1. Introduction
Iron ore sinter is a primary raw material for the production of pig iron and steel in the iron and steel industry. During the sintering process, the iron ore sinter undergoes high-temperature heating followed by cooling. This process plays a crucial role in the formation of the structure and physicochemical properties of the mixed sinter, which, in turn, affect the quality of the resulting pig iron and steel [
1].
Despite numerous studies dedicated to the investigation of iron ore sinter, there is a need for further research on its structure and physicochemical properties, taking into account the characteristics of mixed basicity compositions [
2,
3,
4,
5,
6,
7,
8,
9]. Analyzing the data obtained from studying the structure of mixed iron ore sinter will provide a better understanding of the mechanisms involved in its formation and optimize the sintering process.
The sinter charge was a wet mixture of ferrous materials, fluxes (limestone and dolomite), and fuel. The main components of the charge were ore and iron ore concentrate, returns from previous sintering, coke breeze used as fuel, fluxes (limestone and dolomite), and metallurgical waste containing metals. In the production of sinter, mineral additives can also be used to improve pelletization and activate mineral formation processes during sintering [
10]. Dolomite, (Ca, Mg)CO
3, and other magnesium-bearing materials are increasingly being used as key flux components. The use of magnesium-containing materials has a significant effect on the mineralogy, microstructure, and physical properties of the sinter [
11]. In general, the sinter charge undergoes a complex series of chemical-mineralogical transformations and physical processes during heating. In the first stage, transformations in the solid phases occurred in the zone of intensive heating up to 600–900 °C. Mineralization processes occur between the gaseous and solid phases at 1100–1150 °C. At this stage, feed fuel combustion, hydrate compound decomposition, carbonate dissociation, iron oxide reduction, and sulfur removal occur. In the second stage, processes involving the formation of liquid phases occur in the temperature range of 1150–1450 °C. The third stage involved the impregnation of solid particles with liquid phases and their chemical interactions. At 1250–1350 °C in the combustion zone, the dissociation of hematite occurred with the formation of 10–15% FeO. In the fourth stage, the crystallization of the liquid phases occurs during their cooling in air, oxidation of iron oxide FeO, and formation of the final structure of the sinter. In the crystallization process of the high-base iron ore sinter, complex oxides consisting mainly of Fe, Ca, Al, Si, and Mg are formed.
The basicity of sinter has a significant influence on the microstructure of the final product. The microstructure of the sinter is composed of various phases, such as hematite, magnetite, and calcium ferrite. The evolution of these phases during the sintering process is affected by the basicity of the sintering mix. For example, an increase in basicity can lead to the formation of more calcium ferrite, which can improve the strength and reducibility of the sinter. On the other hand, a decrease in basicity can lead to the formation of more magnetite, which can improve the softening and melting properties of the sinter.
In addition to its effect on the microstructure, the basicity of sinter also affects its physicochemical and metallurgical properties. For instance, an increase in basicity can improve the permeability and gas flow of the sinter bed, which can enhance the efficiency of the blast furnace. Moreover, the basicity of the sinter can affect the slag formation and the quality of the hot metal produced in the blast furnace. Also, alkalis are known to have an adverse effect on sinter quality, and their presence in the blast furnace can lead to operational problems. Alkali metals reduce the resistance to cracking of sinters [
12]. An increase in the basicity of the sinter leads to a decrease in the alkali content of the sinter whereas sulphides inhibit the alkali transfer into the gas phase [
13].
The quality of the agglomerates is closely related to the mineral structure. The process of agglomeration consists of the redistribution and recrystallization of the components of the system [
14]. Complex silicate binders (mainly Fe, Ca, Al, Si, Mg, and O) consist mainly of multicomponent calcium-aluminum silicoferrite (SFCA), two-calcium silicates with various modifications, and their solid solutions.
In industrially produced iron ore sinter, the composition of SFCA phases varies widely [
15]. Although the SFCA phase has been defined as a distinct and unique phase present in iron ore sinter, many researchers have noted that there are many other SFCA-like phases [
16,
17,
18,
19] with different morphologies and chemical compositions. SFCA and SFCA-I, SFCA-II, and SFCA-III phases containing Fe
2O
3, CaO, SiO
2, and Al
2O
3, and insignificantly MgO and TiO
2, are known as multicomponent calcium ferrites in agglomerates. The typical agglomerate and major binding phases of SFCA and SFCA-I have structures related to the mineral phase of the enigmatite group and can be expressed as Me
14+6nO
20+8n (
n = 0, 1). The SFCA phase was A
2M
6T
6O
20, and the SFCA-I phase was A
3BM
8T
8O
28 (where A = Ca
2+; B = Ca
2+; Fe
2+, M (octahedral pores) = Fe
3+; T (tetrahedral pores) = Fe
3+, Al
3+, and Si
4+). The crystal structures of these phases consist of a layered structure of spinel (S) and pyroxene (P), with period SPSP for the SFCA structure and -SSPSSP for SFCA-I [
20,
21]. The lattices of two-calcium silicate are island lattices with [SiO
4] tetrahedrons connected by O
2− to Ca
2+ cations and have several modifications that are stable at different temperatures.
Recently, scientific research on the mineralogical composition of laboratory agglomerates—the formation of two calcium silicate phases CaO-SiO
2, and more complicated phases of SFCA at different modes of heating and cooling—has been carried out worldwide. In the works of Australian scientists [
14,
15,
16,
21,
22,
23] a critical review of the research on silicoferrites of calcium and aluminum (SFCA), the main bound phases found in agglomerates, has been conducted. In particular, the papers focus on the description of the different phases of SFCA-I and the study of their phase and chemical composition, crystalline structures, and formation conditions. In the works of Chinese scientists [
24,
25], correlations were found between the phase components of the agglomerate and their properties based on the composition and characteristics of the concentrate. Mumme W.G. and colleagues [
26] synthesised an SFCA-like phase called SFCA-II, which is thought to be a fusion phase of SFCA and SFCA-I. Kahlenberg V. and colleagues [
20] tried to find the SFCA-III phase in iron ore specks and determined its structure.
Each of the dozens of papers mainly investigated the conditions of formation, chemical composition, and crystal lattice of the SFCA phases. Only a few studies have drawn conclusions about the influence of these phases on the properties of the sinter. To summarize the current state of the art, most studies have been conducted on laboratory samples prepared under different regimes and conditions simulating the agglomeration process. This is true for the investigation of the influence of certain sintering conditions (temperature, pressure, and sintering charge composition) on the composition and shape of the SFCA phases and metallurgical properties. This does not eliminate the lack of correlation between the phase components of industrially sintered samples and their properties.
Numerous studies have been carried out to investigate changes in the phase composition of sinter under different conditions [
27,
28,
29,
30,
31,
32,
33] to determine the compositional range, phase stability, crystal structure, and mechanism of SFCA formation and their influence on the metallurgical properties of the sinter. However, at both the fundamental and practical levels, the knowledge gained is disparate and individual.
The aim of this paper is to investigate the influence of the basicity of a sintered agglomerate on its microstructure, phase composition, and physicochemical and metallurgical properties. For this purpose, samples of agglomerates with different basicities were sintered and investigated under laboratory conditions. Optimum values for the metallurgical properties of the agglomerates were determined. The results of the mineralogical transformations that occurred during the sintering of the agglomerates are also presented.
The originality of this paper lies in the study of laboratory agglomerates obtained under conditions close to industrial.
2. Materials and Methods
An iron ore concentrate was used in this study. The chemical composition of the concentrate is listed in
Table 1. As an example,
Table 2 lists the composition of the sinter charge for sintering with a basicity of 2.0. For the study, both laboratory sintered 25–35 mm fractions, the basicity of which was varied from 1.2 to 3.0 by the addition of lime CaO in the sintering charge, and commercial samples of sintered products (including titanomagnetite sintered products) were examined. The chemical compositions are listed in
Table 3 and
Table 4.
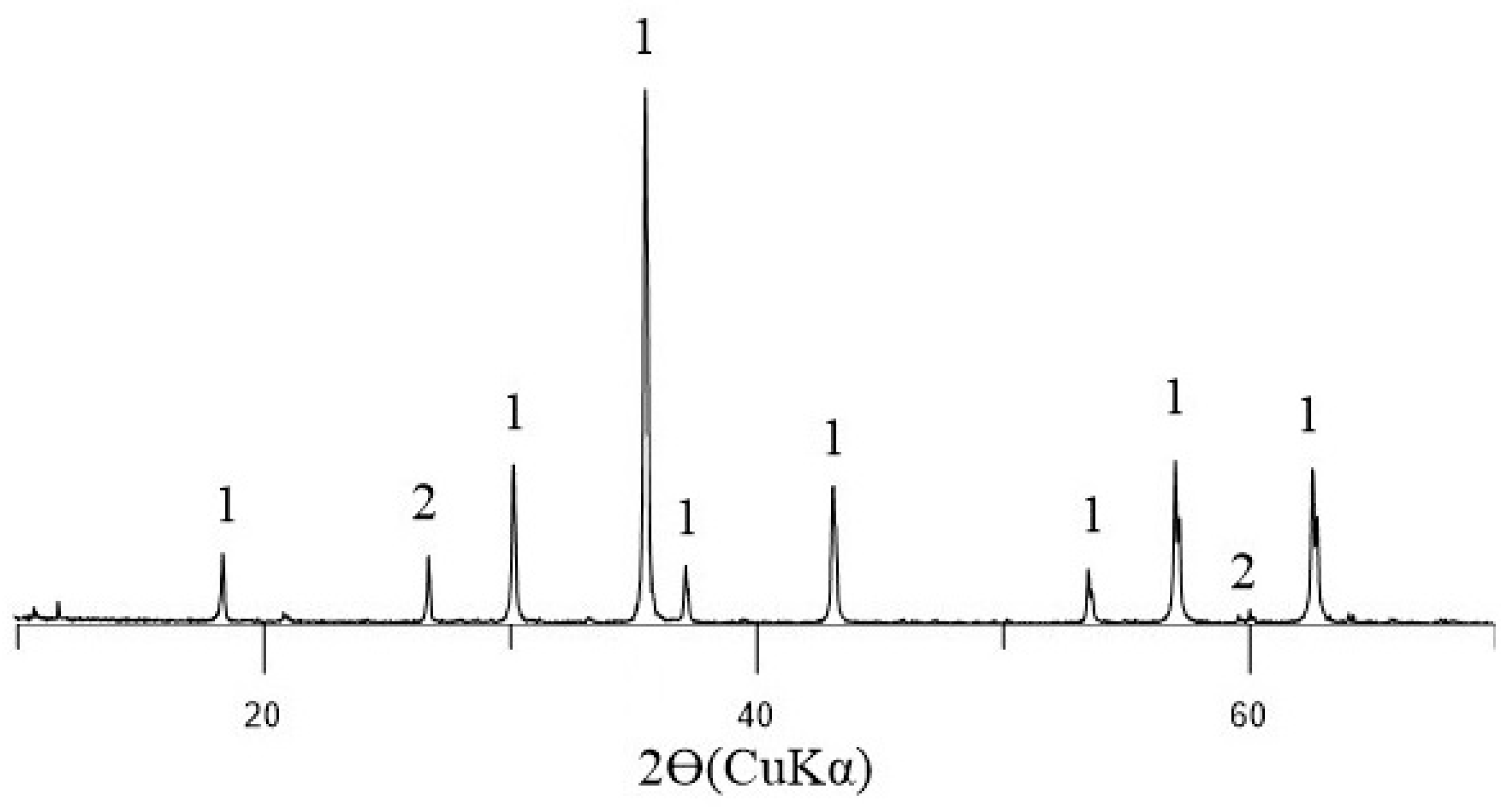
To identify the phases in the sinter, we used an X-ray diffractometer Shimadzu XRD-7000 equipped with an X-ray tube with CuKα radiation in the air in the range of angles 2θ from 10 to 85°. The ICDD PDF4 (International Centre for Diffraction Data, Newtown Square, PA, USA) databases were used to interpret the diffractograms. The structure and elemental composition of the phases in the sinter were studied by scanning electron microscopy (Carl Zeiss Evo 40, Oberkochen, Germany). An Olympus GX-51 optical microscope (Shinjuku, Japan) was used to study mineralogical composition.
The dominant phase, magnetite Fe
3O
4, was observed by the intensity of peaks in the samples of the concentrate. The iron ore concentrate consisted of Fe
3O
4 and SiO
2 (
Figure 1).
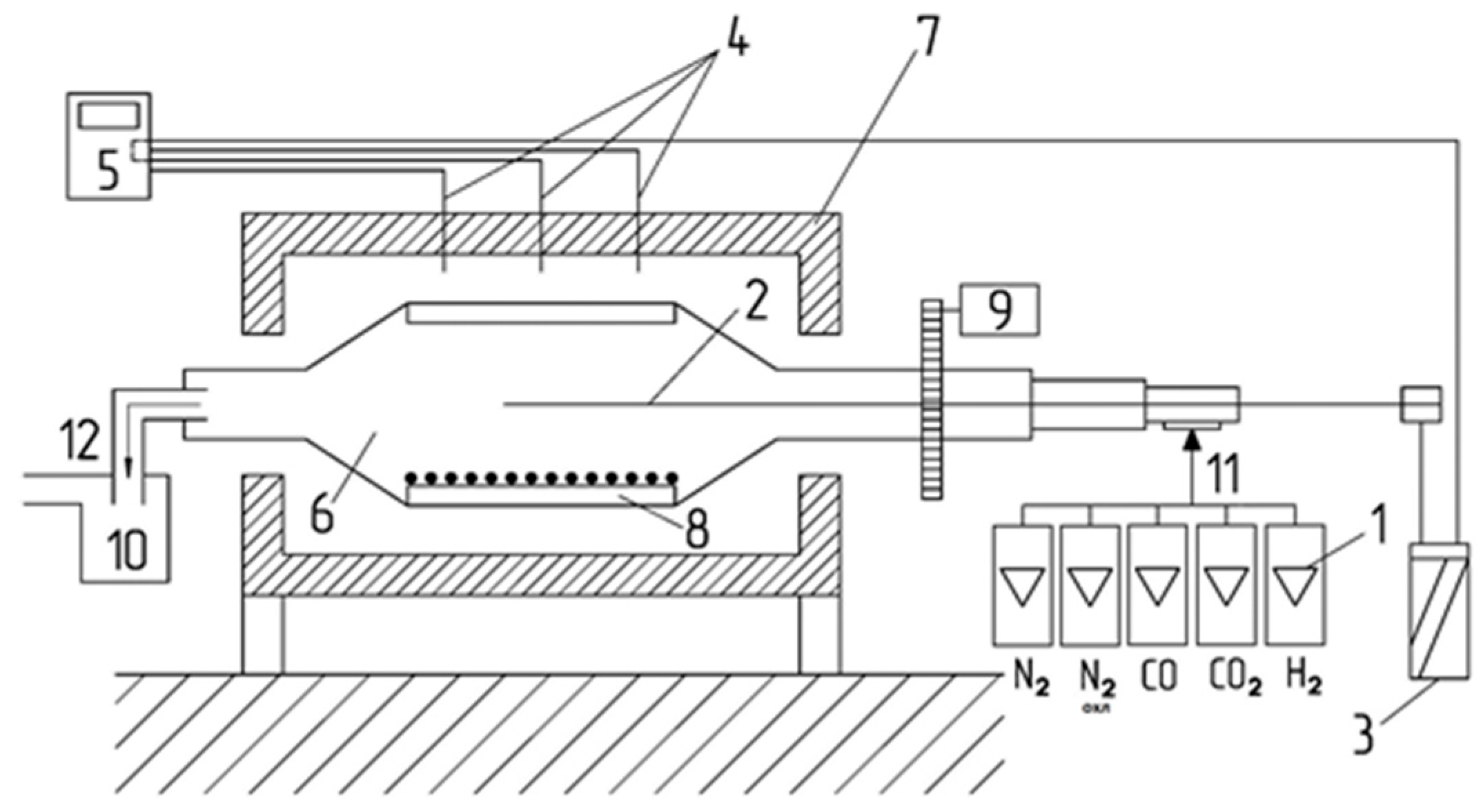
The reduction (thermomechanical) strength LTD (Low-Temperature Disintegration) was studied according to ISO 13930, the temperature ranges of softening of the sinter samples according to the State Standard of the Russian Federation No. 26517-85.
ISO 13930:2015 specifies a method for calculating a relative index to assess the degree of refinement of iron ores and materials during reduction, under conditions similar to those prevailing in the low-temperature reduction zone of a blast furnace.
The essence of the method is the reduction of 500 g of sample with 10–12.5 mm or 12.5–16.0 mm of gaseous reducing agent in a rotating drum at a given temperature regime (heating in nitrogen medium to 500 °C for the first 45 min, 15 min—temperature stabilization, then 60 min—reduction gas, cooling the sample in nitrogen and subsequent sieving of the test material into size classes (+6.3 mm; +3.15 mm; +0.5 mm; −0.5 mm) characterizing its strength properties. The composition of the reducing gas was CO—20%; CO
2—20%; H
2—2%; N
2—58%, flow rate—20 l/min. A schematic of the installation is shown in
Figure 2.
The Low-Temperature Fracture Index (LTD) is calculated as the percentage ratio of the masses of material coarser than 6.3 mm, less than 3.15 mm, and less than 0.5 mm to the total mass of the sample after rotation in the drum, including dust from the dust collector (Equations (1)–(3)):
where m
0— is the mass of the sample after rotation, including dust from the dust collector, g; m
1—is the mass of the sample of the 6.3 mm particle size class, g; m
2— is the mass of the sample of the 3.15 mm particle size class, g; m
3—is the mass of the sample of the 0.5 mm particle size class, g.
The method for determining the temperature range of softening involves heating a sample of the test material in an inert gas and determining the temperature at which softening begins when a rigid rod is immersed in the sample under the effect of external pressure and the temperature range of softening. The installation is illustrated in
Figure 3.
3. Results
3.1. Study of Structure and Phase Composition of Laboratory Agglomerates
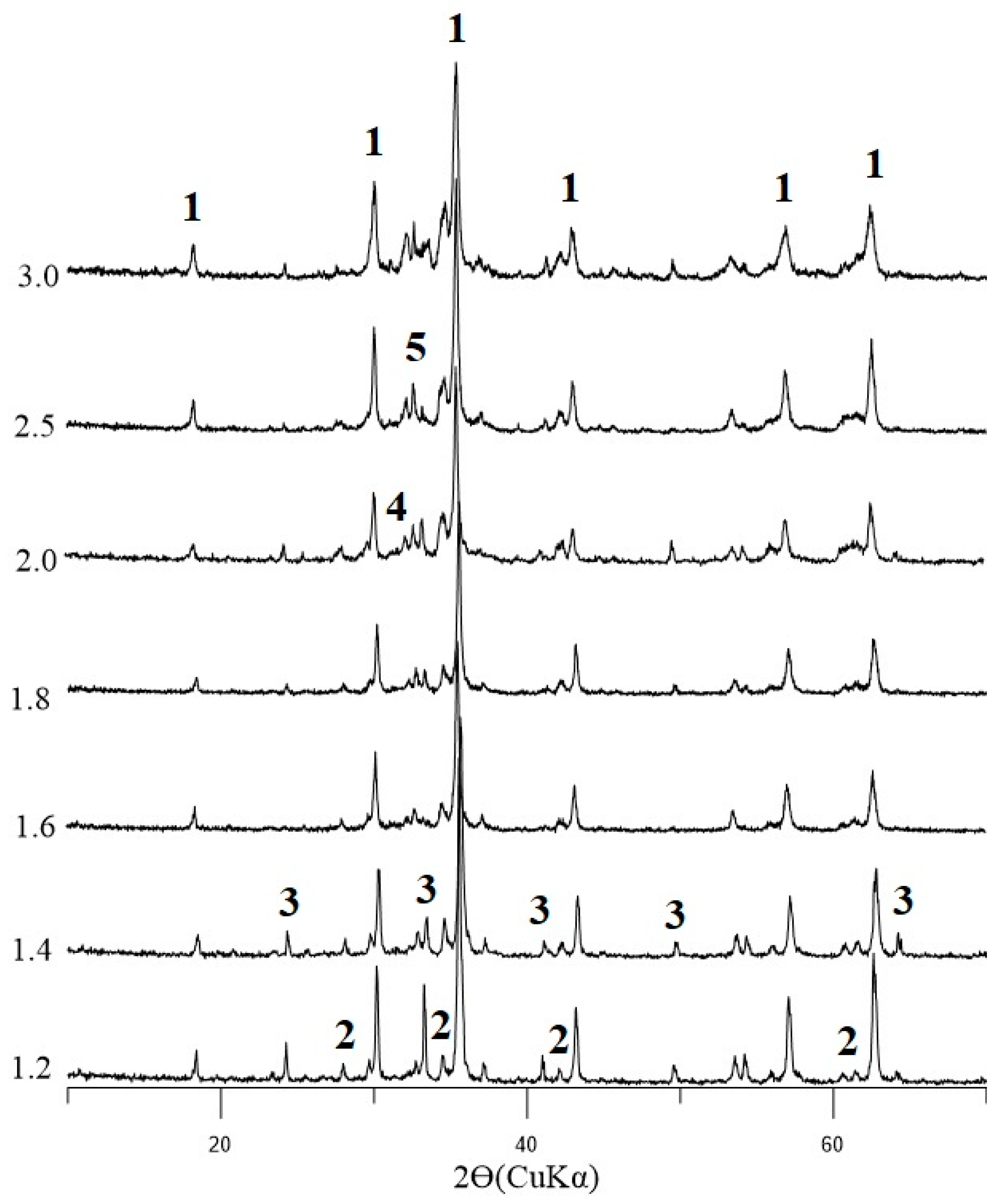
X-ray diffraction results (
Figure 4,
Table 5) show that the ore phase in agglomerates with basicity 1.2–3.0 consists of magnetite Fe
3O
4 and hematite Fe
2O
3. The phase composition of laboratory agglomerates was calculated by the method of corundum numbers from the PDF file. The relative measurement error is 10%.
The binder of the ore phases during the cooling of the iron silicate melt consists of calcium and aluminum silicoferrite (SFCA). The SFCA phase in the studied agglomerates is better described as Ca2.3Mg0.8Al1.5Fe8.3Si1.1O20 [00-046-0037], or Ca1.12Al0.68Fe4.34Mg0.073O10Si0.38 [01-085-2796], or Ca5.03Fe21.77Si1.30O40 [01-085-3749]. These formulas correspond to M14O20, where M is Ca, Si, Fe, Al, and two-calcium silicate in different polymorphic modifications (α-, β- and γ-Ca2SiO4). The amount of SFCA increases from 8 to 23% with the basicity of the agglomerates; the amount of bicalcium silicate increases slightly with the calcium content of the load but remains within the range of 1–3%. For samples with a basicity above 2.5, the vitreous phase increases.
It has been found that the content of phases, such as magnetite and hematite, decreases with increasing basicity. This was due to an increase in the volume of silicate bonding. In agglomerates, the silicate binder fills the space between the ferrous phases and is the main source of strength in finished products. With an increase in basicity above 1.4, two-calcium silicate β-Ca
2SiO
4 was detected by X-ray diffraction analysis, and its amount increased (
Figure 5).
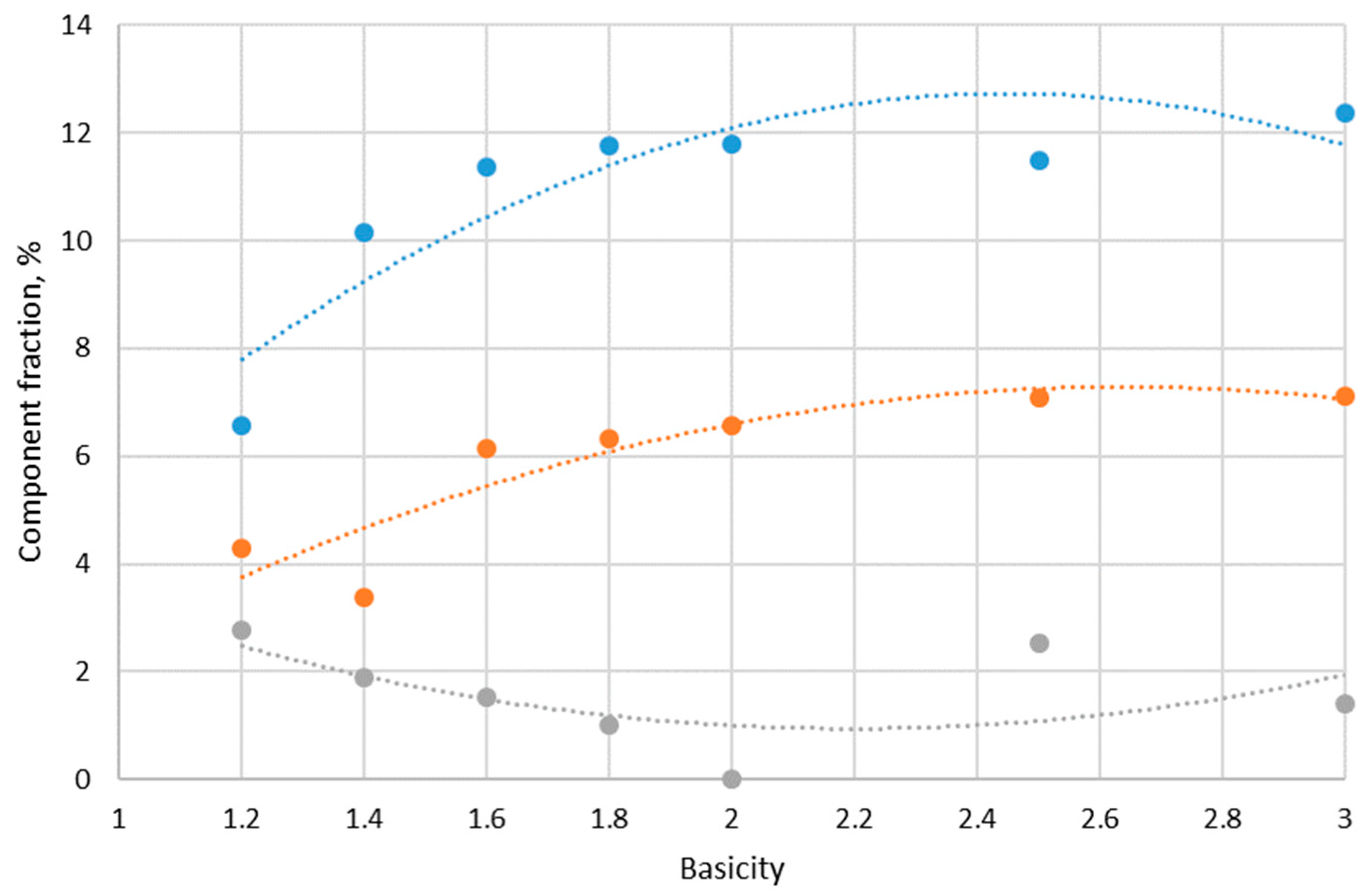
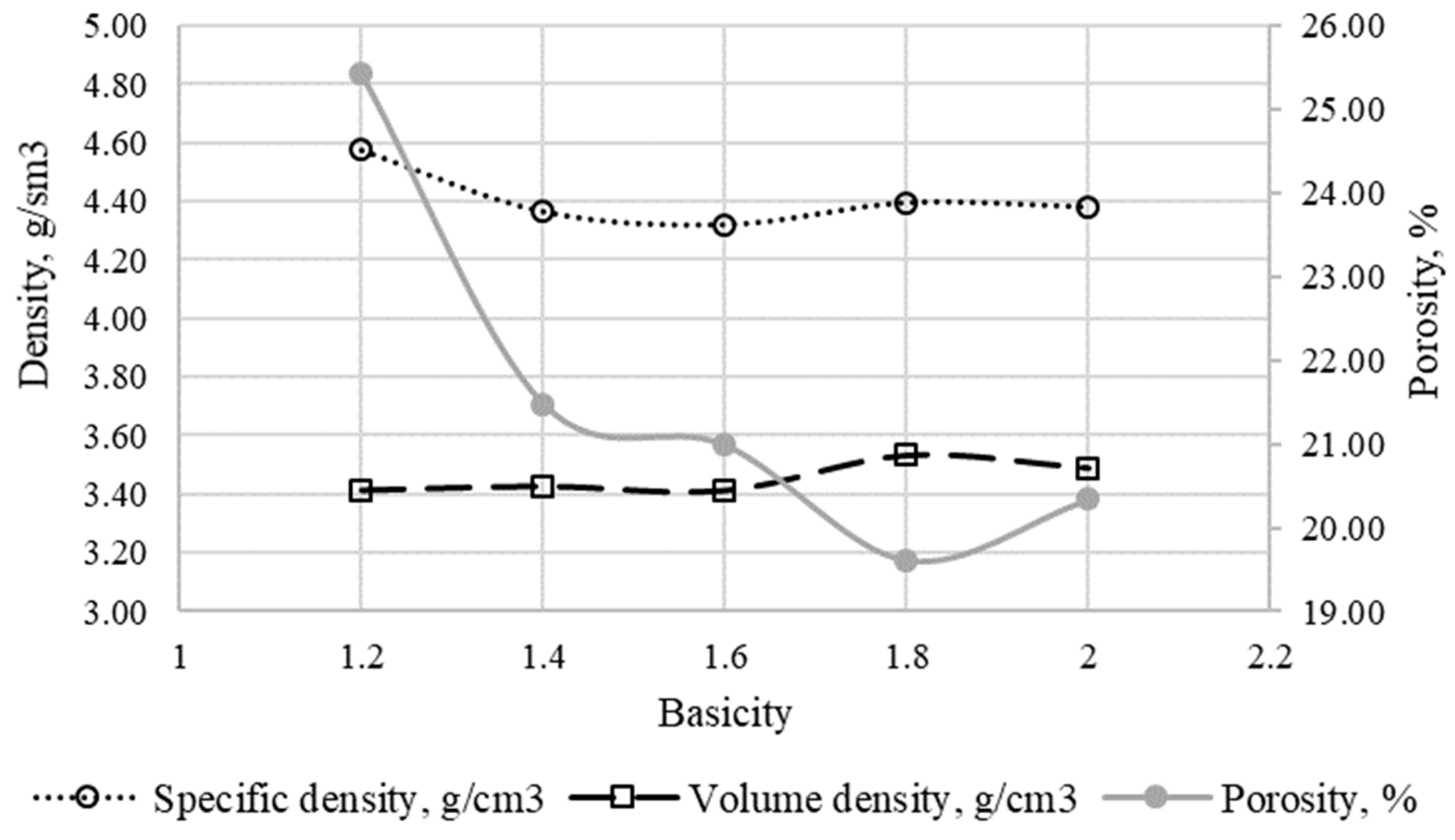
According to optical microscopy data, in agglomerates with basicity from 1.2 to 3.0, the macrostructure is characterized by round, less often oblong pores up to 2 mm, on occasion, there are pores of 4 to 5 mm, and small round pores with sizes from 0.09 to 0.2 mm. The pores are irregularly distributed in the sinter volume (the specific density of agglomerates ranges from 4.4 to 4.6 g/sm
3). According to the specific and bulk density measurements and porosity calculations (
Figure 6), an increase in the basicity of the sinter leads to a decrease in porosity [
34].
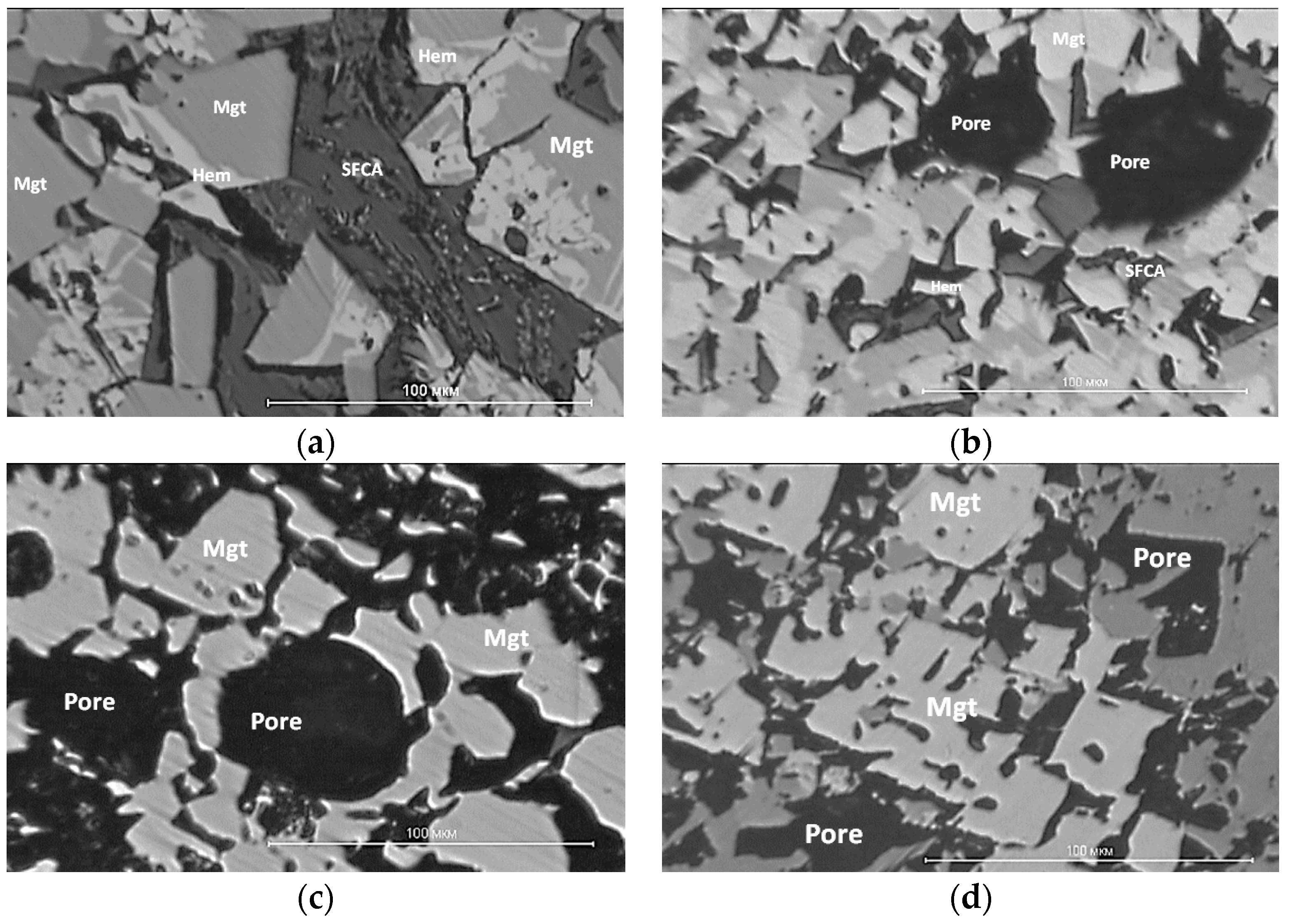
In samples with a hardness of 1.2 to 3.0, the dominant phase was magnetite Fe
3O
4, which was cemented by the silicate matrix and often contained inclusions (
Figure 7a). Magnetite in agglomerates occurs in various morphological forms. On microstructures of thin sections, Fe
3O
4 is presented as dense and coarse crystalline formations from 0.05 to 0.1 mm in size. The shape and grain size of the ore phase of the iron-ore agglomerate did not change with basicity (
Figure 7a–d).
Hematite Fe
2O
3 was also detected in the structure of laboratory agglomerates, isolated in various forms. Hematite occurs along the boundaries of magnetite (1.2 and 1.8 basicity samples) or the boundaries of magnetite (1.4 and 1.8 basicity samples) and also occurs in small amounts at pore margins (1.2, 1.8–2.0 basicity samples). The phase forms are shown in
Figure 8.
The silicate bonding of iron oxides in the agglomerates has a different morphology (
Figure 8). According to scanning electron microscopy data, the silicate bond formed during the cooling of the iron silicate melt is heterogeneous in composition—the grey calcium aluminum silicoferrite (SFCA) phase with small inclusions of acermanite and darker colored small inclusions of bicalcium silicate form the basis. The amount of 2CaO-SiO
2 was small in the sintered structure but did not produce good interlocking between the ore phases; as a result, the sintered sample had many cracks and pores, which reduced the strength of the sinter.
According to the XRD analysis, the crystalline components were predominantly SFCA and β-2CaO-SiO2 phases. It is possible that the α- and γ-Ca2SiO4 phases are present in the structure, but their own set of reflections is minimal in the diffractograms.
3.2. Relationship between Structure and Metallurgical Characteristics of Iron Ore Materials
The reduction in sinter strength is caused by internal stresses due to: a significant temperature gradient in a piece (thermal stress); and a significant difference in the coefficients of thermal expansion of individual phases (structural stress); in flux sinter, the polymorphic transformation β-2CaO∙SiO
2 → γ-2CaO∙SiO
2 occurs at 675 °C with an increase in volume of 11% (phase transformation) according to the authors [
35].
Polymorphic transformation of Ca2SiO4 is one of the main causes of the decrease in the strength of the flux sinter. The stabilization of bicalcium silicate can be achieved by thermal, physical, or crystallographic methods. Thermal and physical stabilization are not of practical importance.
According to the literature [
36,
37] up to a basicity of 0.5 the mineralogical composition and strength of the agglomerate remained almost unchanged, after which a sharp loss in the strength of the product began. This was due to the combined effect of many factors, including the appearance of bicalcium silicate and an increase in the total number of phases present in the sintered structure. The most unfavorable combination of phases from the point of view of internal stresses corresponds to a basicity of 1.3–1.4. At sinter basicity values more than 1.4, a gradual increase in sinter strength was observed. As the basicity increased, the amount of brittle glass in the finished product decreased. The remainder of this phase disappears at a basicity of 3. The Ca-olivine bond was replaced by a ferritic-calcium bond, which is less brittle. The two-calcium silicate partially gives way to a three-calcium silicate that does not undergo polymorphic transformation. The level of internal stress in the sinter lumps is also more favorable at high basicity values. Thus, another method of preventing the polymorphic transformation of two-calcium silicate and loss of strength of fluxed agglomerates may be to receive and use in blast-furnace melting in appropriate proportions of two types of high-strength agglomerates: non-fluxed (basicity 0.4–0.7) and high-fluxed (basicity 2.0–3.5) [
36,
37].
Iron ore materials undergo considerable degradation during premelting. The peculiarity of the medium basicity sinter is that despite its high mechanical strength, it tends to collapse in the upper part of the blast furnace shaft. It has been found that the decisive factor in this destruction is the reduction process: the maximum destruction is observed at a reduction rate of 25–40%, that is, at the stage of reduction Fe
2O
3→Fe
3O
4 (which corresponds to temperatures of 500–700 °C, at higher temperatures, the softening slows down, and at temperatures above 900 °C, the strength even increases) [
38]. Material destruction during reduction by carbon monoxide occurs more strongly than during reduction by hydrogen [
39].
One of the most important causes of sintering strength loss is the rearrangement of the iron crystal lattice during reduction, especially at low degrees of reduction. This is primarily related to the reduction of hematite, which involves significant changes in the specific volume of the crystal lattice. Thus, during the reduction of agglomerates α-Fe
2O
3 (specific volume of 100%) was sequentially converted into maghemite γ-Fe
2O
3 (oxymagnetite, specific volume of 107.7%) and magnetite (specific volume of 98.5%). The crystals exhibit different properties in different directions (crystal anisotropy). This law applies fully to the rate of reduction of magnetite and hematite crystals. However, the degree of anisotropy is substantially different: the reduction rates of magnetite crystals in different directions differ from each other by no more than 20–25%, hematite—by 35–45%, and more [
40].
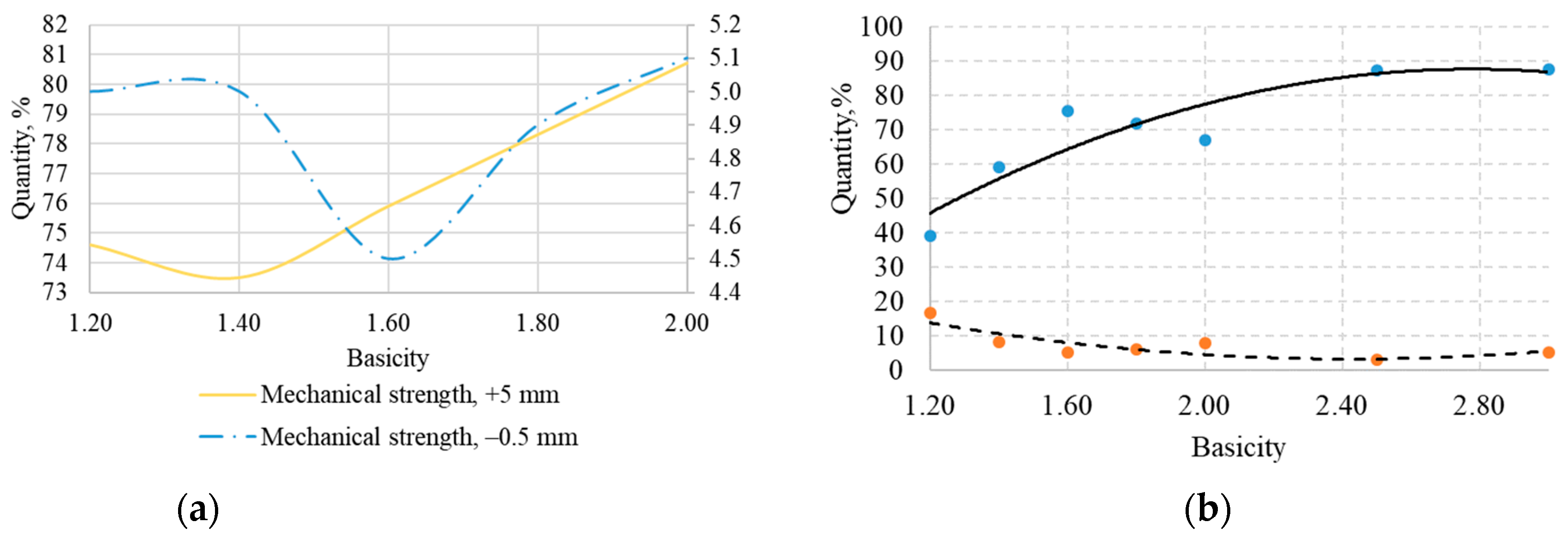
Figure 9 shows diagrams of the mechanical strength, abradability, and thermomechanical strength LTD
+6.3 of the laboratory sinter as a function of its basicity.
Analysis of the X-ray diffraction composition, mechanical and thermomechanical strength, and laboratory sinter structure showed that agglomerate with 1.4 basicity had the lowest value of mechanical strength, and this parameter increased with increasing basicity.
The change in mechanical strength can be explained by the increased formation of β-2CaO-SiO
2, which undergoes a modification transformation β → γ-2CaO-SiO
2 accompanied by a 10–12 fold increase in volume and destroys the structure as sinter cools [
7]. As the basicity increases, crystallochemical stabilization of β-2CaO-SiO
2 occurs, and increasingly complex calcium silicoferrite SFCA is found in the agglomerate structure. Increasing the basicity to 1.6 leads to maximum hot strength values, and the amount of fines in both the mechanical and thermomechanical strength tests was minimal. In the silicate binder structure of sinter with basicity 1.6, the amount of wollastonite (Fe,Ca)SiO
3 is found in dendritic form; with further increase in basicity, no dendritic form is found. A further increase in basicity leads to an increase in the amount of SFCA [
41], an increase in sinter strength, and a reduction in fines in both the thermomechanical and mechanical strength tests.
Thus, there is a direct correlation between the phase, mineral composition of the binder, and strength properties of agglomerates of different basicities. The mineral composition of the binder determines the strength of the sinter.
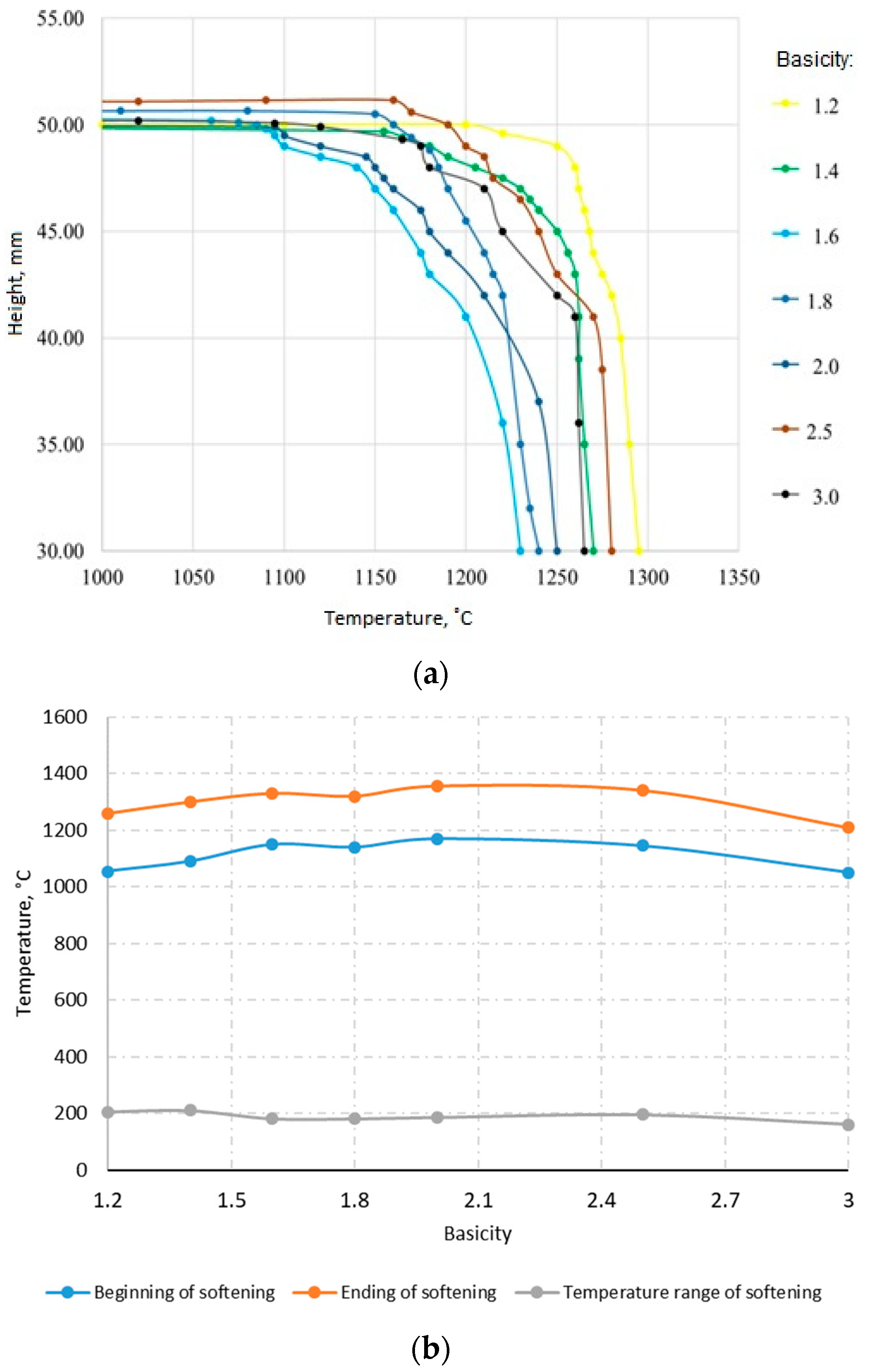
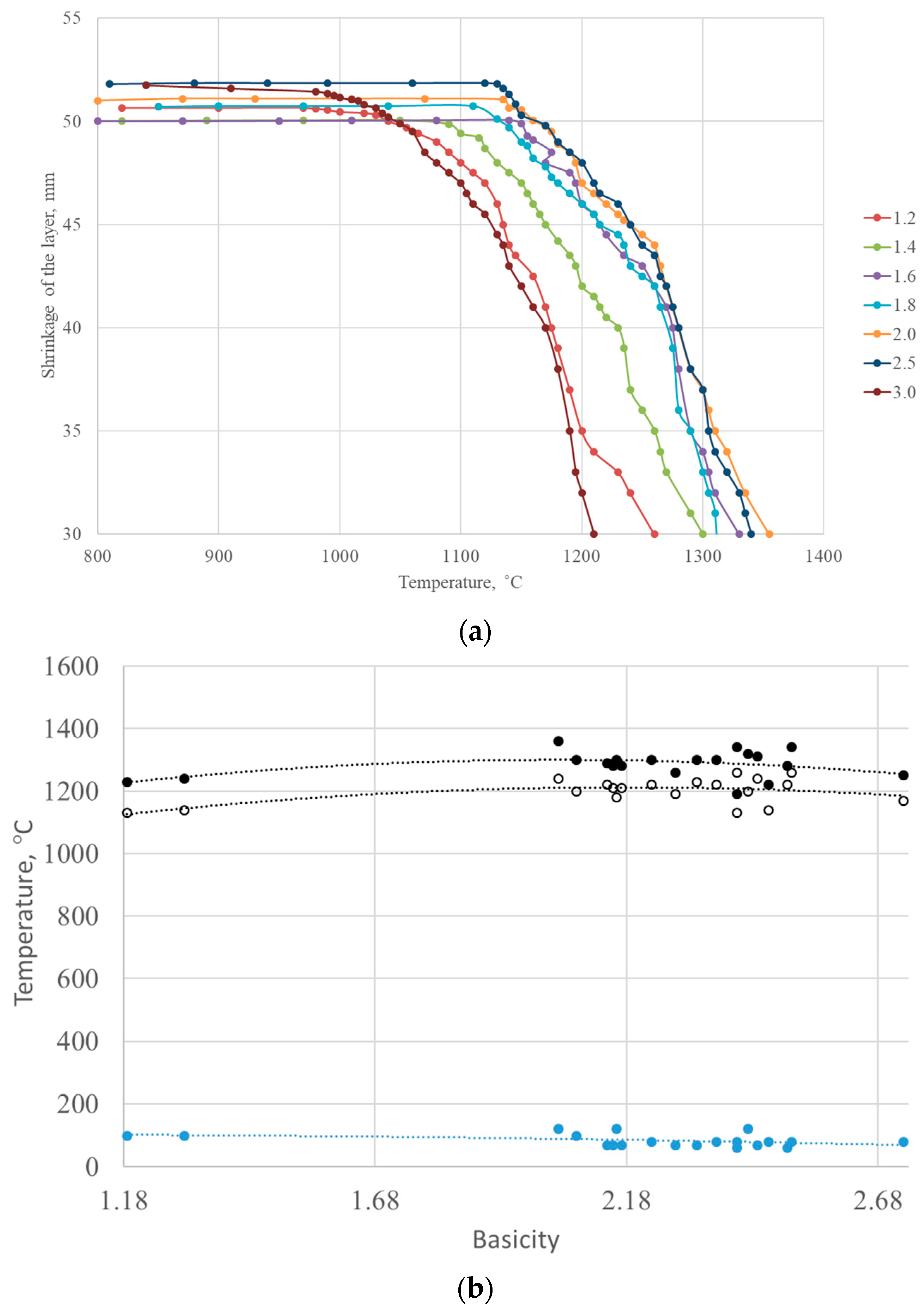
Figure 10. shows the softening curves of the studied agglomerates with different basicities of 1.2–3.0.
The results of the softening temperature measurements of the agglomerates showed that there were two stages in the softening process of the unreduced agglomerates. In the first stage, softening begins at temperatures of 1060–1075 °C for sintering with a basicity of 1.6 and is 1–3% up to temperatures of 1135–1140 °C (the beginning of softening). In the second step, the shrinkage rate increased rapidly upon further heating and reached 40% at 1220–1230 °C (end of softening), corresponding to a softening interval of ~160 °C. According to [
27], the beginning of the intensive increase in shrinkage (temperature of inflection in the shrinkage-temperature curve) corresponds to the values of the melting initiation temperature obtained with a high-temperature microscope. This indicates that in the first stage in the agglomerate, there was softening of the silicate bond and subsequent consolidation of the crystalline phase and reduction of pores (plastic deformation). In the second stage, the first portion of the melt began to appear. The minimum softening point corresponds to the maximum amount of glass in the sinter, as glass, which has no fixed melting point, softens over a wide temperature range.
3.3. Study of the Metallurgical Characteristics of Industrial Agglomerates
The iron ore and titanomagnetite agglomerates obtained under industrial conditions were tested.
Table 6 shows the values of the thermo-mechanical strength LTD
+6.3 (fraction +6.3 mm). The results shown in
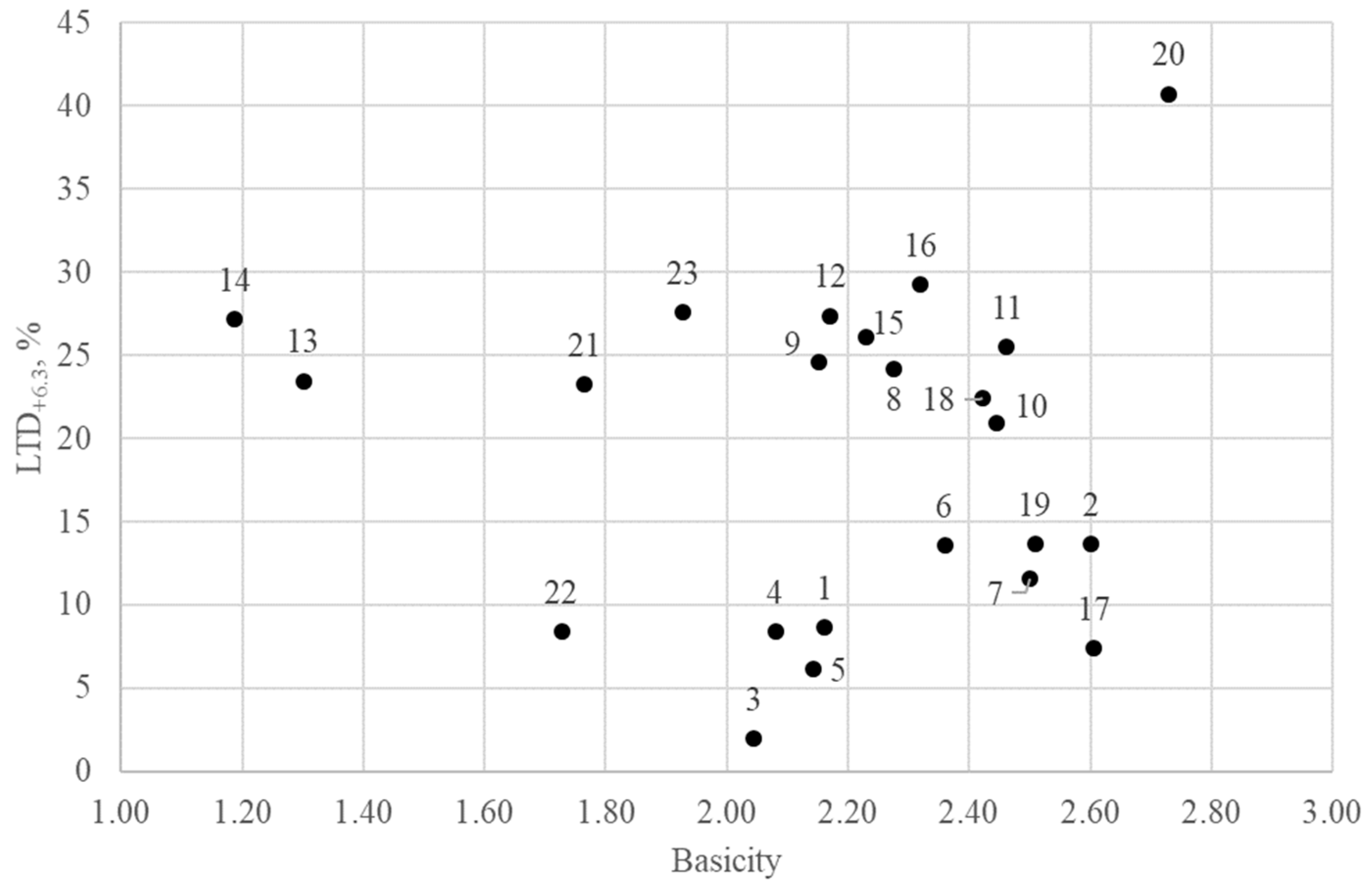
Figure 11 indicate that the strength of the agglomerates has different values, not exceeding 45%. After low-temperature sintering, the fraction of the +6.3 mm ranged from 2 to 41%, and that of the −0.5 mm fraction ranged from 16 to 35%.
All samples obtained were divided into three groups: low-strength samples with LTD+6.3 from 2 to 14%, medium-strength samples with LTD+6.3 from 14 to 27%, and high-strength samples with LTD+6.3 from 27 to 41%.
An X-ray phase analysis of the sintered samples was carried out (as an example, a diffractogram of sintered sample No. 3 is shown in
Figure 12).
According to XRD and X-ray diffraction analyses, agglomerates are composed of ore phases (magnetite Fe3O4, hematite Fe2O3) and silico-ferrite bonding, the composition of which is determined by the basicity of the agglomerate and the content of additional elements in a charge; the microstructure depends on the sintering and cooling conditions of the final products. The magnetite in this structure is a multi-component system containing Al2O3, TiO2, and MgO. In many cases, hematite exists in the form of “skeletal rhomboids” and is formed in the vicinity of large pores. This type of hematite is formed as a result of the transformation of multicomponent magnetite as the temperature is lowered.
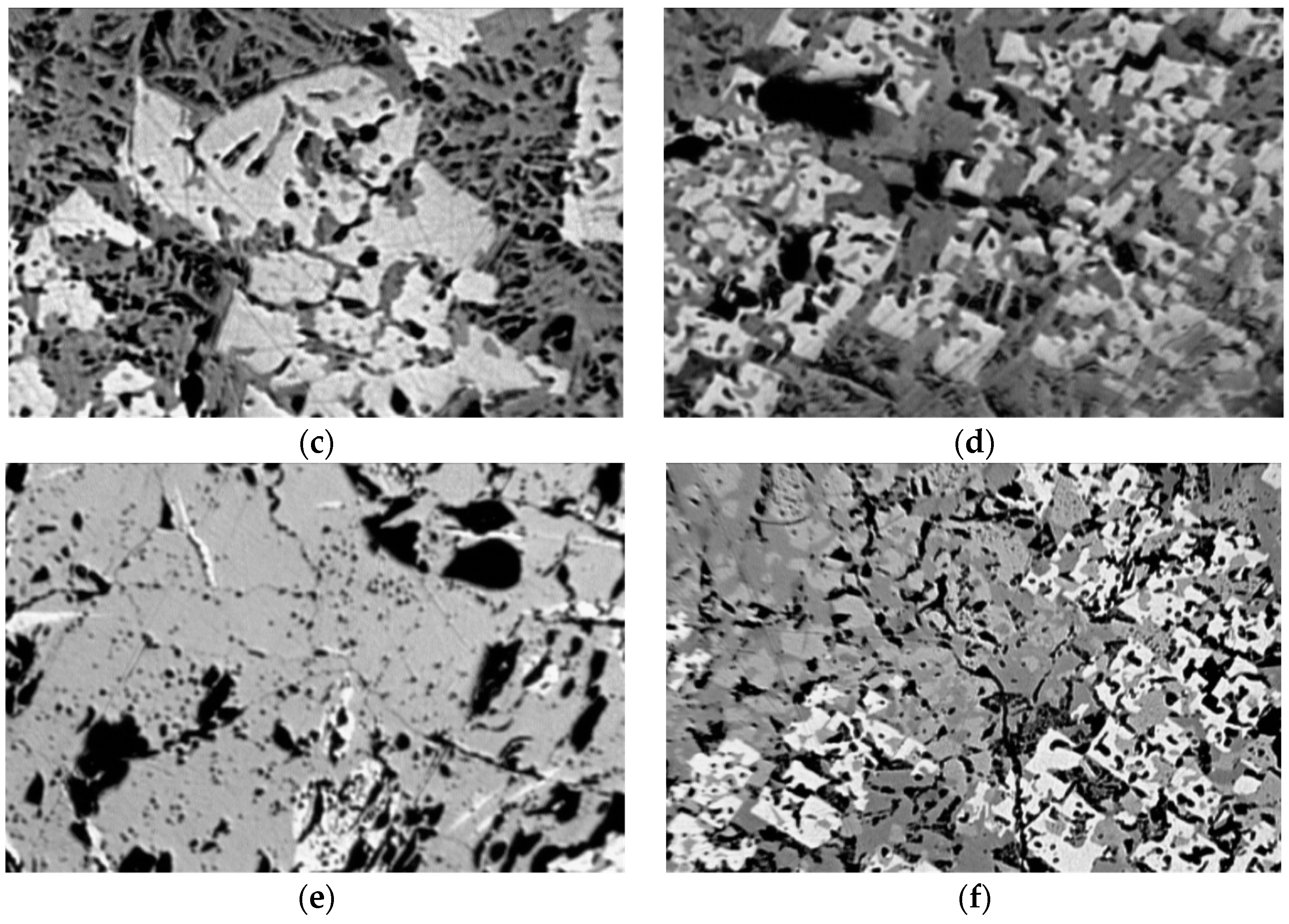
Figure 13 shows the microstructure of sinter samples from different “groups” of thermomechanical strength: a, b—with low strength, c, d—with medium strength, and e, f—with high strength.
The structure of the low-strength samples (
Figure 13a) has an uneven phase distribution over the cross-section and contains a large number of large pores; however, at the same time, it has a reducing power of up to 80%. The main phases of the agglomerates studied were magnetite and hematite, with admixtures of Mg, Ca, Al, and Ti. The bond of the ore phases during the cooling of the iron silicate melt consists of calcium aluminum silicoferrite SFCA and can be described as the compound Ca
2.3Mg
0.8Al
1.5Fe
8.3Si
1.1O
20 with the general formula M
14O
20 (M includes Ca, Si, Fe, Al, and Ti). A small amount of bicalcium silicate was found in sample №22 with a basicity of 1.73, which contributed to the destruction of the sinter. Low iron (9–15 wt.%) in the SFCA phase and high content of silicon (10–18 wt.%) are characteristic features of agglomerate samples with low thermomechanical strength in basicity 2.04 and 1.73.
The group of samples with average hot strength values (
Figure 13c,d) exhibited a more uniform phase distribution across the section. The main ore phases are magnetite, with partial replacement of Fe
2+ ions by Mg
2+, and hematite, with partial replacement of Fe
3+ ions by Al
3+. The bonding of SFCA ore phases can be described by a compound with the general formula M
14O
20. In addition, in the silicate binder, there are extracts of titanium andradite with the general formula M
8O
12, where M is Ca, Fe, Si, and Ti, which in [
42,
43,
44] is thought to be formed by the substitution of Fe
3+ in SFCA with Ti
4+ and Si
4+.
Agglomerate samples with high thermomechanical strength indices (
Figure 13e,f) had an even distribution of phases over the cross-section of the sample, and the pores were small and evenly distributed. The basis of the ore component is magnetite, with the replacement of part of the iron ions by Mg, Ca, and Al ions, and hematite with the replacement of part of the iron ions by Ca, Si, and Al ions. Silicate bond SFCA can also be described by the general formula M
14O
20, where M is Ca, Si, Fe, Al, and Ti. SFCA samples with a high hot strength index are characterized by high iron content (15–22 wt.%) and low silicon content (3–10 wt.%). In sample No. 14 with a low basicity, the Ca
2(Al
0.25Mg
0.75)(Al
0.25Si
1.75)O
7 acermonite phase was detected in the silicate bond.
Figure 14 shows the softening curves of the studied industrial agglomerates with different basicities. With increasing basicity, the temperature softening-melting interval of the agglomerate, which characterizes the cohesion zone, decreased. The softening interval is widest for agglomerates with a basicity of 1.4–1.6 and narrowest for agglomerates with a basicity of 2.5 and higher.
4. Conclusions
During sintering, the magnetite in the concentrate is partially dissolved in the silicate component and fluxed to form a complex silicate SFCA with the general formula M14O20, where M is Ca, Si, Fe, Al, Mg, and Ti. SFCA is a binder for sinter ore phases. The amount of ore binder used depends on the basicity of the sinter. The more CaO in the sinter, the more SFCA phase is formed in the final sinter.
The morphology of the SFCA phase depended on the cooling rate of the sinter. Slow cooling results in the growth of large lamellar and dendritic SFCA phases. Rapid cooling produces fine lamellar or acicular structures.
The main “carrier” for the thermomechanical strength of industrial and laboratory sinters is the SFCA bond. With increasing basicity, the composition of the bond changes from high-silicon and low-iron to high-iron (ferrite) with a small amount of silicon. The change in SFCA from silicate to ferrite with the increasing basicity of the sinter results in an increase in the strength properties of the sinter.
We consider the following agglomerate properties to be optimal for the blast-furnace process: basicity 1.8–2.0 (due to its high thermo-mechanical strength of up to 67%) and silicate binder content of 20–25%.
The softening point and final temperatures decreased with basicity up to 1.6, which may be related to the increase in the amount of β-2CaO-SiO2 and the reduction in porosity, and then began to increase. The softening interval of the investigated sintered particles increased from 70 °C to 140 °C. The increase in basicity has a positive effect on the temperature softening interval of laboratory and industrial agglomerates.
Therefore, it can be concluded that the metallurgical properties of sinter are directly related to the presence of complex silicate SCFA, which should be considered in sintering and blast furnace processes. Future research should seek to establish this relationship at a fundamental level through new experimental studies. It is expected that these results will help sinter producers obtain better products with improved metallurgical properties.
In the development of this topic, the authors plan to further investigate the processes of phase transformation in industrial agglomerates.