Edible Gelatin and Cosmetic Activated Carbon Powder as Biodegradable and Replaceable Materials in the Production of Supercapacitors
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Material for the Electrode
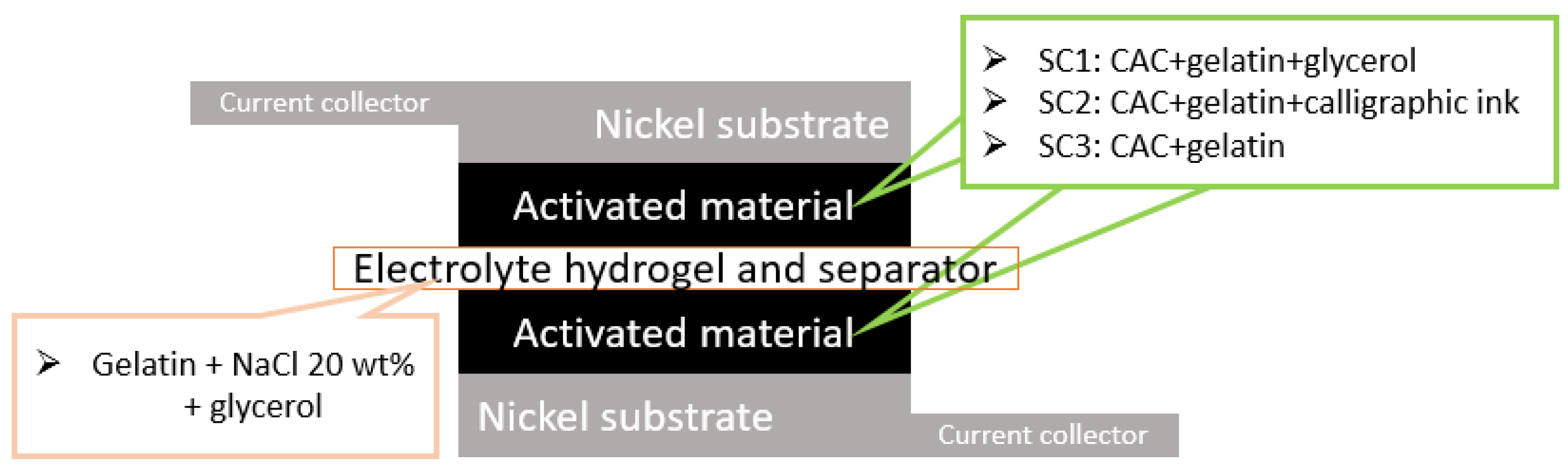
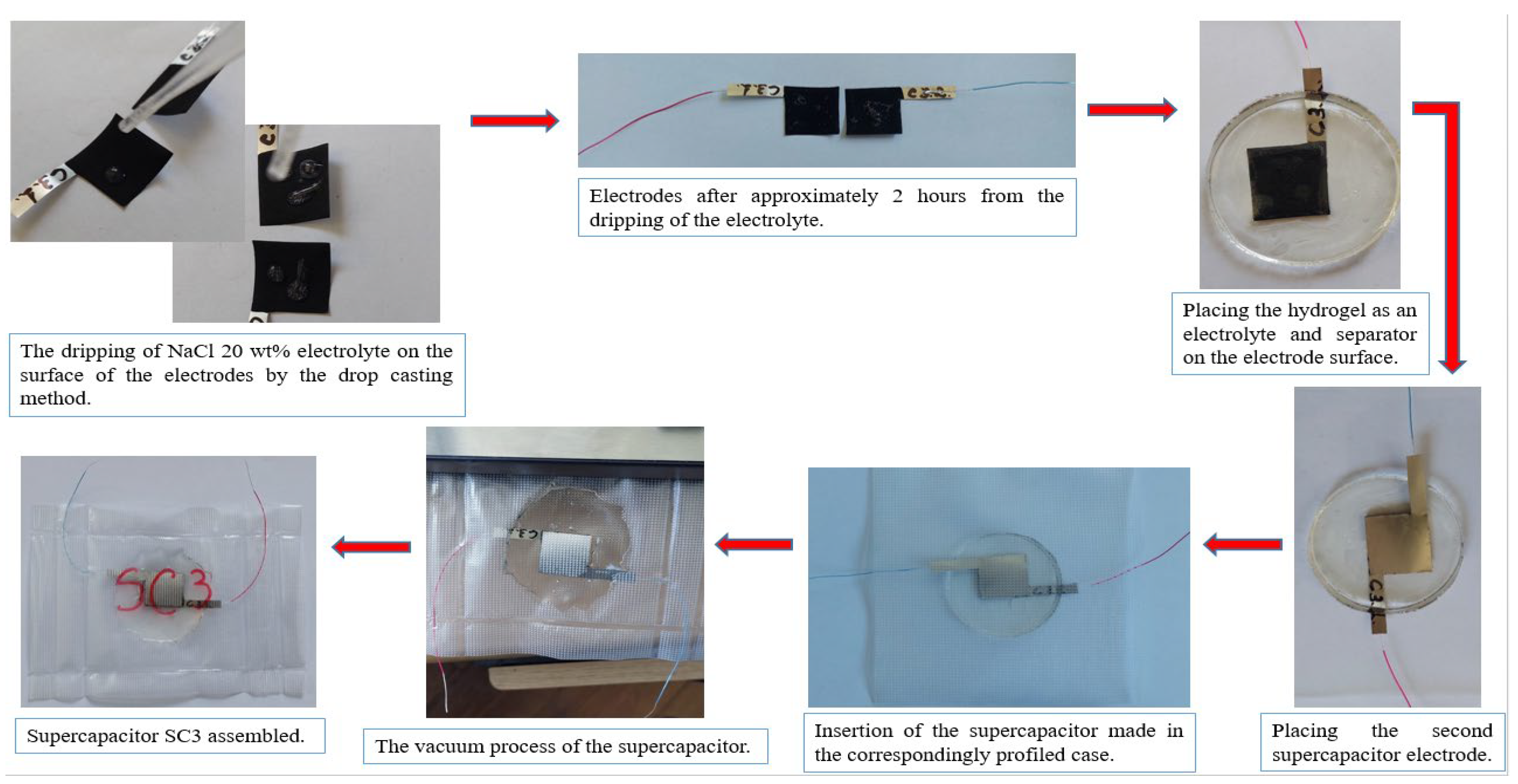
2.2. Joint Preparation of the Electrolyte and the Separator
3. Assembly of Supercapacitor Cells
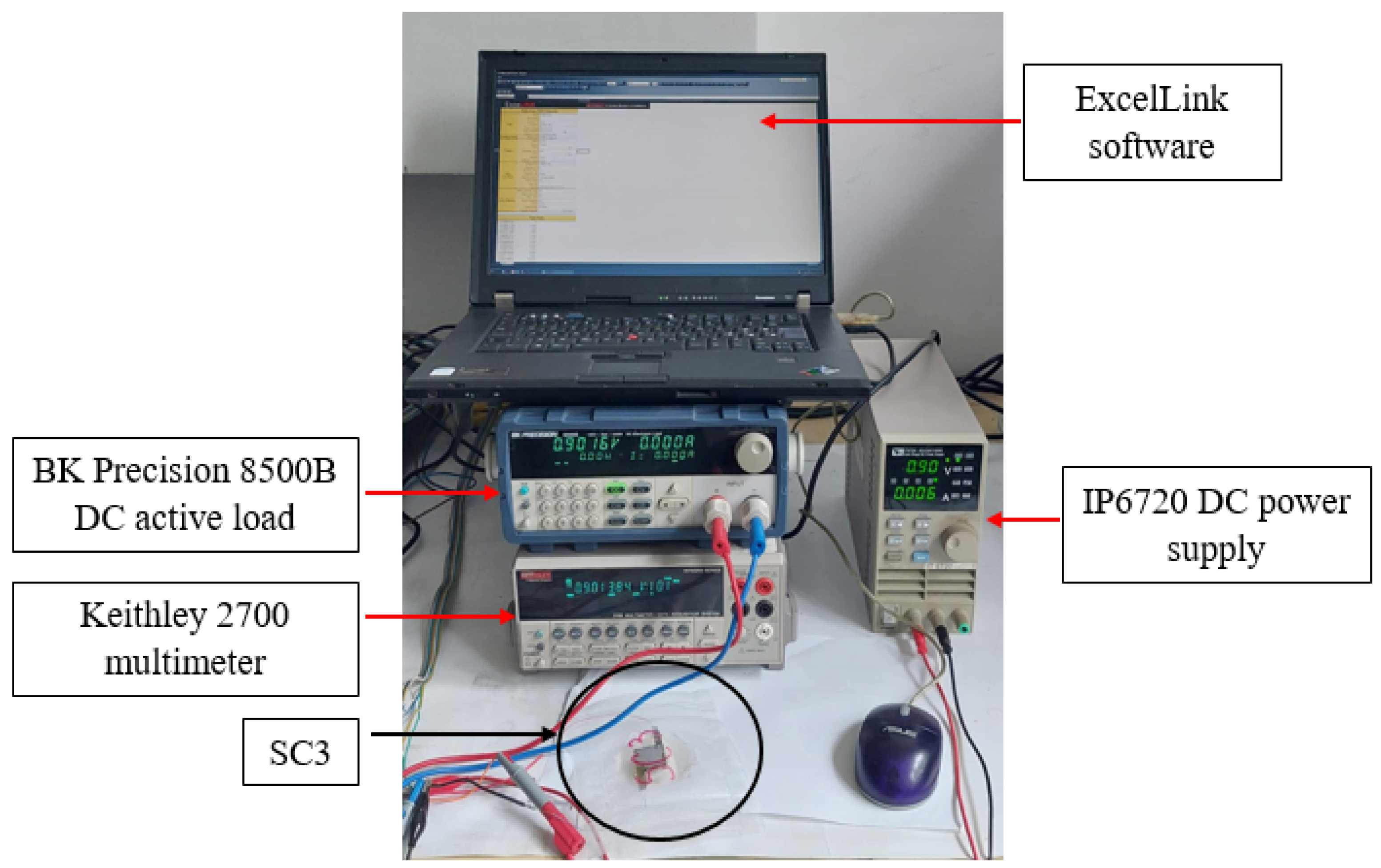
4. Test Methods
4.1. Test Method for Electrodes
4.2. Galvanostatic Testing of Supercapacitors
5. Results and Discussion
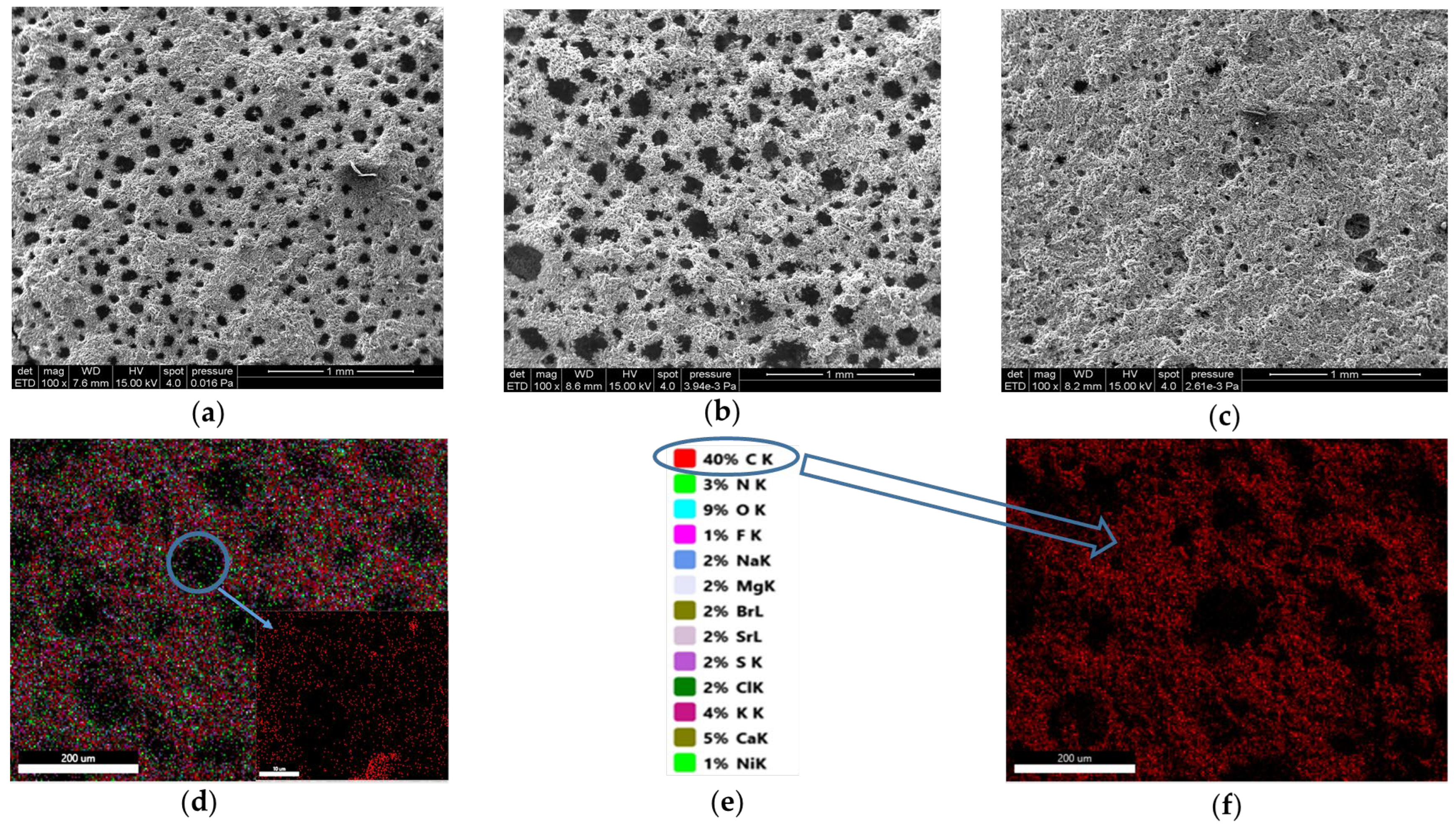
5.1. Morphological Analyses
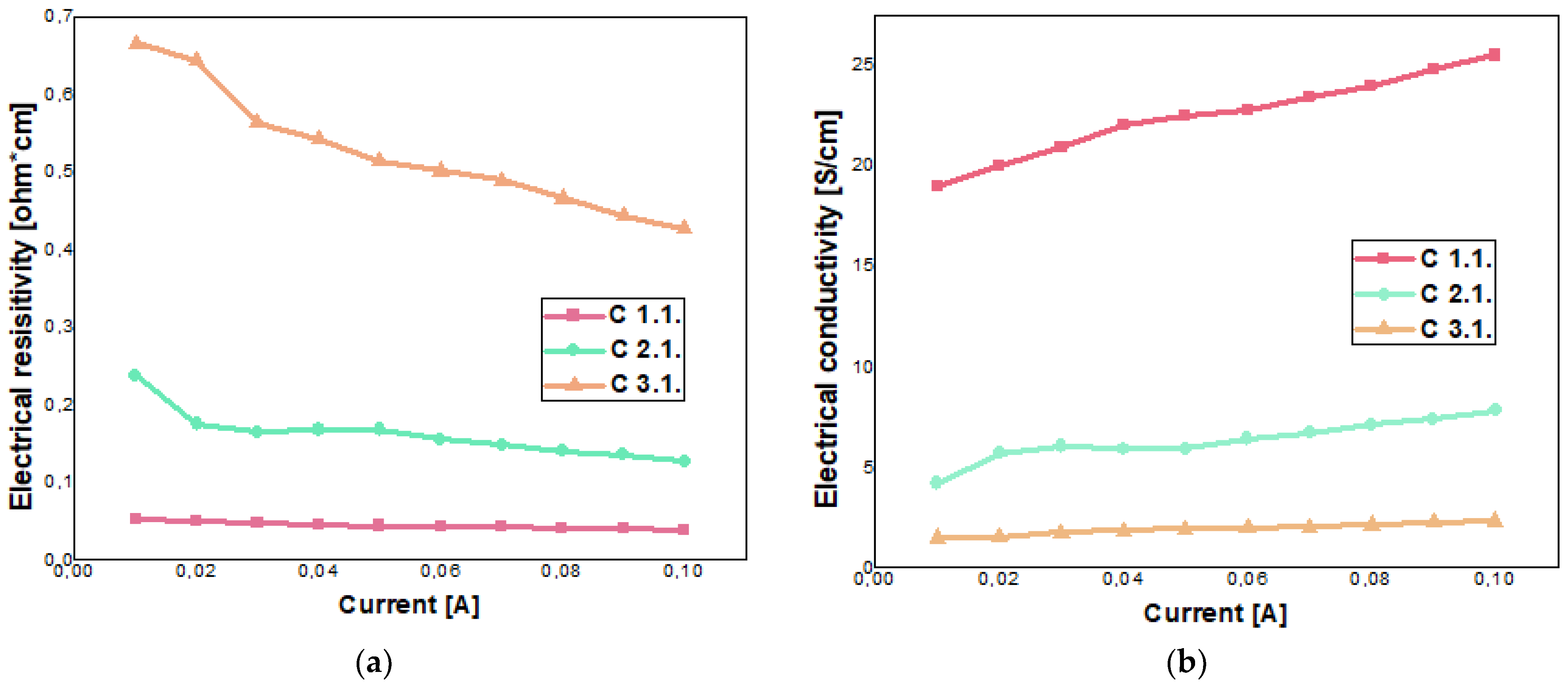
5.2. Electrodes Investigation
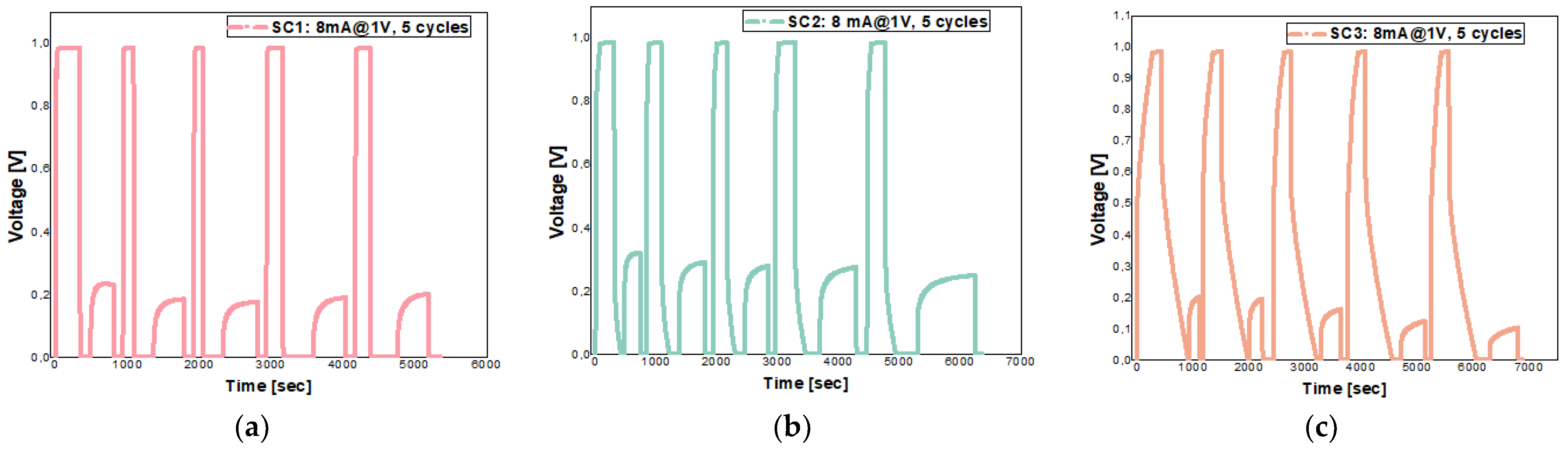
5.3. Galvanostatic Investigation for Supercapacitors Cells
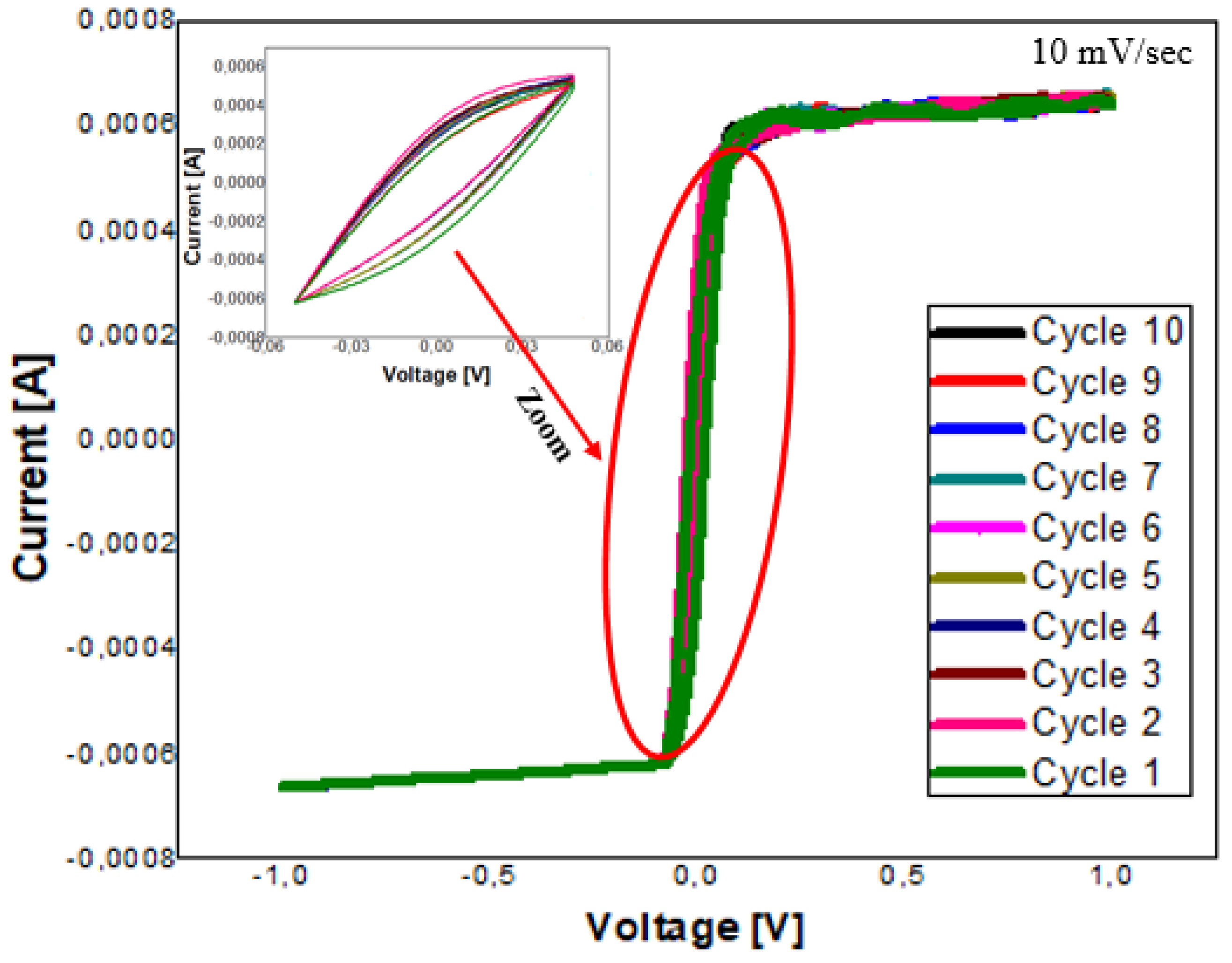
5.4. Cyclic Voltammetry
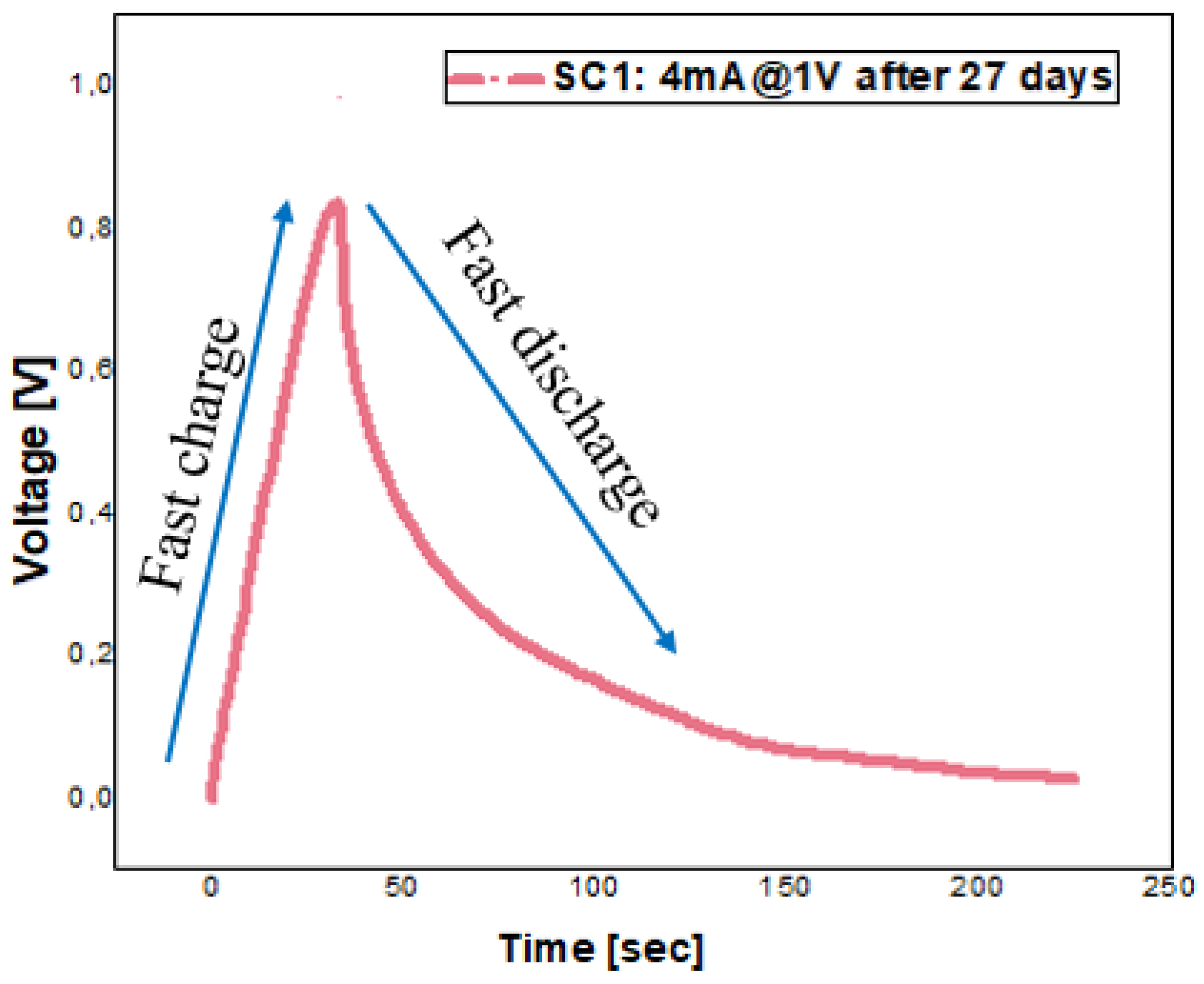
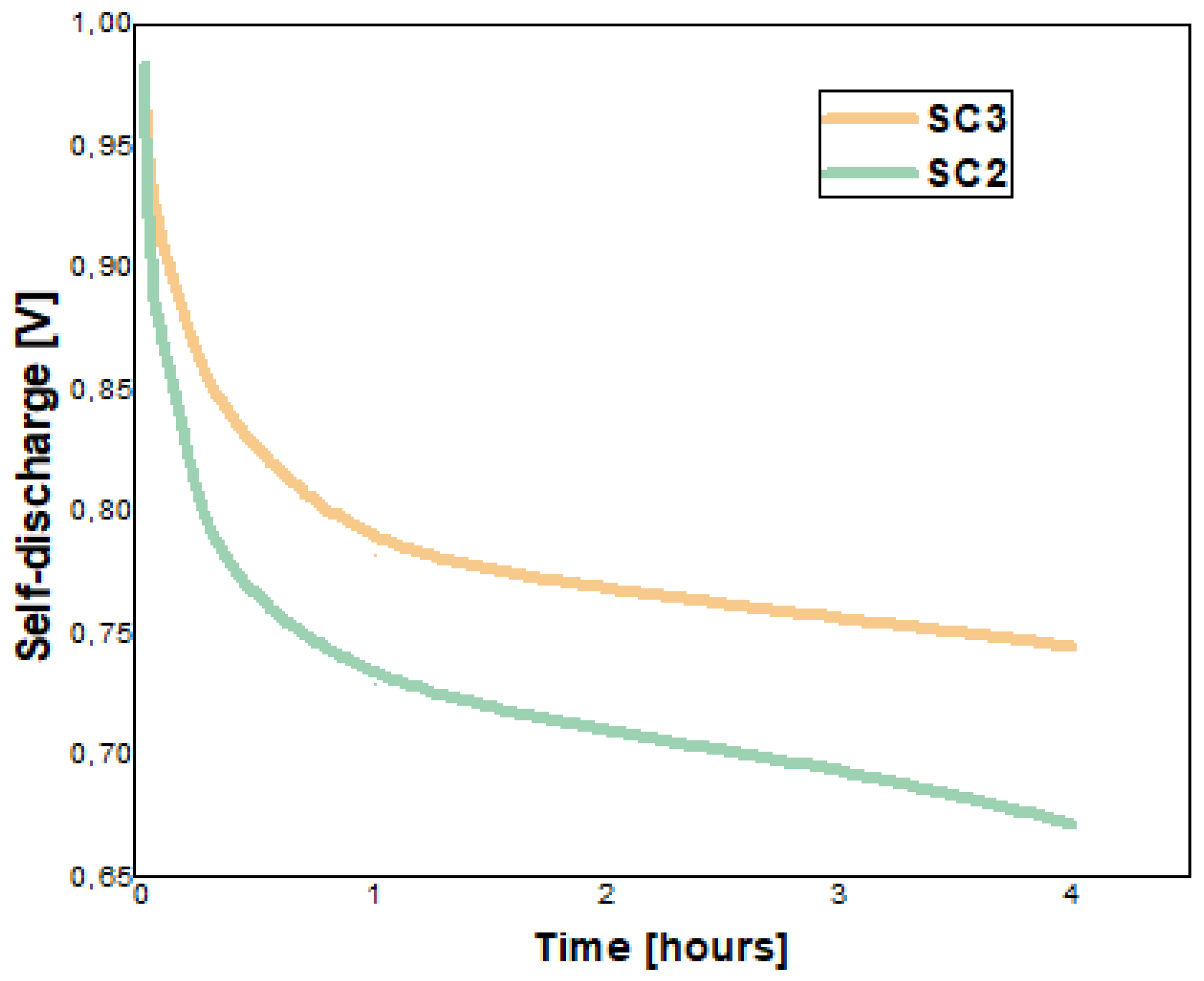
5.5. Stability of Supercapacitors over Time—Galvanostatic Testing after 27 Days
6. Conclusions and Final Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, A.; Chabot, V.; Zhang, J. Electrochemical Supercapacitors for Energy Storage and Delivery Fundamentals and Applications; CRC Press; Taylor & Francis Group: New York, NY, USA, 2013; pp. 51–52. [Google Scholar]
- Kim, I.; San, S.T.; Mendhe, A.C.; Dhas, S.D.; Jeon, S.-B.; Kim, D. Rheological and Electrochemical Properties of Biodegradable Chia Mucilage Gel Electrolyte Applied to Supercapacitor. Batteries 2023, 9, 512. [Google Scholar] [CrossRef]
- TME. Available online: https://www.tme.eu/ro/katalog/elemente-pasive_112309/?queryPhrase=supercondensator&productListOrderBy=1000014 (accessed on 2 May 2024).
- Lv, P.; Song, L.; Li, Y.; Pang, H.; Liu, W. Hybrid ternary rice paper/polypyrrole ink/pen ink nanocomposites as components of flexible supercapacitors. Int. J. Hydrogen Energy 2021, 46, 13219–13229. [Google Scholar] [CrossRef]
- Keawploy, N.; Venkatkarthick, R.; Wangyao, P.; Qin, J. Screen printed textile electrodes using graphene and carbon nanotubes with silver for flexible supercapacitor applications. J. Met. Mater. Miner 2020, 30, 39–44. [Google Scholar] [CrossRef]
- Cholewinski, A.; Si, P.; Uceda, M.; Pope, M.; Zhao, B. Polymer Binders: Characterization and Development toward Aqueous Electrode Fabrication for Sustainability. Polymers 2021, 13, 631. [Google Scholar] [CrossRef] [PubMed]
- European Union. Available online: https://ec.europa.eu/environment/topics/waste-and-recycling/rohs-directive_ro (accessed on 10 April 2024).
- European Union. Available online: https://environment.ec.europa.eu/topics/chemicals/reach-regulation_en (accessed on 10 April 2024).
- European Union. Available online: https://environment.ec.europa.eu/topics/waste-and-recycling/waste-electrical-and-electronic-equipment-weee_en (accessed on 11 April 2024).
- Salleh, N.A.; Kheawhom, S.; Hamid, N.A.A.; Rahiman, W.; Mohamad, A.A. Electrode polymer binders for supercapacitor applications: A review. J. Mater. Res. Technol. 2023, 23, 3470–3491. [Google Scholar] [CrossRef]
- Landi, G.; La Notte, L.; Palma, A.L.; Sorrentino, A.; Maglione, M.G.; Puglisi, G. A comparative evaluation of sustainable binders for environmentally friendly carbon-based supercapacitors. Nanomaterials 2021, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zeng, B.; Jiang, L.; Wu, Y.; Wang, Y.; Wang, J.; Wu, Q.; Sun, X. Tough and self-healable double-network hydrogel for environmentally resistant all-in-one supercapacitors and strain sensors. Chem. Eng. J. 2023, 460, 141787. [Google Scholar] [CrossRef]
- Liang, Y.; Song, Q.; Chen, Y.; Hu, C.; Zhang, S. Stretch-induced robust intrinsic antibacterial thermoplastic gelatin organohydrogel for a thermoenhanced supercapacitor and mono-gauge-factor sensor. ACS Appl. Mater. Interfaces 2023, 15, 20278–20293. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Zhang, Y.; Ni, Y. Recent progress on development of electrolyte and aerogel electrodes applied in supercapacitors. J. Power Sources 2023, 560, 232698. [Google Scholar] [CrossRef]
- Nanografi. Available online: https://nanografi.com/battery-equipment/nickel-foil-purity-99-9-thickness-0-03mm-width-100mm/ (accessed on 2 February 2024).
- Mira Kosmetyki. Available online: https://mirakosmetyki.pl/surowce-kosmetyczne/wegiel-aktywny/50g (accessed on 5 February 2024).
- Xie, R.J.; Cheng, I.C.; Chen, J.Z. East asian calligraphy black ink-coated paper as flexible conducting electrode for supercapacitor. ECS J. Solid State Sci. Technol. 2021, 10, 123013. [Google Scholar] [CrossRef]
- Belineli Barbosa, I.A.; Marcuzzo, J.S.; Cosentino, I.C.; de Faria, R.N., Jr. Binder-Free Textile PAN-Based Electrodes for Aqueous and Glycerol-Based Electrochemical Supercapacitors. Waste Biomass Valorization 2024, 15, 1005–1018. [Google Scholar] [CrossRef]
- Wang, X.; Xing, Z.; Yang, C.; Qin, Y. A Stretchable and Healable Gelatin Hydrogel Assisted by Hofmeister Effect for All-in-One Flexible Supercapacitor. Energy Technol. 2022, 10, 2200897. [Google Scholar] [CrossRef]
- Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications; Kluver Academic: New York, NY, USA, 1999; p. 345. [Google Scholar]
- Negroiu, R.; Svasta, P.; Vasile, A.; Ionescu, C.; Buga, M.R. Realization and Testing of a Supercapacitor, Pouch Type Cell. In Proceedings of the 2020 IEEE 26th International Symposium for Design and Technology in Electronic Packaging (SIITME), Pitesti, Romania, 21–24 October 2020. [Google Scholar]
- Burcea, I.M.; Negroiu, R.; Bonyár, A.; Huszánk, R.; Ionescu, C.; Marghescu, C.; Svasta, P. Comparison of Nanocomposites based on PDMS and Conductive Nanomaterials for the realization of Supercapacitors. In Proceedings of the 2023 46th International Spring Seminar on Electronics Technology (ISSE), Timisoara, Romania, 10–14 May 2023. [Google Scholar]
- Tariq, H.; Awan, S.U.; Hussain, D.; Rizwan, S.; Shah, S.A.; Zainab, S.; Riaz, M.B. Enhancing supercapacitor performance through design optimization of laser-induced graphene and MWCNT coatings for flexible and portable energy storage. Sci. Rep. 2023, 13, 21116. [Google Scholar] [CrossRef] [PubMed]
- Mikhailov, O.V. Gelatin as it is: History and modernity. Int. J. Mol. Sci. 2023, 24, 3583. [Google Scholar] [CrossRef] [PubMed]
- Four Point Probes. Available online: https://inst.eecs.berkeley.edu//~ee143/fa10/lab/four_point_probe.pdf (accessed on 12 April 2024).
- IEC web store. Available online: https://webstore.iec.ch/publication/23581 (accessed on 15 January 2024).
- Railanmaa, A.; Kujala, M.; Keskinen, J.; Kololuoma, T.; Lupo, D. Highly flexible and non-toxic natural polymer gel electrolyte for printed supercapacitors for IoT. Appl. Phys. A 2019, 125, 1–7. [Google Scholar] [CrossRef]
- Wang, J.; Gao, C.; Hou, P.; Liu, Y.; Zhao, J.; Huo, P. All-bio-based, adhesive and low-temperature resistant hydrogel electrolytes for flexible supercapacitors. Chem. Eng. J. 2023, 455, 140952. [Google Scholar] [CrossRef]
- Dalvand, S.; Foroozandeh, A.; Heydarian, A.; Nasab, F.S.; Omidvar, M.; Yazdanfar, N.; Asghari, A. A review on carbon material-metal oxide-conducting polymer and ionic liquid as electrode materials for energy storage in supercapacitors. Ionics 2024, 30, 1–14. [Google Scholar] [CrossRef]
- Goswami, M.; Kumar, S.; Siddiqui, H.; Chauhan, V.; Singh, N.; Sathish, N.; Kumar, S. Hybrid energy storage devices: Li-ion and Na-ion capacitors. In Emerging Trends in Energy Storage Systems and Industrial Applications; Academic Press: Cambridge, MA, USA, 2023; pp. 223–258. [Google Scholar]
- Thalji, M.R.; Ali, G.A.; Shim, J.J.; Chong, K.F. Cobalt-doped tungsten suboxides for supercapacitor applications. Chem. Eng. J. 2023, 473, 145341. [Google Scholar] [CrossRef]
- Cleanenergywiki.org. Available online: https://cleanenergywiki.org/index.php?title=Supercapacitor (accessed on 19 June 2024).





| Materials | Deposited Layer: CAC + Gelatin + Glycerol | Deposited Layer: CAC + Gelatin + Calligraphic Ink | Deposited Layer: CAC + Gelatin | |||
|---|---|---|---|---|---|---|
| C1.1 | C1.2 | C2.1 | C2.2 | C3.1 | C3.2 | |
| Dimensions (length × width) | 2 × 2 cm | |||||
| Undeposited electrode mass [g] | 0.13 | 0.13 | 0.12 | 0.12 | 0.12 | 0.12 |
| Wet-deposited electrode mass [g] | 0.42 | 0.42 | 0.5 | 0.49 | 0.41 | 0.42 |
| Dry-deposited electrode mass [g] | 0.22 | 0.23 | 0.22 | 0.22 | 0.21 | 0.21 |
| Test Conditions | Electrodes | ρ [Ω × cm] | σ [S/cm] |
|---|---|---|---|
| 100 mA @ 20 V | C 1.1 | 39.16 × 10−3 | 25.53 |
| C 2.1 | 127.33 × 10−3 | 7.85 | |
| C 3.1 | 427.25 × 10−3 | 2.34 |
| Supercapacitors | SC 1 | SC 2 | SC 3 | ||||
|---|---|---|---|---|---|---|---|
| Parameters | 0.5 V@4 mA | 1 V@4 mA | 0.5 V@4 mA | 1 V@4 mA | 0.5 V@4 mA | 1 V@4 mA | |
| Test Condition | |||||||
| CSC [F] | 1.985 | 4.819 | 5.626 | 11.663 | 3.730 | 10.181 | |
| ESR [Ω] | 9.254 | 15.671 | 10.542 | 14.574 | 12.239 | 20.834 | |
| Supercapacitors | SC1 | SC2 | SC3 | |
|---|---|---|---|---|
| Parameter | ||||
| Csp [F/g] | 101.46 | 233.26 | 226.25 | |
| Dens (E) [Wh/kg] | 3.52 | 8.09 | 7.85 | |
| Dens (P) [W/kg] | 83.96 | 85.76 | 66.66 | |
| Supercapacitors | SC1 | SC2 | SC3 | |
|---|---|---|---|---|
| Parameters | 1 V@4 mA—after 30 Days | |||
| Test Condition | ||||
| CSC [F] | N/A | 6.79 | 4.13 | |
| ESR [Ω] | N/A | 18.50 | 27.78 | |
| Csp [F/g] | N/A | 135.85 | 91.94 | |
| Dens (E) [Wh/kg] | N/A | 4.71 | 3.19 | |
| Dens (P) [W/kg] | N/A | 67.54 | 49.99 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negroiu, R.-C.; Marghescu, C.-I.; Bacis, I.-B.; Burcea, M.-I.; Drumea, A.; Dinca, L.; Radulescu, I.R. Edible Gelatin and Cosmetic Activated Carbon Powder as Biodegradable and Replaceable Materials in the Production of Supercapacitors. Batteries 2024, 10, 237. https://doi.org/10.3390/batteries10070237
Negroiu R-C, Marghescu C-I, Bacis I-B, Burcea M-I, Drumea A, Dinca L, Radulescu IR. Edible Gelatin and Cosmetic Activated Carbon Powder as Biodegradable and Replaceable Materials in the Production of Supercapacitors. Batteries. 2024; 10(7):237. https://doi.org/10.3390/batteries10070237
Chicago/Turabian StyleNegroiu, Rodica-Cristina, Cristina-Ioana Marghescu, Irina-Bristena Bacis, Madalina-Irina Burcea, Andrei Drumea, Laurentiu Dinca, and Ion Razvan Radulescu. 2024. "Edible Gelatin and Cosmetic Activated Carbon Powder as Biodegradable and Replaceable Materials in the Production of Supercapacitors" Batteries 10, no. 7: 237. https://doi.org/10.3390/batteries10070237
APA StyleNegroiu, R.-C., Marghescu, C.-I., Bacis, I.-B., Burcea, M.-I., Drumea, A., Dinca, L., & Radulescu, I. R. (2024). Edible Gelatin and Cosmetic Activated Carbon Powder as Biodegradable and Replaceable Materials in the Production of Supercapacitors. Batteries, 10(7), 237. https://doi.org/10.3390/batteries10070237







