Magnetic Field Assisted Spark Discharge-Generated Gold Nanostructures: XPS Study of Nitrogen Gas Fate and Chemical Composition of Gold Thin Films
Abstract
1. Introduction
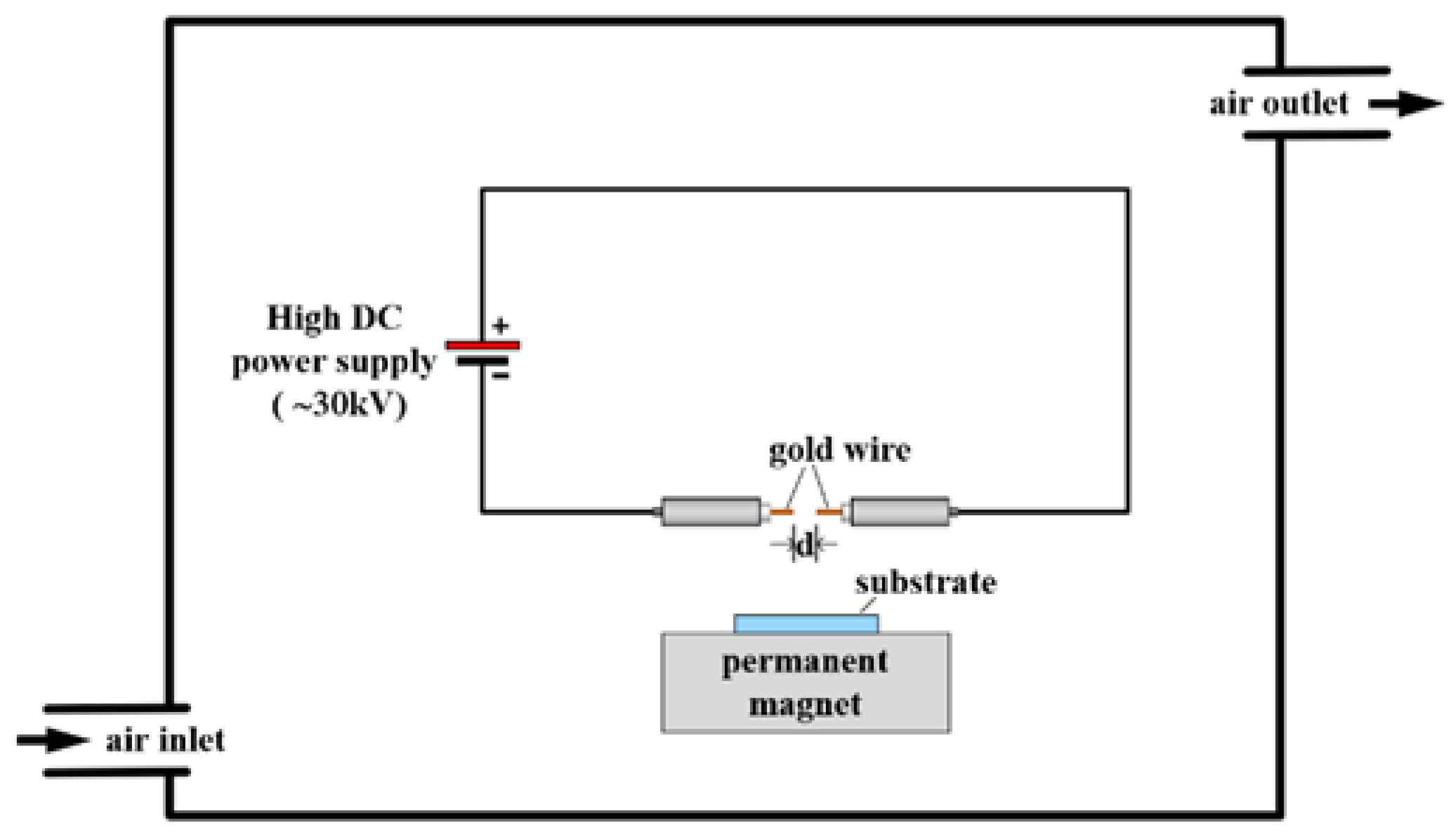
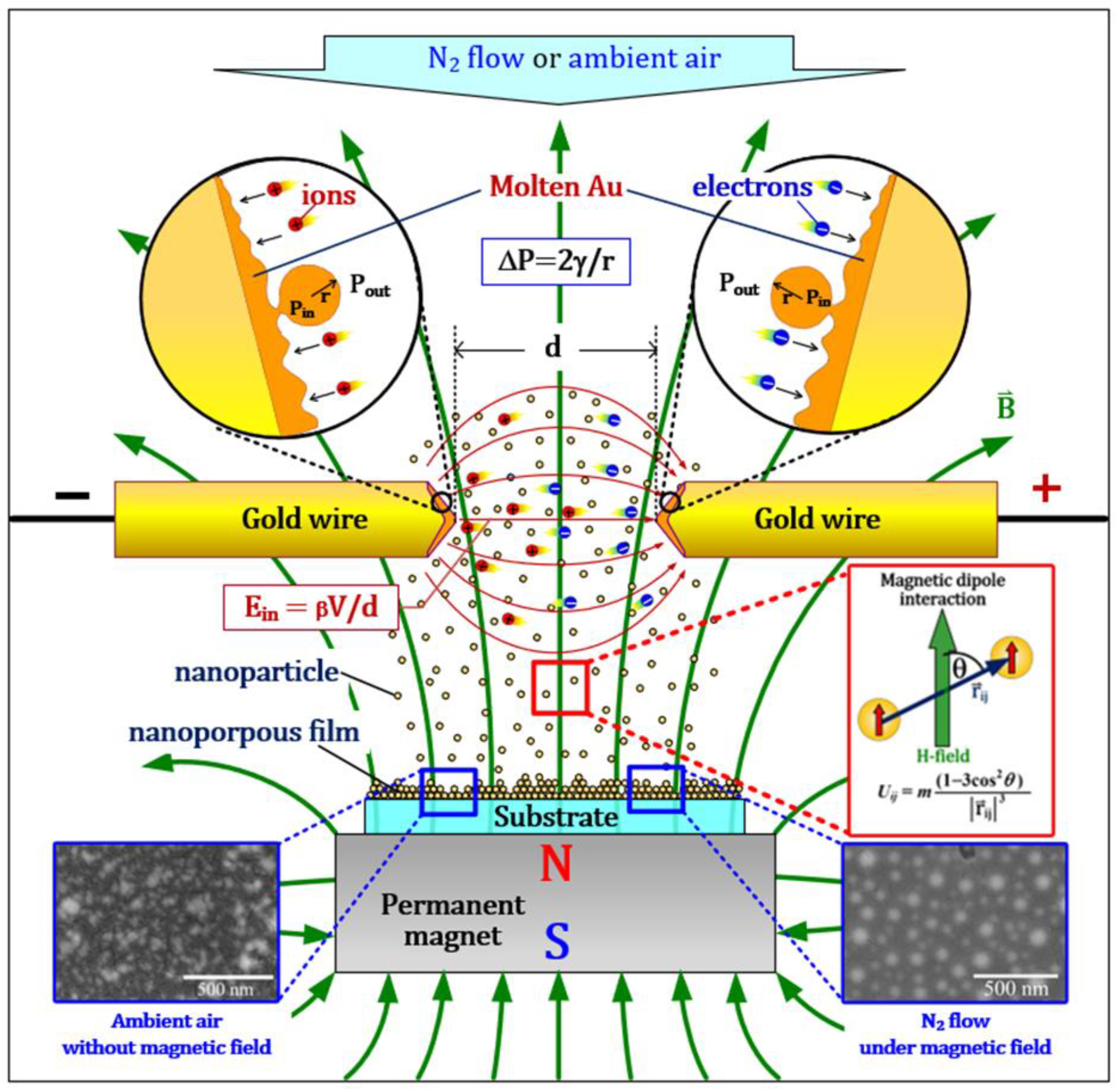
2. Experimental Setup
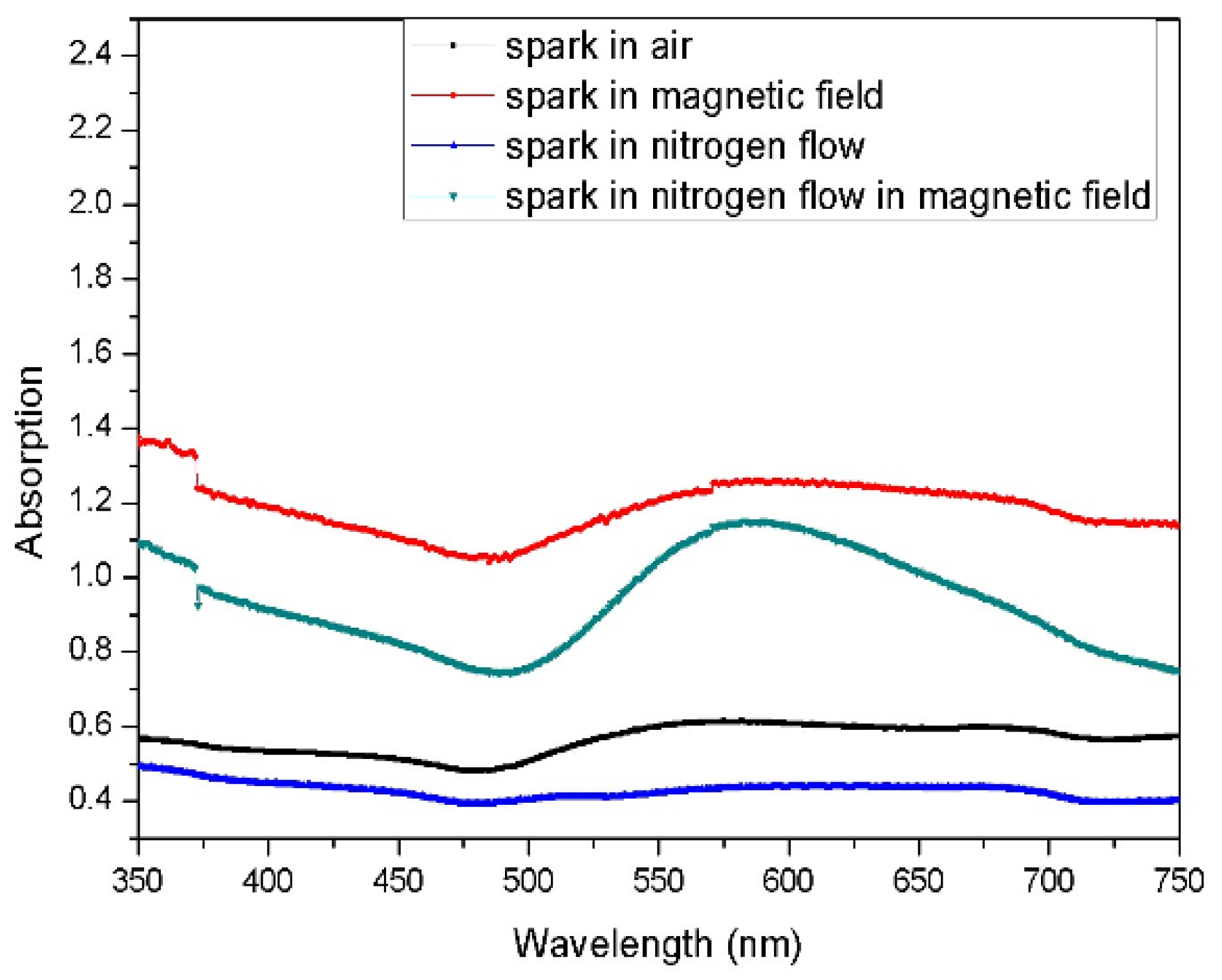
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, X.; Xu, W.; Liu, G.; Panda, D.; Chen, P. Size-Dependent Catalytic Activity and Dynamics of Gold Nanoparticles at the Single-Molecule Level. J. Am. Chem. Soc. 2009, 132, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, Y.; Ding, T.; Liu, J.; Zhao, H. Multifunctional Gold Nanoparticles: A Novel Nanomaterial for Various Medical Applications and Biological Activities. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Mokammel, M.A.; Islam, M.J.; Hasanuzzaman, M.; Hashmi, S. Nanoscale Materials for Self-Cleaning and Antibacterial Applications. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Messing, M.E.; Hillerich, K.; Johansson, J.; Deppert, K.; Dick, K.A. The Use of Gold for Fabrication of Nanowire Structures. Gold Bull. 2009, 42, 172–181. [Google Scholar] [CrossRef]
- Efimov, A.; Arsenov, P.; Borisov, V.; Buchnev, A.; Lizunova, A.; Kornyushin, D.; Tikhonov, S.; Musaev, A.; Urazov, M.; Shcherbakov, M.; et al. Synthesis of Nanoparticles by Spark Discharge as a Facile and Versatile Technique of Preparing Highly Conductive Pt Nano-Ink for Printed Electronics. Nanomaterials 2021, 11, 234. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, N.S.; Ullmann, M.; Vons, V.A.; Lafont, U.; Schmidt-Ott, A. Generation of Nanoparticles by Spark Discharge. J. Nanopart. Res. 2008, 11, 315–332. [Google Scholar] [CrossRef]
- Stefan, R.; Jakmunee, J.; Punyodom, W.; Singjai, P. A novel strategy for longevity prolongation of iron-based nanoparticle thin films by Applied Magnetic Force. New J. Chem. 2018, 42, 4807–4810. [Google Scholar]
- Ručman, S.; Punyodom, W.; Jakmunee, J.; Singjai, P. Inducing Crystallinity of Metal Thin Films with Weak Magnetic Fields without Thermal Annealing. Crystals 2018, 8, 362. [Google Scholar] [CrossRef]
- Kumpika, T.; Ručman, S.; Polin, S.; Kantarak, E.; Sroila, W.; Thongsuwan, W.; Panthawan, A.; Sanmuangmoon, P.; Jhuntama, N.; Singjai, P. Studies on the Characteristics of Nanostructures Produced by Sparking Discharge Process in the Ambient Atmosphere for Air Filtration Application. Crystals 2021, 11, 140. [Google Scholar] [CrossRef]
- Kumar, P.; Mathpal, M.C.; Ghosh, S.; Inwati, G.K.; Maze, J.R.; Duvenhage, M.-M.; Roos, W.D.; Swart, H.C. Plasmonic Au nanoparticles embedded in glass: Study of Tof-Sims, XPS and its enhanced antimicrobial activities. J. Alloys Compd. 2022, 909, 164789. [Google Scholar] [CrossRef]
- Kumar, P.; Mathpal, M.C.; Jagannath, G.; Prakash, J.; Maze, J.-R.; Roos, W.D.; Swart, H.C. Optical limiting applications of resonating plasmonic au nanoparticles in a dielectric glass medium. Nanotechnology 2021, 32, 345709. [Google Scholar] [CrossRef]
- Reinecke, B.N.; Kuhl, K.P.; Ogasawara, H.; Li, L.; Voss, J.; Abild-Pedersen, F.; Nilsson, A.; Jaramillo, T.F. Elucidating the electronic structure of supported gold nanoparticles and its relevance to catalysis by means of hard X-ray photoelectron spectroscopy. Surf. Sci. 2016, 650, 24–33. [Google Scholar] [CrossRef]
- Cuenya, B.R.; Baeck, S.-H.; Jaramillo, T.F.; McFarland, E.W. Size- and Support-Dependent Electronic and Catalytic Properties of Au0/Au3+ Nanoparticles Synthesized from Block Copolymer Micelles. J. Am. Chem. Soc. 2003, 125, 12928–12934. [Google Scholar] [CrossRef]
- Quintero, J.H.; Mariño, A.; Šiller, L.; Restrepo-Parra, E.; Caro-Lopera, F.J. Rocking curves of gold nitride species prepared by arc pulsed - physical assisted plasma vapor deposition. Surf. Coat. Technol. 2017, 309, 249–257. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, A. Absolute work function measurement by using photoelectron spectroscopy. Curr. Appl. Phys. 2021, 31, 52–59. [Google Scholar] [CrossRef]
- Němec, T.; Šonský, J.; Gruber, J.; de Prado, E.; Kupčík, J.; Klementová, M. Platinum and platinum oxide nanoparticles generated by unipolar spark discharge. J. Aerosol Sci. 2020, 141, 105502. [Google Scholar] [CrossRef]
- Borisov, V.I.; Lizunova, A.A.; Malo, D.; Kameneva, E.I.; Ramanenka, A.A.; Ivanov, V.V. Synthesis of gold nanoparticles by the spark discharge method for visible plasmonics. J. Phys. Conf. Ser. 2021, 2086, 012002. [Google Scholar] [CrossRef]
- Svensson, C.R.; Ludvigsson, L.; Meuller, B.O.; Eggersdorfer, M.L.; Deppert, K.; Bohgard, M.; Pagels, J.H.; Messing, M.E.; Rissler, J. Characteristics of airborne gold aggregates generated by spark discharge and high temperature evaporation furnace: Mass–mobility relationship and surface area. J. Aerosol Sci. 2015, 87, 38–52. [Google Scholar] [CrossRef]
- Ghildiyal, P.; Biswas, P.; Herrera, S.; Mulholland, G.W.; Yang, Y.; Abbaschian, R.; Zachariah, M.R. Magnetic-field directed vapor-phase assembly of low fractal dimension metal nanostructures: Experiment and Theory. J. Phys. Chem. Lett. 2021, 12, 4085–4091. [Google Scholar] [CrossRef]
- Biswas, P.; Ghildiyal, P.; Mulholland, G.W.; Zachariah, M.R. Modelling and simulation of field directed linear assembly of aerosol particles. J. Colloid Interface Sci. 2021, 592, 195–204. [Google Scholar] [CrossRef]
- Wu, C.-M.; Li, C.-Y.; Kuo, Y.-T.; Wang, C.-W.; Wu, S.-Y.; Li, W.-H. Quantum Spins in Mackay Icosahedral Gold Nanoparticles. J. Nanopart. Res. 2009, 12, 177–185. [Google Scholar] [CrossRef]
- Thongpan, W.; Kumpika, T.; Kantarak, E.; Panthawan, A.; Pooseekheaw, P.; Singjai, P.; Tuantranont, A.; Thongsuwan, W. External-Electric-Field-Enhanced Uniformity and Deposition Rate of a TiO2 Film Prepared by the Sparking Process. Ukr. J. Phys. 2018, 63, 531. [Google Scholar] [CrossRef]
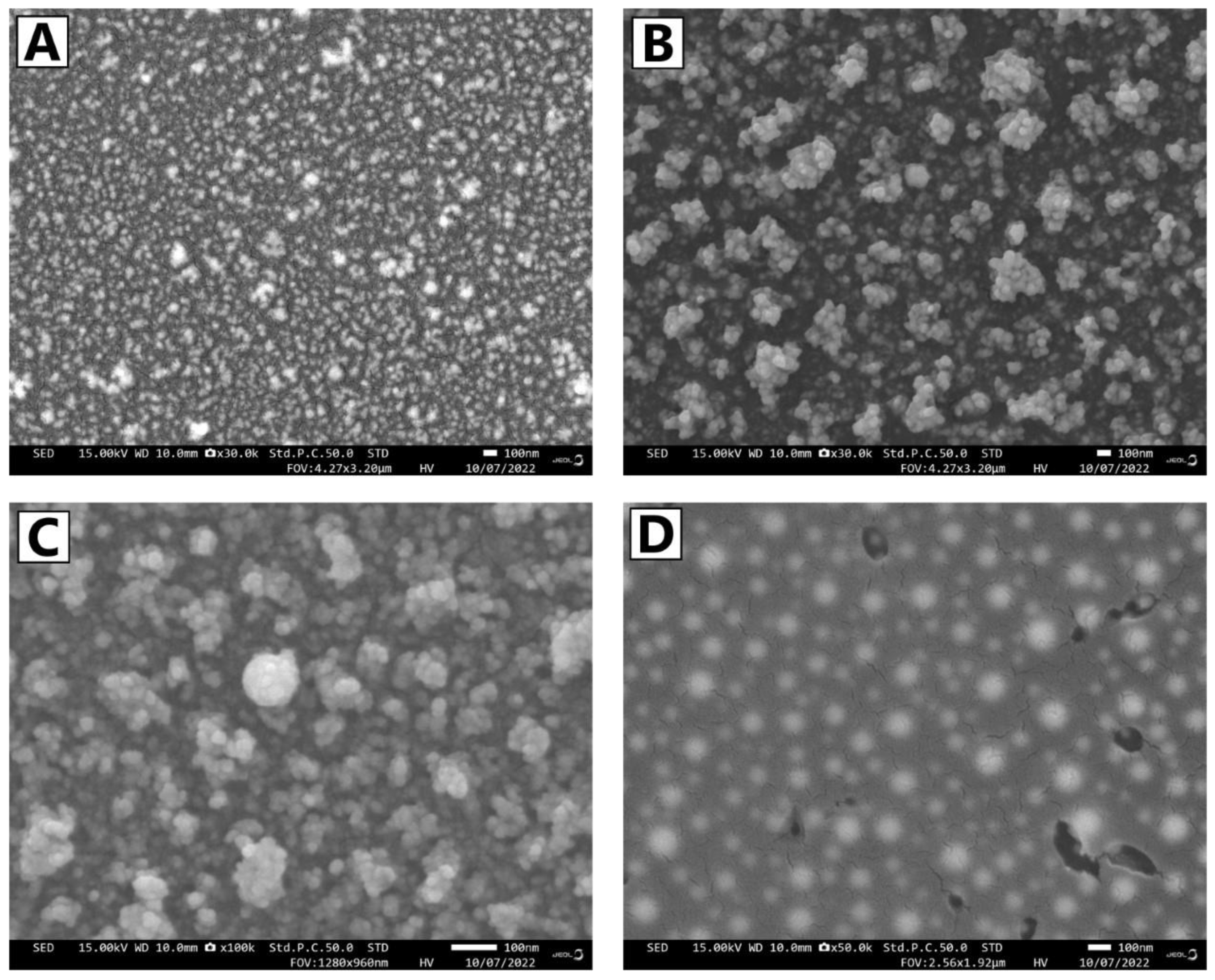
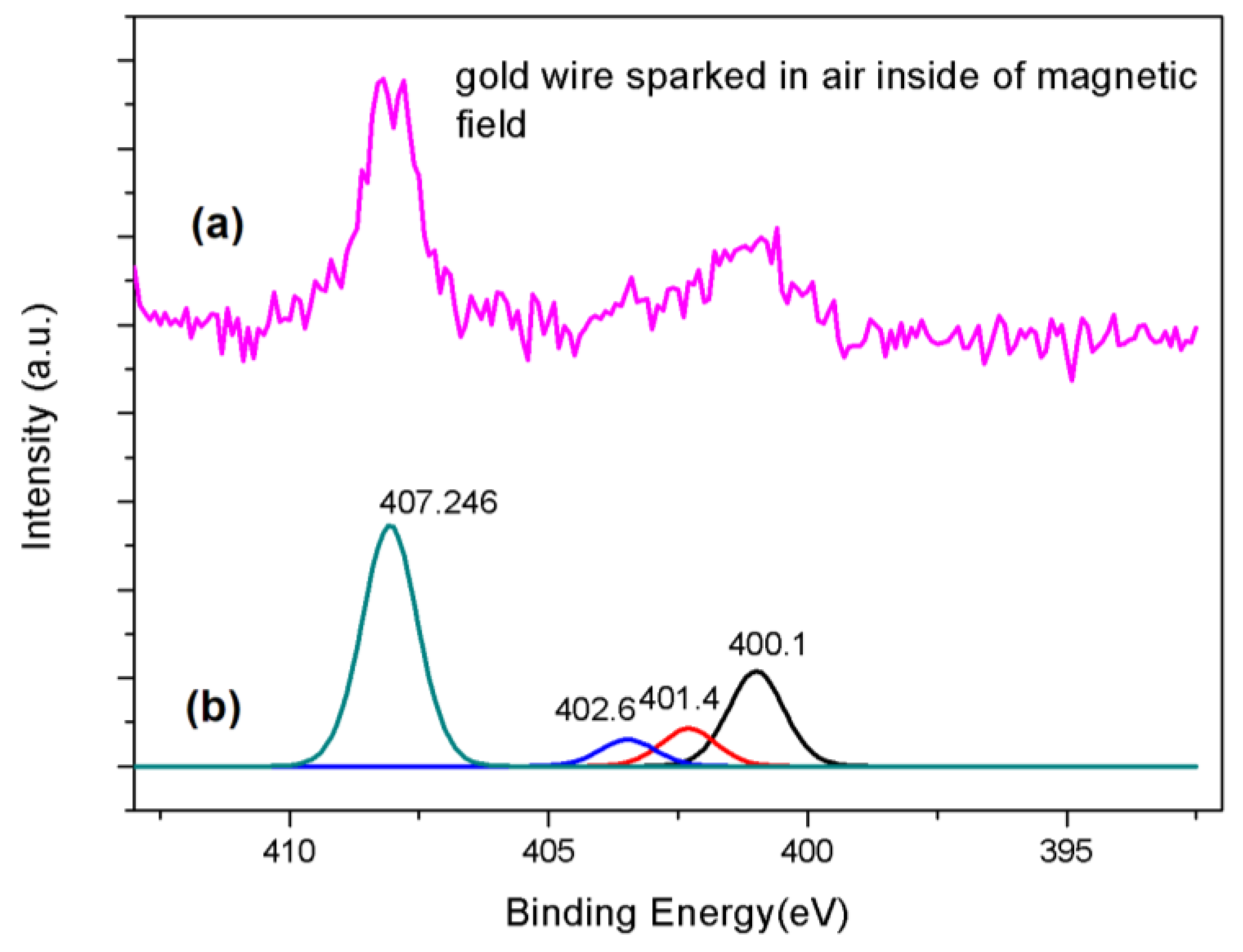
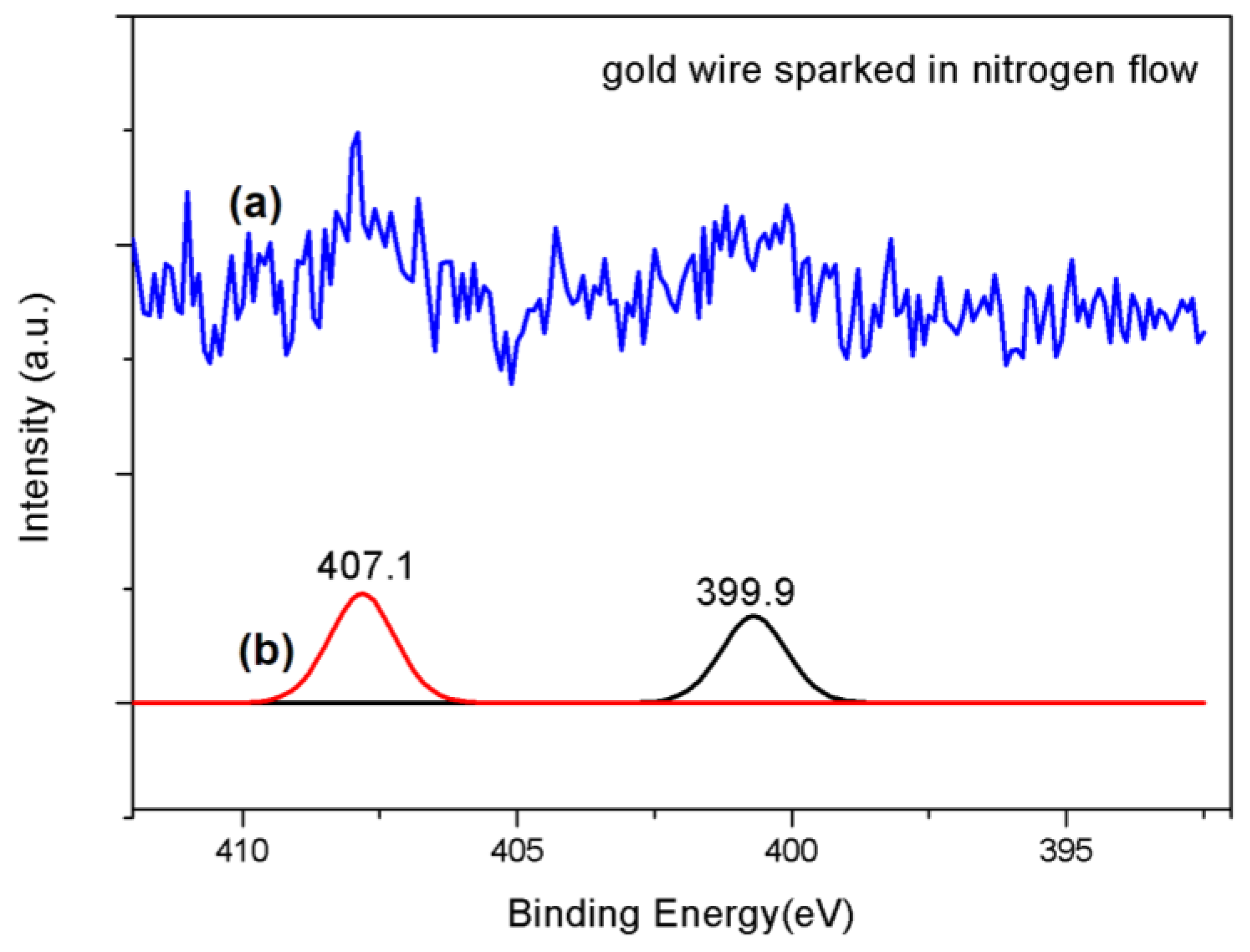

| Name of Sample | Number of Particles | Average Size (nm) | Standard Deviation | Min and Max (nm) |
|---|---|---|---|---|
| Au wire sparked in ambient air | 53 | 65.8 | 24.8 | 25 and 130.3 |
| Au sparked in magnetic field in air | 45 | 79.7 | 24 | 33.3 and 141.5 |
| Au sparked in nitrogen flow | 50 | 37.1 | 14.8 | 12.6 and 76.7 |
| Au in nitrogen flow under magnetic field | 63 | 77.3 | 20.1 | 36.7 and 137.1 |
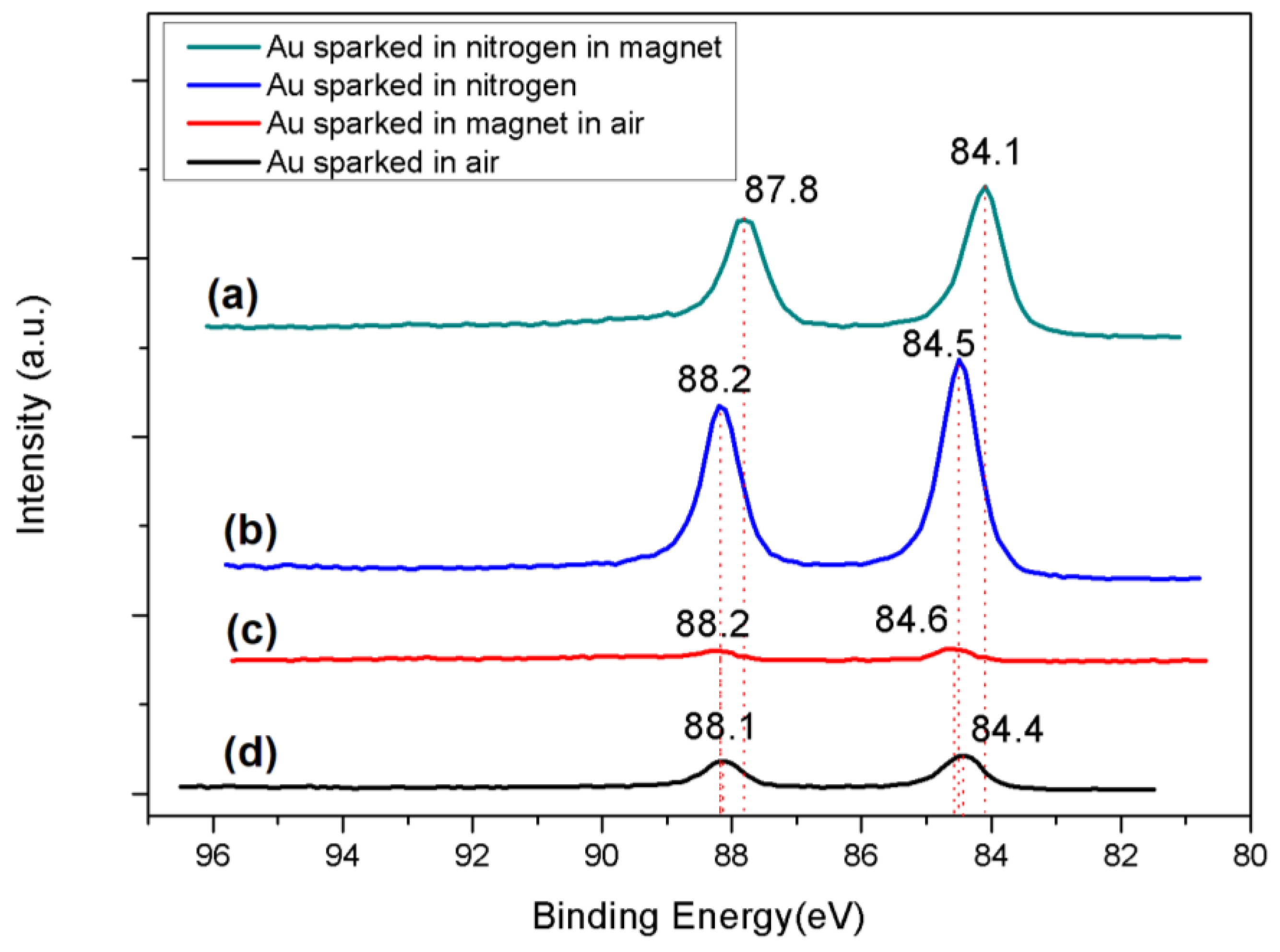
| Name of Sample | O 1s | C 1s | N 1s | Au 4f | Mass Conc of Au 4f (%) |
|---|---|---|---|---|---|
| Gold sparked in ambient air | 532.288 533.593 534.846 | 284.945 285.822 286.807 287.972 289.046 | - | 84.442 88.147 | 11.14% |
| Gold sparked in magnetic field in ambient air | 531.999 532.827 533.702 | 284.900 285.907 286.808 288.015 289.078 | 400.178 401.479 402.654 407.246 | 84.570 88.245 | 4.04% |
| Gold sparked in nitrogen flow | 531.434 532.275 533.236 534.197 | 284.997 286.161 286.949 288.310 289.116 | 399.996 407.127 | 84.483 88.154 | 44.48% |
| Gold sparked in nitrogen flow with magnetic field | 531.287 532.180 533.210 534.394 | 285.010 286.228 287.052 288.127 289.058 | - | 84.091 87.829 | 28.34% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ručman, S.; Thongpan, W.; Sroila, W.; Jhuntama, N.; Singjai, P. Magnetic Field Assisted Spark Discharge-Generated Gold Nanostructures: XPS Study of Nitrogen Gas Fate and Chemical Composition of Gold Thin Films. Magnetochemistry 2022, 8, 178. https://doi.org/10.3390/magnetochemistry8120178
Ručman S, Thongpan W, Sroila W, Jhuntama N, Singjai P. Magnetic Field Assisted Spark Discharge-Generated Gold Nanostructures: XPS Study of Nitrogen Gas Fate and Chemical Composition of Gold Thin Films. Magnetochemistry. 2022; 8(12):178. https://doi.org/10.3390/magnetochemistry8120178
Chicago/Turabian StyleRučman, Stefan, Winai Thongpan, Wattikon Sroila, Niwat Jhuntama, and Pisith Singjai. 2022. "Magnetic Field Assisted Spark Discharge-Generated Gold Nanostructures: XPS Study of Nitrogen Gas Fate and Chemical Composition of Gold Thin Films" Magnetochemistry 8, no. 12: 178. https://doi.org/10.3390/magnetochemistry8120178
APA StyleRučman, S., Thongpan, W., Sroila, W., Jhuntama, N., & Singjai, P. (2022). Magnetic Field Assisted Spark Discharge-Generated Gold Nanostructures: XPS Study of Nitrogen Gas Fate and Chemical Composition of Gold Thin Films. Magnetochemistry, 8(12), 178. https://doi.org/10.3390/magnetochemistry8120178








