeng Y, Jhan C-Y, Sung S-H, Chiou Y-Y. Effects of Crystalline Diamond Nanoparticles on Silicon Thin Films as an Anode for a Lithium-Ion Battery. Batteries. 2024; 10(9):321. https://doi.org/10.3390/batteries10090321
Chicago/Turabian StyleTzeng, Yonhua, Cheng-Ying Jhan, Shi-Hong Sung, and Yu-Yang Chiou. 2024. "Effects of Crystalline Diamond Nanoparticles on Silicon Thin Films as an Anode for a Lithium-Ion Battery" Batteries 10, no. 9: 321. https://doi.org/10.3390/batteries10090321
APA StyleTzeng, Y., Jhan, C.-Y., Sung, S.-H., & Chiou, Y.-Y. (2024). Effects of Crystalline Diamond Nanoparticles on Silicon Thin Films as an Anode for a Lithium-Ion Battery. Batteries, 10(9), 321. https://doi.org/10.3390/batteries10090321








