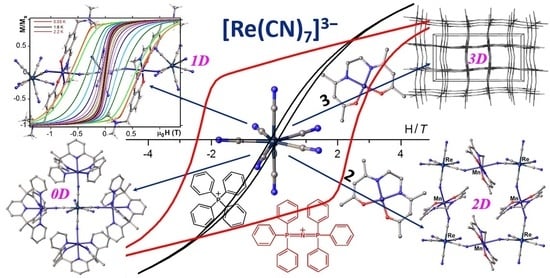
Application of the Heptacyanidorhenate(IV) as a Metalloligand in the Design of Molecular Magnets
Abstract
1. Introduction
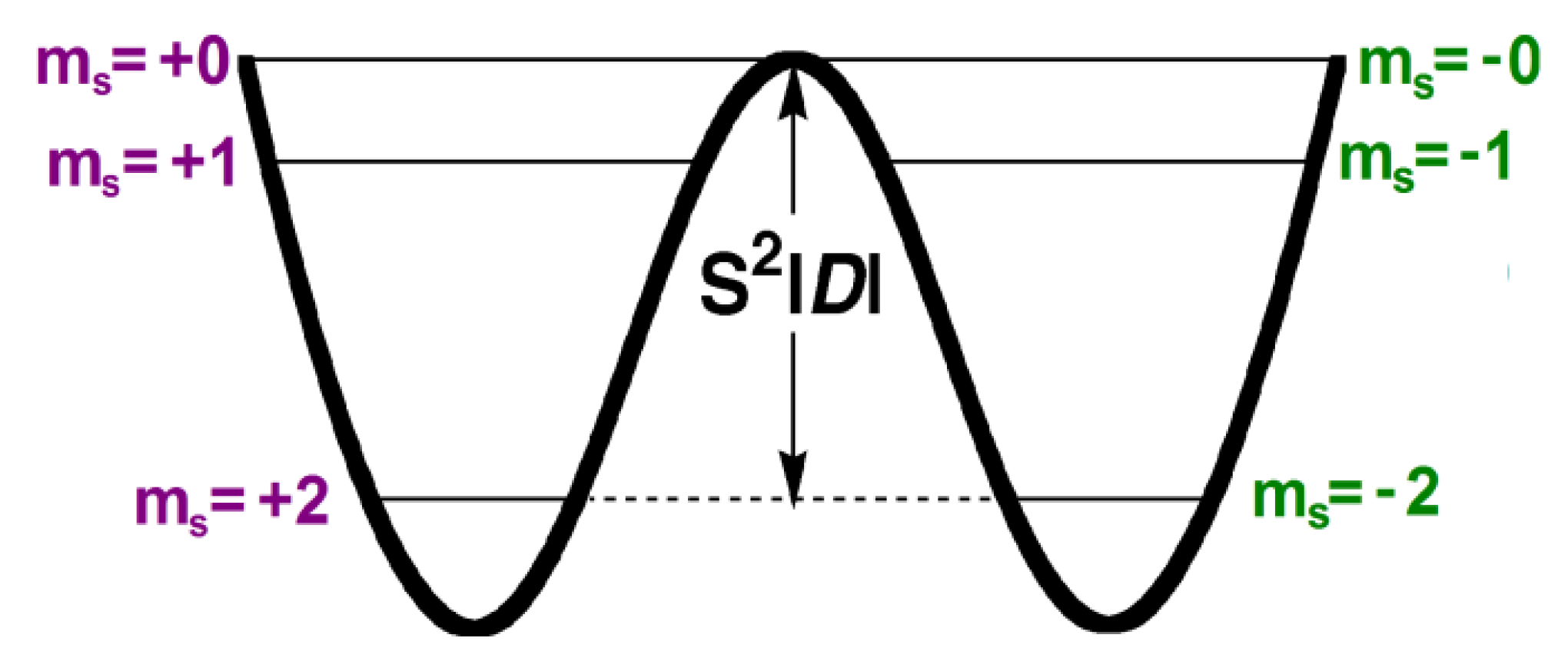
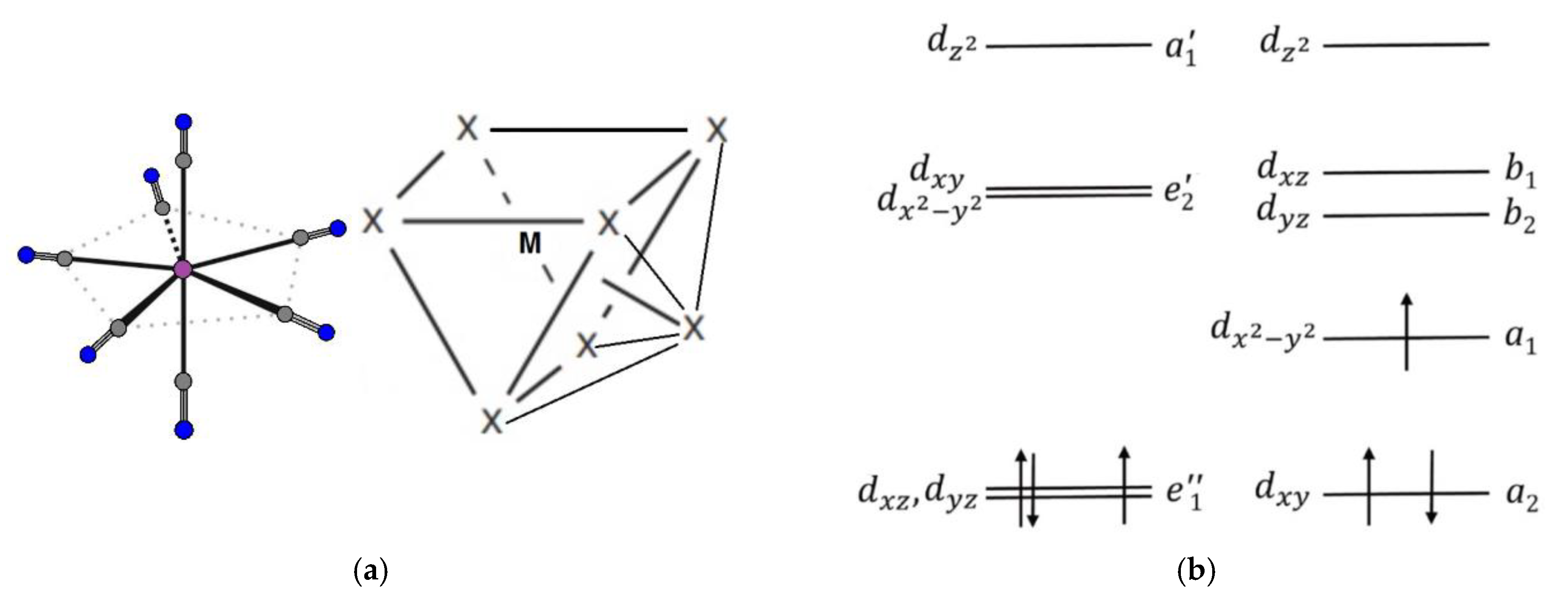
2. Theoretical Background
3. Heterobimetallic Assemblies Based on Pentagonal Bipyramidal Tecton [ReIV(CN)7]3−
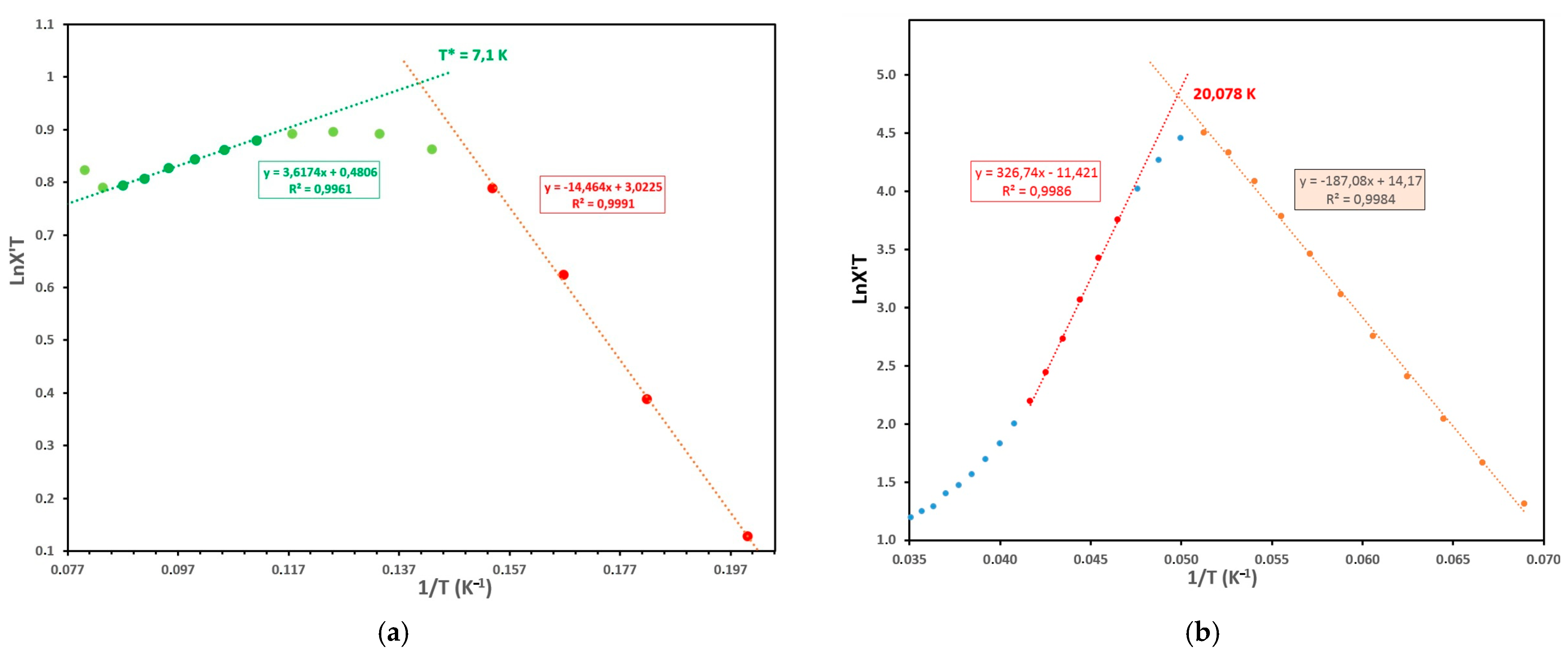
3.1. [ReIV(CN)7]3− Metalloligand
3.2. Reduction to [ReIII(CN)7]3− by MII
3.3. Heterobimetallic Assemblies of [ReIV(CN)7]3− and MIII Shiff Base Complexes
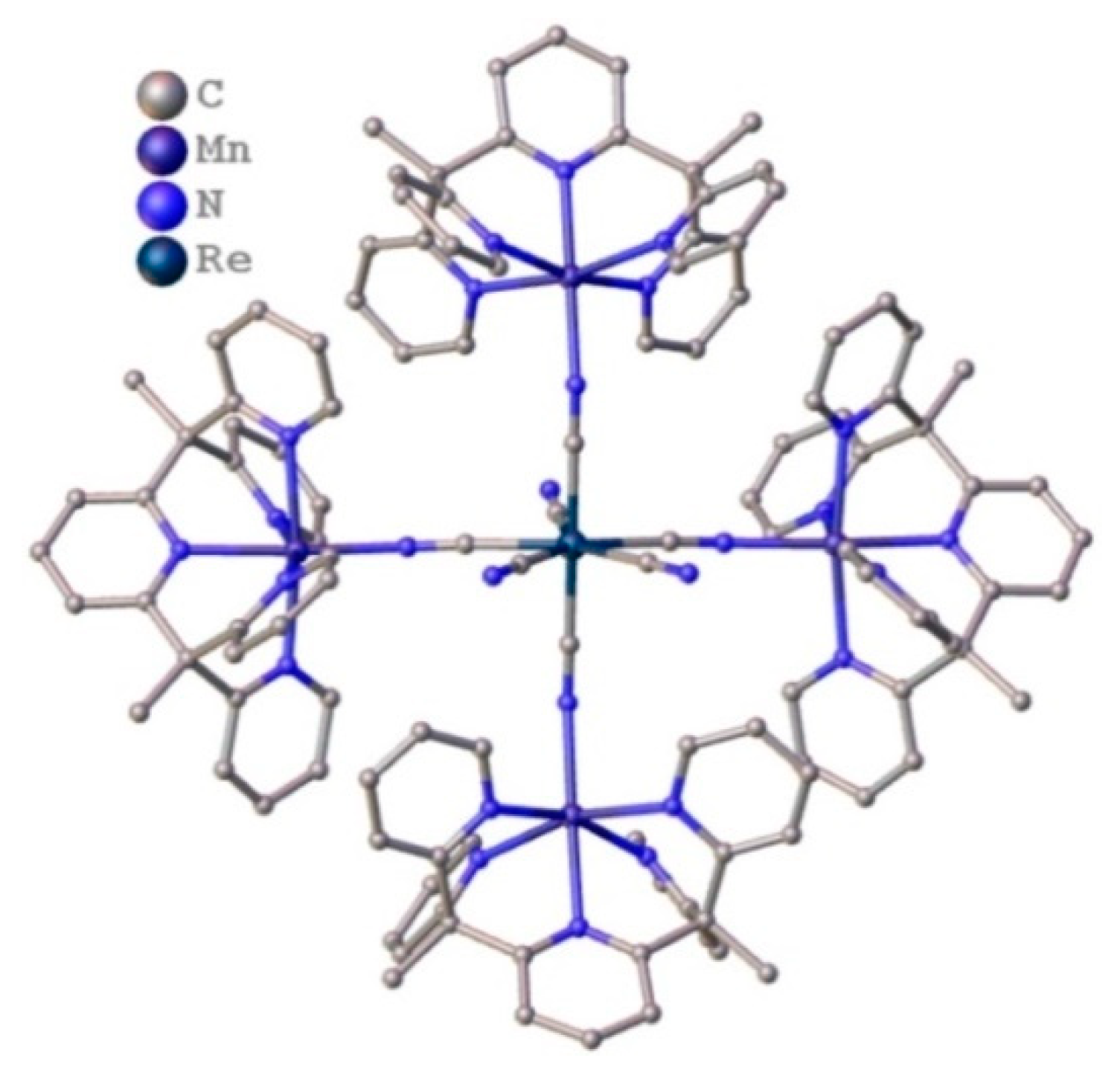
3.3.1. 3D Framework of [Mn(acacen)]3[ReIII(CN)7] (11)
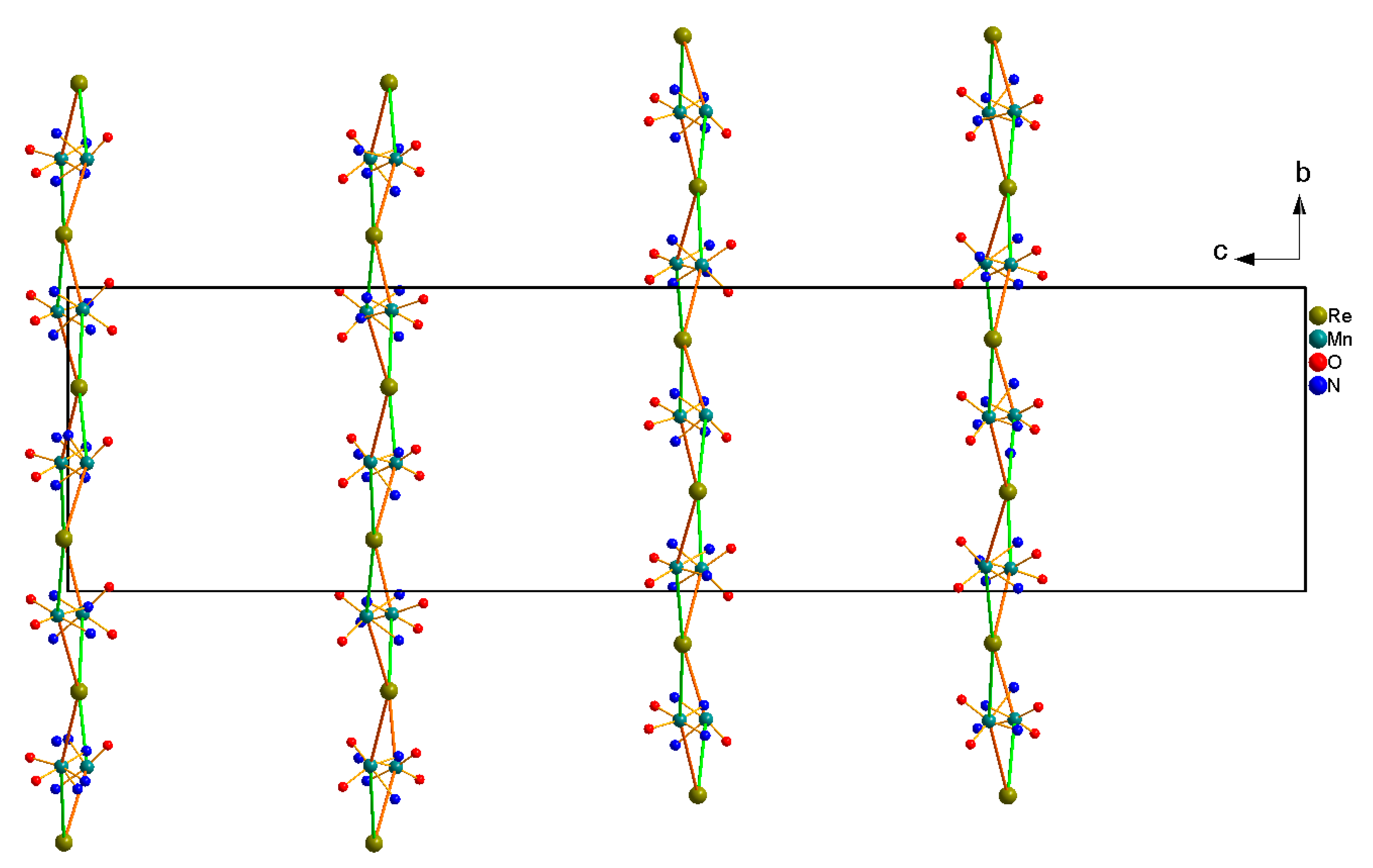
Crystal Structure of [Mn(acacen)]3[ReIII(CN)7]
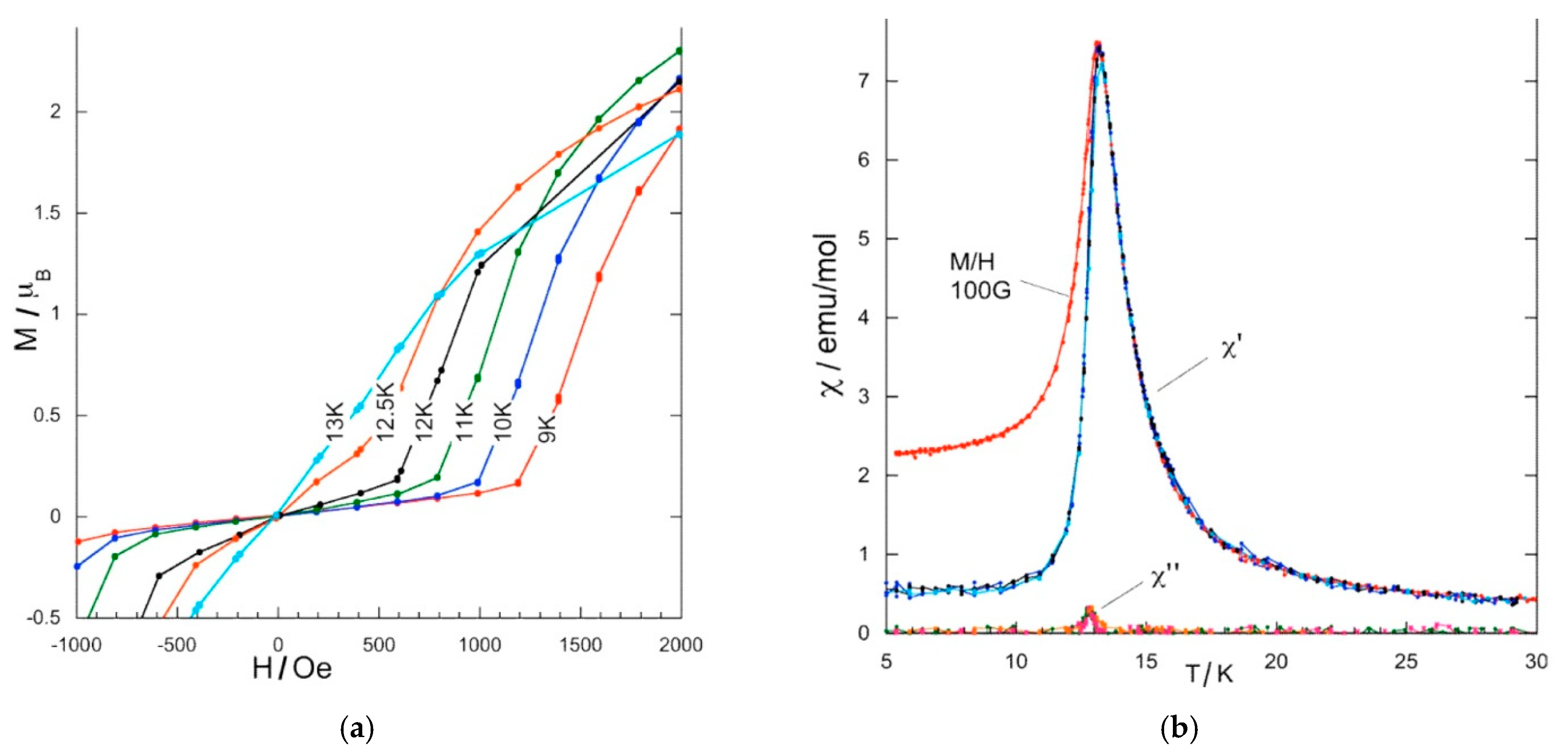
Magnetic Studies of Polycrystalline Samples
Magnetic Studies on a Single Crystal
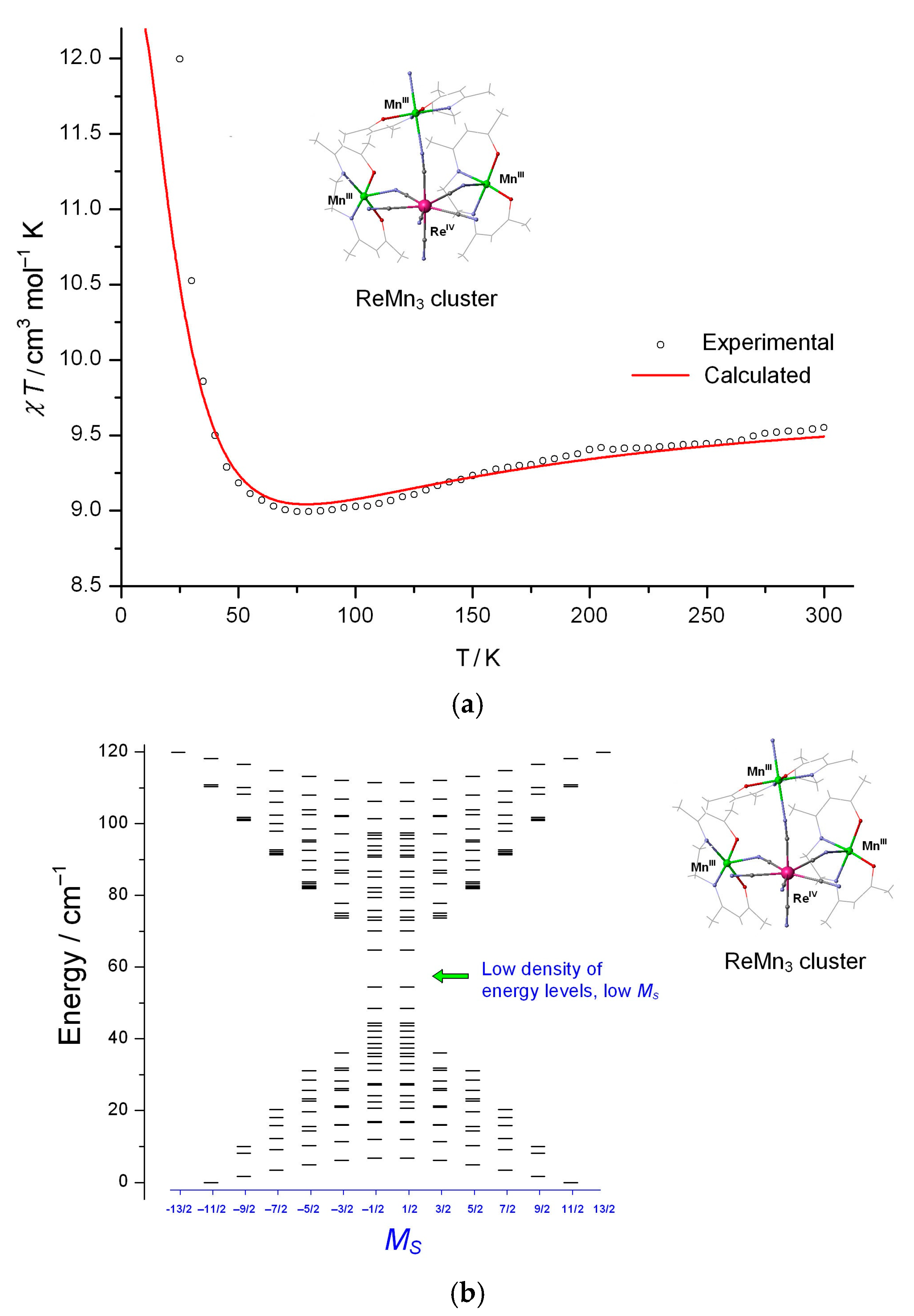
Theoretical Analysis of Anisotropic Spin Coupling in Re–CN–Mn Binding
3.3.2. LD Neutral Assemblies [Mn(SB2+)Re(CN)7]·xH2O
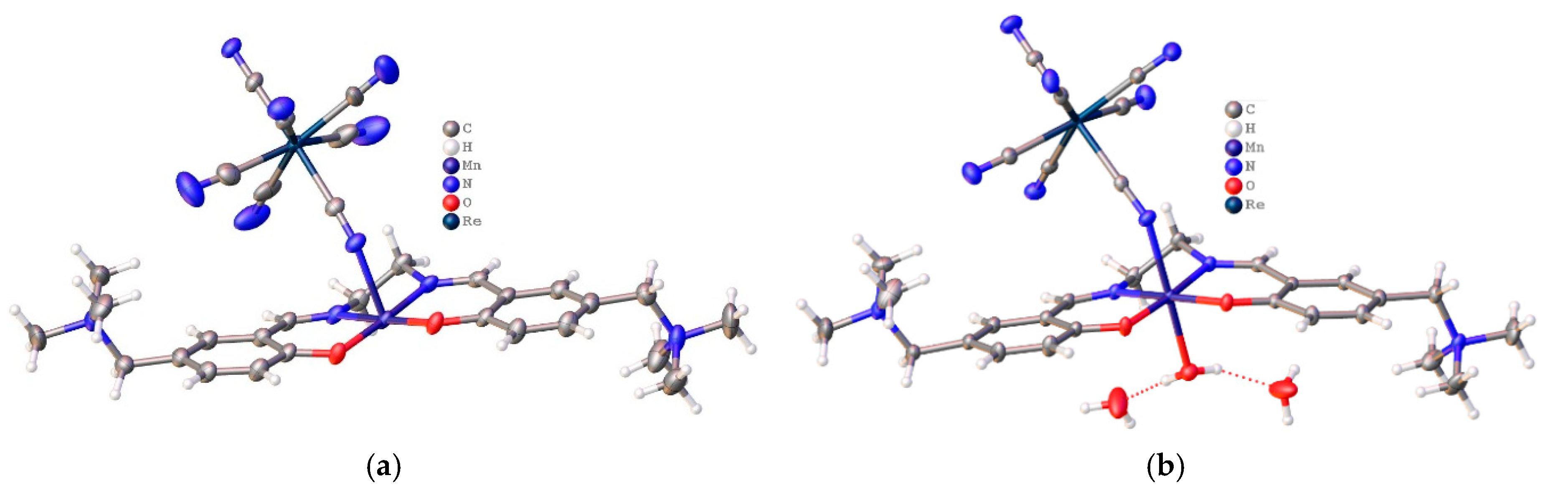
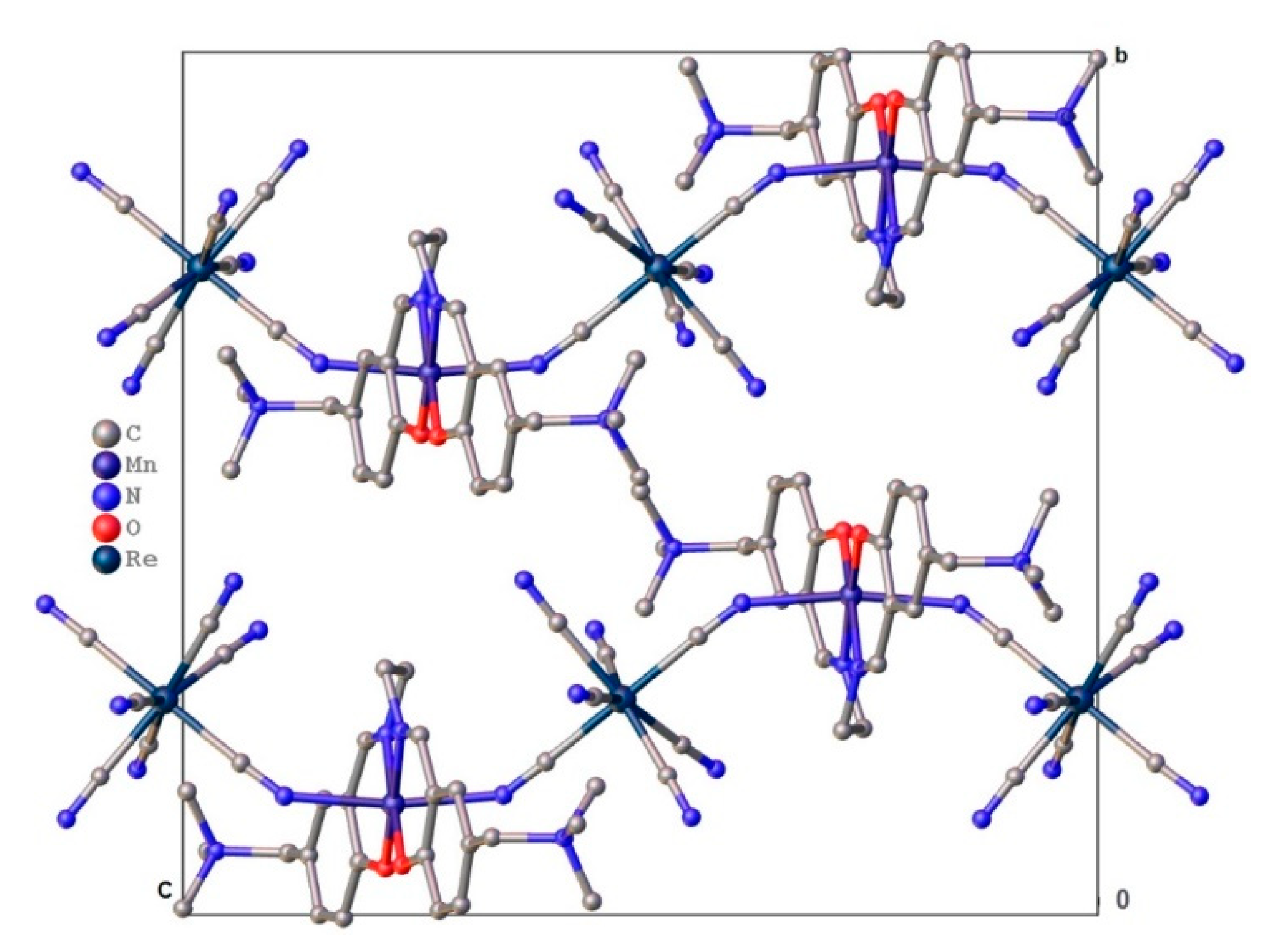
Crystal Structure Description
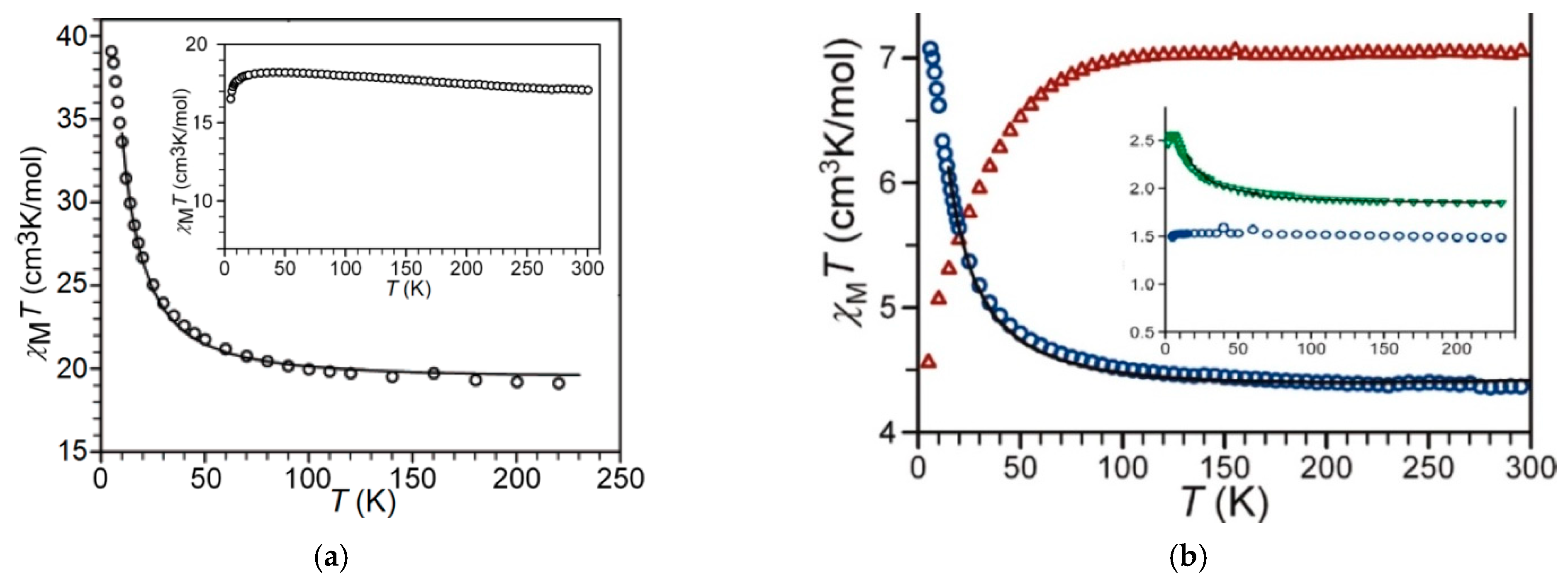
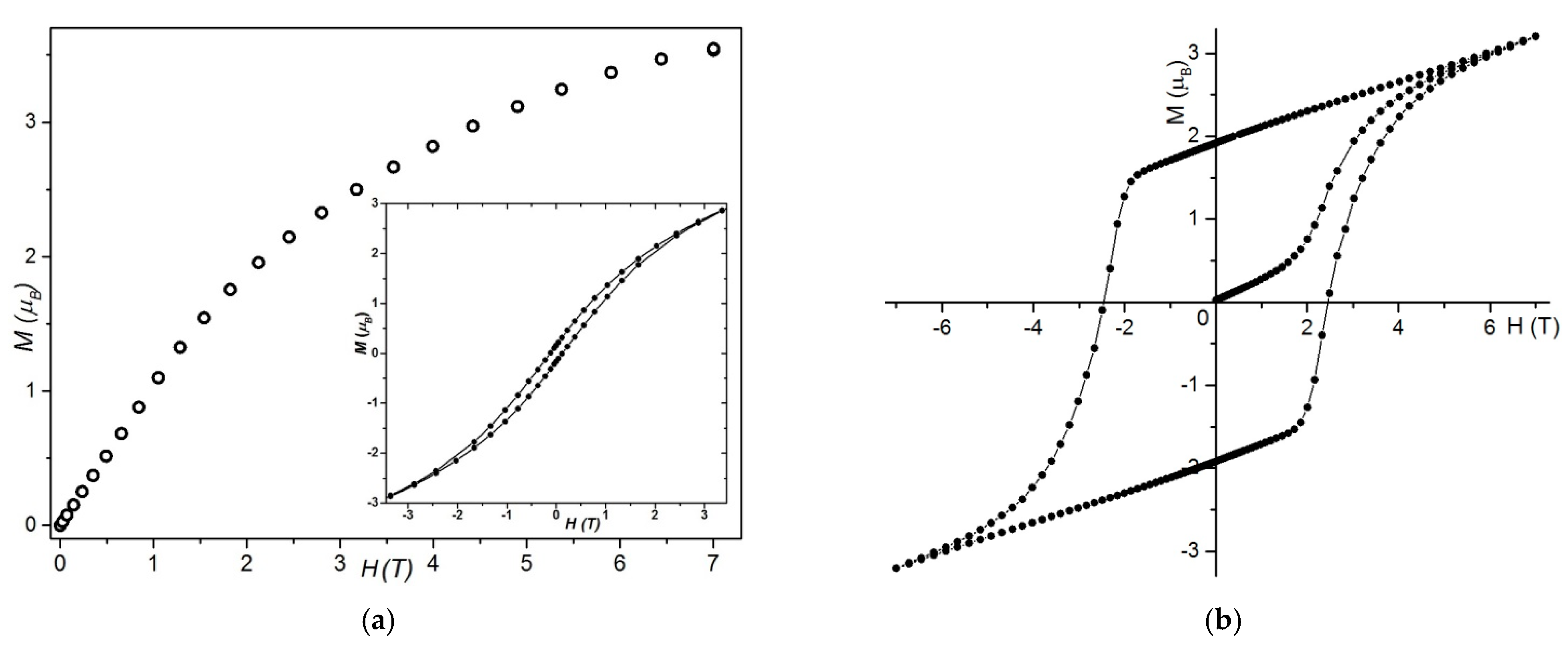
Static Magnetic Behaviors
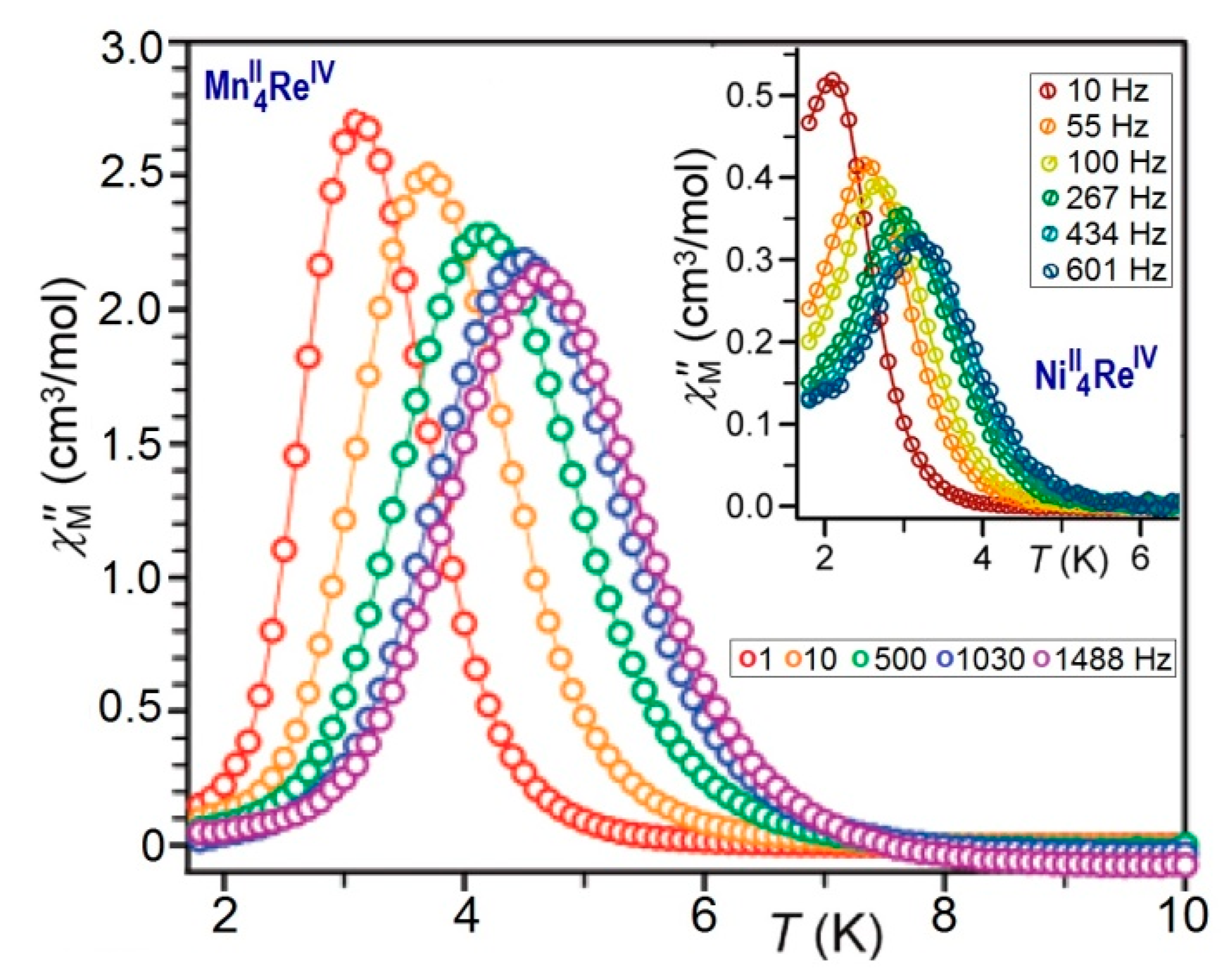
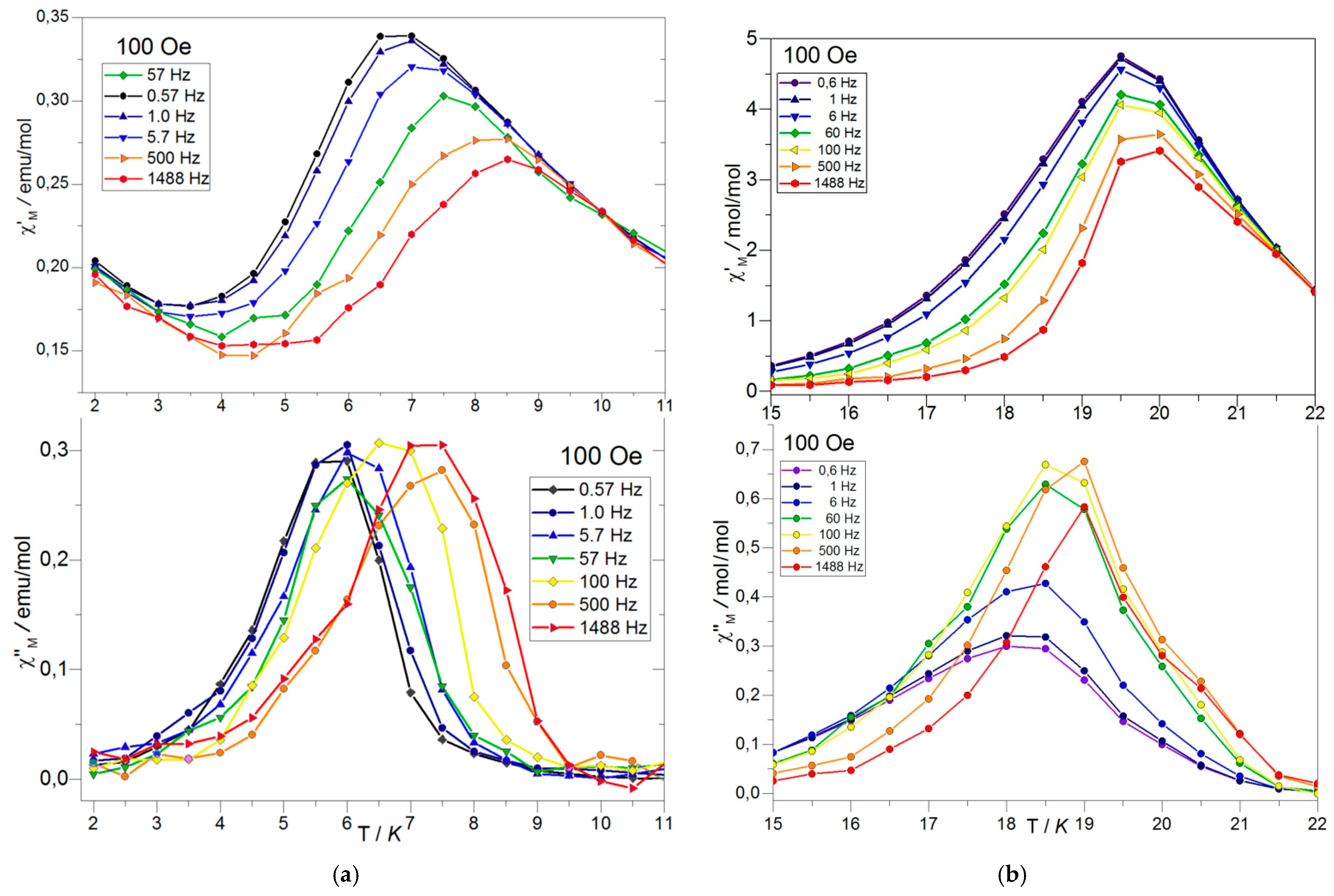
AC Magnetic Measurements
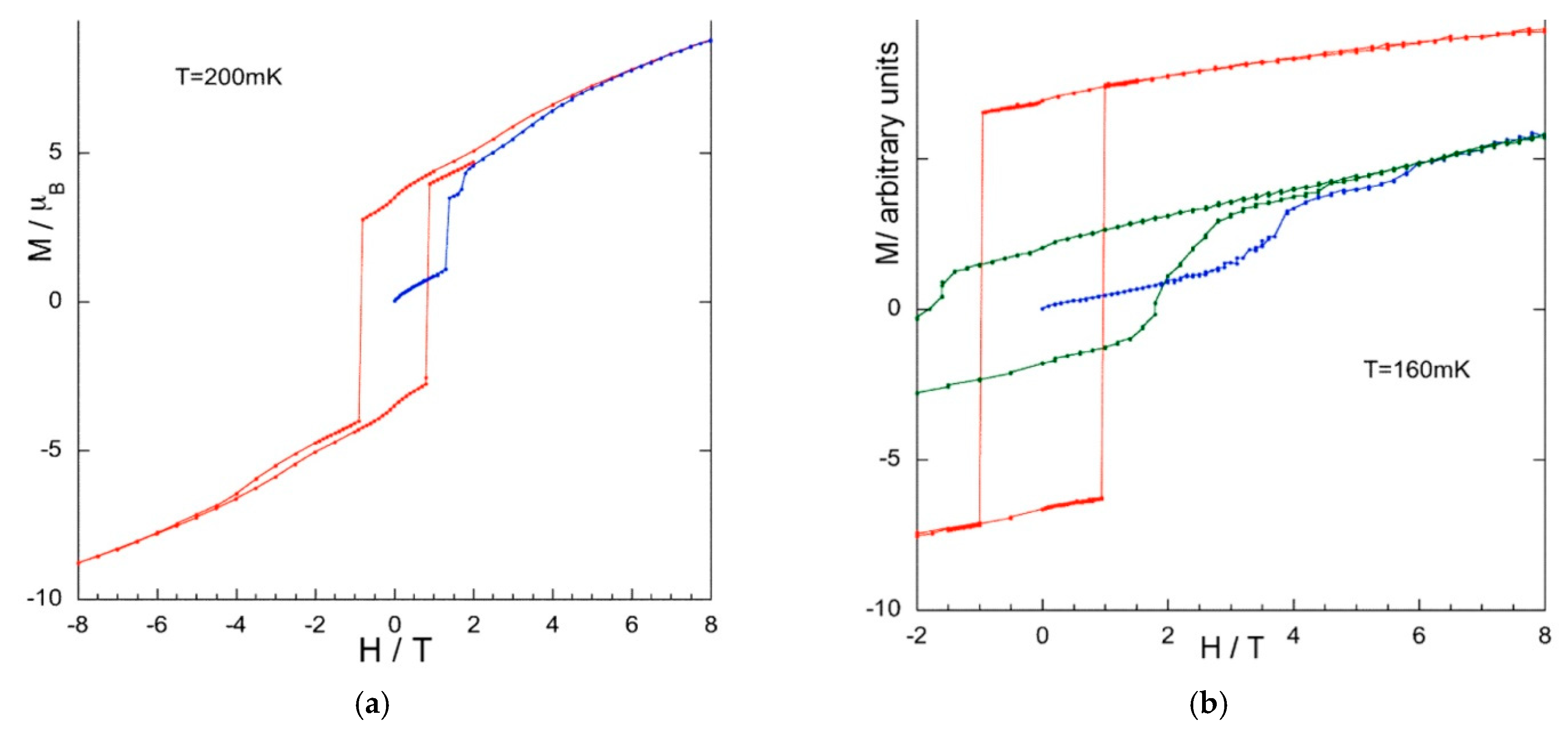
Magnetic Measurements at Very Low Temperatures
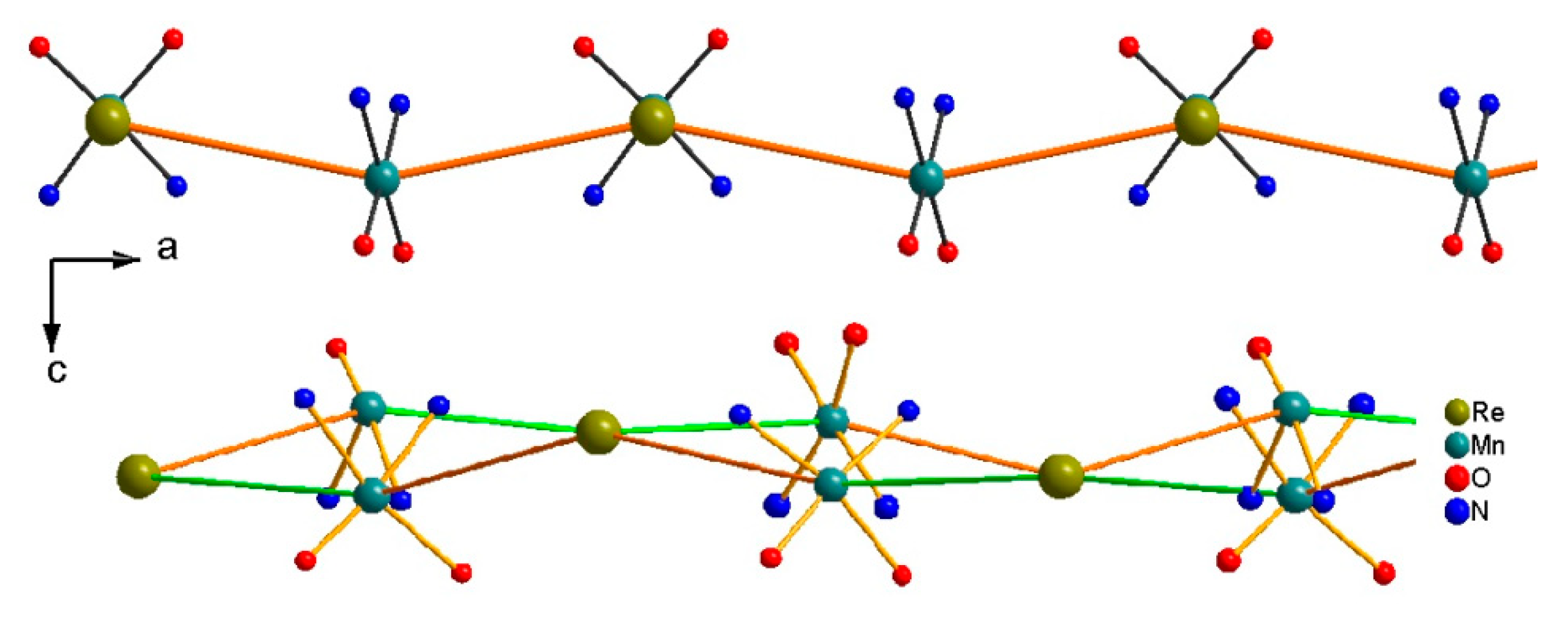
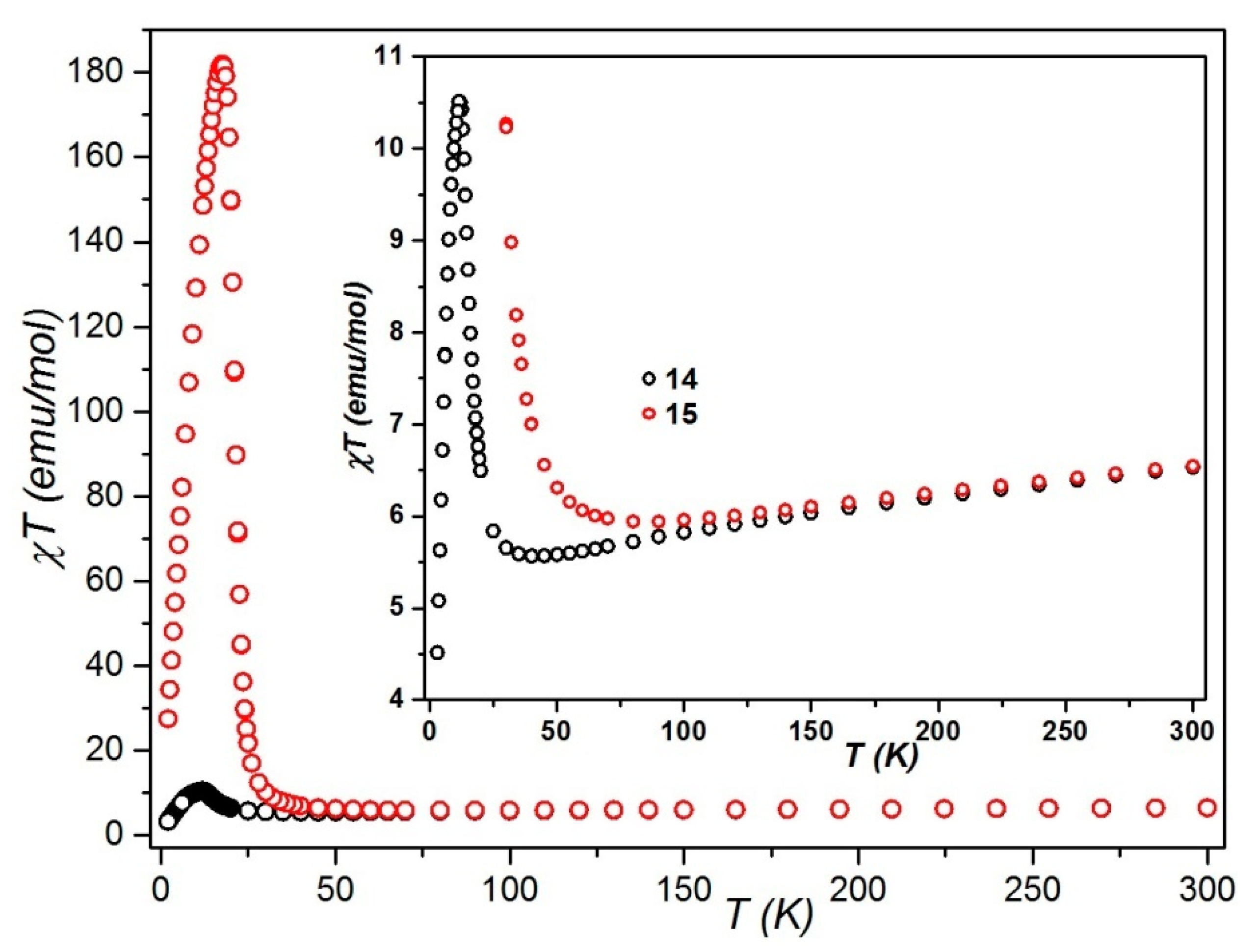
3.3.3. 2D {Cat[{Mn(acacen)}2ReIII(CN)7]}n, Cat = Ph4P+ (14) and PPN+(15)
Crystal Structure Descriptions
Static Magnetic Studies
Dynamic Magnetic Studies
3.3.4. Heterobimetallic Assemblies of [ReIV(CN)7]3− with Some Monocationic Complexes [MnIII(SB)]+ of Salen-Type Schiff Bases
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Mironov, V.S.; Chibotaru, L.F.; Ceulemans, A. Mechanism of a Strongly Anisotropic MoIII−CN−MnII Spin−Spin Coupling in Molecular Magnets Based on the [Mo(CN)7]4− Heptacyanometalate: A New Strategy for Single-Molecule Magnets with High Blocking Temperatures. J. Am. Chem. Soc. 2003, 125, 9750–9760. [Google Scholar] [CrossRef] [PubMed]
- Mironov, V.S. New Approaches to the Problem of High-Temperature Single-Molecule Magnets. Dokl. Phys. Chem. 2006, 408, 130–136. [Google Scholar] [CrossRef]
- Freedman, D.E.; Jenkins, D.M.; Iavarone, A.T.; Long, J.R. A Redox-Switchable Single-Molecule Magnet Incorporating [Re(CN)7]3−. J. Am. Chem. Soc. 2008, 130, 2884–2885. [Google Scholar] [CrossRef] [PubMed]
- Zadrozny, J.M.; Freedman, D.E.; Jenkins, D.M.; Harris, T.D.; Iavarone, A.T.; Mathonière, C.; Clérac, R.; Long, J.R. Slow Magnetic Relaxation and Charge-Transfer in Cyano-Bridged Coordination Clusters Incorporating [Re(CN)7]3−/4−. Inorg. Chem. 2010, 49, 8886–8896. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Y.; Avendaño, C.; Dunbar, K.R. Molecular Magnetic Materials Based on 4d and 5d Transition Metals. Chem. Soc. Rev. 2011, 40, 3213. [Google Scholar] [CrossRef]
- Qian, K.; Huang, X.-C.; Zhou, C.; You, X.-Z.; Wang, X.-Y.; Dunbar, K.R. A Single-Molecule Magnet Based on Heptacyanomolybdate with the Highest Energy Barrier for a Cyanide Compound. J. Am. Chem. Soc. 2013, 135, 13302–13305. [Google Scholar] [CrossRef]
- Wang, Q.-L.; Southerland, H.; Li, J.-R.; Prosvirin, A.V.; Zhao, H.; Dunbar, K.R. Crystal-to-Crystal Transformation of Magnets Based on Heptacyanomolybdate (III) Involving Dramatic Changes in Coordination Mode and Ordering Temperature. Angew. Chem. Int. Ed. 2012, 51, 9321. [Google Scholar] [CrossRef]
- Shi, L.; Wei, X.; Wang, X.; Wu, D. Research Progress in Molecular Magnetic Materials Based on the [Mo(CN)7]4− Unit. Sci. Sin. Chim. 2020, 50, 1637–1653. [Google Scholar] [CrossRef]
- Miyasaka, H.; Saitoh, A.; Abe, S. Magnetic Assemblies Based on Mn(III) Salen Analogues. Coord. Chem. Rev. 2007, 251, 2622–2664. [Google Scholar] [CrossRef]
- Coulon, C.; Miyasaka, H.; Clérac, R. Single-Chain Magnets: Theoretical Approach and Experimental Systems BT. In Single-Molecule Magnets and Related Phenomena; Winpenny, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 163–206. ISBN 978-3-540-33240-4. [Google Scholar]
- Sun, H.-L.; Wang, Z.-M.; Gao, S. Strategies towards Single-Chain Magnets. Coord. Chem. Rev. 2010, 254, 1081–1100. [Google Scholar] [CrossRef]
- Bar, A.K.; Pichon, C.; Sutter, J.-P. Magnetic Anisotropy in Two- to Eight-Coordinated Transition–Metal Complexes: Recent Developments in Molecular Magnetism. Coord. Chem. Rev. 2016, 308, 346–380. [Google Scholar] [CrossRef]
- Coulon, C.; Pianet, V.; Urdampilleta, M.; Clérac, R. Single-Chain Magnets and Related Systems BT. In Molecular Nanomagnets and Related Phenomena; Gao, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 143–184. ISBN 978-3-662-45723-8. [Google Scholar]
- Peresypkina, E.V.; Majcher, A.M.; Rams, M.; Vostrikova, K.E. A Single Chain Magnet Involving Hexacyanoosmate. Chem. Commun. 2014, 50, 7150–7153. [Google Scholar] [CrossRef] [PubMed]
- Majcher, A.M.; Pilet, G.; Mironov, V.S.; Vostrikova, K.E. Neutral Low-Dimensional Assemblies of a Mn(III) Schiff Base Complex and Octacyanotungstate(V): Synthesis, Characterization, and Magnetic Properties. Magnetochemistry 2017, 3, 16. [Google Scholar] [CrossRef]
- Pedersen, K.S.; Bendix, J.; Clérac, R. Single-molecule magnet engineering: Building-block approaches. Chem. Commun. 2014, 50, 4396–4415. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Shao, D.; Wei, X.; Dunbar, K.R.; Wang, X. Enhanced Single-Chain Magnet Behavior via Anisotropic Exchange in a Cyano-Bridged MoIII–MnII Chain. Angew. Chem. Int. Ed. 2020, 59, 10379–10384. [Google Scholar] [CrossRef]
- Charytanowicz, T.; Jankowski, R.; Zychowicz, M.; Chorazy, S.; Sieklucka, B. The Rationalized Pathway from Field-Induced Slow Magnetic Relaxation in CoII–WIV Chains to Single-Chain Magnetism in Isotopological CoII–WV Analogues. Inorg. Chem. Front. 2022, 9, 1152–1170. [Google Scholar] [CrossRef]
- Tan, P.; Yang, Y.; Lv, W.; Jing, R.; Cui, H.; Zheng, S.-J.; Chen, L.; Yuan, A.; Chen, X.-T.; Zhao, Y. A Cyanometallate- and Carbonate-Bridged Dysprosium Chain Complex with a Pentadentate Macrocyclic Ligand: Synthesis, Structure, and Magnetism. N. J. Chem. 2022, 46, 7892–7898. [Google Scholar] [CrossRef]
- Miyasaka, H.; Madanbashi, T.; Saitoh, A.; Motokawa, N.; Ishikawa, R.; Yamashita, M.; Bahr, S.; Wernsdorfer, W.; Clérac, R. Cyano-Bridged MnIII-MIII Single-Chain Magnets with MIII = CoIII, FeIII, MnIII, and CrIII. Chem. Eur. J. 2012, 18, 3942–3954. [Google Scholar] [CrossRef]
- Rams, M.; Peresypkina, E.V.; Mironov, V.S.; Wernsdorfer, W.; Vostrikova, K.E. Magnetic Relaxation of 1D Coordination Polymers X2[Mn(acacen)Fe(CN)6], X = Ph4P+, Et4N+. Inorg. Chem. 2014, 53, 10291–10300. [Google Scholar] [CrossRef]
- Ferbinteanu, M.; Miyasaka, H.; Wernsdorfer, W.; Nakata, K.; Sugiura, K.; Yamashita, M.; Coulon, C.; Clérac, R. Single-Chain Magnet (NEt4)[Mn2(5-MeOsalen)2Fe(CN)6] Made of MnIII−FeIII−MnIII Trinuclear Single-Molecule Magnet with an S = 9/2 Spin Ground State. J. Am. Chem. Soc. 2005, 127, 3090–3099. [Google Scholar] [CrossRef]
- Aguilà, D.; Jeannin, O.; Fourmigué, M.; Jeon, I.-R. MnIII–FeIII Heterometallic Compounds within Hydrogen-Bonded Supramolecular Networks Promoted by an [Fe(CN)5(CNH)]2− Building Block: Structural and Magnetic Properties. Inorg. Chem. 2018, 57, 7892–7903. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.-Q.; Qian, K.; Wei, H.-Y.; Wang, X.-Y. A One-Dimensional Magnet Based on [MoIII(CN)7]4−. Inorg. Chem. 2016, 55, 5107–5109. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Xia, B.; Wang, Q.-L.; Ma, Y.; Liao, D.-Z.; Tang, J. Slow Magnetic Relaxation Based on the Anisotropic Ising-Type Magnetic Coupling between the MoIII and MnII Centers. Dalton Trans. 2017, 46, 1042–1046. [Google Scholar] [CrossRef] [PubMed]
- Larionova, J.; Kahn, O.; Gohlen, S.; Ouahab, L.; Clérac, R. Structure, Ferromagnetic Ordering, Anisotropy, and Spin Reorientation for the Two-Dimensional Cyano-Bridged Bimetallic Compound K2Mn3(H2O)6[Mo(CN)7]2·6H2O. J. Am. Chem. Soc. 1999, 121, 3349–3356. [Google Scholar] [CrossRef]
- Larionova, J.; Clérac, R.; Sanchiz, J.; Kahn, O.; Golhen, S.; Ouahab, L. Ferromagnetic Ordering, Anisotropy, and Spin Reorientation for the Cyano-Bridged Bimetallic Compound Mn2(H2O)5Mo(CN)7·4H2O (α Phase). J. Am. Chem. Soc. 1998, 120, 13088–13095. [Google Scholar] [CrossRef]
- Tomono, K.; Tsunobuchi, Y.; Nakabayashi, K.; Ohkoshi, S. Vanadium(II) Heptacyanomolybdate(III)-Based Magnet Exhibiting a High Curie Temperature of 110 K. Inorg. Chem. 2010, 49, 1298–1300. [Google Scholar] [CrossRef]
- Gatteschi, D.; Sessoli, R.; Villain, J. Molecular Nanomagnets; Oxford University Press: Oxford, UK, 2006. [Google Scholar] [CrossRef]
- Gao, S. Molecular Nanomagnets and Related Phenomena; Springer: New York, NY, USA, 2015; Volume 164. [Google Scholar]
- Juan, B.; Fernando, L.; Fernández, J.F. (Eds.) Molecular Magnets: Physics and Applications. In NanoScience and Technology; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–395. [Google Scholar]
- Dhers, S.; Feltham, H.L.C.; Brooker, S. A Toolbox of Building Blocks, Linkers and Crystallisation Methods Used to Generate Single-Chain Magnets. Coord. Chem. Rev. 2015, 296, 24–44. [Google Scholar] [CrossRef]
- Glauber, R.J. Time-Dependent Statistics of the Ising Model. J. Math. Phys. 1963, 4, 294–307. [Google Scholar] [CrossRef]
- Coulon, C.; Clérac, R.; Lecren, L.; Wernsdorfer, W.; Miyasaka, H. Glauber dynamics in a single-chain magnet: From theory to real systems. Phys. Rev. B 2004, 69, 132408. [Google Scholar] [CrossRef]
- Zhang, W.X.; Breedlove, B.; Ishikawa, R.; Yamashita, M. Single-chain magnets: Beyond the Glauber model. RSC Adv. 2013, 3, 3772–3798. [Google Scholar] [CrossRef]
- Chibotaru, L.F.; Hendrickx, M.F.A.; Clima, S.; Larionova, J.; Ceulemans, A.J. Magnetic Anisotropy of [Mo(CN)7]4- Anions and Fragments of Cyano-Bridged Magnetic Networks. Phys. Chem. A 2005, 109, 7251–7257. [Google Scholar] [CrossRef] [PubMed]
- Manoli, J.M.; Potvin, C.; Bregeault, J.M.J. Crystal structure of potassium heptacyanorhenate(III) dehydrate. Chem. Soc. Dalton Trans. 1980, 1, 192–195. [Google Scholar] [CrossRef]
- Bennett, M.V.; Long, J.R. New Cyanometalate Building Units: Synthesis and Characterization of [Re(CN)7]3− and [Re(CN)8]3−. J. Am. Chem. Soc. 2003, 125, 2394–2395. [Google Scholar] [CrossRef]
- Mironov, V.S. Origin of Dissimilar Single-Molecule Magnet Behavior of Three MnII2MoIII Complexes Based on [MoIII(CN)7]4− Heptacyanomolybdate: Interplay of MoIII–CN–MnII Anisotropic Exchange Interactions. Inorg. Chem. 2015, 54, 11339–11355. [Google Scholar] [CrossRef] [PubMed]
- Rossman, G.R.; Tsay, F.D.; Gray, H.B. Spectroscopic and magnetic properties of heptacyanomolybdate(III). Evidence for pentagonal-bipyramidal and monocapped trigonal-prismatic structures. Inorg. Chem. 1973, 12, 824–829. [Google Scholar] [CrossRef]
- Peresypkina, E.V.; Samsonenko, D.G.; Vostrikova, K.E. Heterobimetallic coordination polymers involving 3d metal complexes and heavier transition metals cyanometallates. J. Solid State Chem. 2015, 224, 107–114. [Google Scholar] [CrossRef]
- Bonadies, J.A.; Kirk, M.L.; Lah, M.S.; Kessissoglou, D.P.; Hatfield, W.E.; Pecoraro, V.L. Structurally diverse manganese(III) Schiff base complexes: Chains, dimers, and cages. Inorg. Chem. 1989, 28, 2037–2044. [Google Scholar] [CrossRef]
- Peresypkina, E.V.; Vostrikova, K.E. 2[Mn(acacen)]+ + [Fe(CN)5NO]2− Polynuclear Heterobimetallic Coordination Compounds of Different Dimensionality in the Solid State. Dalton Trans. 2012, 41, 4100. [Google Scholar] [CrossRef]
- Samsonenko, D.G.; Paulsen, C.; Lhotel, E.; Mironov, V.S.; Vostrikova, K.E. [MnIII(SchiffBase)]3[ReIV(CN)7], Highly Anisotropic 3D Coordination Framework: Synthesis, Crystal Structure, Magnetic Investigations, and Theoretical Analysis. Inorg. Chem. 2014, 53, 10217–10231. [Google Scholar] [CrossRef]
- Wu, D.-Q.; Shao, D.; Wei, X.-Q.; Shen, F.-X.; Shi, L.; Kempe, D.; Zhang, Y.-Z.; Dunbar, K.R.; Wang, X.-Y. Reversible On–Off Switching of a Single-Molecule Magnet via a Crystal-to-Crystal Chemical Transformation. J. Am. Chem. Soc. 2017, 139, 11714–11717. [Google Scholar] [CrossRef]
- Sukhikh, T.; Wernsdorfer, W.; Vostrikova, K. Slow Magnetic Relaxation in Neutral 0D and 1D Assemblies of a Mn(III) Schiff Base Complex and Heptacyanorhenate(IV). Magnetochemistry 2022, 8, 126. [Google Scholar] [CrossRef]
- Ishikawa, R.; Nakano, M.; Breedlove, B.K.; Yamashita, M. Syntheses, Structures, and Magnetic Properties of Discrete Cyano-Bridged Heterodinuclear Complexes Composed of MnIII(Salen)-Type Complex and MIII(CN)6 Anion (MIII = Fe, Mn, and Cr). Polyhedron 2013, 64, 346–351. [Google Scholar] [CrossRef]
- Vostrikova, K.E. Homoleptic Osmium Cyanide Complexes: Synthesis and Perspective Application in Molecular Magnetism. In Osmium: Synthesis Characterization and Applications; Wise, G., Ed.; Nova Science Publishers: New York, NY, USA, 2015; pp. 43–78. ISBN 978-1-63483-517-6. [Google Scholar]
- Lescouëzec, R.; Toma, L.M.; Vaissermann, J.; Verdaguer, M.; Delgado, F.S.; Ruiz-Pérez, C.; Lloret, F.; Julve, M. Design of Single Chain Magnets through Cyanide-Bearing Six-Coordinate Complexes. Coord. Chem. Rev. 2005, 249, 2691–2729. [Google Scholar] [CrossRef]
- Sakamoto, F.; Sumiya, T.; Fujita, M.; Tada, T.; Tan, X.S.; Suzuki, E.; Okura, I.; Fujii, Y. T-Site Selective Photocleavage of DNA by Cationic Schiff Base Complex of Manganese(III). Chem. Lett. 1998, 27, 1127–1128. [Google Scholar] [CrossRef]
- Pinkowicz, D.; Podgajny, R.; Nowicka, B.; Chorazy, S.; Reczyński, M.; Sieklucka, B. Magnetic Clusters Based on Octacyanidometallates. Inorg. Chem. Front. 2015, 2, 10–27. [Google Scholar] [CrossRef]
- Harris, T.D.; Bennett, M.V.; Clérac, R.; Long, J.R. [ReCl4(CN)2]2−: A High Magnetic Anisotropy Building Unit Giving Rise to the Single-Chain Magnets (DMF)4MReCl4(CN)2 (M = Mn, Fe, Co, Ni). J. Am. Chem. Soc. 2010, 132, 3980–3988. [Google Scholar] [CrossRef]
- Miyasaka, H.; Ieda, H.; Re, N.; Crescenzi, R.; Floriani, C. Assembling Bi-, Tri- and Pentanuclear Complexes into Extended Structures Using a Desolvation Reaction: Synthesis, Structure, and Magnetic Properties of Manganese(III)−Schiff-Base−Hexacyanoferrate Polymeric Compounds and Their Derived Extended Structures. Inorg. Chem. 1998, 37, 255–263. [Google Scholar] [CrossRef]
- Nakamura, K.; Sasada, T. Statistical Mechanics of Classical One-Dimensional Heisenberg Ferromagnets with Single-Site Anisotropy. J. Phys. C Solid State Phys. 1978, 11, 331–343. [Google Scholar] [CrossRef]
- Miyasaka, H.; Julve, M.; Yamashita, M.; Clérac, R. Slow Dynamics of the Magnetization in One-Dimensional Coordination Polymers: Single-Chain Magnets. Inorg. Chem. 2009, 48, 3420–3437. [Google Scholar] [CrossRef]
- Moreno Pineda, E.; Wernsdorfer, W.; Vostrikova, K.E. Very Anisotropic 2D Molecular Magnetic Materials Based on Pentagonal Bipyramidal Heptacyanidorhenate(IV). Materials 2022, 15, 8324. [Google Scholar] [CrossRef]
- Miyasaka, H.; Okawa, H.; Miyazaki, A.; Enoki, T. Synthesis, Crystal and Network Structures, and Magnetic Properties of a Hybrid Layered Compound: [K(18-Cr)(2-PrOH)2][{Mn(Acacen)}2{Fe(CN)6}] (18-Cr = 18-Crown-6-Ether, Acacen = N,N‘-Ethylenebis(Acetylacetonylideneiminate)). Inorg. Chem. 1998, 37, 4878–4883. [Google Scholar] [CrossRef] [PubMed]
- Kou, H.-Z.; Ni, Z.-H.; Zhou, B.C.; Wang, R.-J. A Cyano-Bridged Molecule-Based Magnet Containing Manganese(III) Schiff Base and Octacyanotungstate(V) Building Blocks. Inorg. Chem. Commun. 2004, 7, 1150–1153. [Google Scholar] [CrossRef]
- Sukhikh, T.; Vostrikova, K. Assembly of Mn(III) Schiff Base Complexes with Heptacyanorhenate(IV). Inorganics 2017, 5, 59. [Google Scholar] [CrossRef]
- Zhang, C.-G.; Tian, G.-H.; Ma, Z.-F.; Yan, D.-Y. Synthesis, crystal structure and properties of a novel di-μ2-aqua bridged binuclear manganese(III) Schiff base complex [Mn(vanen)(H2O)2]2(ClO4)2·2H2O. Trans. Met. Chem. 2000, 25, 270–273. [Google Scholar] [CrossRef]
- Manakin, Y.V.; Mironov, V.S.; Bazhenova, T.A.; Lyssenko, K.A.; Gilmutdinov, I.F.; Bikbaev, K.S.; Masitov, A.A.; Yagubskii, E.B. (Et4N)[MoIII(DAPBH)Cl2], the first pentagonal-bipyramidal Mo(III) complex with a N3O2-type Schiff-base ligand: Manifestation of unquenched orbital momentum and Ising-type magnetic anisotropy. Chem. Commun. 2018, 54, 10084–10087. [Google Scholar] [CrossRef]














Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vostrikova, K.E. Application of the Heptacyanidorhenate(IV) as a Metalloligand in the Design of Molecular Magnets. Magnetochemistry 2022, 8, 189. https://doi.org/10.3390/magnetochemistry8120189
Vostrikova KE. Application of the Heptacyanidorhenate(IV) as a Metalloligand in the Design of Molecular Magnets. Magnetochemistry. 2022; 8(12):189. https://doi.org/10.3390/magnetochemistry8120189
Chicago/Turabian StyleVostrikova, Kira E. 2022. "Application of the Heptacyanidorhenate(IV) as a Metalloligand in the Design of Molecular Magnets" Magnetochemistry 8, no. 12: 189. https://doi.org/10.3390/magnetochemistry8120189
APA StyleVostrikova, K. E. (2022). Application of the Heptacyanidorhenate(IV) as a Metalloligand in the Design of Molecular Magnets. Magnetochemistry, 8(12), 189. https://doi.org/10.3390/magnetochemistry8120189