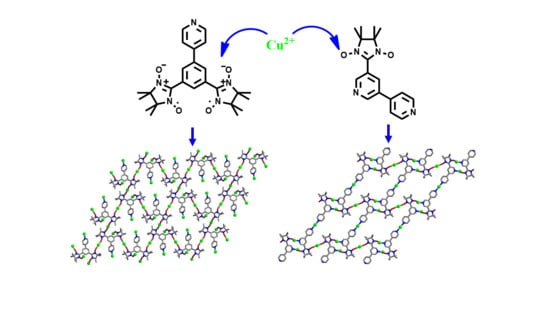
Two-Dimensional Nitronyl Nitroxide–Cu Networks Based on Multi-Dentate Nitronyl Nitroxides: Structures and Magnetic Properties
Abstract
1. Introduction
2. Results and Discussion
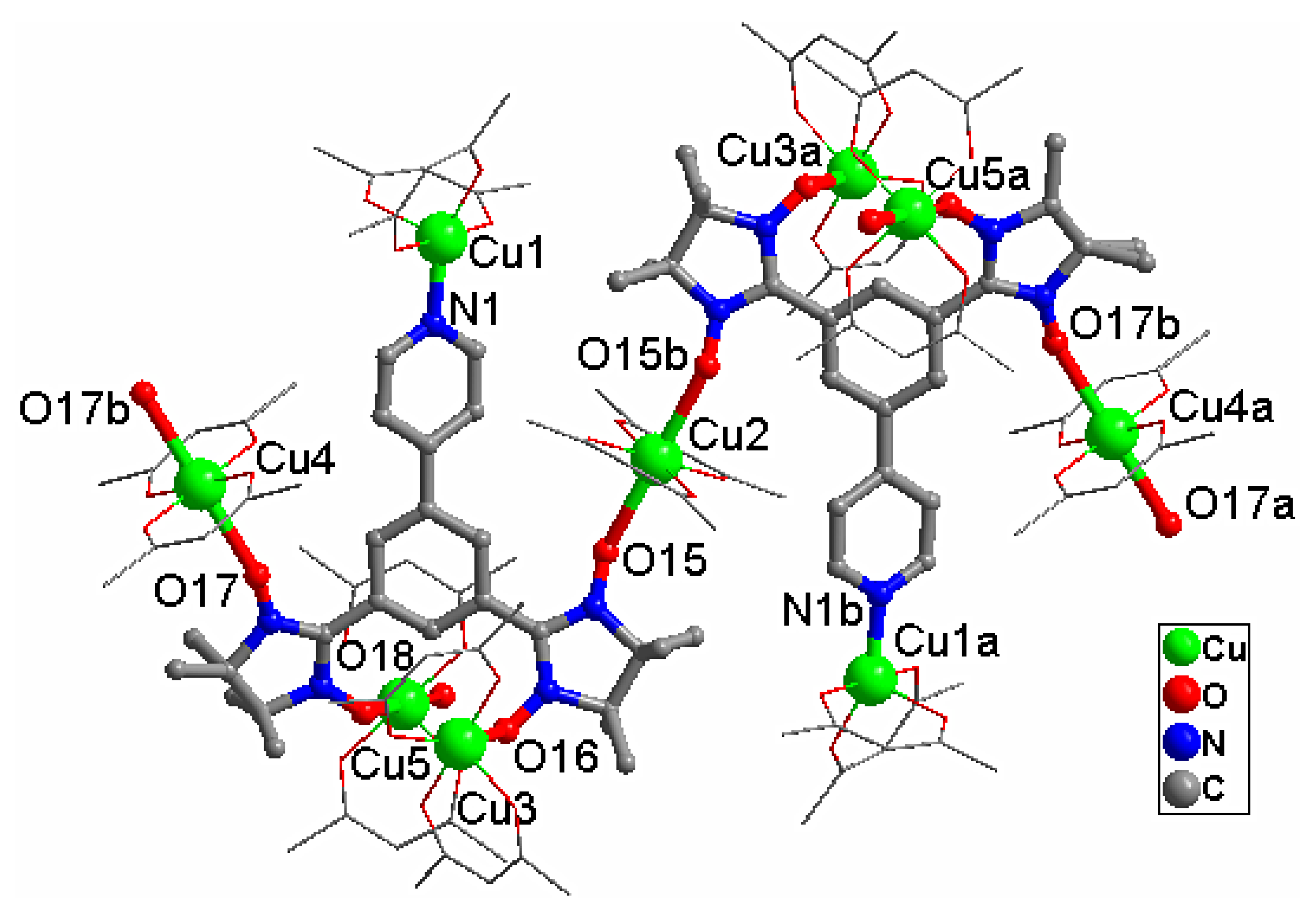
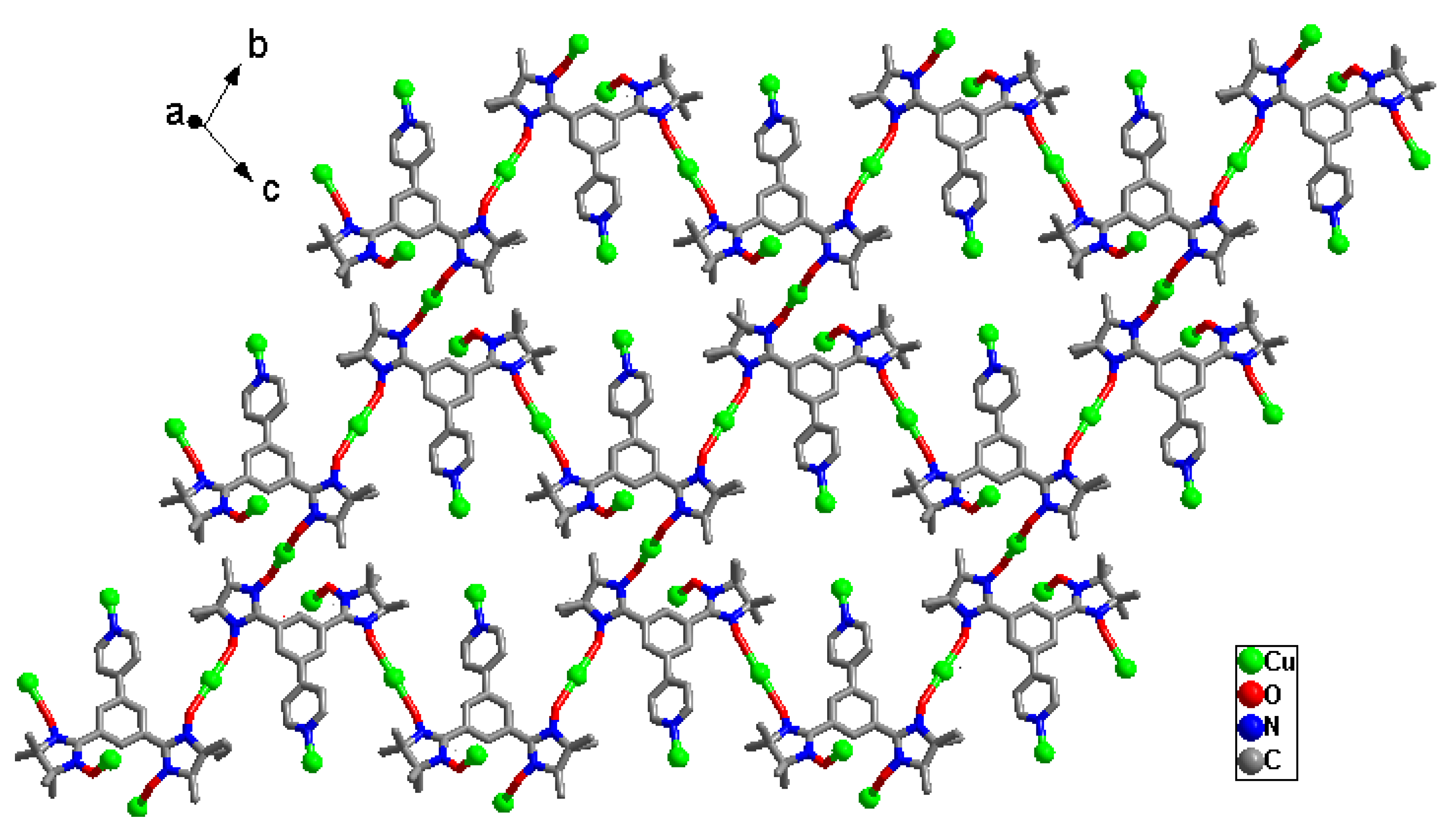
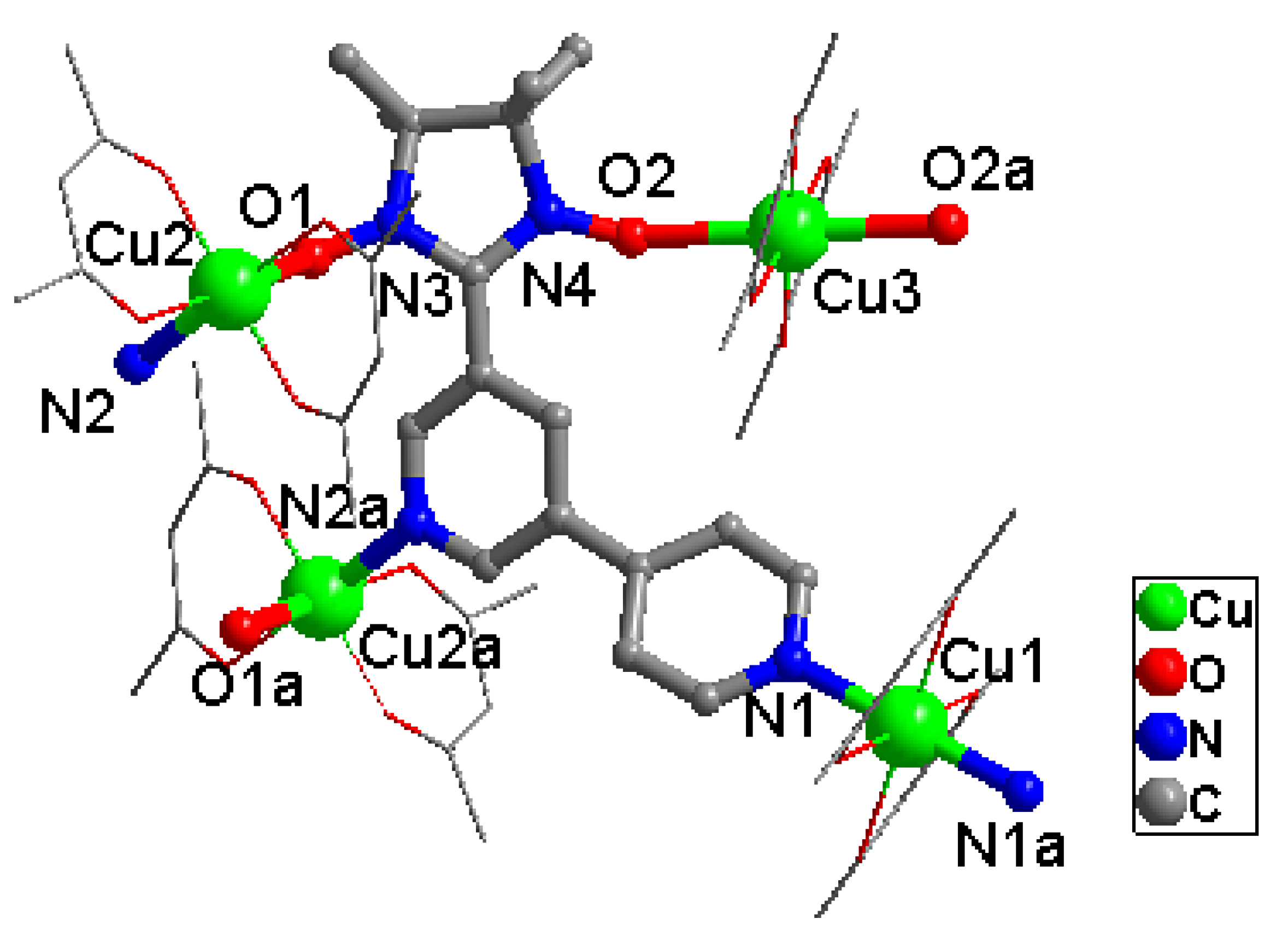
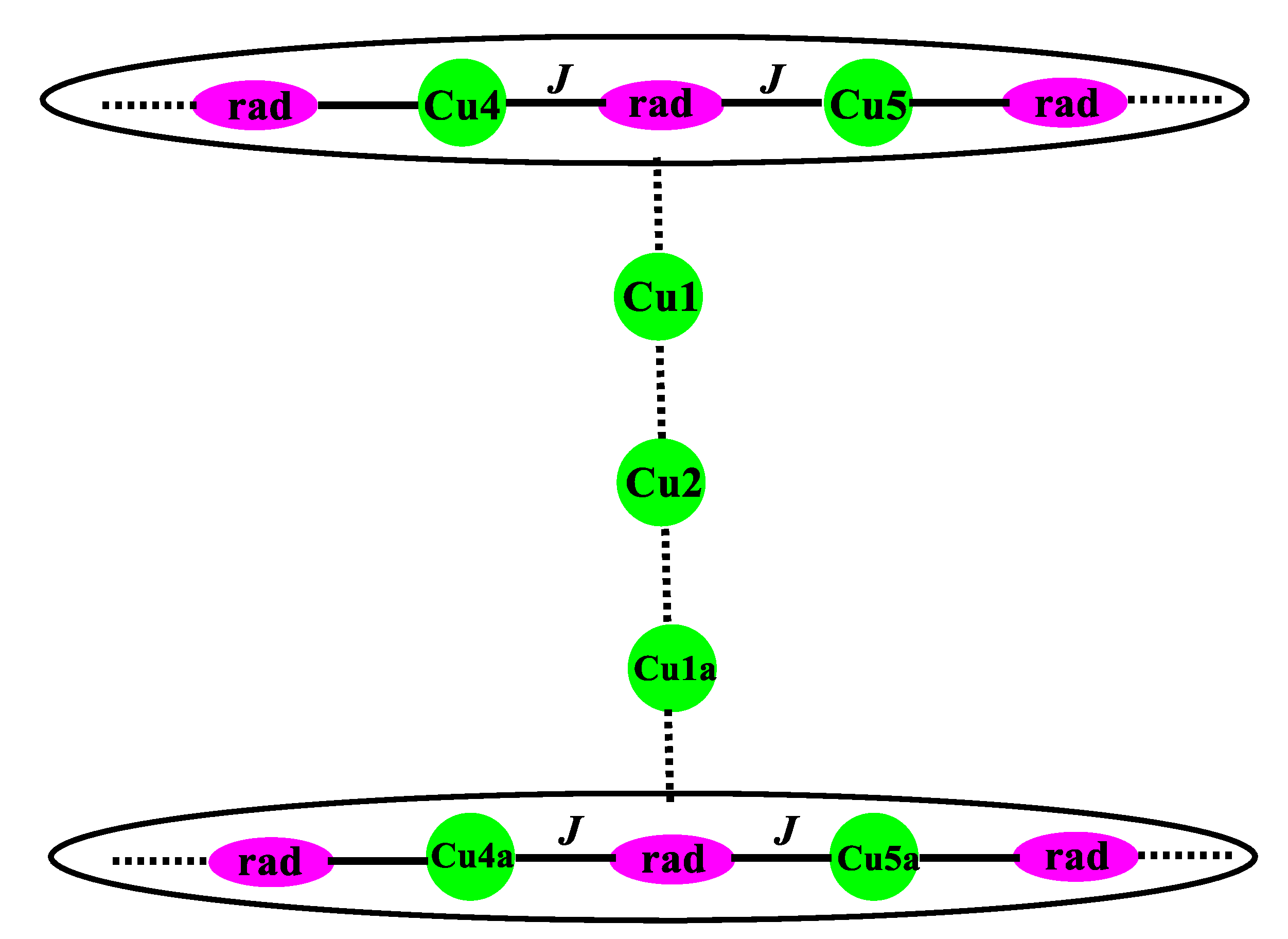
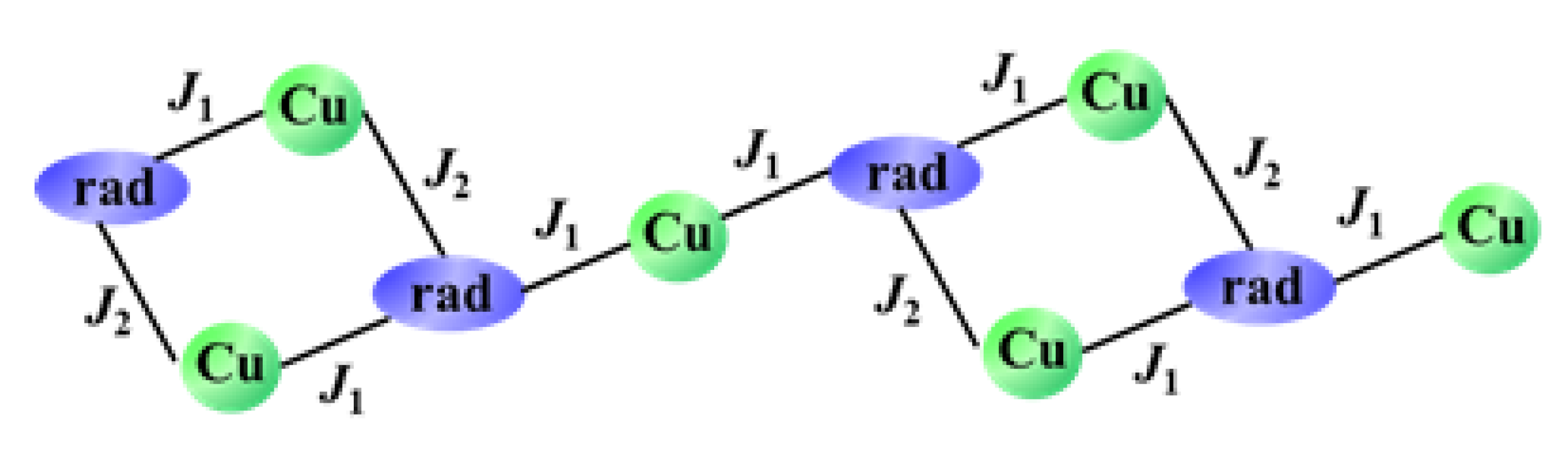
2.1. Structural Investigation and Description
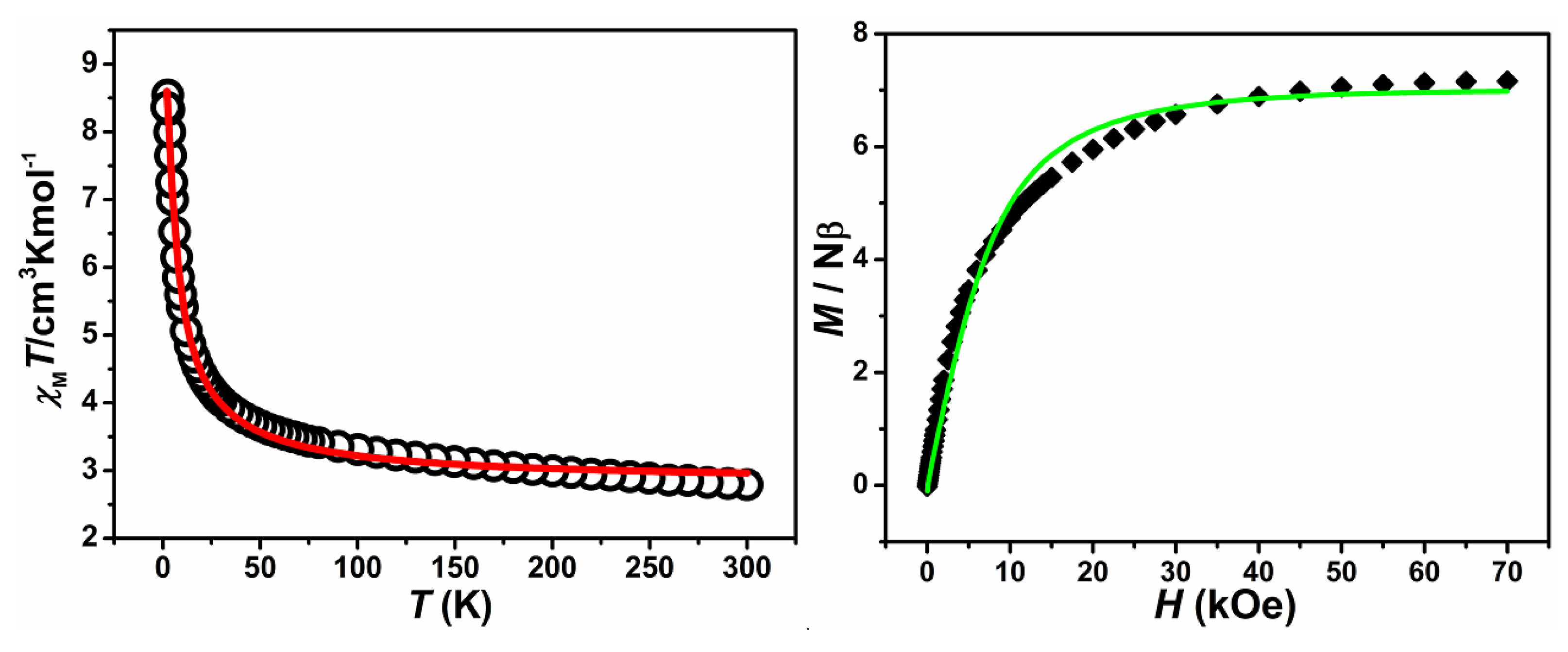
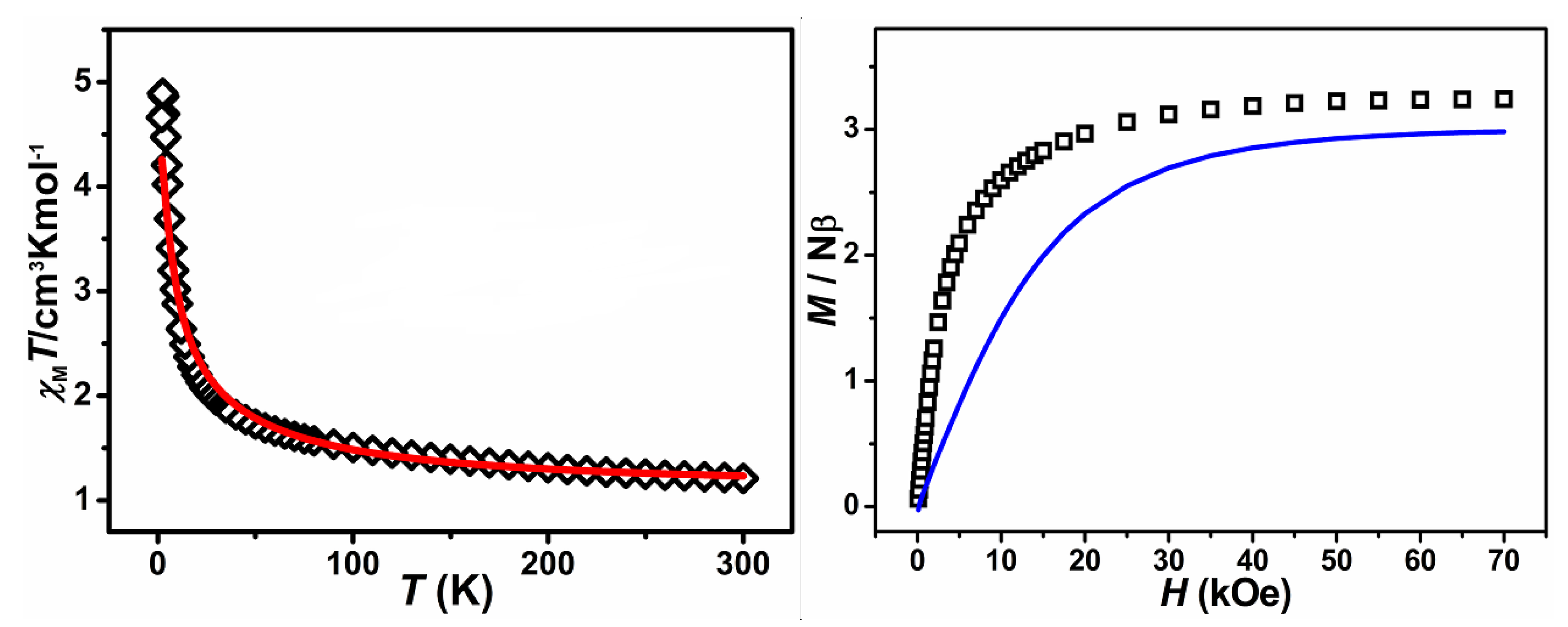
2.2. Magnetic Properties
3. Experimental Section
3.1. Raw Materials and Physical Investigation
3.2. Preparation of [Cu7(hfac)14(bisNITPhPy)2]n (1)
3.3. Preparation of [Cu2(hfac)4(NIT-3Py-5-4Py)]n (2)
3.4. Crystallographic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vostrikova, K.E. High-spin molecules based on metal complexes of organic free radicals. Coord. Chem. Rev. 2008, 252, 1409–1419. [Google Scholar] [CrossRef]
- Traina, C.; Norel, L.; Baumgarten, M. Organic radicals, a promising route towards original molecule-based magnetic materials. Coord. Chem. Rev. 2009, 253, 2342–2351. [Google Scholar] [CrossRef]
- Fedin, M.V.; Veber, S.L.; Bagryanskaya, E.G.; Ovcharenko, V.I. Electron paramagnetic resonance of switchable copper-nitroxide-based molecular magnets: An indispensable tool for intriguing systems. Coord. Chem. Rev. 2015, 289, 341–356. [Google Scholar] [CrossRef]
- Woodruff, D.N.; Winpeeny, R.E.P.; Layfield, R.A. Lanthanide Single-Molecule Magnets. Chem. Rev. 2013, 113, 5110–5148. [Google Scholar] [CrossRef] [PubMed]
- Demir, S.; Jeon, I.R.; Long, J.R.; Harris, T.D. Radical ligand-containing single-molecule magnets. Coord. Chem. Rev. 2015, 289, 149–176. [Google Scholar] [CrossRef]
- Luneau, D.; Rey, P. Magnetism of metal-nitroxide compounds involving bis-chelating imidazole and benzimidazole substituted nitronyl nitroxide free radicals. Coord. Chem. Rev. 2005, 249, 2591–2611. [Google Scholar] [CrossRef]
- Romero, F.M.; Ziessel, R.; Luneau, D. Structural control of ferromagnetic interactions in nickel(II) complexes based on a tetradentate biradical. Chem. Commun. 1998, 551–552. [Google Scholar] [CrossRef]
- Field, L.M.; Lahti, P.M.; Palacio, F.; Paduan-Filho, A. Manganese(II) and Copper(II) Hexafluoroacetylacetonate 1:1 Complexes with 5-(4-[N-tert-Butyl-N-aminoxyl]phenyl)pyrimidine: Regiochemical Parity Analysis for Exchange Behavior of Complexes between Radicals and Paramagnetic Cations. J. Am. Chem. Soc. 2003, 125, 10110–10118. [Google Scholar] [CrossRef] [PubMed]
- Kanegawa, S.; Karasawa, S.; Nakano, M.; Koga, N. Magnetic behavior of tetrakis [4-(N-tert-butyl-N-oxylamino)pyridine]bis(isocyanato-N)cobalt(II) in frozen solution. Chem. Commun. 2004, 1750–1751. [Google Scholar] [CrossRef] [PubMed]
- Bogani, L.; Sangregorio, C.; Sessoli, R.; Gatteschi, D. Molecular Engineering for Single-Chain-Magnet Behavior in a One-Dimensional Dysprosium-Nitronyl Nitroxide Compound. Angew. Chem. Int. Ed. 2005, 44, 5817–5821. [Google Scholar] [CrossRef] [PubMed]
- Bernot, K.; Bogani, L.; Caneschi, A.; Gatteschi, D.; Sessoli, R. A Family of Rare-Earth-Based Single Chain Magnets: Playing with Anisotropy. J. Am. Chem. Soc. 2006, 128, 7947–7956. [Google Scholar] [CrossRef]
- Demir, S.; Zadrozny, J.M.; Nippe, M.; Long, J.R. Exchange Coupling and Magnetic Blocking in Bipyrimidyl Radical-Bridged Dilanthanide Complexes. J. Am. Chem. Soc. 2012, 134, 18546–18549. [Google Scholar] [CrossRef] [PubMed]
- Poneti, G.; Bernot, K.; Bogani, L.; Caneschi, A.; Sessoli, R.; Wernsdorfer, W.; Gatteschi, D. A rational approach to the modulation of the dynamics of the magnetisation in a dysprosium-nitronyl-nitroxide radical complex. Chem. Commun. 2007, 1807–1809. [Google Scholar] [CrossRef]
- Fatila, E.M.; Rouzieres, M.; Jennings, M.C.; Lough, A.J.; Clérac, R.; Preuss, K.E. Fine-Tuning the Single-Molecule Magnet Properties of a [Dy(III)-Radical]2 Pair. J. Am. Chem. Soc. 2013, 135, 9596–9599. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, J.D.; Fang, M.; Evans, W.J.; Long, J.R. A N23– Radical-Bridged Terbium Complex Exhibiting Magnetic Hysteresis at 14 K. J. Am. Chem. Soc. 2011, 133, 14236–14239. [Google Scholar] [CrossRef]
- Demir, S.; Gonzalez, M.I.; Darago, L.E.; Evans, W.J.; Long, J.R. Giant coercivity and high magnetic blocking temperatures for N23− radical-bridged dilanthanide complexes upon ligand dissociation. Nat. Commun. 2017, 8, 2144. [Google Scholar] [CrossRef] [PubMed]
- Benelli, C.; Gatteschi, D. Magnetism of Lanthanides in Molecular Materials with Transition-Metal Ions and Organic Radicals. Chem. Rev. 2002, 102, 2369–2388. [Google Scholar] [CrossRef] [PubMed]
- Madalan, A.M.; Roesky, H.W.; Andruh, M.; Noltemeyer, M.; Stanica, N. The first coordination compound containing three different types of spin carriers: 2p-3d-4f (TCNQ˙−, Cu2+ and Gd3+). Chem. Commun. 2002, 1638–1639. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Hu, P.; Li, Y.G.; Li, L.C. Construction of Nitronyl Nitroxide-Based 3d-4f Clusters: Structure and Magnetism. Chem. Asian J. 2015, 10, 325–328. [Google Scholar] [CrossRef]
- Escobar, L.B.L.; Guedes, G.P.; Soriano, S.; Speziali, N.L.; Jordao, A.K.; Cunha, A.C.; Ferreira, V.F.; Maxim, C.; Novak, M.A.; Andruh, M.; et al. New Families of Hetero-tri-spin 2p-3d-4f Complexes: Synthesis, Crystal Structures, and Magnetic Properties. Inorg. Chem. 2014, 53, 7508–7517. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Zhang, X.; Zhang, Y.Z.; Li, M.X.; Zhao, H.H.; Andruh, M.; Dunbar, K.R. Single-Chain Magnetic Behavior in a Hetero-Tri-Spin Complex Mediated by Supramolecular Interactions with TCNQF•− Radicals. Angew. Chem. Int. Ed. 2014, 53, 11567–11570. [Google Scholar] [CrossRef]
- Novitchi, G.; Shov, S.; Lan, Y.H.; Wernsdorfer, W.; Train, C. Verdazyl Radical, a Building Block for a Six-Spin-Center 2p-3d-4f Single-Molecule Magnet. Inorg. Chem. 2016, 55, 12122–12125. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Li, L.C.; Sutter, J.P. 2p-3d-4f hetero-tri-spin molecule-based magnetic compounds. Inorg. Chem. Front. 2016, 3, 994–1003. [Google Scholar] [CrossRef]
- Gould, C.A.; Darago, L.E.; Gonzalez, M.I.; Demir, S.; Long, J.R. A Trinuclear Radical-Bridged Lanthanide Single-Molecule Magnet. Angew. Chem. Int. Ed. 2017, 56, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pointillart, F.; Guennic, B.L.; Golhen, S.; Cador, O.; Ouahab, L. Slow magnetic relaxation in radical cation tetrathiafulvalene-based lanthanide(III) dinuclear complexes. Chem. Commun. 2013, 49, 11632–11634. [Google Scholar] [CrossRef] [PubMed]
- Caneschi, A.; Gatteschi, D.; Lalioti, N.; Sangregorio, C.; Sessoli, R.; Venturi, G.; Vindigni, A.; Rettori, A.; Pini, M.G.; Novak, M.A. Cobalt(II)-Nitronyl Nitroxide Chains as Molecular Magnetic Nanowires. Angew. Chem. Int. Ed. 2001, 40, 1760–1763. [Google Scholar] [CrossRef]
- Liu, X.Q.; Feng, X.W.; Meihaus, K.R.; Meng, X.X.; Zhang, Y.; Li, L.; Liu, J.L.; Shi, W.; Zhang, Y.Q.; Cheng, P.; et al. Coercive Fields Above 6 T in Two Cobalt(II)-Radical Chain Compounds. Angew. Chem. Int. Ed. 2020, 132, 10697–10705. [Google Scholar] [CrossRef]
- Liu, R.N.; Ma, Y.; Yang, P.P.; Song, X.Y.; Xu, G.F.; Tang, J.K.; Li, L.C.; Liao, D.Z.; Yan, S.P. Dynamic magnetic behavior and magnetic ordering in one-dimensional Tb-nitronyl nitroxide radical chain. Dalton Trans. 2010, 39, 3321–3325. [Google Scholar] [CrossRef]
- Liu, R.N.; Li, L.C.; Wang, X.L.; Yang, P.P.; Wang, C.; Liao, D.Z.; Sutter, J.P. Smooth transition between SMM and SCM-type slow relaxing dynamics for a 1-D assemblage of {Dy(nitronyl nitroxide)2} units. Chem. Commun. 2010, 46, 2566–2568. [Google Scholar] [CrossRef]
- Bernot, K.; Pointillart, F.; Rosa, P.; Etienne, M.; Sessoli, R.; Gatteschi, D. Single molecule magnet behaviour in robust dysprosium-biradical complexes. Chem. Commun. 2010, 46, 6458–6460. [Google Scholar] [CrossRef]
- Li, H.D.; Sun, J.; Yang, M.; Sun, Z.; Tang, J.K.; Ma, Y.; Li, L.C. Functionalized Nitronyl Nitroxide Biradicals for the Construction of 3d-4f Heterometallic Compounds. Inorg. Chem. 2018, 57, 9757–9765. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.X.; Miao, H.; Shao, D.; Wei, H.Y.; Zhang, Y.Q.; Wang, X.Y. Single-molecule magnet behaviour in a dysprosium-triradical complex. Chem. Commun. 2018, 54, 9726–9729. [Google Scholar] [CrossRef] [PubMed]
- Souza, D.A.; Florencio, A.S.; Soriano, S.; Calvo, R.; Sartoris, R.P.; Carneiro, J.W.M.; Sangregorio, C.; Novak, M.A.; Vaz, M.G.F. New copper(II)-radical one dimensional chain: Synthesis, crystal structure, EPR, magnetic properties and DFT calculations. Dalton Trans. 2009, 6816–6824. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Li, L.C.; Liao, D.Z. Slow Magnetic Relaxation in Lanthanide Complexes with Chelating Nitronyl Nitroxide Radical. Inorg. Chem. 2010, 49, 4735–4737. [Google Scholar] [CrossRef]
- Lannes, A.; Intissar, M.; Suffren, Y.; Reber, C.; Luneau, D. Terbium(III) and Yttrium(III) Complexes with Pyridine-Substituted Nitronyl Nitroxide Radical and Different β-Diketonate Ligands. Crystal Structures and Magnetic and Luminescence Properties. Inorg. Chem. 2014, 53, 9548–9560. [Google Scholar] [CrossRef]
- Xi, L.; Li, H.D.; Sun, J.; Ma, Y.; Tang, J.K.; Li, L.C. Designing Multicoordinating Nitronyl Nitroxide Radical Toward Multinuclear Lanthanide Aggregates. Inorg. Chem. 2020, 59, 443–451. [Google Scholar] [CrossRef]
- Shi, J.Y.; Wu, M.Z.; Chen, P.Y.; Li, T.; Tian, L.; Zhang, Y.Q. Terbium Triangle Bridged by a Triazole Nitronyl Nitroxide Radical with Single-Molecule-Magnet Behavior. Inorg. Chem. 2019, 58, 14285–14288. [Google Scholar] [CrossRef]
- Tretyakov, E.; Fokin, S.; Romanenko, G.; Ikorskii, V.; Vasilevsky, S.; Ovcharenko, V. 2D and 3D Cu(hfac)2 Complexes with Nitronyl Nitroxide Biradicals. Inorg. Chem. 2006, 45, 3671–3678. [Google Scholar] [CrossRef]
- Zhu, M.; Li, Y.G.; Ma, Y.; Li, L.C.; Liao, D.Z. Unprecedented Nitronyl Nitroxide Bridged 3d-4f Complexes: Structure and Magnetic Properties. Inorg. Chem. 2013, 52, 12326–12328. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, J.; Yang, M.; Ma, Y.; Li, L.C. Slow magnetic relaxation in two-dimensional 3d-4f complexes based on phenyl pyrimidyl substituted nitronyl nitroxide radicals. Dalton Trans. 2015, 44, 9815–9822. [Google Scholar] [CrossRef]
- Sun, G.; Guo, J.; Yang, M.; Xi, L.; Li, L.; Sutter, J.P. Two-dimensional Co-Ln networks bridged by phenyl pyrimidyl substituted nitronyl nitroxides: Structural and magnetic properties. Dalton Trans. 2018, 47, 4672–4677. [Google Scholar] [CrossRef] [PubMed]
- Lanfranc de Panthou, F.; Belorizky, E.; Calemzuk, R.; Luneau, D.; Marcenat, C.; Ressouche, E.; Turek, P.; Rey, P. A New Type of Thermally Induced Spin Transition Associated with an Equatorial.dblarw. Axial Conversion in a Copper(II)-Nitroxide Cluster. J. Am. Chem. Soc. 1995, 117, 11247–11253. [Google Scholar] [CrossRef]
- Wang, H.M.; Liu, Z.L.; Liu, C.M.; Zhang, D.Q.; Lu, Z.L.; Geng, H.; Shuai, Z.G.; Zhu, D.B. Coordination Complexes of 2-(4-Quinolyl) nitronyl Nitroxide with M(hfac)2 [M = Mn(II), Co(II), and Cu(II)]: Syntheses, Crystal Structures, and Magnetic Characterization. Inorg. Chem. 2004, 43, 4091–4098. [Google Scholar] [CrossRef] [PubMed]
- Casanova, D.; Llunell, M.; Alemany, P.; Alvarez, S. The Rich Stereochemistry of Eight-Vertex Polyhedra: A Continuous Shape Measures Study. Chem. Eur. J. 2005, 11, 1479–1494. [Google Scholar] [CrossRef] [PubMed]
- Caneschi, A.; Gatteschi, D.; Sessoli, R.; Hoffmann, S.K. Crystal and molecular structure, magnetic properties and EPR spectra of a trinuclear copper(II) complex with bridging nitronyl nitroxides. Inorg. Chem. 1988, 27, 2390–2392. [Google Scholar] [CrossRef]
- Caneschi, A.; Gatteschi, D.; Sessoli, R.; Rey, P. Toward molecular magnets: The metal-radical approach. Acc. Chem. Res. 1989, 22, 392–398. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.L.; Ma, Y.; Wang, Q.L.; Li, L.C.; Liao, D.Z. A Four-spin Cobalt-radical Complex: Structure and Magnetic Properties. Chem. Lett. 2012, 41, 1523–1525. [Google Scholar] [CrossRef]
- Baker, G.; Rushbrooke, G.; Gilbert, H. High-temperature series expansions for the spin-½ Heisenberg model by the method of irreducible representations of the symmetric group. Phys. Rev. 1964, 135, A1272. [Google Scholar] [CrossRef]
- Gatteschi, D.; Laugier, J.; Rey, P.; Zanchini, C. Crystal and molecular structure and magnetic properties of the adducts of copper(II) hexafluoroacetylacetonate with the nitroxide ligand 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide. Inorg. Chem. 1987, 26, 938–943. [Google Scholar] [CrossRef]
- Cabello, C.I.; Caneschi, A.; Carlin, R.L.; Gatteschi, D.; Rey, P.; Sessoli, R. Structure and magnetic properties of ferromagnetic alternating spin chains. Inorg. Chem. 1990, 29, 2582–2587. [Google Scholar] [CrossRef]
- Caneschi, A.; Gatteschi, D.; Laugier, J.; Rey, P. Ferromagnetic alternating spin chains. J. Am. Chem. Soc. 1987, 109, 2191–2192. [Google Scholar] [CrossRef]
- Wang, X.F.; Hu, P.; Li, L.C.; Sutter, J.P. [(Cu-Radical)2-Ln]: Structure and Magnetic Properties of a Hetero-trispin Chain of Rings (Ln = YIII, GdIII, TbIII, DyIII). Inorg. Chem. 2015, 54, 9664–9669. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Jing, P.; Jin, C.Y.; Xie, J.F.; Li, L.C. Modulating the magnetization dynamics in Ln–Cu-Rad hetero-tri-spin complexes through cis/trans coordination of nitronyl nitroxide radicals around the metal center. Dalton Trans. 2021, 50, 3280–3288. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXS-2014, Program. for Structure Solution; Universität of Göttingen: Gottingen, Germany, 2014. [Google Scholar]
- Sheldrick, G.M. SHELXL-2014, Program. for the Refinement of Crystal Structures; Universität of Göttingen: Göttingen, Germany, 2014. [Google Scholar]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. 2009, D65, 148–155. [Google Scholar] [CrossRef] [PubMed]


| Complex | 1 | 2 |
|---|---|---|
| Formula | C120H76Cu7F84N10O36 | C53H38F36Tb2N6O17 |
| M (g·mol−1) | 4274.76 | 1266.67 |
| T (K) | 113(2) | 113(2) |
| Crystal system | Triclinic | Triclinic |
| Space group | Pī | Pī |
| a (Å) | 13.4647(13) | 11.172(2) |
| b (Å) | 16.7473(18) | 15.205(3) |
| c (Å) | 18.793(2) | 15.890(3) |
| α (°) | 109.221(2) | 78.94(3) |
| β (°) | 96.022(2) | 85.98(3) |
| γ (°) | 98.901(2) | 70.21(3) |
| Z | 1 | 2 |
| Dcalcd (g·cm–3) | 1.821 | 1.688 |
| μ (mm–1) | 1.113 | 0.998 |
| θ (°) | 3.00–25.00 | 1.93–25.00 |
| F(000) | 2113 | 1254 |
| Reflns collected | 36,087 | 24,124 |
| Unique reflns/Rint | 13,715/0.0429 | 8784/0.0598 |
| GOF (F2) | 0.983 | 1.007 |
| R1/wR2 (I > 2σ(I)) | 0.0497, 0.1412 | 0.0927, 0.2159 |
| R1/wR2 (all data) | 0.0663, 0.1511 | 0.1149, 0.2363 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Lu, J.; Xie, J.; Jing, P.; Li, L. Two-Dimensional Nitronyl Nitroxide–Cu Networks Based on Multi-Dentate Nitronyl Nitroxides: Structures and Magnetic Properties. Magnetochemistry 2021, 7, 73. https://doi.org/10.3390/magnetochemistry7050073
Li H, Lu J, Xie J, Jing P, Li L. Two-Dimensional Nitronyl Nitroxide–Cu Networks Based on Multi-Dentate Nitronyl Nitroxides: Structures and Magnetic Properties. Magnetochemistry. 2021; 7(5):73. https://doi.org/10.3390/magnetochemistry7050073
Chicago/Turabian StyleLi, Hongdao, Jiao Lu, Jing Xie, Pei Jing, and Licun Li. 2021. "Two-Dimensional Nitronyl Nitroxide–Cu Networks Based on Multi-Dentate Nitronyl Nitroxides: Structures and Magnetic Properties" Magnetochemistry 7, no. 5: 73. https://doi.org/10.3390/magnetochemistry7050073
APA StyleLi, H., Lu, J., Xie, J., Jing, P., & Li, L. (2021). Two-Dimensional Nitronyl Nitroxide–Cu Networks Based on Multi-Dentate Nitronyl Nitroxides: Structures and Magnetic Properties. Magnetochemistry, 7(5), 73. https://doi.org/10.3390/magnetochemistry7050073