Tuning the Spin-Crossover Behaviour in Fe(II) Polymeric Composites for Food Packaging Applications
Abstract
:1. Introduction
2. Results and Discussion
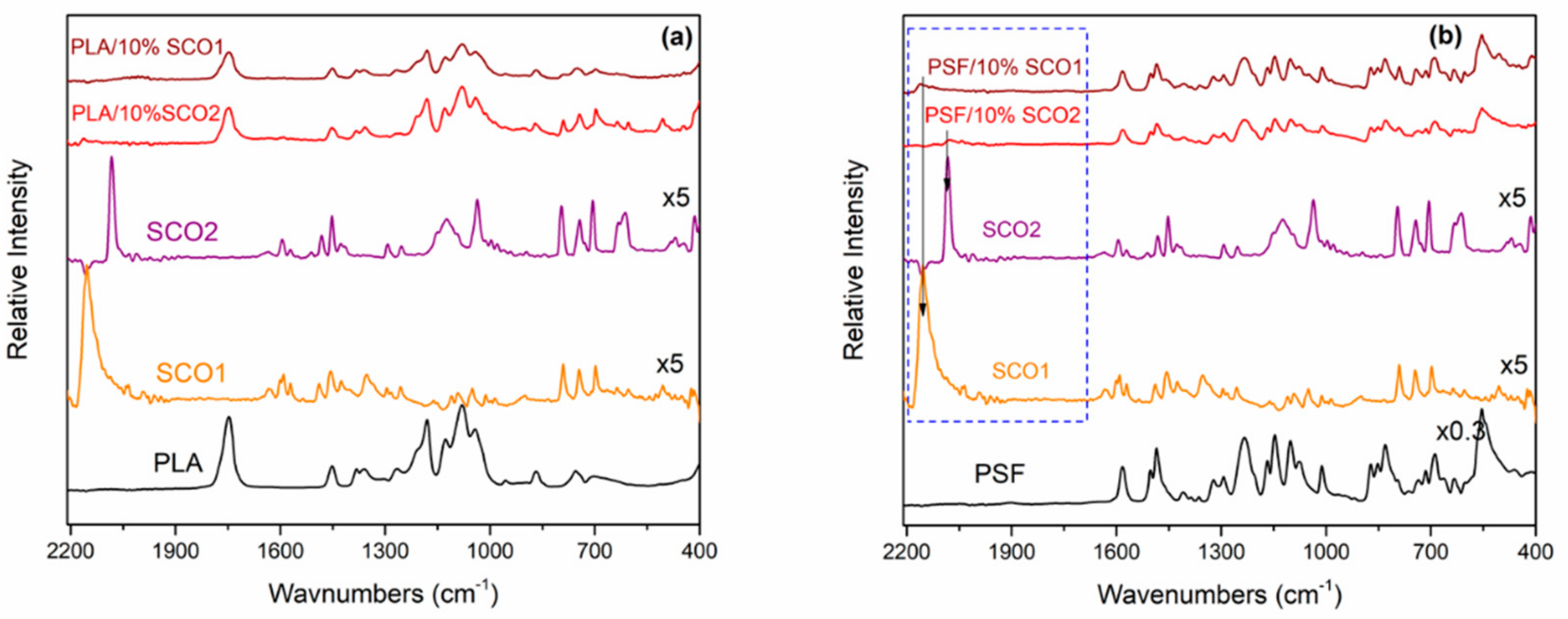
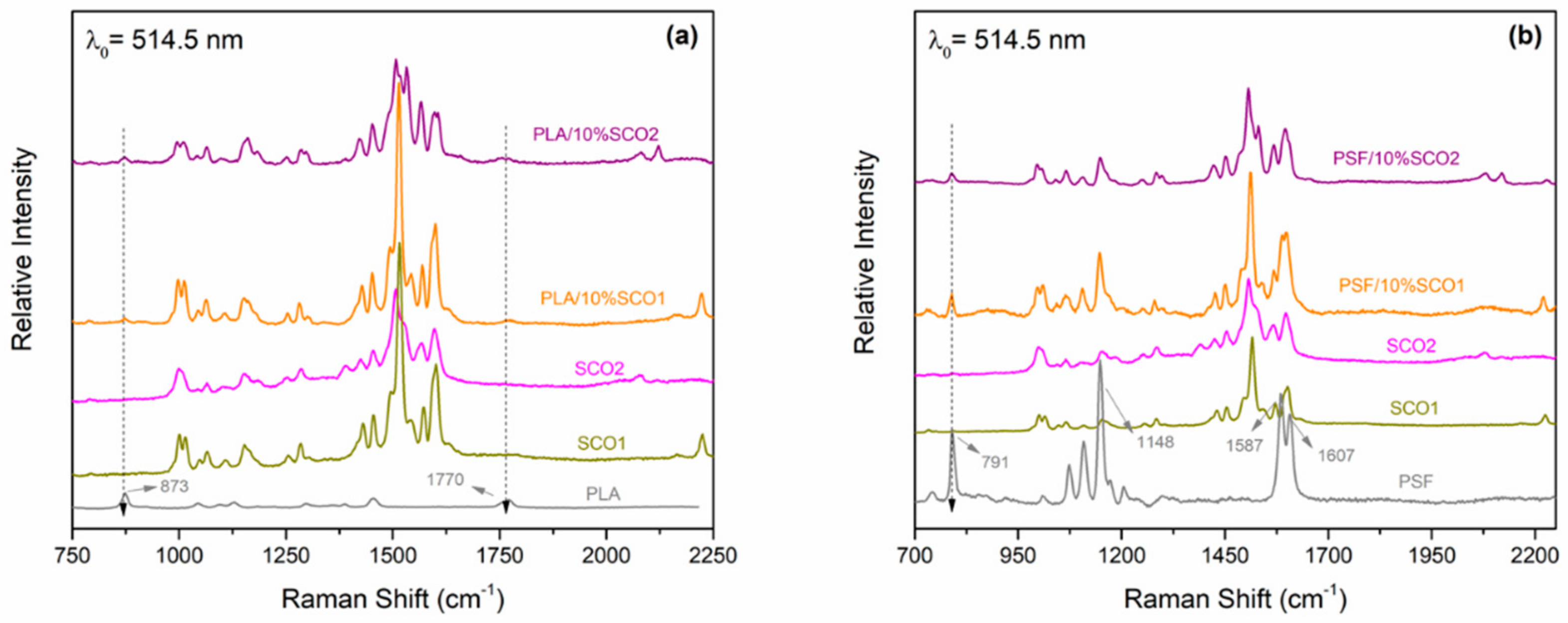
2.1. Morphological and Spectroscopic Characterisation
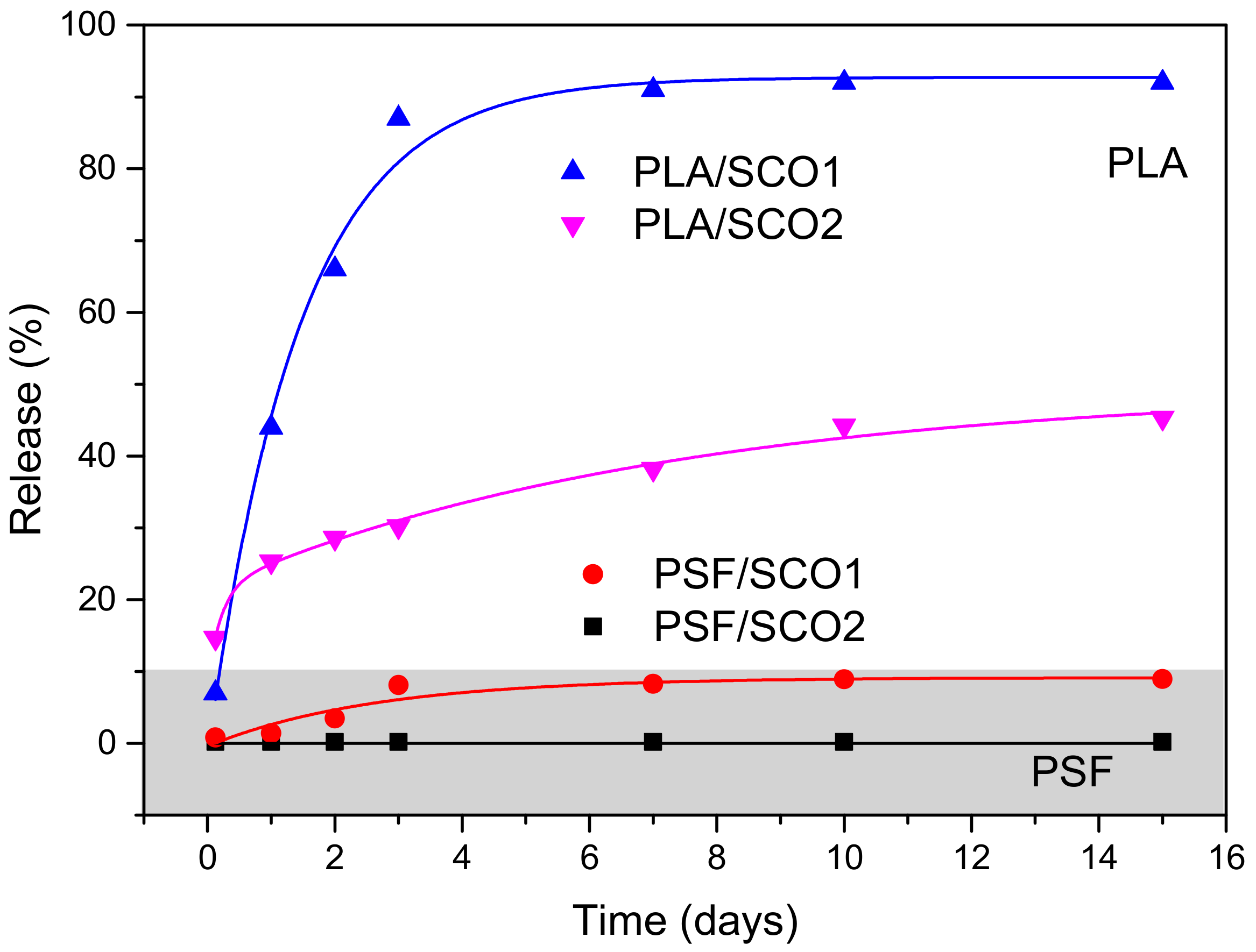
2.2. Migration/Release Studies (MRS)
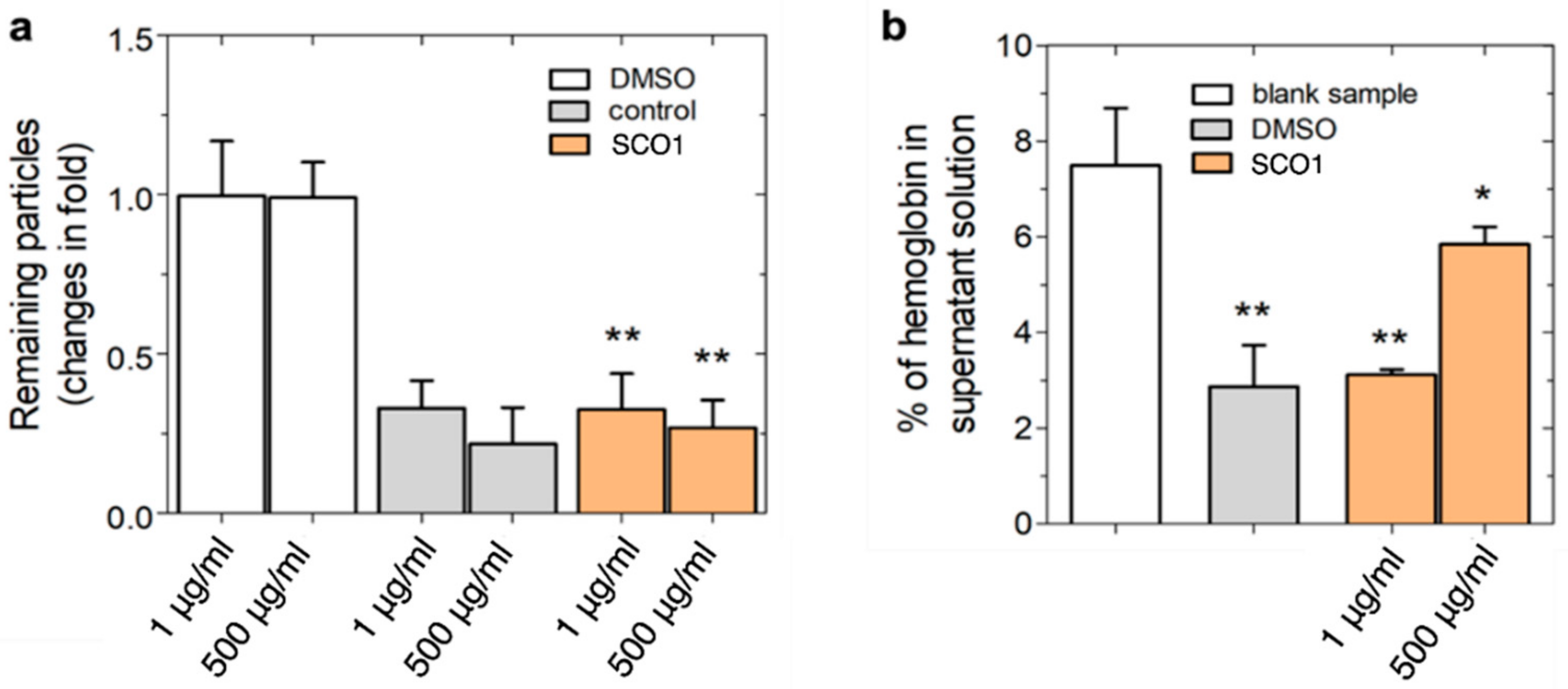
2.3. Toxicity Evaluation
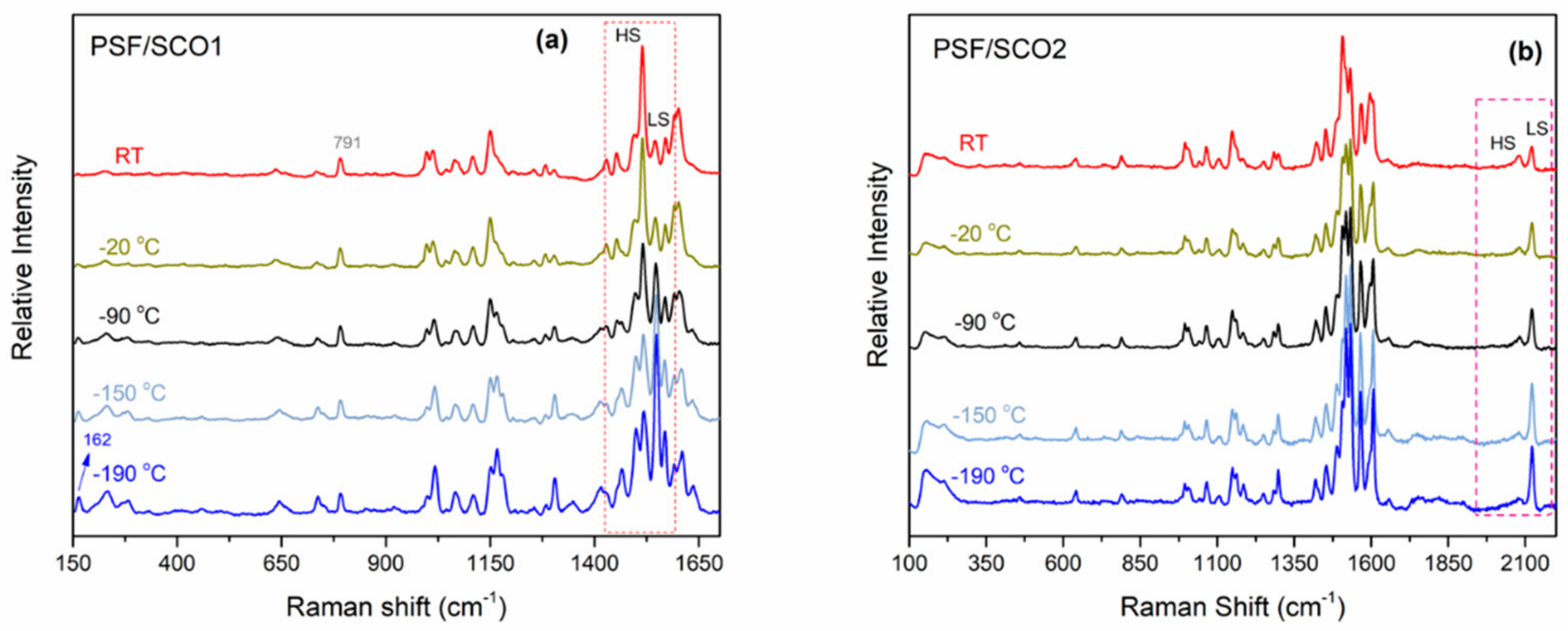
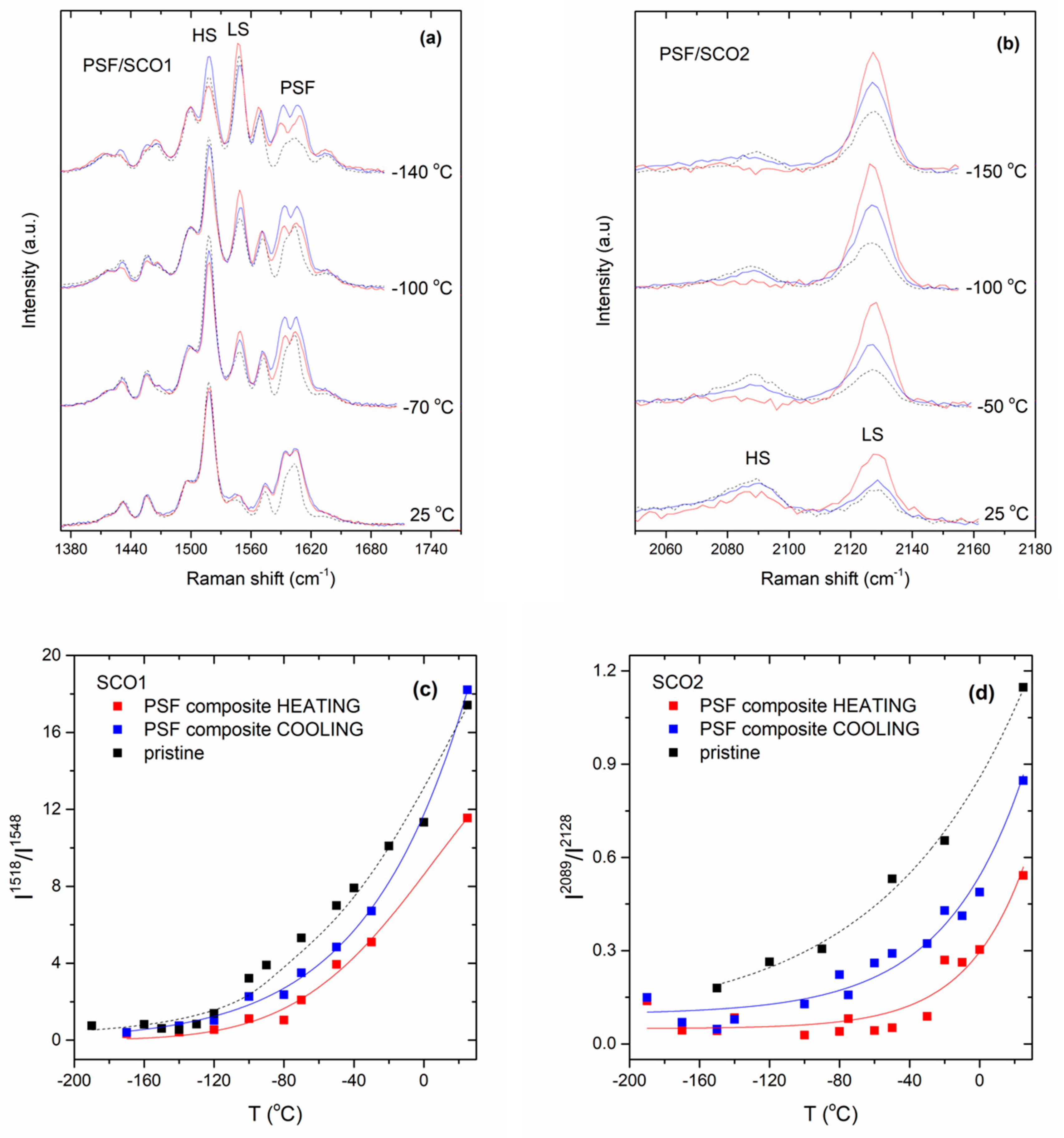
2.4. Monitoring of the SCO Behaviour
3. Materials and Methods
3.1. Materials and Procedures
3.2. Experimental Techniques
3.3. Migration Release Study
3.4. Toxicological Assessment of Nanomaterial
3.4.1. Biochemicals and Reagents
3.4.2. Cell Cultures and Conditions
3.4.3. WST-1 Cell Viability Assay
3.4.4. Reactive Oxygen Species (ROS) Detection
3.4.5. Immunofluorescence Microscopy
Blood Assay
3.4.6. In vitro Digestion Study
Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Real, J.A.; Gaspar, A.B.; Munoz, M.C. Thermal, pressure and light switchable spin-crossover materials. Dalton Trans. 2005, 2062–2079. [Google Scholar] [CrossRef]
- Gamez, P.; Costa, J.S.; Quesada, M.; Aromí, G. Iron Spin-Crossover compounds: From fundamental studies to practical applications. Dalton Trans. 2009, 7845–7853. [Google Scholar] [CrossRef]
- Bousseksou, A.; Molnár, G.; Salmon, L.; Nicolazzi, W. Molecular spin crossover phenomenon: Recent achievements and prospects. Chem. Soc. Rev. 2011, 40, 3313–3335. [Google Scholar] [CrossRef]
- Senthil Kumar, K.; Ruben, M. Emerging trends in spin crossover (SCO) based functional materials and devices. Coord. Chem. Rev. 2017, 346, 176–205. [Google Scholar] [CrossRef]
- Olikrom. Available online: https://www.olikrom.com/en/nos-produits/quelques-applications/ (accessed on 12 November 2020).
- Brooker, S. Spin crossover with thermal hysteresis: Practicalities and lessons learnt. Chem. Soc. Rev. 2015, 44, 2880–2892. [Google Scholar] [CrossRef] [Green Version]
- Spiering, H. Elastic Interaction in Spin-Crossover Compounds. In Spin Crossover in Transition Metal Compounds III; Springer: Berlin, Germany, 2004; pp. 171–195. [Google Scholar]
- Vela, S.; Paulsen, H. Cooperativity in Spin Crossover Systems. An Atomistic Perspective on the Devil’s Staircase. Inorg. Chem. 2018, 57, 9478–9488. [Google Scholar] [CrossRef]
- Murray, K.S.; Kepert, C.J. Cooperativity in Spin Crossover Systems: Memory, Magnetism and Microporosity. In Spin Crossover in Transition Metal Compounds I; Gütlich, P., Goodwin, H.A., Eds.; Springer: Berlin, Germany, 2004; pp. 195–228. [Google Scholar]
- Lada, Z.G.; Chrissanthopoulos, A.; Perlepes, S.; Andrikopoulos, K.S.; Voyiatzis, G.A. Wet-Chemistry Assembly of One-Dimensional Nanowires: Switching Characteristics of a Known Spin-Crossover Iron(II) Complex Through Raman Spectroscopy. Chem. Commun. 2022, 58, 521–524. [Google Scholar] [CrossRef]
- Lada, Z.G.; Andrikopoulos, K.S.; Polyzou, C.D.; Tangoulis, V.; Voyiatzis, G.A. Monitoring the spin crossover phenomenon of [Fe(2-mpz)2Ni(CN)4] 2D Hofmann-type polymer nanoparticles via temperature-dependent Raman spectroscopy. J. Raman Spectr. 2020, 51, 2171–2181. [Google Scholar] [CrossRef]
- Enriquez-Cabrera, A.; Rapakousiou, A.; Piedrahita Bello, M.; Molnár, G.; Salmon, L.; Bousseksou, A. Spin crossover polymer composites, polymers and related soft materials. Coord. Chem. Rev. 2020, 419, 213396. [Google Scholar] [CrossRef]
- Cuéllar, M.P.; Lapresta-Fernández, A.; Herrera, J.M.; Salinas-Castillo, A.; Pegalajar, M.d.C.; Titos-Padilla, S.; Colacio, E.; Capitán-Vallvey, L.F. Thermochromic sensor design based on Fe(II) spin crossover/polymers hybrid materials and artificial neural networks as a tool in modelling. Sens. Actuators B Chem. 2015, 208, 180–187. [Google Scholar] [CrossRef]
- Hauser, A.; Adler, J.; Gütlich, P. Light-induced excited spin state trapping (LIESST) in [Fe(2-mephen)3]2+ embedded in polymer matrices. Chem. Phys. Lett. 1988, 152, 468–472. [Google Scholar] [CrossRef]
- Salmon, L.; Catala, L. Spin-crossover nanoparticles and nanocomposite materials. Compt. Rend. Chim. 2018, 21, 1230–1269. [Google Scholar] [CrossRef]
- Wang, W.; Ji, B.; Zhang, C.; Cao, X. New spin crossover polymeric composite and another way to describe the result. Inorg. Chem. Commun. 2016, 67, 55–59. [Google Scholar] [CrossRef]
- Lada, Z.G.; Soto Beobide, A.; Mathioudakis, G.N.; Voyiatzis, G.A. Fe(II) Spin Crossover/Polymer Hybrid Materials: Investigation of the SCO Behavior via Temperature-Dependent Raman Spectroscopy, Physicochemical Characterization and Migration Release Study. Molecules 2021, 26, 201. [Google Scholar] [CrossRef]
- Lada, Z.G.; Andrikopoulos, K.S.; Chrissanthopoulos, A.; Perlepes, S.P.; Voyiatzis, G.A. A Known Iron(II) Complex in Different Nanosized Particles: Variable-Temperature Raman Study of its Spin-Crossover Behavior. Inorg. Chem. 2019, 58, 5183–5195. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Brant, J.; Hotze, M.; Sempf, J.; Oberley, T.; Sioutas, C.; Yeh, J.I.; Wiesner, M.R.; Nel, A.E. Comparison of the Abilities of Ambient and Manufactured Nanoparticles To Induce Cellular Toxicity According to an Oxidative Stress Paradigm. Nano Lett. 2006, 6, 1794–1807. [Google Scholar] [CrossRef]
- Zhao, F.; Zhao, Y.; Liu, Y.; Chang, X.; Chen, C.; Zhao, Y. Cellular Uptake, Intracellular Trafficking, and Cytotoxicity of Nanomaterials. Small 2011, 7, 1322–1337. [Google Scholar] [CrossRef]
- Singh, S.; Nalwa, H.S. Nanotechnology and Health Safety — Toxicity and Risk Assessments of Nanostructured Materials on Human Health. J. Nanosci. Nanotech. 2007, 7, 3048–3070. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic Potential of Materials at the Nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [Green Version]
- Trohalaki, S.; Zellmer, R.J.; Pachter, R.; Hussain, S.M.; Frazier, J.M. Risk Assessment of High-Energy Chemicals by in Vitro Toxicity Screening and Quantitative Structure-Activity Relationships. Toxicol. Sci. 2002, 68, 498–507. [Google Scholar] [CrossRef]
- Yuniarto, K.; Purwanto, Y.A.; Purwanto, S.; Welt, B.A.; Purwadaria, H.K.; Sunarti, T.C. Infrared and Raman studies on polylactide acid and polyethylene glycol-400 blend. AIP Conf. Proc. 2016, 1725, 020101. [Google Scholar]
- Singh, K.; Devi, S.; Bajaj, H.C.; Ingole, P.; Choudhari, J.; Bhrambhatt, H. Optical Resolution of Racemic Mixtures of Amino Acids through Nanofiltration Membrane Process. Sep. Sci. Technol. 2014, 49, 2630–2641. [Google Scholar] [CrossRef]
- Restuccia, D.; Spizzirri, U.G.; Parisi, O.I.; Cirillo, G.; Curcio, M.; Iemma, F.; Puoci, F.; Vinci, G.; Picci, N. New EU regulation aspects and global market of active and intelligent packaging for food industry applications. Food Control 2010, 21, 1425–1435. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come into Contact with Food Text with EEA Relevance. OJ L 2011, 338, 1–89. (updated 02011R0010—EN—23.09.2020—015.001, on September 2020). Available online: http://data.europa.eu/eli/reg/2011/10/oj (accessed on 20 December 2021).
- Marquis, B.J.; Love, S.A.; Braun, K.L.; Haynes, C.L. Analytical methods to assess nanoparticle toxicity. Analyst 2009, 134, 425–439. [Google Scholar] [CrossRef]
- Harington, J.S.; Miller, K.; Macnab, G. Hemolysis by asbestos. Environ. Res. 1971, 4, 95–117. [Google Scholar] [CrossRef]
- Thomas, K.; Aalbers, M.; Bannon, G.A.; Bartels, M.; Dearman, R.J.; Esdaile, D.J.; Fu, T.J.; Glatt, C.M.; Hadfield, N.; Hatzos, C.; et al. A multi-laboratory evaluation of a common in vitro pepsin digestion assay protocol used in assessing the safety of novel proteins. Regul. Toxicol. Pharmacol. 2004, 39, 87–98. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lada, Z.G.; Andrikopoulos, K.S.; Mathioudakis, G.N.; Piperigkou, Z.; Karamanos, N.; Perlepes, S.P.; Voyiatzis, G.A. Tuning the Spin-Crossover Behaviour in Fe(II) Polymeric Composites for Food Packaging Applications. Magnetochemistry 2022, 8, 16. https://doi.org/10.3390/magnetochemistry8020016
Lada ZG, Andrikopoulos KS, Mathioudakis GN, Piperigkou Z, Karamanos N, Perlepes SP, Voyiatzis GA. Tuning the Spin-Crossover Behaviour in Fe(II) Polymeric Composites for Food Packaging Applications. Magnetochemistry. 2022; 8(2):16. https://doi.org/10.3390/magnetochemistry8020016
Chicago/Turabian StyleLada, Zoi G., Konstantinos S. Andrikopoulos, Georgios N. Mathioudakis, Zoi Piperigkou, Nikos Karamanos, Spyros P. Perlepes, and George A. Voyiatzis. 2022. "Tuning the Spin-Crossover Behaviour in Fe(II) Polymeric Composites for Food Packaging Applications" Magnetochemistry 8, no. 2: 16. https://doi.org/10.3390/magnetochemistry8020016
APA StyleLada, Z. G., Andrikopoulos, K. S., Mathioudakis, G. N., Piperigkou, Z., Karamanos, N., Perlepes, S. P., & Voyiatzis, G. A. (2022). Tuning the Spin-Crossover Behaviour in Fe(II) Polymeric Composites for Food Packaging Applications. Magnetochemistry, 8(2), 16. https://doi.org/10.3390/magnetochemistry8020016










