Characterization and Magnetic Properties of Sintered Glass-Ceramics from Dispersed Fly Ash Microspheres
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Methods
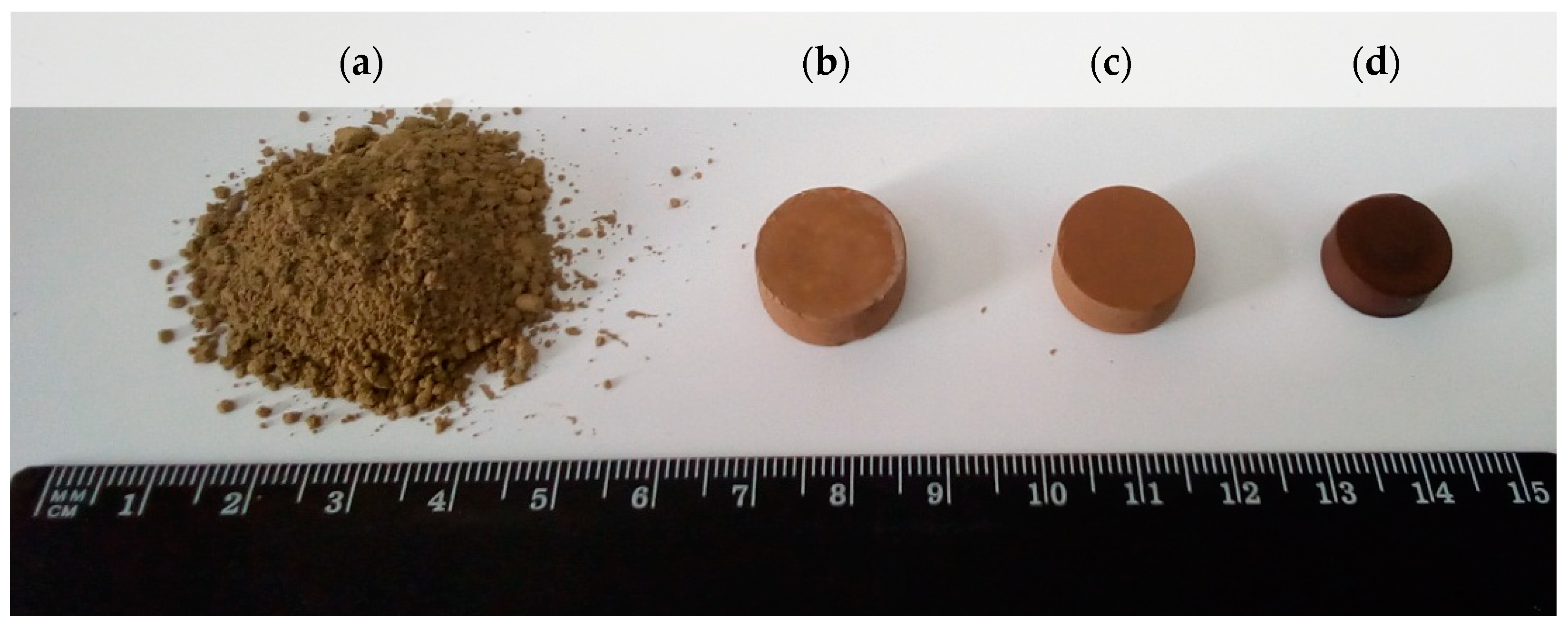
2.3. Glass-Ceramic Preparation and Characterization
- Sintering coefficient: k = V2/V1 is a dimensionless value, which is determined by the ratio of the sample volume after sintering (V2 = πr22h2) to the volume of the compacted sample (V1 = πr12h1).
- Linear shrinkage (%): changes in the height (Δh) and diameter (Δd) of the as-compacted sample resulting from drying and sintering.
- Apparent density (g/cm3): this is determined as the ratio of the sample weight (g) to its total volume (cm3) [40].
- Water absorption (%): this characterizes the ability of the material to absorb and retain moisture in the pores of capillaries; it is determined by the ratio of the water volume absorbed by the sample during vacuum pumping to the weight of the initial sample [40].
- Open porosity (%): this is the ratio of the volume of available pores in the sample to its total volume; the volume of available pores is determined by the water saturation of the material [41].
- Compressive strength (MPa): σ = F/S is the compressive strength corresponding to the compressive load at which the test sample is fractured; it is calculated as the ratio of the breaking load F (H) to the cross-sectional area of the sample S (mm2) [42]. The F value was determined on a laboratory hydraulic press #4350 (Carver, Wabash, IN, USA), S = 2Rh.
3. Results and Discussion
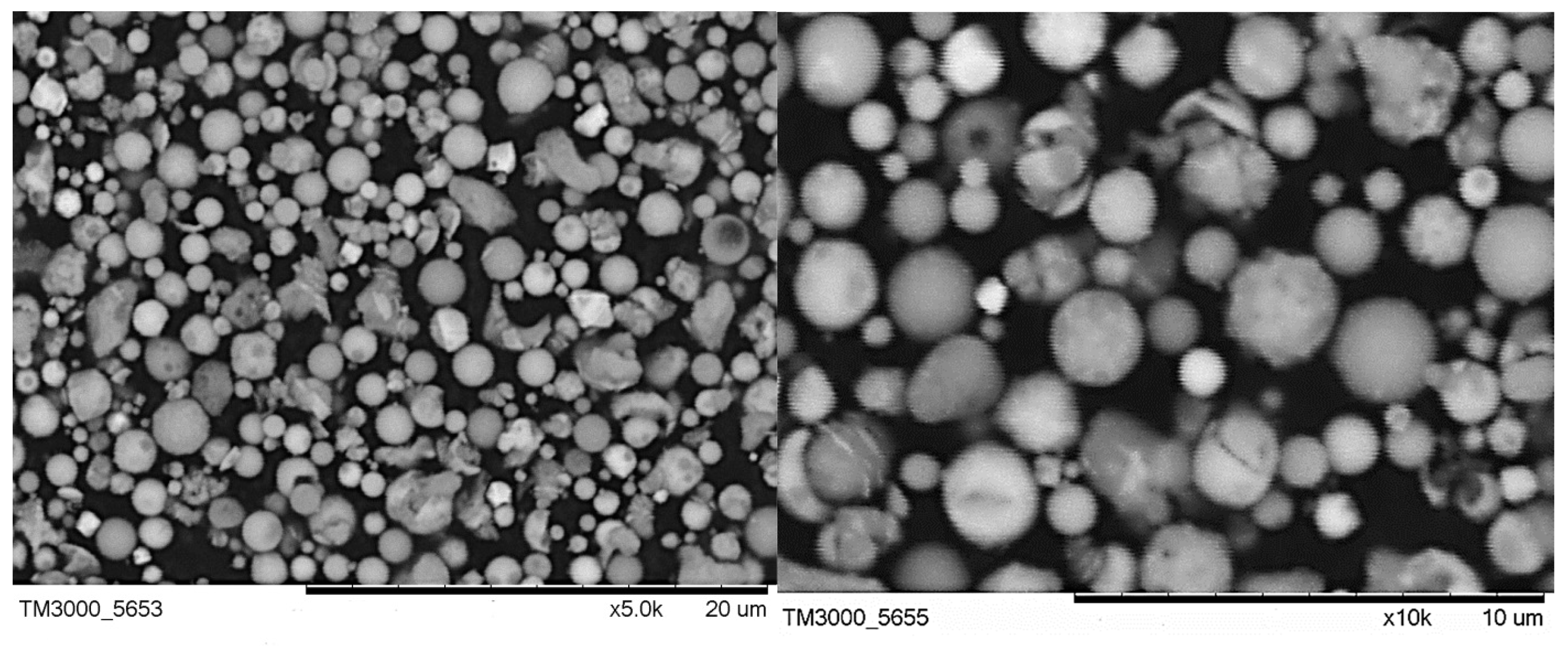
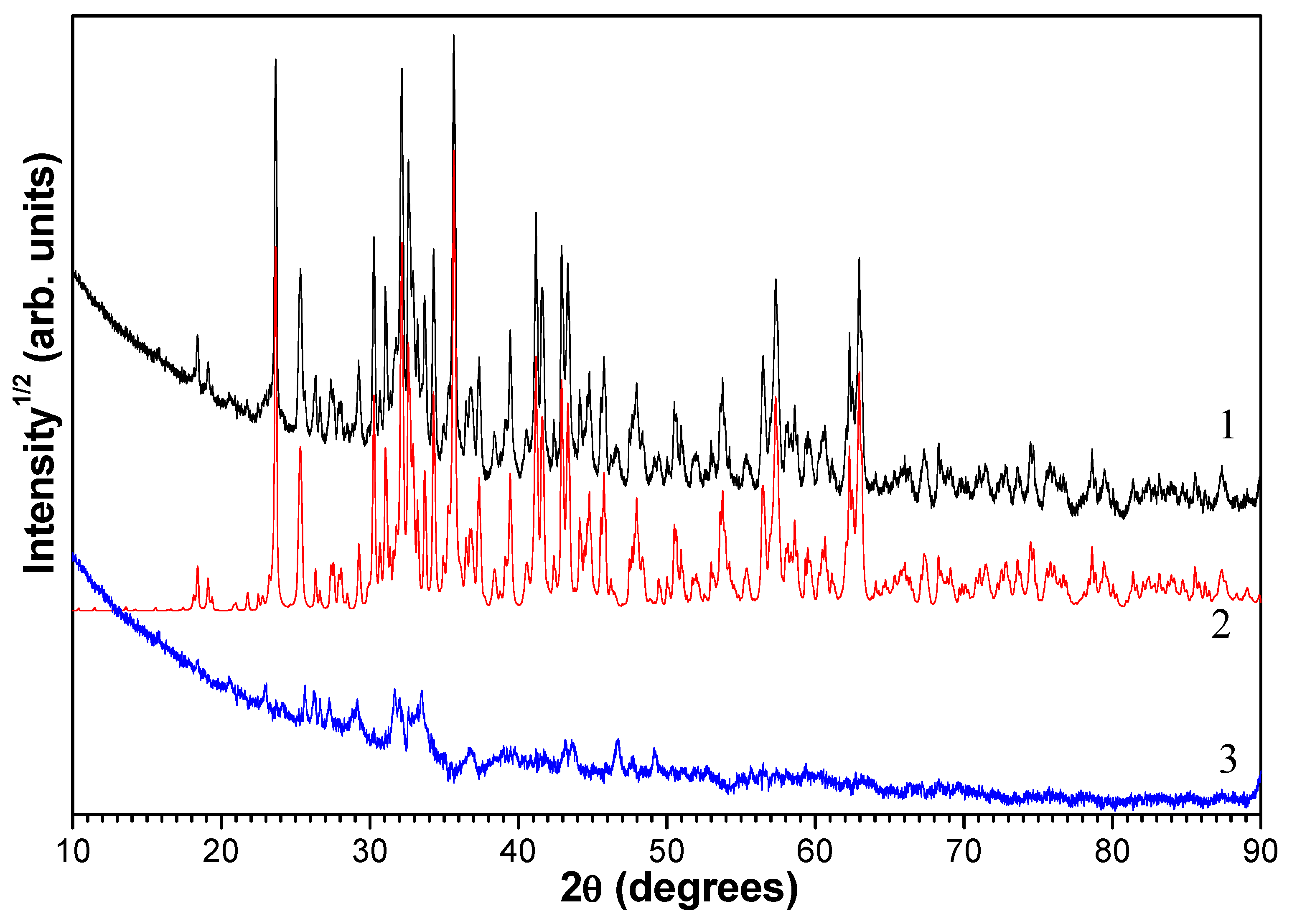
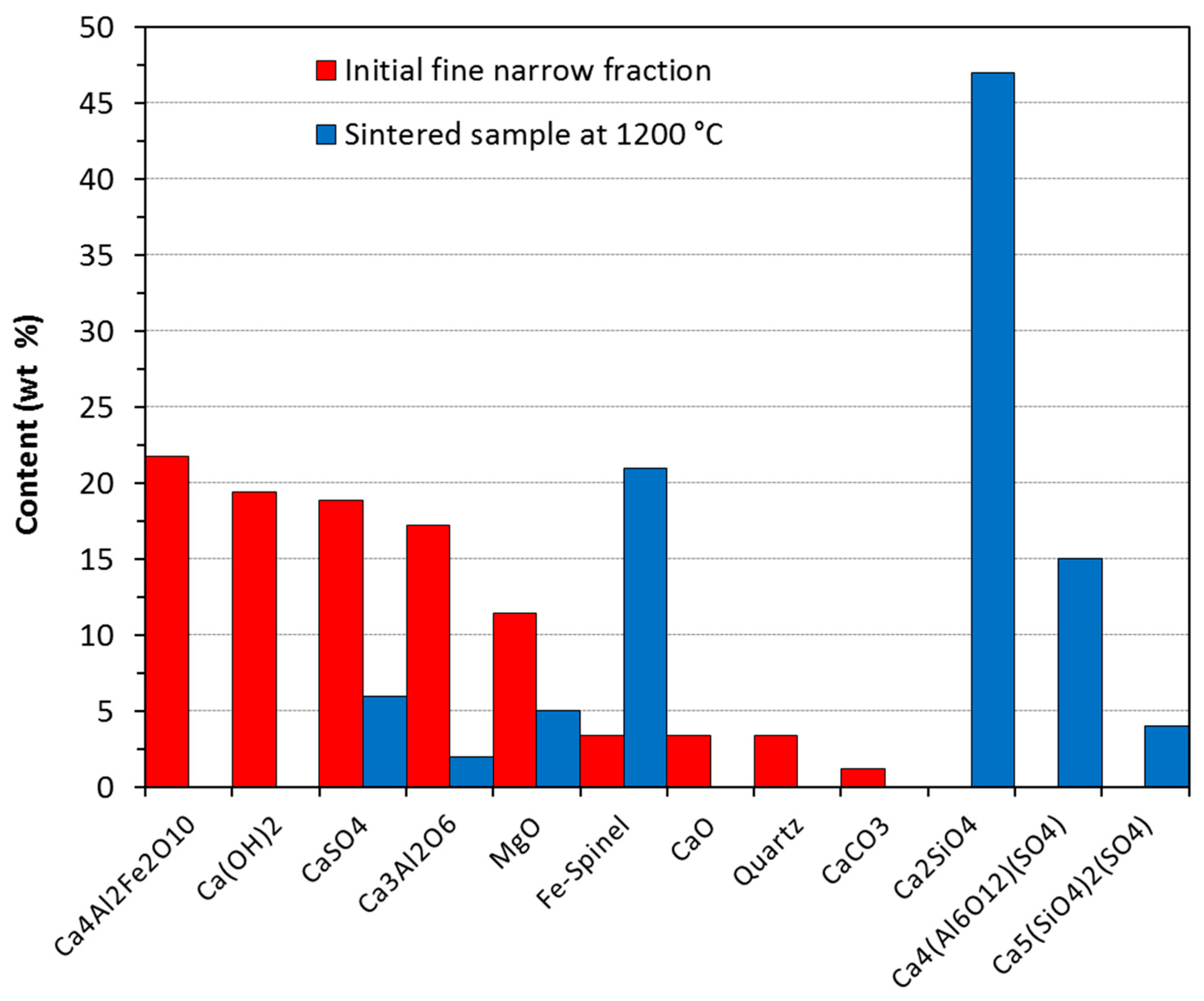
3.1. Characterization of the Fine Narrow Fraction
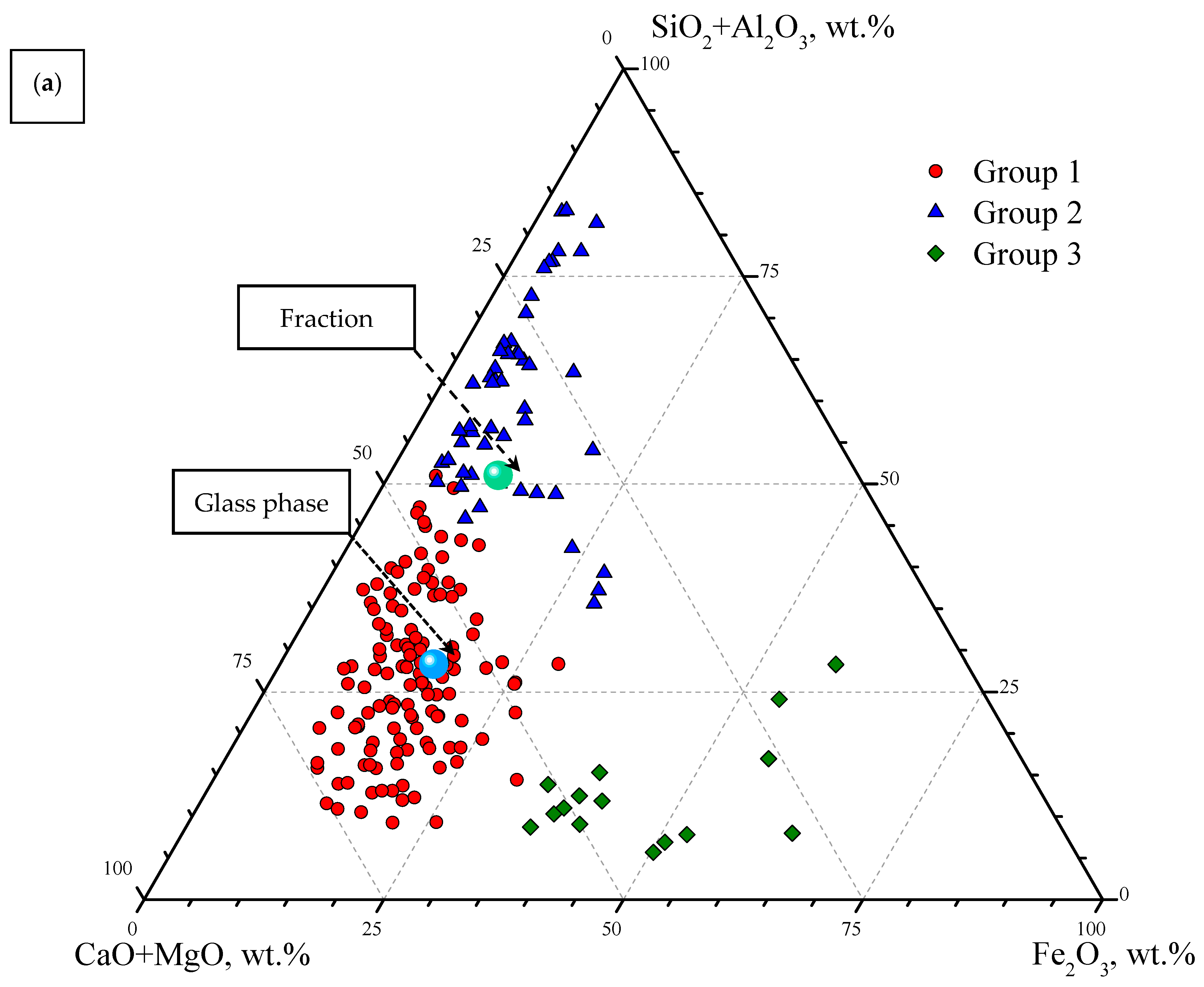
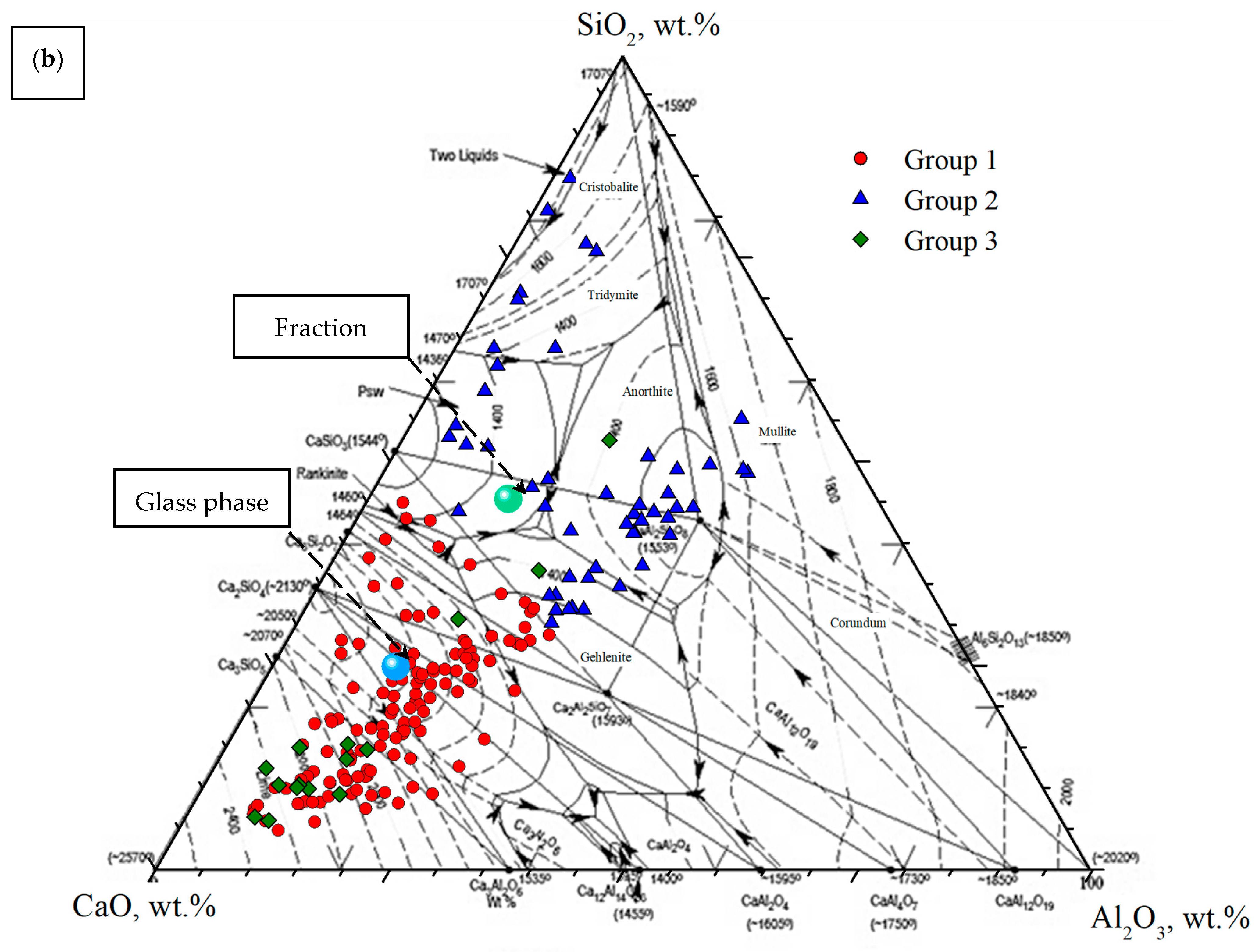
3.2. Single-Particle SEM-EDS Analysis
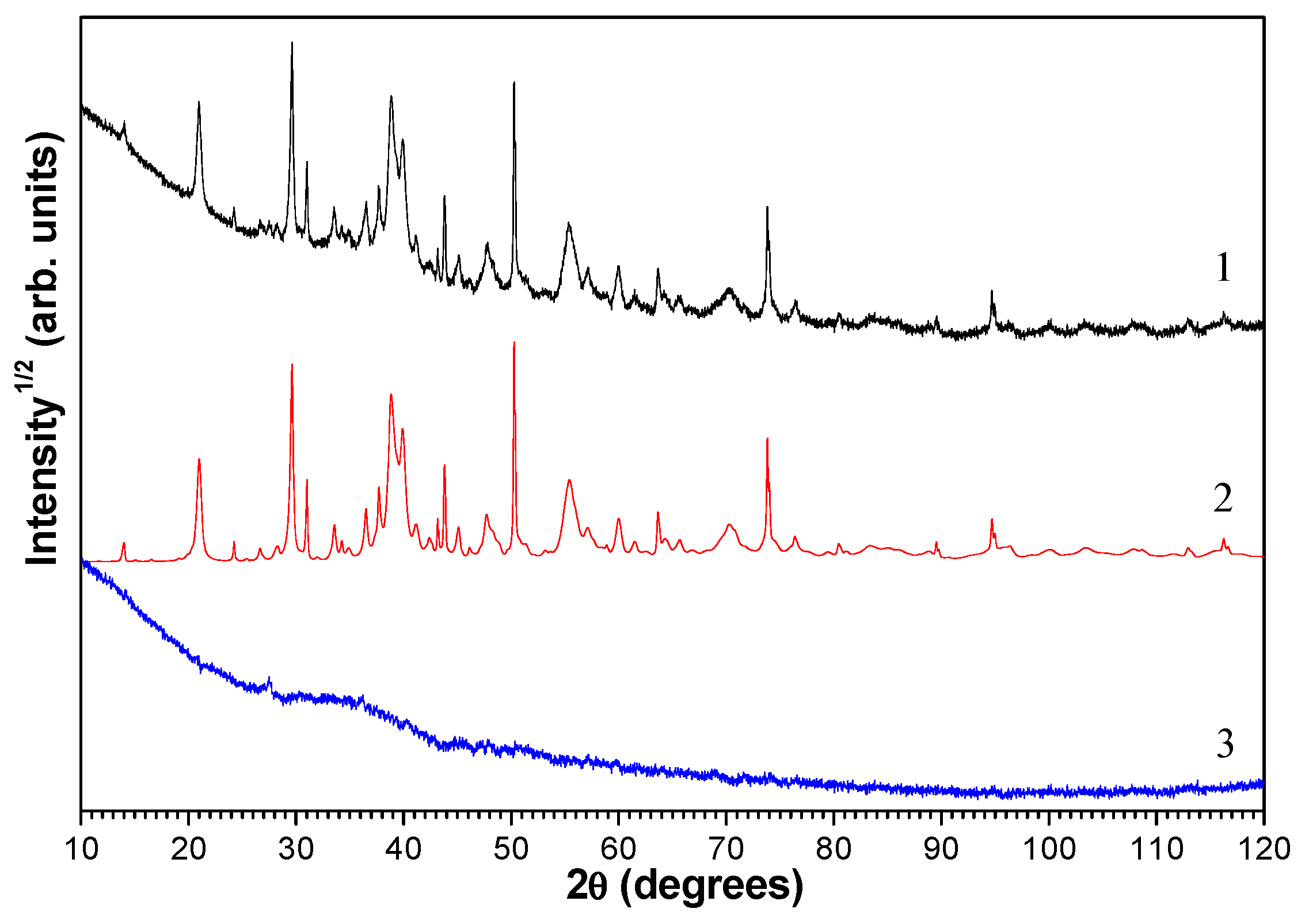
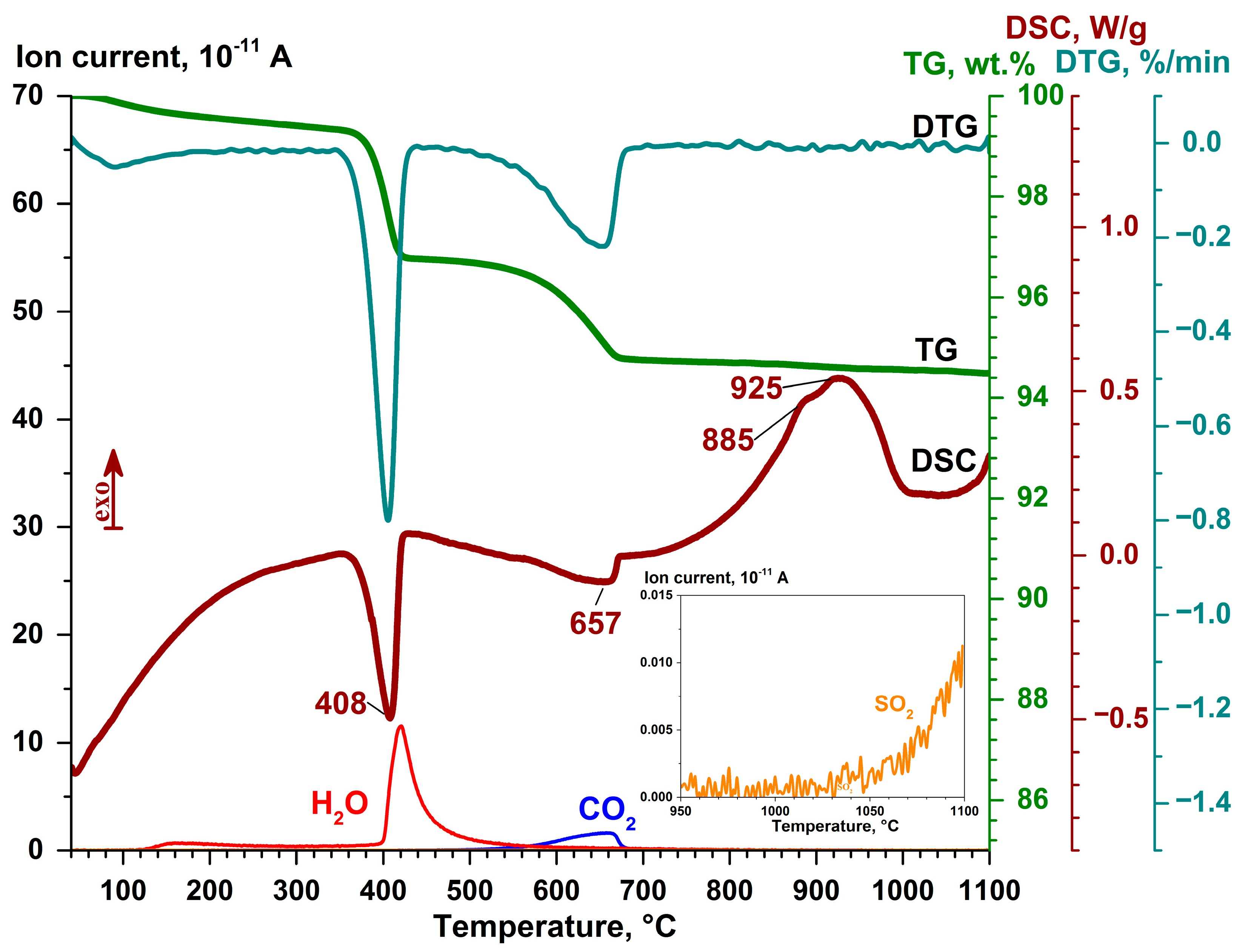
3.3. Thermochemical Transformations
- The endothermic effect in the temperature range of 40–244 °C corresponds to the dehydration of calcium compounds and/or thermal desorption of hygroscopic moisture, accompanied by a weight loss of 0.50 wt %.
- The endothermic effect in the temperature range of 244–435 °C with the maximum at 408 °C corresponds to the dissociation reaction Ca(OH)2 = CaO + H2O with a weight loss of 2.74 wt.%.
- The endothermic effect in the temperature range of 435–700 °C with the main maximum at 657 °C and the local one at 624 °C corresponds to the dissociation reaction of calcium carbonate CaCO3 = CaO + CO2 (or that of the solid solution Ca1−x−yMgxFeyCO3) with a weight loss of 2.01 wt %.
- The endothermic effect expressed as a bimodal DSC peak with the main maximum at 925 °C and the local one near 885 °C in the temperature range of 700–1050 °C corresponds to the crystallization of a new phase, presumably, wollastonite, and it is characterized by a slight weight loss of 0.21 wt %, which is due to the continuous emission of CO2.
- The endothermic effect in the temperature range of T > 1050 °C compensates for the endothermic process of phase dissociation of anhydrite CaSO4 = CaO + SO2 and corresponds to the onset of crystallization of new calcium silicate/aluminosilicate phases.
- CaO (glass matrix) + SiO2 (glass matrix) → CaSiO3;
- Fe2O3 (glass matrix) + Al2O3 (glass matrix) + MgO (glass matrix) → Fe-spinel;
- Ca3(Al3+, Fe3+)O6 + CaSO4 → Ca4[(Al3+, Fe3+)3O12]SO4;
- Ca2(Al3+, Fe3+)O5 + CaSO4 + CaO (portlandite, calcite) → Ca4[(Al3+, Fe3+)3O12]SO4;
- CaSiO3 + CaO (portlandite, calcite, anhydrite) → Ca2SiO4;
- Ca2SiO4 + CaSO4 → Ca5(SiO4)2SO4.
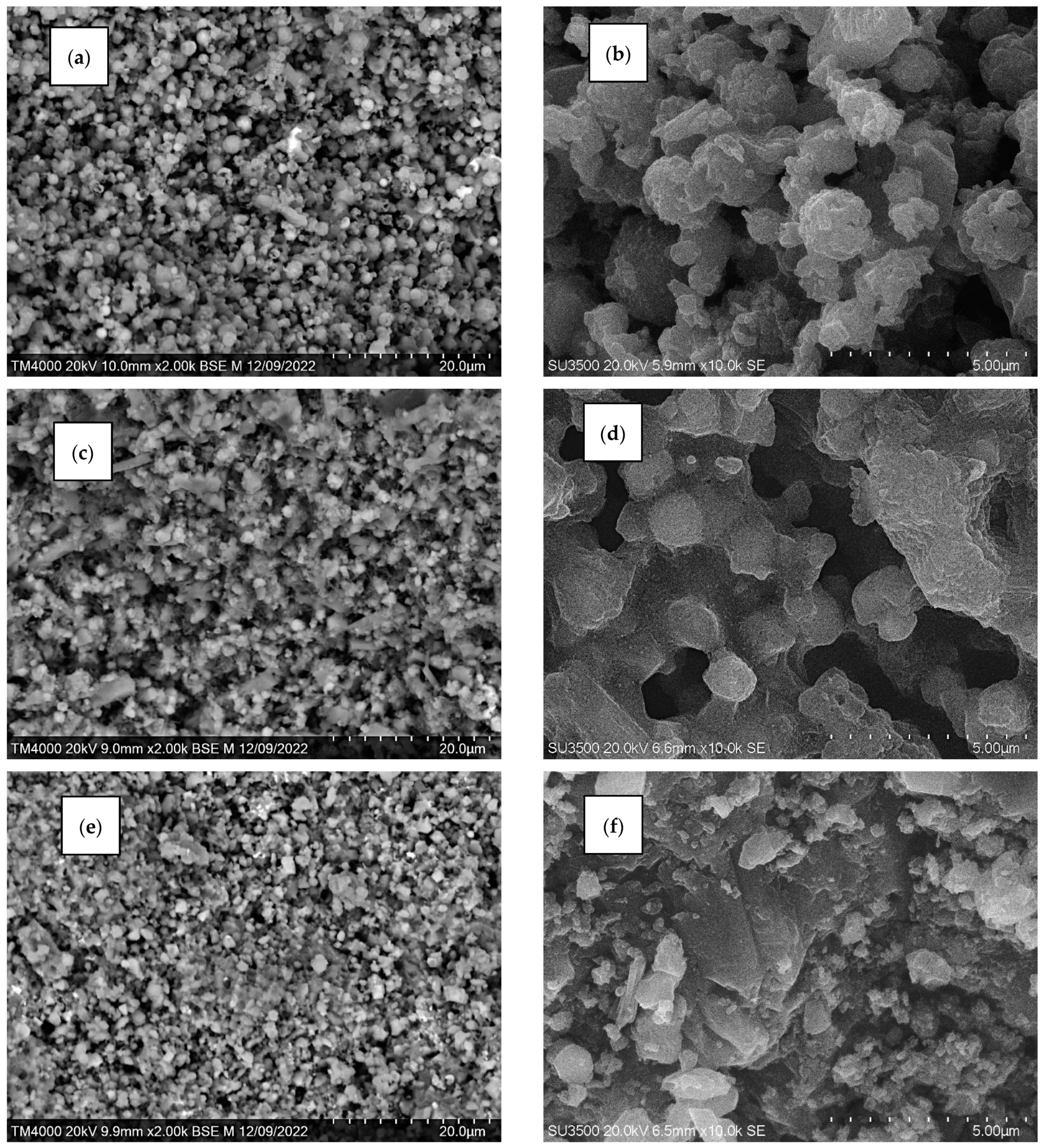
3.4. Characterization of Glass-Ceramic Materials
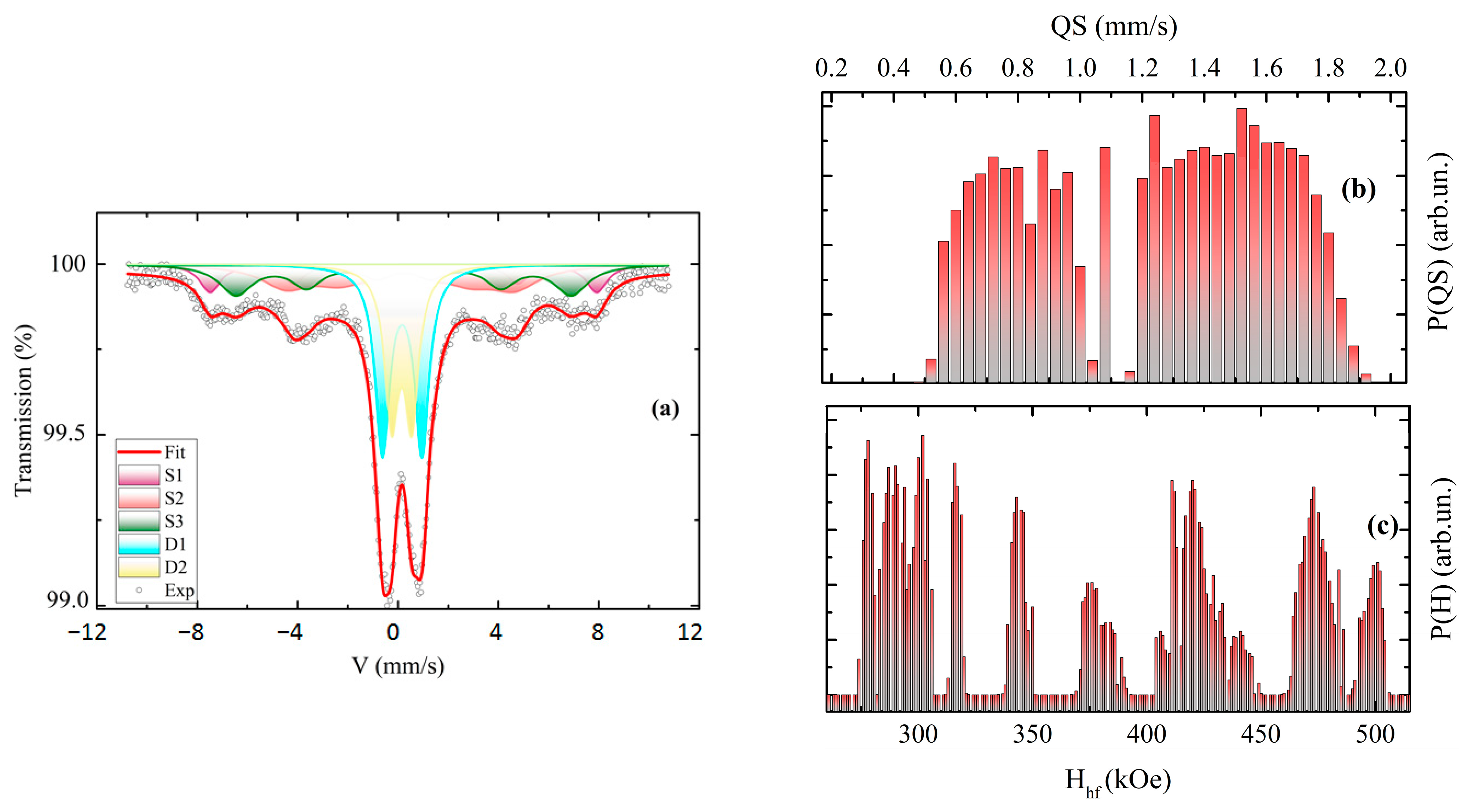
3.5. Mössbauer Spectroscopy
3.6. Magnetization Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coal & Electricity. Available online: https://www.worldcoal.org/coal-facts/coal-electricity/ (accessed on 15 March 2023).
- Yao, Z.T.; Ji, X.S.; Sarker, P.K.; Tang, J.H.; Ge, L.Q.; Xia, M.S.; Xi, Y.Q. A comprehensive review on the applications of coal fly ash. Earth-Sci. Rev. 2015, 141, 105–121. [Google Scholar] [CrossRef]
- Belviso, C. State-of-the-art applications of fly ash from coal and biomass: A focus on zeolite synthesis processes and issues. Prog. Energy Combust. Sci. 2018, 65, 109–135. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Blissett, R.S.; Rowson, N.A. A review of the multi-component utilization of coal fly ash. Fuel 2012, 97, 1–23. [Google Scholar] [CrossRef]
- Kotelnikova, A.D.; Rogova, O.B.; Karpukhina, E.A.; Solopov, A.B.; Levin, I.S.; Levkina, V.V.; Proskurnin, M.A.; Volkov, D.S. Assessment of the structure, composition, and agrochemical properties of fly ash and ash-and-slug waste from coal-fired power plants for their possible use as soil ameliorants. J. Clean. Prod. 2022, 333, 130088. [Google Scholar] [CrossRef]
- ASTM Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete (C618-05) Annual Book of ASTM Standards, Concrete and Aggregates, Volume 04.02, American Society for Testing Materials 2005. Available online: www.astm.org (accessed on 15 March 2023).
- Vassilev, S.V.; Vassileva, C.G. A new approach for the classification of coal fly ashes based on their origin, composition, properties, and behaviour. Fuel 2007, 86, 1490–1512. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, C.; Chen, J. Utilization of coal fly ash for the production of glass-ceramics with unique performances: A brief review. J. Mater. Sci. Technol. 2014, 30, 1208–1212. [Google Scholar] [CrossRef]
- Erol, M.; Kücükbayrak, S.; Ersoy-Mericboyu, A. Comparison of the properties of glass, glass-ceramic and ceramic materials produced from coal fly ash. J. Hazard. Mater. 2008, 153, 418–425. [Google Scholar] [CrossRef]
- Yoon, S.D.; Lee, J.U.; Lee, J.H.; Yun, Y.H.; Yoon, W.J. Characterization of Wollastonite Glass-ceramics Made from Waste Glass and Coal Fly Ash. J. Mater. Sci. Technol. 2013, 29, 149–153. [Google Scholar] [CrossRef]
- Choo, T.F.; Mohd Salleh, M.A.; Kok, K.Y.; Matori, K.A.; Abdul Rashid, S. A Study on the Utilization of Coal Fly Ash Derived Grog in Clay Ceramics. Materials 2020, 13, 5218. [Google Scholar] [CrossRef]
- Balapour, M.; Thway, T.; Rao, R.; Moser, N.; Garboczi, E.J.; Hsuan, Y.G.; Farnam, Y. A thermodynamics-guided framework to design lightweight aggregate from waste coal combustion fly ash. Resour. Conserv. Recycl. 2022, 178, 106050. [Google Scholar] [CrossRef]
- Kourti, I.; Cheeseman, C.R. Properties and microstructure of lightweight aggregate produced from lignite coal fly ash and recycled glass. Resour. Conserv. Recycl. 2010, 54, 769–775. [Google Scholar] [CrossRef]
- Abdullayev, A.; Bekheet, M.F.; Hanaor, D.A.H.; Gurlo, A. Materials and Applications for Low-Cost Ceramic Membranes. Membranes 2019, 9, 105. [Google Scholar] [CrossRef]
- Huang, J.; Chen, H.; Yang, J.; Zhou, T.; Zhang, H. Effects of particle size on microstructure and mechanical strength of a fly ash based ceramic membrane. Ceram. Int. 2023, 49, 15655–15664. [Google Scholar] [CrossRef]
- Húlan, T.; Štubňa, I.; Ondruška, J.; Trník, A. The influence of fly ash on mechanical properties of clay-based ceramics. Minerals 2020, 10, 930. [Google Scholar] [CrossRef]
- Sokolar, R.; Nguyen, M. The Effect of Class C Fly Ash on the Plasticity and Ageing of Ceramic Mixtures Based on Kaolin. Materials 2021, 14, 2761. [Google Scholar] [CrossRef]
- Arshad, M.T.; Ahmad, S.; Khitab, A.; Hanif, A. Synergistic use of fly ash and silica fume to produce high-strength self-compacting cementitious composites. Crystals 2021, 11, 915. [Google Scholar] [CrossRef]
- Ambrus, M.; Mucsi, G. Advanced processing of high Ca fly ash for enhanced reactivity and improved high value-added application possibilities. Case Stud. Constr. Mater. 2023, 18, e02214. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, J.; Lu, M.; Chen, B.; Gao, S.; Bai, J.; Zhang, H.; Yang, Y. Study on the effect of calcium and sulfur content on the properties of fly ash based geopolymer. Constr. Build. Mater. 2022, 314, 125650. [Google Scholar] [CrossRef]
- Kumar, S.; Mucsi, G.; Kristály, F.; Pekker, P. Mechanical activation of fly ash and its influence on micro and nano-structural behaviour of resulting geopolymers. Adv. Powder Technol. 2017, 28, 805–813. [Google Scholar] [CrossRef]
- DeGuire, E.J.; Risbud, S.H. Crystallization and properties of glasses prepared from Illinois coal fly ash. J. Mater. Sci. 1984, 19, 1760–1766. [Google Scholar] [CrossRef]
- Angjusheva, B.; Jovanov, V.; Fidanchevski, E. Conversion of coal fly ash glass into glass-ceramics by controlled thermal treatment. Maced. J. Chem. Chem. Eng. 2021, 40, 307–319. [Google Scholar] [CrossRef]
- Acar, I.; Atalay, M.U. Characterization of sintered class F fly ashes. Fuel 2013, 106, 195–203. [Google Scholar] [CrossRef]
- Zeng, L.; Sun, H.J.; Peng, T.J.; Zheng, W.M. The sintering kinetics and properties of sintered glass-ceramics from coal fly ash of different particle size. Results Phys. 2019, 15, 102774. [Google Scholar] [CrossRef]
- Ilic, M.; Cheeseman, C.; Sollars, C.; Knight, J. Mineralogy and microstructure of sintered lignite coal fly ash. Fuel 2003, 82, 331–336. [Google Scholar] [CrossRef]
- Moreno, N.; Querol, X.; Andrés, J.M.; Stanton, K.; Towler, M.; Nugteren, H.; Janssen-Jurkovicová, M.; Jones, R. Physico-chemical characteristics of European pulverized coal combustion fly ashes. Fuel 2005, 84, 1351–1363. [Google Scholar] [CrossRef]
- State Report “On the State and Protection of the Environment in the Krasnoyarsk Territory in 2020”—Krasnoyarsk, 2021. Available online: http://www.mpr.krskstate.ru/dat/bin/art_attach/17690_gosdoklad_2020.pdf (accessed on 15 March 2023). (In Russian).
- Sokolar, R.; Nguyen, M. Influence of Class C Fly Ash on the Properties of Plastic Clay and Fired Brick Body. Mater. Technol. 2020, 54, 107–111. [Google Scholar] [CrossRef]
- Biffi, G. Book for the Production of Ceramic Tiles, 1st ed.; Gruppo Editionale: Faenza, Italy, 2003. [Google Scholar]
- Zimmer, A.; Bergmann, C.P. Fly ash of mineral coal as ceramic tiles raw material. Waste Manag. 2007, 27, 59–68. [Google Scholar] [CrossRef]
- Tatsky, L.N.; Lokhova, N.A.; Gershanovich, G.L.; Senichak, E.B. Raw Mixture for the Manufacture of Wall Ceramic Products. RU Patent No. 2086517C1, 10 August 1997. Available online: https://patents.google.com/patent/RU2086517C1/ru (accessed on 15 March 2023). (In Russian).
- Fomenko, E.V.; Anshits, N.N.; Kushnerova, O.A.; Akimochkina, G.V.; Kukhtetskiy, S.V.; Anshits, A.G. Separation of nonmagnetic fine narrow fractions of PM10 from coal fly ash and their characteristics and mineral precursors. Energy Fuels 2019, 33, 3584–3593. [Google Scholar] [CrossRef]
- Fomenko, E.V.; Akimochkina, G.V.; Anshits, A.G. Narrow dispersed fractions of high-calcium fly ash produced from the pulverized combustion of irsha-borodinsky coal. Therm. Eng. 2019, 66, 560–568. [Google Scholar] [CrossRef]
- Akimochkina, G.V.; Rogovenko, E.S.; Gareeva, A.S.; Fomenko, E.V. Aerodynamic separation of dispersed microspheres PM2.5, PM10 from fly ash of lignite combustion for production of new materials. J. Sib. Fed. Univ. Chem. 2022, 15, 387–397. Available online: https://elib.sfu-kras.ru/bitstream/handle/2311/148510/09_Akimochkina.pdf?sequence=1 (accessed on 15 March 2023).
- GOST 5382-2019; Cements and Materials for Cement Production. Chemical Analysis Methods. Publishing House of Standards: Moscow, Russia, 2019. Available online: https://docs.cntd.ru/document/1200168999 (accessed on 15 March 2023). (In Russian)
- Fomenko, E.; Anshits, N.; Solovyov, L.; Mikhaylova, O.A.; Anshits, A. Composition and morphology of fly ash cenospheres produced from the combustion of kuznetsk coal. Energy Fuels 2013, 27, 5440–5448. [Google Scholar] [CrossRef]
- Glass, S.J.; Ewsuk, K.G. Ceramic Powder Compaction. MRS Bull. 1997, 22, 24–28. [Google Scholar] [CrossRef]
- GOST 7025–91; Ceramic and Calcium Silicate Bricks and Stones. Methods for Water Absorption and Density Determination and Frost Resistance Control. Standards Inform: Moscow, Russia, 2006. Available online: https://docs.cntd.ru/document/901700526 (accessed on 15 March 2023). (In Russian)
- GOST 2409-14; Refractories. Method for Determination of Bulk Density, Apparent and True Porosity, Water Absorption. Standards Inform: Moscow, Russia, 2014. Available online: https://docs.cntd.ru/document/1200114732 (accessed on 15 March 2023). (In Russian)
- GOST 24409-80; Ceramic Electrotechnical Materials. Methods of Testing. Standards Inform: Moscow, Russia, 2005. Available online: https://docs.cntd.ru/document/1200011905 (accessed on 15 March 2023). (In Russian)
- Osborn, E.F.; Muan, A. Phase Equilibrium Diagrams of Oxide Systems, Plate 1. The System CaO–Al2O3–SiO2; American Ceramic Society and Edward Orton, Ir., Ceramic Foundation: Columbus, OH, USA, 1960. [Google Scholar]
- Kaminskas, R.; Kubiliūtė, R. The effect of coal ash on synthesis and properties of tricalcium silicate. Mater. Sci. 2010, 16, 236–241. [Google Scholar]
- Giergiczny, Z. Effect of some additives on the reactions in fly ASH-Ca(OH)2 system. J. Therm. Anal. Calorim. 2004, 76, 747–754. [Google Scholar] [CrossRef]
- Gou, Z.; Chang, J.; Zhai, W. Preparation and characterization of novel bioactive dicalcium silicate ceramics. J. Eur. Ceram. Soc. 2005, 25, 1507–1514. [Google Scholar] [CrossRef]
- Venkatraman, S.K.; Choudhary, R.; Krishnamurithy, G.; Raghavendran, H.R.B.; Murali, M.R.; Kamarul, T.; Suresh, A.; Abraham, J.; Swamiappan, S. Biomineralization, mechanical, antibacterial and biological investigation of larnite and rankinite bioceramics. Mater. Sci. Eng. C 2021, 118, 111466. [Google Scholar] [CrossRef]
- Jansen, F.; Wei, X.H.; Dorfman, M.R.; Peters, J.A.; Nagy, D.R. Performance of dicalcium silicate coatings in hot-corrosive environment. Surf. Coat. Technol. 2002, 149, 57–61. [Google Scholar] [CrossRef]
- Rungchet, A.; Poon, C.S.; Chindaprasirt, P.; Pimraksa, K. Synthesis of low-temperature calcium sulfoaluminate-belite cements from industrial wastes and their hydration: Comparative studies between lignite fly ash and bottom ash. Cem. Concr. Compos. 2017, 83, 10–19. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, X.; Li, J.; Wang, P.; Qian, J. Preparation and Performance of Ternesite–Ye’elimite Cement. Materials 2022, 15, 4369. [Google Scholar] [CrossRef]
- Morrell, R. Handbook of Properties of Technical and Engineering Ceramics. Part 1. An Introduction for the Engineer and Designer; HMSO: London, UK, 1989. [Google Scholar]
- Asokan, P.; Saxena, M.; Asolekar, S.R. Coal combustion residues—Environmental implications and recycling potentials. Resour. Conserv. Recycl. 2005, 43, 239–262. [Google Scholar] [CrossRef]
- Gütlich, P.; Bill, E.; Trautwein, A.X. Mössbauer Spectroscopy and Transition Metal Chemistry: Fundamentals and Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Bayukov, O.A.; Anshits, N.N.; Balaev, A.D.; Sharonova, O.M.; Rabchevskii, E.V.; Petrov, M.I.; Anshits, A.G. Mössbauer study of magnetic microspheres isolated from power plant fly ash. Inorg. Mater. 2005, 41, 50–59. [Google Scholar] [CrossRef]
- Murray, P.J.; Linnett, J.W. Mössbauer studies in the spinel system coxfe3−xo4. J. Phys. Chem. Solids 1976, 37, 619–624. [Google Scholar] [CrossRef]
- De Boer, C.B.; Dekkers, M.J. Unusual thermomagnetic behaviour of haematites: Neoformation of a highly magnetic spinel phase on heating in air. Geophys. J. Int. 2001, 144, 481–494. [Google Scholar] [CrossRef]
- Na, J.G.; Lee, T.D.; Park, S.J. Effects of cation distribution on magnetic properties in cobalt ferrite. J. Mater. Sci. Lett. 1993, 12, 961–962. [Google Scholar] [CrossRef]
- Hu, P.; Yang, H.; Pan, D.; Wang, H.; Tian, J.; Zhang, S.; Wang, X.; Volinsky, A.A. Heat treatment effects on microstructure and magnetic properties of Mn–Zn ferrite powders. J. Magn. Magn. Mater. 2010, 322, 173–177. [Google Scholar] [CrossRef]



| Chemical Composition, wt % | |||||||||
| LOI | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | SO3 | TiO2 |
| 5.50 | 15.90 | 8.42 | 13.78 | 39.52 | 8.25 | 0.30 | 0.14 | 7.64 | 0.25 |
| Phase Composition, wt % | |||||||||
| Glass phase | Ca4Al2Fe2O10 | Ca3Al2O6 | CaSO4 | CaCO3 | CaO | Ca(OH)2 | MgO | Quartz | Fe-spinel |
| 40.7 | 12.9 | 10.2 | 11.2 | 0.7 | 2.0 | 11.5 | 6.8 | 2.0 | 2.0 |
| Group | d, μm | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | TiO2 | MnO | P2O5 | SO3 | BaO | SiO2/Al2O3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | SiO2 + Al2O3 < 40 wt %; 129 microspheres | |||||||||||||
| min | 0.6 | 3.0 | 2.9 | 3.8 | 23.3 | 4.1 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 1.6 | <0.01 | 0.4 |
| max | 2.3 | 33.5 | 18.7 | 27.5 | 56.9 | 28.3 | 0.8 | 0.8 | 2.5 | 2.9 | 1.6 | 23.2 | 3.9 | 10.4 |
| 2 | SiO2 + Al2O3 > 40 wt %; 51 microspheres | |||||||||||||
| min | 0.7 | 21.2 | 1.3 | 1.9 | 7.6 | 2.1 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 1.9 | 0.00 | 1.08 |
| max | 2.4 | 70.1 | 33.5 | 17.8 | 36.4 | 9.1 | 8.1 | 3.61 | 1.9 | 0.83 | 0.9 | 13.2 | 2.7 | 55.2 |
| 4 | Fe2O3 > 30 wt %; 15 microspheres | |||||||||||||
| min | 0.9 | 2.4 | 1.9 | 31.2 | 9.00 | 3.8 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 2.1 | <0.01 | 0.6 |
| max | 1.8 | 18.7 | 8.8 | 58.1 | 42.8 | 14.2 | 2.2 | 1.1 | 1.4 | 1.3 | 0.4 | 11.8 | 1.6 | 2.4 |
| Parameter | Thermal Treatment Temperature | ||
|---|---|---|---|
| 1000 °C | 1100 °C | 1200 °C | |
| Sintering coefficient | 1.1 | 0.8 | 0.5 |
| Linear shrinkage (%) | |||
| 0 | −12.5 | −25.0 |
| 6.2 | −6.3 | −21.9 |
| Apparent density (g/cm3) | 1.3 | 1.9 | 3.2 |
| Water absorption (%) | 42.5 | 22.1 | 1.8 |
| Open porosity (%) | 32.7 | 27.4 | 4.3 |
| Compressive strength (MPa) | 7.2 | 46.7 | 100.6 |
| δ, mm/s ±0.005 | Hhf, kOe, ±3 | Δ/2, mm/s ±0.01 | W, mm/s ±0.01 | dW, mm/s ±0.01 | A, % ±3% | Origin | |
|---|---|---|---|---|---|---|---|
| Initial fine narrow fraction (χ2 = 1.128) | |||||||
| S1 | 0.312 | 480 | −0.18 | 0.80 | 0.20 | 12 | Fe3+(B) |
| S2 | 0.353 | 425 | 0 | 0.79 | 0.0 | 20 | Fe3+(B) |
| S3 | 0.291 | 283 | −0.37 | 0.53 | 0.33 | 21 | Fe3+(A) |
| D1 | 0.232 | -- | 0.79 | 0.66 | -- | 20 | Fe3+(A) |
| D2 | 0.274 | -- | 1.57 | 0.70 | -- | 27 | Fe3+(A) |
| Sintered sample at 1200 °C fraction (χ2 = 1.346) | |||||||
| S1 | 0.302 | 264 | 0.00 | 0.16 | 2.20 | 29 | Fe3+(B) |
| S2 | 0.300 | 396 | 0.14 | 0.39 | 0.60 | 20 | Fe3+(B) |
| S3 | 0.284 | 359 | 0.00 | 0.39 | 0.63 | 31 | Fe3+(A) |
| S4 | 0.239 | 320 | −0.06 | 0.38 | 0.36 | 10 | Fe3+(A) |
| D1 | 0.163 | -- | 0.73 | 0.71 | -- | 9 | Fe3+(A) |
| Parameter | Sample | |
|---|---|---|
| Initial Fine Narrow Fraction | Sintered Sample at 1200 °C | |
| HC (Oe) | 125 | 25 |
| HCR (Oe) | 1168 | 69 |
| HCR/HC | 9.344 | 2.76 |
| MR (emu/g) | 0.185 | 0.366 |
| MS (emu/g) | 1.136 | 3.450 |
| MR/MS | 0.163 | 0.106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fomenko, E.V.; Akimochkina, G.V.; Knyazev, Y.V.; Semenov, S.V.; Yumashev, V.V.; Solovyov, L.A.; Anshits, A.G. Characterization and Magnetic Properties of Sintered Glass-Ceramics from Dispersed Fly Ash Microspheres. Magnetochemistry 2023, 9, 177. https://doi.org/10.3390/magnetochemistry9070177
Fomenko EV, Akimochkina GV, Knyazev YV, Semenov SV, Yumashev VV, Solovyov LA, Anshits AG. Characterization and Magnetic Properties of Sintered Glass-Ceramics from Dispersed Fly Ash Microspheres. Magnetochemistry. 2023; 9(7):177. https://doi.org/10.3390/magnetochemistry9070177
Chicago/Turabian StyleFomenko, Elena V., Galina V. Akimochkina, Yuriy V. Knyazev, Sergey V. Semenov, Vladimir V. Yumashev, Leonid A. Solovyov, and Alexander G. Anshits. 2023. "Characterization and Magnetic Properties of Sintered Glass-Ceramics from Dispersed Fly Ash Microspheres" Magnetochemistry 9, no. 7: 177. https://doi.org/10.3390/magnetochemistry9070177
APA StyleFomenko, E. V., Akimochkina, G. V., Knyazev, Y. V., Semenov, S. V., Yumashev, V. V., Solovyov, L. A., & Anshits, A. G. (2023). Characterization and Magnetic Properties of Sintered Glass-Ceramics from Dispersed Fly Ash Microspheres. Magnetochemistry, 9(7), 177. https://doi.org/10.3390/magnetochemistry9070177






