Biodiesel Production from Macroalgae Oil from Fucus vesiculosus Using Magnetic Catalyst in Unconventional Reactor Assisted by Magnetic Field
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
2.2.1. Cobalt Ferrites Synthesis
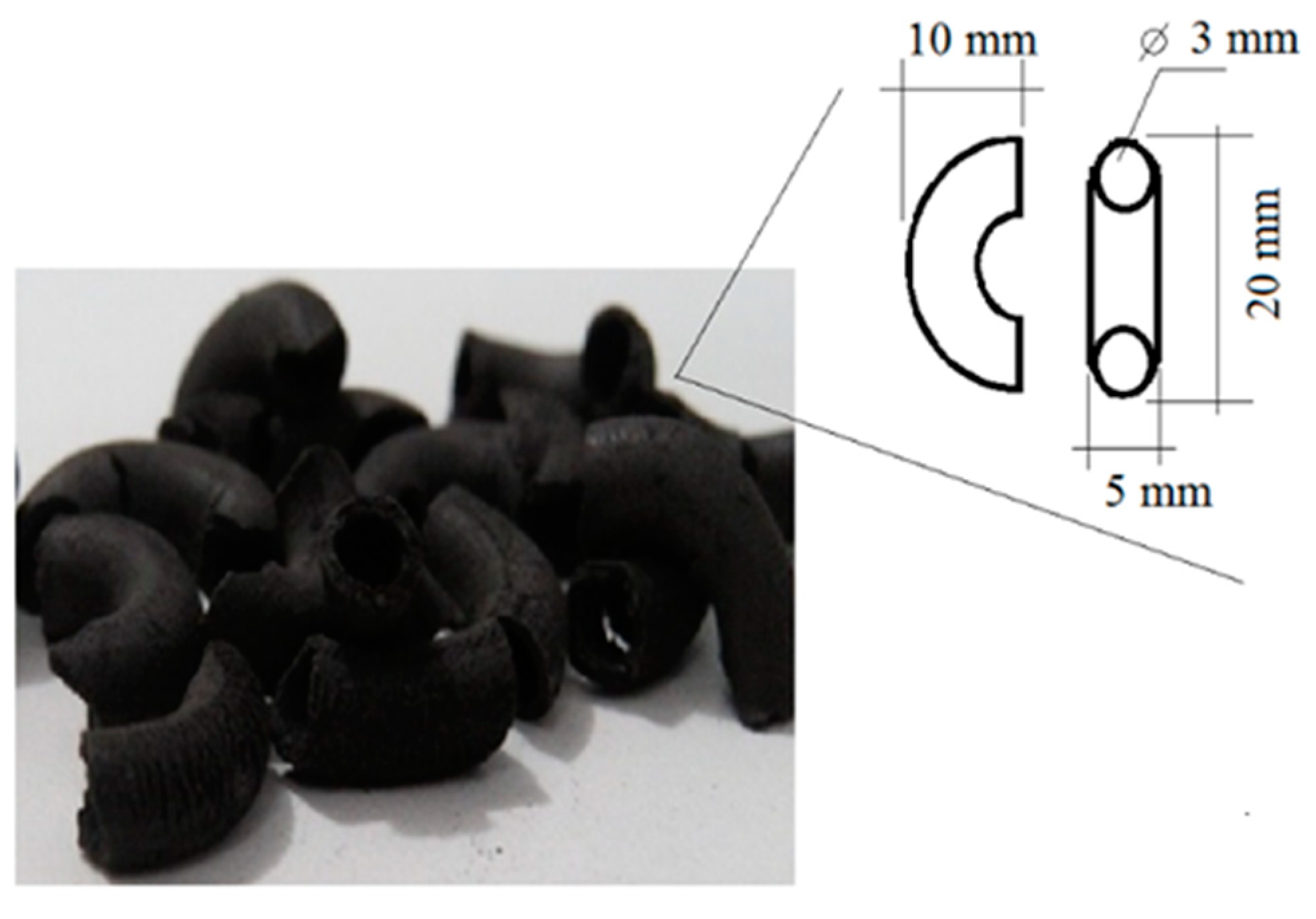
2.2.2. Preparation of Magnetic Catalysts
2.2.3. Biodiesel Synthesis by Chemical Transesterification
2.3. Analytical Methods
2.3.1. Magnetic Catalyst Characterization
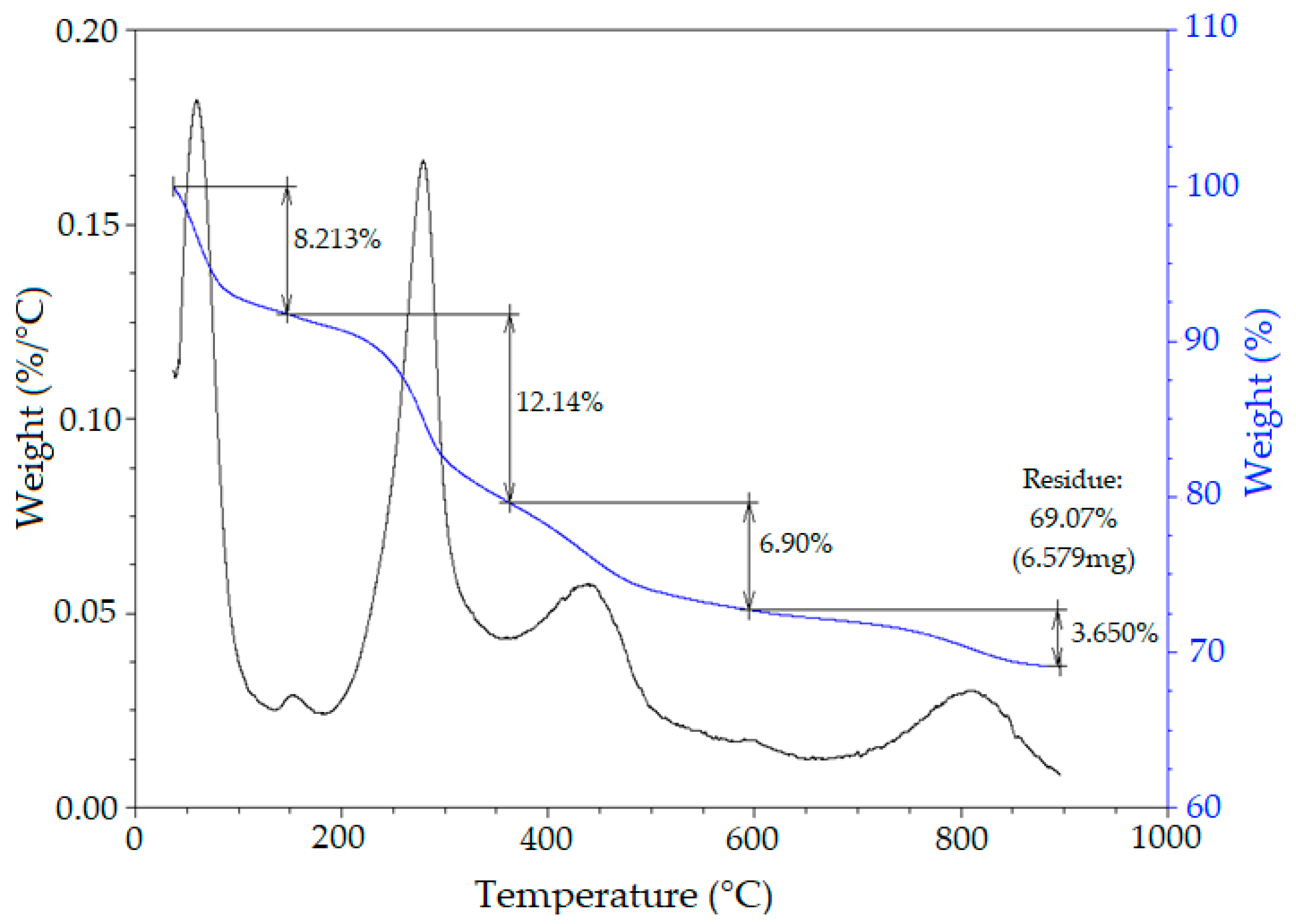
Thermogravimetric Analysis (TG/DTG)
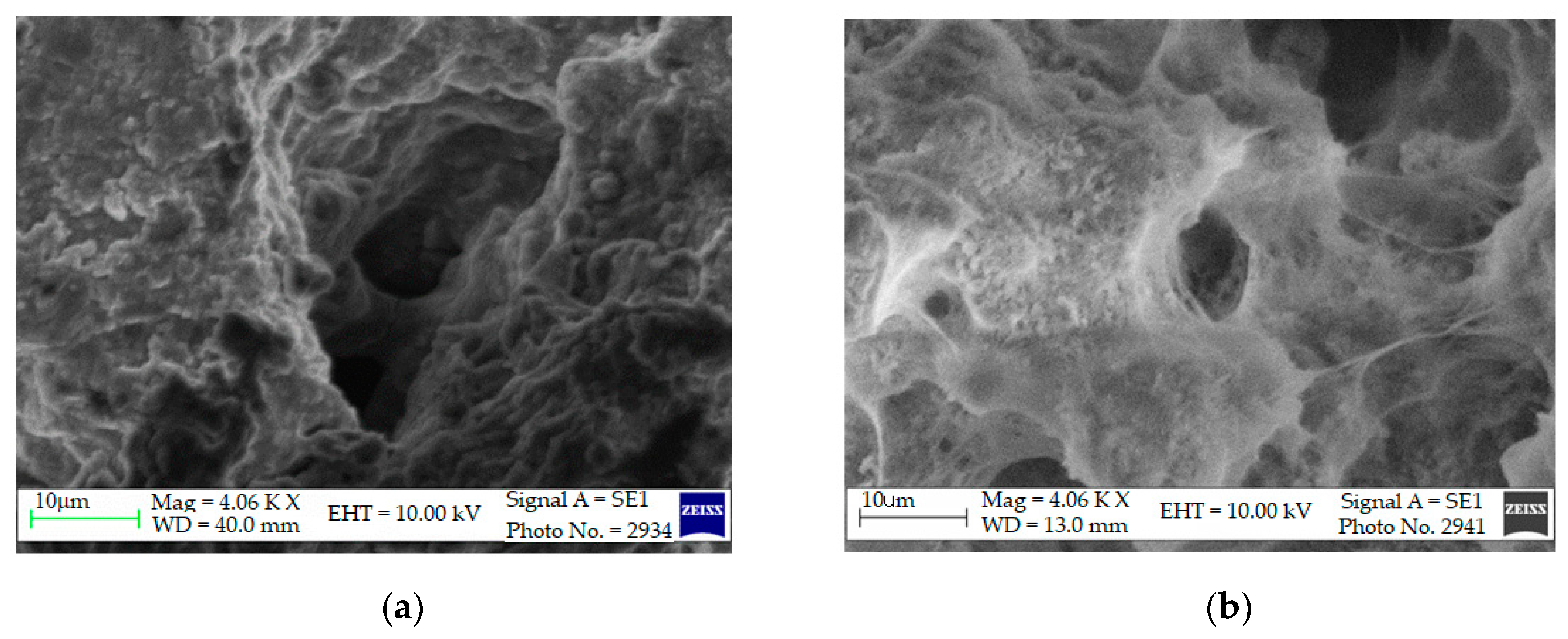
Scanning Electron Microscopy (SEM)
X-ray Diffraction
Magnetic Properties Analysis of the Magnetic Catalysts
Mechanical Strength
B.E.T Analysis
Chemisorption Analysis (CO2-TPD)
2.3.2. Characterization of Macroalgae Oil and Biodiesel Quality
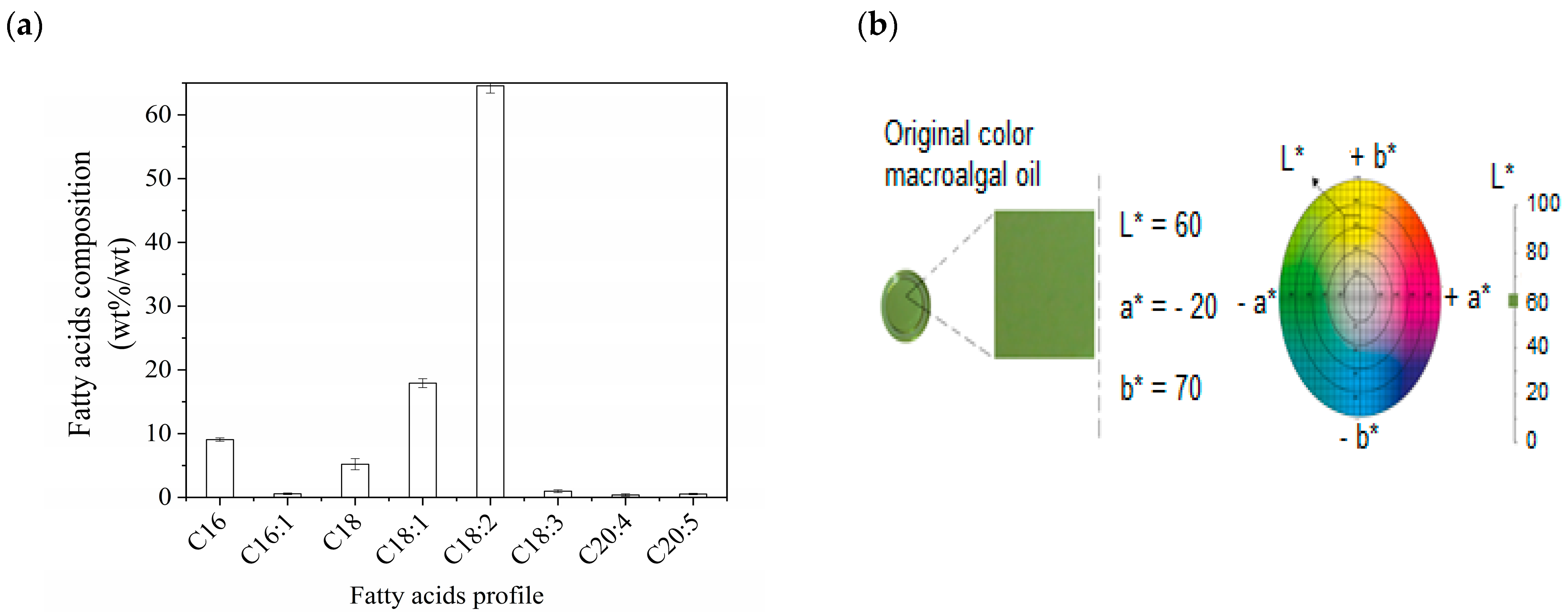
Color Analysis of Macroalgae Oil
Macroalgae Oil Composition
Macroalgae Oil and Biodiesel Quality
3. Results
| Feedstock | Catalyst | Reactor Type | Fluid Flow Rate and a Magnetic Field Intensity | Biodiesel Yield (%) | Ref. |
|---|---|---|---|---|---|
| Macroalgae Fucus vesiculosus oil | K2CO3/γ-Al2O3/Sepiolite/CoFe2O4 (hollow cylindrical noodle geometry) | Reactor assisted by magnetic field | 750 mL/min and 12 mT | 99.8 | This study |
| Soybean oil | K2CO3/γ-Al2O3/Sepiolite/γ-Fe2O3 (magnetic monolithic catalyst) | Reactor assisted by magnetic field | 16.6 mL/min and 2.5 mT | 98.07 | [44] |
| Sunflower oil | K2CO3/γ-Al2O3/Sepiolite/Fe3O4 (pellets) | *Reactor assisted by magnetic field | *The reactor assisted by magnetic field was used for magnetic catalyst separation. | 88 | [45] |
| Coconut oil | Pseudomonas fluorescens immobilized on chitosan with magnetic particles (microspheres) | Bioreactor assisted by electromagnetic field (differential mode reactor) | 10.3 mL/min and 9.7 mT | 12 | [41] |
| Waste cooking oil | Pseudomonas mendocina immobilized in magnetic microspheres | magnetically fluidized bed reactor | 16.97 mL/min and 136.63 Oe (13.67 mT) | 91.8 | [59] |
| Soybean oil | Rhizopus oryzae immobilized on chitosan with magnetic particles (microspheres) | Magnetically stabilized fluidized bed reactor (MSFBR) | 25 mL/min and 150 Oe (15 mT) | 91.3 | [72] |
| Properties | Standard Methods | Macroalgal Oil | Biodiesel |
|---|---|---|---|
| Density (kg/m3) at 20 °C | ASTM D1298 | 922 | 870 |
| Kinematic viscosity (mm2/s) at 40 °C | ASTM D445 | 42.3 | 4.3 |
| Oxidative stability, 110 °C | EN 14112 | -- | 1 h |
| Acid number (mg NaOH/g) | ASTM D664 | 2.66 | 0.5 |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IPCC. Global Warming of 1.5 °C; An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V., Zhai, P., Pörtner, H.-O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2018; p. 616. [Google Scholar] [CrossRef]
- REN21. Renewables 2021 Global Status Report (Paris: REN21 Secretariat); REN21: Paris, France, 2021; ISBN 978-3-948393-03-8. [Google Scholar]
- Silveira Junior, E.G.; Perez, V.H.; Reyero, I.; Serrano-Lotina, A.; Justo, O.R. Biodiesel production from heterogeneous catalysts based K2CO3 supported on extruded γ-Al2O3. Fuel 2019, 241, 311–318. [Google Scholar] [CrossRef]
- Silveira, E.G.; Barcelos, L.F.T.; Perez, V.H.; Justo, O.R.; Ramirez, L.C.; Filho, L.d.M.R.; de Castro, M.P.P. Biodiesel production from non-edible forage turnip oil by extruded catalyst. Ind. Crops Prod. 2019, 139, 111503. [Google Scholar] [CrossRef]
- Roschat, W.; Siritanon, T.; Yoosuk, B.; Sudyoadsuk, T.; Promarak, V. Rubber seed oil as potential non-edible feedstock for biodiesel production using heterogeneous catalyst in Thailand. Renew. Energy 2017, 101, 937–944. [Google Scholar] [CrossRef]
- Carvalho, A.K.F.; da Rós, P.C.M.; Teixeira, L.F.; Andrade, G.S.S.; Zanin, G.M.; de Castro, H.F. Assessing the potential of non-edible oils and residual fat to be used as a feedstock source in the enzymatic ethanolysis reaction. Ind. Crops Prod. 2013, 50, 485–493. [Google Scholar] [CrossRef]
- Moreira, M.A.C.; Arrúa, M.E.; Antunes, A.; Fiuza, T.; Costa, B.J.; Neto, P.W.; Antunes, S.R.M. Characterization of Syagrus romanzoffiana oil aiming at biodiesel production. Ind. Crops Prod. 2013, 48, 57–60. [Google Scholar] [CrossRef]
- Zullaikah, S.; Lai, C.-C.; Vali, S.R.; Ju, Y.-H. A two-step acid-catalyzed process for the production of biodiesel from rice bran oil. Bioresour. Technol. 2005, 96, 1889–1896. [Google Scholar] [CrossRef]
- Ahmad, S.; Chaudhary, S.; Pathak, V.V.; Kothari, R.; Tyagi, V.V. Optimization of direct transesterification of Chlorella pyrenoidosa catalyzed by waste egg shell based heterogenous nano—CaO catalyst. Renew. Energy 2020, 160, 86–97. [Google Scholar] [CrossRef]
- Das, V.; Tripathi, A.M.; Borah, M.J.; Dunford, N.T.; Deka, D. Cobalt-doped CaO catalyst synthesized and applied for algal biodiesel production. Renew. Energy 2020, 161, 1110–1119. [Google Scholar] [CrossRef]
- Li, Y.; Lian, S.; Tong, D.; Song, R.; Yang, W.; Fan, Y.; Qing, R.; Hu, C. One-step production of biodiesel from Nannochloropsis sp. on solid base Mg–Zr catalyst. Appl. Energy 2011, 88, 3313–3317. [Google Scholar] [CrossRef]
- Teo, S.H.; Islam, A.; Taufiq-Yap, Y.H. Algae derived biodiesel using nanocatalytic transesterification process. Chem. Eng. Res. Des. 2016, 111, 362–370. [Google Scholar] [CrossRef]
- Turkkul, B.; Deliismail, O.; Seker, E. Ethyl esters biodiesel production from Spirulina sp. and Nannochloropsis oculata microalgal lipids over alumina-calcium oxide catalyst. Renew. Energy 2020, 145, 1014–1019. [Google Scholar] [CrossRef]
- Umdu, E.S.; Tuncer, M.; Seker, E. Transesterification of Nannochloropsis oculata microalga’s lipid to biodiesel on Al2O3 supported CaO and MgO catalysts. Bioresour. Technol. 2009, 100, 2828–2831. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.Y.; Chia, W.Y.; Ong, W.-J.; Cheah, W.Y.; Lim, S.S.; Chew, K.W. Advances in catalytic transesterification routes for biodiesel production using microalgae. Sustain. Energy Technol. Assess. 2022, 52, 102336. [Google Scholar] [CrossRef]
- Manzoor, M.; Hussain, A.; Ahmad, Q.-u.-A.; Chaudhary, A.; Schenk, P.M.; Deepanraj, B.; Show, P.L. Biodiesel quality assessment of microalgae cultivated mixotrophically on sugarcane bagasse. Sustain. Energy Technol. Assess. 2022, 53, 102359. [Google Scholar] [CrossRef]
- Draaisma, R.B.; Wijffels, R.H.; Slegers, P.; Brentner, L.B.; Roy, A.; Barbosa, M.J. Food commodities from microalgae. Curr. Opin. Biotechnol. 2013, 24, 169–177. [Google Scholar] [CrossRef] [PubMed]
- van Hal, J.; Huijgen, W.; López-Contreras, A.M. Opportunities and challenges for seaweed in the biobased economy. Trends Biotechnol. 2014, 32, 231–233. [Google Scholar] [CrossRef]
- Gruduls, A.; Maurers, R.; Romagnoli, F. Baltic Sea seaweed biomass pretreatment: Effect of combined CO2 and thermal treatment on biomethane potential. Energy Procedia 2018, 147, 607–613. [Google Scholar] [CrossRef]
- Ometto, F.; Steinhovden, K.; Kuci, H.; Lunnbäck, J.; Berg, A.; Karlsson, A.; Handå, A.; Hallan, H.; Ejlertsson, J. Seasonal variation of elements composition and biomethane in brown macroalgae. Biomass Bioenergy 2018, 109, 31–38. [Google Scholar]
- Romagnoli, F.; Pastare, L.; Sabūnas, A.; Bāliņa, K.; Blumberga, D. Effects of pre-treatment on Biochemical Methane Potential (BMP) testing using Baltic Sea Fucus vesiculosus feedstock. Biomass Bioenergy 2017, 105, 23–31. [Google Scholar] [CrossRef]
- Sheng, Y.; Shanmugam, S.; Chinnathambi, A.; Salmen, S.H.; Ge, S.; Xia, C.; Brindhadevi, K. Feasibility of microalgal and macroalgal biomass co-digestion on biomethane production. Int. J. Hydrog. Energy 2022, 47, 37394–37400. [Google Scholar] [CrossRef]
- Jaiswal, K.K.; Kumar, V.; Vlaskin, M.S.; Nanda, M.; Verma, M.; Ahmad, W.; Kim, H. Hydropyrolysis of freshwater macroalgal bloom for bio-oil and biochar production: Kinetics and isotherm for removal of multiple heavy metals. Environ. Technol. Innov. 2021, 22, 101440. [Google Scholar] [CrossRef]
- Jazie, A.A.; Haydary, J.; Abed, S.A.; Al-Dawody, M.F. Hydrothermal liquefaction of Fucus vesiculosus algae catalyzed by Hβ zeolite catalyst for Biocrude oil production. Algal Res. 2022, 61, 102596. [Google Scholar] [CrossRef]
- Vimali, E.; Gunaseelan, S.; Devi, V.C.; Mothil, S.; Arumugam, M.; Ashokkumar, B.; Moorthy, I.M.G.; Pugazhendhi, A.; Varalakshmi, P. Comparative study of different catalysts mediated FAME conversion from macroalga Padina tetrastromatica biomass and hydrothermal liquefaction facilitated bio-oil production. Chemosphere 2022, 292, 133485. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, A.; Gigli, E.; Romagnoli, F.; Simoni, S.; Blumberga, D.; Palerno, M.; Guerriero, E. Co-digestion of Macroalgae for Biogas Production: An LCA-based Environmental Evaluation. Energy Procedia 2015, 72, 3–10. [Google Scholar] [CrossRef]
- Dave, N.; Selvaraj, R.; Varadavenkatesan, T.; Vinayagam, R. A critical review on production of bioethanol from macroalgal biomass. Algal Res. 2019, 42, 101606. [Google Scholar] [CrossRef]
- Dave, N.; Varadavenkatesan, T.; Selvaraj, R.; Vinayagam, R. Modelling of fermentative bioethanol production from indigenous Ulva prolifera biomass by Saccharomyces cerevisiae NFCCI1248 using an integrated ANN-GA approach. Sci. Total Environ. 2021, 791, 148429. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, K.; Chiou, T.-Y.; Konishi, M. Torulaspora quercuum shows great potential for bioethanol production from macroalgal hydrolysate. Bioresour. Technol. Rep. 2022, 17, 100952. [Google Scholar] [CrossRef]
- Ruangrit, K.; Chaipoot, S.; Phongphisutthinant, R.; Kamopas, W.; Jeerapan, I.; Pekkoh, J.; Srinuanpan, S. Environmental-friendly pretreatment and process optimization of macroalgal biomass for effective ethanol production as an alternative fuel using Saccharomyces cerevisiae. Biocatal. Agric. Biotechnol. 2021, 31, 101919. [Google Scholar] [CrossRef]
- Ashokkumar, V.; Salim, M.R.; Salam, Z.; Sivakumar, P.; Chong, C.T.; Elumalai, S.; Suresh, V.; Ani, F.N. Production of liquid biofuels (biodiesel and bioethanol) from brown marine macroalgae Padina tetrastromatica. Energy Convers. Manag. 2017, 135, 351–361. [Google Scholar] [CrossRef]
- Kalavathy, G.; Baskar, G. Synergism of clay with zinc oxide as nanocatalyst for production of biodiesel from marine Ulva lactuca. Bioresour. Technol. 2019, 281, 234–238. [Google Scholar] [CrossRef]
- Khan, A.; Fatima, N. Biodiesel synthesis via metal oxides and metal chlorides catalysis from marine alga Melanothamnus afaqhusainii. Chin. J. Chem. Eng. 2016, 24, 388–393. [Google Scholar] [CrossRef]
- Khan, A.M.; Fatima, N.; Hussain, M.S.; Yasmeen, K. Biodiesel production from green seaweed Ulva fasciata catalyzed by novel waste catalysts from Pakistan Steel Industry. Chin. J. Chem. Eng. 2016, 24, 1080–1086. [Google Scholar] [CrossRef]
- Khan, A.M.; Obaid, M.; Shah, M.R. Nanocatalyzed biodiesel synthesis from the oily contents of Marine brown alga Dictyota dichotoma. Int. J. Green Energy 2017, 14, 925–933. [Google Scholar] [CrossRef]
- Saengsawang, B.; Bhuyar, P.; Manmai, N.; Ponnusamy, V.K.; Ramaraj, R.; Unpaprom, Y. The optimization of oil extraction from macroalgae, Rhizoclonium sp. by chemical methods for efficient conversion into biodiesel. Fuel 2020, 274, 117841. [Google Scholar] [CrossRef]
- Sivaprakash, G.; Mohanrasu, K.; Ananthi, V.; Jothibasu, M.; Nguyen, D.D.; Ravindran, B.; Chang, S.W.; Nguyen-Tri, P.; Tran, N.H.; Sudhakar, M.; et al. Biodiesel production from Ulva linza, Ulva tubulosa, Ulva fasciata, Ulva rigida, Ulva reticulate by using Mn2ZnO4 heterogenous nanocatalysts. Fuel 2019, 255, 115744. [Google Scholar] [CrossRef]
- Suganya, T.; Gandhi, N.N.; Renganathan, S. Production of algal biodiesel from marine macroalgae Enteromorpha compressa by two step process: Optimization and kinetic study. Bioresour. Technol. 2013, 128, 392–400. [Google Scholar] [CrossRef]
- Xu, X.; Kim, J.Y.; Oh, Y.R.; Park, J.M. Production of biodiesel from carbon sources of macroalgae, Laminaria japonica. Bioresour. Technol. 2014, 169, 455–461. [Google Scholar] [CrossRef]
- Kostas, E.T.; Adams, J.M.M.; Ruiz, H.A.; Durán-Jiménez, G.; Lye, G.J. Macroalgal biorefinery concepts for the circular bioeconomy: A review on biotechnological developments and future perspectives. Renew. Sustain. Energy Rev. 2021, 151, 111553. [Google Scholar] [CrossRef]
- Cubides-Román, D.C.; Perez, V.H.; Castro, H.F.d.; Orrego, C.E.; Giraldo, O.; Silveira, E.G.; David, G.F. Ethyl esters (biodiesel) production by Pseudomonas fluorescens lipase immobilized on chitosan with magnetic properties in a bioreactor assisted by electromagnetic field. Fuel 2017, 196, 481–487. [Google Scholar] [CrossRef]
- de Andrade, C.M.; Cogo, A.J.D.; Perez, V.H.; Santos, N.F.d.; Okorokova-Façanha, A.L.; Justo, O.R.; Façanha, A.R. Increases of bioethanol productivity by S. cerevisiae in unconventional bioreactor under ELF-magnetic field: New advances in the biophysical mechanism elucidation on yeasts. Renew. Energy 2021, 169, 836–842. [Google Scholar] [CrossRef]
- Dussan, K.; Justo, O.; Perez, V.; David, G.; Junior, E.S.; da Silva, S. Bioethanol Production From Sugarcane Bagasse Hemicellulose Hydrolysate by Immobilized S. shehatae in a Fluidized Bed Fermenter Under Magnetic Field. Bio. Energy Res. 2019, 12, 338–346. [Google Scholar] [CrossRef]
- Junior, E.G.S.; Justo, O.R.; Perez, V.H.; Melo, F.d.; Reyero, I.; Serrano-Lotina, A.; Mompean, F.J. Biodiesel synthesis using a novel monolithic catalyst with magnetic properties (K2CO3/γ-Al2O3/Sepiolite/γ-Fe2O3) by ethanolic route. Fuel 2020, 271, 117650. [Google Scholar] [CrossRef]
- Junior, E.G.S.; Justo, O.R.; Perez, V.H.; Reyero, I.; Serrano-Lotina, A.; Ramirez, L.C.; dos Santos Dias, D.F. Extruded Catalysts with Magnetic Properties for Biodiesel Production. Adv. Mater. Sci. Eng. 2018, 2018, 3980967. [Google Scholar]
- Guiochon, G.; Guillemin, C.L. Chapter 13 Quantitative Analysis By Gas Chromatography Basic Problems, Fundamental Relatio ships, Measurement of the Sample Size. In Journal of Chromatography Library; Guiochon, G., Guillemin, C.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 563–586. [Google Scholar]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Veeranan, T.; Kasirajaan, R.; Gurunathan, B.; Sahadevan, R. A novel approach for extraction of algal oil from marine macroalgae Ulva fasciata. Renew. Energy 2018, 127, 64–73. [Google Scholar] [CrossRef]
- Habibi, M.H.; Parhizkar, J. Cobalt ferrite nano-composite coated on glass by Doctor Blade method for photo-catalytic degradation of an azo textile dye Reactive Red 4: XRD, FESEM and DRS investigations, Spectrochimica acta. Part A. Mol. Biomol. Spectrosc. 2015, 150, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Kooti, M.; Sedeh, A.N.; Gheisari, K.; Figuerola, A. Synthesis, characterization, and performance of nanocomposites containing reduced graphene oxide, polyaniline, and cobalt ferrite. Phys. B Condens. Matter 2021, 612, 412974. [Google Scholar] [CrossRef]
- Helmi, F.; Helmi, M.; Hemmati, A. Phosphomolybdic acid/chitosan as acid solid catalyst using for biodiesel production from pomegranate seed oil via microwave heating system: RSM optimization and kinetic study. Renew. Energy 2022, 189, 881–898. [Google Scholar] [CrossRef]
- Salim, S.M.; Izriq, R.; Almaky, M.M.; Al-Abbassi, A.A. Synthesis and characterization of ZnO nanoparticles for the production of biodiesel by transesterification: Kinetic and thermodynamic studies. Fuel 2022, 321, 124135. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, Y.; Cong, W.; Zhao, X.; Janaun, J.; Wei, T.; Fang, Z. Soybean biodiesel production using synergistic CaO/Ag nano catalyst: Process optimization, kinetic study, and economic evaluation. Ind. Crop. Prod. 2021, 166, 113479. [Google Scholar] [CrossRef]
- Kamran, E.; Mashhadi, H.; Mohammadi, A.; Ghobadian, B. Biodiesel production from Elaeagnus angustifolia.L seed as a novel waste feedstock using potassium hydroxide catalyst. Biocatal. Agric. Biotechnol. 2020, 25, 101578. [Google Scholar] [CrossRef]
- Liu, J.-Z.; Cui, Q.; Kang, Y.-F.; Meng, Y.; Gao, M.-Z.; Efferth, T.; Fu, Y.-J. Euonymus maackii Rupr. Seed oil as a new potential non-edible feedstock for biodiesel. Renew. Energy 2019, 133, 261–267. [Google Scholar] [CrossRef]
- Xu, W.; Ge, X.-d.; Yan, X.-h.; Shao, R. Optimization of methyl ricinoleate synthesis with ionic liquids as catalysts using the response surface methodology. Chem. Eng. J. 2015, 275, 63–70. [Google Scholar] [CrossRef]
- Zhao, R.; Yang, X.; Li, M.; Peng, X.; Wei, M.; Zhang, X.; Yang, L.; Li, J. Biodiesel preparation from Thlaspi arvense L. seed oil utilizing a novel ionic liquid core-shell magnetic catalyst. Ind. Crops Prod. 2021, 162, 113316. [Google Scholar] [CrossRef]
- Chen, G.; Liu, J.; Yao, J.; Qi, Y.; Yan, B. Biodiesel production from waste cooking oil in a magnetically fluidized bed reactor using whole-cell biocatalysts. Energy Convers. Manag. 2017, 138, 556–564. [Google Scholar] [CrossRef]
- Jnaneshwara, D.; Avadhani, D.; Prasad, B.D.; Nagabhushana, B.; Nagabhushana, H.; Sharma, S.; Shivakumara, C.; Rao, J.; Gopal, N.; Ke, S.-C.; et al. Electron paramagnetic resonance, magnetic and electrical properties of CoFe2O4 nanoparticles. J. Magn. Magn. Mater. 2013, 339, 40–45. [Google Scholar] [CrossRef]
- Krishnan, S.G.; Pua, F.-L.; Zhang, F. A review of magnetic solid catalyst development for sustainable biodiesel production. Biomass Bioenergy 2021, 149, 106099. [Google Scholar] [CrossRef]
- Bauer, L.M.; Costa, J.A.V.; da Rosa, A.P.C.; Santos, L.O. Growth stimulation and synthesis of lipids, pigments and antioxidants with magnetic fields in Chlorella kessleri cultivations. Bioresour. Technol. 2017, 244, 1425–1432. [Google Scholar] [CrossRef]
- Chu, F.-J.; Wan, T.-J.; Pai, T.-Y.; Lin, H.-W.; Liu, S.-H.; Huang, C.-F. Use of magnetic fields and nitrate concentration to optimize the growth and lipid yield of Nannochloropsis oculata. J. Environ. Manag. 2020, 253, 109680. [Google Scholar] [CrossRef]
- Costa, S.S.; Peres, B.P.; Machado, B.R.; Costa, J.A.V.; Santos, L.O. Increased lipid synthesis in the culture of Chlorella homosphaera with magnetic fields application. Bioresour. Technol. 2020, 315, 123880. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Chen, Y.; Lv, J.; Han, S.; Tu, R.; Zhou, X.; Jin, W.; Ren, N. Enhanced Lipid Production by Chlorella pyrenoidosa through Magnetic Field Pretreatment of Wastewater and Treatment of Microalgae-wastewater Culture Solution: Magnetic Field Treatment Modes and Conditions. Bioresour. Technol. 2020, 306, 123102. [Google Scholar] [CrossRef] [PubMed]
- Small, D.P.; Hüner, N.P.A.; Wan, W. Effect of static magnetic fields on the growth, photosynthesis and ultrastructure of Chlorella kessleri microalgae. Bioelectromagnetics 2012, 33, 298–308. [Google Scholar] [CrossRef]
- Lau, C.H.; Gan, S.; Lau, H.L.N.; Lee, L.Y.; Thangalazhy-Gopakumar, S.; Ng, H.K. Insights into the effectiveness of synthetic and natural additives in improving biodiesel oxidation stability. Sustain. Energy Technol. Assess. 2022, 52, 102296. [Google Scholar] [CrossRef]
- Rajamohan, S.; Gopal, A.H.; Muralidharan, K.R.; Huang, Z.; Paramasivam, B.; Ayyasamy, T.; Nguyen, X.P.; Le, A.T.; Hoang, A.T. Evaluation of oxidation stability and engine behaviors operated by Prosopis juliflora biodiesel/diesel fuel blends with presence of synthetic antioxidant. Sustain. Energy Technol. Assess. 2022, 52, 102086. [Google Scholar] [CrossRef]
- Silva, W.C.; Castro, M.P.P.; Perez, V.H.; Machado, F.A.; Mota, L.; Sthel, M.S. Thermal degradation of ethanolic biodiesel: Physicochemical and thermal properties evaluation. Energy 2016, 114, 1093–1099. [Google Scholar] [CrossRef]
- da Silva, J.C.M.; Nicolau, C.L.; Cabral, M.R.P.; Costa, E.R.; Stropa, J.M.; Silva, C.A.A.; Scharf, D.R.; Simionatto, E.L.; Fiorucci, A.R.; de Oliveira, L.C.S.; et al. Thermal and oxidative stabilities of binary blends of esters from soybean oil and non-edible oils (Aleurites moluccanus, Terminalia catappa, and Scheelea phalerata). Fuel 2020, 262, 116644. [Google Scholar] [CrossRef]
- Nicolau, C.L.; Klein, A.F.V.; Silva, C.A.A.; Fiorucci, A.R.; Stropa, J.M.; Santos, E.; Simionatto, E. Thermal Properties of the Blends of Methyl and Ethyl Esters Prepared from Babassu and Soybean Oils. J. Braz. Chem. Soc. 2018, 29, 1672–1679. [Google Scholar] [CrossRef]
- Zhou, G.-x.; Chen, G.-y.; Yan, B.-b. Biodiesel production in a magnetically-stabilized, fluidized bed reactor with an immobilized lipase in magnetic chitosan microspheres. Biotechnol. Lett. 2014, 36, 63–68. [Google Scholar] [CrossRef]

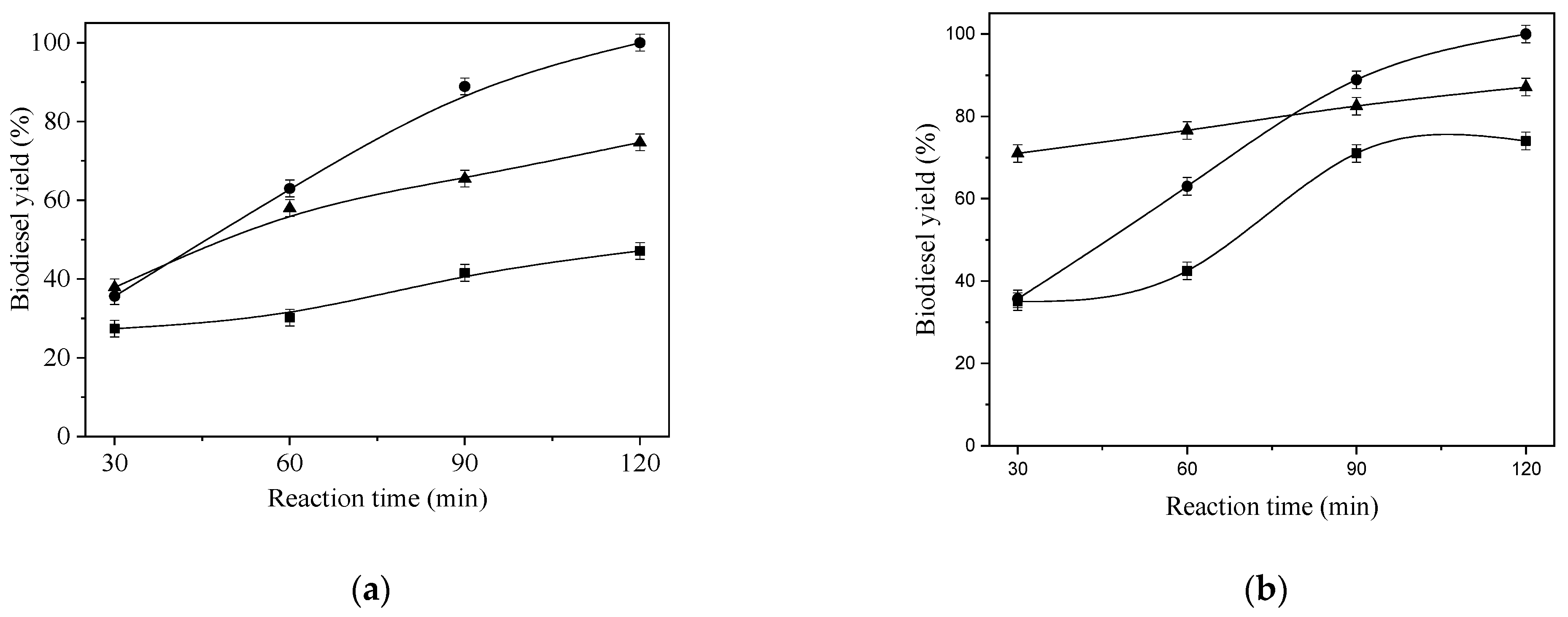
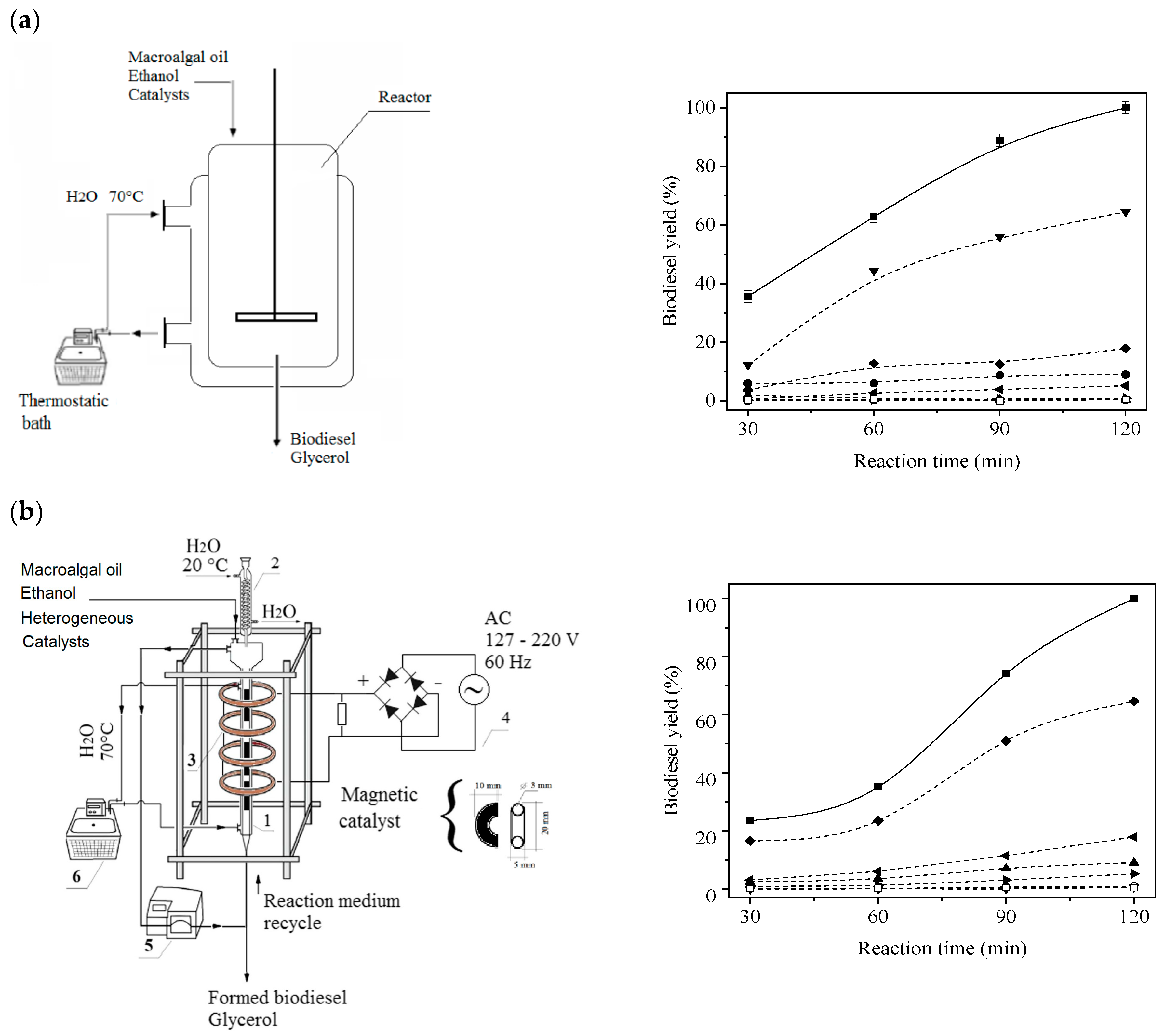
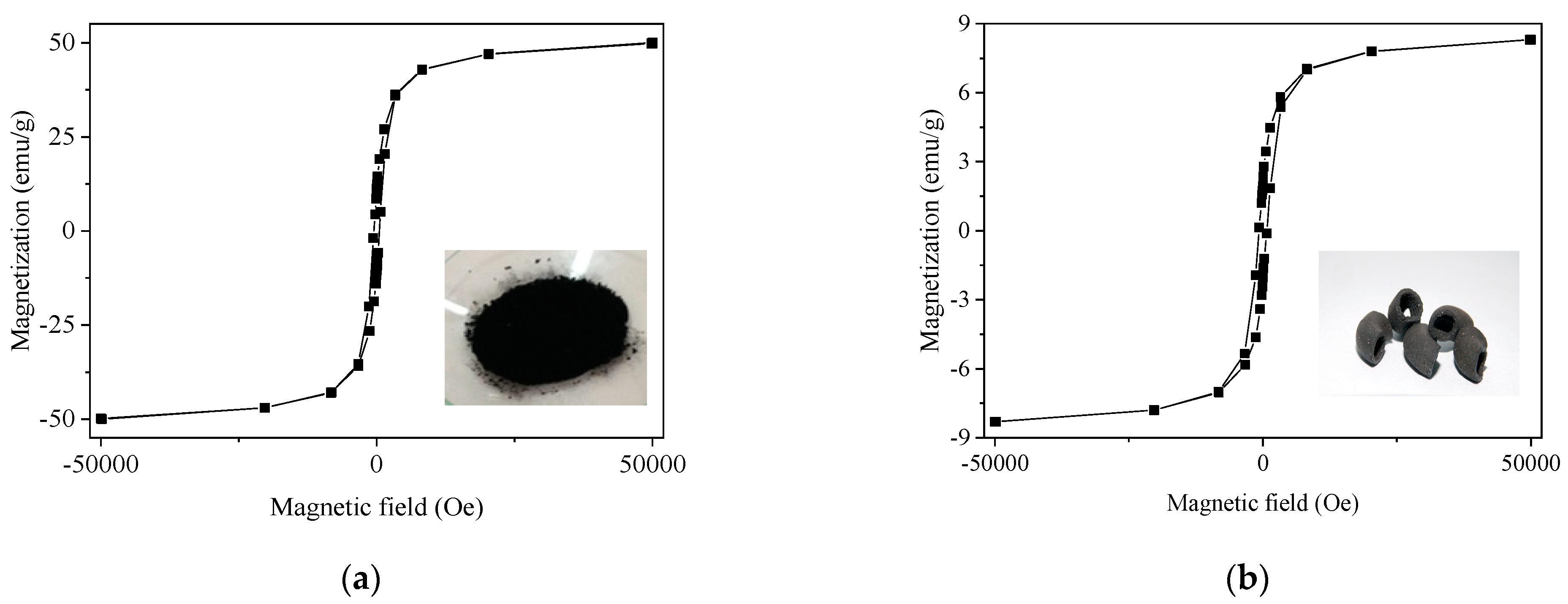
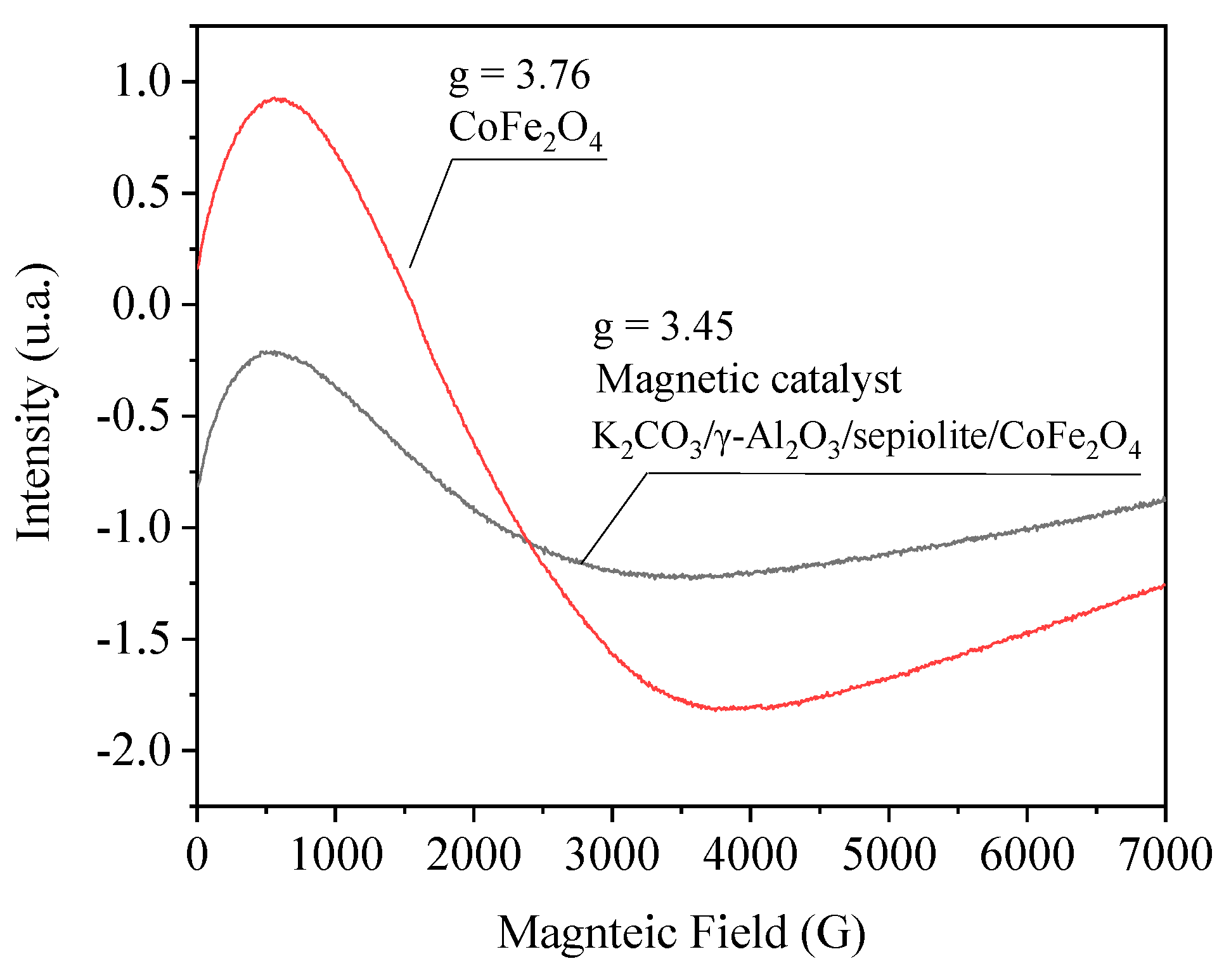
| Physico-Chemical Properties | Values |
|---|---|
| Mechanical strength (kgf/cm) | 3.12 |
| Specific area (m2/g) determined by BET analysis | 30 |
| Pore volume (cm3/g) determined by BET analysis | 0.11 |
| Pore size (nm) determined by BET analysis | 15.3 |
| Basicity (mmol/g of CO2) determined by CO2-TPD | 5.68 |
| Density of basic sites (mmol/m2) | 0.15 |
| Macro Algae Oil | Catalysts | Reaction Parameters | Biodiesel Yield (%) | Ref. |
|---|---|---|---|---|
| Oil: Alcohol Molar Ratio; Catalyst Weight (wt% or %); Temperature (°C); Stirring (rpm) or Flux (mL/min); Time (min) | ||||
| Rhizoclonium sp. | NaOH | Oil: methanol (1:1); 1 wt% catalyst; 45 °C; 300 rpm; 180 min | 82.2 | [36] |
| Ulva linza | Mn2ZnO4 | Oil: ethanol (1:12); 6 wt.% catalyst; 80 °C; magnetic stirrer; 240 min | 72.3 | [37] |
| Ulva tubulosa | Mn2ZnO4 | Oil: ethanol (1:12); 6 wt.% catalyst; 80 °C; magnetic stirrer; 240 min | 72 | [37] |
| Ulva rigida | Mn2ZnO4 | Oil: ethanol (1:12); 6 wt.% catalyst; 80 °C; magnetic stirrer; 240 min | 70.4 | [37] |
| Ulva reticulado | Mn2ZnO4 | Oil: ethanol (1:12); 6 wt.% catalyst; 80 °C; magnetic stirrer; 240 min | 71.5 | [37] |
| Ulva lactuca | Clay-ZnO doped | Oil: methanol (1:9) 8% catalyst; 55 °C; continuous stirring; 50 min | 97.43 | [32] |
| Padina tetrastromatica | H2SO4 and CH3ONa (two step reaction) | Oil: methanol (1:12); 1.5 wt% catalyst; 65 °C; 600 rpm; 120 min | 96.2 | [31] |
| Dictyota dichotoma | CaO, MgO, ZnO e TiO2 | Oil: methanol (1:18); 5 wt% catalyst; 65 °C; 600 rpm; 180 | 93.2 | [35] |
| Melanothamnus afaqhusainii | CaO | Oil (5 g), methanol (10 mL) and catalyst (0.5 g); 100–110 °C; hot plate stirrer; 1080 min | 80 | [33] |
| Ulva fasciata | Waste industrial dusts containing CaO, MgO and ZnO | Oil: methanol (1:9) and catalyst (2 g); 80–100 °C; fast stirring; 360 min | 88 | [34] |
| Enteromorpha compressa | NaOH | Oil: methanol (1:9); 1% catalyst; 60 °C; 600 rpm; 90 min | 90.6 | [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silveira Junior, E.G.; de Souza, L.F.B.; Perez, V.H.; Justo, O.R.; Simionatto, E.; de Oliveira, L.C.S. Biodiesel Production from Macroalgae Oil from Fucus vesiculosus Using Magnetic Catalyst in Unconventional Reactor Assisted by Magnetic Field. Magnetochemistry 2022, 8, 177. https://doi.org/10.3390/magnetochemistry8120177
Silveira Junior EG, de Souza LFB, Perez VH, Justo OR, Simionatto E, de Oliveira LCS. Biodiesel Production from Macroalgae Oil from Fucus vesiculosus Using Magnetic Catalyst in Unconventional Reactor Assisted by Magnetic Field. Magnetochemistry. 2022; 8(12):177. https://doi.org/10.3390/magnetochemistry8120177
Chicago/Turabian StyleSilveira Junior, Euripedes Garcia, Lilian Fiori Boechat de Souza, Victor Haber Perez, Oselys Rodriguez Justo, Euclésio Simionatto, and Lincoln Carlos Silva de Oliveira. 2022. "Biodiesel Production from Macroalgae Oil from Fucus vesiculosus Using Magnetic Catalyst in Unconventional Reactor Assisted by Magnetic Field" Magnetochemistry 8, no. 12: 177. https://doi.org/10.3390/magnetochemistry8120177
APA StyleSilveira Junior, E. G., de Souza, L. F. B., Perez, V. H., Justo, O. R., Simionatto, E., & de Oliveira, L. C. S. (2022). Biodiesel Production from Macroalgae Oil from Fucus vesiculosus Using Magnetic Catalyst in Unconventional Reactor Assisted by Magnetic Field. Magnetochemistry, 8(12), 177. https://doi.org/10.3390/magnetochemistry8120177







