Crystal Structures and Magnetic Properties of Diaquatetrapyridinenickel(II) and Diaquatetrapyridinecobalt(II) Complexes
Abstract
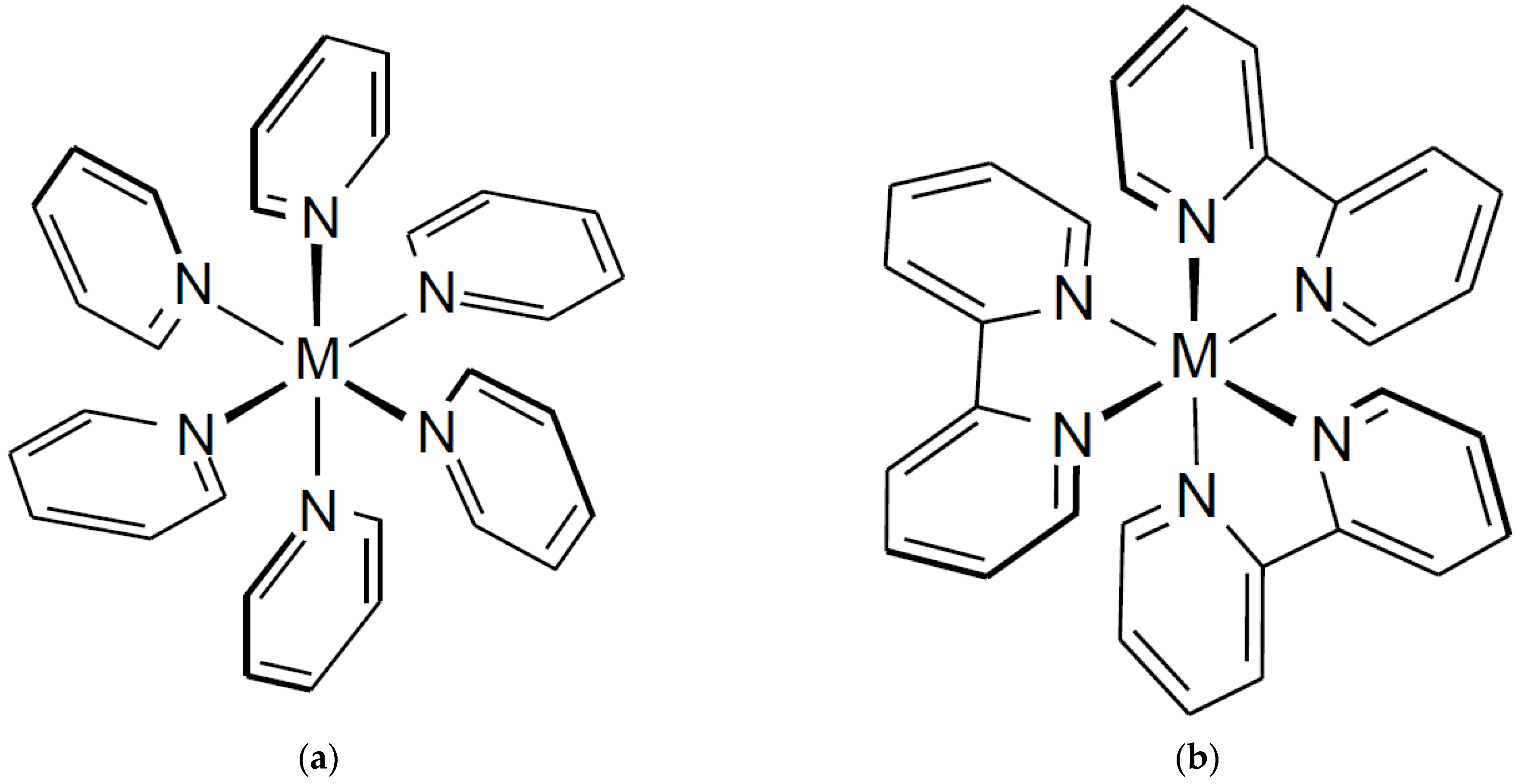
1. Introduction
2. Materials and Methods
2.1. Measurements
2.2. Materials
2.3. Preparations
2.4. Crystallography
2.5. Computation
3. Results and Discussion
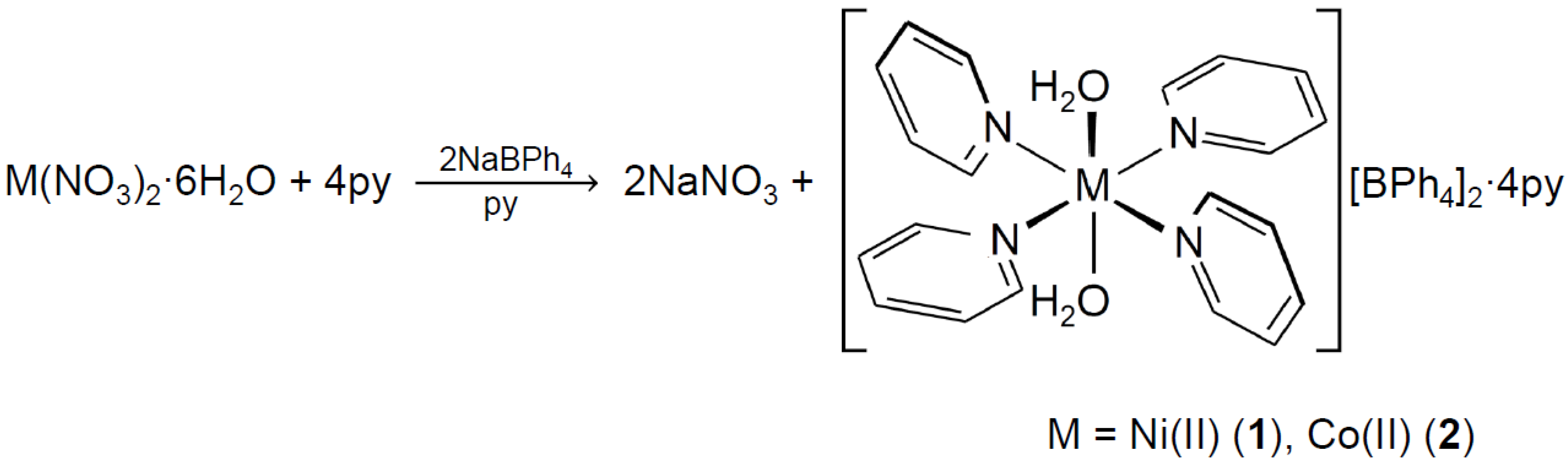
3.1. Preparation of Complexes 1 and 2
3.2. Crystal Structures of Complexes 1 and 2
3.2.1. Crystal Structure of [Ni(H2O)2(py)4][BPh4]2·4py (1)
3.2.2. Crystal Structure of [Co(H2O)2(py)4][BPh4]2·4py (2)
3.3. Magnetic Properties of Complexes 1 and 2
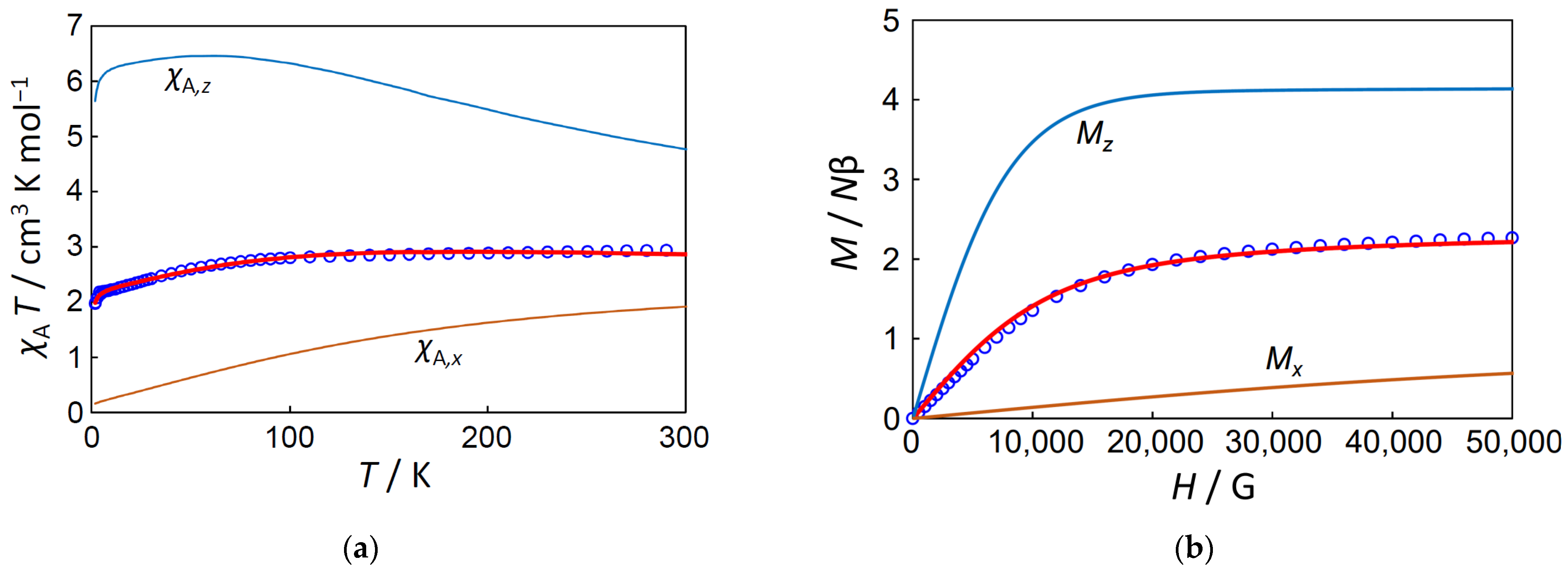
3.3.1. Magnetic Properties of 1
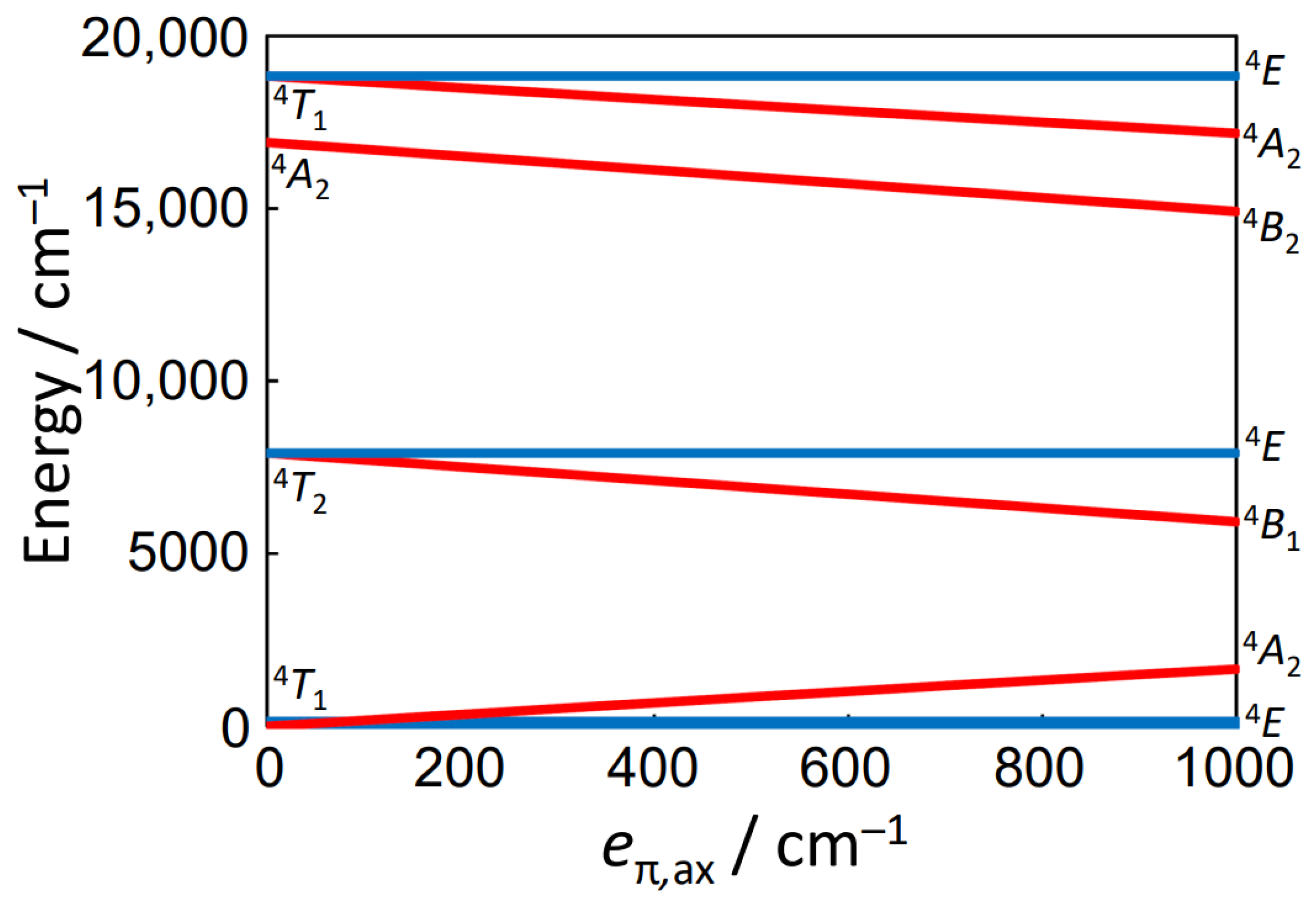
3.3.2. Magnetic Properties of [Co(H2O)2(py)4][BPh4]2·4py (2)
3.4. Structural Consideration for Diaquatetrapyridine Complexes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pal, S. Pyridine: A useful ligand in transition metal complexes. In Pyridine; Pandey, P.P., Ed.; IntechOpen Limited: London, UK, 2018; Chapter 5; pp. 57–74. [Google Scholar]
- Raj, D.; Padhi, S.K. The sporadic l-pyridine bridge in transition metal complexes: A real bond or an interaction? Coord. Chem. Rev. 2022, 450, 214238. [Google Scholar] [CrossRef]
- Pardey, A.J.; Longo, C. Catalysis by rhodium complexes bearing pyridine ligands: Mechanistic aspects. Coord. Chem. Rev. 2010, 254, 254–272. [Google Scholar] [CrossRef]
- Doedens, R.J.; Dahl, L.F. Structure of the hexapyridineiron(II) salt of the tetranuclear iron carbonyl anion, [Fe4(CO)13]–2, with comments concerning the nonisolation of the corresponding neutral tetranuclear iron carbonyl, Fe4(CO)14. J. Am. Chem. Soc. 1966, 88, 4847–4855. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E. The early years of 2,2′-bipyridine—A ligand in its own lifetime. Molecules 2019, 24, 3951. [Google Scholar] [CrossRef] [PubMed]
- Wada, A.; Sakabe, N.; Tanaka, J. The crystal structure of tris(2,2′-bipyridyl)nickel(II) sulphate hydrate, [Ni(C10H8N2)3]SO4.7.5H2O. Acta Cryst. 1976, B32, 1121–1127. [Google Scholar] [CrossRef]
- Stebler, M.; Gutierrez, A.; Ludi, A.; Buergi, H.B. Synthesis and crystal structure of tris(2,2′-bipyridine)rhenium(2+) perrhenate. Inorg. Chem. 1987, 26, 1449–1451. [Google Scholar] [CrossRef]
- Constable, E.C.; Raithby, P.R.; Smit, D.N. The X-ray crystal structure of tris (2,2′-bipyridine)osmium(II) hexafluorophosphate. Polyhedron 1989, 8, 367–369. [Google Scholar] [CrossRef]
- Harrowfield, J.M.; Sobolev, A.N. The crystal structure of tris (2,2′-bipyridine) ruthenium (II) perchlorate. Aust. J. Chem. 1994, 47, 763–767. [Google Scholar] [CrossRef]
- Dick, S. Crystal structure of tris(2,2′-bipyridine)iron(II) bis(hexafluorophosphate), (C10H8N2)3Fe(PF6)2. Z. Krist.-New Cryst. Struct. 1998, 213, 356. [Google Scholar] [CrossRef][Green Version]
- Benabdallah, J.; Setifi, Z.; Setifi, F.; Boughzala, H.; Titi, A. Crystal structure of tris(2,2′-bipyridine)cobalt(II)bis(1,1,3,3-tetracyano-2-ethoxypropenide). Acta Cryst. 2019, E75, 142–145. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sakiyama, H. Development of MagSaki software for magnetic analysis of dinuclear high-spin cobalt(II) complexes in an axially distorted octahedral field. J. Chem. Softw. 2001, 7, 171–178. [Google Scholar] [CrossRef][Green Version]
- Sakiyama, H. Development of MagSaki(A) software for the magnetic analysis of dinuclear high-spin cobalt(II) complexes considering anisotropy in exchange interaction. J. Comput. Chem. Jpn. 2007, 6, 123–134. [Google Scholar] [CrossRef][Green Version]
- Sakiyama, H. Development of MagSaki(Tri) software for the magnetic analysis of trinuclear high-spin cobalt(II) complexes. J. Comput. Chem. Jpn. Int. Ed. 2015, 1, 9–13. [Google Scholar] [CrossRef][Green Version]
- Sakiyama, H. Development of MagSaki(Tetra) software for the magnetic analysis of tetranuclear high-spin cobalt(II) complexes. J. Comput. Chem. Jpn. Int. Ed. 2016, 2, 1–4. [Google Scholar] [CrossRef]
- Bondi, A. van der Waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Sakiyama, H. Theoretical equations of Zeeman energy levels for distorted metal complexes with 3T1 ground terms. Magnetochemistry 2019, 5, 17. [Google Scholar] [CrossRef]
- Sakiyama, H.; Abiko, T.; Yoshida, K.; Shomura, K.; Mitsuhashi, R.; Koyama, Y.; Mikuriya, M.; Koikawa, M.; Mitsumi, M. Detailed magnetic analysis and successful deep-neural-network-based conformational prediction for [VO(dmso)5][BPh4]2. RSC Adv. 2020, 10, 9678. [Google Scholar] [CrossRef]
- Boča, R. Zero-field splitting in metal complexes. Coord. Chem. Rev. 2004, 248, 757–815. [Google Scholar] [CrossRef]
- Kennedy, B.J.; Murray, K.S.; Hitchman, M.A.; Rowbottom, G.L. Metal–ligand bonding parameters and magnetic properties of some previously reported tetragonal nickel(II) complexes. J. Chem. Soc. Dalton Trans. 1987, 4, 825–830. [Google Scholar] [CrossRef]
- Sakiyama, H.; Sudo, R.; Abiko, T.; Yoshioka, D.; Mitsuhashi, R.; Omote, M.; Mikuriya, M.; Yoshitake, M.; Koikawa, M. Magneto-structural correlation of hexakis-dmso cobalt(II) complex. Dalton Trans. 2017, 46, 16306. [Google Scholar] [CrossRef] [PubMed]
- Kahn, O. Molecular Magnetism; VCH Publishers, Inc.: New York, NY, USA, 1993; pp. 38–41. [Google Scholar]
- Sakiyama, H.; Ito, R.; Kumagai, H.; Inoue, K.; Sakamoto, M.; Nishida, Y.; Yamasaki, M. Dinuclear cobalt(II) complexes of an acyclic phenol-based dinucleating ligand with four methoxyethyl chelating arms—First magnetic analysis in an axially distorted octahedral field. Eur. J. Inorg. Chem. 2001, 2001, 2027–2032. [Google Scholar] [CrossRef]
- Mikuriya, M.; Naka, Y.; Inaoka, A.; Okayama, M.; Yoshioka, D.; Sakiyama, H.; Handa, M.; Tsuboi, M. Mixed-Valent Trinuclear CoIII-CoII-CoIII Complex with 1,3-Bis(5-chlorosalicylideneamino)-2-propanol. Molecules 2022, 27, 4211. [Google Scholar] [CrossRef]
- Figgis, B.N.; Gerloch, M.; Lewis, J.; Mabbs, F.E.; Webb, G.A. The magnetic behaviour of cubic-field 4T1g terms in lower symmetry. J. Chem. Soc. A 1968, 2086–2093. [Google Scholar] [CrossRef]
- Figgis, B.N.; Hitchman, M.A. Ligand Field Theory and Its Application; Wiley-VCH: New York, NY, USA, 2000; pp. 70–73. [Google Scholar]
- Lever, A.B.P. Inorganic Electronic Spectroscopy, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Hoshikawa, R.; Waki, K.; Sakiyama, H. Enumeration of conformers for octahedral [M(AB2)6] complexes and conformational prediction for a related metal complex. MATCH Commun. Math. Comput. Chem. 2021, 85, 499–508. [Google Scholar]
- Sakiyama, H.; Waki, K. Enumeration of edge-orienting conformers for octahedral [MX6–n(AB2)n] complexes (n = 1–5). MATCH Commun. Math. Comput. Chem. 2021, 85, 509–522. [Google Scholar]



| Compound | Complex 1 | Complex 2 |
|---|---|---|
| Empirical formula | C88H84B2N8NiO2 | C88H84B2CoN8O2 |
| Formula weight | 1365.96 | 1366.18 |
| Crystal system | orthorhombic | orthorhombic |
| Space group | Pna21 | Pna21 |
| a/Å | 28.4603(10) | 28.5839(14) |
| b/Å | 11.4004(5) | 11.3675(5) |
| c/Å | 22.6229(6) | 22.6811(8) |
| V/Å3 | 7340.2(5) | 7369.7(6) |
| Z | 4 | 4 |
| Crystal dimensions/mm | 0.24 × 0.14 × 0.12 | 0.22 × 0.17 × 0.10 |
| T/K | 100 | 100 |
| λ/Å | 0.71073 | 0.71073 |
| ρcalcd/g cm−3 | 1.236 | 1.231 |
| µ/mm−1 | 0.320 | 0.288 |
| F(000) | 2888 | 2884 |
| 2θmax/◦ | 55 | 55 |
| No. of reflections measured | 15,854 | 14,855 |
| No. of independent reflections | 11,529 (Rint = 0.0651) | 9696 (Rint = 0.1526) |
| Data/restraints/parameters | 15,854/5/926 | 14,855/5/926 |
| R1 (I > 2.00σ(I)) 1 | 0.0467 | 0.0786 |
| wR2 (All reflections) 2 | 0.1012 | 0.1816 |
| Goodness of fit indicator | 1.006 | 0.992 |
| Highest peak, deepest hole/e Å−3 | 0.250, −0.446 | 0.569, −0.873 |
| CCDC deposition number | 2219439 | 2219440 |
| Atom–Atom | Distance/Å | Atom–Atom | Distance/Å |
|---|---|---|---|
| Ni(1)–O(1) | 2.052(3) | Ni(1)–O(2) | 2.028(3) |
| Ni(1)–N(1) | 2.163(3) | Ni(1)–N(2) | 2.157(3) |
| Ni(1)–N(3) | 2.164(3) | Ni(1)–N(4) | 2.157(3) |
| O(1)∙∙∙N(5) | 2.726(4) | O(1)∙∙∙N(6) | 2.763(5) |
| O(2)∙∙∙N(7) | 2.687(4) | O(2)∙∙∙N(8) | 2.676(4) |
| Atom–Atom–Atom | Angle/° | Atom–Atom–Atom | Angle/° |
|---|---|---|---|
| O(1)–Ni(1)–O(2) | 178.58(13) | O(1)–Ni(1)–N(1) | 90.40(12) |
| O(1)–Ni(1)–N(2) | 90.39(13) | O(1)–Ni(1)–N(3) | 87.88(13) |
| O(1)–Ni(1)–N(4) | 90.54(13) | O(2)–Ni(1)–N(1) | 90.99(12) |
| O(2)–Ni(1)–N(2) | 89.94(12) | O(2)–Ni(1)–N(3) | 90.73(12) |
| O(2)–Ni(1)–N(4) | 89.16(12) | N(1)–Ni(1)–N(2) | 89.21(10) |
| N(1)–Ni(1)–N(3) | 178.18(12) | N(1)–Ni(1)–N(4) | 89.35(12) |
| N(2)–Ni(1)–N(3) | 91.37(12) | N(2)–Ni(1)–N(4) | 178.29(13) |
| N(3)–Ni(1)–N(4) | 90.09(10) |
| Atom–Atom | Distance/Å | Atom–Atom | Distance/Å |
|---|---|---|---|
| Co(1)–O(1) | 2.048(4) | Co(1)–O(2) | 2.016(4) |
| Co(1)–N(1) | 2.218(6) | Co(1)–N(2) | 2.213(6) |
| Co(1)–N(3) | 2.221(6) | Co(1)–N(4) | 2.190(6) |
| O(1)∙∙∙N(5) | 2.761(8) | O(1)∙∙∙N(6) | 2.716(8) |
| O(2)∙∙∙N(7) | 2.676(8) | O(2)∙∙∙N(8) | 2.682(8) |
| Atom–Atom–Atom | Angle/° | Atom–Atom–Atom | Angle/° |
|---|---|---|---|
| O(1)–Co(1)–O(2) | 178.2(3) | O(1)–Co(1)–N(1) | 87.9(2) |
| O(1)–Co(1)–N(2) | 90.1(2) | O(1)–Co(1)–N(3) | 90.0(2) |
| O(1)–Co(1)–N(4) | 90.6(2) | O(2)–Co(1)–N(1) | 90.4(2) |
| O(2)–Co(1)–N(2) | 89.6(2) | O(2)–Co(1)–N(3) | 91.7(2) |
| O(2)–Co(1)–N(4) | 89.7(2) | N(1)–Co(1)–N(2) | 91.9(2) |
| N(1)–Co(1)–N(3) | 177.88(19) | N(1)–Co(1)–N(4) | 89.64(18) |
| N(2)–Co(1)–N(3) | 88.64(18) | N(2)–Co(1)–N(4) | 178.4(2) |
| N(3)–Co(1)–N(4) | 89.9(2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakiyama, H.; Yamamoto, Y.; Hoshikawa, R.; Mitsuhashi, R. Crystal Structures and Magnetic Properties of Diaquatetrapyridinenickel(II) and Diaquatetrapyridinecobalt(II) Complexes. Magnetochemistry 2023, 9, 14. https://doi.org/10.3390/magnetochemistry9010014
Sakiyama H, Yamamoto Y, Hoshikawa R, Mitsuhashi R. Crystal Structures and Magnetic Properties of Diaquatetrapyridinenickel(II) and Diaquatetrapyridinecobalt(II) Complexes. Magnetochemistry. 2023; 9(1):14. https://doi.org/10.3390/magnetochemistry9010014
Chicago/Turabian StyleSakiyama, Hiroshi, Yuya Yamamoto, Ryusei Hoshikawa, and Ryoji Mitsuhashi. 2023. "Crystal Structures and Magnetic Properties of Diaquatetrapyridinenickel(II) and Diaquatetrapyridinecobalt(II) Complexes" Magnetochemistry 9, no. 1: 14. https://doi.org/10.3390/magnetochemistry9010014
APA StyleSakiyama, H., Yamamoto, Y., Hoshikawa, R., & Mitsuhashi, R. (2023). Crystal Structures and Magnetic Properties of Diaquatetrapyridinenickel(II) and Diaquatetrapyridinecobalt(II) Complexes. Magnetochemistry, 9(1), 14. https://doi.org/10.3390/magnetochemistry9010014







