Single-Use Vape Batteries: Investigating Their Potential as Ignition Sources in Waste and Recycling Streams
Abstract
1. Introduction
2. Materials and Methods
2.1. Preliminaries
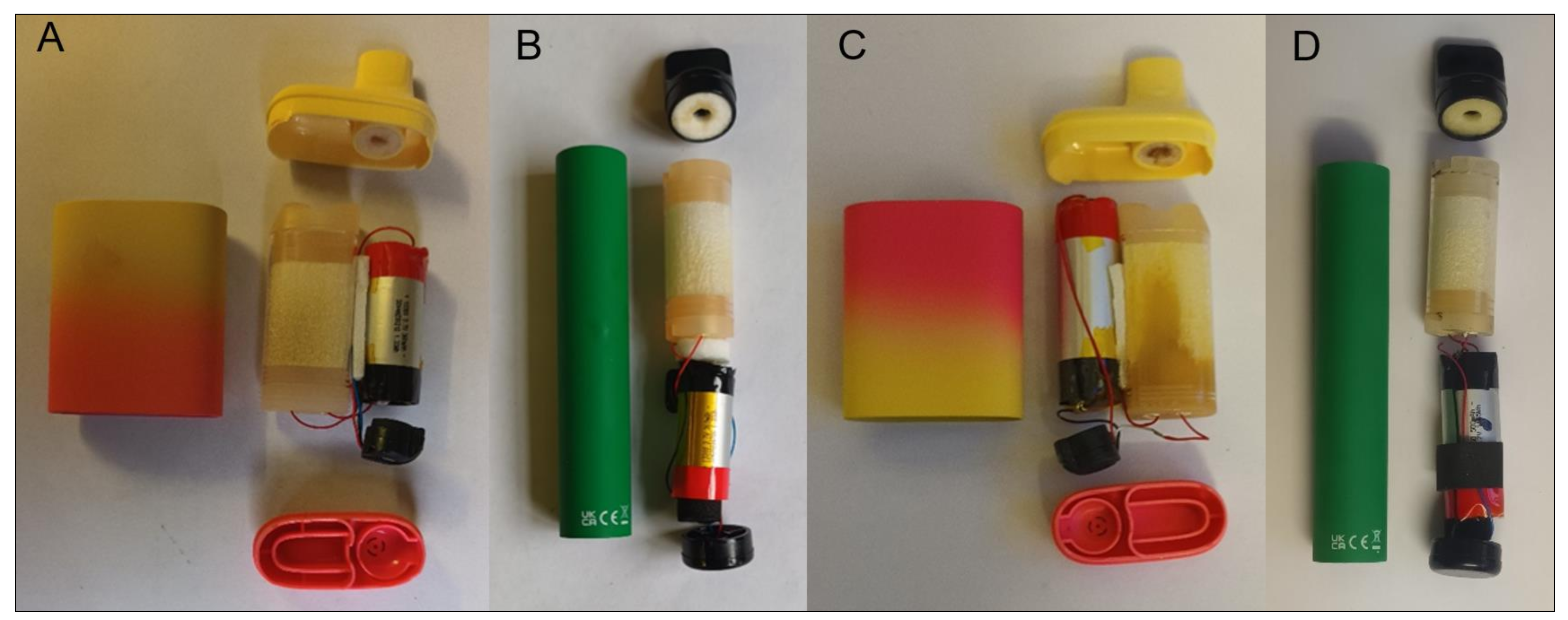
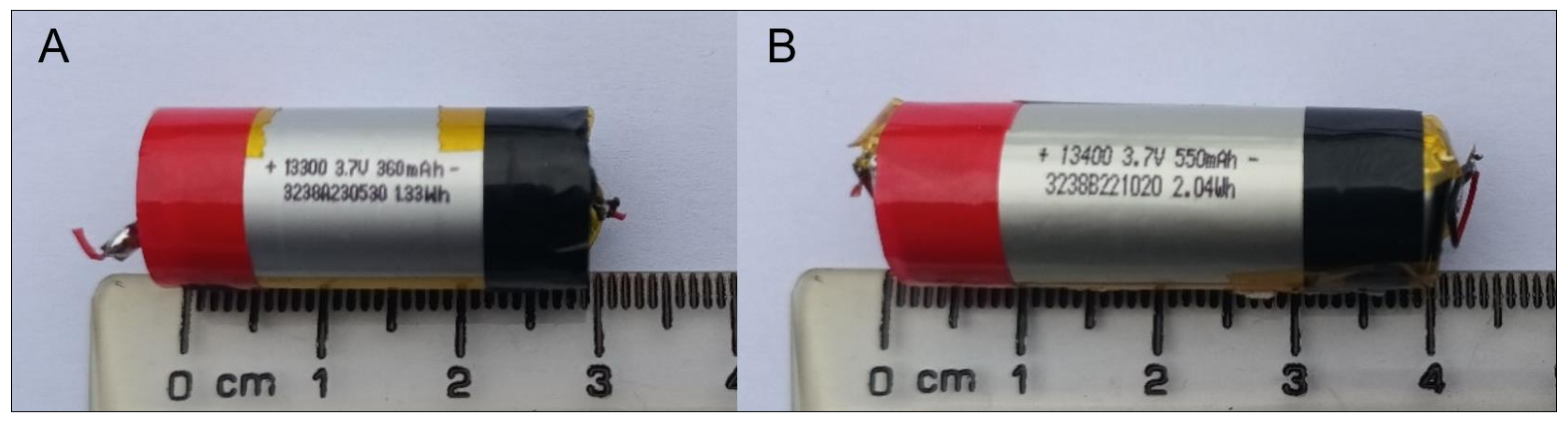
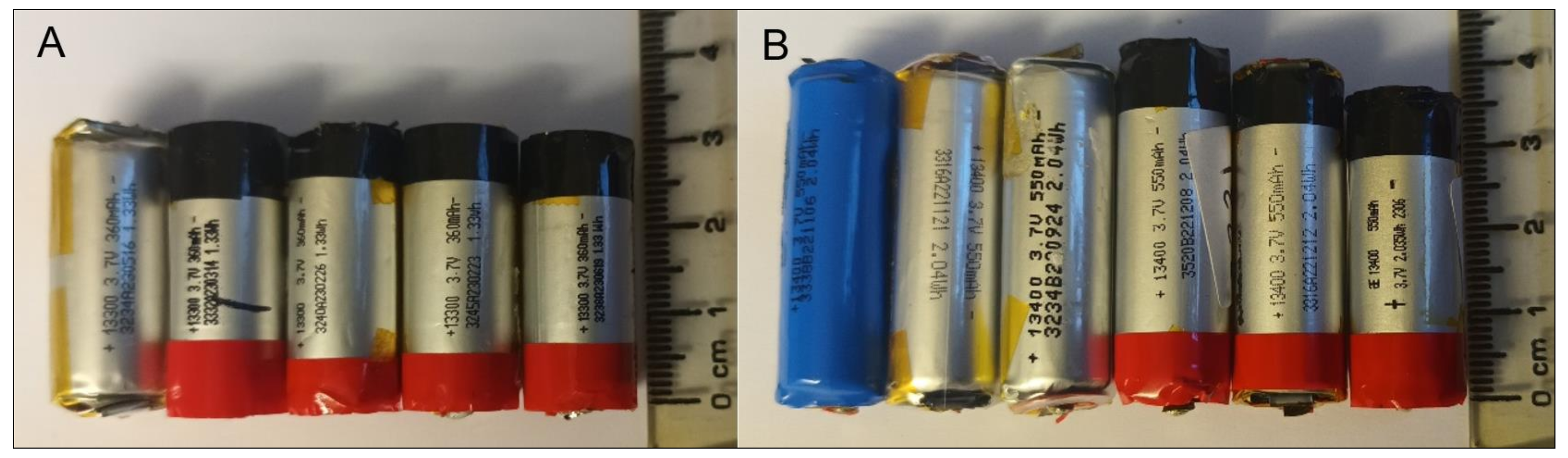
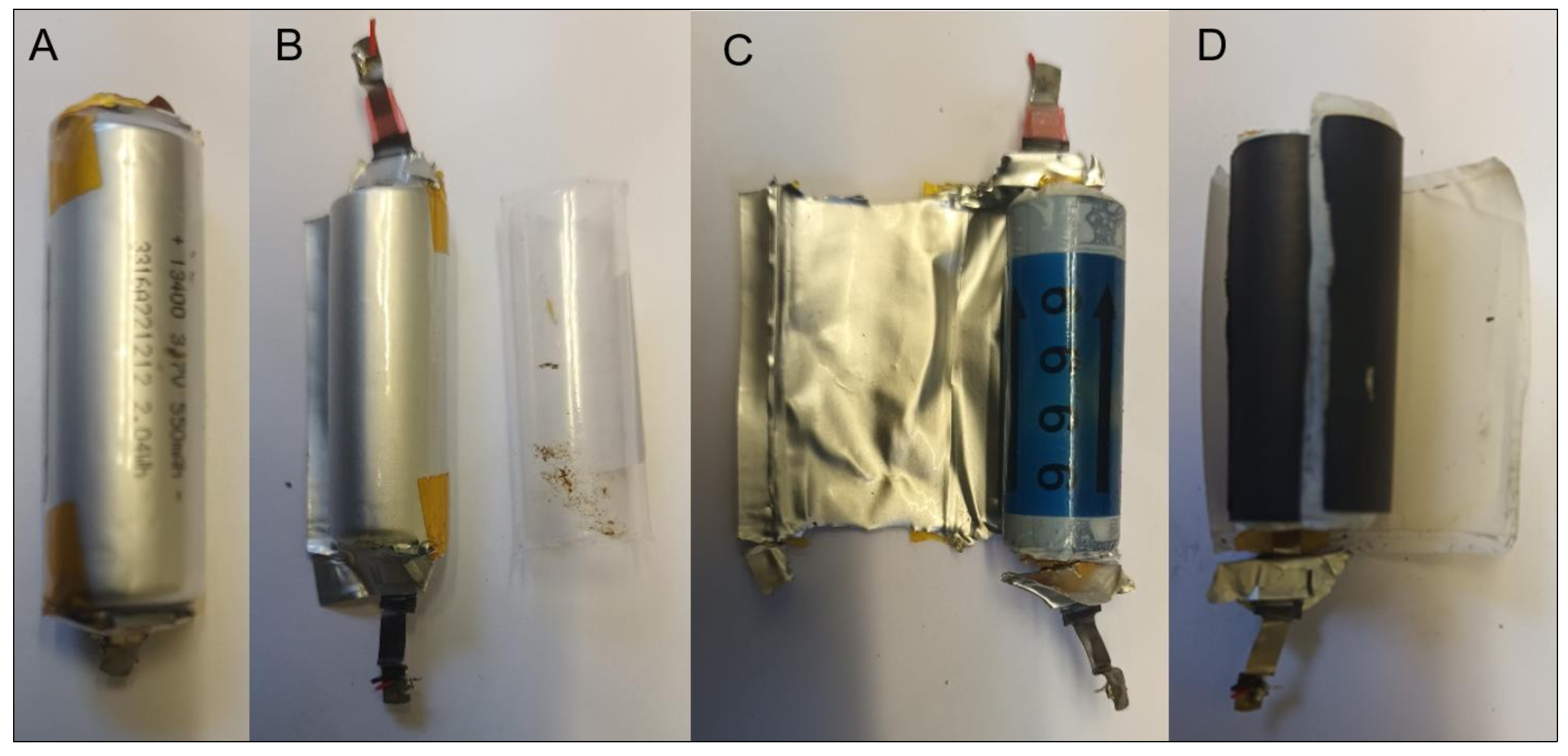
2.2. Target SUVs
2.3. Testing Rationale
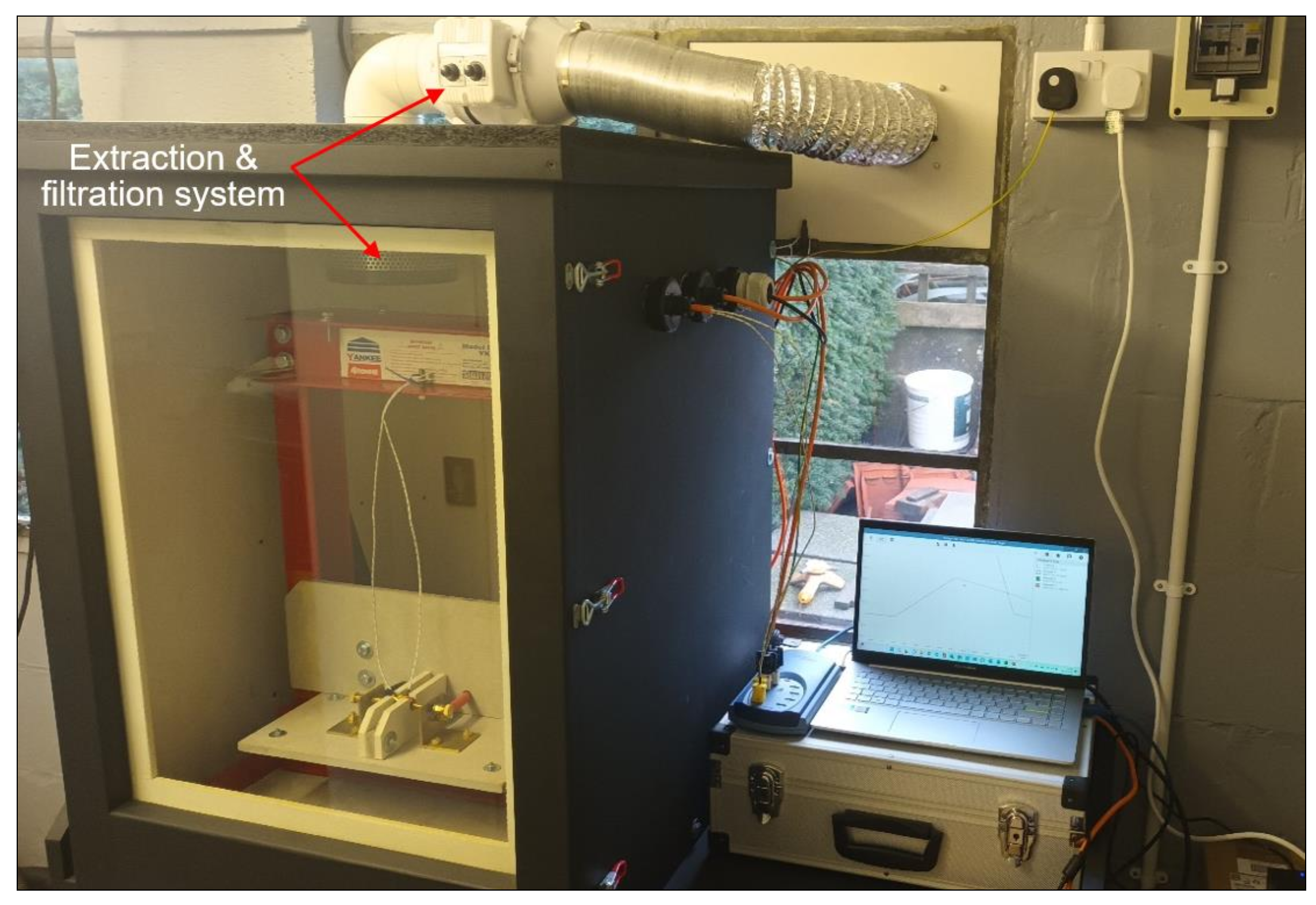
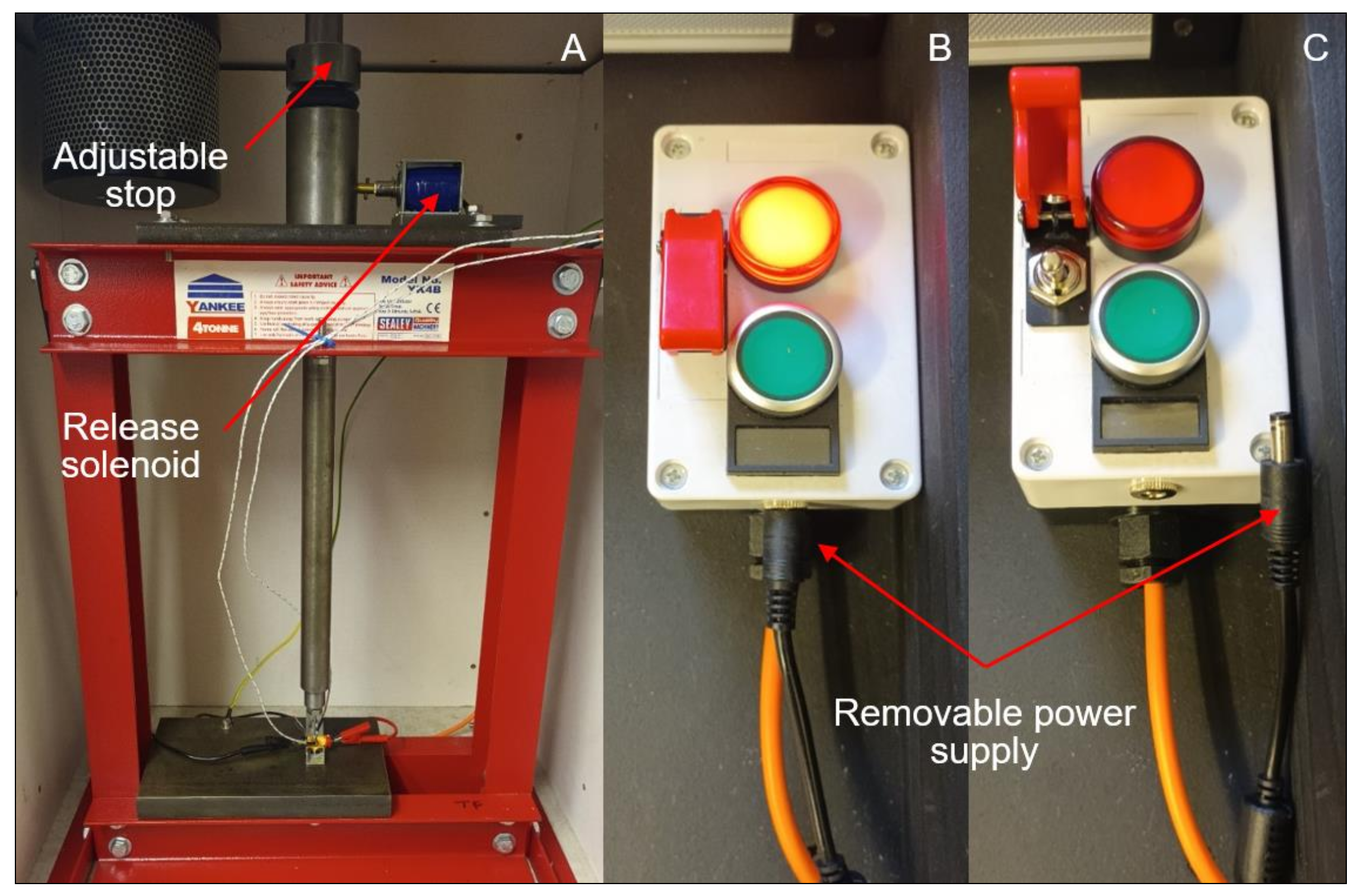
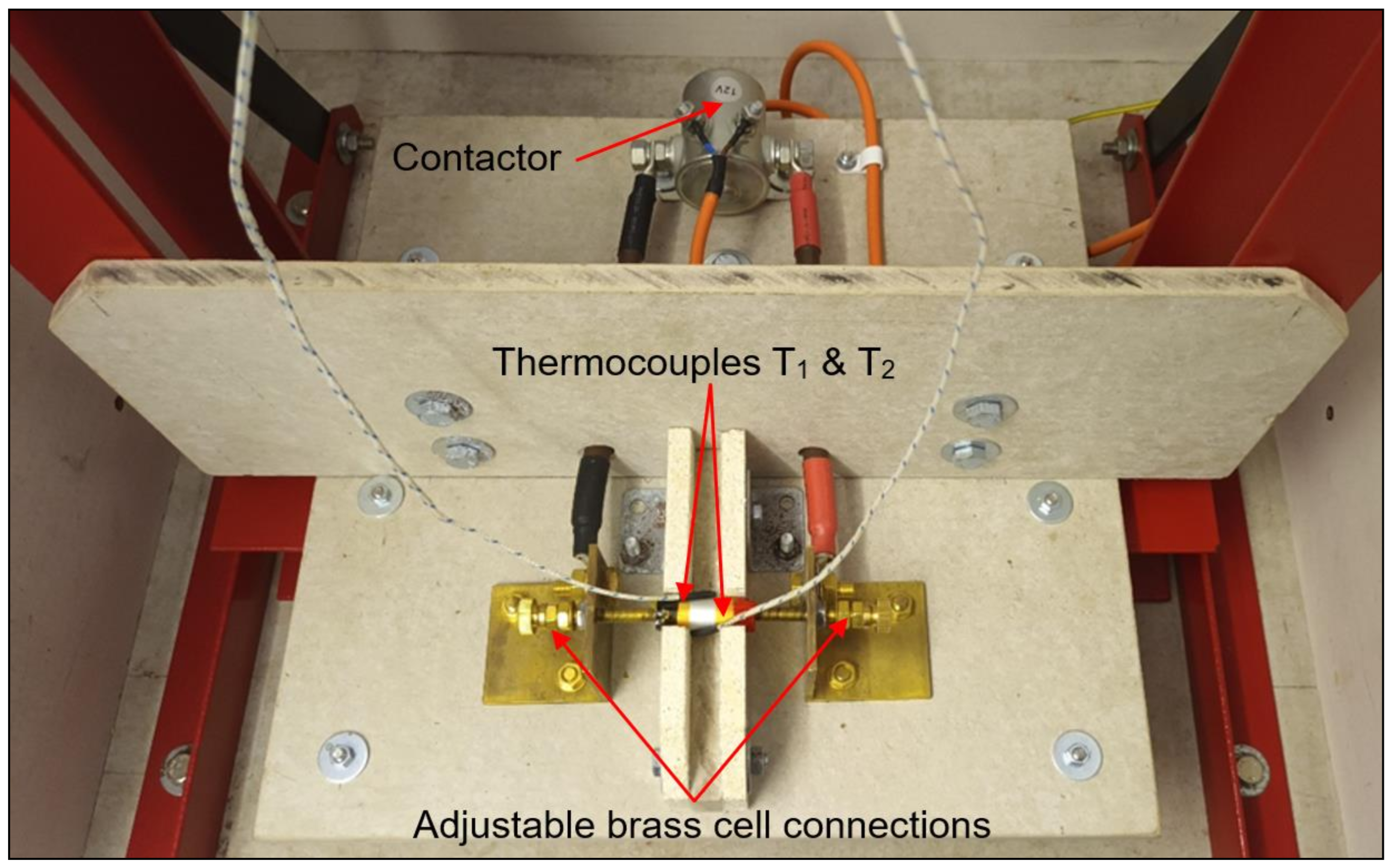
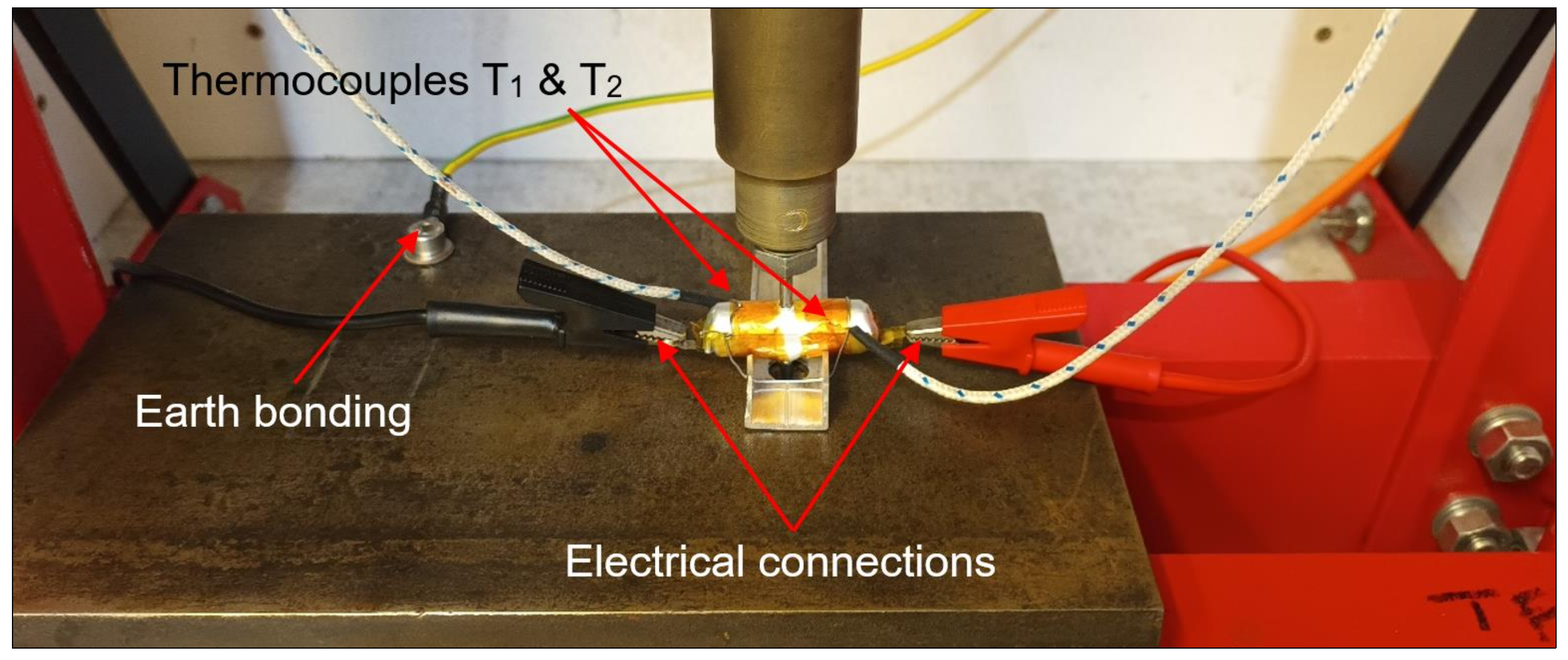
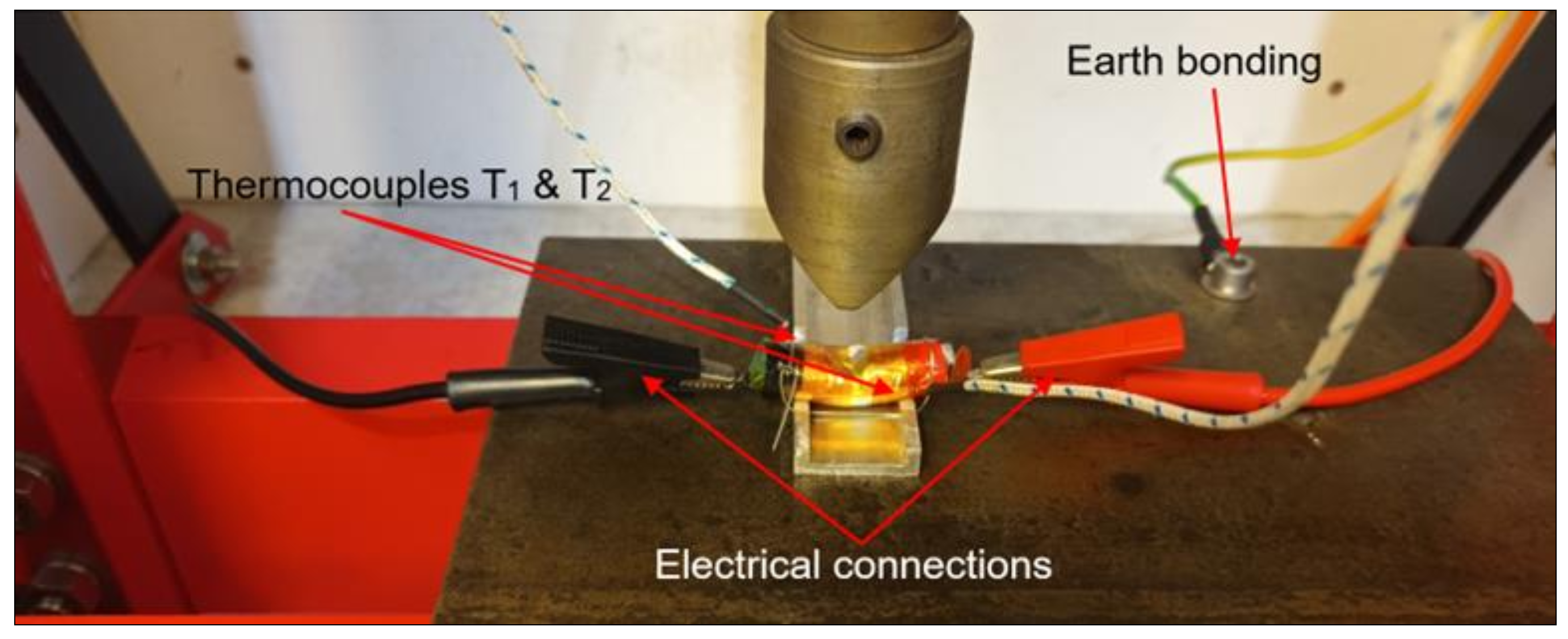
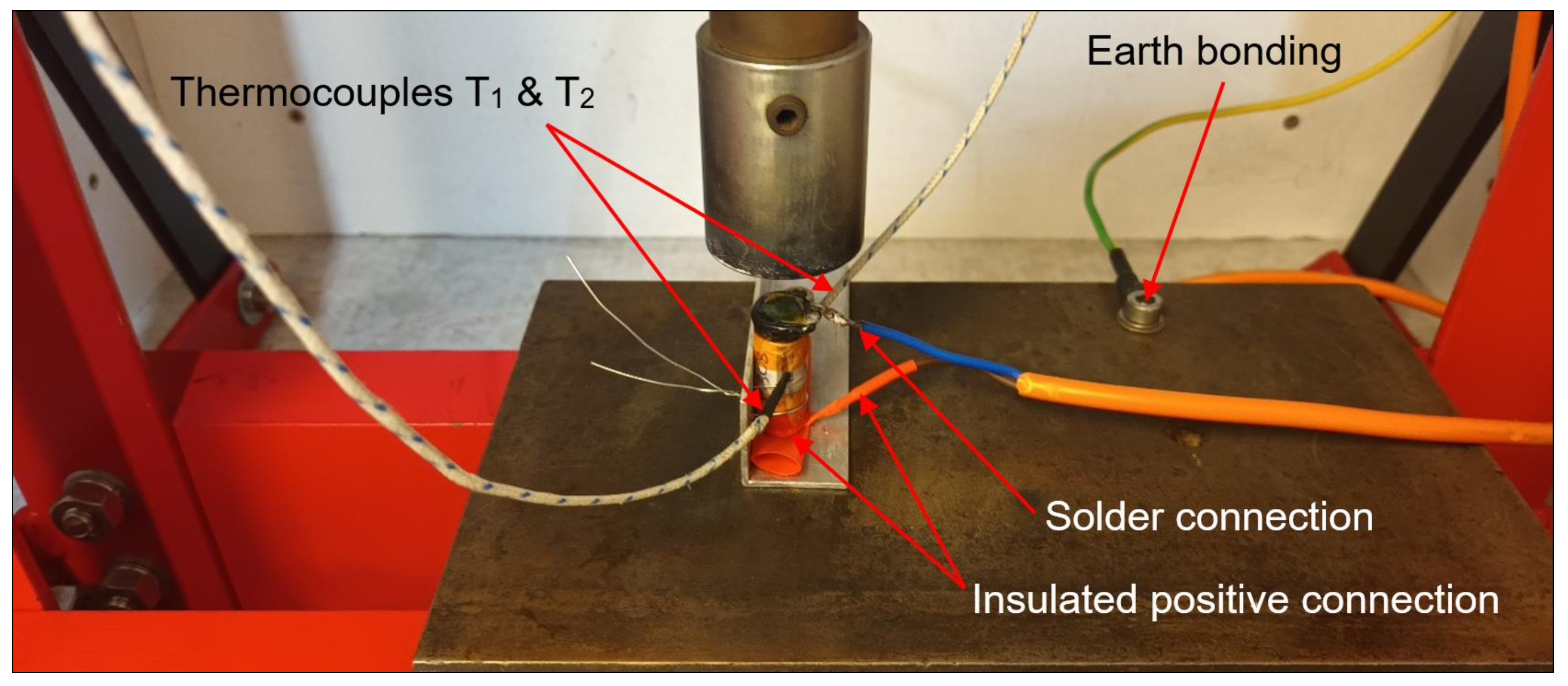
2.4. Test Rig Design
2.5. Testing Approach
2.5.1. Short-Circuit Tests
2.5.2. Nail Tests
2.5.3. Impact Tests
2.5.4. Crush Tests
3. Results and Discussion
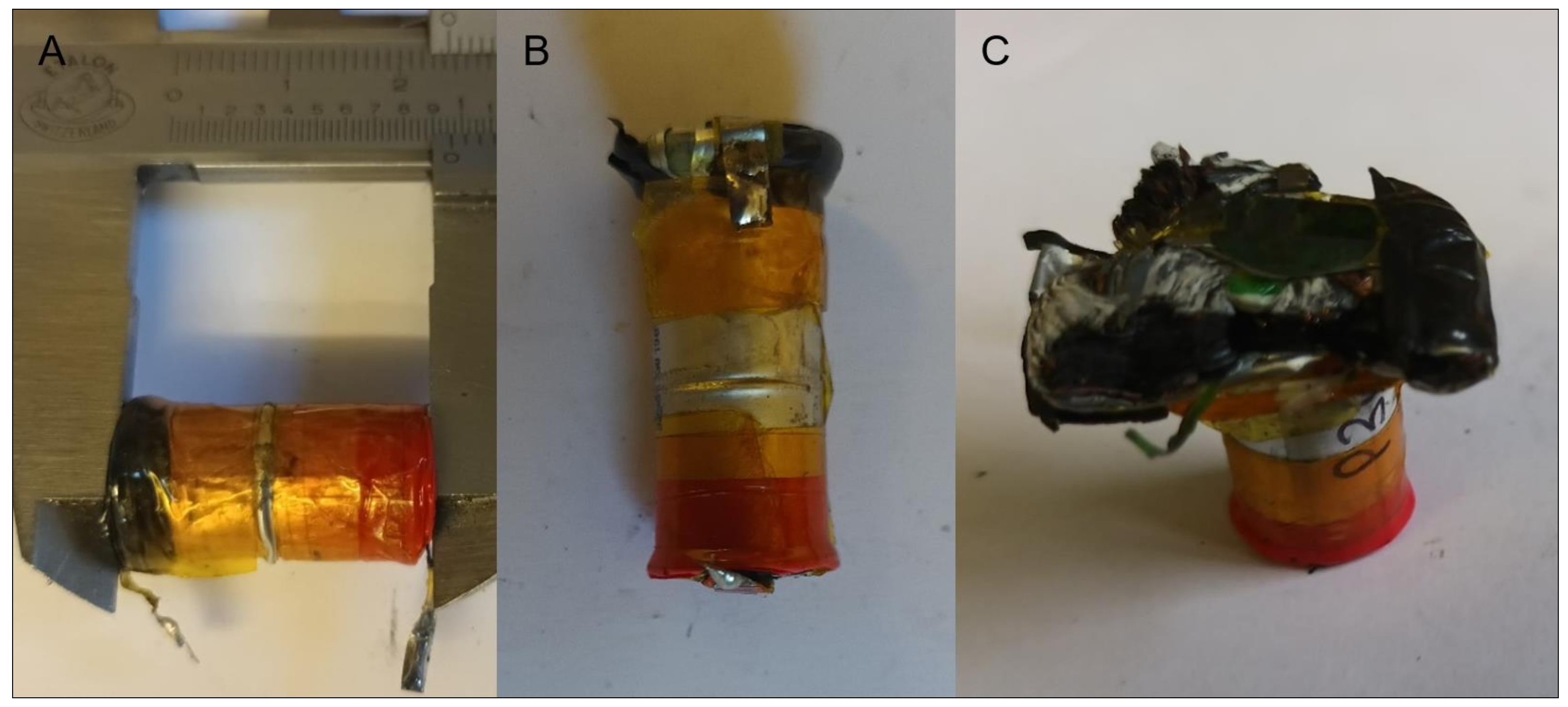
3.1. Initial Cell Assessment
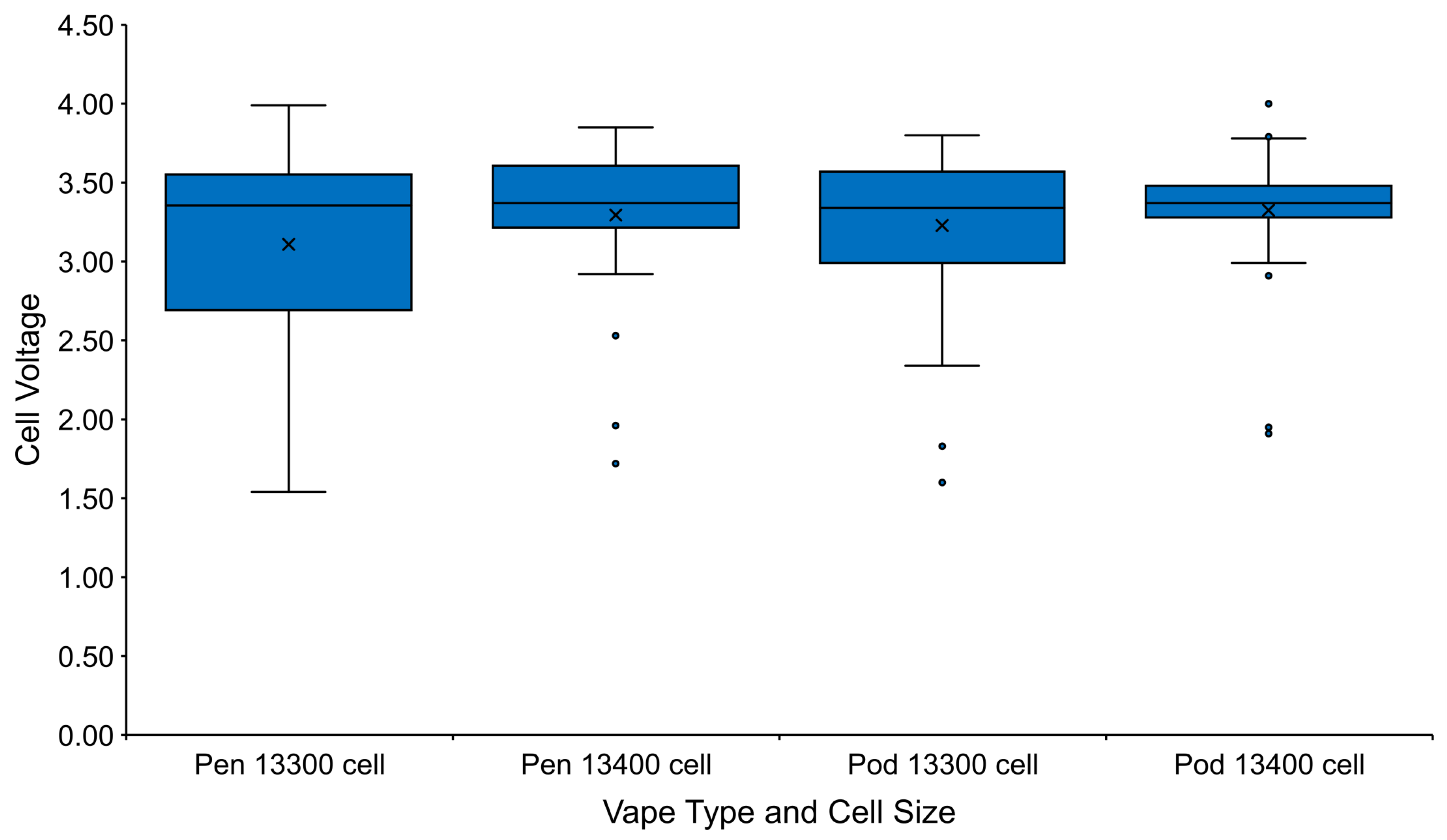
3.1.1. Open Circuit Voltage
3.1.2. Initial State of Charge
3.1.3. Cell Total Capacity
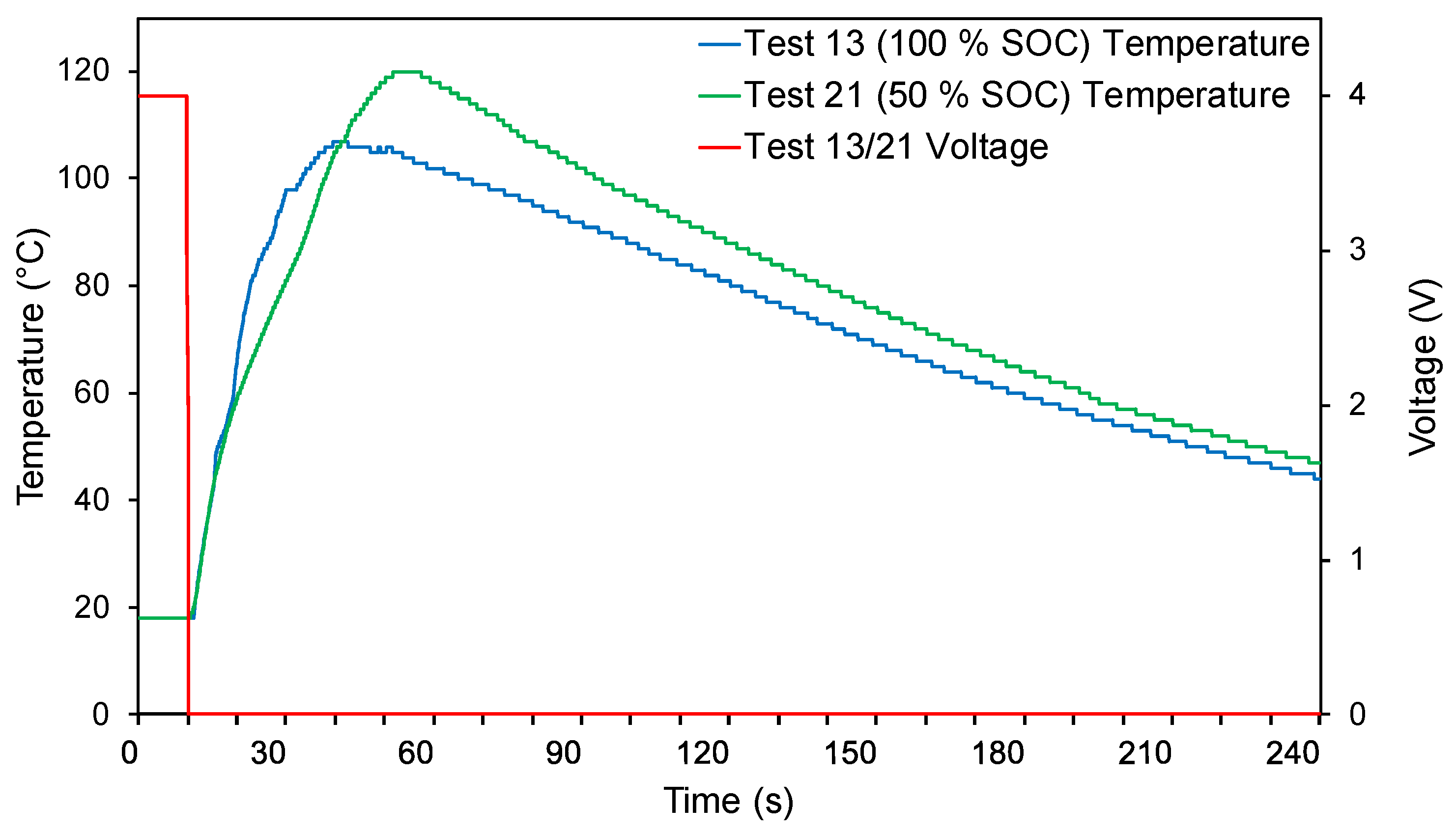
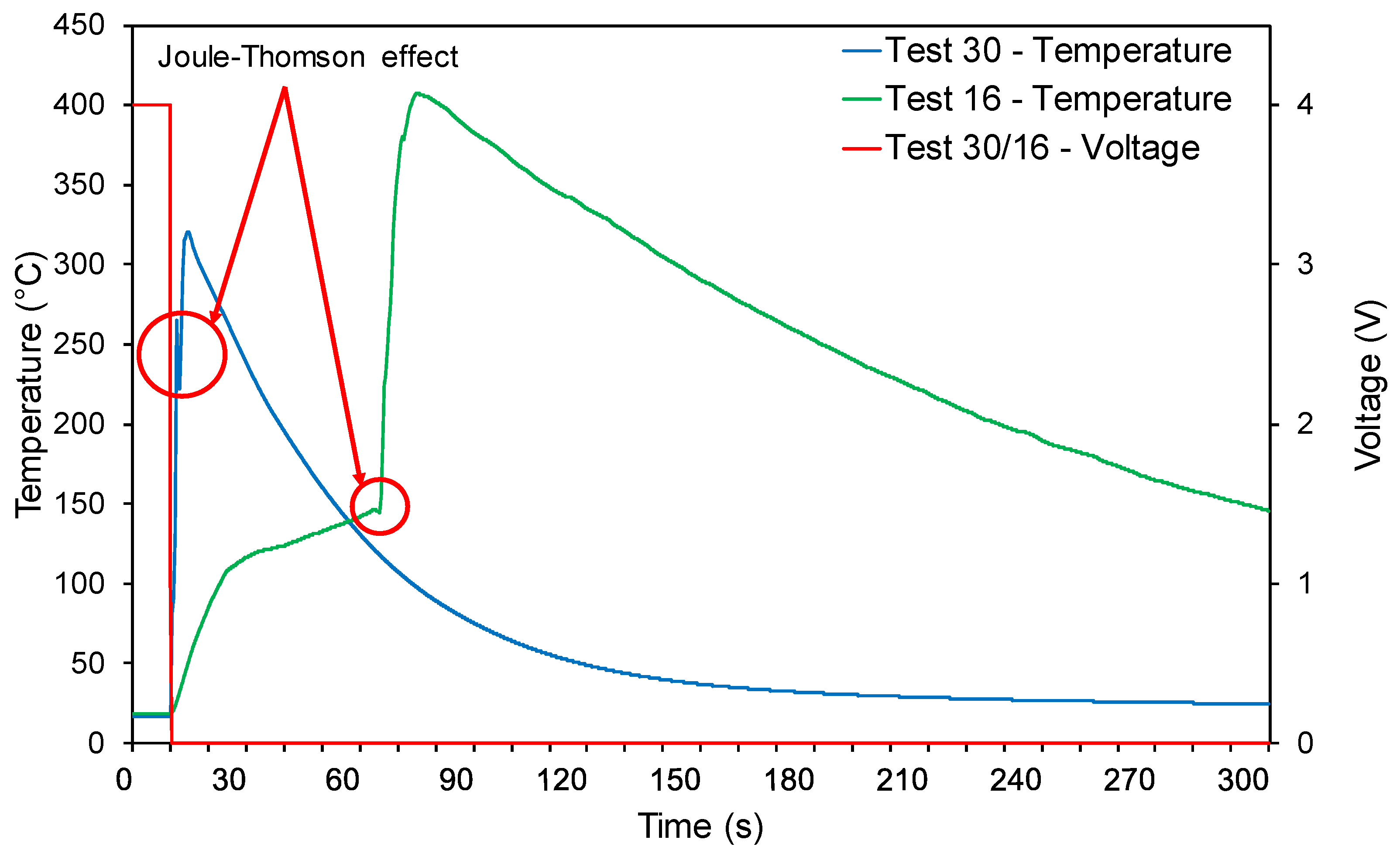
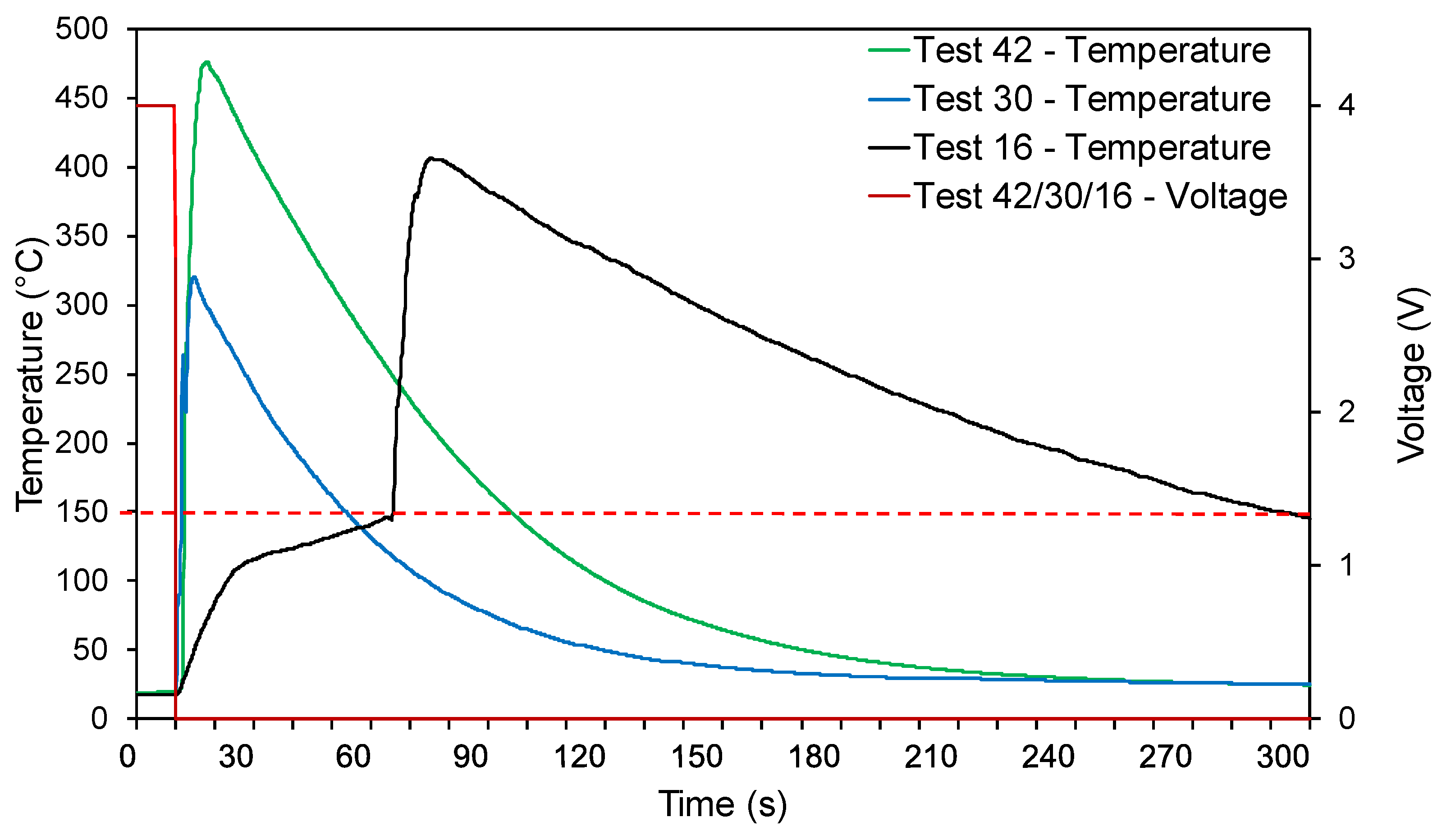
3.2. Electrical Abuse Tests
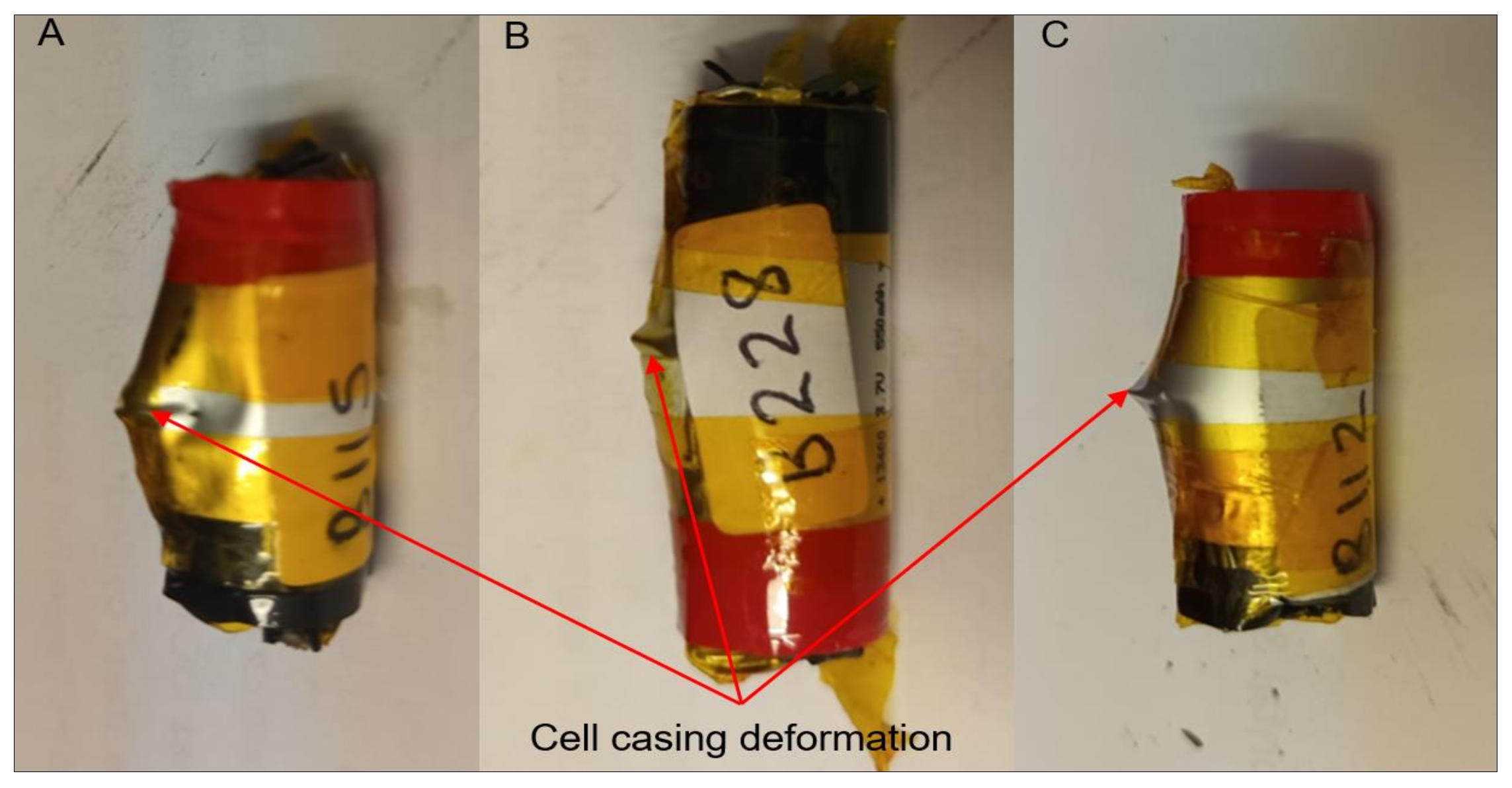
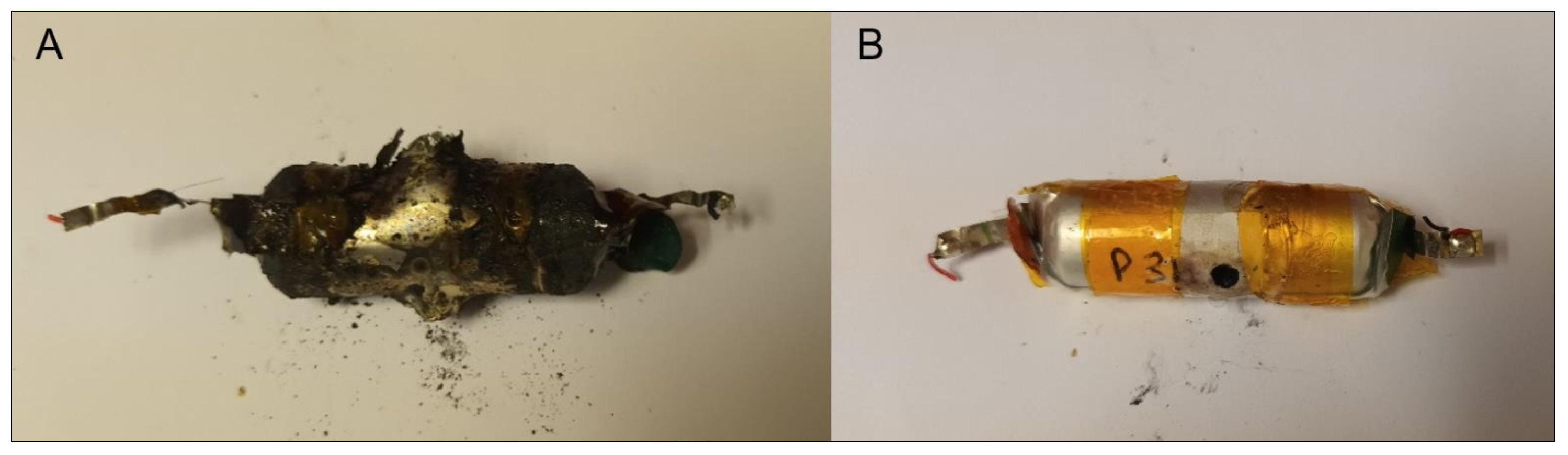
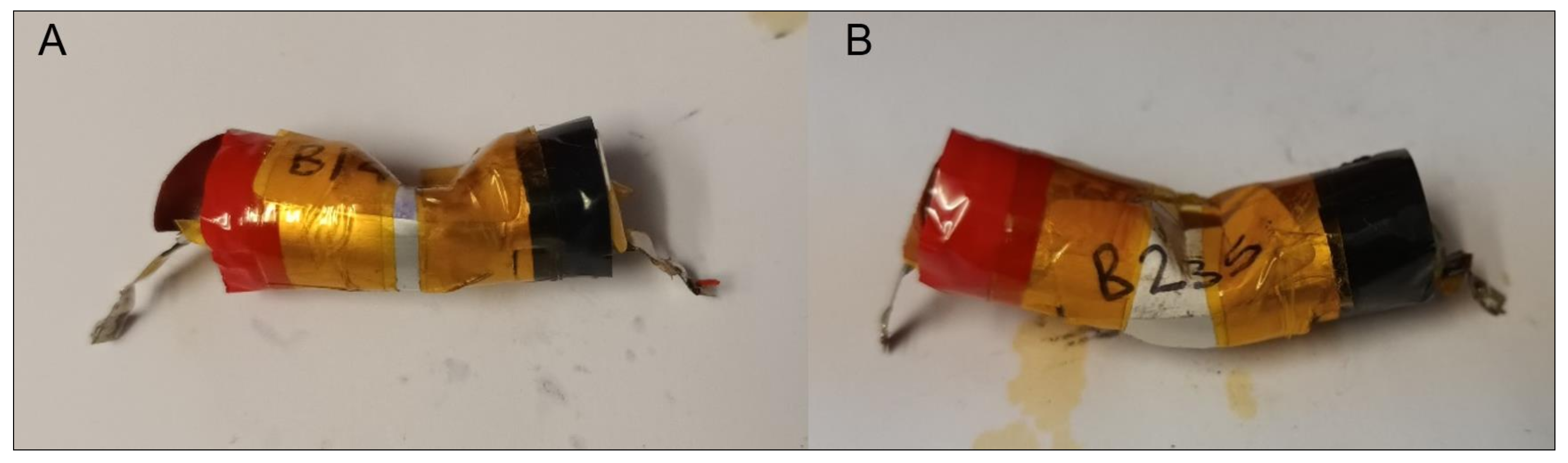
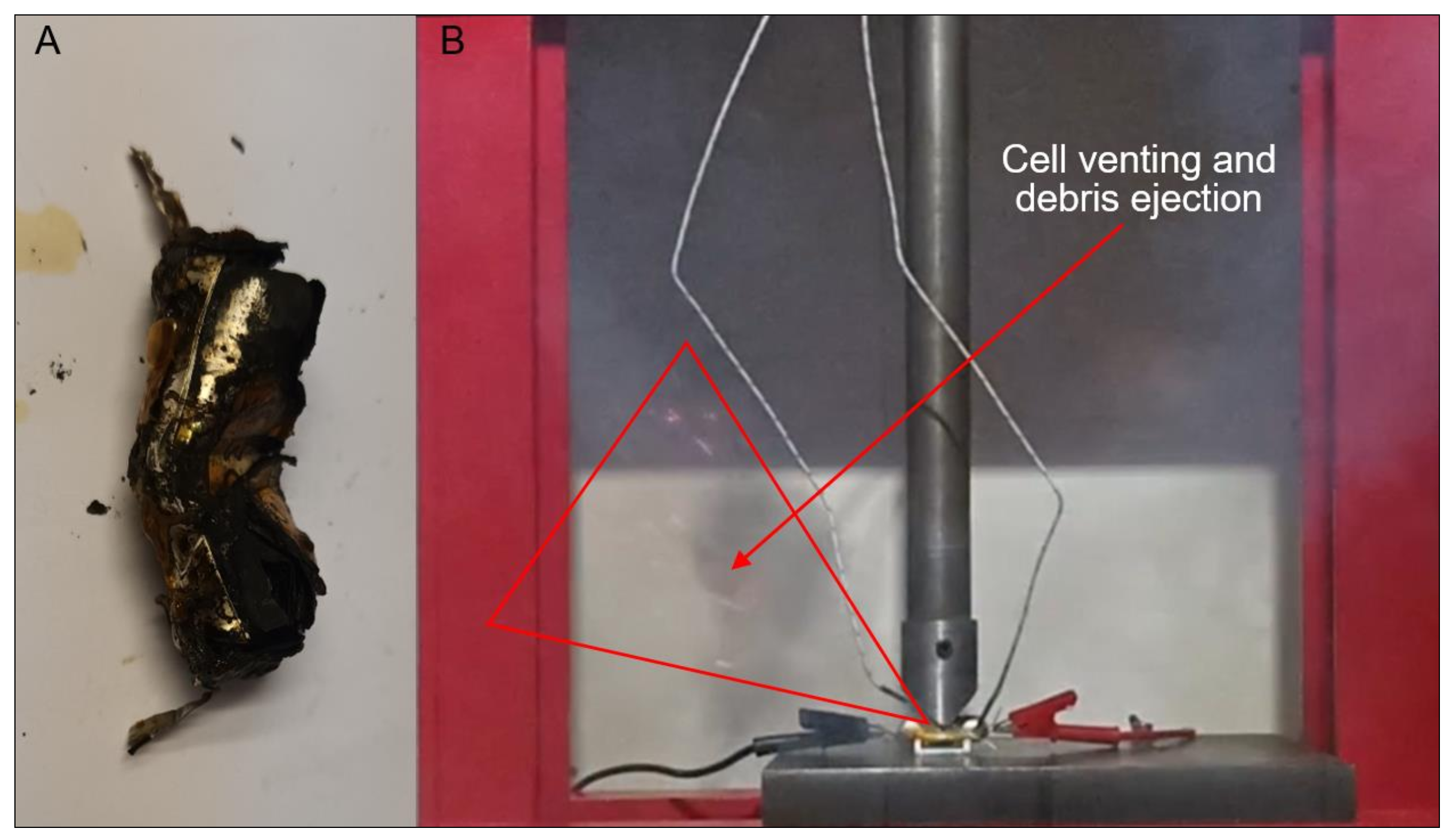
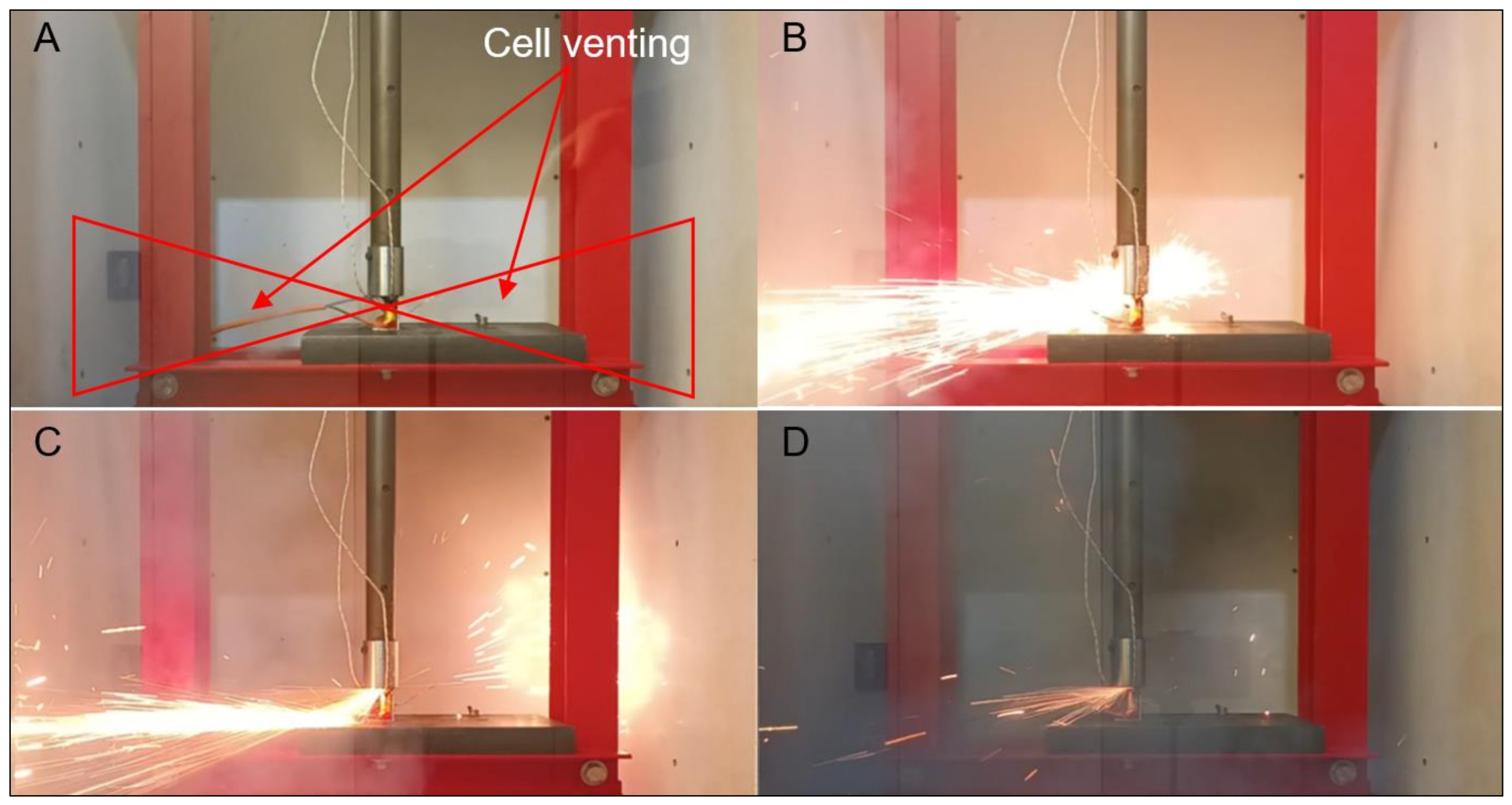
3.3. Mechanical Abuse Tests
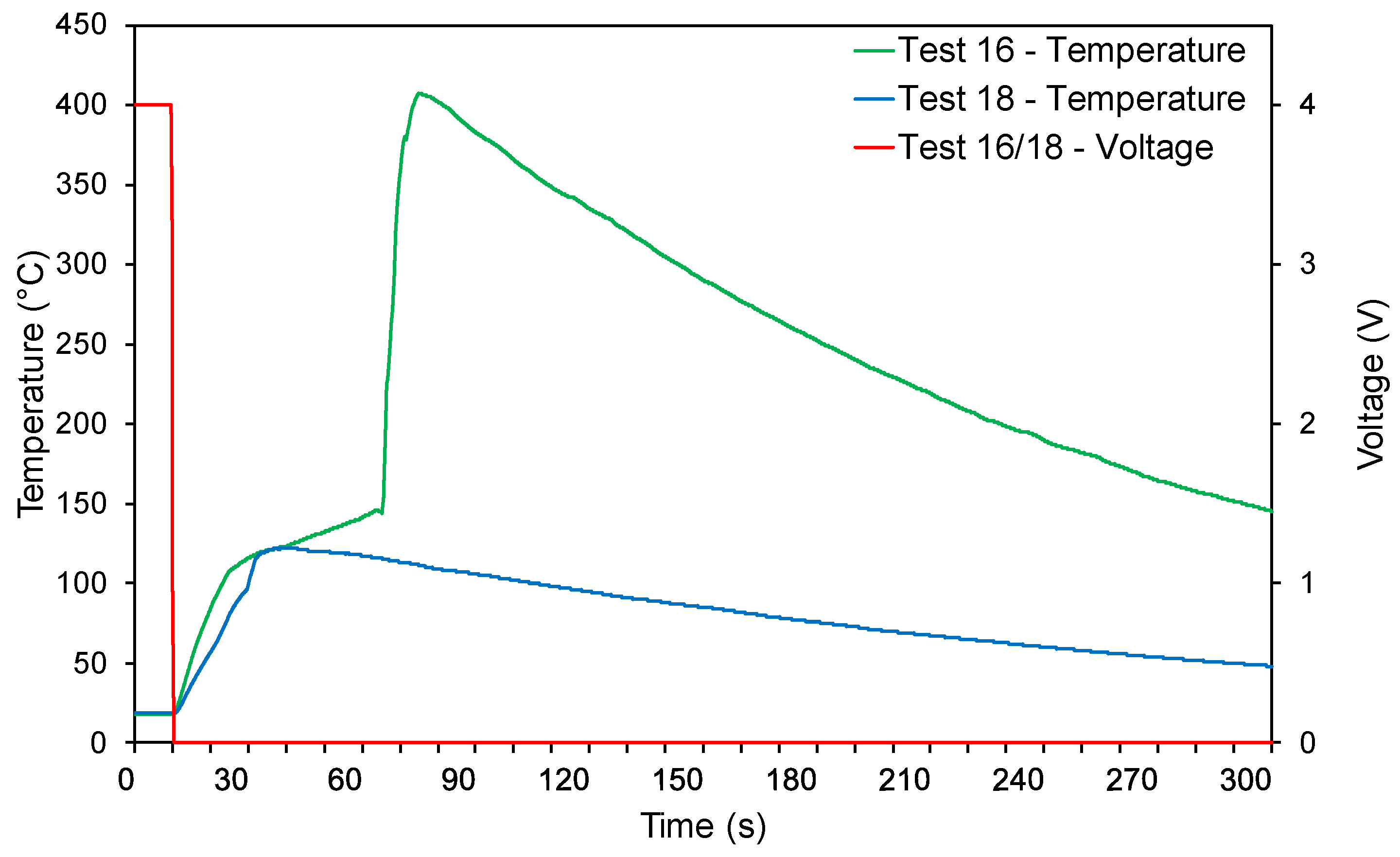
3.3.1. Nail Tests
3.3.2. Impact Tests
3.3.3. Crush Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| C | Cell Rated Capacity |
| CC | Constant Current |
| CV | Constant Voltage |
| CID | Current Interruption Device |
| DOD | Depth of Discharge |
| FPS | Frame Per Second |
| ISC | Internal Short-Circuit |
| LFL | Lower Flammable Limit |
| LiPo | Lithium Polymer |
| OCV | Open Circuit Voltage |
| PTC | Positive Temperature Coefficient |
| SOC | State of Charge |
| SUV | Single Use Vape |
| TR | Thermal Runaway |
References
- Brown, M.; Hilton, M.; Horton, I.; Hickman, M.; Mason, R.; Brown, A. Cutting Lithium-Ion Battery Fires in the Waste Industry; Technical Report; Environmental Services Association: London, UK, 2021. [Google Scholar]
- Nigl, T.; Rübenbauer, W.; Pomberger, R. Cause-oriented investigation of the fire incidents in Austrian waste management systems. Detritus 2019, 9, 213–220. [Google Scholar] [CrossRef]
- Anta, M.; Herreras, L.; Ollion, L. Characterisation of Fires Caused by Batteries in WEEE: Survey Results from the WEEE Management Chain-Part A; Technical report; WEEE Forum: Schaerbeek, Belgium, 2020. [Google Scholar]
- Material Focus. Number of Disposable Single-Use Vapes Thrown away Have in a Year Quadrupled to 5 Million per Week; Material Focus: London, UK, 2023. [Google Scholar]
- Lisbona, D.; Snee, T. A review of hazards associated with primary lithium and lithium-ion batteries. Process Saf. Environ. Prot. 2011, 89, 434–442. [Google Scholar] [CrossRef]
- Larsson, F.; Andersson, P.; Blomqvist, P.; Mellander, B.E. Toxic fluoride gas emissions from lithium-ion battery fires. Sci. Rep. 2017, 7, 10018. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. Thermal runaway caused fire and explosion of lithium ion battery. J. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- SAE International. Electric and Hybrid Electric Vehicle Rechargeable Energy Storage System (RESS) Safety and Abuse Testing; Technical Report SAE J2464; SAE International: Warrendale, PA, USA, 2021. [Google Scholar]
- Bubbico, R.; Greco, V.; Menale, C. Hazardous scenarios identification for Li-ion secondary batteries. Saf. Sci. 2018, 108, 72–88. [Google Scholar] [CrossRef]
- Nigl, T.; Baldauf, M.; Hohenberger, M.; Pomberger, R. Lithium-Ion batteries as ignition sources in waste treatment processes: A semi-quantitate risk analysis and assessment of battery-caused waste fires. Processes 2020, 9, 49. [Google Scholar] [CrossRef]
- Abbott, K.C.; Buston, J.E.H.; Gill, J.; Goddard, S.L.; Howard, D.; Howard, G.E.; Read, E.; Williams, R.C.E. Experimental study of three commercially available 18650 lithium ion batteries using multiple abuse methods. J. Energy Storage 2023, 65, 107293. [Google Scholar] [CrossRef]
- An, Z.; Li, W.; Du, X.; Jia, L.; Li, Q.; Zhang, D. Experimental study on behaviors of lithium-ion cells experiencing internal short circuit and thermal runaway under nail penetration abuse condition. Appl. Therm. Eng. 2024, 247, 123058. [Google Scholar] [CrossRef]
- Diekmann, J.; Doose, S.; Weber, S.; Münch, S.; Haselrieder, W.; Kwade, A. Development of a new procedure for nail penetration of lithium-ion cells to obtain meaningful and reproducible results. J. Electrochem. Soc. 2020, 167, 090504. [Google Scholar] [CrossRef]
- Mao, B.; Chen, H.; Cui, Z.; Wu, T.; Wang, Q. Failure mechanism of the lithium ion battery during nail penetration. Int. J. Heat Mass Transf. 2018, 122, 1103–1115. [Google Scholar] [CrossRef]
- Shelke, A.V.; Buston, J.E.H.; Gill, J.; Howard, D.; Abbott, K.C.; Goddard, S.L.; Read, E.; Howard, G.E.; Abaza, A.; Cooper, B.; et al. Characterizing and predicting 21700 NMC lithium-ion battery thermal runaway induced by nail penetration. Appl. Therm. Eng. 2022, 209, 118278. [Google Scholar] [CrossRef]
- An, Z.; Shi, T.; Du, X.; An, X.; Zhang, D.; Bai, J. Experimental study on the internal short circuit and failure mechanism of lithium-ion batteries under mechanical abuse conditions. J. Energy Storage 2024, 89, 111819. [Google Scholar] [CrossRef]
- Chen, W.C.; De Li, J.; Shu, C.M.; Wang, Y.W. Effects of thermal hazard on 18650 lithium-ion battery under different states of charge. J. Therm. Anal. Calorim. 2015, 121, 525–531. [Google Scholar] [CrossRef]
- Lai, Y.W.; Chi, K.H.; Chung, Y.H. Thermal runaway characteristics of 18650 lithium-ion batteries in various states of charge. J. Therm. Anal. Calorim. 2024, 1–10. [Google Scholar] [CrossRef]
- Willstrand, O.; Pushp, M.; Andersson, P.; Brandell, D. Impact of different Li-ion cell test conditions on thermal runaway characteristics and gas release measurements. J. Energy Storage 2023, 68, 107785. [Google Scholar] [CrossRef]
- Quintiere, J.G. On a method to mitigate thermal runaway and propagation in packages of lithium ion batteries. Fire Saf. J. 2022, 130, 103573. [Google Scholar] [CrossRef]
- Wang, Q.; Mao, B.; Stoliarov, S.I.; Sun, J. A review of lithium ion battery failure mechanisms and fire prevention strategies. Prog. Energy Combust. Sci. 2019, 73, 95–131. [Google Scholar] [CrossRef]
- Juan, W.Y.; Wu, C.L.; Liu, F.W.; Chen, W.S. Fires in Waste Treatment Facilities: Challenges and Solutions from a Fire Investigation Perspective. Sustainability 2023, 15, 9756. [Google Scholar] [CrossRef]
- BS EN 62133-2:2017+A1:2021; Secondary Cells and Batteries Containing Alkaline or Other Non-Acid Electrolytes. Safety Requirements for Portable Sealed Secondary Cells, and for Batteries Made from Them, for Use in Portable Applications—Lithium Systems. British Standards Institution: London, UK, 2021.
- Zou, K.; Jiang, M.; Ning, T.; Tan, L.; Zheng, J.; Wang, J.; Ji, X.; Li, L. Thermodynamics-directed bulk/grain-boundary engineering for superior electrochemical durability of Ni-rich cathode. J. Energy Chem. 2024, 97, 321–331. [Google Scholar] [CrossRef]
- Bugryniec, P.J.; Resendiz, E.G.; Nwophoke, S.M.; Khanna, S.; James, C.; Brown, S.F. Review of gas emissions from lithium-ion battery thermal runaway failure—Considering toxic and flammable compounds. J. Energy Storage 2024, 87, 111288. [Google Scholar] [CrossRef]
- Xu, C.; Fan, Z.; Zhang, M.; Wang, P.; Wang, H.; Jin, C.; Peng, Y.; Jiang, F.; Feng, X.; Ouyang, M. A comparative study of the venting gas of lithium-ion batteries during thermal runaway triggered by various methods. Cell Rep. Phys. Sci. 2023, 4, 101705. [Google Scholar] [CrossRef]
- Christensen, P.; Mrozik, W.; Weaving, J. Improving the Safety of Lithium-Ion Battery Cells; Technical report; The Faraday Institution: Cambridge, UK, 2023. [Google Scholar]
- Mikolajczak, C.; Kahn, M.; White, K.; Long, R.T. Lithium-Ion Batteries Hazard and Use Assessment; Springer: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Zhang, M.; Du, J.; Liu, L.; Stefanopoulou, A.; Siegel, J.; Lu, L.; He, X.; Xie, X. Internal Short Circuit Trigger Method for Lithium-Ion Battery Based on Shape Memory Alloy. J. Electrochem. Soc. 2017, 164, A326–A333. [Google Scholar] [CrossRef]
- Radaš, I.; Pilat, N.; Gnjatović, D.; Šunde, V.; Ban, Ž. Estimating the state of charge of lithium-ion batteries based on the transfer function of the voltage response to the current pulse. Energies 2022, 15, 6495. [Google Scholar] [CrossRef]
- Ng, K.S.; Moo, C.S.; Chen, Y.P.; Hsieh, Y.C. Enhanced coulomb counting method for estimating state-of-charge and state-of-health of lithium-ion batteries. Appl. Energy 2009, 86, 1506–1511. [Google Scholar] [CrossRef]
- Nigl, T.; Back, T.; Stuhlpfarrer, S.; Pomberger, R. The fire risk of portable batteries in their end-of-life: Investigation of the state of charge of waste lithium-ion batteries in Austria. Waste Manag. Res. J. A Sustain. Circ. Econ. 2021, 39, 0734242X2110106. [Google Scholar] [CrossRef]
- Pistoia, G. (Ed.) Electric and Hybrid Vehicles; Elsevier Science: London, UK, 2010. [Google Scholar] [CrossRef]
- Deng, J.; Yang, X.; Zhang, G. Simulation study on internal short circuit of lithium ion battery caused by lithium dendrite. Mater. Today Commun. 2022, 31, 103570. [Google Scholar] [CrossRef]
- Buston, J.; Goddard, S.; Howard, G. Testing of Vape Batteries The Development and Review of Tests for Assessing the Safety of 18650 Batteries for Vaping; Technical Report; Office for Product Safety and Standards: Birmingham, UK, 2023. [Google Scholar]
- Xu, B.; Lee, J.; Kwon, D.; Kong, L.; Pecht, M. Mitigation strategies for li-ion battery thermal runaway: A review. Renew. Sustain. Energy Rev. 2021, 150, 111437. [Google Scholar] [CrossRef]
- Bandhauer, T.M.; Garimella, S.; Fuller, T.F. A Critical Review of Thermal Issues in Lithium-Ion Batteries. J. Electrochem. Soc. 2011, 158, R1. [Google Scholar] [CrossRef]




| Facility Area/Process | Possible Hazards and Threats | Risk Level |
|---|---|---|
| Collection bins | Damage due to external short-circuit | low |
| Loading activity | Damage due to external short-circuit | low-medium |
| Collection vehicle | Mechanical damage due to compaction | medium |
| Unloading activity | Mechanical damage due to tip-off | low |
| Waste bunker/input storage | Damage due to external heating (self-heating of waste) | medium-high |
| Waste transfer activity | Mechanical damage due to (wheel) loader or gripper | medium |
| Treatment facility | Mechanical damage due to pre-shredding and post-shredding processes; Dangerous heat generation after damage; Carry-over through the processing facility | high-very high |
| Output storage | Damage due to external short-circuit; Damage due to external heating (self-heating of waste); Dangerous heat generation after damage | low-medium |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gausden, A.; Cerik, B.C. Single-Use Vape Batteries: Investigating Their Potential as Ignition Sources in Waste and Recycling Streams. Batteries 2024, 10, 236. https://doi.org/10.3390/batteries10070236
Gausden A, Cerik BC. Single-Use Vape Batteries: Investigating Their Potential as Ignition Sources in Waste and Recycling Streams. Batteries. 2024; 10(7):236. https://doi.org/10.3390/batteries10070236
Chicago/Turabian StyleGausden, Andrew, and Burak Can Cerik. 2024. "Single-Use Vape Batteries: Investigating Their Potential as Ignition Sources in Waste and Recycling Streams" Batteries 10, no. 7: 236. https://doi.org/10.3390/batteries10070236
APA StyleGausden, A., & Cerik, B. C. (2024). Single-Use Vape Batteries: Investigating Their Potential as Ignition Sources in Waste and Recycling Streams. Batteries, 10(7), 236. https://doi.org/10.3390/batteries10070236







