Doxorubicin-Loaded Magnetic Nanoparticles: Enhancement of Doxorubicin’s Effect on Breast Cancer Cells (MCF-7)
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanoparticle Synthesis and Characterization
2.1.1. Materials
2.1.2. Magnetic Nanoparticle Synthesis
2.1.3. Silica-Coated Iron Oxide Nanoparticle Synthesis
2.1.4. X-ray Diffraction
2.1.5. FTIR Spectroscopy
2.1.6. Zeta Potential Determination
2.1.7. Vibrating-Sample Magnetometer (VSM)
2.1.8. Doxorubicin Loading
2.2. Cell Lines
2.3. Cytotoxicity Assay–MTT
2.4. Membrane Integrity Analysis by Flow Cytometry
2.5. Scanning Electron Microscopy (SEM)
2.6. Transmission Electron Microscopy (TEM)
3. Results and Discussions
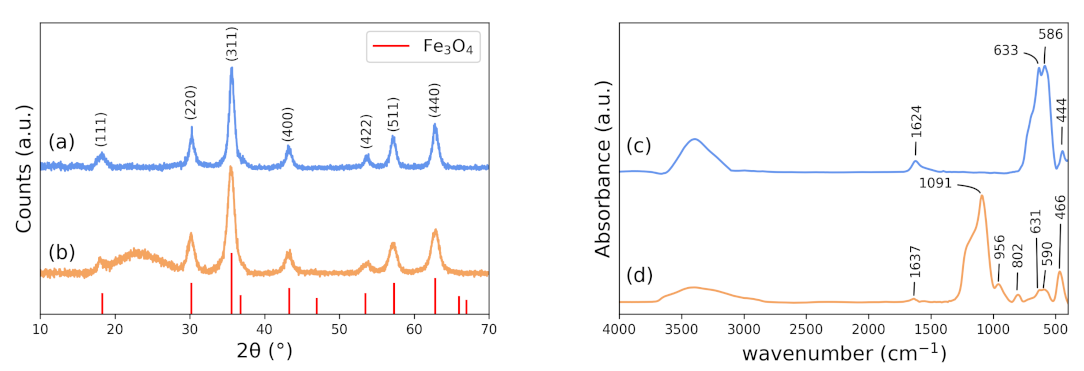
3.1. X-ray Diffraction (XRD)
3.2. FTIR
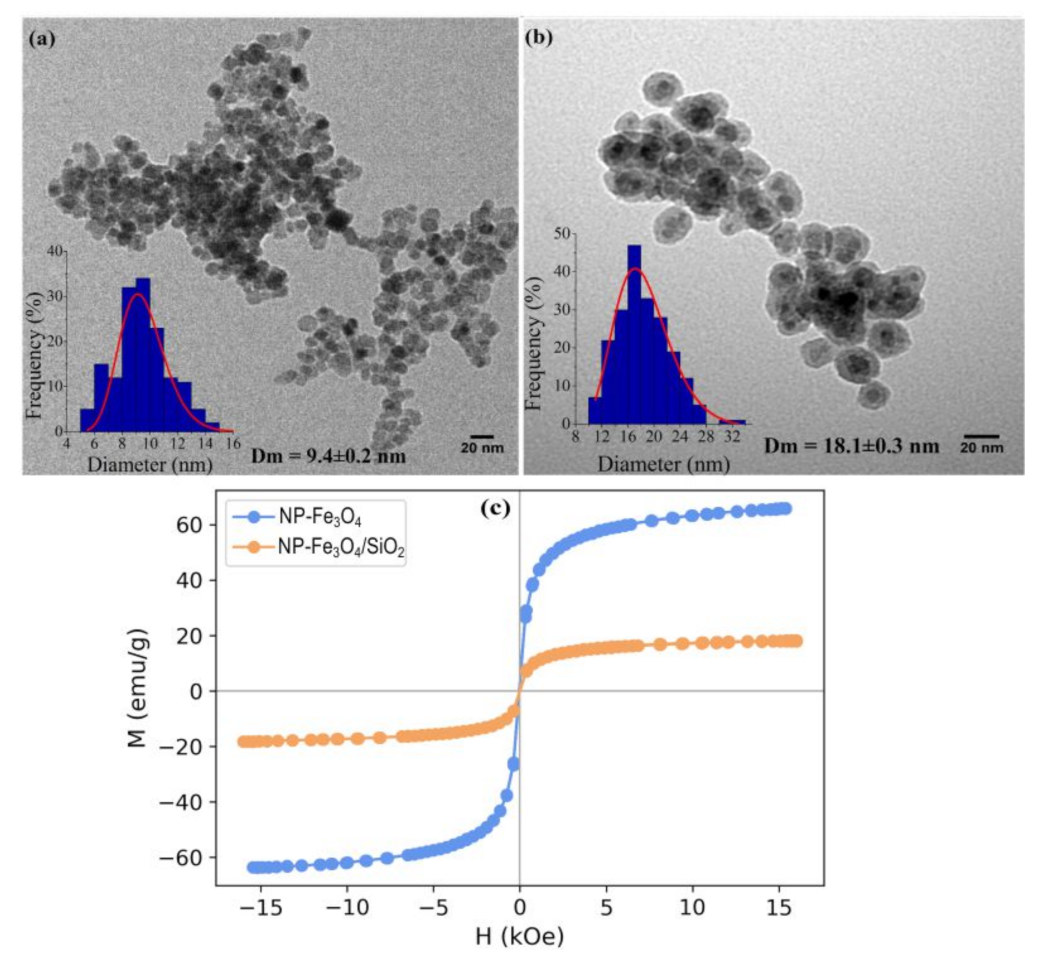
3.3. Transmission Electron Microscopy (TEM)
3.4. Zeta Potential Analysis
3.5. Vibrating Sample Magnometometer
3.6. Drug-Loading Efficiency
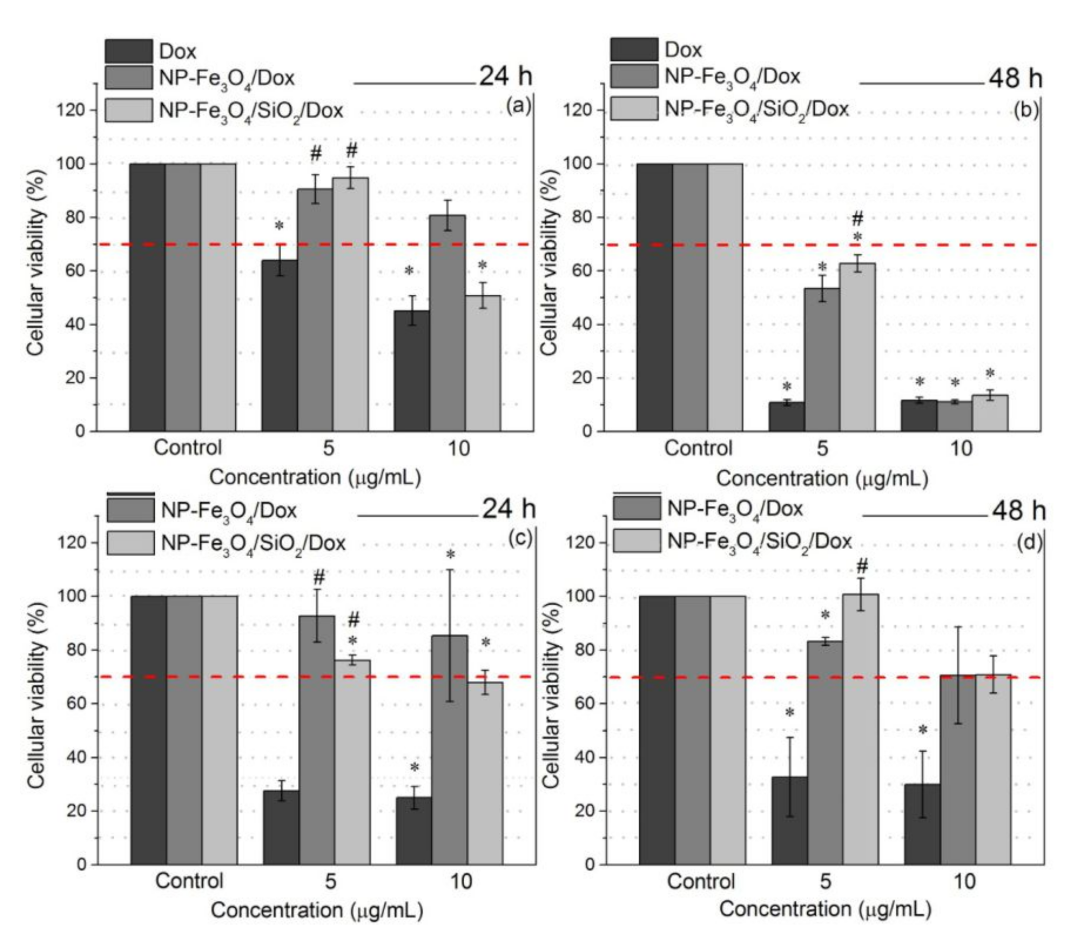
3.7. Cell Viability Assay
3.7.1. Plasma Membrane Integrity
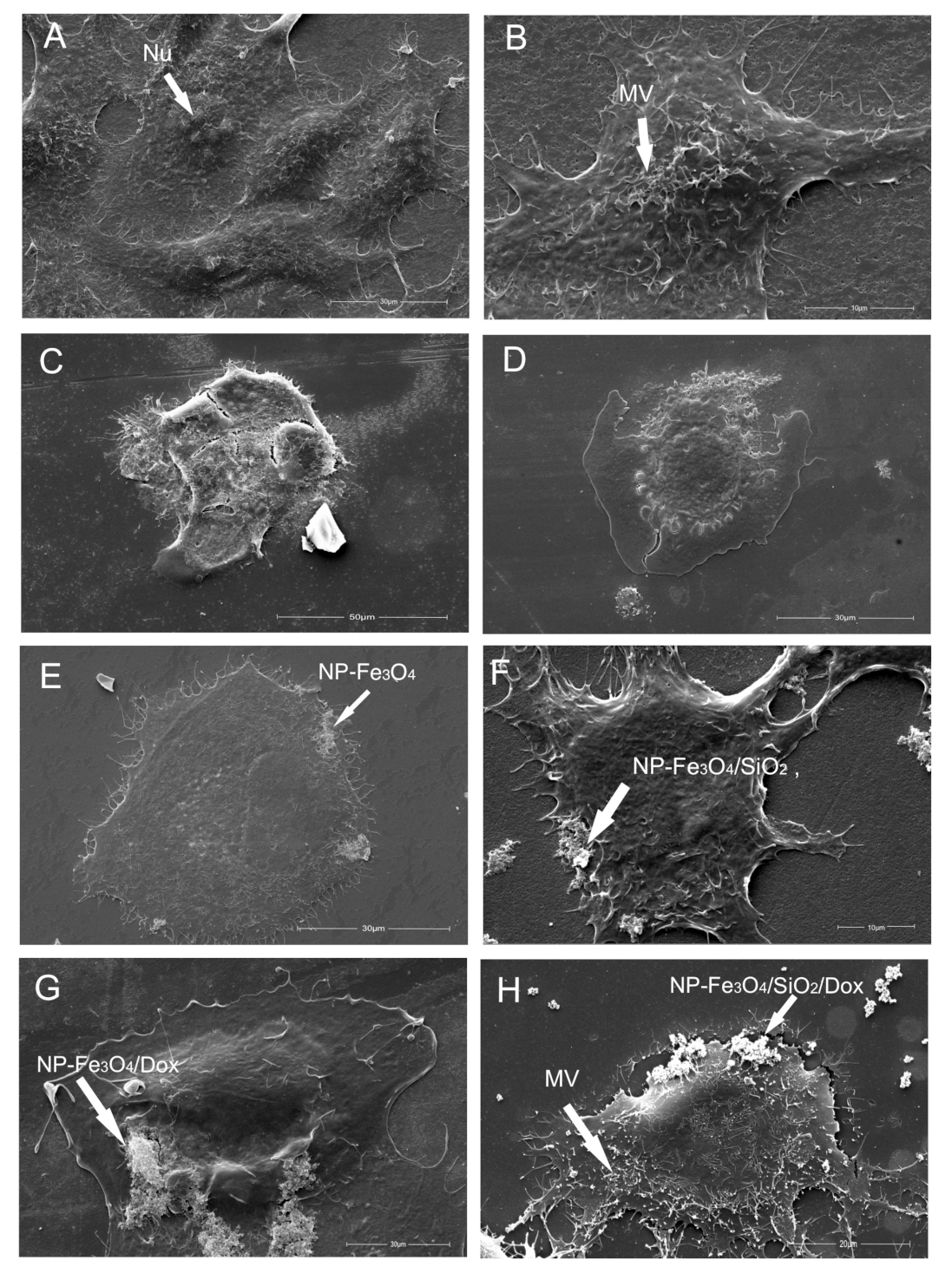
3.7.2. Scanning Electron Microscopy—Cell Morphology
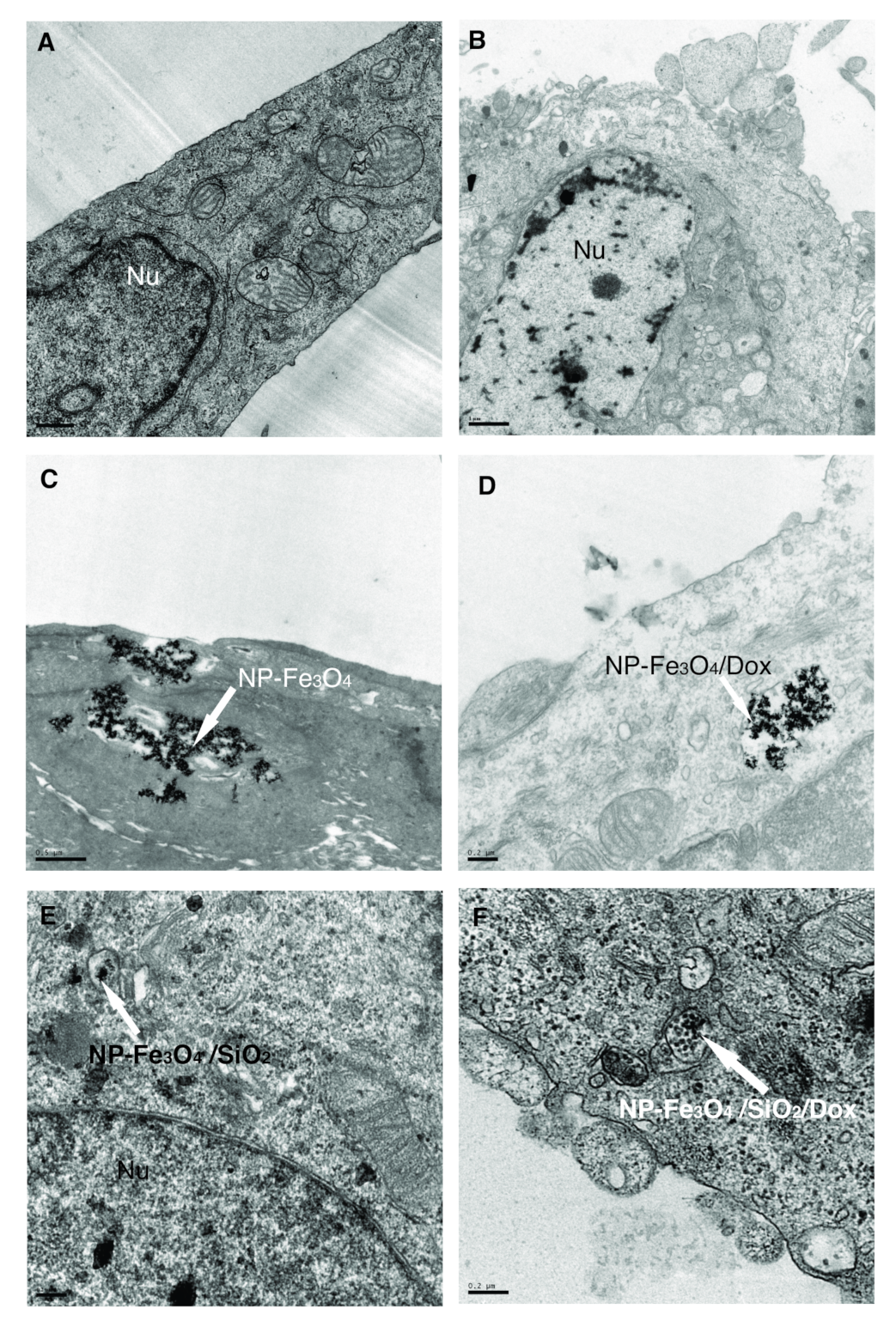
3.7.3. Transmission Electron Microscopy—NP Distribution Inside Cells
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Li, N.; Wang, K.; Deng, Q.; Lei, Z.; Sun, J.; Chen, L. Design, synthesis and biological activity evaluation of novel scopoletin-NO donor derivatives against MCF-7 human breast cancer in vitro and in vivo. Eur. J. Med. Chem. 2021, 224, 113701. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, H.F.; Zarghi, A.; Kobarfard, F.; Zendehdel, R.; Nakhjavani, M.; Arfaiee, S.; Zebardast, T.; Mohebi, S.; Anjidani, N.; Ashtarinezhad, A.; et al. Remarks in Successful Cellular Investigations for Fighting Breast Cancer Using Novel Synthetic Compounds. In Breast Cancer—Focusing Tumor Microenvironment, Stem Cells and Metastasis; Gunduz, M., Gunduz, E., Eds.; InTech: London, UK, 2011. [Google Scholar]
- Comşa, Ş.; Cîmpean, A.M.; Raica, M. The Story of MCF-7 Breast Cancer Cell Line: 40 years of Experience in Research. Anticancer Res. 2015, 35, 3147–3154. [Google Scholar]
- Fernandes Cardoso, V.; Francesko, A.; Ribeiro, C.; Bañobre-López, M.; Martins, P.; Lanceros-Mendez, S. Advances in Magnetic Nanoparticles for Biomedical Applications. Adv. Healthc. Mater. 2017, 7, 1700845. [Google Scholar] [CrossRef]
- Dobosz, B.; Krzyminiewski, R.; Schroeder, G.; Kurczewska, J. Electron paramagnetic resonance as an effective method for a characterization of functionalized iron oxide. J. Phys. Chem. Solids 2014, 75, 594–598. [Google Scholar] [CrossRef]
- Anik, M.I.; Hossain, M.K.; Hossain, I.; Mahfuz, A.M.U.B.; Rahman, M.T.; Ahmed, I. Recent progress of magnetic nanoparticles in biomedical applications: A review. Nano Select 2021, 2, 1146–1186. [Google Scholar] [CrossRef]
- Martins, P.M.; Lima, A.C.; Ribeiro, S.; Lanceros-Mendez, S.; Martins, P. Magnetic Nanoparticles for Biomedical Applications: From the Soul of the Earth to the Deep History of Ourselves. Appl. Bio Mater. 2021, 4, 5839–5870. [Google Scholar] [CrossRef] [PubMed]
- Behrens, S.; Appel, I. Magnetic nanocomposites. Curr. Opin. Biotechnol. 2016, 39, 89–96. [Google Scholar] [CrossRef]
- Zhao, Z.; Huang, D.; Yin, Z.; Chi, X.; Wang, X.; Gao, J. Magnetite nanoparticles as smart carriers to manipulate the cytotoxicity of anticancer drugs: Magnetic control and pH-responsive release. J. Mater. Chem. 2012, 22, 15717. [Google Scholar] [CrossRef]
- Patil-Sen, Y.; Torino, E.; De Sarno, F.; Ponsiglione, A.M.; Chhabria, V.; Ahmed, W.; Mercer, T. Biocompatible superparamagnetic core-shell nanoparticles for potential use in hyperthermia-enabled drug release and as an enhanced contrast agent. Nanotechnology 2020, 31, 375102. [Google Scholar] [CrossRef]
- Vallejo-Fernandez, G.; Whear, O.; Roca, A.G.; Hussain, S.; Timmis, J.; Patel, V.; O’Grady, K. Mechanisms of hyperthermia in magnetic nanoparticles. J. Phys. Appl. Phys. 2013, 46, 312001. [Google Scholar] [CrossRef]
- Thomas, R.; Park, I.K.; Jeong, Y. Magnetic Iron Oxide Nanoparticles for Multimodal Imaging and Therapy of Cancer. Int. J. Mol. Sci. 2013, 14, 15910–15930. [Google Scholar] [CrossRef] [PubMed]
- Popescu, R.C.; Savu, D.; Dorobantu, I.; Vasile, B.S.; Hosser, H.; Boldeiu, A.; Temelie, M.; Straticiuc, M.; Iancu, D.A.; Andronescu, E.; et al. Efficient uptake and retention of iron oxide-based nanoparticles in HeLa cells leads to an effective intracellular delivery of doxorubicin. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Avasthi, A.; Caro, C.; Pozo-Torres, E.; Leal, M.P.; García-Martín, M.L. Magnetic Nanoparticles as MRI Contrast Agents. Top. Curr. Chem. 2020, 378, 40. [Google Scholar]
- Kianfar, E. Magnetic Nanoparticles in Targeted Drug Delivery: A Review. J. Supercond. Nov. Magn. 2021, 34, 1709–1735. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 24–46. [Google Scholar] [CrossRef]
- Wadajkar, A.S.; Bhavsar, Z.; Ko, C.Y.; Koppolu, B.; Cui, W.; Tang, L.; Nguyen, K.T. Multifunctional particles for melanoma-targeted drug delivery. Acta Biomater. 2012, 8, 2996–3004. [Google Scholar] [CrossRef]
- Long, N.V.; Yang, Y.; Teranishi, T.; Thi, C.M.; Cao, Y.; Nogami, M. Biomedical Applications of Advanced Multifunctional Magnetic Nanoparticles. J. Nanosci. Nanotechnol. 2015, 15, 10091–10107. [Google Scholar] [CrossRef]
- Li, G.; Cao, L.; Zhou, Z.; Chen, Z.; Huang, Y.; Zhao, Y. Rapamycin loaded magnetic Fe3O4/carboxymethylchitosan nanoparticles as tumor-targeted drug delivery system: Synthesis and in vitro characterization. Colloids Surf. B 2015, 128, 379–388. [Google Scholar] [CrossRef]
- Natarajan, S.; Harini, K.; Gajula, G.P.; Sarmento, B.; Neves-Petersen, M.T.; Thiagarajan, V. Multifunctional magnetic iron oxide nanoparticles: Diverse synthetic approaches, surface modifications, cytotoxicity towards biomedical and industrial applications. Materials 2019, 1, 1–22. [Google Scholar] [CrossRef]
- Estelrich, J.; Escribano, E.; Queralt, J.; Busquets, M. Iron Oxide Nanoparticles for Magnetically-Guided and Magnetically-Responsive Drug Delivery. Int. J. Mol. Sci. 2015, 16, 8070–8101. [Google Scholar] [CrossRef] [PubMed]
- Majeed, M.I.; Lu, Q.; Yan, W.; Li, Z.; Hussain, I.; Tahir, M.N.; Tremel, W.; Tan, B. Highly water-soluble magnetic iron oxide (Fe3O4) nanoparticles for drug delivery: Enhanced in vitro therapeutic efficacy of doxorubicin and MION conjugates. J. Mater. Chem. B 2013, 1, 2874. [Google Scholar] [CrossRef] [PubMed]
- Poller, J.; Zaloga, J.; Schreiber, E.; Unterweger, H.; Janko, C.; Radon, P.; Eberbeck, D.; Trahms, L.; Alexiou, C.; Friedrich, R. Selection of potential iron oxide nanoparticles for breast cancer treatment based on in vitro cytotoxicity and cellular uptake. Int. J. Nanomed. 2017, 12, 3207–3220. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; Koo, H.; Sun, I.C.; Ryu, J.H.; Kim, K.; Kwon, I.C. Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem. Soc. Rev. 2012, 41, 2656–2672. [Google Scholar] [CrossRef]
- Aşik, E.; Akpinar, Y.; Güray, N.T.; Işcan, M.; Demircigil, G.Ç.; Volkan, M. Cellular uptake, genotoxicity and cytotoxicity of cobalt ferrite magnetic nanoparticles in human breast cells. Toxicol. Res. 2016, 5, 1649–1662. [Google Scholar] [CrossRef] [PubMed]
- Hola, K.; Markova, Z.; Zoppellaro, G.; Tucek, J.; Zboril, R. Tailored functionalization of iron oxide nanoparticles for MRI, drug delivery, magnetic separation and immobilization of biosubstances. Biotechnol. Adv. 2015, 33, 1162–1176. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Du, Y.; Shi, J.; Pong, P.W. Magnetic iron oxide nanoparticle-hollow mesoporous silica Spheres:Fabrication and potential application in drug delivery. Curr. Appl. Phys. 2020, 20, 320–325. [Google Scholar] [CrossRef]
- Basu, P.; De, K.; Das, S.; Mandal, A.K.; Kumar, A.; Jana, T.K.; Chatterjee, K. Silica-Coated Metal Oxide Nanoparticles: Magnetic and Cytotoxicity Studies. ChemistrySelect 2018, 3, 7346–7353. [Google Scholar] [CrossRef]
- Singh, R.K.; Kim, T.H.; Patel, K.D.; Knowles, J.C.; Kim, H.W. Biocompatible magnetite nanoparticles with varying silica-coating layer for use in biomedicine: Physicochemical and magnetic properties, and cellular compatibility. J. Biomed. Mater. Res. Part A 2012, 100A, 1734–1742. [Google Scholar] [CrossRef]
- Janßen, H.C.; Angrisani, N.; Kalies, S.; Hansmann, F.; Kietzmann, M.; Warwas, D.P.; Behrens, P.; Reifenrath, J. Biodistribution, biocompatibility and targeted accumulation of magnetic nanoporous silica nanoparticles as drug carrier in orthopedics. J. Nanobiotechnol. 2020, 18, 14. [Google Scholar] [CrossRef]
- Siminzar, P.; Omidi, Y.; Golchin, A.; Aghanejad, A.; Barar, J. Targeted delivery of doxorubicin by magnetic mesoporous silica nanoparticles armed with mucin-1 aptamer. J. Drug Target. 2019, 28, 92–101. [Google Scholar] [CrossRef]
- Rubia, G.G.; Peigneux, A.; Jabalera, Y.; Puerma, J.; Oltolina, F.; Elert, K.; Colangelo, D.; Morales, J.G.; Prat, M.; Jimenez-Lopez, C. pH-Dependent Adsorption Release of Doxorubicin on MamC-Biomimetic Magnetite Nanoparticles. Langmuir 2018, 34, 13713–13724. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Shen, S.; Wu, L.; Qi, X.; Ni, H.; Ge, Y. Doxorubicin-loaded Fe3O4@SiO2 Nanoparticles as Magnetic Targeting Agents for Combined Photothermal-chemotherapy of Cancer. Chem. Lett. 2015, 44, 858–860. [Google Scholar] [CrossRef]
- Eslami, P.; Albino, M.; Scavone, F.; Chiellini, F.; Morelli, A.; Baldi, G.; Cappiello, L.; Doumett, S.; Lorenzi, G.; Ravagli, C.; et al. Smart Magnetic Nanocarriers for Multi-Stimuli On-Demand Drug Delivery. Nanomaterials 2022, 12, 303. [Google Scholar] [CrossRef]
- Cagel, M.; Grotz, E.; Bernabeu, E.; Moretton, M.A.; Chiappetta, D.A. Doxorubicin: Nanotechnological overviews from bench to bedside. Drug Discov. Today 2017, 22, 270–281. [Google Scholar] [CrossRef]
- Sritharan, S.; Sivalingam, N. A comprehensive review on time-tested anticancer drug doxorubicin. Life Sci. 2021, 278, 119527. [Google Scholar] [CrossRef] [PubMed]
- Kankala, R.K.; Liu, C.G.; Chen, A.Z.; Wang, S.B.; Xu, P.Y.; Mende, L.K.; Liu, C.L.; Lee, C.H.; Hu, Y.F. Overcoming Multidrug Resistance through the Synergistic Effects of Hierarchical pH-Sensitive, ROS-Generating Nanoreactors. Biomater. Sci. Eng. 2017, 3, 2431–2442. [Google Scholar] [CrossRef]
- Kankala, R.K.; Liu, C.G.; Yang, D.Y.; Wang, S.B.; Chen, A.Z. Ultrasmall platinum nanoparticles enable deep tumor penetration and synergistic therapeutic abilities through free radical species-assisted catalysis to combat cancer multidrug resistance. Chem. Eng. J. 2020, 383, 123138. [Google Scholar] [CrossRef]
- Norouzi, M.; Yathindranath, V.; Thliveris, J.A.; Kopec, B.M.; Siahaan, T.J.; Miller, D.W. Doxorubicin-loaded iron oxide nanoparticles for glioblastoma therapy: A combinational approach for enhanced delivery of nanoparticles. Sci. Rep. 2020, 10, 11292. [Google Scholar] [CrossRef]
- Kovrigina, E.; Chubarov, A.; Dmitrienko, E. High Drug Capacity Doxorubicin-Loaded Iron Oxide Nanocomposites for Cancer Therapy. Magnetochemistry 2022, 8, 54. [Google Scholar] [CrossRef]
- Lungu, I.I.; Nistorescu, S.; Badea, M.A.; Petre, A.M.; Udrea, A.M.; Banici, A.M.; Fleacă, C.; Andronescu, E.; Dinischiotu, A.; Dumitrache, F.; et al. Doxorubicin-Conjugated Iron Oxide Nanoparticles Synthesized by Laser Pyrolysis: In Vitro Study on Human Breast Cancer Cells. Polymers 2020, 12, 2799. [Google Scholar] [CrossRef]
- Nieciecka, D.; Celej, J.; Żuk, M.; Majkowska-Pilip, A.; Żelechowska Matysiak, K.; Lis, A.; Osial, M. Hybrid System for Local Drug Delivery and Magnetic Hyperthermia Based on SPIONs Loaded with Doxorubicin and Epirubicin. Pharmaceutics 2021, 13, 480. [Google Scholar] [CrossRef]
- Araújo-Neto, R.P.; Silva-Freitas, E.L.; Carvalho, J.F.; Pontes, T.R.F.; Silva, K.L.; Damasceno, I.H.M.; Egito, E.S.T.; Dantas, A.L.; Morales, M.A.; Carriço, A.S. Monodisperse sodium oleate coated magnetite high susceptibility nanoparticles for hyperthermia applications. J. Magn. Magn. Mater. 2014, 364, 72–79. [Google Scholar] [CrossRef]
- Chang, E.P.; Hatton, T.A. Membrane Emulsification and Solvent Pervaporation Processes for the Continuous Synthesis of Functional Magnetic and Janus Nanobeads. Langmuir 2012, 28, 9748–9758. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.G.S.; Beck, W.; Varanda, L.C. Multifunctional luminomagnetic FePt@Fe3O4/SiO2/Rhodamine B/SiO2 nanoparticles with high magnetic emanation for biomedical applications. J. Nanoparticle Res. 2013, 15, 1545. [Google Scholar] [CrossRef]
- Cótica, L.F.; Estrada, F.R.; Freitas, V.F.; Dias, G.S.; Santos, I.A.; Eiras, J.A.; Garcia, D. Ferroic states in La doped BiFeO3-PbTiO3 multiferroic compounds. J. Appl. Phys. 2012, 111, 114105. [Google Scholar] [CrossRef]
- Kayal, S.; Ramanujan, R.V. Doxorubicin loaded PVA coated iron oxide nanoparticles for targeted drug delivery. Mater. Sci. Eng. C 2010, 30, 484–490. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- ImageJ. Available online: https://imagej.net/software/imagej/ (accessed on 31 January 2022).
- Bini, R.A.; Marques, R.F.C.; Santos, F.J.; Chaker, J.A.; Jafelicci, M. Synthesis and functionalization of magnetite nanoparticles with different amino-functional alkoxysilanes. J. Magn. Magn. Mater. 2012, 324, 534–539. [Google Scholar] [CrossRef]
- Ding, H.L.; Zhang, Y.X.; Wang, S.; Xu, J.M.; Xu, S.C.; Li, G.H. Fe3O4@SiO2 Core/Shell Nanoparticles: The Silica Coating Regulations with a Single Core for Different Core Sizes and Shell Thicknesses. Chem. Mater. 2012, 24, 4572–4580. [Google Scholar] [CrossRef]
- Langford, J.I.; Wilson, A.J.C. Scherrer after sixty years: A survey and some new results in the determination of crystallite size. J. Appl. Crystallogr. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Bui, T.Q.; Ngo, H.T.M.; Tran, H.T. Surface-protective assistance of ultrasound in synthesis of superparamagnetic magnetite nanoparticles and in preparation of mono-core magnetite-silica nanocomposites. J. Sci. Adv. Mater. Devices 2018, 3, 323–330. [Google Scholar] [CrossRef]
- Li, C.; Ma, C.; Wang, F.; Xi, Z.; Wang, Z.; Deng, Y.; He, N. Preparation and Biomedical Applications of Core—Silica/Magnetic Nanoparticle Composites. J. Nanosci. Nanotechnol. 2012, 12, 2964–2972. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Khan, S.A.; Yamani, Z.H.; Qamar, M.T.; Morsy, M.A.; Sarfraz, S. Shape- and size-controlled superparamagnetic iron oxide nanoparticles using various reducing agents and their relaxometric properties by Xigo acorn area. Appl. Nanosci. 2018, 9, 479–489. [Google Scholar] [CrossRef]
- Sun, Y.; Duan, L.; Guo, Z.; DuanMu, Y.; Ma, M.; Xu, L.; Zhang, Y.; Gu, N. An improved way to prepare superparamagnetic magnetite-silica core-shell nanoparticles for possible biological application. J. Magn. Magn. Mater. 2005, 285, 65–70. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurences and Uses, 2nd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003. [Google Scholar]
- Meng, C.; Zhikun, W.; Qiang, L.; Chunling, L.; Shuangqing, S.; Songqing, H. Preparation of amino-functionalized Fe3O4@mSiO2 core-shell magnetic nanoparticles and their application for aqueous Fe3+ removal. J. Hazard. Mater. 2018, 341, 198–206. [Google Scholar] [CrossRef]
- Oliveira, P.N.; Bini, R.D.; Dias, G.S.; Alcouffe, P.; Santos, I.A.; David, L.; Cótica, L.F. Magnetite nanoparticles with controlled sizes via thermal degradation of optimized PVA/Fe(III) complexes. J. Magn. Magn. Mater. 2018, 460, 381–390. [Google Scholar] [CrossRef]
- Kimoto, S.; Dick, W.D.; Hunt, B.; Szymanski, W.W.; McMurry, P.H.; Roberts, D.L.; Pui, D.Y.H. Characterization of nanosized silica size standards. Aerosol Sci. Technol. 2017, 51, 936–945. [Google Scholar] [CrossRef]
- Green, D.L.; Lin, J.S.; Lam, Y.F.; Hu, M.Z.C.; Schaefer, D.W.; Harris, M.T. Size, volume fraction, and nucleation of Stober silica nanoparticles. J. Colloid Interface Sci. 2003, 266, 346–358. [Google Scholar] [CrossRef]
- Paufler, P. Landolt-Börnstein, New Series, K.H. Hellwege (Ed.). Group III: Crystal and Solid State Physics, Vol. 12: Magnetic and Other Properties of Oxides and Related Compounds, Part c: Hexagonal Ferrites. Special Lanthanide and Actinide Compounds. Springer-Verlag Berlin, Heidelberg, New York: 1982, XI + 604 Seiten, 1034 Figuren Leinen DM 980.–, etwa. US $392.00. Cryst. Res. Technol. 1983, 18, 1546. [Google Scholar]
- Saepudin, E.; Fadhilah, H.R.; Khalil, M. The influence of carboxylate moieties for efficient loading and pH-controlled release of doxorubicin in Fe3O4 magnetic nanoparticles. Colloids Surfaces Physicochem. Eng. Asp. 2020, 602, 125137. [Google Scholar] [CrossRef]
- Rana, S.; Shetake, N.G.; Barick, K.C.; Pandey, B.N.; Salunke, H.G.; Hassan, P.A. Folic acid conjugated Fe3O4 magnetic nanoparticles for targeted delivery of doxorubicin. Dalton Trans. 2016, 45, 17401–17408. [Google Scholar] [CrossRef]
- Wolf, F.A.D.; Nicolay, K.; Kruijff, B.D. Effect of doxorubicin on the order of the acyl chains of anionic and zwitterionic phospholipids in liquid-crystalline mixed model membranes: Absence of drug-induced segregation of lipids into extended domains. Biochemistry 1992, 31, 9252–9262. [Google Scholar] [CrossRef] [PubMed]
- Nigam, S.; Barick, K.C.; Bahadur, D. Development of citrate-stabilized Fe3O4 nanoparticles: Conjugation and release of doxorubicin for therapeutic applications. J. Magn. Magn. Mater. 2011, 323, 237–243. [Google Scholar] [CrossRef]
- Roik, N.V.; Belyakova, L.; Dziazko, M.O. Kinetic and equilibrium studies of doxorubicin adsorption on MCM-41-type silica surface. Him. Fiz. Tehnol. Poverhni 2016, 7, 405–412. [Google Scholar] [CrossRef]
- Helal-Neto, E.; da Silva de Barros, A.O.; Saldanha-Gama, R.; Brandão-Costa, R.; Alencar, L.M.R.; dos Santos, C.C.; Martínez-Máñez, R.; Ricci-Junior, E.; Alexis, F.; Morandi, V.; et al. Molecular and Cellular Risk Assessment of Healthy Human Cells and Cancer Human Cells Exposed to Nanoparticles. Int. J. Mol. Sci. 2019, 21, 230. [Google Scholar] [CrossRef]
- Racles, C.; Zaltariov, M.F.; Peptanariu, D.; Vasiliu, T.; Cazacu, M. Functionalized Mesoporous Silica as Doxorubicin Carriers and Cytotoxicity Boosters. Nanomaterials 2022, 12, 1823. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; He, Q.; Gao, Y.; Shi, J.; Li, Y. Mesoporous silica nanoparticles loading doxorubicin reverse multidrug resistance: Performance and mechanism. Nanoscale 2011, 3, 4314. [Google Scholar] [CrossRef] [PubMed]
- Sadighian, S.; Rostamizadeh, K.; Hosseini-Monfared, H.; Hamidi, M. Doxorubicin-conjugated core– magnetite nanoparticles as dual-targeting carriers for anticancer drug delivery. Colloids Surf. B 2014, 117, 406–413. [Google Scholar] [CrossRef]
- Mu, Q.; Lin, G.; Jeon, M.; Wang, H.; Chang, F.C.; Revia, R.A.; Yu, J.; Zhang, M. Iron oxide nanoparticle targeted chemo-immunotherapy for triple negative breast cancer. Mater. Today 2021, 50, 149–169. [Google Scholar] [CrossRef]
- El-Boubbou, K.; Ali, R.; Al-Zahrani, H.; Trivilegio, T.; Alanazi, A.H.; Khan, A.L.; Boudjelal, M.; AlKushi, A. Preparation of iron oxide mesoporous magnetic microparticles as novel multidrug carriers for synergistic anticancer therapy and deep tumor penetration. Sci. Rep. 2019, 9, 9481. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Jenkins, G.J.; Asadi, R.; Doak, S.H. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 2010, 1, 5358. [Google Scholar] [CrossRef] [PubMed]
- Malvindi, M.A.; De Matteis, V.; Galeone, A.; Brunetti, V.; Anyfantis, G.C.; Athanassiou, A.; Cingolani, R.; Pompa, P.P. Toxicity Assessment of Silica Coated Iron Oxide Nanoparticles and Biocompatibility Improvement by Surface Engineering. PLoS ONE 2014, 9, e85835. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandes, E.P.; Bini, R.D.; Endo, K.M.; de Oliveira Junior, V.A.; de Almeida, I.V.; Dias, G.S.; dos Santos, I.A.; de Oliveira, P.N.; Vicentini, V.E.P.; Cotica, L.F. Doxorubicin-Loaded Magnetic Nanoparticles: Enhancement of Doxorubicin’s Effect on Breast Cancer Cells (MCF-7). Magnetochemistry 2022, 8, 114. https://doi.org/10.3390/magnetochemistry8100114
Hernandes EP, Bini RD, Endo KM, de Oliveira Junior VA, de Almeida IV, Dias GS, dos Santos IA, de Oliveira PN, Vicentini VEP, Cotica LF. Doxorubicin-Loaded Magnetic Nanoparticles: Enhancement of Doxorubicin’s Effect on Breast Cancer Cells (MCF-7). Magnetochemistry. 2022; 8(10):114. https://doi.org/10.3390/magnetochemistry8100114
Chicago/Turabian StyleHernandes, Elisa Parcero, Raquel Dosciatti Bini, Karina Midori Endo, Verci Alves de Oliveira Junior, Igor Vivian de Almeida, Gustavo Sanguino Dias, Ivair Aparecido dos Santos, Paula Nunes de Oliveira, Veronica Elisa Pimenta Vicentini, and Luiz Fernando Cotica. 2022. "Doxorubicin-Loaded Magnetic Nanoparticles: Enhancement of Doxorubicin’s Effect on Breast Cancer Cells (MCF-7)" Magnetochemistry 8, no. 10: 114. https://doi.org/10.3390/magnetochemistry8100114
APA StyleHernandes, E. P., Bini, R. D., Endo, K. M., de Oliveira Junior, V. A., de Almeida, I. V., Dias, G. S., dos Santos, I. A., de Oliveira, P. N., Vicentini, V. E. P., & Cotica, L. F. (2022). Doxorubicin-Loaded Magnetic Nanoparticles: Enhancement of Doxorubicin’s Effect on Breast Cancer Cells (MCF-7). Magnetochemistry, 8(10), 114. https://doi.org/10.3390/magnetochemistry8100114






