Diffusion-Equation-Based Electrical Modeling for High-Power Lithium Titanium Oxide Batteries
Abstract
1. Introduction
2. Related Work
2.1. Equivalent Circuit Model
2.2. Solid-Phase Diffusion Equation
3. Experimental Setup and Procedures
- (1)
- 1 C charging for 60 s;
- (2)
- 0.1 C discharging until the discharged capacity equals the charging capacity of the previous step;
- (3)
- Resting for 10 min;
- (4)
- 1 C discharging for 60 s;
- (5)
- 0.1 C charging until the charging capacity equals the charging capacity of the previous step;
- (6)
- Repeat steps (1) to (5) after replacing 1 C with 2 C, 4 C, 6 C, and 8 C, respectively.
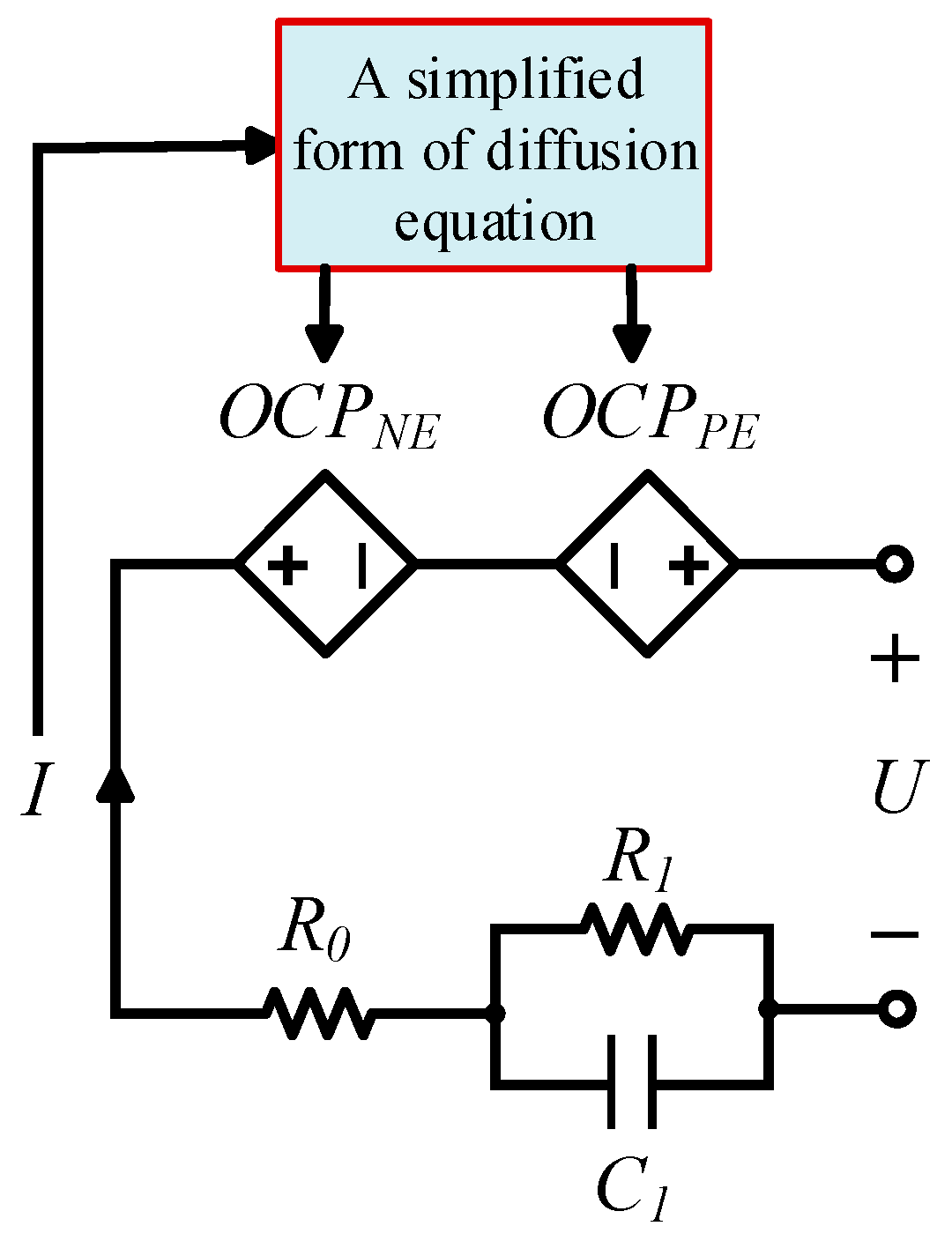
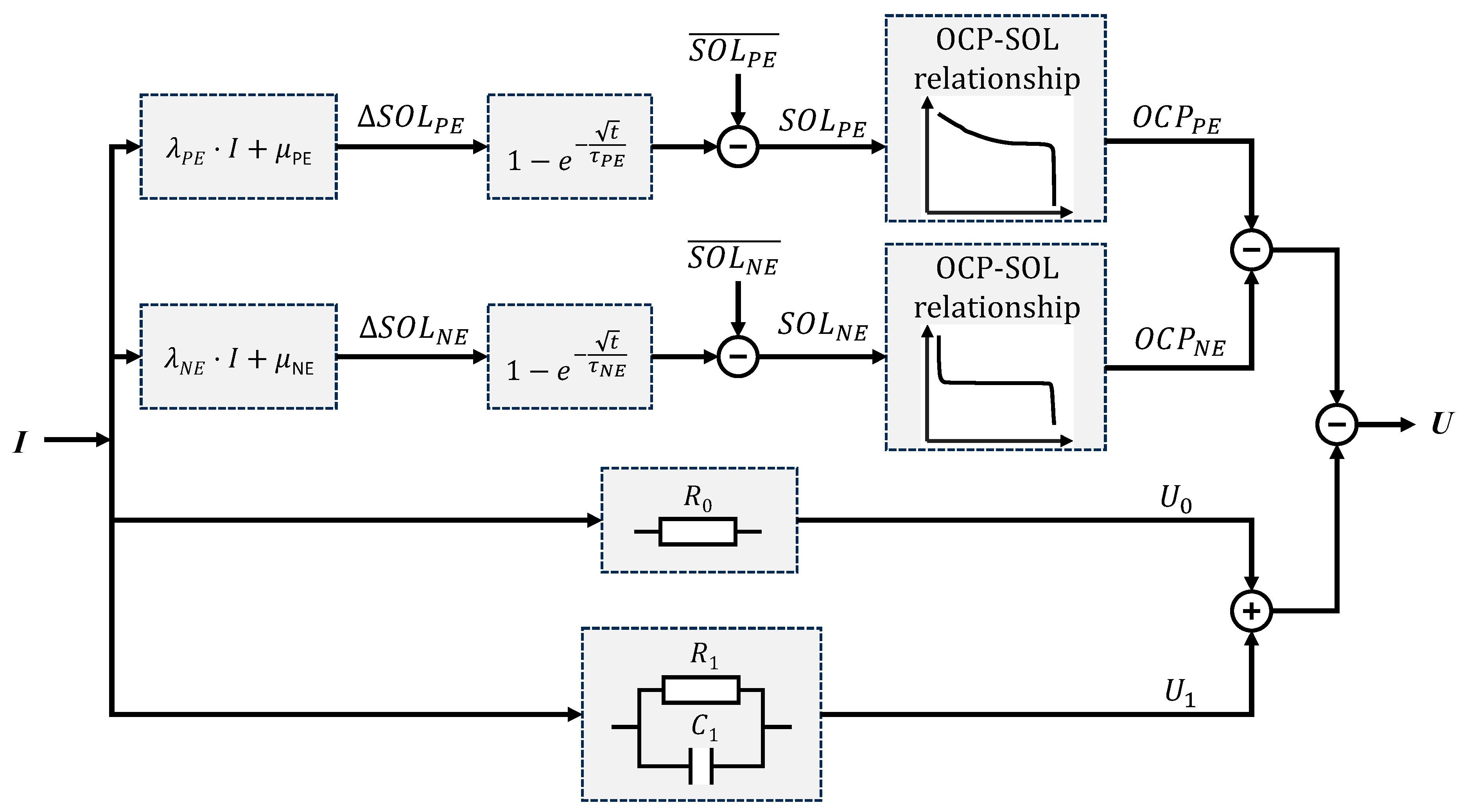
4. Diffusion Equation Based Electrical Model
5. Model Extraction
5.1. Full Cell OCV-SOC and Electrode OCP-SOL
5.2. Parameter Identification
- (1)
- The OCV curves of the full cell were reconstructed using the OCP data of the half-cells based on the methodology presented in the literature [33,38], to obtain the correspondence parameters between the electrode and the full cell (, , and ), as well as the ohmic resistance (). These parameters and Equation (10) were then used to calculate the capacity of the electrode (, ).
- (2)
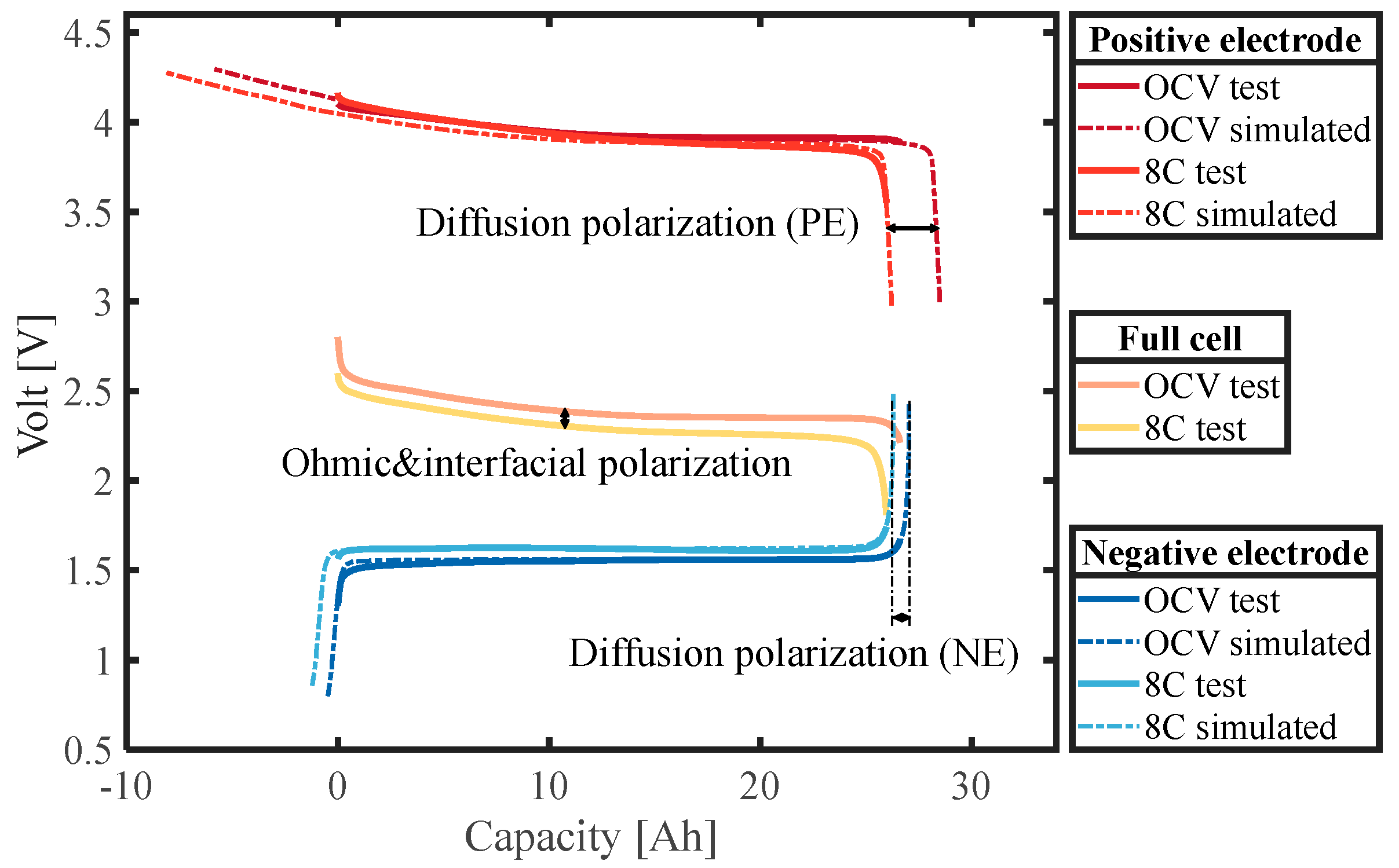
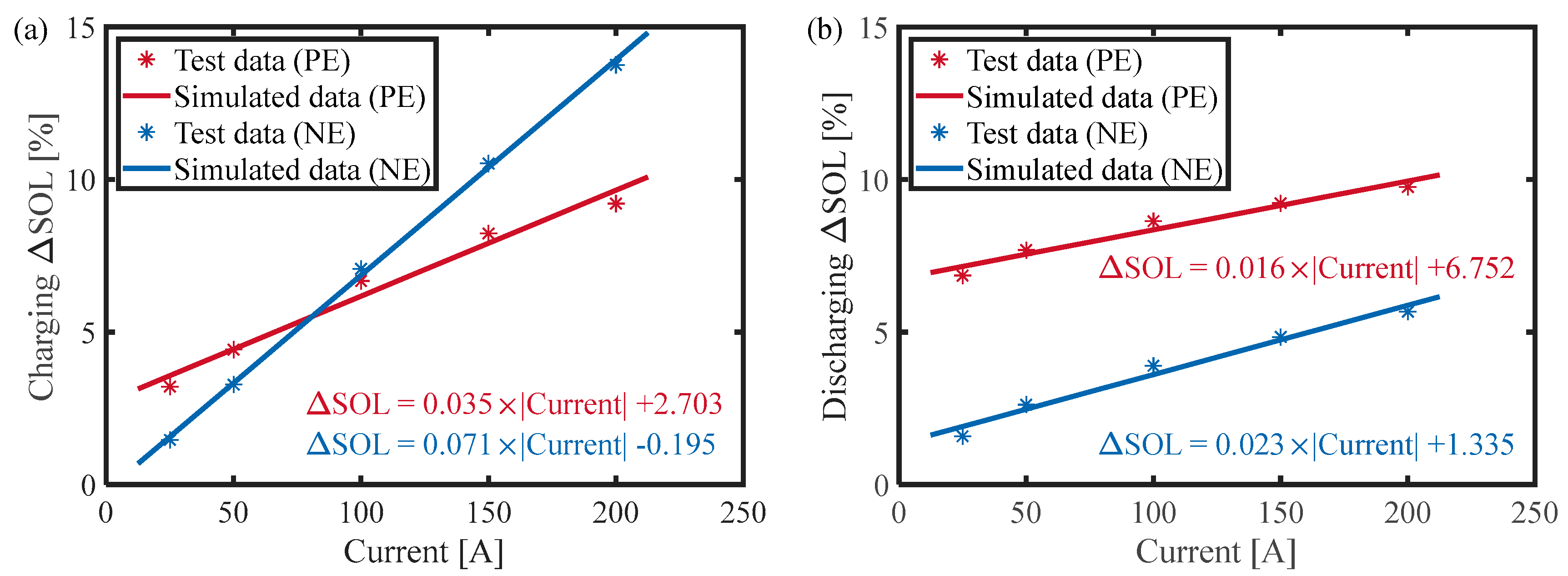
- Based on the three-electrode battery configuration, the battery was subjected to constant-current charge/discharge experiments at different C-rates. The parameters () in the diffusion Equation (9) were identified based on the difference between the diffusion polarization of the positive and negative electrodes. In addition, the parameter was determined by ohmic and interfacial polarization.
- (3)
- Based on the JEVS experiments with different SOCs, the parameters () were obtained by least-squares fitting of different pulses according to the different magnitudes of the time constant. The final parameters were obtained by averaging the parameters at different SOCs.
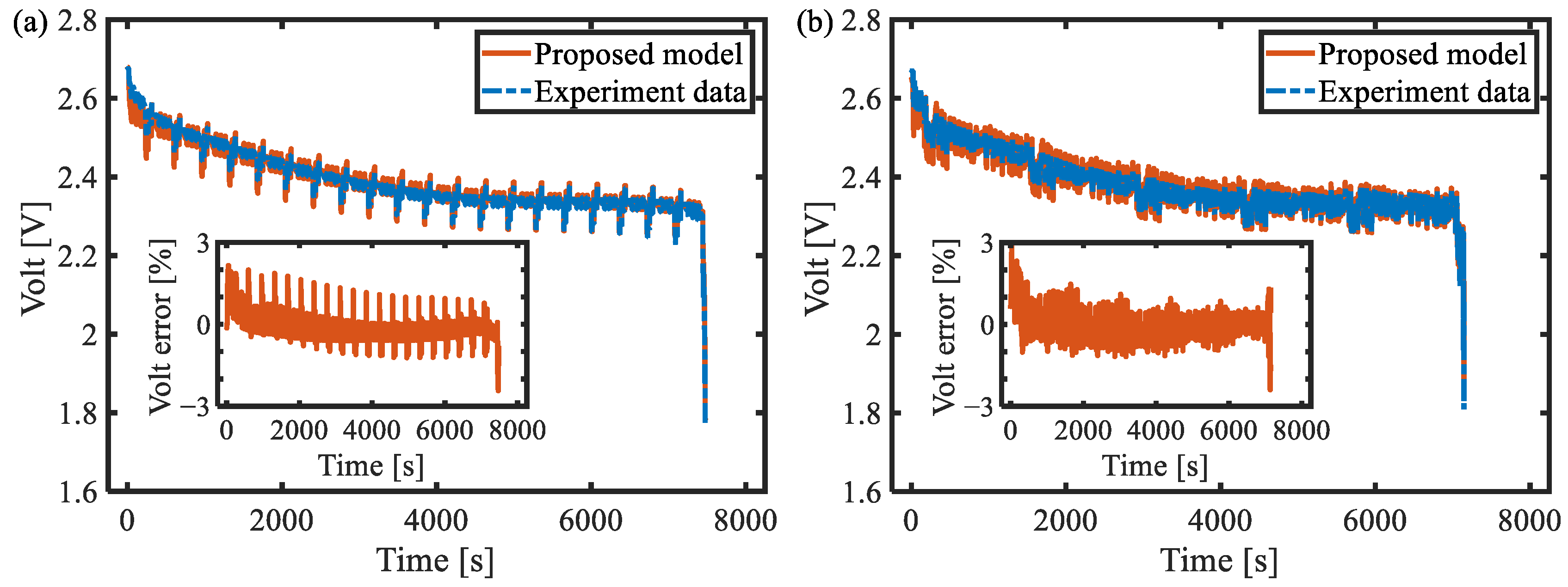
6. Model Verification
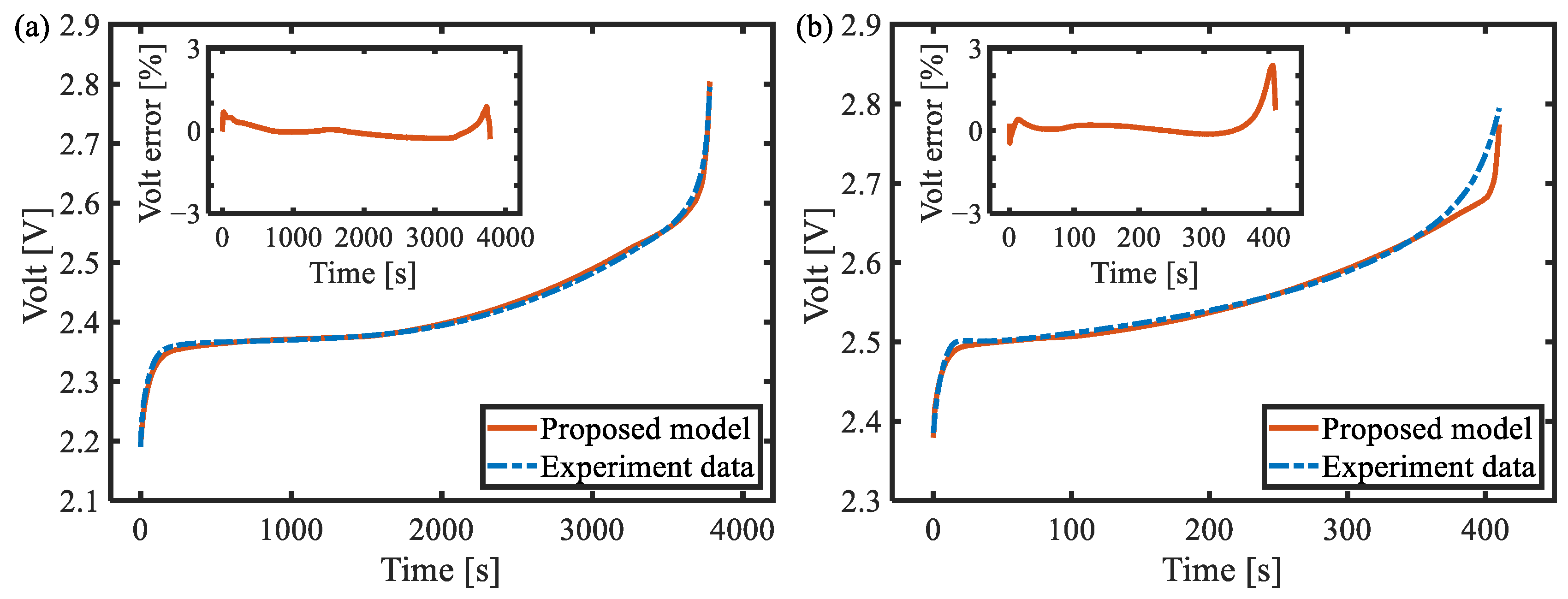
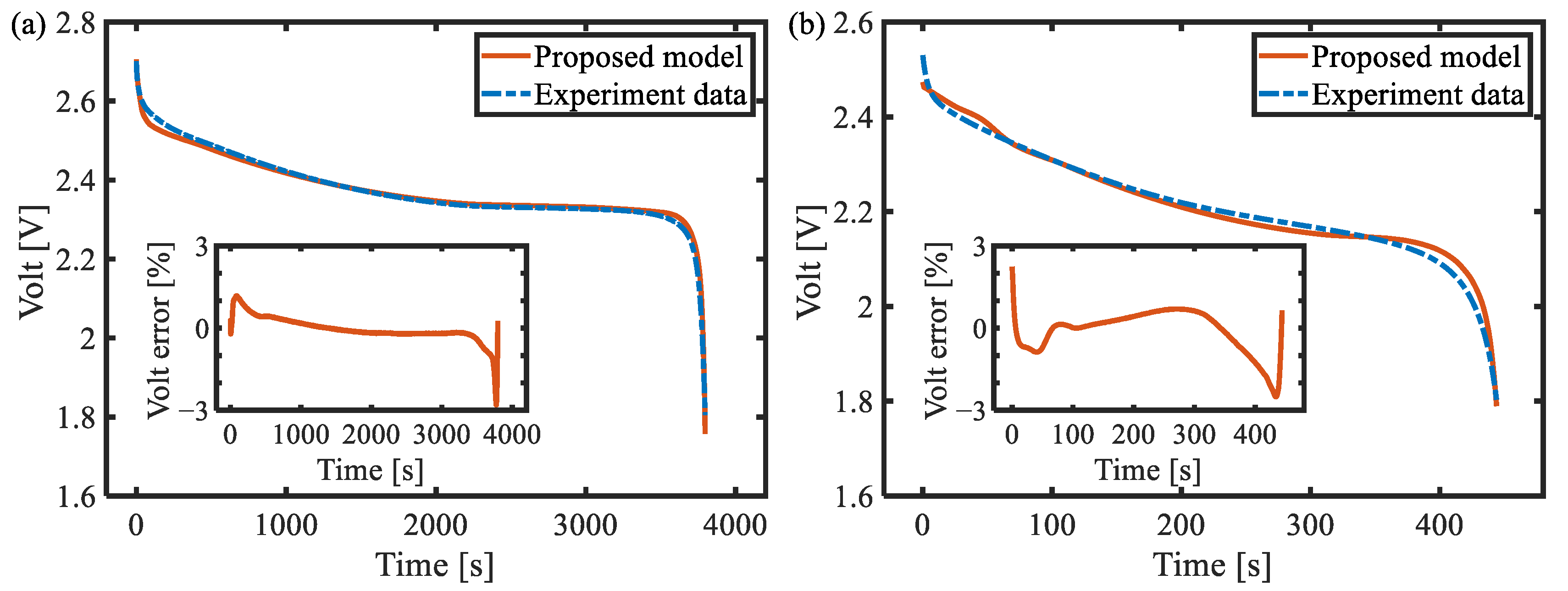
6.1. Galvanostatic Test at Different Rates
6.2. Dynamic Current Testing Using DST and FUDS Profiles
6.3. Comparison against Second-Order RC Model
7. Conclusions
- (1)
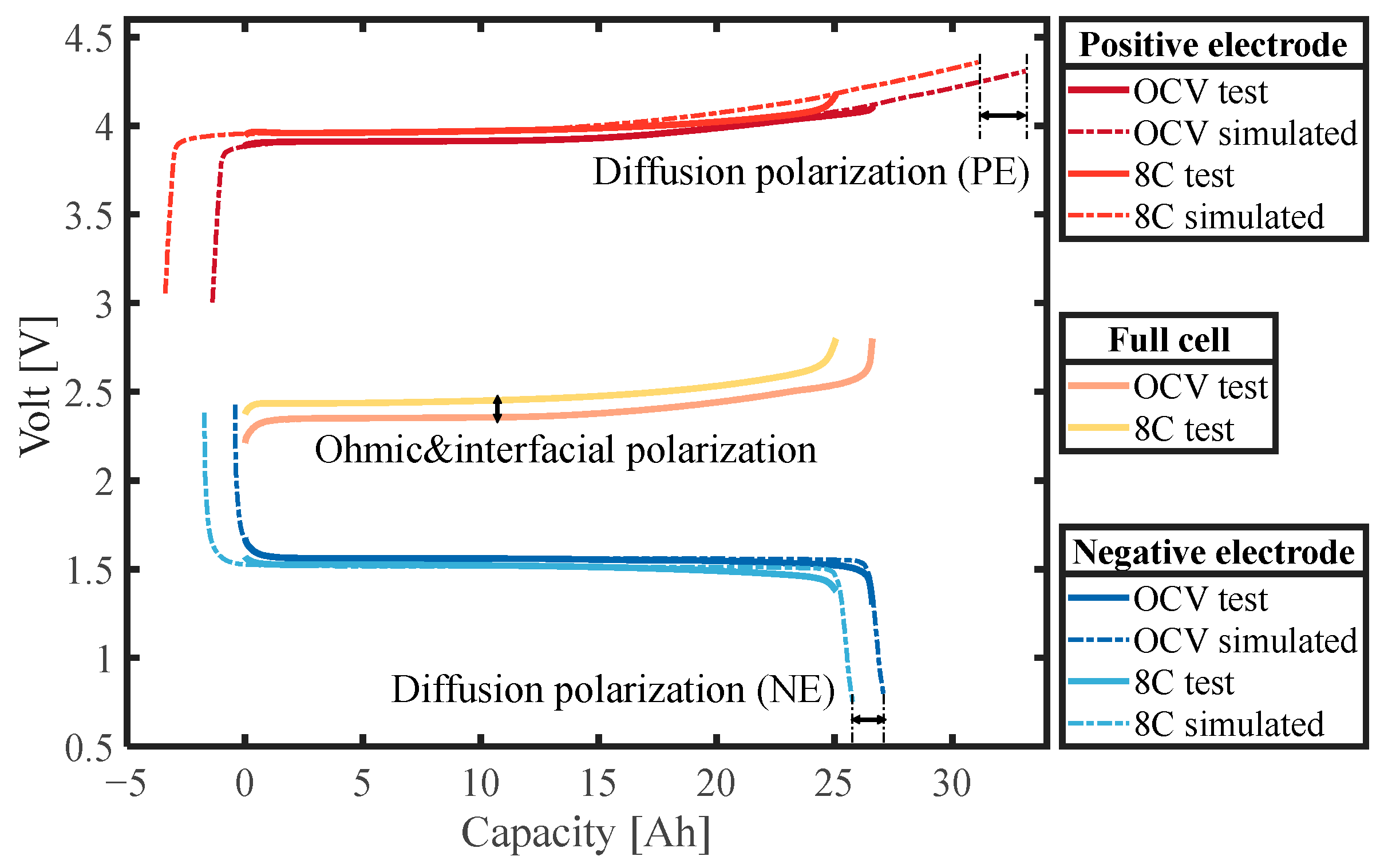
- By comparing the polarization distribution of the battery, it was found that the effect of diffusion polarization on the LTO battery is more serious than that of interfacial polarization, and it is the diffusion polarization rather than the interfacial polarization that will change with SOC. Specifically, the effect of diffusion polarization is 1.07 to 1.75 times that of interfacial polarization.
- (2)
- A simplified diffusion equation related to SOL that can be directly added to the circuit model was elaborately derived, which has more physical significance than the mathematical solution, and gives an ideal expression for the lithiation state of the positive and negative electrodes in the steady state.
- (3)
- Through the three-electrode battery architecture, the degree of diffusion polarization of the positive and negative electrodes under high-power conditions was decoupled and analyzed, and the model based on the simplified diffusion equation was established with high accuracy in the full SOC range, with a maximum voltage error of less than 3%.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| C-rate | Current rate |
| DST | Dynamic stress test |
| EIS | Electrochemical impedance spectroscopy |
| FUDS | Federal urban driving schedule |
| JEVS | Japanese electric vehicle standard |
| LFP | Lithium iron phosphate |
| LTO | Lithium titanium oxide |
| NE | Negative electrode |
| OCP | Open-circuit potential |
| OCV | Open-circuit voltage |
| PE | Positive electrode |
| RC | Resistance–capacitance |
| SOC | State of charge |
| SOL | State of lithiation |
References
- Khan, F.M.N.U.; Rasul, M.G.; Sayem, A.S.M.; Mandal, N.K. Design and Optimization of Lithium-Ion Battery as an Efficient Energy Storage Device for Electric Vehicles: A Comprehensive Review. J. Energy Storage 2023, 71, 108033. [Google Scholar] [CrossRef]
- Islam, S.; Ghazal, T.M. Lithium-Ion Battery Management System for Electric Vehicles: Constraints, Challenges, and Recommendations. Batteries 2023, 9, 152. [Google Scholar] [CrossRef]
- Larcher, D.; Tarascon, J.M. Towards Greener and More Sustainable Batteries for Electrical Energy Storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ahn, J.; Kang, T.; Park, S.; Kim, Y.; Cho, I.; Kim, J. Review of State-of-the-Art Battery State Estimation Technologies for Battery Management Systems of Stationary Energy Storage Systems. J. Power Electron. 2020, 20, 1526–1540. [Google Scholar] [CrossRef]
- Wegmann, R.; Döge, V.; Sauer, D.U. Assessing the Potential of a Hybrid Battery System to Reduce Battery Aging in an Electric Vehicle by Studying the Cycle Life of a Graphite∣NCA High Energy and a LTO∣metal Oxide High Power Battery Cell Considering Realistic Test Profiles. Appl. Energy 2018, 226, 197–212. [Google Scholar] [CrossRef]
- Farmann, A.; Waag, W.; Sauer, D.U. Application-Specific Electrical Characterization of High Power Batteries with Lithium Titanate Anodes for Electric Vehicles. Energy 2016, 112, 294–306. [Google Scholar] [CrossRef]
- Bank, T.; Alsheimer, L.; Löffler, N.; Uwe, D. State of Charge Dependent Degradation Effects of Lithium Titanate Oxide Batteries at Elevated Temperatures: An in-Situ and Ex-Situ Analysis. J. Energy Storage 2022, 51, 104201. [Google Scholar] [CrossRef]
- Chahbaz, A.; Meishner, F.; Li, W.; Ünlübayir, C.; Uwe Sauer, D. Non-Invasive Identification of Calendar and Cyclic Ageing Mechanisms for Lithium-Titanate-Oxide Batteries. Energy Storage Mater. 2021, 42, 794–805. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, C.; Jiang, J.; Zhang, W.; Zhang, L.; Wang, Y. Review on State-of-Health of Lithium-Ion Batteries: Characterizations, Estimations and Applications. J. Clean. Prod. 2021, 314, 128015. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, C.; Chen, H.; Wang, J.; Chen, D.; Zhang, L.; Zhang, W.; Yang, S.; Yang, S.; Zhang, C.; et al. A Hierarchical Enhanced Data-Driven Battery Pack Capacity Estimation Framework for Real-World Operating Conditions with Fewer Labeled Data. J. Energy Chem. 2023, 91, 417–423. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Li, K.; Zhao, G.; Chen, Z. Perspectives and Challenges for Future Lithium-Ion Battery Control and Management. eTransportation 2023, 18, 100260. [Google Scholar] [CrossRef]
- Liu, W.; Placke, T.; Chau, K.T. Overview of Batteries and Battery Management for Electric Vehicles. Energy Rep. 2022, 8, 4058–4084. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, C.; Jiang, J.; Zhang, W.; Gao, Y.; Zhang, L. A Voltage Reconstruction Model Based on Partial Charging Curve for State-of-Health Estimation of Lithium-Ion Batteries. J. Energy Storage 2021, 35, 102271. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, C.; Jiang, J.; Zhang, W.; Chen, H.; Jiang, Y.; Sauer, D.U.; Li, W. Fast Screening of Lithium-Ion Battery Packs for Second Use with Machine Learning. eTransportation 2023, 17, 100255. [Google Scholar] [CrossRef]
- Zhang, C.; Li, K.; McLoone, S.; Yang, Z. Battery Modelling Methods for Electric Vehicles—A Review. In Proceedings of the 2014 European Control Conference (ECC), Strasbourg, France, 24–27 June 2014; pp. 2673–2678. [Google Scholar] [CrossRef]
- Miguel, E.; Plett, G.L.; Trimboli, M.S.; Lopetegi, I.; Oca, L.; Iraola, U.; Bekaert, E. Electrochemical Model and Sigma Point Kalman Filter Based Online Oriented Battery Model. IEEE Access 2021, 9, 98072–98090. [Google Scholar] [CrossRef]
- Hu, X.; Li, S.; Peng, H. A Comparative Study of Equivalent Circuit Models for Li-Ion Batteries. J. Power Sources 2012, 198, 359–367. [Google Scholar] [CrossRef]
- Kim, J.; Chun, H.; Kim, M.; Han, S.; Lee, J.W.; Lee, T.K. Effective and Practical Parameters of Electrochemical Li-Ion Battery Models for Degradation Diagnosis. J. Energy Storage 2021, 42, 103077. [Google Scholar] [CrossRef]
- Watrin, N.; Roche, R.; Ostermann, H.; Blunier, B.; Miraoui, A. Multiphysical Lithium-Based Battery Model for Use in State-of-Charge Determination. IEEE Trans. Veh. Technol. 2012, 61, 3420–3429. [Google Scholar] [CrossRef]
- Greenleaf, M.; Li, H.; Zheng, J.P. Modeling of LixFePO4 Cathode Li-Ion Batteries Using Linear Electrical Circuit Model. IEEE Trans. Sustain. Energy 2013, 4, 1065–1070. [Google Scholar] [CrossRef]
- Low, W.Y.; Aziz, J.A.; Idris, N.R.N.; Saidur, R. Electrical Model to Predict Current-Voltage Behaviours of Lithium Ferro Phosphate Batteries Using a Transient Response Correction Method. J. Power Sources 2013, 221, 201–209. [Google Scholar] [CrossRef]
- Schröer, P.; van Faassen, H.; Nemeth, T.; Kuipers, M.; Sauer, D.U. Challenges in Modeling High Power Lithium Titanate Oxide Cells in Battery Management Systems. J. Energy Storage 2020, 28, 101189. [Google Scholar] [CrossRef]
- Madani, S.S.; Schaltz, E. An Electrical Equivalent Circuit Model of a Lithium Titanate Oxide Battery. Batteries 2019, 5, 31. [Google Scholar] [CrossRef]
- Schröer, P.; Khoshbakht, E.; Nemeth, T.; Kuipers, M.; Zappen, H.; Sauer, D.U. Adaptive Modeling in the Frequency and Time Domain of High-Power Lithium Titanate Oxide Cells in Battery Management Systems. J. Energy Storage 2020, 32, 101966. [Google Scholar] [CrossRef]
- Maria, E.D.; Dallapiccola, M.; Aloisio, D.; Brunaccini, G.; Sergi, F.; Moser, D.; Barchi, G. A Data-Driven Equivalent Circuit Model’s Parameter Estimation Method Applied to Lithium-Titanate Battery. J. Energy Storage 2023, 74, 109497. [Google Scholar] [CrossRef]
- Gao, L.; Liu, S.; Dougal, R.A. Dynamic Lithium-Ion Battery Model for System Simulation. IEEE Trans. Components Packag. Technol. 2002, 25, 495–505. [Google Scholar] [CrossRef]
- Zhang, J.; Ci, S.; Sharif, H.; Alahmad, M. An Enhanced Circuit-Based Model for Single-Cell Battery. In Proceedings of the 2010 Twenty-Fifth Annual IEEE Applied Power Electronics Conference and Exposition (APEC), Palm Springs, CA, USA, 21–25 February 2010; pp. 672–675. [Google Scholar] [CrossRef]
- Kim, T.; Qiao, W. A Hybrid Battery Model Capable of Capturing Dynamic Circuit Characteristics and Nonlinear Capacity Effects. IEEE Trans. Energy Convers. 2011, 26, 1172–1180. [Google Scholar] [CrossRef]
- Lam, L.; Bauer, P.; Kelder, E. A Practical Circuit-Based Model for Li-Ion Battery Cells in Electric Vehicle Applications. In Proceedings of the 2011 IEEE 33rd International Telecommunications Energy Conference, Amsterdam, The Netherlands, 9–13 October 2011; pp. 1–9. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, J.; Shi, W.; Ma, Z.; Wang, L.Y.; Guo, H. Butler-Volmer-Equation-Based Electrical Model for High-Power Lithium Titanate Batteries Used in Electric Vehicles. IEEE Trans. Ind. Electron. 2015, 62, 7557–7568. [Google Scholar] [CrossRef]
- Chen, A.; Zhang, W.; Zhang, C.; Huang, W.; Liu, S. A Temperature and Current Rate Adaptive Model for High-Power Lithium-Titanate Batteries Used in Electric Vehicles. IEEE Trans. Ind. Electron. 2020, 67, 9492–9502. [Google Scholar] [CrossRef]
- Ouyang, M.; Liu, G.; Lu, L.; Li, J.; Han, X. Enhancing the Estimation Accuracy in Low State-of-Charge Area: A Novel Onboard Battery Model through Surface State of Charge Determination. J. Power Sources 2014, 270, 221–237. [Google Scholar] [CrossRef]
- Chen, H.; Chahbaz, A.; Yang, S.; Zhang, W.; Sauer, D.U.; Li, W. Thermodynamic and Kinetic Degradation of LTO Batteries: Impact of Different SOC Intervals and Discharge Voltages in Electric Train Applications. eTransportation 2024, 21, 100340. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, J.; Pan, Z.; Ouyang, M. Impedance Characterization of Lithium-Ion Batteries Aging under High-Temperature Cycling: Importance of Electrolyte-Phase Diffusion. J. Power Sources 2019, 426, 216–222. [Google Scholar] [CrossRef]
- Newman, J.; Tiedemann, W. Porous-electrode Theory with Battery Applications. AIChE J. 1975, 21, 25–41. [Google Scholar] [CrossRef]
- Wang, C.Y.; Srinivasan, V. Computational Battery Dynamics (CBD)—Electrochemical/Thermal Coupled Modeling and Multi-Scale Modeling. J. Power Sources 2002, 110, 364–376. [Google Scholar] [CrossRef]
- Dubarry, M.; Truchot, C.; Liaw, B.Y. Synthesize Battery Degradation Modes via a Diagnostic and Prognostic Model. J. Power Sources 2012, 219, 204–216. [Google Scholar] [CrossRef]
- Lu, D.; Scott Trimboli, M.; Fan, G.; Zhang, R.; Plett, G.L. Implementation of a Physics-Based Model for Half-Cell Open-Circuit Potential and Full-Cell Open-Circuit Voltage Estimates: Part II. Processing Full-Cell Data. J. Electrochem. Soc. 2021, 168, 070533. [Google Scholar] [CrossRef]







| Battery Parameters | Characteristics |
|---|---|
| Nominal capacity | 25 Ah |
| Voltage range | 1.8~2.8 V |
| Max. charge current | 8 C (200 A) |
| Max. discharge current | 12 C (300 A) |
| Cathode material | LiCoO2 |
| Anode material | Li4Ti5O12 |
| Parameters | Current Direction | Positive Electrode | Negative Electrode | Full Cell |
|---|---|---|---|---|
| - | - | - | 0.8 mΩ | |
| - | - | - | 0.4 mΩ | |
| - | - | - | 27,732 F | |
| - | 1.239 | 1.023 | - | |
| - | −0.064 | −0.003 | - | |
| Charge | 0.009 | 0.018 | - | |
| Discharge | 0.004 | 0.006 | - | |
| Charge | 0.027 | −0.002 | - | |
| Discharge | 0.068 | 0.013 | - | |
| Charge | 116.6 s | 106.1 s | - | |
| Discharge | 123.2 s | 102.0 s | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Zhang, W.; Zhang, C.; Sun, B.; Yang, S.; Chen, D. Diffusion-Equation-Based Electrical Modeling for High-Power Lithium Titanium Oxide Batteries. Batteries 2024, 10, 238. https://doi.org/10.3390/batteries10070238
Chen H, Zhang W, Zhang C, Sun B, Yang S, Chen D. Diffusion-Equation-Based Electrical Modeling for High-Power Lithium Titanium Oxide Batteries. Batteries. 2024; 10(7):238. https://doi.org/10.3390/batteries10070238
Chicago/Turabian StyleChen, Haoze, Weige Zhang, Caiping Zhang, Bingxiang Sun, Sijia Yang, and Dinghong Chen. 2024. "Diffusion-Equation-Based Electrical Modeling for High-Power Lithium Titanium Oxide Batteries" Batteries 10, no. 7: 238. https://doi.org/10.3390/batteries10070238
APA StyleChen, H., Zhang, W., Zhang, C., Sun, B., Yang, S., & Chen, D. (2024). Diffusion-Equation-Based Electrical Modeling for High-Power Lithium Titanium Oxide Batteries. Batteries, 10(7), 238. https://doi.org/10.3390/batteries10070238












