ESR Investigations of the Submicron LiFe1−xMnxPO4 Systems
Abstract
:1. Introduction
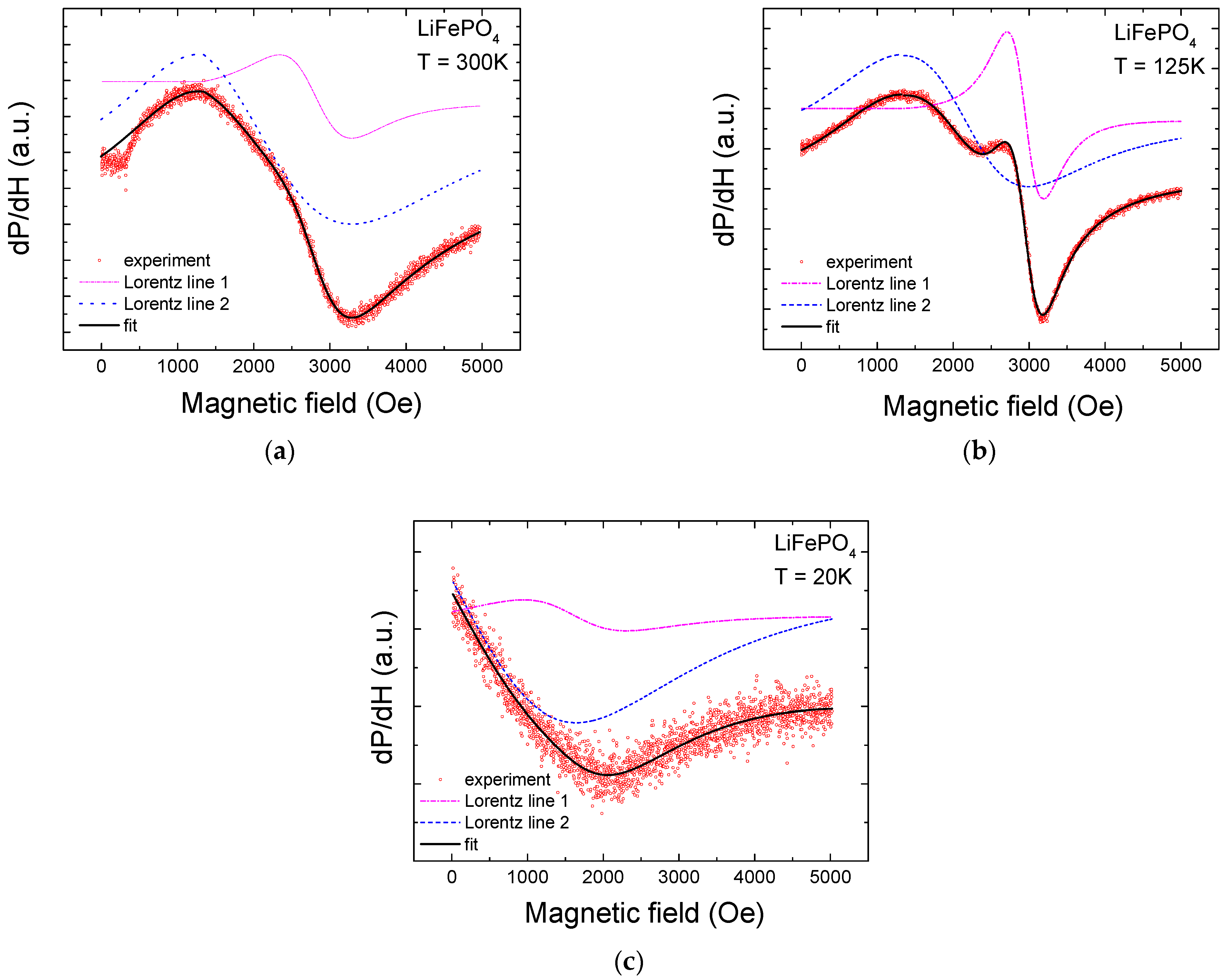
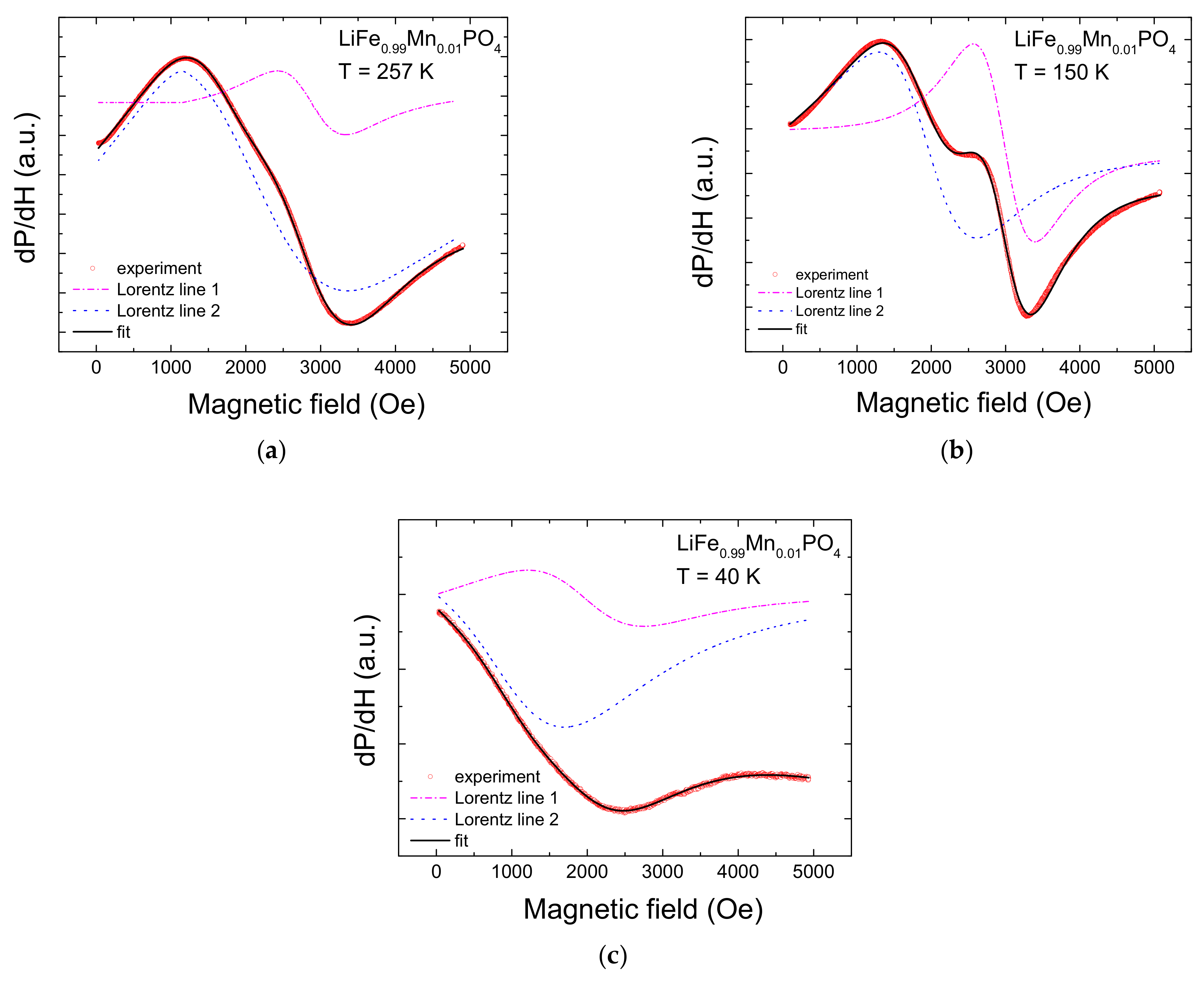
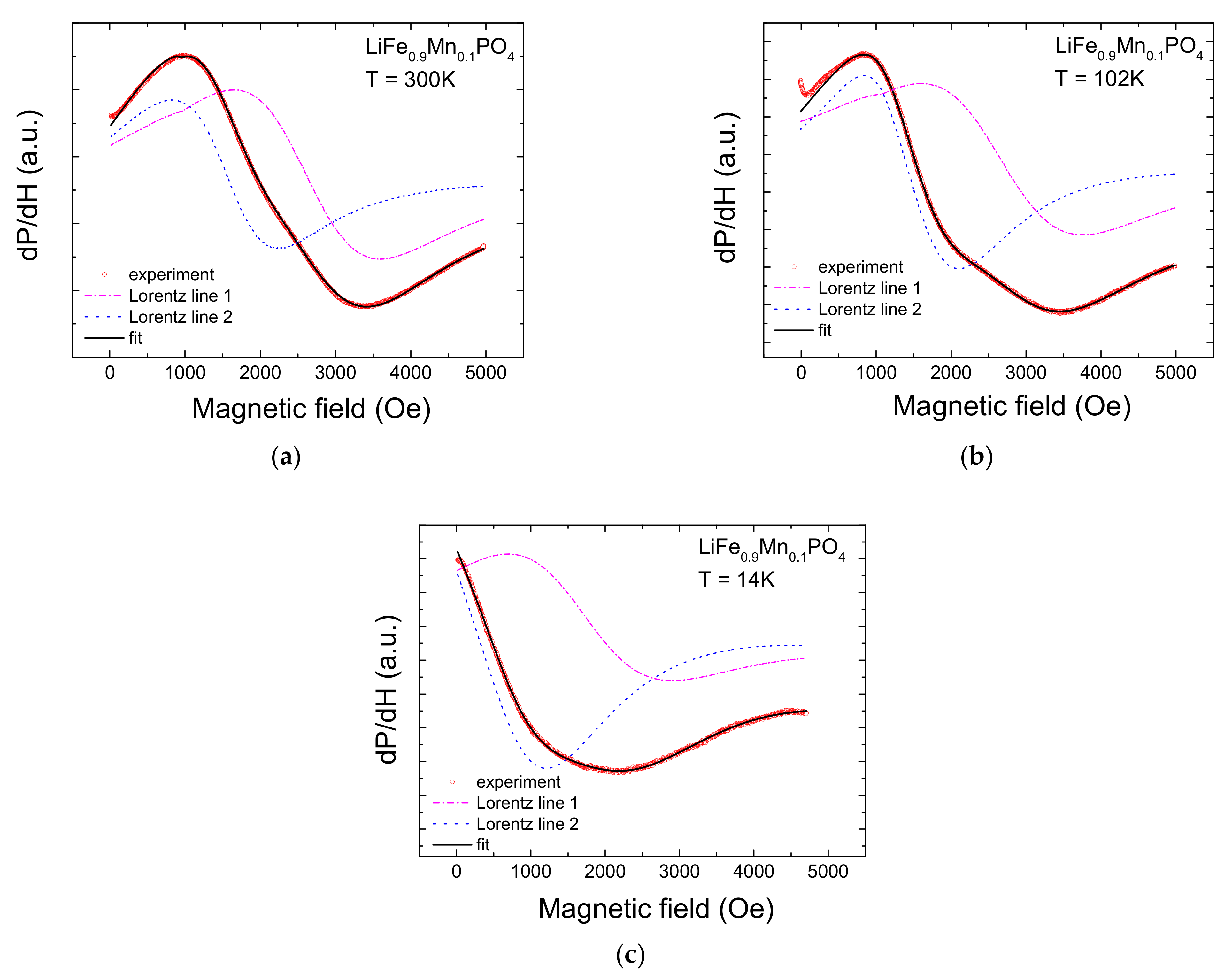
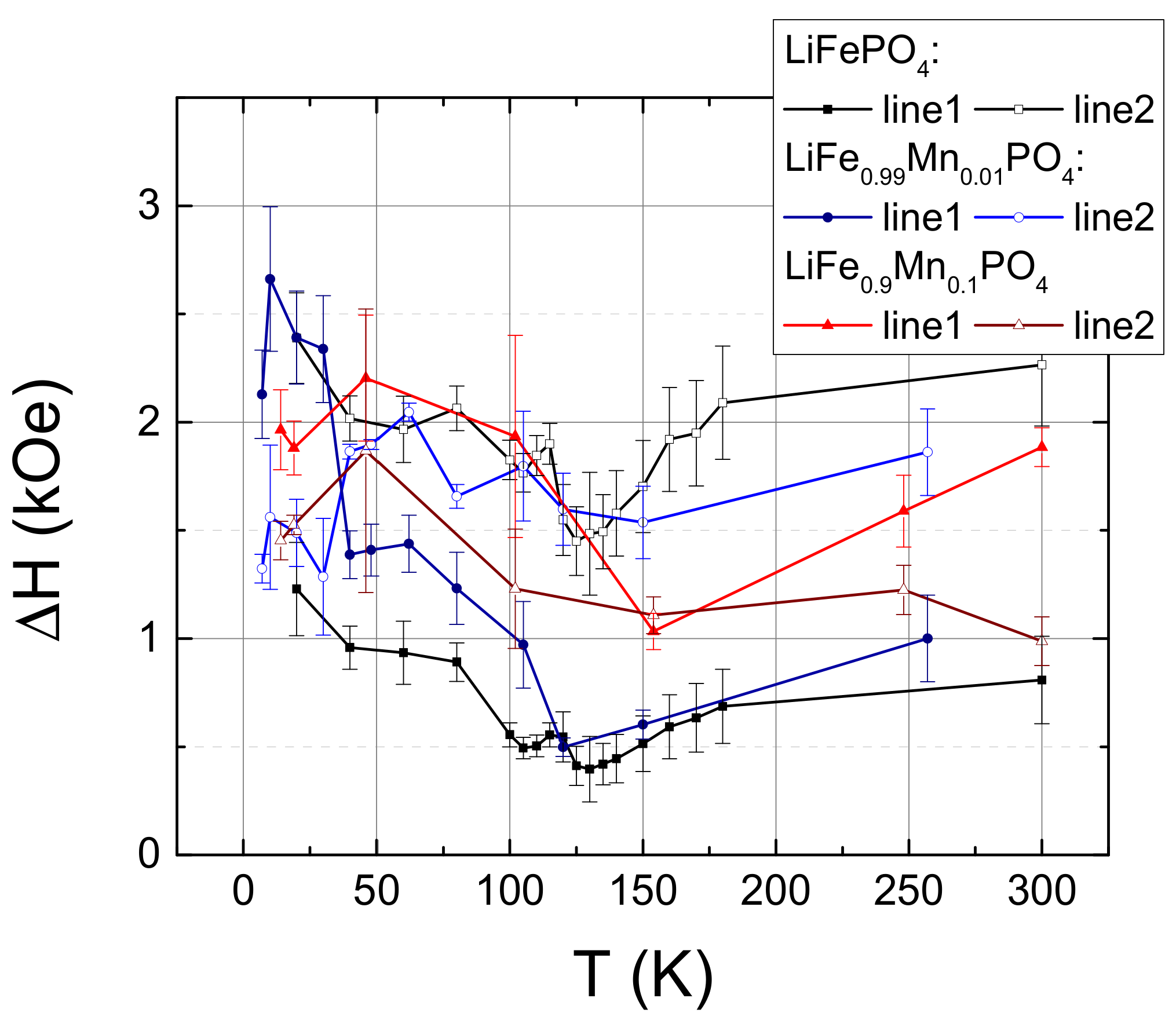
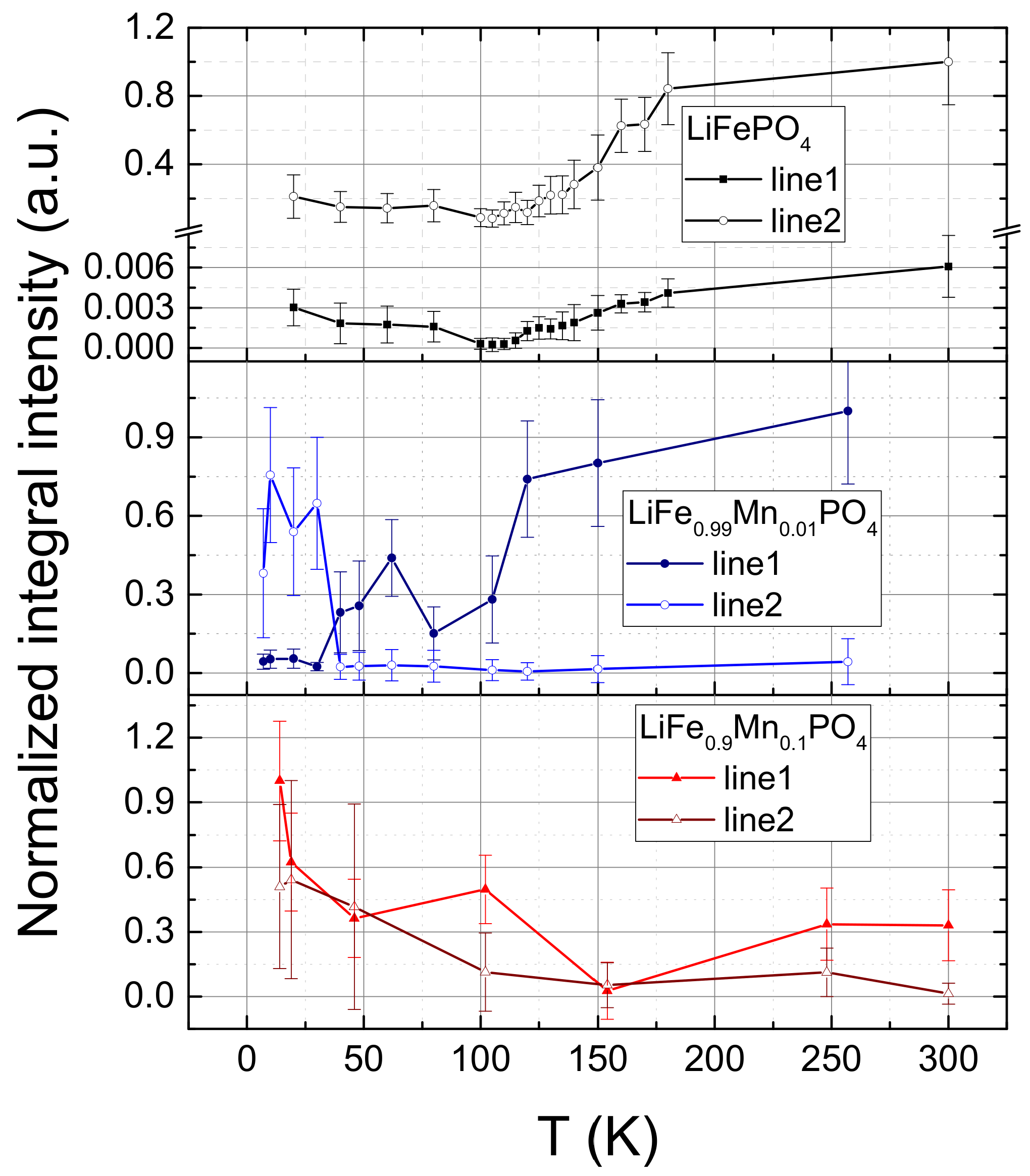
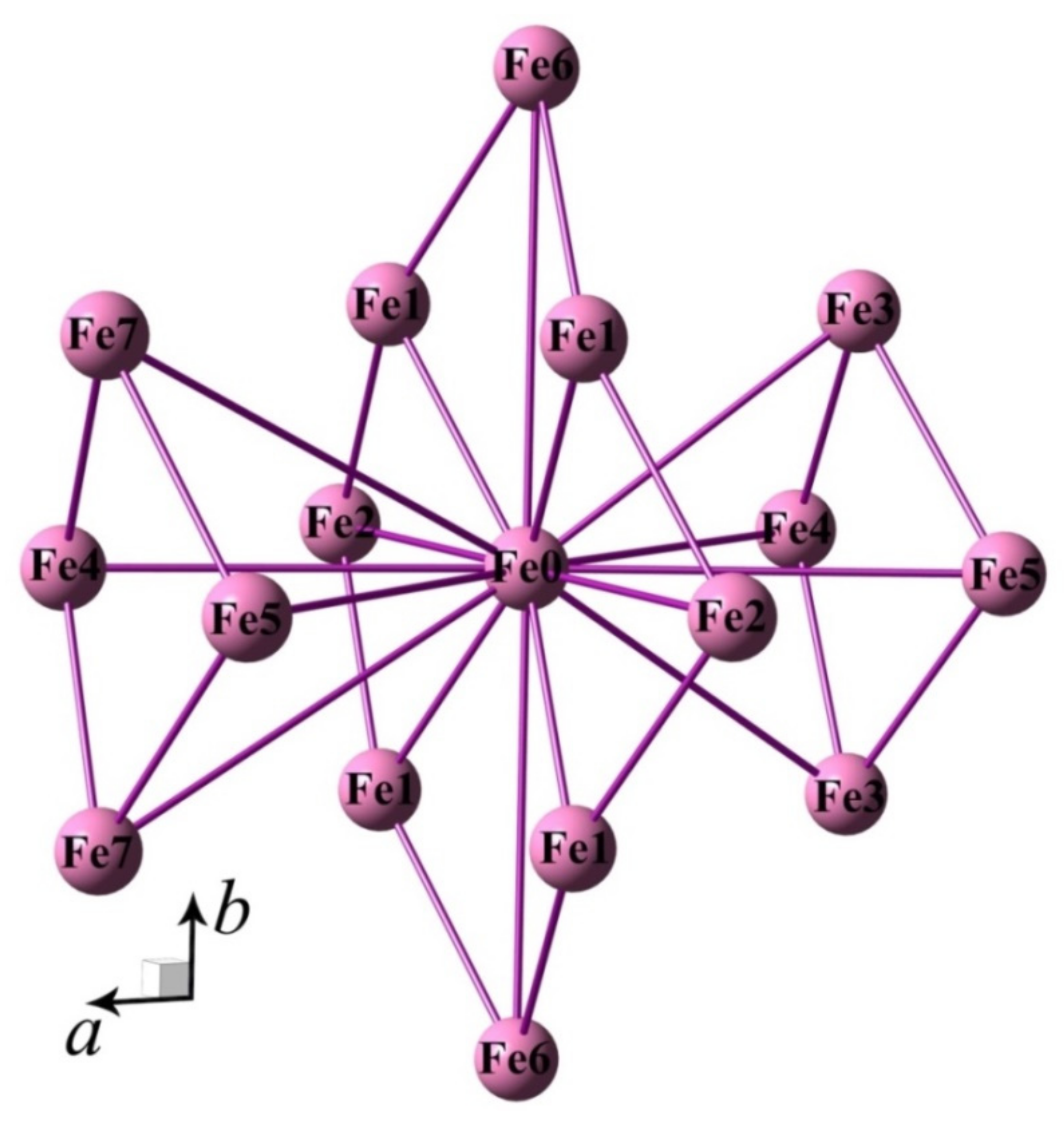
2. Experimental Results
3. Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Padhi, A.K.; Nanjundaswamy, K.S.; Goodenough, J.B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J. Electrochem. Soc. 1997, 144, 1188–1194. [Google Scholar] [CrossRef]
- Yamada, A.; Chung, S.C.; Hinokuma, K. Optimized LiFePO4 for Lithium Battery Cathodes. J. Electrochem. Soc. 2001, 148, A224–A229. [Google Scholar] [CrossRef]
- Huang, H.; Yin, S.-C.; Nazarz, L.F. Approaching Theoretical Capacity of LiFePO4 at Room Temperature at High Rates. Electrochem. Solid-State Lett. 2001, 4, A170–A172. [Google Scholar] [CrossRef]
- Yuan, L.X.; Wang, Z.H.; Zhang, W.X.; Hu, X.L.; Chen, J.T.; Huang, Y.H.; Goodenough, J.B. Development and challenges of LiFePO4 cathode material for lithium-ion batteries. Energy Environ. Sci. 2011, 4, 269–284. [Google Scholar] [CrossRef]
- Zhu, S.; Huang, A.; Xu, Y. Improving Methods for better Performance of Commercial LiFePO4/C Batteries. Int. J. Electrochem. Sci. 2021, 16, 210564. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, S.; Hu, M.; Wang, C.; Jiang, G. Recent Advances in LiFePO4 Cathode Materials for Lithium-Ion Batteries. First-Principles Research. Int. J. Electrochem. Sci. 2021, 16, 211226. [Google Scholar] [CrossRef]
- Hebert, A.; McCalla, E. The role of metal substitutions in the development of Li batteries, part I: Cathodes. Mater. Adv. 2021, 2, 3474–3518. [Google Scholar] [CrossRef]
- Islam, M.S.; Driscoll, D.J.; Fisher, C.A.J.; Slater, P.R. Atomic-Scale Investigation of Defects, Dopants, and Lithium Transport in the LiFePO4 Olivine-Type Battery Material. Chem. Mater. 2005, 17, 5085–5092. [Google Scholar] [CrossRef]
- Deng, Y.; Yang, C.; Zou, K.; Qin, X.; Zhao, Z.; Chen, G. Recent Advances of Mn-Rich LiFe1−yMnyPO4 (0.5 ≤ y <1.0) Cathode Materials for High Energy Density Lithium Ion Batteries. Adv. Energy Mater. 2017, 7, 1601958. [Google Scholar] [CrossRef]
- Bograchev, D.A.; Volfkovich, Y.M.; Sosenkin, V.E.; Podgornova, O.A.; Kosova, N.V. The Influence of Porous Structure on the Electrochemical Properties of LiFe0.5Mn0.5PO4 Cathode Material Prepared by Mechanochemically Assisted Solid-State Synthesis. Energies 2020, 13, 542. [Google Scholar] [CrossRef] [Green Version]
- Hagen, R.V.; Lorrmann, H.; Möller, K.-C.; Mathur, S. Electrospun LiFe1−yMnyPO4/C Nanofiber Composites as Self-Supporting Cathodes in Li-Ion Batteries. Adv. Energy Mater. 2012, 2, 553–559. [Google Scholar] [CrossRef]
- Kosova, N.V.; Podgornova, O.A.; Volfkovich, Y.M.; Sosenkin, V.E. Optimization of the cathode porosity via mechanochemical synthesis with carbon black. J. Solid State Electrochem. 2021, 25, 1029–1037. [Google Scholar] [CrossRef]
- Kosova, N.V.; Podgornova, O.A.; Gutakovskii, A.K. Different electrochemical responses of LiFe0.5Mn0.5PO4 prepared by mechanochemical and solvothermal methods. J. Alloys Compd. 2018, 742, 454–465. [Google Scholar] [CrossRef]
- Liu, L.; Cao, Z.; Cui, Y.; Ke, X.; Zeng, G.; Liu, J.; Liu, D.; Li, Q.; Lai, J.; Shi, Z.; et al. Nanocomposites LiMnxFe1−xPO4/C synthesized via freeze drying assisted sol-gel routine and their magnetic and electrochemical properties. J. Alloys Compd. 2019, 779, 339–346. [Google Scholar] [CrossRef]
- Liu, L.; Chen, G.; Du, B.; Cui, Y.; Ke, X.; Liu, J.; Guo, Z.; Shi, Z.; Zhang, H.; Chou, S. Nano-sized cathode material LiMn0.5Fe0.5PO4/C synthesized via improved sol-gel routine and its magnetic and electrochemical properties. Electrochim. Acta 2017, 255, 205–211. [Google Scholar] [CrossRef]
- Shen, H.; Xiang, W.; Shi, X.; Zhong, B.; Liu, H. Hierarchical LiMn0.5Fe0.5PO4/C nanorods with excellent electrochemical performance synthesized by rheological phase method as cathode for lithium ion battery. Ionics 2016, 22, 193–200. [Google Scholar] [CrossRef]
- Huang, W.; Tao, S.; Zhou, J.; Si, C.; Chen, X.; Huang, W.; Jin, C.; Chu, W.; Song, L.; Wu, Z. Phase Separations in LiFe1−xMnxPO4: A Random Stack Model for Efficient Cathode Materials. J. Phys. Chem. C 2014, 118, 796–803. [Google Scholar] [CrossRef]
- Phan, A.T.; Gheribi, A.E.; Chartrand, P. Coherent and para-equilibrium phase transformations in Mn-doped-LiFePO4 cathode materials: Implications for lithium ion battery performances. J. Alloys Compd. 2020, 838, 155550. [Google Scholar] [CrossRef]
- Novikova, S.; Yaroslavtsev, S.; Rusakov, V.; Chekannikov, A.; Kulova, T.; Skundin, A.; Yaroslavtsev, A. Behavior of LiFe1-yMnyPO4/C cathode materials upon electrochemical lithium intercalation/deintercalation. J. Power Sources 2015, 300, 444–452. [Google Scholar] [CrossRef]
- Kosova, N.V.; Devyatkina, E.T.; Slobodyuk, A.B.; Petrov, S.A. Submicron LiFe1−yMnyPO4 solid solutions prepared by mechanochemically assisted carbothermal reduction: The structure and properties. Electrochim. Acta 2012, 59, 404–411. [Google Scholar] [CrossRef]
- Kosova, N.V.; Devyatkina, E.T.; Petrov, S.A. Fast and Low Cost Synthesis of LiFePO4 Using Fe3+ Precursor. J. Electrochem. Soc. 2010, 157, A1247–A1252. [Google Scholar] [CrossRef]
- Joshi, J.P.; Bhat, S.V. On the analysis of broad Dysonian electron paramagnetic resonance spectra. J. Magn. Reson. 2004, 168, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Bykov, E.O.; Gavrilova, T.P.; Yatsyk, I.V.; Gilmutdinov, I.F.; Parfenov, V.V.; Kurbakov, A.I.; Eremina, R.M. Structural and magnetic properties of Yb1−xSrxMnO3. Ceram. Int. 2019, 45, 10286–10294. [Google Scholar] [CrossRef]
- Nigmatullina, I.I.; Parfenov, V.V.; Eremina, R.M.; Gavrilova, T.P.; Yatsyk, I.V. ESR and Mössbauer Spectroscopic Study of Sr-Doped Ytterbium Ferromanganites. Phys. Solid State 2018, 60, 936–942. [Google Scholar] [CrossRef]
- Abragam, A.; Bleaney, B. Electron Paramagnetic Resonance of Transition Ions; Oxford University Press: Oxford, UK, 1970; Mir: Moscow, Russia, 1972; Volume 1. [Google Scholar]
- Low, W.; Weger, M. Paramagnetic Resonance and Optical Spectra of Divalent Iron in Cubic Fields. II. Experimental Results. Phys. Rev. 1960, 118, 1130–1136. [Google Scholar] [CrossRef]
- Low, W.; Weger, M. Paramagnetic Resonance and Optical Spectra of Divalent Iron in Cubic Fields. I. Theory. Phys. Rev. 1960, 118, 1119–1130. [Google Scholar] [CrossRef]
- Arčon, D.; Zorko, A.; Dominko, R.; Jagličič, Z. A comparative study of magnetic properties of LiFePO4 and LiMnPO4. J. Phys. Condens. Matter 2004, 16, 5531–5548. [Google Scholar] [CrossRef]
- Van Vleck, J.H. The Dipolar Broadening of Magnetic Resonance Lines in Crystals. Phys. Rev. 1948, 74, 1168–1183. [Google Scholar] [CrossRef]
- Andersson, A.S.; Kalska, B.; Häggström, L.; Thomas, J.O. Lithium extraction/insertion in LiFePO: An X-ray diffraction and Mössbauer spectroscopy study. Solid State Ion. 2000, 130, 41–52. [Google Scholar] [CrossRef]
- Rousse, G.; Rodriguez-Carvajal, J.; Patoux, S.; Masquelier, C. Magnetic Structures of the Triphylite LiFePO4 and of Its Delithiated Form FePO4. Chem. Mater. 2003, 15, 4082–4090. [Google Scholar] [CrossRef]
- Nizamov, F.A.; Togulev, P.N.; Abdullin, D.R.; Khantimerov, S.M.; Balaya, P.; Suleimanov, N.M. Antisite Defects and Valence State of Vanadium in Na3V2(PO4)3. Phys. Solid State 2016, 58, 475–480. [Google Scholar] [CrossRef]
- Anderson, P.W.; Weiss, P.R. Exchange Narrowing in Paramagnetic Resonance. Rev. Mod. Phys. 1953, 25, 269–276. [Google Scholar] [CrossRef]
- Ait-Salah, A.; Dodd, J.; Mauger, A.; Yazamib, R.; Gendron, F.; Julien, C.M. Structural and Magnetic Properties of LiFePO4 and Lithium Extraction Effects. Z. Anorg. Allg. Chem. 2006, 632, 1598–1605. [Google Scholar] [CrossRef]
- Gavrilova, T.; Khantimerov, S.; Cherosov, M.; Batulin, R.; Lyadov, N.; Yatsyk, I.; Deeva, Y.; Turkin, D.; Chupakhina, T.; Suleimanov, N. Magnetic properties of Li3V2(PO4)3/Li3PO4 composite. Magnetochemistry 2021, 7, 64. [Google Scholar] [CrossRef]
- Gavrilova, T.P.; Khantimerov, S.M.; Fatykhov, R.R.; Yatsyk, I.V.; Cherosov, M.A.; Lee, H.S.; Vishwanathan, R.; Saravanan, K.; Suleimanov, N.M. Magnetic properties and vanadium oxidation state in α-Li3V2(PO4)3/C composite: Magnetization and ESR measurements. Solid State Commun. 2021, 323, 114108. [Google Scholar] [CrossRef]
- Deisenhofer, J.; Eremina, R.M.; Pimenov, A.; Gavrilova, T.; Berger, H.; Johnsson, M.; Lemmens, P.; Krug von Nidda, H.-A.; Loidl, A.; Lee, K.-S.; et al. Structural and magnetic dimers in the spin-gapped system CuTe2O5. Phys. Rev. B 2006, 74, 174421. [Google Scholar] [CrossRef] [Green Version]
- Seidov, Z.; Gavrilova, T.P.; Eremina, R.M.; Svistov, L.E.; Bush, A.A.; Loidl, A.; Krug von Nidda, H.-A. Anisotropic exchange in LiCu2O2. Phys. Rev. B 2017, 95, 224411. [Google Scholar] [CrossRef] [Green Version]
- Gavrilova, T.P.; Yagfarova, A.R.; Deeva, Y.A.; Yatsyk, I.V.; Gilmutdinov, I.F.; Cherosov, M.A.; Vagizov, F.G.; Chupakhina, T.I.; Eremina, R.M. Iron oxidation state in La0.7Sr1.3Fe0.7Ti0.3O4 and La0.5Sr1.5Fe0.5Ti0.5O4 layered perovskites: Magnetic properties. J. Phys. Chem. Solids 2021, 153, 109994. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavrilova, T.; Yagfarova, A.; Khantimerov, S.; Abdullin, D.; Kosova, N.; Suleimanov, N. ESR Investigations of the Submicron LiFe1−xMnxPO4 Systems. Magnetochemistry 2022, 8, 74. https://doi.org/10.3390/magnetochemistry8070074
Gavrilova T, Yagfarova A, Khantimerov S, Abdullin D, Kosova N, Suleimanov N. ESR Investigations of the Submicron LiFe1−xMnxPO4 Systems. Magnetochemistry. 2022; 8(7):74. https://doi.org/10.3390/magnetochemistry8070074
Chicago/Turabian StyleGavrilova, Tatiana, Adilya Yagfarova, Sergey Khantimerov, Dinar Abdullin, Nina Kosova, and Nail Suleimanov. 2022. "ESR Investigations of the Submicron LiFe1−xMnxPO4 Systems" Magnetochemistry 8, no. 7: 74. https://doi.org/10.3390/magnetochemistry8070074
APA StyleGavrilova, T., Yagfarova, A., Khantimerov, S., Abdullin, D., Kosova, N., & Suleimanov, N. (2022). ESR Investigations of the Submicron LiFe1−xMnxPO4 Systems. Magnetochemistry, 8(7), 74. https://doi.org/10.3390/magnetochemistry8070074







