New Spin-Crossover Compounds Containing the [Ni(mnt)] Anion (mnt = Maleonitriledithiolate)
Abstract
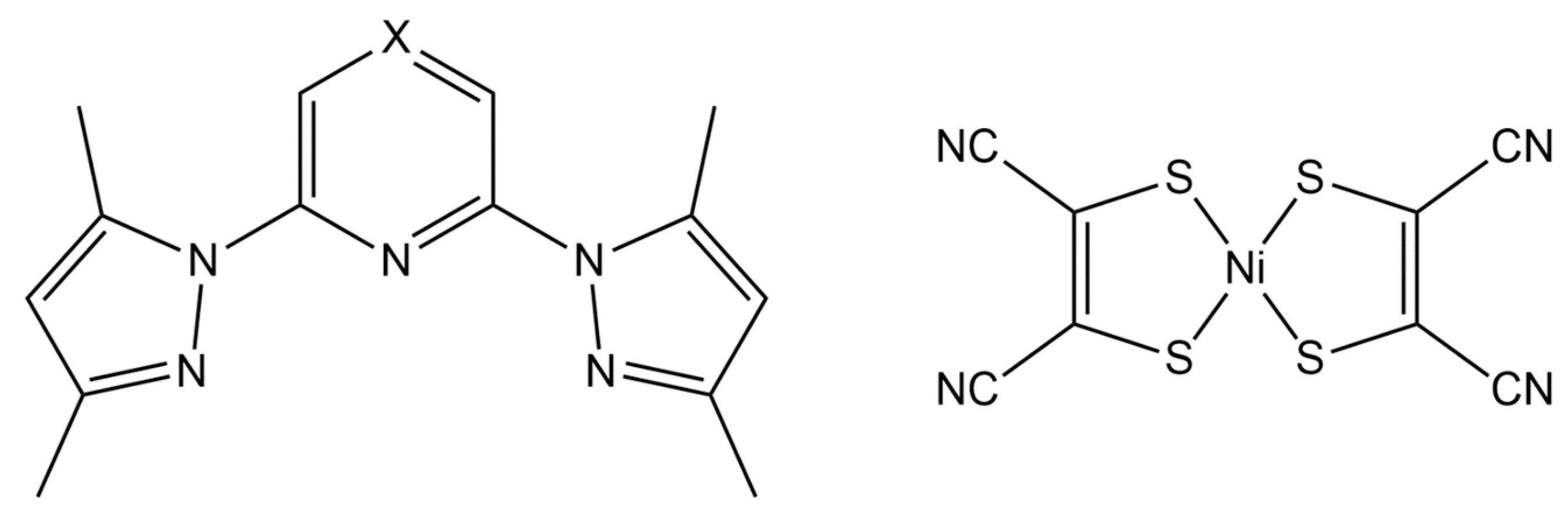
1. Introduction
2. Results and Discussion
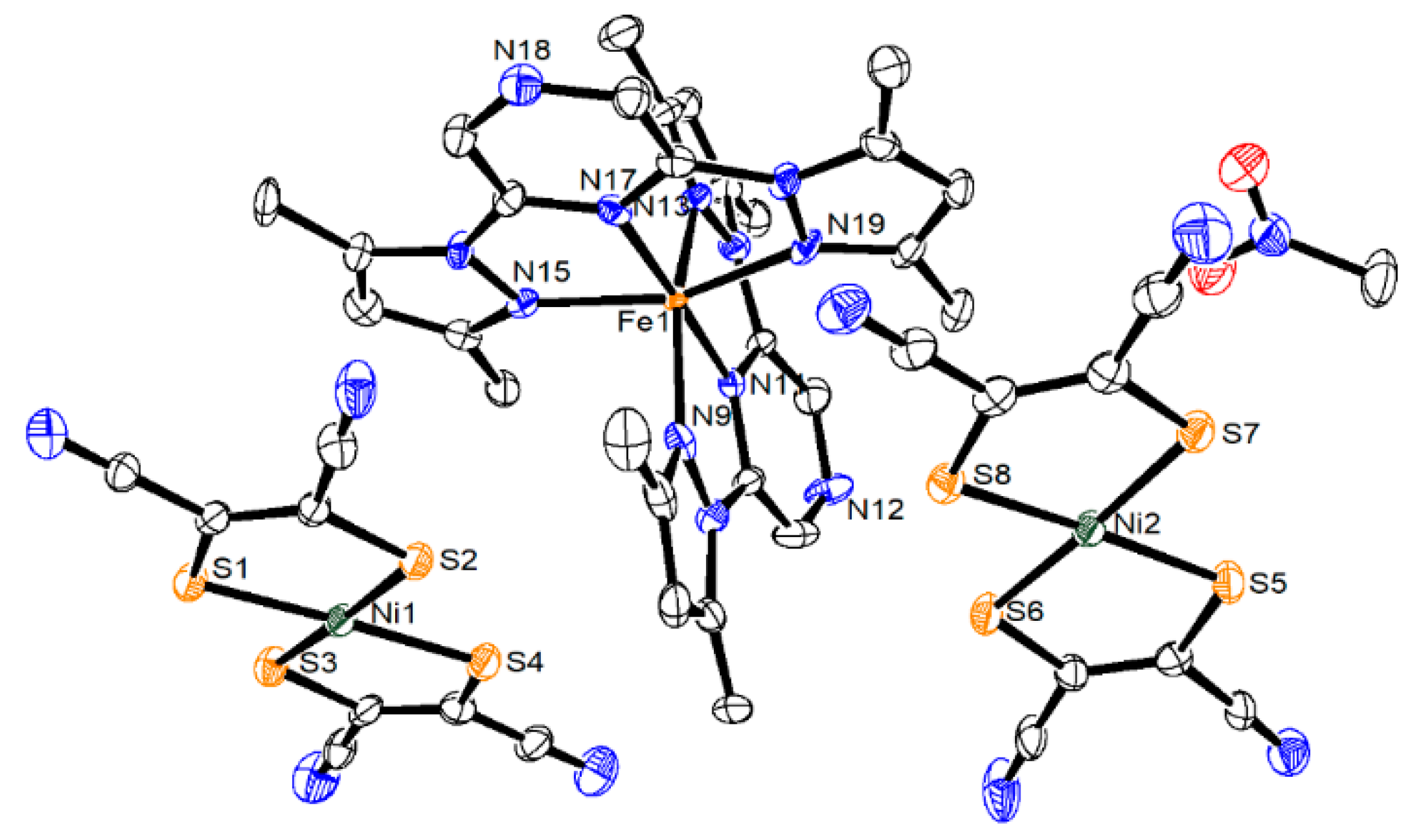
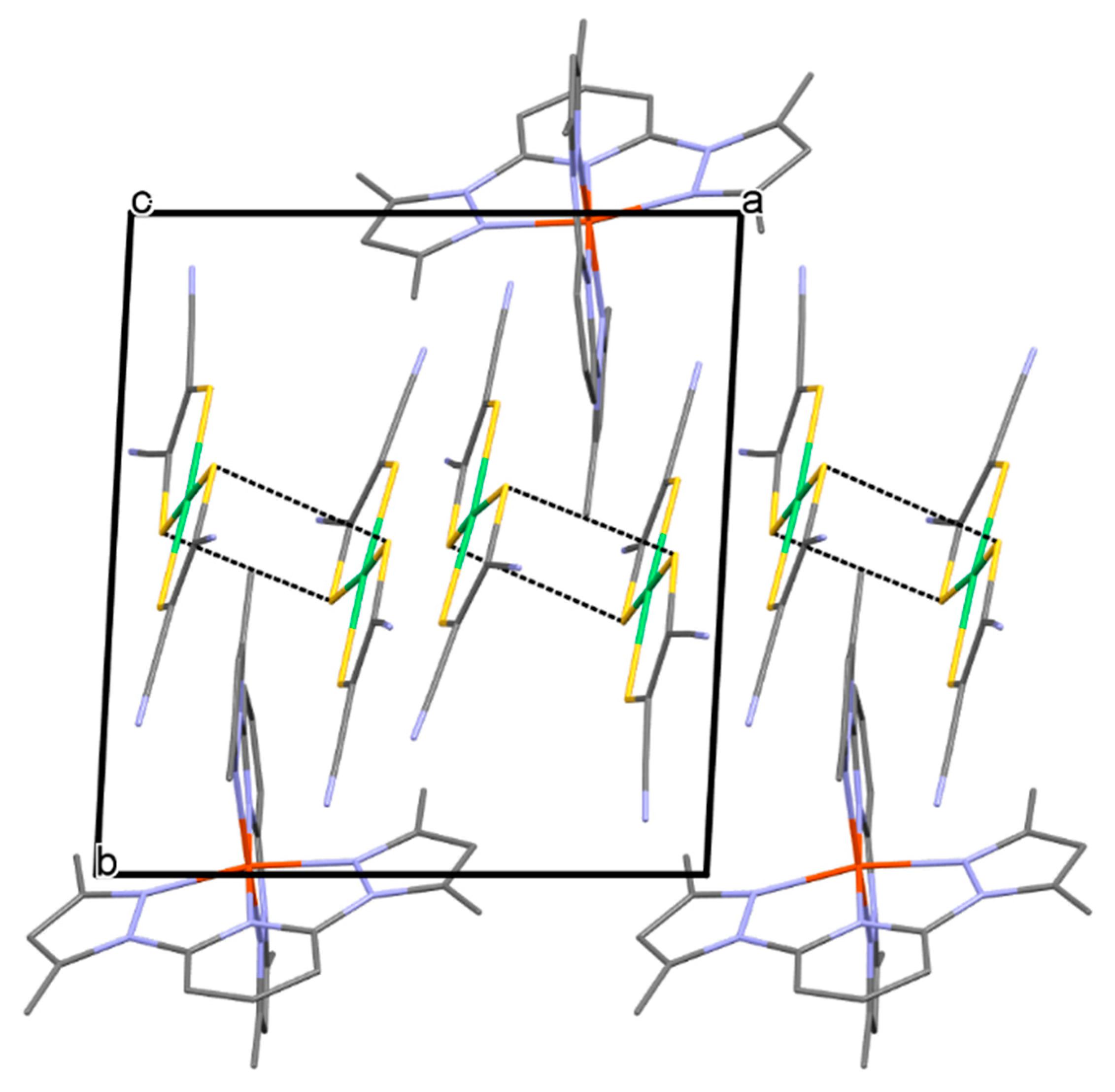
2.1. Description of Structures of Compounds 1 and 2
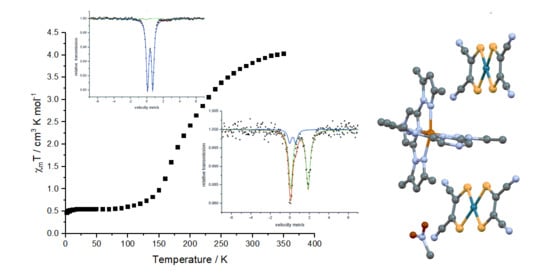
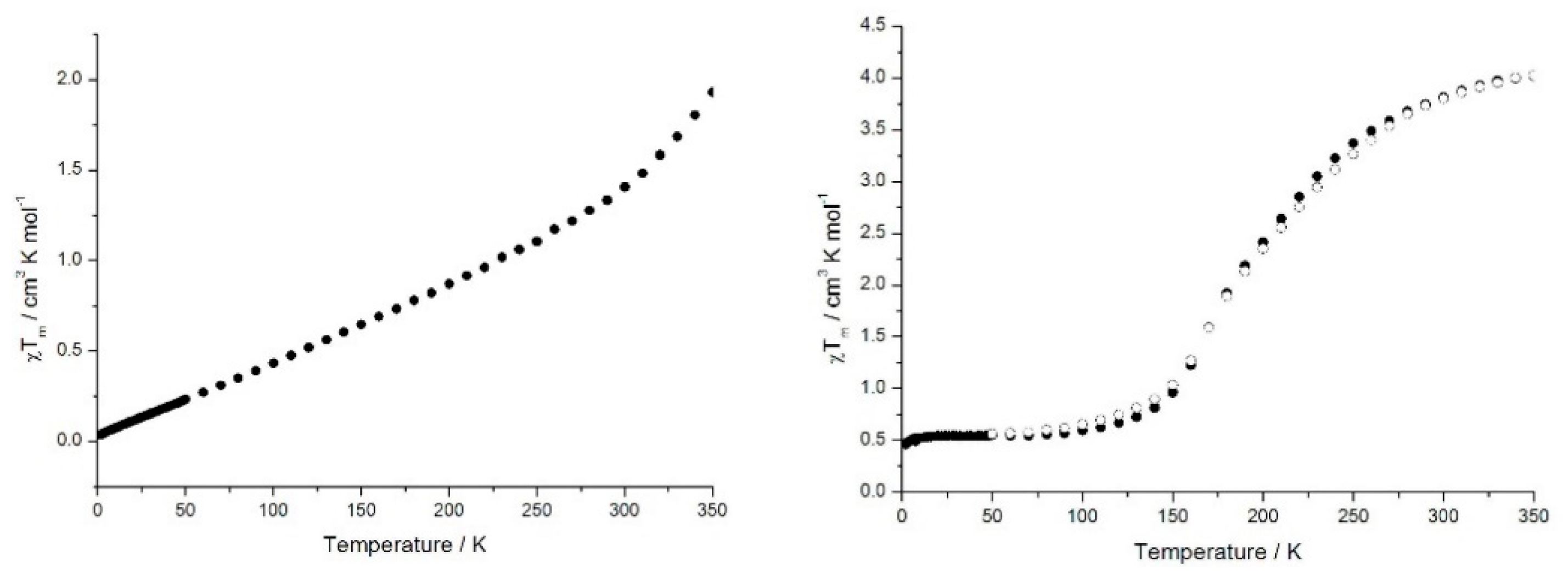
2.2. Magnetic Properties
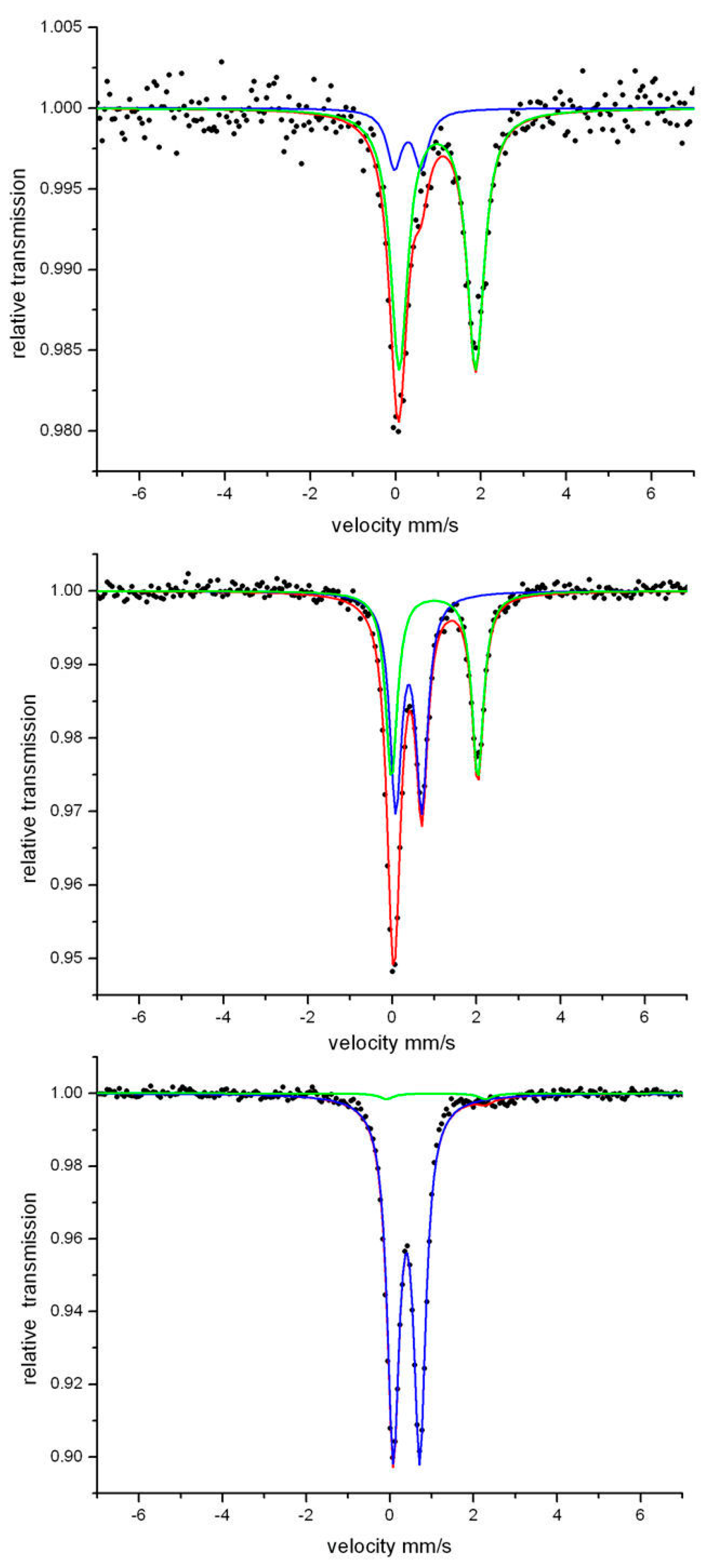
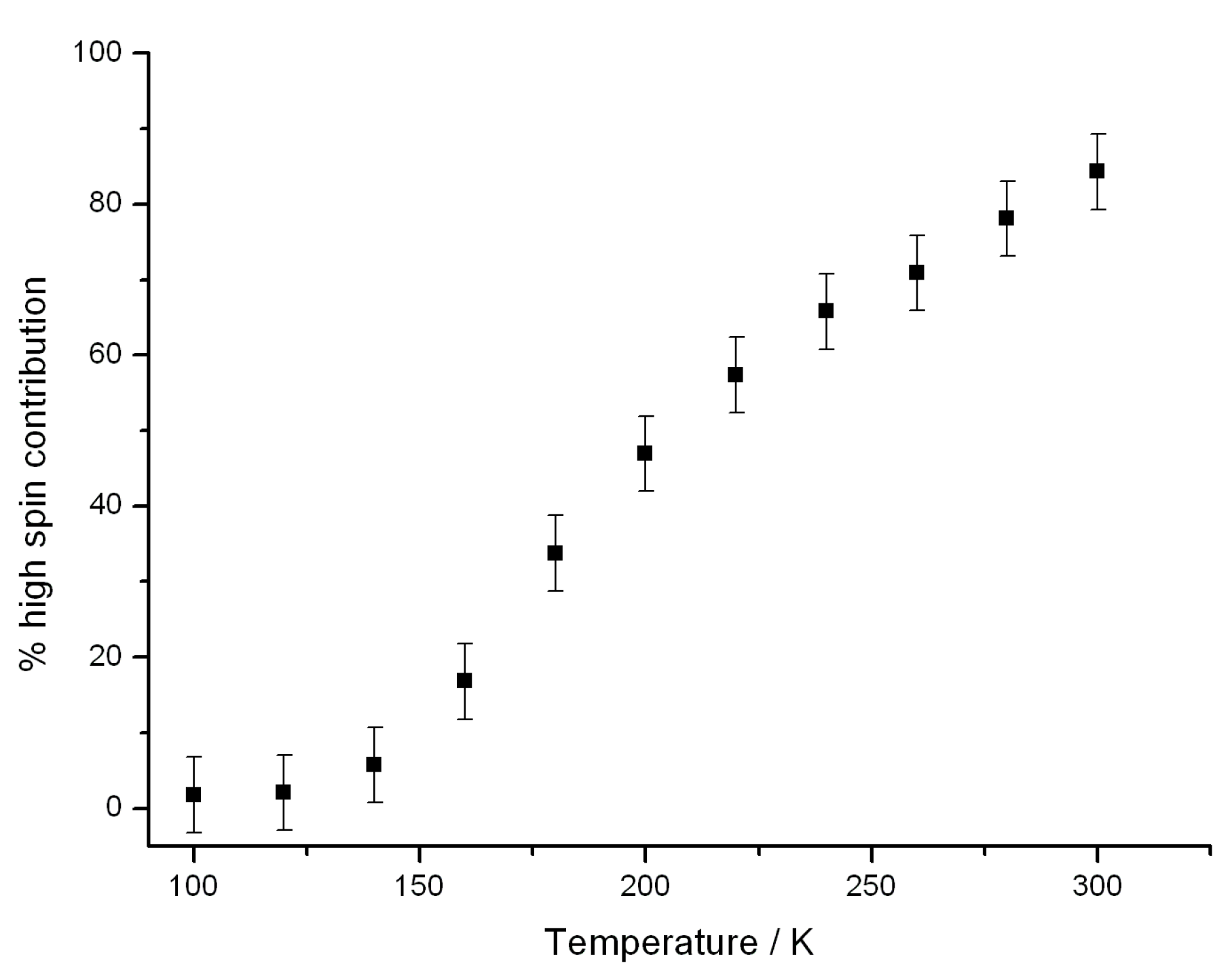
2.3. Mössbauer Spectroscopy
3. Materials and Methods
3.1. Synthesis of Ligands and Fe(II) Complexes
3.2. Synthesis of Compounds 1 and 2
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cambi, L.; Szegö, L. The magnetic susceptibility of complex compounds. Ber. Deutsch. Chem. Ges. 1931, 64, 2591–2598. [Google Scholar] [CrossRef]
- Cambi, L.; Malatesta, L. Magnetism and polymorphy of internal complex salts—Iron salts ofdithio-carbonicamide acids. Ber. Deutsch. Chem. Ges. 1937, 70, 2067–2078. [Google Scholar] [CrossRef]
- Kahn, O.; Martinez, C.J. Spin transition polymers: From materials towards memory devices. Science 1998, 179, 44–48. [Google Scholar] [CrossRef]
- Hayami, S.; Holmes, S.M.; Halcrow, M.A. Spin-state switching in molecular materials. J. Mater. Chem. C. 2015, 3, 7775–7778. [Google Scholar] [CrossRef]
- Gütlich, P.; Gaspar, A.B.; Garcia, Y. Spin-state switching in iron coordination compounds. Beilstein J. Org. Chem. 2013, 9, 342–381. [Google Scholar] [CrossRef]
- Bonhommeau, S.; Guillon, T.; Daku, L.M.L.; Demont, P.; Costa, J.S.; Létard, J.F.; Molnár, G.; Bousseksou, A. Photoswitching of the dielectric constant of the spin-crossover complex [Fe(L)(CN)2].H2O. Angew. Chem. Int. Ed. 2006, 45, 1625–1629. [Google Scholar] [CrossRef]
- Guilon, T.; Bonhommeau, S.; Costa, J.S.; Zwick, A.; Létard, J.F.; Demont, P.; Molnár, G.; Bousseksou, A. On the dielectric properties of the spin-crossover complex [Fe(bpp)2][BF4]2. Phys. Status Solidi A Appl. Mat. Sci. 2006, 203, 2974–2980. [Google Scholar] [CrossRef]
- Schäfer, B.; Bauer, T.; Faus, I.; Wolny, J.A.; Dahms, F.; Fuhr, O.; Lebedkin, S.; Wille, H.C.; Schlage, K.; Chevalier, K.; et al. A luminescent Pt2Fe spin-crossover complex. Dalton Trans. 2017, 46, 2289–2302. [Google Scholar] [CrossRef]
- Wang, C.F.; Li, R.F.; Chen, X.Y.; Wei, R.J.; Zheng, L.S.; Tao, J. Synergistic spin-crossover and fluorescence in one-dimensional hybrid complexes. Angew. Chem. Int. Ed. 2015, 54, 1574–1577. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhu, J.P.; Guo, Y.; He, W.J.; Guo, Z.J. Synergetic effect between spin-crossover and luminescence in the [Fe(bpp)2][BF4]2 (bpp=2,6-bis(pyrazol-1-yl)pyridine complex. J. Mater. Chem. C 2017, 5, 5214–5222. [Google Scholar] [CrossRef]
- Nihei, M.; Takahashi, H.; Nishikawa, H.; Oshio, H. Spin-crossover behavior and electrical conduction property in iroj(II) complexes with tetrathiafulvalene derivatives. Dalton Trans. 2011, 40, 2154–2156. [Google Scholar] [CrossRef]
- Halcrow, M.A. Structure-function relationships in molecular spin-crossover complexes. Chem. Soc. Rev. 2011, 40, 4119–4142. [Google Scholar] [CrossRef] [PubMed]
- Boillot, M.L.; Weber, B. Mononuclear ferrous and ferric complexes. Compte. Rendus. Chim. 2018, 1196–1208. [Google Scholar] [CrossRef]
- Kumar, K.S.; Ruben, M. Emerging trends in spin-crossover (SCO) based functional materials. Coord. Chem. Rev. 2017, 346, 176–205. [Google Scholar] [CrossRef]
- Halcrow, M.A. The synthesis and coordination chemistry of 2, 6-bis(pyrazolyl)pyridines and related ligands—Versatile terpyridine analogues. Coord. Chem. Rev. 2005, 249, 2880–2908. [Google Scholar] [CrossRef]
- Halcrow, M.A. Iron(II) complexes of 2,6-di(pyrazol-1-yl)pyridines—A versatile system for spin-crossover research. Coord. Chem. Rev. 2009, 253, 2493–2514. [Google Scholar] [CrossRef]
- Cook, L.J.K.; Mohammed, R.; Sherborne, G.; Roberts, T.D.; Alvarez, S.; Halcrow, M.A. Spin state behavior of iron(II)/dipyrazolylpyridine complexes. New insights from crystallographic and solution measurements. Coord. Chem. Rev. 2015, 289, 2–12. [Google Scholar] [CrossRef]
- Coronado, E.; Galán-Mascarós, J.R.; Giménez-López, M.C.; Almeida, M.; Waerenborgh, J.C. Spin-crossover Fe(II) complexes as templates for bimetallic oxalate-based 3D magnets. Polyhedron 2007, 26, 1838–1844. [Google Scholar] [CrossRef]
- Cassoux, P.; Valade, L.; Kobayashi, H.; Kobayashi, A.; Clark, R.A.; Underhill, A.E. Molecular metals and superconductors derived from metal complexes of 1,3-dithiol-2-thione-4,5-dithiolate (dmit). Coord. Chem. Rev. 1991, 110, 115–160. [Google Scholar] [CrossRef]
- Robertson, N.; Cronin, L. Metal bis-1,2-dithiolene complexes in conducting or magnetic crystalline assemblies. Coord. Chem. Rev. 2002, 227, 93–127. [Google Scholar] [CrossRef]
- Cassoux, P. Molecular (super)conductors derived from bis-dithiolate metal complexes. Coord. Chem. Rev. 1999, 185, 213–232. [Google Scholar] [CrossRef]
- Dorbes, S.; Valade, L.; Real, J.A.; Faulmann, C. [Fe(sal2-trien)][Ni(dmit)2]; towards switchable spin crossover molecular conductors. Chem. Comm. 2005, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Szilágyi, P.A.; Dorbes, S.; Molnár, G.; Real, J.A.; Homonnay, Z.; Faulmann, C.; Bousseksou, A. Temperature and pressure effects on the spin state of ferric ions in the [Fe(sal2-trien)][Ni(mnt)2] spin crossover complex. J. Phys. Chem. Solids 2008, 69, 2681–2686. [Google Scholar] [CrossRef]
- Faulmann, C.; Szilágyi, P.A.; Jacob, K.; Chahine, J.; Valade, L. Polymorphism and its effects on the magnetic behaviour of the [Fe(sal2-trien)][Ni(dmit)2] spin-crossover complex. New J. Chem. 2009, 33, 1268–1276. [Google Scholar] [CrossRef]
- Faulmann, C.; Dorbes, S.; Real, J.A.; Valade, L. Electrical conductivity and spin crossover: Towards the first achievement with a metal bis-dithiolene complex. J. Low Temp. Phys. 2006, 142, 261–266. [Google Scholar] [CrossRef]
- Faulmann, C.; Dorbes, S.; de Bonneval, W.G.; Molnár, G.; Bousseksou, A.; Gomez-Garcia, C.J.; Coronado, E.; Valade, L. Towards molecular conductors with a spin-crossover phenomenon: Crystal structures, magnetic properties and Mössbauer spectra of [Fe(salten)Mepepy][M(dmit)2]. Eur. J. Inorg. Chem. 2005, 16, 3261–3270. [Google Scholar] [CrossRef]
- Takahashi, K.; Cui, H.B.; Okano, Y.; Kaboyashi, H.; Einaga, Y.; Sato, O. Electrical conductivity modulation coupled to a high-spin-low-spin conversion in the molecular system [Fe-III(qsal)2][Ni(dmit)2]3.CH3CN.H2O. Inorg. Chem. 2006, 45, 5739–5741. [Google Scholar] [CrossRef] [PubMed]
- Nihei, M.; Tahira, H.; Takahashi, N.; Otake, Y.; Yamamura, Y.; Saito, K.; Oshio, H. Multiple bistability and tristability with dual spin-state conversions in [Fe(dpp)2][Ni(mnt)2].MeNO2. J. Am. Chem. Soc. 2010, 132, 3553–3560. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Ketkaew, R.; Tabtirungrotechai, Y.; Harding, P.; Chastanet, G.; Guionneau, P.; Machivie, M.; Harding, D.J. OctaDist: A tool for calculating distortion parameters in spin crossover and coordination complexes. Dalton Trans. 2021, 50, 1086–1096. [Google Scholar] [CrossRef]
- Ribera, A.; Rovira, C.; Veciana, J.; Tarrés, J.; Canadell, E.; Rousseau, R.; Molins, E.; Mas, M.; Schoeffel, J.P.; Pouget, J.P.; et al. The [(DT-TTF)2M(mnt)2] family of radical ion salts: From a spin ladder to delocalized conduction electrons that interact with localized magnetic moments. Chem. Eur. J. 1999, 5, 2025. [Google Scholar] [CrossRef]
- Elhaїk, J.; Money, V.A.; Barrett, S.A.; Kilner, C.A.; Evans, I.R.; Halcrow, M.A. The spin states and spin-crossover behaviour of iron(II) complexes of 2,6-dipyrazol-1-ylpyrazine derivatives. Dalton Trans. 2003, 10, 2053–2060. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic corrections and Pascal’s constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta. Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Betteridge, P.W.; Carruthers, J.R.; Cooper, R.I.; Prout, K.; Watkin, D.J. CRYSTALS version 12: Software for guided crystal structure analysis. J. Appl. Cryst. 2003, 36, 1487. [Google Scholar] [CrossRef]
- Jameson, D.L.; Goldsby, K.A. 2,6-bis(N-pyrazolyl)pyridines—The convenient synthesis of a family of planar tridentate N3 ligands that are terpyridine analogs. J. Org. Chem. 1990, 55, 4992–4994. [Google Scholar] [CrossRef]


| Parameter | Compound 1 | Compound 2 | ||
|---|---|---|---|---|
| Empirical formula | C46H34N18S8FeNi2 | C45H35N21S8O2FeNi2 | ||
| Molecular Mass | 1268.66 | 1331.64 | ||
| T/K | 100(2) | 293(2) | 100(2) | 290(2) |
| CCDC number | 2080108 | 2080109 | 2080110 | 2080111 |
| Crystal color & shape | Brown Plate | Black Prism | ||
| Crystal system | Triclinic | Triclinic | ||
| Space Group | P-1 (2) | P-1 (2) | ||
| a/Å | 11.1318(3) | 11.2229(5) | 13.3643(6) | 13.4983(8) |
| b/Å | 12.1322(4) | 12.3046(6) | 14.7900(6) | 15.1017(10) |
| c/Å | 19.4594(6) | 19.6208(7) | 14.9875(8) | 15.1633(9) |
| α/° | 94.543(2) | 93.625(3) | 79.731(6) | 79.756(6) |
| β/° | 90.216(2) | 89.349(3) | 81.844(6) | 81.882(6) |
| γ/° | 92.986(2) | 90.932(4) | 69.962(5) | 68.796(3) |
| Volume/Å3 | 2616.16(14) | 2703.6(2) | 2728.01(15) | 2825.8(3) |
| Wavelength/Å | 0.71075 | 0.71075 | ||
| Radiation Type | Mo Kα | Mo Kα | ||
| Z | 2 | 2 | 2 | 2 |
| μ/mm−1 | 1.360 | 1.316 | 1.312 | 1.267 |
| Measured reflections | 33346 | 35619 | 26321 | 27746 |
| Independent reflections | 11866 | 12326 | 12418 | 12853 |
| Reflections I ≥ 2 σ(I) | 7764 | 6982 | 7709 | 6893 |
| wR1 (all data) | 0.0956 | 0.1109 | 0.0828 | 0.1435 |
| R1, I ≥ 2 σ(I) | 0.0496 | 0.0528 | 0.0456 | 0.0772 |
| Temperature/K | Spin State | δ | ΔEQ | Γ |
|---|---|---|---|---|
| 300 | HS | 0.98 | 1.78 | 0.49 |
| LS | 0.29 | 0.64 | 0.42 | |
| 200 | HS | 1.01 | 2.05 | 0.34 |
| LS | 0.39 | 0.63 | 0.34 | |
| 100 | HS | 1.10 | 2.38 | 0.40 |
| LS | 0.40 | 0.64 | 0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turner, S.S.; Daniell, J.; Akutsu, H.; Horton, P.N.; Coles, S.J.; Schünemann, V. New Spin-Crossover Compounds Containing the [Ni(mnt)] Anion (mnt = Maleonitriledithiolate). Magnetochemistry 2021, 7, 72. https://doi.org/10.3390/magnetochemistry7050072
Turner SS, Daniell J, Akutsu H, Horton PN, Coles SJ, Schünemann V. New Spin-Crossover Compounds Containing the [Ni(mnt)] Anion (mnt = Maleonitriledithiolate). Magnetochemistry. 2021; 7(5):72. https://doi.org/10.3390/magnetochemistry7050072
Chicago/Turabian StyleTurner, Scott S., Joanna Daniell, Hiroki Akutsu, Peter N. Horton, Simon J. Coles, and Volker Schünemann. 2021. "New Spin-Crossover Compounds Containing the [Ni(mnt)] Anion (mnt = Maleonitriledithiolate)" Magnetochemistry 7, no. 5: 72. https://doi.org/10.3390/magnetochemistry7050072
APA StyleTurner, S. S., Daniell, J., Akutsu, H., Horton, P. N., Coles, S. J., & Schünemann, V. (2021). New Spin-Crossover Compounds Containing the [Ni(mnt)] Anion (mnt = Maleonitriledithiolate). Magnetochemistry, 7(5), 72. https://doi.org/10.3390/magnetochemistry7050072