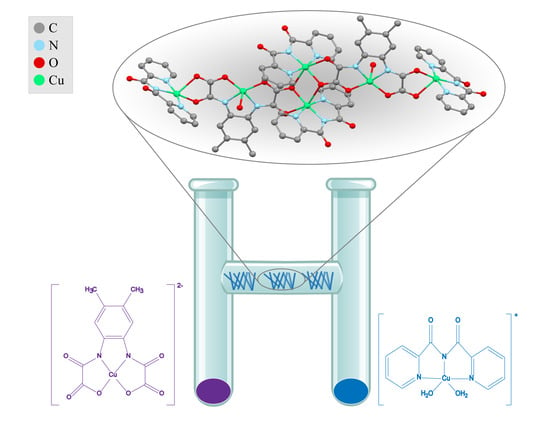
Solid-State Self-Assembly of a Linear Hexanuclear Copper(II) Oxamate Complex with Alternating Antiferro- and Ferromagnetic Coupling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of (n-Bu4N)2[Cu(dmopba)]
2.2. Synthesis of {[Cubpca)]2[Cu(dmopba)(H2O)]}2·4H2O (1)
2.3. Crystallographic Data Collection and Refinement
2.4. Magnetic Measurements
3. Results and Discussion
3.1. Synthesis and Characterization of 1
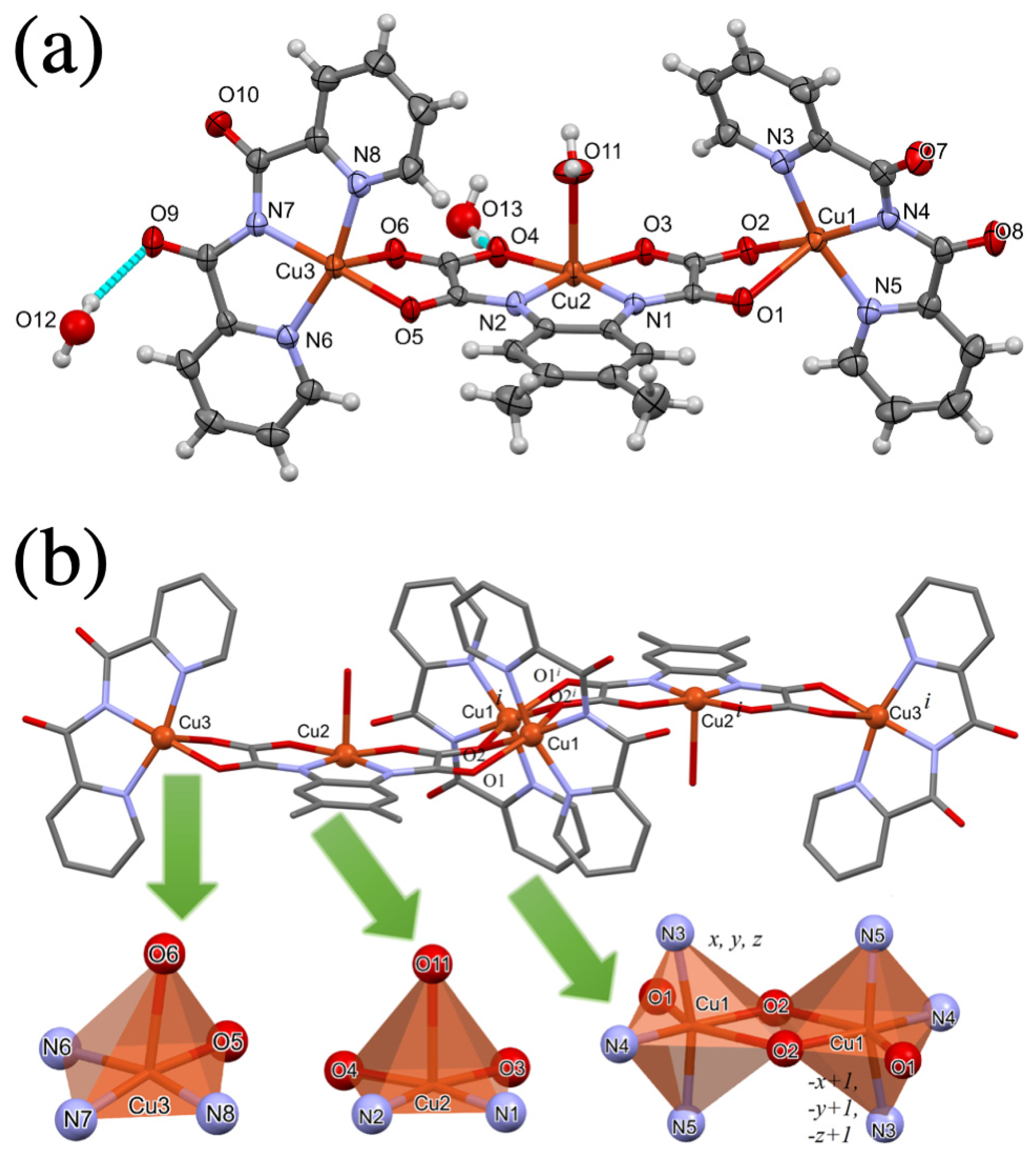
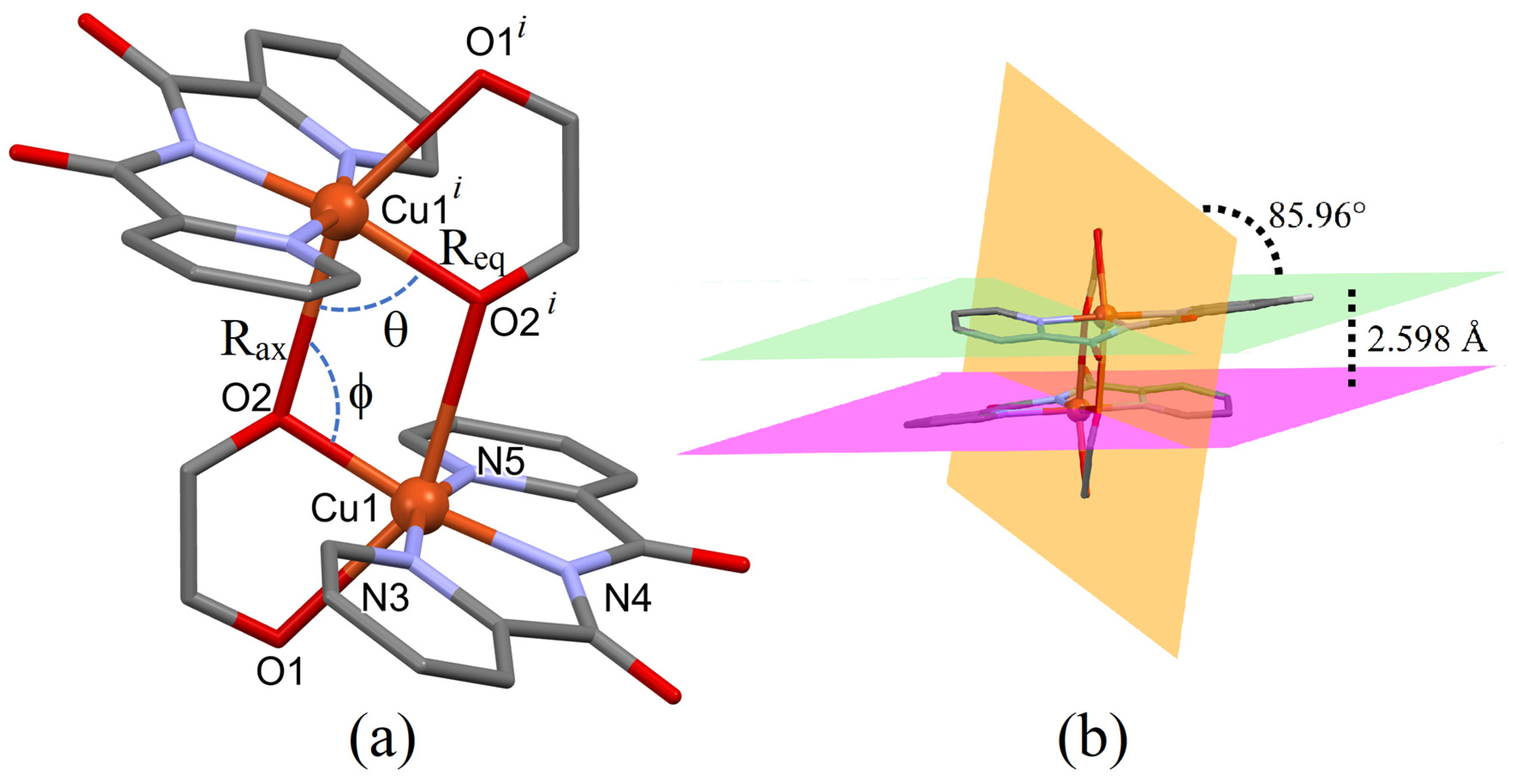
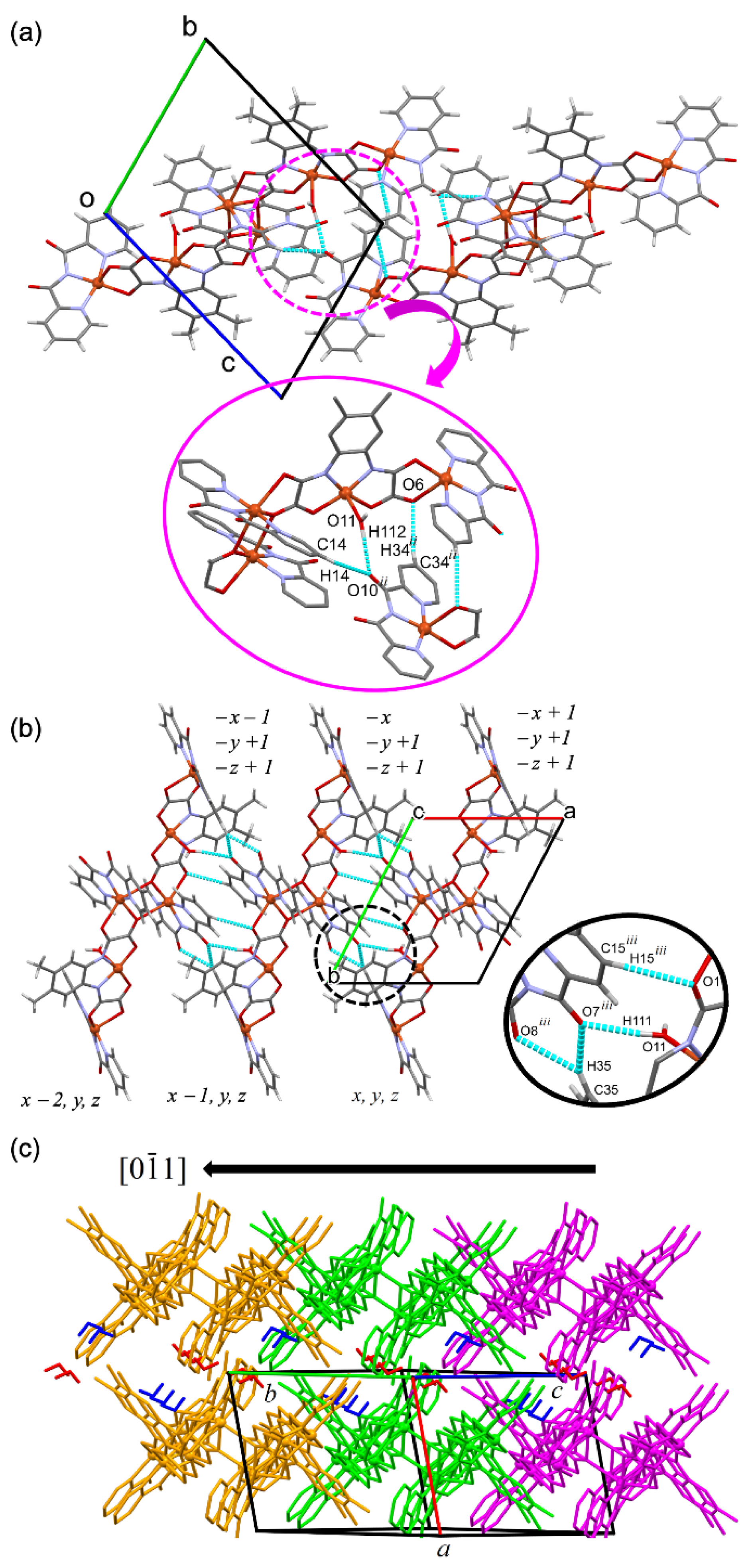
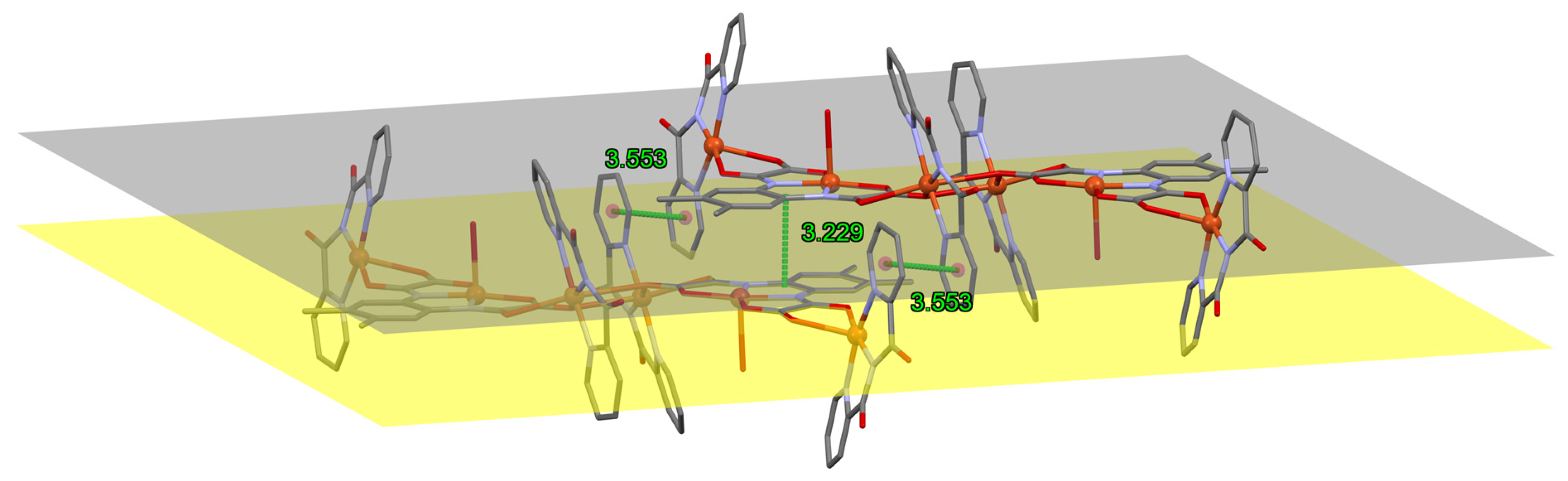
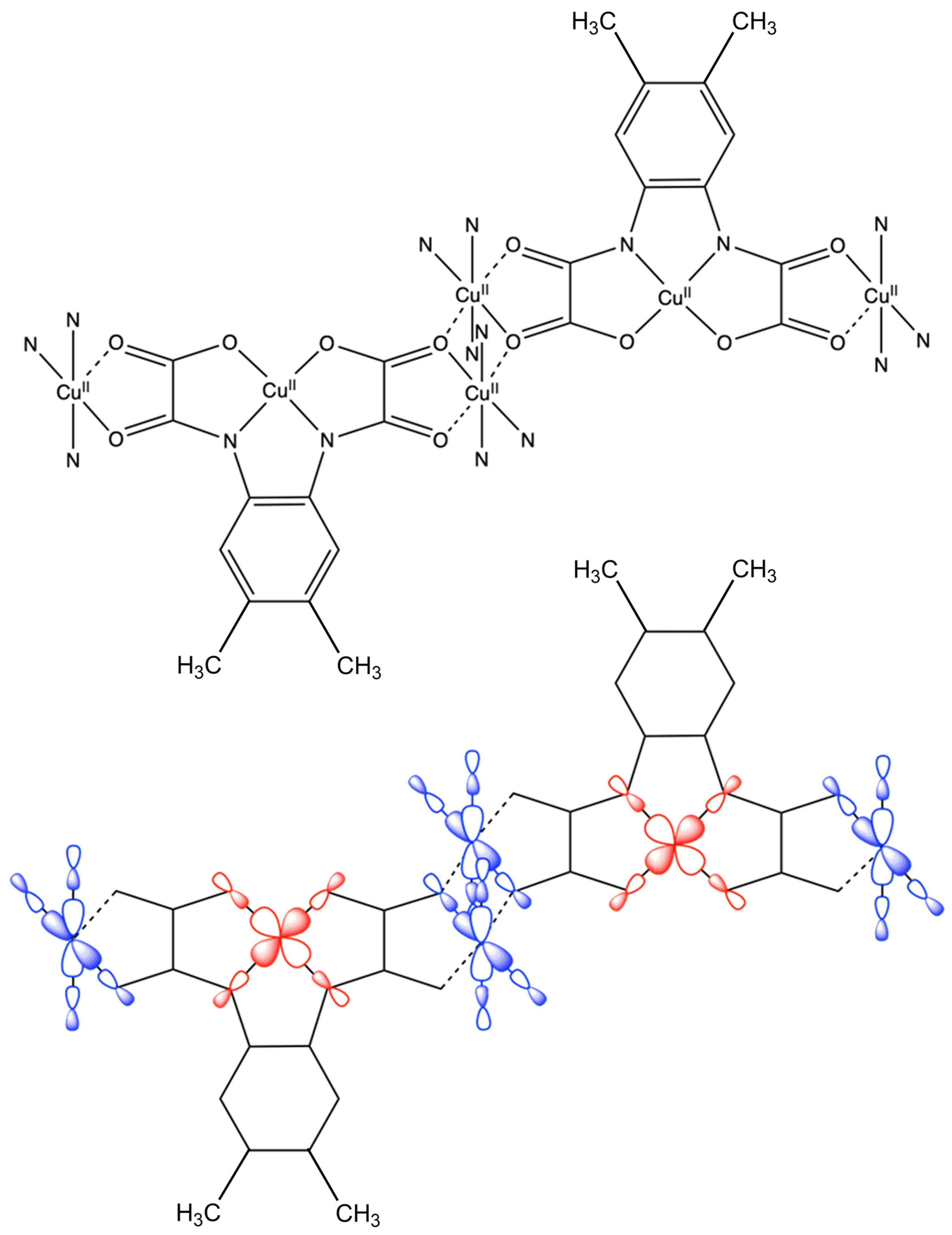
3.2. Description of the Crystal Structure of 1
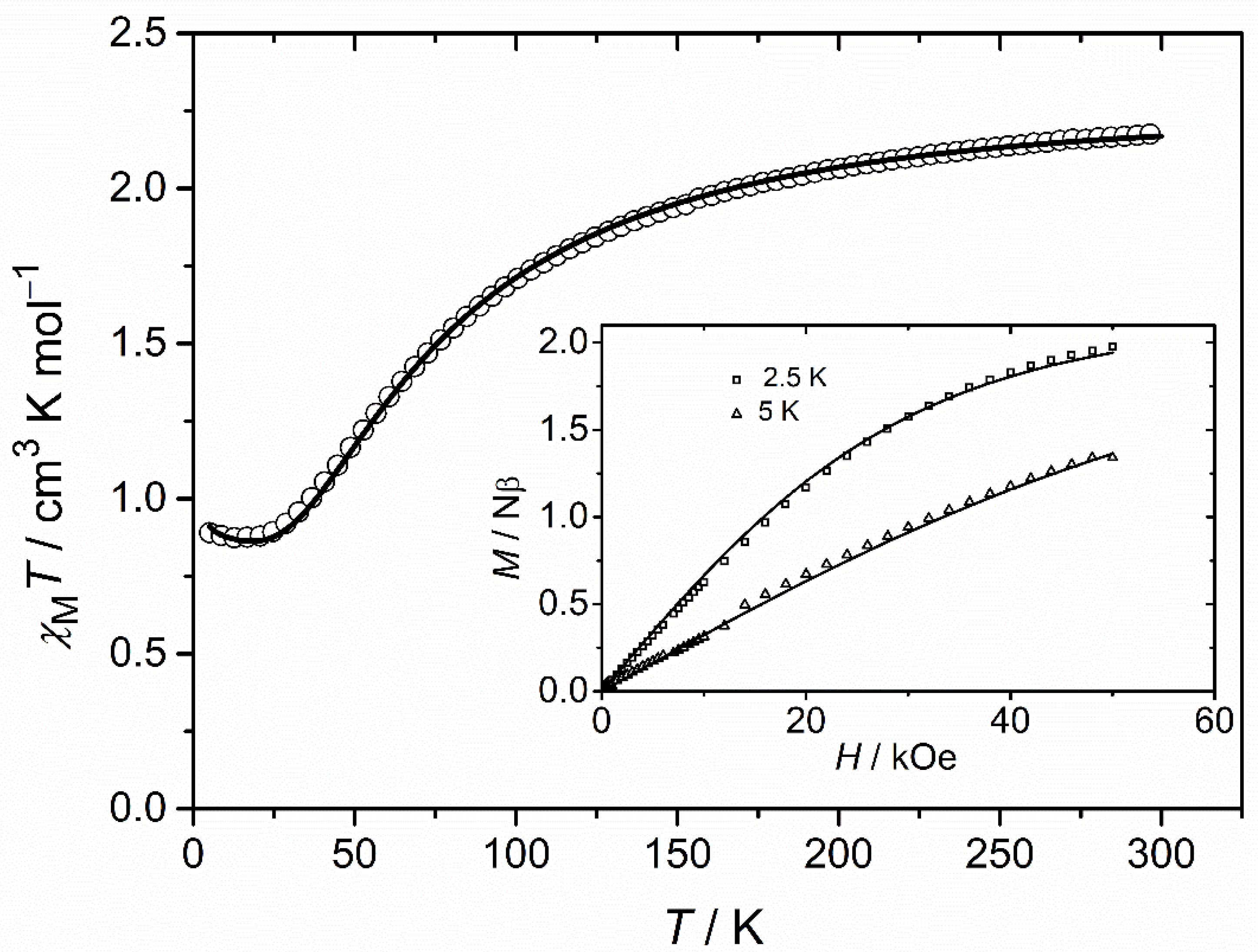
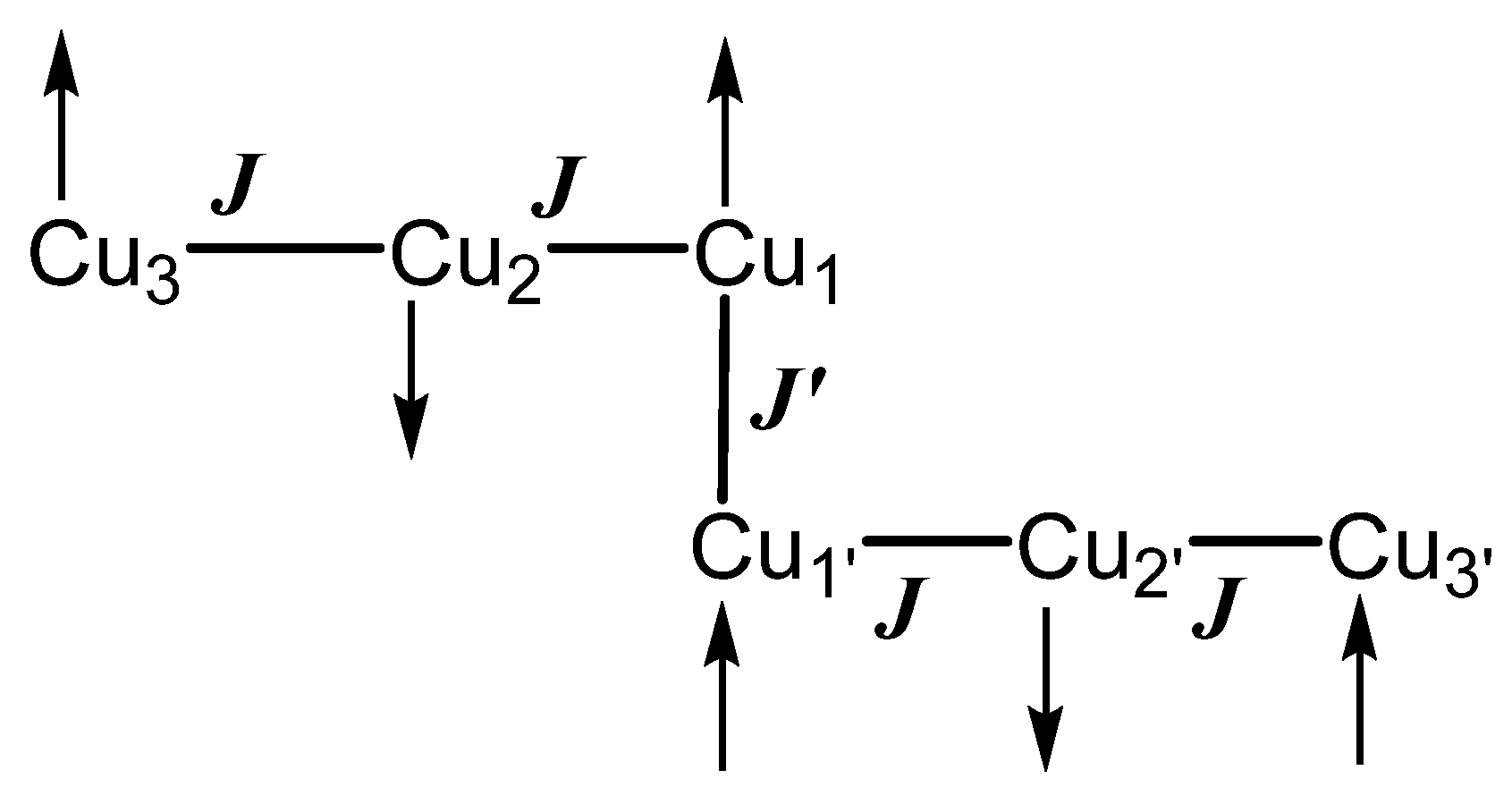
3.3. Magnetic Properties of 1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mako, T.L.; Racicot, J.M.; Levine, M. Supramolecular Luminescent Sensors. Chem. Rev. 2019, 119, 322–477. [Google Scholar] [CrossRef]
- Tang, Y.-Y.; Zeng, Y.-L.; Xiong, R.-G. Contactless Manipulation of Write–Read–Erase Data Storage in Diarylethene Ferroelectric Crystals. J. Am. Chem. Soc. 2022, 144, 8633–8640. [Google Scholar] [CrossRef]
- Olivo, G.; Capocasa, G.; del Giudice, D.; Lanzalunga, O.; di Stefano, S. New Horizons for Catalysis Disclosed by Supramolecular Chemistry. Chem. Soc. Rev. 2021, 50, 7681–7724. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Z.; Zhao, H.; Xu, J.-F.; Sun, Z.; Zhang, X. Supramolecular Chemotherapy: Cooperative Enhancement of Antitumor Activity by Combining Controlled Release of Oxaliplatin and Consuming of Spermine by Cucurbit[7]Uril. ACS Appl. Mater. Interfaces 2017, 9, 8602–8608. [Google Scholar] [CrossRef]
- Dumele, O.; Chen, J.; Passarelli, J.v.; Stupp, S.I. Supramolecular Energy Materials. Adv. Mater. 2020, 32, 1907247. [Google Scholar] [CrossRef]
- Irie, M.; Fukaminato, T.; Matsuda, K.; Kobatake, S. Photochromism of Diarylethene Molecules and Crystals: Memories, Switches, and Actuators. Chem. Rev. 2014, 114, 12174–12277. [Google Scholar] [CrossRef]
- Geng, W.; Zheng, Z.; Guo, D. Supramolecular Design Based Activatable Magnetic Resonance Imaging. View 2021, 2, 20200059. [Google Scholar] [CrossRef]
- Moreno-Pineda, E.; Wernsdorfer, W. Measuring Molecular Magnets for Quantum Technologies. Nat. Rev. Phys. 2021, 3, 645–659. [Google Scholar] [CrossRef]
- Fyfe, M.C.T.; Stoddart, J.F. Synthetic Supramolecular Chemistry. Acc. Chem. Res. 1997, 30, 393–401. [Google Scholar] [CrossRef]
- Dhers, S.; Feltham, H.L.C.; Brooker, S. A Toolbox of Building Blocks, Linkers and Crystallisation Methods Used to Generate Single-Chain Magnets. Coord. Chem. Rev. 2015, 296, 24–44. [Google Scholar] [CrossRef]
- Journaux, Y.; Ferrando-Soria, J.; Pardo, E.; Ruiz-Garcia, R.; Julve, M.; Lloret, F.; Cano, J.; Li, Y.; Lisnard, L.; Yu, P.; et al. Design of Magnetic Coordination Polymers Built from Polyoxalamide Ligands: A Thirty Year Story. Eur. J. Inorg. Chem. 2018, 2018, 228–247. [Google Scholar] [CrossRef]
- Dul, M.-C.; Pardo, E.; Lescouëzec, R.; Journaux, Y.; Ferrando-Soria, J.; Ruiz-García, R.; Cano, J.; Julve, M.; Lloret, F.; Cangussu, D.; et al. Supramolecular Coordination Chemistry of Aromatic Polyoxalamide Ligands: A Metallosupramolecular Approach toward Functional Magnetic Materials. Coord. Chem. Rev. 2010, 254, 2281–2296. [Google Scholar] [CrossRef]
- Ferrando-Soria, J.; Vallejo, J.; Castellano, M.; Martínez-Lillo, J.; Pardo, E.; Cano, J.; Castro, I.; Lloret, F.; Ruiz-García, R.; Julve, M. Molecular Magnetism, Quo Vadis? A Historical Perspective from a Coordination Chemist Viewpoint. Coord. Chem. Rev. 2017, 339, 17–103. [Google Scholar] [CrossRef]
- Da Cunha, T.T.; da Silveira, C.O.C.; Barbosa, V.M.M.; Oliveira, W.X.C.; da Silva Júnior, E.N.; Ferreira, F.F.; Pedroso, E.F.; Pereira, C.L.M. Ferromagnetic Coupling in a Dicopper(II) Oxamate Complex Bridged by Carboxylate Groups. CrystEngComm 2021, 23, 1885–1897. [Google Scholar] [CrossRef]
- Da Cunha, T.T.; Barbosa, V.M.M.; Oliveira, W.X.C.; Pedroso, E.F.; García, D.M.A.; Nunes, W.C.; Pereira, C.L.M. Field-Induced Slow Magnetic Relaxation of a Six-Coordinate Mononuclear Manganese(II) and Cobalt(II) Oxamate Complexes. Inorg. Chem. 2020, 59, 12983–12987. [Google Scholar] [CrossRef]
- Da Cunha, T.T.; Barbosa, V.M.M.; Oliveira, W.X.C.; Pinheiro, C.B.; Pedroso, E.F.; Nunes, W.C.; Pereira, C.L.M. Slow Magnetic Relaxation in Mononuclear Gadolinium(III) and Dysprosium(III) Oxamato Complexes. Polyhedron 2019, 169, 102–113. [Google Scholar] [CrossRef]
- Do Pim, W.D.; Oliveira, W.X.C.; Ribeiro, M.A.; de Faria, É.N.; Teixeira, I.F.; Stumpf, H.O.; Lago, R.M.; Pereira, C.L.M.; Pinheiro, C.B.; Figueiredo-Júnior, J.C.D.; et al. A PH-Triggered Bistable Copper(Ii) Metallacycle as a Reversible Emulsion Switch for Biphasic Processes. Chem. Commun. 2013, 49, 10778–10780. [Google Scholar] [CrossRef]
- Barros, W.P.; da Silva, B.C.; Reis, N.V.; Pereira, C.L.M.; Doriguetto, A.C.; Cano, J.; Pirota, K.R.; Pedroso, E.F.; Julve, M.; Stumpf, H.O. Discrete Trinuclear Copper(Ii) Compounds as Building Blocks: The Influence of the Peripheral Substituents on the Magnetic Coupling in Oxamato-Bridged Complexes. Dalton Trans. 2014, 43, 14586–14595. [Google Scholar] [CrossRef]
- Do Pim, W.D.; de Faria, É.N.; Oliveira, W.X.C.; Pinheiro, C.B.; Nunes, W.C.; Cano, J.; Lloret, F.; Julve, M.; Stumpf, H.O.; Pereira, C.L.M. A Heterobimetallic [MnII5CuII5] Nanowheel Modulated by a Flexible Bis-Oxamate Type Ligand. Dalton Trans. 2015, 44, 10939–10942. [Google Scholar] [CrossRef]
- Do Pim, W.D.; Silva, I.F.; da Silva Júnior, E.N.; Stumpf, H.O.; Oliveira, W.X.C.; Pedroso, E.F.; Pinheiro, C.B.; Journaux, Y.; Fantuzzi, F.; Krummenacher, I.; et al. Unexpected Formation of a Dodecanuclear {CoII6CuII6} Nanowheel under Ambient Conditions: Magneto-Structural Correlations. Dalton Trans. 2021, 50, 12430–12434. [Google Scholar] [CrossRef]
- Da Cunha, T.; Oliveira, W.X.C.; Pedroso, E.F.; Lloret, F.; Julve, M.; Pereira, C.L.M. Heterobimetallic One-Dimensional Coordination Polymers MICuII (M = Li and K) Based on Ferromagnetically Coupled Di- and Tetracopper(II) Metallacyclophanes. Magnetochemistry 2018, 4, 38. [Google Scholar] [CrossRef]
- Marinho, M.V.; Simões, T.R.G.; Ribeiro, M.A.; Pereira, C.L.M.; Machado, F.C.; Pinheiro, C.B.; Stumpf, H.O.; Cano, J.; Lloret, F.; Julve, M. A Two-Dimensional Oxamate- and Oxalate-Bridged CuIIMnII Motif: Crystal Structure and Magnetic Properties of (Bu4N)2[Mn2{Cu(opba)}2ox]. Inorg. Chem. 2013, 52, 8812–8819. [Google Scholar] [CrossRef]
- Pardo, E.; Cangussu, D.; Dul, M.-C.; Lescouëzec, R.; Herson, P.; Journaux, Y.; Pedroso, E.F.; Pereira, C.L.M.; Muñoz, M.C.; Ruiz-García, R.; et al. A Metallacryptand-Based Manganese(II)-Cobalt(II) Ferrimagnet with a Three-Dimensional Honeycomb Open-Framework Architecture. Angew. Chem. Int. Ed. 2008, 47, 4279–4284. [Google Scholar] [CrossRef]
- Viciano-Chumillas, M.; Mon, M.; Ferrando-Soria, J.; Corma, A.; Leyva-Pérez, A.; Armentano, D.; Pardo, E. Metal–Organic Frameworks as Chemical Nanoreactors: Synthesis and Stabilization of Catalytically Active Metal Species in Confined Spaces. Acc. Chem. Res. 2020, 53, 520–531. [Google Scholar] [CrossRef]
- Desiraju, G.R. Chemistry beyond the Molecule. Nature 2001, 412, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Lehn, J.-M. Perspectives in Chemistry-Steps towards Complex Matter. Angew. Chem. Int. Ed. 2013, 52, 2836–2850. [Google Scholar] [CrossRef]
- Oliveira, W.X.C.; da Costa, M.M.; Fontes, A.P.S.; Pinheiro, C.B.; de Paula, F.C.S.; Jaimes, E.H.L.; Pedroso, E.F.; de Souza, P.P.; Pereira-Maia, E.C.; Pereira, C.L.M. Palladium(II) and Platinum(II) Oxamate Complexes as Potential Anticancer Agents: Structural Characterization and Cytotoxic Activity. Polyhedron 2014, 76, 16–21. [Google Scholar] [CrossRef]
- Kovač, V.; Kodrin, I.; Radošević, K.; Molčanov, K.; Adhikari, B.; Kraatz, H.-B.; Barišić, L. Oxalamide-Bridged Ferrocenes: Conformational and Gelation Properties and In Vitro Antitumor Activity. Organometallics 2022, 41, 920–936. [Google Scholar] [CrossRef]
- Altinoz, M.A.; Ozpinar, A. Oxamate Targeting Aggressive Cancers with Special Emphasis to Brain Tumors. Biomed. Pharmacother. 2022, 147, 112686. [Google Scholar] [CrossRef]
- Fortea-Pérez, F.R.; Armentano, D.; Julve, M.; de Munno, G.; Stiriba, S.E. Bis(Oxamato)Palladate(II) Complexes: Synthesis, Crystal Structure and Application to Catalytic Suzuki Reaction. J. Coord. Chem. 2014, 67, 4003–4015. [Google Scholar] [CrossRef]
- Da Cunha, T.T.; de Souza, T.E.; do Pim, W.D.; de Almeida, L.D.; do Nascimento, G.M.; García-España, E.; Inclán, M.; Julve, M.; Stumpf, H.O.; Oliveira, L.C.A.; et al. A Hybrid Catalyst for Decontamination of Organic Pollutants Based on a Bifunctional Dicopper(II) Complex Anchored over Niobium Oxyhydroxide. Appl. Catal. B 2017, 209, 339–345. [Google Scholar] [CrossRef]
- Ferrando-Soria, J.; Serra-Crespo, P.; de Lange, M.; Gascon, J.; Kapteijn, F.; Julve, M.; Cano, J.; Lloret, F.; Pasán, J.; Ruiz-Pérez, C.; et al. Selective Gas and Vapor Sorption and Magnetic Sensing by an Isoreticular Mixed-Metal-Organic Framework. J. Am. Chem. Soc. 2012, 134, 15301–15304. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.F.; Teixeira, I.F.; Barros, W.P.; Pinheiro, C.B.; Ardisson, J.D.; do Nascimento, G.M.; Pradie, N.A.; Teixeira, A.P.C.; Stumpf, H.O. An FeIII Dinuclear Metallacycle Complex as a Size-Selective Adsorbent for Nitrogenous Compounds and a Potentially Effective Ammonia Storage Material. J. Mater. Chem. A Mater. 2019, 7, 15225–15232. [Google Scholar] [CrossRef]
- Ferrando-Soria, J.; Khajavi, H.; Serra-Crespo, P.; Gascon, J.; Kapteijn, F.; Julve, M.; Lloret, F.; Pasán, J.; Ruiz-Pérez, C.; Journaux, Y.; et al. Highly Selective Chemical Sensing in a Luminescent Nanoporous Magnet. Adv. Mater. 2012, 24, 5625–5629. [Google Scholar] [CrossRef] [PubMed]
- Vaz, R.C.A.; Esteves, I.O.; Oliveira, W.X.C.; Honorato, J.; Martins, F.T.; Marques, L.F.; dos Santos, G.L.; Freire, R.O.; Jesus, L.T.; Pedroso, E.F.; et al. Mononuclear Lanthanide(III)-Oxamate Complexes as New Photoluminescent Field-Induced Single-Molecule Magnets: Solid-State Photophysical and Magnetic Properties. Dalton Trans. 2020, 49, 16106–16124. [Google Scholar] [CrossRef]
- Costa, R.; Garcia, A.; Ribas, J.; Mallah, T.; Journaux, Y.; Sletten, J.; Solans, X.; Rodriguez, V. Tailored Magnetic Properties in Trinuclear Copper(II) Complexes: Synthesis, Structure, and Magnetic Properties of Complexes Derived from [1,3-propanediylbis(oxamato)]cuprate(II) ([Cu(pba)]2−). Inorg. Chem. 1993, 32, 3733–3742. [Google Scholar] [CrossRef]
- Tercero, J.; Diaz, C.; el Fallah, M.S.; Ribas, J.; Solans, X.; Maestro, M.A.; Mahía, J. Synthesis, Characterization, and Magnetic Properties of Self-Assembled Compounds Based on Discrete Homotrinuclear Complexes of Cu(II). Inorg. Chem. 2001, 40, 3077–3083. [Google Scholar] [CrossRef] [PubMed]
- Gao, E.-Q.; Zhao, Q.-H.; Tang, J.-K.; Liao, D.-Z.; Jiang, Z.-H.; Yan, S.-P. A New One-Dimensional Coordination Polymer and a New Supramolecular Dimer Made of Trinuclear Copper(II) Complexes: Crystal Structure and Magnetic Properties. J. Chem. Soc. Dalton Trans. 2001, 2001, 1537–1540. [Google Scholar] [CrossRef]
- Tercero, J.; Diaz, C.; Ribas, J.; Mahía, J.; Maestro, M.A. New Oxamato-Bridged Trinuclear CuII−CuII−CuII Complexes with Hydrogen-Bond Supramolecular Structures: Synthesis and Magneto−Structural Studies. Inorg. Chem. 2002, 41, 5373–5381. [Google Scholar] [CrossRef]
- Pardo, E.; Bernot, K.; Julve, M.; Lloret, F.; Cano, J.; Ruiz-García, R.; Delgado, F.S.; Ruiz-Pérez, C.; Ottenwaelder, X.; Journaux, Y. Spin Control in Ladderlike Hexanuclear Copper(II) Complexes with Metallacyclophane Cores. Inorg. Chem. 2004, 43, 2768–2770. [Google Scholar] [CrossRef]
- Pardo, E.; Ruiz-García, R.; Lloret, F.; Julve, M.; Cano, J.; Pasán, J.; Ruiz-Pérez, C.; Filali, Y.; Chamoreau, L.-M.; Journaux, Y. Molecular-Programmed Self-Assembly of Homo- and Heterometallic Penta- and Hexanuclear Coordination Compounds: Synthesis, Crystal Structures, and Magnetic Properties of Ladder-Type CuII2MIIx(M = Cu, Ni; x = 3, 4) Oxamato Complexes with CuII2 Metallacyclophane Cores. Inorg. Chem. 2007, 46, 4504–4514. [Google Scholar] [CrossRef] [PubMed]
- Rabelo, R.; Castellano, M.; Barros, W.P.; Carbonell-Vilar, J.M.; Viciano-Chumillas, M.; Lloret, F.; Julve, M.; Pasán, J.; Cañadillas-Delgado, L.; Ruiz-García, R.; et al. Molecular Engineering of an Inverse Hexacopper(II) Coordination Complex with a Photoactive Metallacyclophane Centroligand as Prototype of a Magnetic Photoswitch. Polyhedron 2022, 217, 115732. [Google Scholar] [CrossRef]
- Pereira, C.L.M.; Pedroso, E.F.; Doriguetto, A.C.; Ellena, J.A.; Boubekeur, K.; Filali, Y.; Journaux, Y.; Novak, M.A.; Stumpf, H.O. Design of 1D and 2D Molecule-Based Magnets with the Ligand 4,5-Dimethyl-1,2-Phenylenebis(Oxamato). Dalton Trans. 2011, 40, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Fettouhi, M.; Ouahab, L.; Boukhari, A.; Cador, O.; Mathonière, C.; Kahn, O. New Metal Oxamates as Precursors of Low-Dimensional Heterobimetallics. Inorg. Chem. 1996, 35, 4932–4937. [Google Scholar] [CrossRef] [PubMed]
- Castro, I.; Faus, J.; Julve, M.; Amigó, J.M.; Sletten, J.; Debaerdemaeker, T. Copper(II)-Assisted Hydrolysis of 2,4,6-Tris(2-Pyridyl)-1,3,5-Triazine. Part 3. Crystal Structures of Diaqua[Bis(2-Pyridylcarbonyl)Amido]Copper(II) Nitrate Dihydrate and Aquabis(Pyridine-2-Carboxamide)Copper(II) Nitrate Monohydrate. J. Chem. Soc. Dalton Trans. 1990, 1990, 891–897. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- CrysAlis, P.R.O. Xcalibur CCD System CrysAlis Software System; Version 1.171.37.31; Rigaku Corporation: Tokyo, Japan, 2014. [Google Scholar]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An Update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New Features for the Visualization and Investigation of Crystal Structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal′s Constants. J. Chem. Educ. 2008, 85, 532. [Google Scholar] [CrossRef]
- Oliveira, W.X.C.; Pinheiro, C.B.; da Costa, M.M.; Fontes, A.P.S.; Nunes, W.C.; Lloret, F.; Julve, M.; Pereira, C.L.M. Crystal Engineering Applied to Modulate the Structure and Magnetic Properties of Oxamate Complexes Containing the [Cu(bpca)]+ Cation. Cryst. Growth Des. 2016, 16, 4094–4107. [Google Scholar] [CrossRef]
- Simões, T.R.G.; Mambrini, R.V.; Reis, D.O.; Marinho, M.V.; Ribeiro, M.A.; Pinheiro, C.B.; Ferrando-Soria, J.; Déniz, M.; Ruiz-Pérez, C.; Cangussu, D.; et al. Copper(II) Assembling with Bis(2-Pyridylcarbonyl)Amidate and N,N′-2,2-Phenylenebis(Oxamate). Dalton Trans. 2013, 42, 5778–5795. [Google Scholar] [CrossRef]
- Llunell, M.; Alemany, P.; Alvarez, S. SHAPE, Version 2.1; The Electronic Structure Groups: Barcelona, Spain, 2013.
- Oliveira, W.X.C.; Ribeiro, M.A.; Pinheiro, C.B.; da Costa, M.M.; Fontes, A.P.S.; Nunes, W.C.; Cangussu, D.; Julve, M.; Stumpf, H.O.; Pereira, C.L.M. Palladium(II)–Copper(II) Assembling with Bis(2-Pyridylcarbonyl)Amidate and Bis(Oxamate) Type Ligands. Cryst. Growth Des. 2015, 15, 1325–1335. [Google Scholar] [CrossRef]
- Castro, I.; Calatayud, M.L.; Orts-Arroyo, M.; Marino, N.; de Munno, G.; Lloret, F.; Ruiz-García, R.; Julve, M. Oxalato as Polyatomic Coordination Center and Magnetic Coupler in Copper(II)-Polypyrazole Inverse Polynuclear Complexes and Coordination Polymers. Coord. Chem. Rev. 2022, 471, 214730. [Google Scholar] [CrossRef]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A Powerful New Program for the Analysis of Anisotropic Monomeric and Exchange-Coupled Polynuclear d- and f-Block Complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism, 1st ed.; Wiley-VCH: New York, NY, USA, 1993; ISBN 978-0471188384. [Google Scholar]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, Structure, and Spectroscopic Properties of Copper(II) Compounds Containing Nitrogen–Sulphur Donor Ligands; the Crystal and Molecular Structure of Aqua[1,7-Bis(N-Methylbenzimidazol-2′-Yl)-2,6-Dithiaheptane]Copper(II) Perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1984, 1349–1356. [Google Scholar] [CrossRef]
- Baggio, R.; Garland, M.T.; Manzur, J.; Peña, O.; Perec, M.; Spodine, E.; Vega, A. A Dinuclear Copper(II) Complex Involving Monoatomic O-Carboxylate Bridging and Cu–S(Thioether) Bonds: [Cu(tda)(phen)]2·H2tda (tda = thiodiacetate, phen = phenanthroline). Inorg. Chim. Acta 1999, 286, 74–79. [Google Scholar] [CrossRef]
- Greenaway, A.M.; O′Connor, C.J.; Overman, J.W.; Sinn, E. Magnetic Properties and Crystal Structure of an Acetate-Bridged Ferromagnetic Copper(II) Dimer. Inorg. Chem. 1981, 20, 1508–1513. [Google Scholar] [CrossRef]
- Christou, G.; Perlepes, S.P.; Libby, E.; Folting, K.; Huffman, J.C.; Webb, R.J.; Hendrickson, D.N. Preparation and Properties of the Triply Bridged, Ferromagnetically Coupled Dinuclear Copper(II) Complexes [Cu2(OAc)3(bpy)2](ClO4) and [Cu2(OH)(H2O)(OAc)(bpy)2](ClO4)2. Inorg. Chem. 1990, 29, 3657–3666. [Google Scholar] [CrossRef]
- Baggio, R.; Calvo, R.; Garland, M.T.; Peña, O.; Perec, M.; Slep, L.D. A New Copper(II) Di-Μ2-Carboxylato Bridged Dinuclear Complex: [Cu(oda)phen]2·6H2O (oda = oxydiacetate, phen = phenanthroline). Inorg. Chem. Commun. 2007, 10, 1249–1252. [Google Scholar] [CrossRef]

Publisher′s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lage, A.L.A.; Ribeiro, L.A.; Doriguetto, A.C.; Pinheiro, C.B.; Nunes, W.C.; Pedroso, E.F.; Pereira, C.L.M. Solid-State Self-Assembly of a Linear Hexanuclear Copper(II) Oxamate Complex with Alternating Antiferro- and Ferromagnetic Coupling. Magnetochemistry 2022, 8, 116. https://doi.org/10.3390/magnetochemistry8100116
Lage ALA, Ribeiro LA, Doriguetto AC, Pinheiro CB, Nunes WC, Pedroso EF, Pereira CLM. Solid-State Self-Assembly of a Linear Hexanuclear Copper(II) Oxamate Complex with Alternating Antiferro- and Ferromagnetic Coupling. Magnetochemistry. 2022; 8(10):116. https://doi.org/10.3390/magnetochemistry8100116
Chicago/Turabian StyleLage, Ana Luísa A., Luísa A. Ribeiro, Antônio C. Doriguetto, Carlos B. Pinheiro, Wallace C. Nunes, Emerson F. Pedroso, and Cynthia L. M. Pereira. 2022. "Solid-State Self-Assembly of a Linear Hexanuclear Copper(II) Oxamate Complex with Alternating Antiferro- and Ferromagnetic Coupling" Magnetochemistry 8, no. 10: 116. https://doi.org/10.3390/magnetochemistry8100116
APA StyleLage, A. L. A., Ribeiro, L. A., Doriguetto, A. C., Pinheiro, C. B., Nunes, W. C., Pedroso, E. F., & Pereira, C. L. M. (2022). Solid-State Self-Assembly of a Linear Hexanuclear Copper(II) Oxamate Complex with Alternating Antiferro- and Ferromagnetic Coupling. Magnetochemistry, 8(10), 116. https://doi.org/10.3390/magnetochemistry8100116