Molarity Effects of Fe and NaOH on Synthesis and Characterisation of Magnetite (Fe3O4) Nanoparticles for Potential Application in Magnetic Hyperthermia Therapy
Abstract
1. Introduction
2. Methodology
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Häring, M.; Schiller, J.; Mayr, J.; Grijalvo, S.; Eritja, R.; Díaz, D. Magnetic Gel Composites for Hyperthermia Cancer Therapy. Gels 2015, 1, 135–161. [Google Scholar] [CrossRef] [PubMed]
- Giustini, A.J.; Petryk, A.A.; Cassim, S.M.; Tate, J.A.; Baker, I.; Hoopes, P.J. Magnetic Nanoparticle Hyperthermia in Cancer Treatment. Nano LIFE 2010, 1, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Moradiya, M.A.; Ladani, A.; Ladani, J.; Raiyani, C.; Markna, J.H. New Way to Treat Cancer: Magnetic Nanoparticle based Hyperthermia. J. Chem. Sci. Eng. JCSE 2019, 2, 58–60. [Google Scholar]
- Azcona, P.; Zysler, R.; Lassalle, V. Simple and novel strategies to achieve shape and size control of magnetite nanoparticles intended for biomedical applications. Colloids Surf. A Physicochem. Eng. Asp. 2016, 504, 320–330. [Google Scholar] [CrossRef]
- Daoush, W.M. Co-Precipitation and Magnetic Properties of Magnetite Nanoparticles for Potential Biomedical Applications. J. Nanomed. Res. 2017, 5, 00118. [Google Scholar] [CrossRef]
- Bonvin, D.; Alexander, D.T.L.; Millán, A.; Piñol, R.; Sanz, B.; Goya, G.F.; Martínez, A.; Bastiaansen, J.A.M.; Stuber, M.; Schenk, K.J.; et al. Tuning properties of iron oxide nanoparticles in aqueous synthesis without ligands to improve MRI relaxivity and SAR. Nanomaterials 2017, 7, 225. [Google Scholar] [CrossRef]
- Macías-Martínez, B.I.; Cortés-Hernández, D.A.; Zugasti-Cruz, A.; Cruz-Ortíz, B.R.; Múzquiz-Ramos, E.M. Heating ability and hemolysis test of magnetite nanoparticles obtained by a simple co-precipitation method. J. Appl. Res. Technol. 2016, 14, 239–244. [Google Scholar] [CrossRef]
- Kobayashi, T. Cancer hyperthermia using magnetic nanoparticles. Biotechnol. J. 2011, 6, 1342–1347. [Google Scholar] [CrossRef]
- Estelrich, J.; Escribano, E.; Queralt, J.; Busquets, M.A. Iron oxide nanoparticles for magnetically-guided and magnetically-responsive drug delivery. Int. J. Mol. Sci. 2015, 16, 8070–8101. [Google Scholar] [CrossRef]
- Estelrich, J.; Antònia Busquets, M. Iron oxide nanoparticles in photothermal therapy. Molecules 2018, 23, 1567. [Google Scholar] [CrossRef]
- Lagrow, A.P.; Besenhard, M.O.; Hodzic, A.; Sergides, A.; Bogart, L.K.; Gavriilidis, A.; Thanh, N.T.K. Unravelling the growth mechanism of the co-precipitation of iron oxide nanoparticles with the aid of synchrotron X-ray diffraction in solution. Nanoscale 2019, 11, 6620–6628. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Pu, X.; Yin, G.; Chen, X.; Yin, J.; Wu, Y. Biomimetic mineralization of magnetic iron oxide nanoparticles mediated by bi-functional copolypeptides. Molecules 2019, 24, 1401. [Google Scholar] [CrossRef] [PubMed]
- Karim, S.S.A.; Dee, C.F.; Majlis, B.Y.; Mohamed, M.A. Recent progress on fabrication of zinc oxide nanorod-based field effect transistor biosensors. Sains Malays. 2019, 48, 1301–1310. [Google Scholar] [CrossRef]
- Piñeiro, Y.; Vargas, Z.; Rivas, J.; Lõpez-Quintela, M.A. Iron Oxide Based Nanoparticles for Magnetic Hyperthermia Strategies in Biological Applications. Eur. J. Inorg. Chem. 2015, 2015, 4495–4509. [Google Scholar] [CrossRef]
- Surowiec, Z.; Miaskowski, A.; Budzyński, M. Investigation of magnetite Fe3O4 nanoparticles for magnetic hyperthermia. Nukleonika 2017, 62, 183–186. [Google Scholar] [CrossRef]
- Cassim, S.M.; Giustini, A.J.; Baker, I.; Hoopes, P.J. Development of novel magnetic nanoparticles for hyperthermia cancer therapy. Energy-Based Treat. Tissue Assess. VI 2011, 7901, 365–374. [Google Scholar] [CrossRef]
- Das, R.; Alonso, J.; Nemati Porshokouh, Z.; Kalappattil, V.; Torres, D.; Phan, M.H.; Garaio, E.; García, J.Á.; Sanchez Llamazares, J.L.; Srikanth, H. Tunable High Aspect Ratio Iron Oxide Nanorods for Enhanced Hyperthermia. J. Phys. Chem. C 2016, 120, 10086–10093. [Google Scholar] [CrossRef]
- Engelmann, U.; Buhl, E.M.; Baumann, M.; Schmitz-Rode, T.; Slabu, I. Agglomeration of magnetic nanoparticles and its effects on magnetic hyperthermia. Curr. Dir. Biomed. Eng. 2017, 3, 457–460. [Google Scholar] [CrossRef]
- Kafrouni, L.; Savadogo, O. Recent progress on magnetic nanoparticles for magnetic hyperthermia. Prog. Biomater. 2016, 5, 147–160. [Google Scholar] [CrossRef]
- Li, Z.; Kawashita, M.; Araki, N.; Mitsumori, M.; Hiraoka, M.; Doi, M. Magnetite nanoparticles with high heating efficiencies for application in the hyperthermia of cancer. Mater. Sci. Eng. C 2010, 30, 990–996. [Google Scholar] [CrossRef]
- Soleymani, M.; Khalighfard, S.; Khodayari, S.; Khodayari, H.; Kalhori, M.R.; Hadjighassem, M.R.; Shaterabadi, Z.; Alizadeh, A.M. Effects of multiple injections on the efficacy and cytotoxicity of folate-targeted magnetite nanoparticles as theranostic agents for MRI detection and magnetic hyperthermia therapy of tumor cells. Sci. Rep. 2020, 10, 1695. [Google Scholar] [CrossRef] [PubMed]
- do Carmo Paresque, M.C.; de Oliveira, E.M.; Nogueira, D.A.; de Castro, J.A.; de Campos, M.F. Magnetite nanoparticles study applied to magnetic hyperthermia treatment. Mater. Sci. Forum 2017, 899, 543–548. [Google Scholar] [CrossRef]
- Saravana Achari, D.; Santhosh, C.; Deivasegamani, R.; Nivetha, R.; Bhatnagar, A.; Jeong, S.K.; Grace, A.N. A non-enzymatic sensor for hydrogen peroxide based on the use of α-Fe2O3 nanoparticles deposited on the surface of NiO nanosheets. Microchim. Acta 2017, 184, 3223–3229. [Google Scholar] [CrossRef]
- Yusoff, A.H.M.; Salimi, M.N.; Jamlos, M.F. A review: Synthetic strategy control of magnetite nanoparticles production. Adv. Nano Res. 2018, 6, 1–19. [Google Scholar] [CrossRef]
- Ganapathe, L.S.; Mohamed, M.A.; Yunus, R.M.; Berhanuddin, D.D. Magnetite (Fe3O4) nanoparticles in biomedical application: From synthesis to surface functionalisation. Magnetochemistry 2020, 6, 68. [Google Scholar] [CrossRef]
- de Oliveira, P.N.; Moussa, A.; Milhau, N.; Bini, R.D.; Prouillac, C.; de Oliveira, B.F.; Dias, G.S.; Santos, I.A.; Morfin, I.; Guillaume Sudre, P.; et al. In situ synthesis of Fe3O4 nanoparticles coated by chito-oligosaccharides: Physico-chemical characterizations and cytotoxicity evaluation for biomedical applications. Nanotechnology 2020, 31, 122890. [Google Scholar] [CrossRef]
- Radoń, A.; Łoński, S.; Kądziołka-Gaweł, M.; Gębara, P.; Lis, M.; Łukowiec, D.; Babilas, R. Influence of magnetite nanoparticles surface dissolution, stabilization and functionalization by malonic acid on the catalytic activity, magnetic and electrical properties. Colloids Surf. A Physicochem. Eng. Asp. 2020, 607, 125446. [Google Scholar] [CrossRef]
- da Silva, F.A.S.; Rojas, E.E.G.; Rodrigues, G.F.; da Silva, B.F.A.; de Campos, M.F. Synthesis and characterization of biocompatible Fe3O4 for use in cell hyperthermia. Mater. Sci. Forum 2014, 775–776, 476–481. [Google Scholar] [CrossRef]
- Radoń, A.; Kubacki, J.; Kądziołka-Gaweł, M.; Gębara, P.; Hawełek, Ł.; Topolska, S.; Łukowiec, D. Structure and magnetic properties of ultrafine superparamagnetic Sn-doped magnetite nanoparticles synthesized by glycol assisted co-precipitation method. J. Phys. Chem. Solids 2020, 145, 109530. [Google Scholar] [CrossRef]
- Cursaru, L.M.; Piticescu, R.M.; Dragut, D.V.; Tudor, I.A.; Kuncser, V.; Iacob, N.; Stoiciu, F. The influence of synthesis parameters on structural and magnetic properties of iron oxide nanomaterials. Nanomaterials 2020, 10, 85. [Google Scholar] [CrossRef]
- Radoń, A.; Kądziołka-Gaweł, M.; Łukowiec, D.; Gębara, P.; Cesarz-Andraczke, K.; Kolano-Burian, A.; Włodarczyk, P.; Polak, M.; Babilas, R. Influence of magnetite nanoparticles shape and spontaneous surface oxidation on the electron transport mechanism. Materials 2021, 14, 5241. [Google Scholar] [CrossRef] [PubMed]
- Rahmawati, R.; Permana, M.G.; Harison, B.; Nurcha; Yuliarto, B.; Suyatman; Kurniadi, D. Optimization of Frequency and Stirring Rate for Synthesis of Magnetite (Fe3O4) Nanoparticles by Using Coprecipitation- Ultrasonic Irradiation Methods. Procedia Eng. 2017, 170, 55–59. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Aziz, A.A.; Jameel, M.S.; Noqta, O.A.; Khaniabadi, P.M.; Mehrdel, B. Simple rapid stabilization method through citric acid modification for magnetite nanoparticles. Sci. Rep. 2020, 10, 10793. [Google Scholar] [CrossRef] [PubMed]
- Akhirudin, N.H.M.; Shamsudin, R.; Othman, N.K. The effect of different concentrations of calcium silicate-maghemite coating towards magnetic behavior and bioactivity. Sains Malays. 2020, 49, 653–660. [Google Scholar] [CrossRef]
- Fadli, A.; Komalasari; Adnan, A.; Iwantono; Rahimah; Addabsi, A.S. Synthesis of Magnetite Nanoparticles via Co-precipitation Method. IOP Conf. Ser. Mater. Sci. Eng. 2019, 622, 012013. [Google Scholar] [CrossRef]
- Iacovita, C.; Florea, A.; Dudric, R.; Pall, E.; Moldovan, A.I.; Tetean, R.; Stiufiuc, R.; Lucaciu, C.M. Small versus large iron oxidemagnetic nanoparticles: Hyperthermia and cell uptake properties. Molecules 2016, 21, 1357. [Google Scholar] [CrossRef]
- Kandasamy, G.; Sudame, A.; Luthra, T.; Saini, K.; Maity, D. Functionalized Hydrophilic Superparamagnetic Iron Oxide Nanoparticles for Magnetic Fluid Hyperthermia Application in Liver Cancer Treatment. ACS Omega 2018, 3, 3991–4005. [Google Scholar] [CrossRef]
- Khairy, M. Synthesis, Characterization and Magnetic Properties of γ-irradiated and Unirradiated Magnetite Nanopowders. Int. J. Mater. Chem. 2013, 3, 106–111. [Google Scholar] [CrossRef]
- Tajabadi, M.; Khosroshahi, M.E. Effect of Alkaline Media Concentration and Modification of Temperature on Magnetite Synthesis Method Using FeSO4/NH4OH. Int. J. Chem. Eng. Appl. 2012, 3, 206–210. [Google Scholar] [CrossRef]
- Wroblewski, C.; Volford, T.; Martos, B.; Samoluk, J.; Martos, P. High yield synthesis and application of magnetite nanoparticles (Fe3O4). Magnetochemistry 2020, 6, 22. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, Q.; Xiong, Q.; Li, W.; Liu, J.; Yang, X. Shape-evolution and growth mechanism of fepolyhedrons. Adv. Mater. Sci. Eng. 2015, 2015, 763124. [Google Scholar] [CrossRef]
- Lim, Y.S.; Lai, C.W.; Hamid, S.B.A.; Julkapli, N.M.; Yehya, W.A.; Karim, M.Z.; Tai, M.F.; Lau, K.S. A study on growth formation of nano-sized magnetite Fe3O4 via co-precipitation method. Acta Crystallogr. Sect. A Found. Crystallogr. 2014, 18, S6-457–S6-461. [Google Scholar] [CrossRef]
- Javed, M.; Khan, A.A.; Kazmi, J.; Mohamed, M.A.; Ahmed, M.S.; Iqbal, Y. Impedance spectroscopic study of charge transport and relaxation mechanism in MnCr2O4 ceramic chromite. J. Alloys Compd. 2021, 854, 156996. [Google Scholar] [CrossRef]
- Javed, M.; Khan, A.A.; Ahmed, M.S.; Khisro, S.N.; Kazmi, J.; Bilkees, R.; Khan, M.N.; Mohamed, M.A. Temperature dependent impedance spectroscopy and electrical transport mechanism in sol-gel derived MgCr2O4 spinel oxide. Phys. B Condens. Matter 2020, 599, 412377. [Google Scholar] [CrossRef]
- Karim, S.S.A.; Takamura, Y.; Tue, P.T.; Tung, N.T.; Kazmi, J.; Dee, C.F.; Majlis, B.Y.; Mohamed, M.A. Developing conductive highly ordered zinc oxide nanorods by acetylacetonate-assisted growth. Materials 2020, 13, 1136. [Google Scholar] [CrossRef]
- Javed, M.; Khan, A.A.; Kazmi, J.; Mohamed, M.A.; Khan, M.N.; Hussain, M.; Bilkees, R. Dielectric relaxation and small polaron hopping transport in sol-gel-derived NiCr2O4 spinel chromite. Mater. Res. Bull. 2021, 138, 111242. [Google Scholar] [CrossRef]
- Kazmi, J.; Ooi, P.C.; Raza, S.R.A.; Goh, B.T.; Karim, S.S.A.; Samat, M.H.; Lee, M.K.; Razip Wee, M.F.M.; Taib, M.F.M.; Mohamed, M.A. Appealing stable room-temperature ferromagnetism by well-aligned 1D Co-doped Zinc Oxide Nanowires. J. Alloys Compd. 2021, 872, 159741. [Google Scholar] [CrossRef]
- Kazmi, J.; Ooi, P.C.; Goh, B.T.; Lee, M.K.; Razip Wee, M.F.M.; Karim, S.A.S.; Ali Raza, S.R.; Mohamed, M.A. Bi-doping improves the magnetic properties of zinc oxide nanowires. RSC Adv. 2020, 10, 23297–23311. [Google Scholar] [CrossRef]
- Guo, S.; Li, D.; Zhang, L.; Li, J.; Wang, E. Monodisperse mesoporous superparamagnetic single-crystal magnetite nanoparticles for drug delivery. Biomaterials 2009, 30, 1881–1889. [Google Scholar] [CrossRef]
- Schwaminger, S.P.; Syhr, C.; Berensmeier, S. Controlled synthesis of magnetic iron oxide nanoparticles: Magnetite or maghemite? Crystals 2020, 10, 214. [Google Scholar] [CrossRef]
- Deivasegamani, R.; Karunanidhi, G.; Santhosh, C.; Gopal, T.; Saravana Achari, D.; Neogi, A.; Nivetha, R.; Pradeep, N.; Venkatraman, U.; Bhatnagar, A.; et al. Chemoresistive sensor for hydrogen using thin films of tin dioxide doped with cerium and palladium. Microchim. Acta 2017, 184, 4765–4773. [Google Scholar] [CrossRef]
- Johari, M.H.; Sirat, M.S.; Mohd Ambri Mohamed, S.; Nasir, N.F.M.; Mohd Asri Mat Teridi, A.R.M. Effects of Mo vapor concentration on the morphology of vertically standing MoS2 nanoflakes. Nanotechnology 2020, 31, 305710. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, G.; Maity, D. Recent advances in superparamagnetic iron oxide nanoparticles (SPIONs) for in vitro and in vivo cancer nanotheranostics. Int. J. Pharm. 2015, 496, 191–218. [Google Scholar] [CrossRef]
- Sun, S.N.; Wei, C.; Zhu, Z.Z.; Hou, Y.L.; Venkatraman, S.S.; Xu, Z.C. Magnetic iron oxide nanoparticles: Synthesis and surface coating techniques for biomedical applications. Chin. Phys. B 2014, 23, 037503. [Google Scholar] [CrossRef]
- Moacă, E.-A.; Coricovac, E.D.; Soica, C.M.; Pinzaru, I.A.; Păcurariu, C.S.; Dehelean, C.A. Preclinical Aspects on Magnetic Iron Oxide Nanoparticles and Their Interventions as Anticancer Agents: Enucleation, Apoptosis and Other Mechanism. In Iron Ores and Iron Oxide Materials; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Ghazanfari, M.R.; Kashefi, M.; Shams, S.F.; Jaafari, M.R. Perspective of Fe3O4 nanoparticles role in biomedical applications. Biochem. Res. Int. 2016, 2016, 7840161. [Google Scholar] [CrossRef]
- Reyes-ortega, F.; Delgado, Á.V.; Iglesias, G.R. Modulation of the magnetic hyperthermia response using different superparamagnetic iron oxide nanoparticle morphologies. Nanomaterials 2021, 11, 627. [Google Scholar] [CrossRef]
- Nemati, Z.; Alonso, J.; Rodrigo, I.; Das, R.; Garaio, E.; García, J.Á.; Orue, I.; Phan, M.H.; Srikanth, H. Improving the Heating Efficiency of Iron Oxide Nanoparticles by Tuning Their Shape and Size. J. Phys. Chem. C 2018, 122, 2367–2381. [Google Scholar] [CrossRef]
- Nemati, Z.; Alonso, J.; Martinez, L.M.; Khurshid, H.; Garaio, E.; Garcia, J.A.; Phan, M.H.; Srikanth, H. Enhanced Magnetic Hyperthermia in Iron Oxide Nano-Octopods: Size and Anisotropy Effects. J. Phys. Chem. C 2016, 120, 8370–8379. [Google Scholar] [CrossRef]
- El Ghandoor, H.; Zidan, H.M.; Khalil, M.M.H.; Ismail, M.I.M. Synthesis and some physical properties of magnetite (Fe3O4) nanoparticles. Int. J. Electrochem. Sci. 2012, 7, 5734–5745. [Google Scholar]
- Mohapatra, J.; Zeng, F.; Elkins, K.; Xing, M.; Ghimire, M.; Yoon, S.; Mishra, S.R.; Liu, J.P. Size-dependent magnetic and inductive heating properties of Fe3O4 nanoparticles: Scaling laws across the superparamagnetic size. Phys. Chem. Chem. Phys. 2018, 20, 12879–12887. [Google Scholar] [CrossRef]
- Goya, G.F.; Lima, E.; Arelaro, A.D.; Torres, T.; Rechenberg, H.R.; Rossi, L.; Marquina, C.; Ibarra, M.R. Magnetic hyperthermia with Fe3O4 nanoparticles: The influence of particle size on energy absorption. IEEE Trans. Magn. 2008, 44, 4444–4447. [Google Scholar] [CrossRef]
- Tong, S.; Quinto, C.A.; Zhang, L.; Mohindra, P.; Bao, G. Size-Dependent Heating of Magnetic Iron Oxide Nanoparticles. ACS Nano 2017, 11, 6808–6816. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.T.; Kim, K.-S. Controlled synthesis of monodisperse magnetite nanoparticles for hyperthermia-based treatments. Powder Technol. 2016, 301, 1112–1118. [Google Scholar] [CrossRef]
- Cervadoro, A.; Giverso, C.; Pande, R.; Sarangi, S.; Preziosi, L.; Wosik, J.; Brazdeikis, A.; Decuzzi, P. Design Maps for the Hyperthermic Treatment of Tumors with Superparamagnetic Nanoparticles. PLoS ONE 2013, 8, e57332. [Google Scholar] [CrossRef]
- Shi, D.; Sadat, M.E.; Dunn, A.W.; Mast, D.B. Photo-fluorescent and magnetic properties of iron oxide nanoparticles for biomedical applications. Nanoscale 2015, 7, 8209–8232. [Google Scholar] [CrossRef]

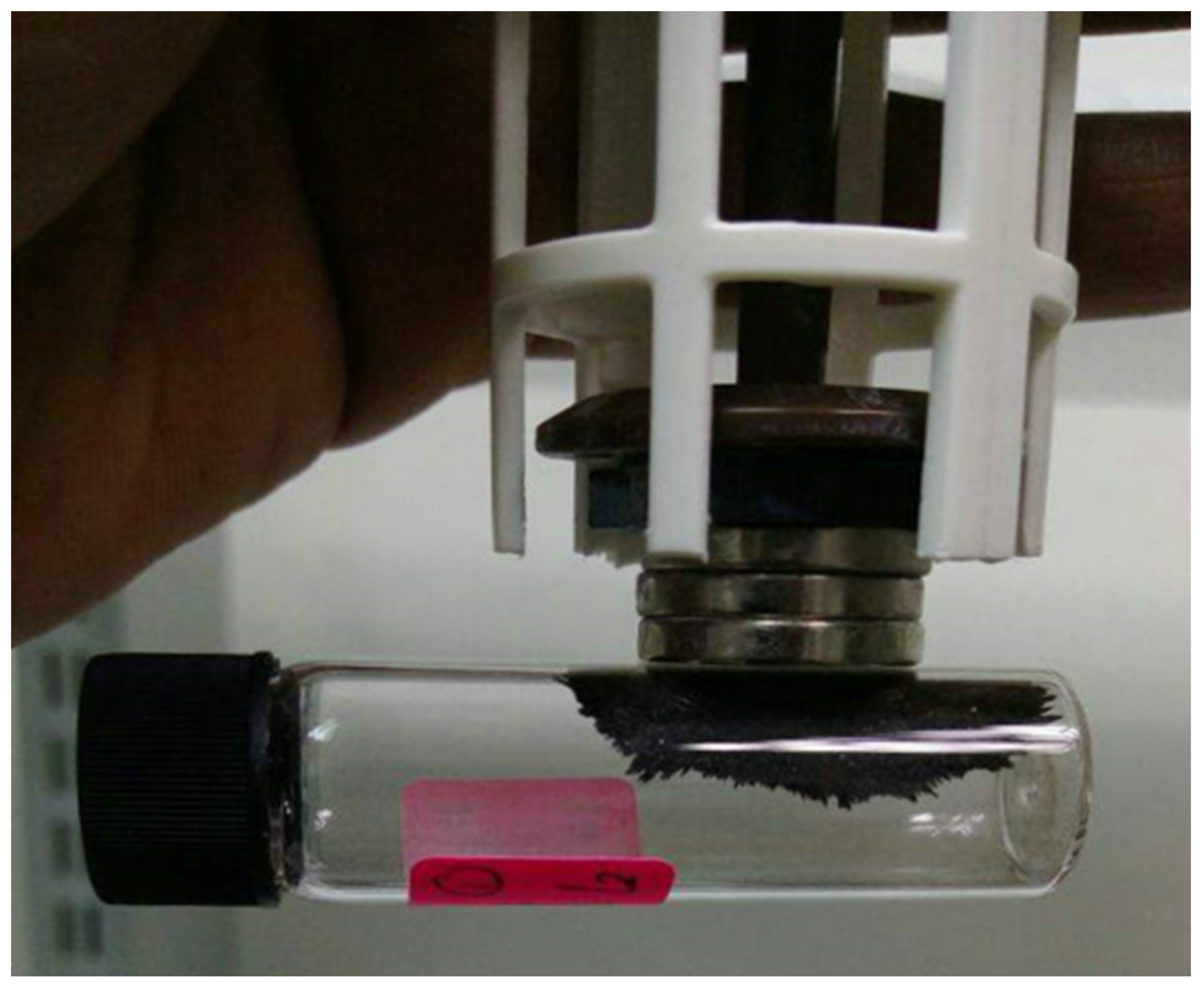
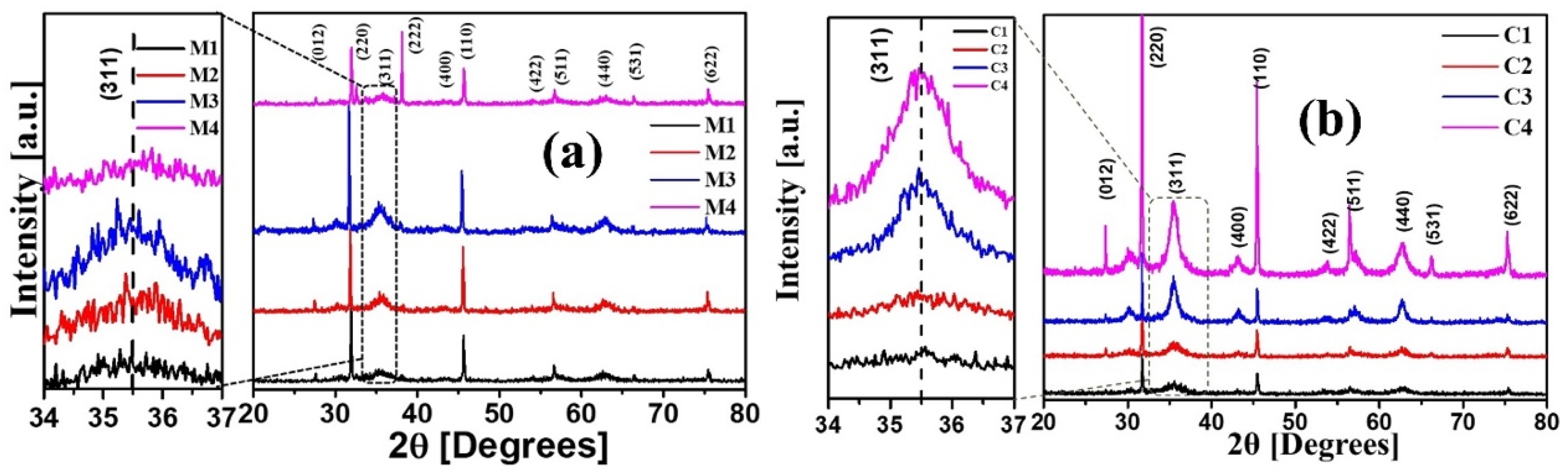
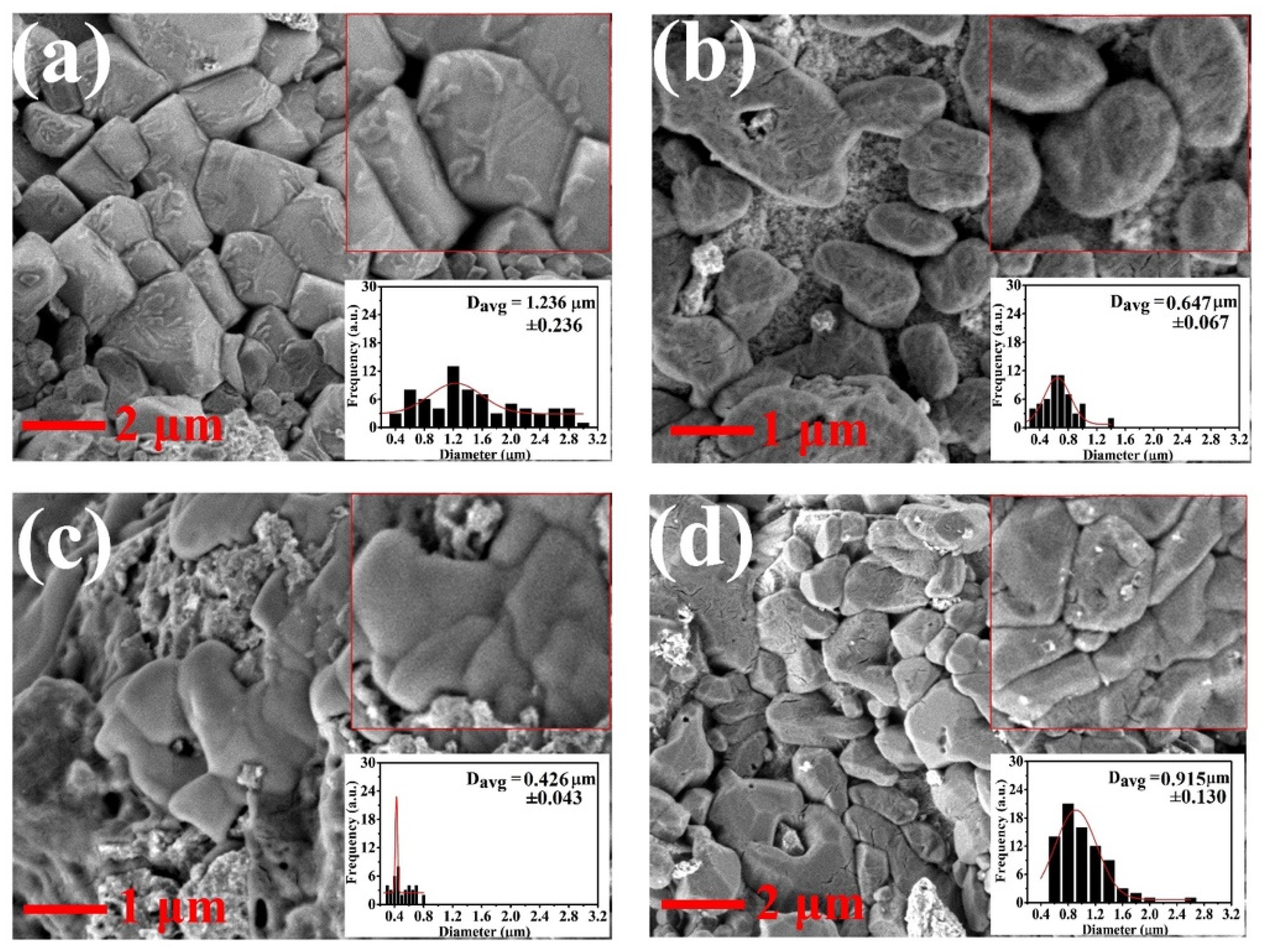
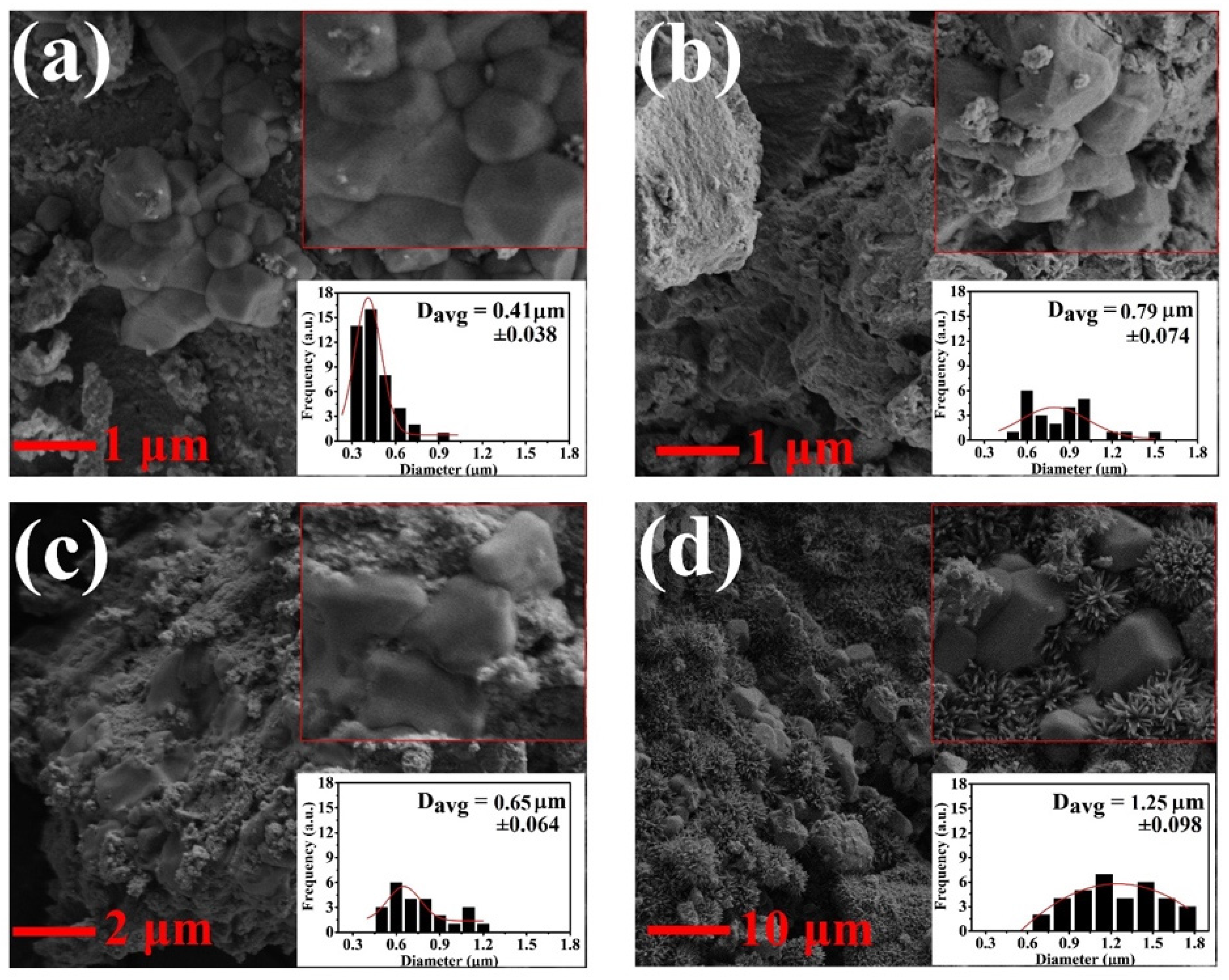
| (a) | |||
| Samples | Iron Source | Base | |
| FeCl3·6H2O | FeCl2·4H2O | NaOH | |
| M1 | 1.28 | 0.64 | 1.5 |
| M2 | 1.75 | 0.875 | 2.0 |
| M3 | 2.00 | 1.00 | 2.5 |
| M4 | 2.56 | 1.28 | 3.0 |
| (b) | |||
| Samples | Iron Source | Base | |
| FeCl3·6H2O | FeCl2·4H2O | NaOH | |
| C1 | 1.75 | 0.875 | 1.5 |
| C2 | 2.00 | 1.00 | 1.5 |
| C3 | 2.56 | 1.28 | 1.5 |
| C4 | 3.2 | 1.6 | 1.5 |
| (a) | |||||
| Samples | 2θ (°) | θ (Rad) | FWHM | Crystallite Size (nm) | (h k l) |
| M1 | 35.56 | 0.3103 | 1.974 | 3.832 | (3 1 1) |
| M2 | 35.52 | 0.3099 | 1.674 | 4.520 | (3 1 1) |
| M3 | 35.45 | 0.3094 | 2.103 | 3.599 | (3 1 1) |
| M4 | 35.69 | 0.3115 | 1.042 | 7.260 | (3 1 1) |
| (b) | |||||
| Samples | 2θ (°) | θ (Rad) | FWHM | Crystallite Size (nm) | (h k l) |
| C1 | 35.48 | 0.3096 | 1.859 | 4.070 | (3 1 1) |
| C2 | 35.63 | 0.3109 | 1.604 | 4.716 | (3 1 1) |
| C3 | 35.51 | 0.3099 | 0.9694 | 7.805 | (3 1 1) |
| C4 | 35.52 | 0.3100 | 1.282 | 5.902 | (3 1 1) |
| (a) | ||||
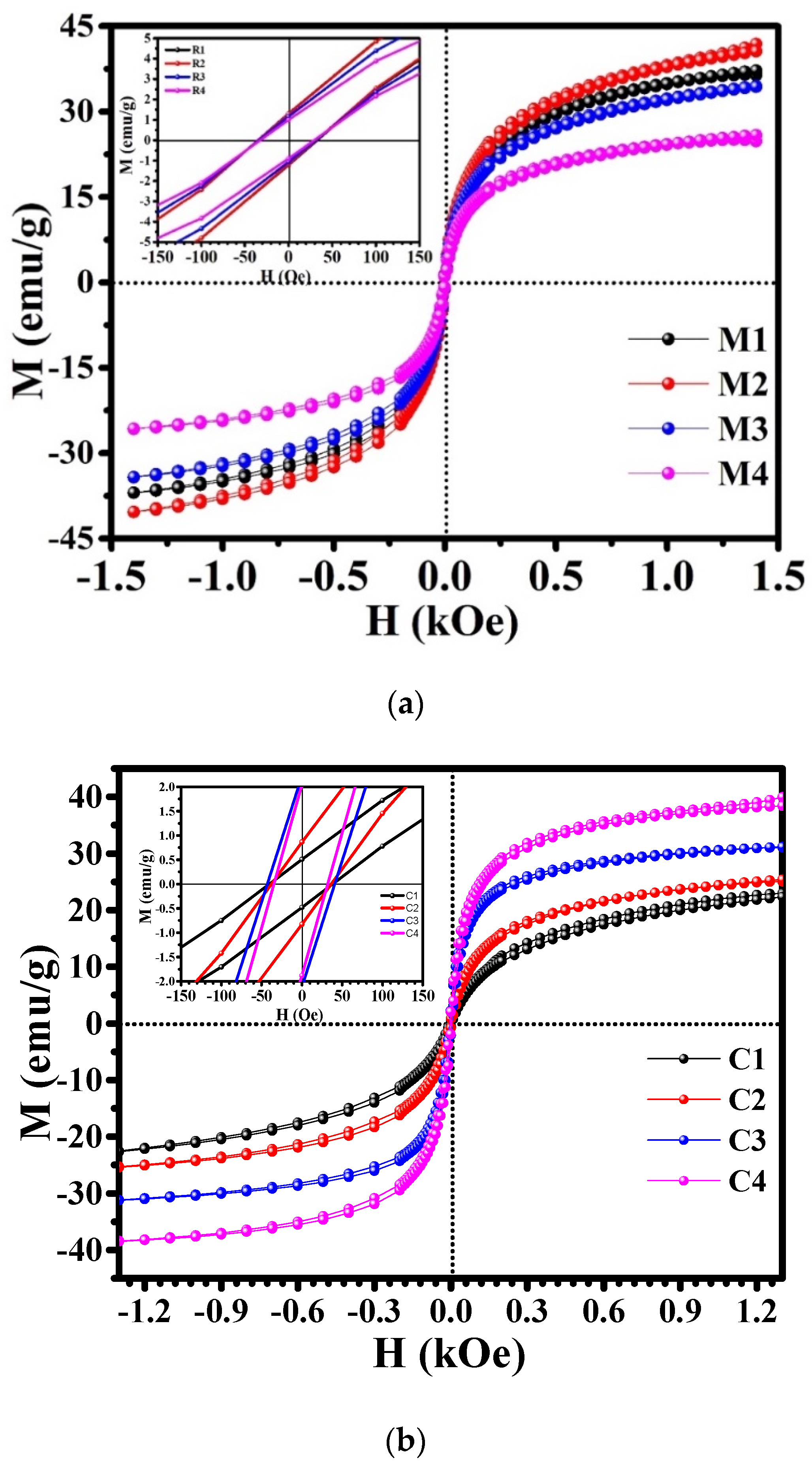
| Samples | Saturation Magnetisation, Ms (emu/g) | Coercivity, Hc (Oe) | Retentivity, Mr (emu/g) | Power Loss, P (W/g) |
| M1 | 37.090 | 33.933 | 1.2623 | 0.1239 |
| M2 | 41.095 | 33.313 | 1.2383 | 0.173 |
| M3 | 34.318 | 32.532 | 1.1104 | 0.1169 |
| M4 | 25.804 | 30.841 | 0.94617 | 0.4056 |
| (b) | ||||
| Samples | Saturation Magnetisation, Ms (emu/g) | Coercivity, Hc (Oe) | Retentivity, Mr (emu/g) | Power Loss, P (W/g) |
| C1 | 22.977 | 39.165 | 0.49263 | 0.0946 |
| C2 | 25.705 | 37.044 | 0.84604 | 0.15222 |
| C3 | 31.411 | 41.741 | 2.1619 | 0.0703 |
| C4 | 40.531 | 33.286 | 1.9561 | 0.1645 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganapathe, L.S.; Kazmi, J.; Mohamed, M.A.; Berhanuddin, D.D. Molarity Effects of Fe and NaOH on Synthesis and Characterisation of Magnetite (Fe3O4) Nanoparticles for Potential Application in Magnetic Hyperthermia Therapy. Magnetochemistry 2022, 8, 161. https://doi.org/10.3390/magnetochemistry8110161
Ganapathe LS, Kazmi J, Mohamed MA, Berhanuddin DD. Molarity Effects of Fe and NaOH on Synthesis and Characterisation of Magnetite (Fe3O4) Nanoparticles for Potential Application in Magnetic Hyperthermia Therapy. Magnetochemistry. 2022; 8(11):161. https://doi.org/10.3390/magnetochemistry8110161
Chicago/Turabian StyleGanapathe, Lokesh Srinath, Jamal Kazmi, Mohd Ambri Mohamed, and Dilla Duryha Berhanuddin. 2022. "Molarity Effects of Fe and NaOH on Synthesis and Characterisation of Magnetite (Fe3O4) Nanoparticles for Potential Application in Magnetic Hyperthermia Therapy" Magnetochemistry 8, no. 11: 161. https://doi.org/10.3390/magnetochemistry8110161
APA StyleGanapathe, L. S., Kazmi, J., Mohamed, M. A., & Berhanuddin, D. D. (2022). Molarity Effects of Fe and NaOH on Synthesis and Characterisation of Magnetite (Fe3O4) Nanoparticles for Potential Application in Magnetic Hyperthermia Therapy. Magnetochemistry, 8(11), 161. https://doi.org/10.3390/magnetochemistry8110161







