Ferromagnetic Coupling and Single-Ion Magnet Phenomenon in Mononuclear Ruthenium(III) Complexes Based on Guanine Nucleobase
Abstract
:1. Introduction
2. Results and Discussion
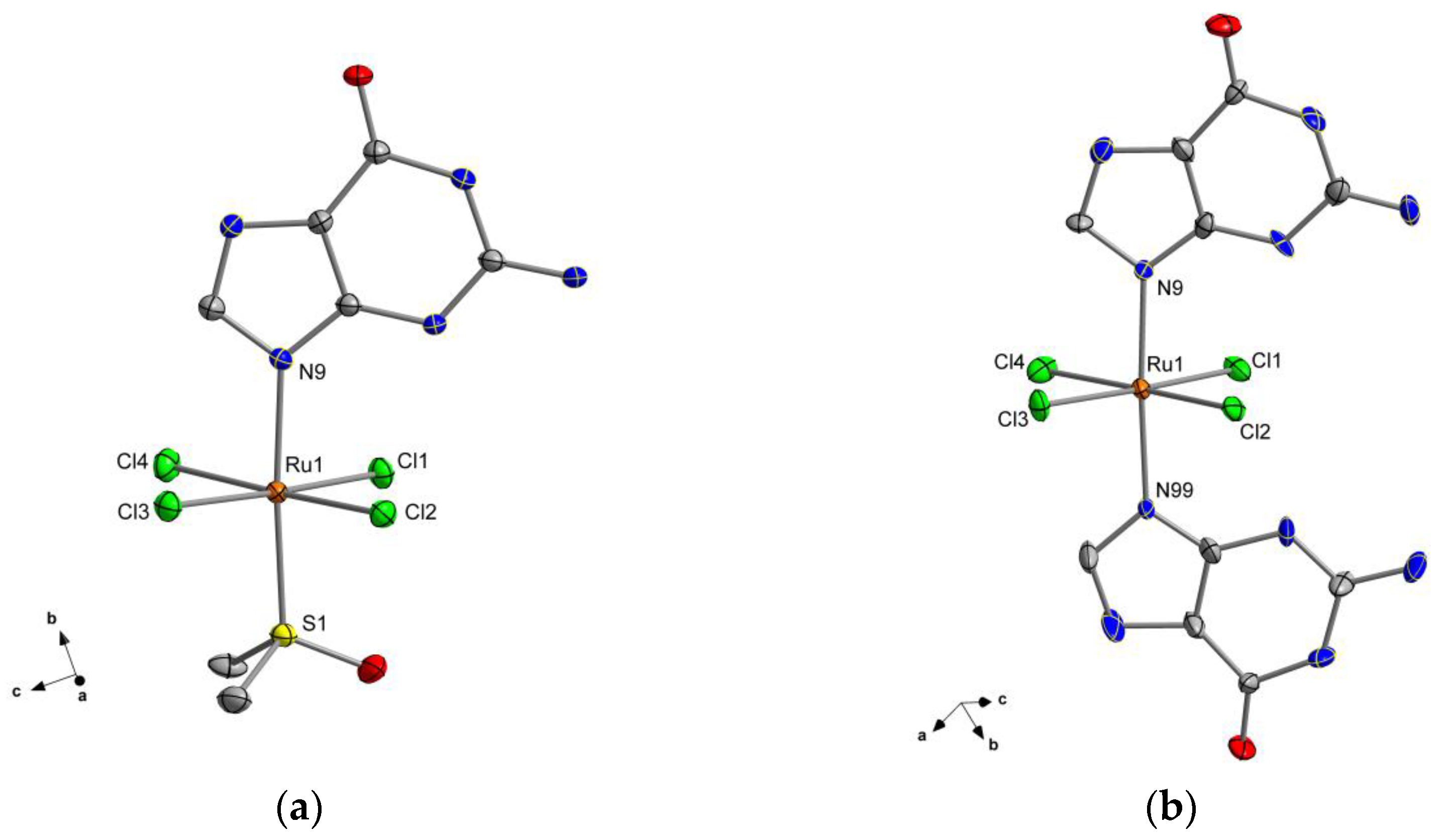
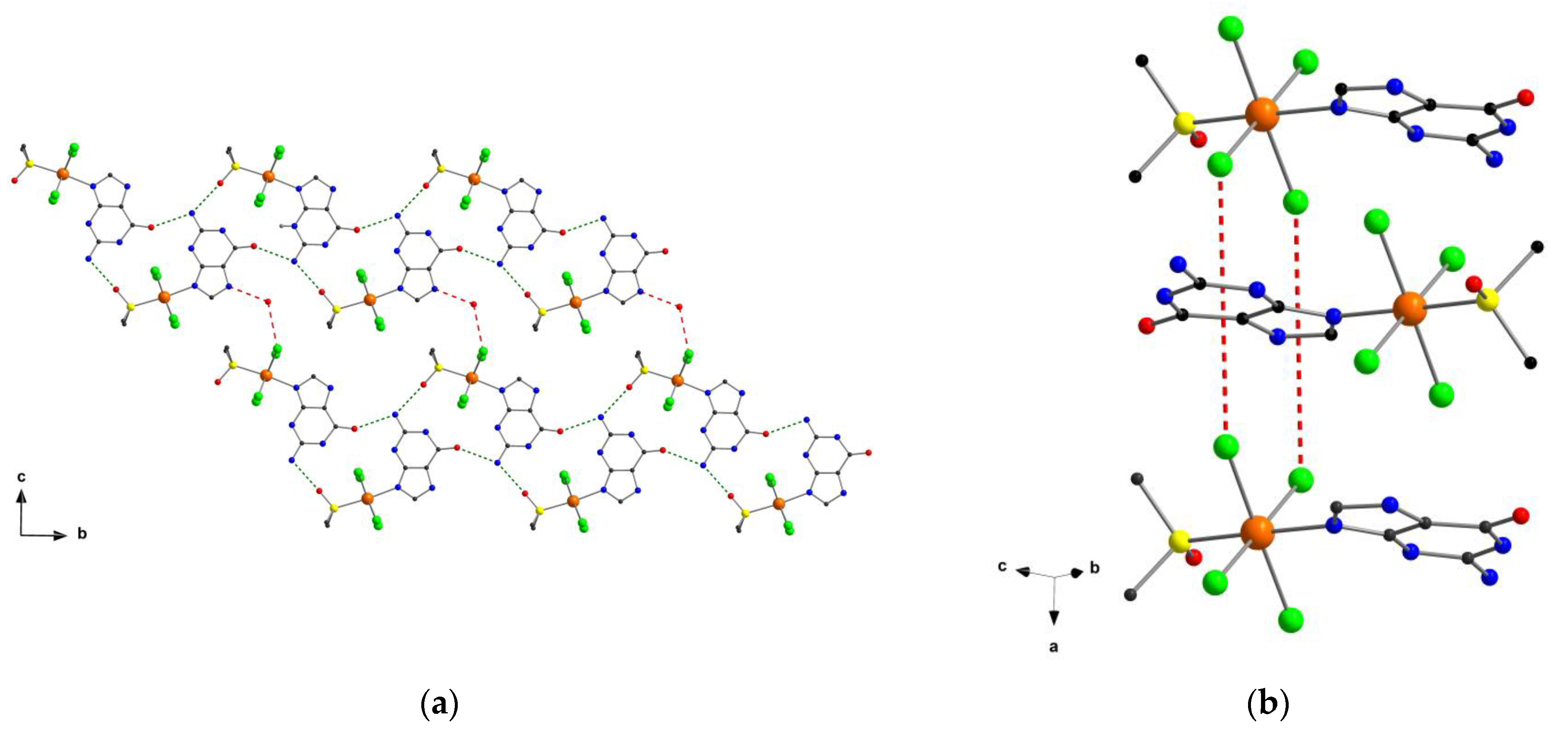
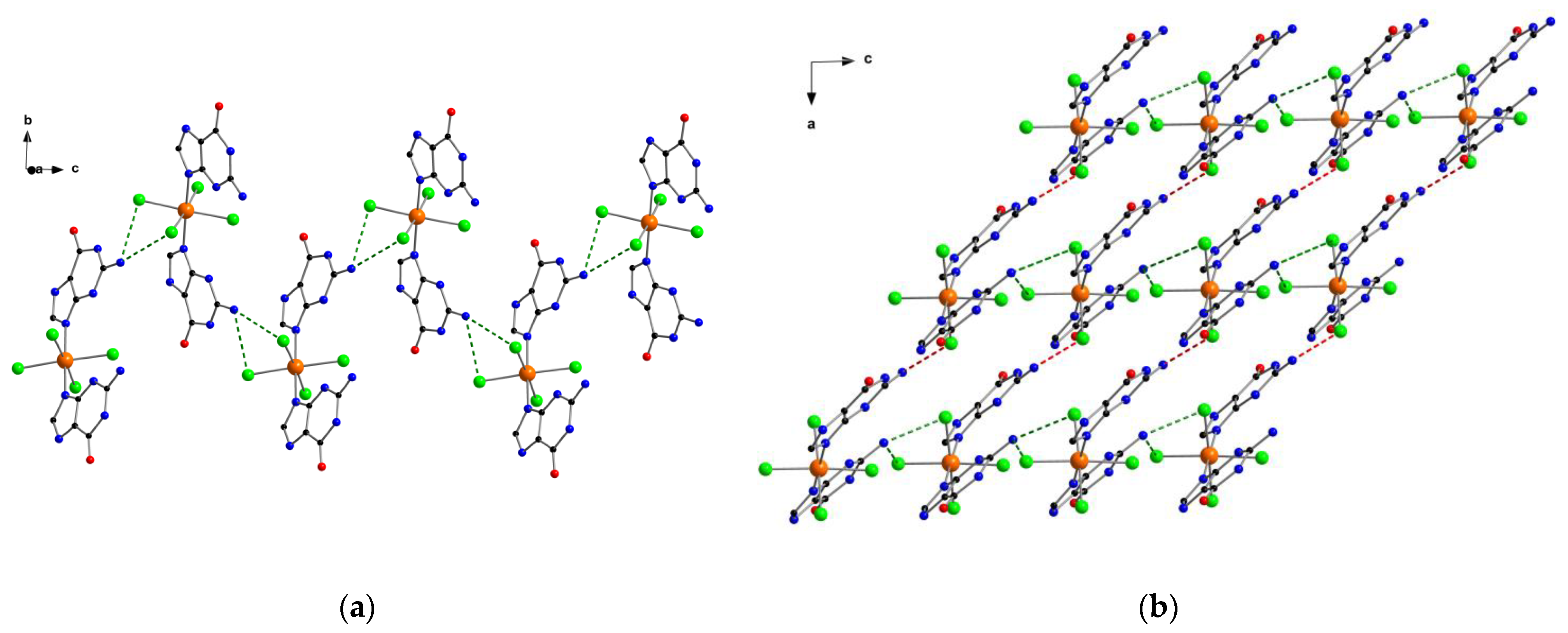
2.1. Description of the Crystal Structures
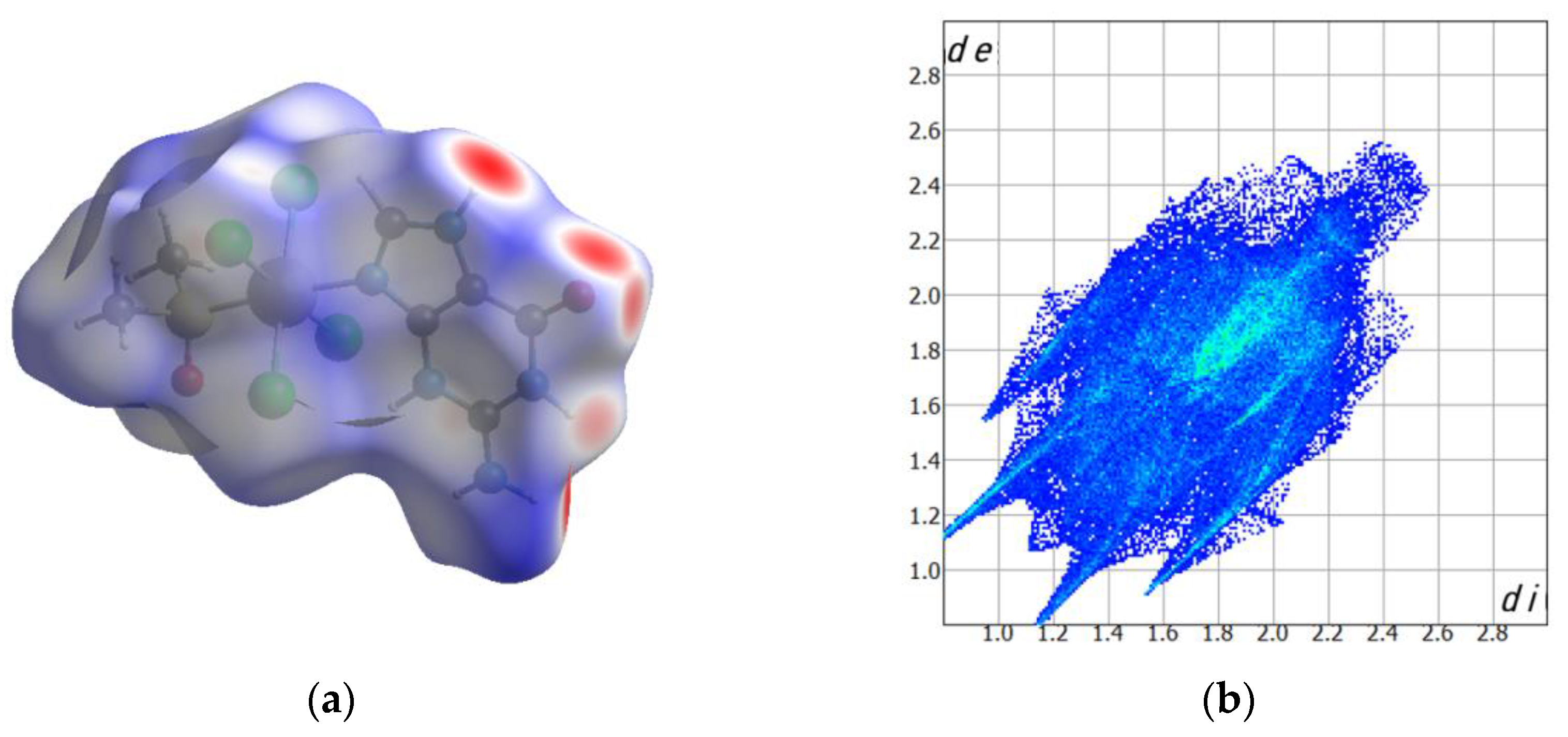
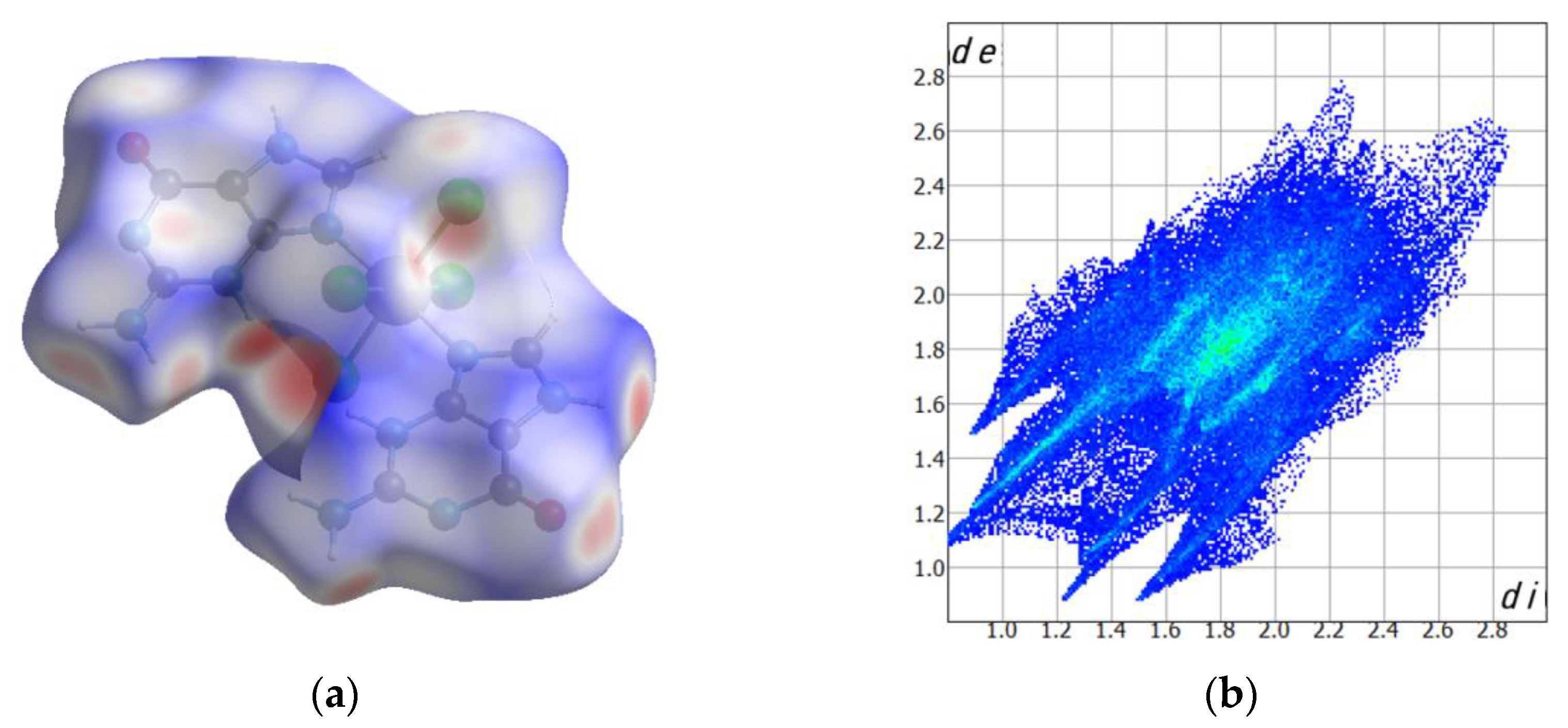
2.2. Computed Hirshfeld Surfaces
2.3. Magnetic Properties
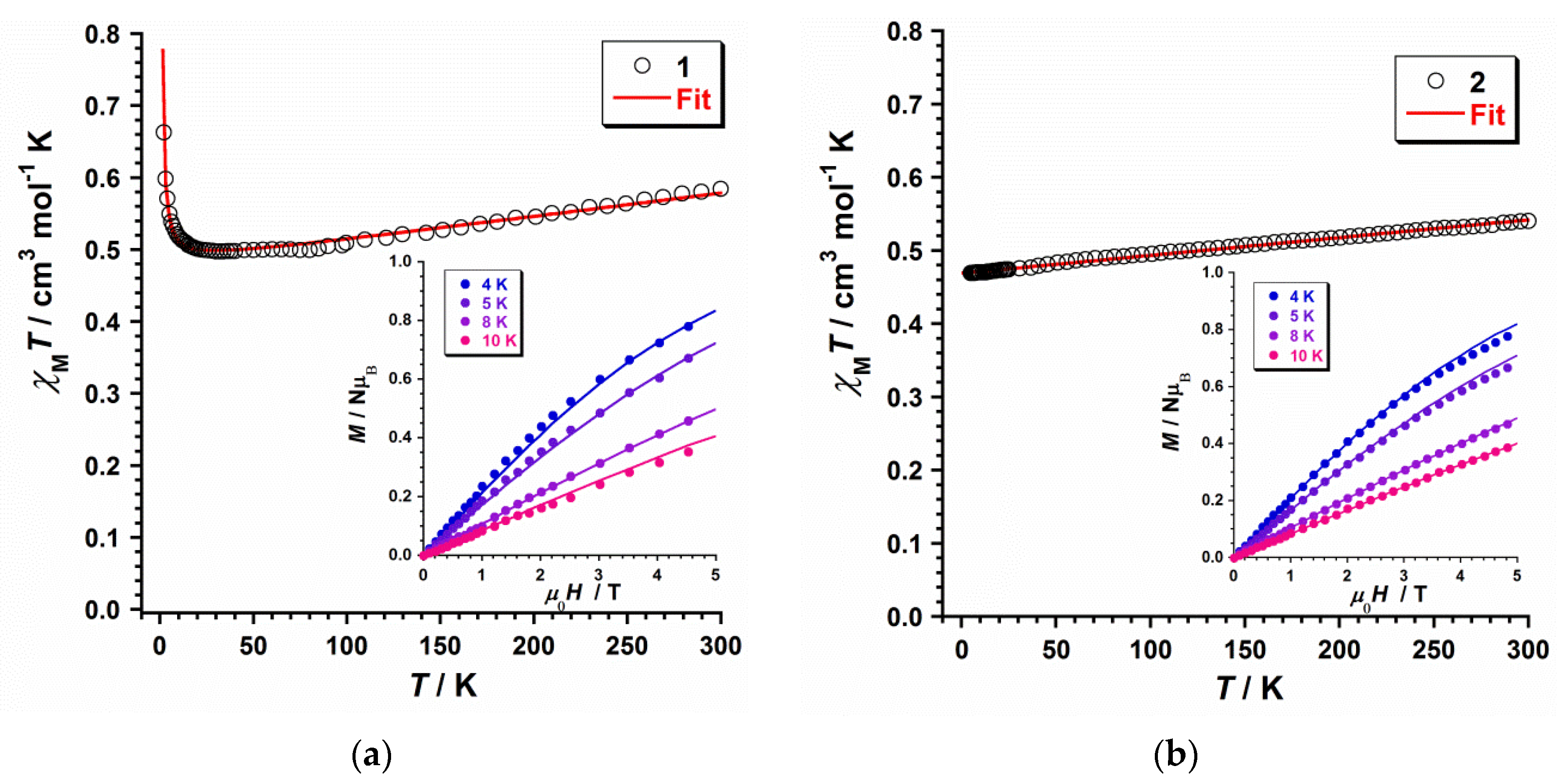
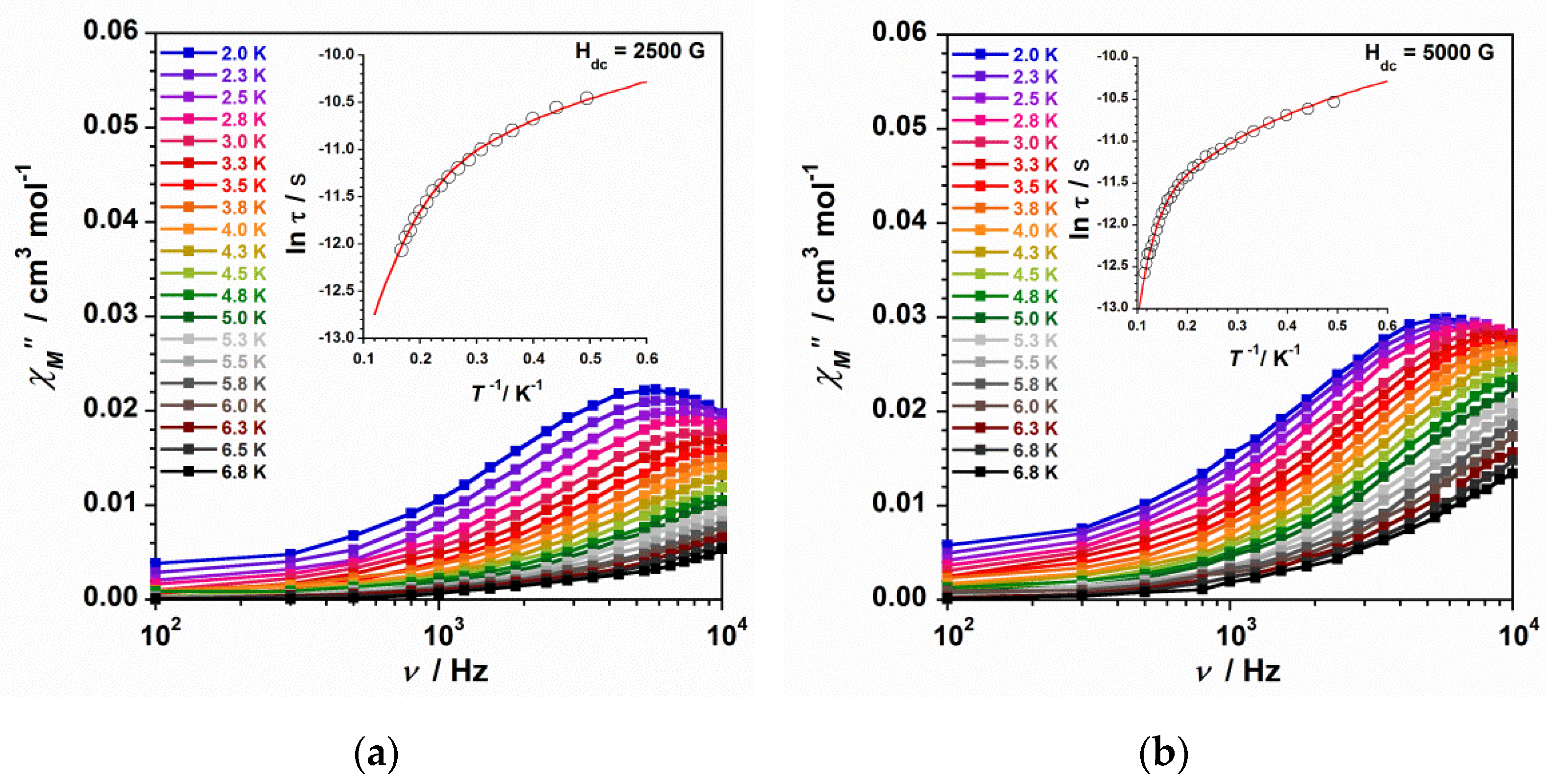
2.3.1. Dc Magnetic Susceptibility
2.3.2. Ac Magnetic Susceptibility
3. Materials and Methods
3.1. Reagents and Instruments
3.2. Preparation of the Compounds
3.2.1. Synthesis of [RuCl4(Hgua)(dmso)]·2H2O (1)
3.2.2. Synthesis of [RuCl4(Hgua)(gua)]·3H2O (2)
3.3. X-ray Data Collection and Structure Refinement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, F.; Collins, J.G.; Keene, F.R. Ruthenium complexes as antimicrobial agents. Chem. Soc. Rev. 2015, 44, 2529–2542. [Google Scholar] [CrossRef]
- Hartinger, C.G.; Zorbas-Seifried, S.; Jakupec, M.A.; Kynast, B.; Zorbas, H.; Keppler, B.K. From bench to bedside-preclinical and early clinical development of the anticancer agent indazolium trans-[tetrachlorobis(1H-indazole)ruthenate(III)] (KP1019 or FFC14A). J. Inorg. Biochem. 2006, 100, 891–904. [Google Scholar] [CrossRef]
- Hartinger, C.G.; Jakupec, M.A.; Zorbas-Seifried, S.; Groessl, M.; Egger, A.; Berger, W.; Zorbas, H.; Dyson, P.J.; Keppler, B.K. KP1019, A New Redox-Active Anticancer Agent—Preclinical Development and Results of a Clinical Phase I Study in Tumor Patients. Chem. Biodivers. 2008, 5, 2140–2155. [Google Scholar] [CrossRef]
- Ang, W.H.; Casini, A.; Sava, G.; Dyson, P.J. Organometallic ruthenium-based antitumor compounds with novel modes of action. J. Organomet. Chem. 2011, 696, 989–998. [Google Scholar] [CrossRef]
- Turel, I.; Pečanac, M.; Golobič, A.; Alessio, E.; Serli, B.; Bergamo, A.; Sava, G. Solution, solid state and biological characterization of ruthenium(III)-DMSO complexes with purine base derivatives. J. Inorg. Biochem. 2004, 98, 393–401. [Google Scholar] [CrossRef]
- Correa, R.S.; Freire, V.; Barbosa, M.I.F.; Bezerra, D.P.; Bomfim, L.M.; Moreira, D.R.M.; Soares, M.B.P.; Ellena, J.; Batista, A.A. Ru(II)–thyminate complexes: New metallodrug candidates against tumor cells. N. J. Chem. 2018, 42, 6794–6802. [Google Scholar] [CrossRef]
- Silva, S.L.R.; Baliza, I.R.S.; Dias, R.B.; Sales, C.B.S.; Rocha, C.A.G.; Soares, M.B.P.; Correa, R.S.; Batista, A.A.; Bezerra, D.P. Ru(II)-thymine complex causes DNA damage and apoptotic cell death in human colon carcinoma HCT116 cells mediated by JNK/p38/ERK1/2 via a p53-independent signaling. Sci. Rep. 2019, 9, 11094. [Google Scholar] [CrossRef] [PubMed]
- Orts-Arroyo, M.; Gutiérrez, F.; Gil-Tebar, A.; Ibarrola-Villava, M.; Jiménez-Martí, E.; Silvestre-Llora, A.; Castro, I.; Ribas, G.; Martínez-Lillo, J. A novel adenine-based diruthenium(III) complex: Synthesis, crystal structure, electrochemical properties and evaluation of the anticancer activity. J. Inorg. Biochem. 2022, 232, 111812. [Google Scholar] [CrossRef]
- Wu, S.-Q.; Miyazaki, Y.; Nakano, M.; Su, S.-Q.; Yao, Z.S.; Kou, H.-Z.; Sato, O. Slow Magnetic Relaxation in a Mononuclear Ruthenium(III) Complex. Chem. Eur. J. 2017, 23, 10028–10033. [Google Scholar] [CrossRef]
- Colacio, E.; Crespo, O.; Cuesta, R.; Kivekas, R.; Laguna, A. [Au2(μ-G)(μ-dmpe)]·(KBr)0.75·2H2O, a cyclic dinuclear gold(I) complex with an N3,N9-bridging coordination mode of guanine and aurophilic interactions: Synthesis, X-ray crystal structure and luminescence properties (dmpe=1,2-bis(dimethylphosphino)ethane and G=guaninato dianion). J. Inorg. Biochem. 2004, 98, 595–600. [Google Scholar]
- Gaballa, A.S.; Schmidt, H.; Wagner, C.; Steinborn, D. Structure and characterization of platinum(II) and platinum(IV) complexes with protonated nucleobase ligands. Inorg. Chim. Acta 2008, 361, 2070–2080. [Google Scholar] [CrossRef]
- Gupta, D.; Nowak, R.; Lippert, B. Pt(II) complexes of unsubstituted guanine and 7-methylguanine. Dalton Trans. 2010, 39, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Orts-Arroyo, M.; Silvestre-Llora, A.; Castro, I.; Martínez-Lillo, J. Molecular Self-Assembly of an Unusual Dinuclear Ruthenium(III) Complex Based on the Nucleobase Guanine. Crystals 2022, 12, 448. [Google Scholar] [CrossRef]
- Martínez-Lillo, J.; Armentano, D.; De Munno, G.; Marino, N.; Lloret, F.; Julve, M.; Faus, J. A self-assembled tetrameric water cluster stabilized by the hexachlororhenate(IV) anion and diprotonated 2,2′-biimidazole: X-ray structure and magnetic properties. Cryst. Eng. Comm. 2008, 10, 1284–1287. [Google Scholar] [CrossRef]
- Martínez-Lillo, J.; Pedersen, A.H.; Faus, J.; Julve, M.; Brechin, E.K. Effect of Protonated Organic Cations and Anion−π Interactions on the Magnetic Behavior of Hexabromorhenate(IV) Salts. Cryst. Growth Des. 2015, 15, 2598–2601. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. Cryst. Eng. Comm. 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer 17; University of Western Australia: Perth, Australia, 2017. [Google Scholar]
- Orts-Arroyo, M.; Castro, I.; Lloret, F.; Martínez-Lillo, J. Molecular Self-Assembly in a Family of Oxo-Bridged Dinuclear Ruthenium(IV) Systems. Cryst. Growth Des. 2020, 20, 2044–2056. [Google Scholar] [CrossRef]
- Sanchis-Perucho, A.; Orts-Arroyo, M.; Camús-Hernández, J.; Rojas-Dotti, C.; Escrivà, E.; Lloret, F.; Martínez-Lillo, J. Hexahalorhenate(IV) salts of protonated ciprofloxacin: Antibiotic-based single-ion magnets. Cryst. Eng. Comm. 2021, 23, 8579–8587. [Google Scholar] [CrossRef]
- Orts-Arroyo, M.; Ten-Esteve, A.; Ginés-Cárdenas, S.; Castro, I.; Martí-Bonmatí, L.; Martínez-Lillo, J. A gadolinium(III) complex based on the thymine nucleobase with properties suitable for magnetic resonance imaging. Int. J. Mol. Sci. 2021, 22, 4586. [Google Scholar] [CrossRef]
- Yeung, W.F.; Man, W.-L.; Wong, W.-T.; Lau, T.-C.; Gao, S. Ferromagnetic Ordering in a Diamond-Like Cyano-Bridged MnIIRuIII Bimetallic Coordination Polymer. Angew. Chem. Int. Ed. 2001, 40, 3031–3033. [Google Scholar] [CrossRef]
- Toma, L.M.; Toma, L.D.; Delgado, F.S.; Ruiz-Pérez, C.; Sletten, J.; Cano, J.; Clemente-Juan, J.M.; Lloret, F.; Julve, M. Trans-dicyanobis(acetylacetonato)ruthenate(III) as a precursor to build novel cyanide-bridged RuIII–MII bimetallic compounds [M = Co and Ni]. Coord. Chem. Rev. 2006, 250, 2176–2193. [Google Scholar] [CrossRef]
- Armentano, D.; Martínez-Lillo, J. Hexachlororhenate(IV) salts of ruthenium(III) cations: X-ray structure and magnetic properties. Inorg. Chim. Acta 2012, 380, 118–124. [Google Scholar] [CrossRef]
- Palacios, M.A.; Mota, A.J.; Ruiz, J.; Hänninen, M.M.; Sillanpää, R.; Colacio, E. Diphenoxo-Bridged NiIILnIII Dinuclear Complexes as Platforms for Heterotrimetallic (LnIIINiII)2RuIII Systems with a High-Magnetic-Moment Ground State: Synthesis, Structure, and Magnetic Properties. Inorg. Chem. 2012, 51, 7010–7012. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, M.; Cuevas, A.; González-Platas, J.; Faccio, R.; Lloret, F.; Julve, M.; Kremer, C. Synthesis, crystal structure and magnetic properties of the Re(II) complexes NBu4[Re(NO)Br4(L)] (L = pyridine and diazine type ligands). Dalton Trans. 2013, 42, 15361–15371. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, M.; Cuevas, A.; González-Platas, J.; Lloret, F.; Julve, M.; Kremer, C. The crystal structure and magnetic properties of 3-pyridinecarboxylate-bridged Re(II)M(II) complexes (M = Cu, Ni, Co and Mn). Dalton Trans. 2015, 44, 11636–11648. [Google Scholar] [CrossRef]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear d- and f-block complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef]
- González, R.; Chiozzone, R.; Kremer, C.; Guerra, F.; De Munno, G.; Lloret, F.; Julve, M.; Faus, J. Magnetic Studies on Hexaiodorhenate(IV) Salts of Univalent Cations. Spin Canting and Magnetic Ordering in K2[ReI6] with Tc = 24 K. Inorg. Chem. 2004, 43, 3013–3019. [Google Scholar] [CrossRef]
- Martínez-Lillo, J.; Armentano, D.; De Munno, G.; Lloret, F.; Julve, M.; Faus, J. A Two-Dimensional ReIVAgI Compound: X-ray Structure and Magnetic Properties. Cryst. Growth Des. 2006, 6, 2204–2206. [Google Scholar] [CrossRef]
- Martínez-Lillo, J.; Armentano, D.; De Munno, G.; Faus, J. Magneto-structural study on a series of rhenium(IV) complexes containing biimH2, pyim and bipy ligands. Polyhedron 2008, 27, 1447–1454. [Google Scholar] [CrossRef]
- Martínez-Lillo, J.; Faus, J.; Lloret, F.; Julve, M. Towards multifunctional magnetic systems through molecular-programmed self assembly of Re(IV) metalloligands. Coord. Chem. Rev. 2015, 289-290, 215–237. [Google Scholar] [CrossRef]
- Martínez-Lillo, J.; Kong, J.; Julve, M.; Brechin, E.K. Self-Assembly of the Hexabromorhenate(IV) Anion with Protonated Benzotriazoles: X-ray Structure and Magnetic Properties. Cryst. Growth Des. 2014, 14, 5985–5990. [Google Scholar] [CrossRef]
- Armentano, D.; Barquero, M.A.; Rojas-Dotti, C.; Moliner, N.; De Munno, G.; Brechin, E.K.; Martínez-Lillo, J. Enhancement of Intermolecular Magnetic Exchange through Halogen···Halogen Interactions in Bisadeninium Rhenium(IV) Salts. Cryst. Growth Des. 2017, 17, 5342–5348. [Google Scholar] [CrossRef]
- Armentano, D.; Sanchis-Perucho, A.; Rojas-Dotti, C.; Martínez-Lillo, J. Halogen⋯halogen interactions in the self-assembly of one-dimensional 2,2′-bipyrimidine-based CuIIReIV systems. Cryst. Eng. Comm. 2018, 20, 4575–4581. [Google Scholar] [CrossRef]
- Rojas-Dotti, C.; Sanchis-Perucho, A.; Orts-Arroyo, M.; Moliner, N.; González, R.; Lloret, F.; Martínez-Lillo, J. Field-Induced Single-Ion Magnet Phenomenon in Hexabromo- and Hexaiodorhenate(IV) Complexes. Magnetochemistry 2020, 6, 20. [Google Scholar] [CrossRef]
- Sanchis-Perucho, A.; Martínez-Lillo, J.J. Ferromagnetic exchange interaction in a new Ir(IV)–Cu(II) chain based on the hexachloroiridate(IV) anion. Dalton Trans. 2019, 48, 13925–13930. [Google Scholar] [CrossRef]
- Sanchis-Perucho, A.; Martínez-Lillo, J.J. A new family of one-dimensional bromo-bridged Ir(IV)–Cu(II) complexes based on the hexabromoiridate(IV) metalloligand. Dalton Trans. 2022, 51, 3323–3330. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
- SHELXTL-2018/1; Bruker Analytical X-ray Instruments. Bruker: Madison, WI, USA, 2018.
- DIAMOND 4.5.0; Crystal Impact GbR. Crystal Impact: Bonn, Germany, 2018.
| Compound | 1 | 2 |
|---|---|---|
| Formula | C7H16N5O4SCl4Ru | C10H11N10O5Cl4Ru |
| Mr/g mol−1 | 509.18 | 594.16 |
| Crystal system | monoclinic | monoclinic |
| Space group | P21/n | Pc |
| a/Å | 9.836(1) | 7.319(1) |
| b/Å | 13.326(1) | 11.433(1) |
| c/Å | 12.886(1) | 11.402(1) |
| α/° | 90(1) | 90(1) |
| β/° | 93.04(1) | 91.64(1) |
| γ/° | 90(1) | 90(1) |
| V/Å3 | 1686.6(1) | 953.7(1) |
| Z | 4 | 2 |
| Dc/g cm−3 | 2.005 | 2.069 |
| μ(Mo-Kα)/mm−1 | 1.708 | 1.433 |
| F(000) | 1012 | 586 |
| Goodness-of-fit on F2 | 1.435 | 1.073 |
| R1 [I > 2σ(I)] | 0.0340 | 0.0591 |
| wR2 [I > 2σ(I)] | 0.0350 | 0.1552 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orts-Arroyo, M.; Moliner, N.; Lloret, F.; Martínez-Lillo, J. Ferromagnetic Coupling and Single-Ion Magnet Phenomenon in Mononuclear Ruthenium(III) Complexes Based on Guanine Nucleobase. Magnetochemistry 2022, 8, 93. https://doi.org/10.3390/magnetochemistry8080093
Orts-Arroyo M, Moliner N, Lloret F, Martínez-Lillo J. Ferromagnetic Coupling and Single-Ion Magnet Phenomenon in Mononuclear Ruthenium(III) Complexes Based on Guanine Nucleobase. Magnetochemistry. 2022; 8(8):93. https://doi.org/10.3390/magnetochemistry8080093
Chicago/Turabian StyleOrts-Arroyo, Marta, Nicolás Moliner, Francesc Lloret, and José Martínez-Lillo. 2022. "Ferromagnetic Coupling and Single-Ion Magnet Phenomenon in Mononuclear Ruthenium(III) Complexes Based on Guanine Nucleobase" Magnetochemistry 8, no. 8: 93. https://doi.org/10.3390/magnetochemistry8080093
APA StyleOrts-Arroyo, M., Moliner, N., Lloret, F., & Martínez-Lillo, J. (2022). Ferromagnetic Coupling and Single-Ion Magnet Phenomenon in Mononuclear Ruthenium(III) Complexes Based on Guanine Nucleobase. Magnetochemistry, 8(8), 93. https://doi.org/10.3390/magnetochemistry8080093