The Internal Field in a Ferromagnetic Crystal with Chiral Molecular Packing of Achiral Organic Radicals
Abstract
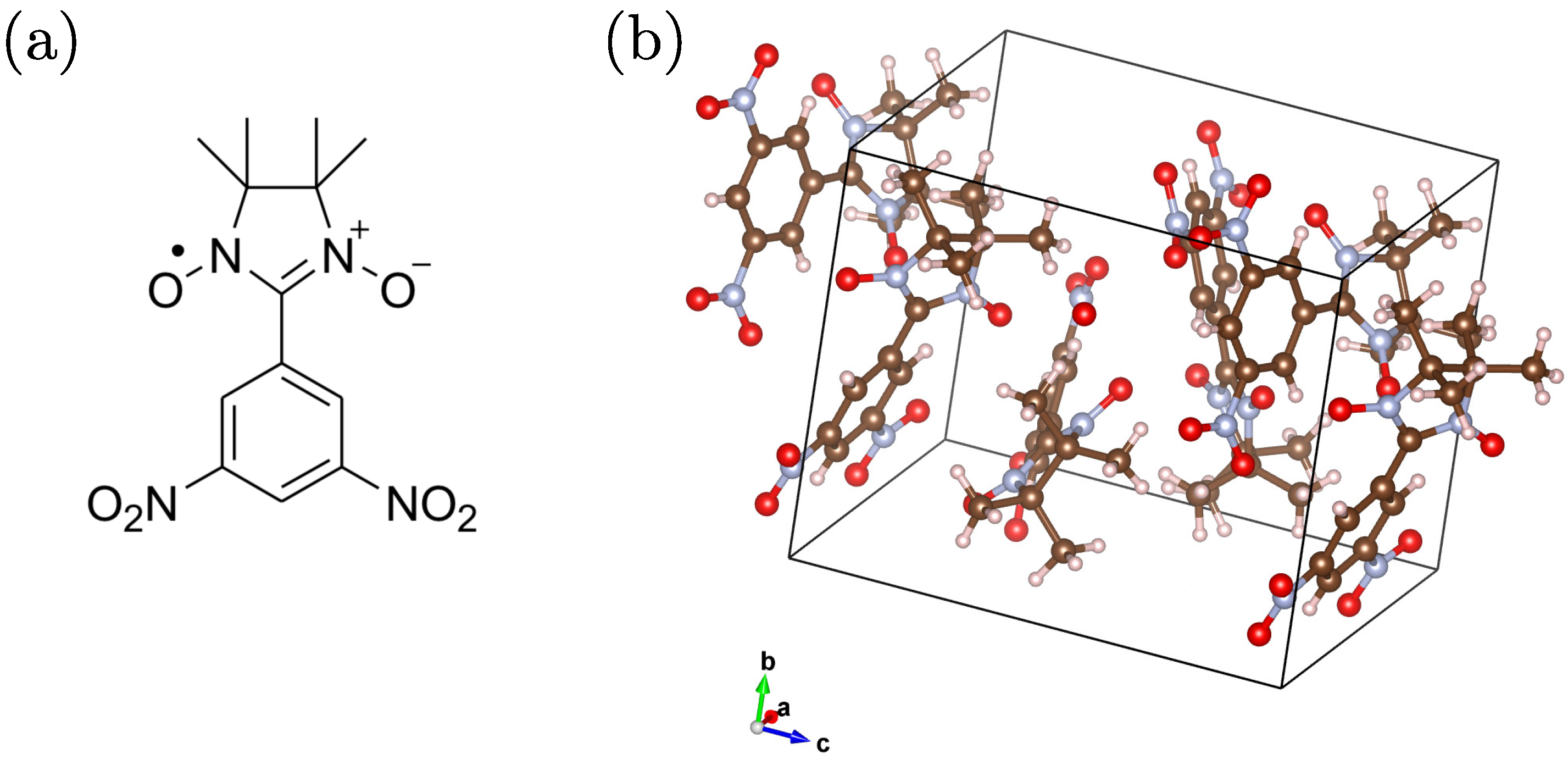
1. Introduction
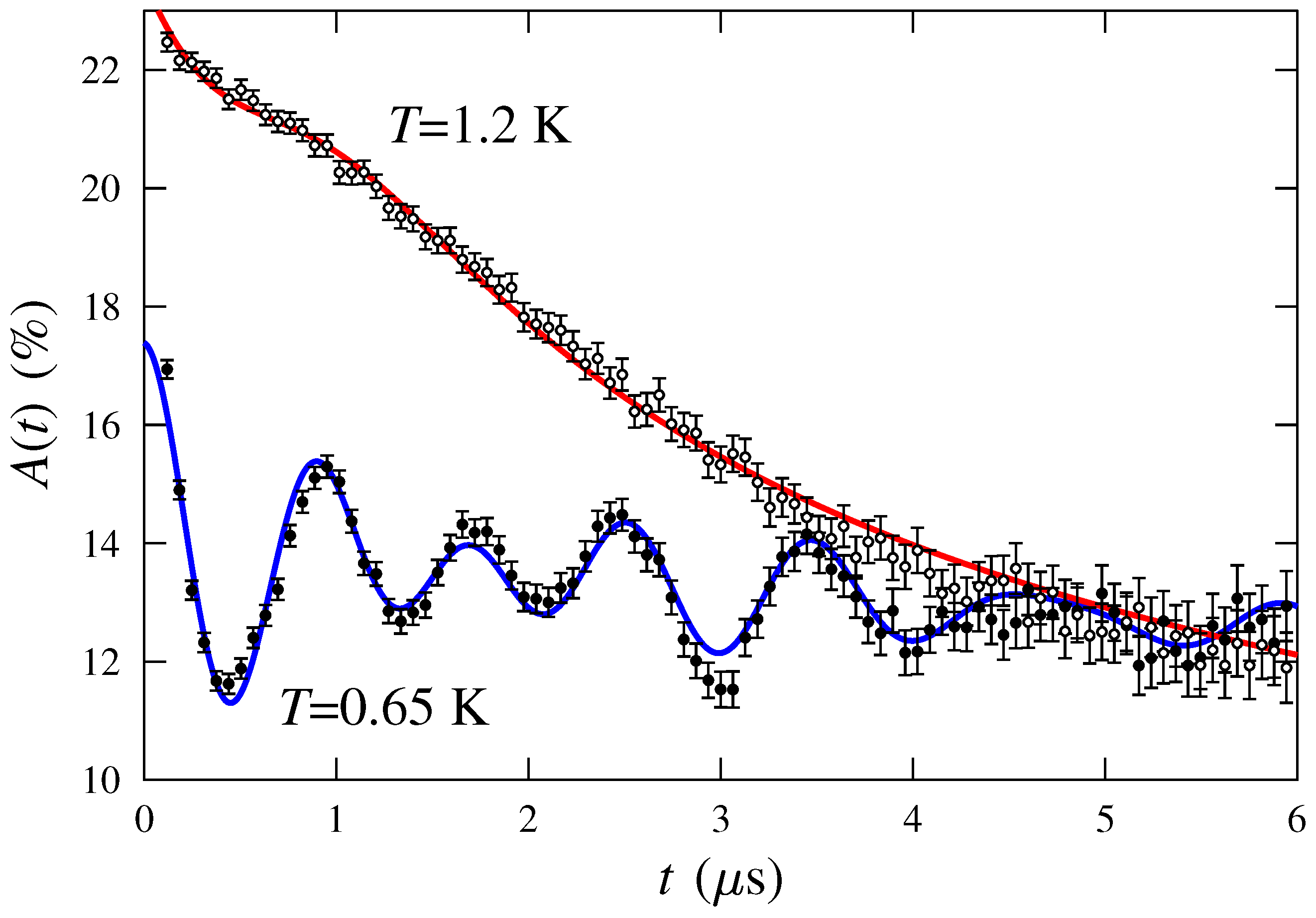
2. SR Experiment
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lahti, P.M. (Ed.) Magnetic Properties of Organic Materials; Dekker: New York, NY, USA, 1999. [Google Scholar]
- Blundell, S.J.; Pratt, F.L. Organic and molecular magnets. J. Phys. Condens. Matter 2004, 16, R771–R828. [Google Scholar] [CrossRef]
- Heisenberg, W. Zur Theorie des Ferromagnetismus. Z. Phys. 1928, 49, 619–636. [Google Scholar] [CrossRef]
- Tamura, M.; Nakazawa, Y.; Shiomi, D.; Nozawa, K.; Hosokoshi, Y.; Ishikawa, M.; Takahashi, M.; Kinoshita, M. Bulk ferromagnetism in the β-phase crystal of the p-nitrophenyl nitronyl nitroxide radical. Chem. Phys. Lett. 1991, 186, 401–404. [Google Scholar] [CrossRef]
- Kinoshita, M. p-nitrophenyl nitronyl nitroxideL the first organic ferromagnet. Phil. Trans. R. Soc. Lond. A 1999, 357, 2855–2872. [Google Scholar] [CrossRef]
- Nakazawa, Y.; Tamura, M.; Shirakawa, N.; Shiomi, D.; Takahashi, M.; Kinoshita, M.; Ishikawa, M. Low-temperature magnetic properties of the ferromagnetic organic radical, p-nitrophenyl nitronyl nitroxide radical. Phys. Rev. B 1992, 46, 8906–8914. [Google Scholar] [CrossRef] [PubMed]
- Le, L.P.; Keren, A.; Luke, G.M.; Wu, W.D.; Uemura, Y.J.; Tamura, M.; Ishikawa, M.; Kinoshita, M. Searching for spontaneous magnetic order in an organic ferromagnet. μSR studies of β-phase p-NPNN. Chem. Phys. Lett. 1993, 206, 405–408. [Google Scholar] [CrossRef]
- Blundell, S.J.; Pattenden, P.A.; Pratt, F.L.; Valladares, R.M.; Sugano, T.; Hayes, W. μ+SR of the organic ferromagnet p-NPNN: Diamagnetic and paramagnetic states. Europhys. Lett. 1995, 31, 573–578. [Google Scholar] [CrossRef]
- Blundell, S.J.; Sugano, T.; Pattenden, P.A.; Pratt, F.L.; Valladares, R.M.; Chow, K.H.; Uekusa, H.; Ohashi, Y.; Hayes, W. Magnetism in the nitronyl nitroxide isomers 1-NAPNN and 2-NAPNN studied by μ+SR. J. Phys. Condens. Matter 1996, 8, L1–L6. [Google Scholar] [CrossRef]
- Blundell, S.J.; Pattenden, P.A.; Pratt, F.L.; Chow, K.H.; Hayes, W.; Sugano, T. Organic magnetism in nitronyl nitroxides studied by μSR. Hyp. Int. 1997, 104, 251–256. [Google Scholar] [CrossRef]
- Blundell, S.J. μSR studies of organic magnets. Phil. Trans. R. Soc. Lond. A 1999, 357, 2923–2937. [Google Scholar] [CrossRef]
- Blundell, S.J.; Husmann, A.; Jestadt, T.; Pratt, F.L.; Marshall, I.M.; Lovett, B.W.; Kurmoo, M.; Sugano, T.; Hayes, W. Muon studies of molecular magnetism. Physica B 2000, 289, 115–118. [Google Scholar] [CrossRef]
- Blundell, S.J.; Marshall, I.M.; Lovett, B.W.; Pratt, F.L.; Husmann, A.; Hayes, W.; Takagi, S.; Sugano, T. Organic magnetic materials studied by positive muons. Hyp. Int. 2001, 133, 169–177. [Google Scholar] [CrossRef]
- Barron, L.D. Symmetry and molecular chirality. Chem. Soc. Rev. 1986, 15, 189–223. [Google Scholar] [CrossRef]
- Yoon, T.P.; Jacobsen, E.N. Privileged Chiral Catalysts. Science 2003, 299, 1691–1693. [Google Scholar] [CrossRef]
- Bullard, G.; Tassinari, F.; Ko, C.-H.; Mondal, A.K.; Wang, R.; Mishra, S.; Naaman, R.; Therien, M.J. Low-Resistance Molecular Wires Propagate Spin-Polarized Currents. J. Am. Chem. Soc. 2019, 141, 14707–14711. [Google Scholar] [CrossRef]
- Naaman, R.; Waldeck, D.H. Chiral Supramolecular Structures as Spin Filters. Annu. Rev. Phys. Chem. 2015, 66, 263–281. [Google Scholar] [CrossRef]
- Michaeli, K.; Naaman, R. Origin of spin-dependent tunneling through chiral molecules. J. Phys. Chem. C 2019, 123, 17043–17048. [Google Scholar] [CrossRef]
- Mondal, A.K.; Brown, N.; Mishra, S.; Makam, P.; Wing, D.; Gilead, S.; Wiesenfeld, Y.; Leitus, G.; Shimon, L.J.W.; Carmieli, R.; et al. Long-Range Spin-Selective Transport in Chiral Metal–Organic Crystals with Temperature-Activated Magnetization. ACS Nano 2020, 14, 16624–16633. [Google Scholar] [CrossRef] [PubMed]
- Rikken, G.L.J.A.; Raupach, E. Enantioselective magnetochiral photochemistry. Nature 2000, 405, 932–935. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, D.; Kanzaki, Y.; Okada, S.; Arima, R.; Miyazaki, Y.; Inaba, A.; Tanaka, R.; Sato, K.; Takui, T. An Enantiopair of Organic Ferromagnet Crystals Based on Helical Molecular Packing of Achiral Organic Radicals. J. Phys. Chem. Lett. 2011, 2, 3036–3039. [Google Scholar] [CrossRef]
- Cox, S.F.J. Implanted muon studies in condensed matter science. J. Phys. C 1987, 20, 3187–3319. [Google Scholar] [CrossRef]
- Blundell, S.J. Spin-polarized muons in condensed matter physics. Contemp. Phys. 1999, 40, 175–192. [Google Scholar] [CrossRef]
- Dalmas de Réotier, P.; Yaouanc, A. Muon spin rotation and relaxation in magnetic materials. J. Phys. C 1997, 9, 9113–9166. [Google Scholar]
- Blundell, S.J.; De Renzi, R.; Lancaster, T.; Pratt, F.L. (Eds.) Introduction to Muon Spectroscopy; Oxford University Press: Oxford, UK, 2021; to be published. [Google Scholar]
- Lancaster, T.; Blundell, S.J.; Pratt, F.L. Another dimension: Investigations of molecular magnetism using muon-spin relaxation. Phys. Scr. 2013, 88, 068506. [Google Scholar] [CrossRef][Green Version]
- Blundell, S.J. Molecular magnets. Contemp. Phys. 2007, 48, 275–290. [Google Scholar] [CrossRef]
- Lancaster, T.; Blundell, S.J.; Brooks, M.L.; Baker, P.J.; Pratt, F.L.; Manson, J.L.; Landee, C.P.; Baines, C. Magnetic order in the quasi-one-dimensional spin-1/2 molecular chain compound copper pyrazine dinitrate. Phys. Rev. B 2006, 73, R020410. [Google Scholar] [CrossRef]
- Manson, J.L.; Lancaster, T.; Chapon, L.C.; Blundell, S.J.; Schlueter, J.A.; Brooks, M.L.; Pratt, F.L.; Nygren, C.L.; Qualls, J.S. Cu(HCO2)2(pym) (pym=pyridine): Low-dimensional magnetic behavior and long-range ordering in a quantum-spin lattice. Inorg. Chem. 2005, 44, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Blundell, S.J.; Pattenden, P.A.; Valladares, R.M.; Pratt, F.L.; Sugano, T.; Hayes, W. Observation of a magnetic transition in para-pyridyl nitronyl nitroxide using zero-field μSR. Solid State Commun. 1994, 92, 569–572. [Google Scholar] [CrossRef]
- Steele, A.J.; Lancaster, T.; Blundell, S.J.; Baker, P.J.; Pratt, F.L.; Baines, C.; Conner, M.M.; Southerland, H.I.; Manson, J.L.; Schlueter, J.A. Magnetic order in quasi-two-dimensional molecular magnets investigated with muon-spin relaxation. Phys. Rev. B 2011, 84, 064412. [Google Scholar] [CrossRef]
- Blundell, S.J.; Lancaster, T.; Pratt, F.L.; Baker, P.J.; Brooks, M.L.; Baines, C.; Manson, J.L.; Landee, C.P. μ+SR as a probe of anisotropy in low-dimensional molecular magnets. J. Phys. Chem. Solids 2007, 68, 2039–2043. [Google Scholar] [CrossRef]
- Pelissetto, A.; Vicari, E. Critical phenomena and renormalization-group theory. Phys. Rep. 2002, 368, 549–727. [Google Scholar] [CrossRef]
- Pratt, F.L.; Baker, P.J.; Blundell, S.J.; Lancaster, T.; Green, M.A.; Kurmoo, M. Chiral-Like Critical Behavior in the Antiferromagnet Cobalt Glycerolate. Phys. Rev. Lett. 2007, 99, 017202. [Google Scholar] [CrossRef]
- Mukamel, D. Physical Realizations of n 4 Vector Models. Phys. Rev. Lett. 1975, 34, 481–485. [Google Scholar] [CrossRef]
- Möller, J.S.; Bonfá, P.; Ceresoli, D.; Bernardini, F.; Blundell, S.J.; Lancaster, T.; De Renzi, R.; Marzari, N.; Watanabe, I.; Sulaiman, S.; et al. Playing quantum hide-and-seek with the muon: Localizing muon stopping sites. Phys. Scr. 2013, 88, 068510. [Google Scholar] [CrossRef]
- Huddart, B.M. A Program to Classify and Analyse Muon Stopping Sites. 2020. Available online: https://gitlab.com/BenHuddart/mufinder/ (accessed on 17 May 2021).
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.J.; Refson, K.; Payne, M. First principles methods using CASTEP. Z. Kristall. 2005, 220, 567. [Google Scholar] [CrossRef]
- Algra, H.A.; de Jongh, L.J.; Huiskamp, W.J.; Carlin, R.L. Magnetic behavior of [(CH3)3NH]CuCl3·2H2O. Evidence for lattice-dimensionality crossovers in a quasi one-dimensional ferromagnet. Physica 1977, 92B, 187–200. [Google Scholar] [CrossRef]
- Swank, D.D.; Landee, C.P.; Willett, R.D. Crystal structure and magnetic susceptibility of copper (II) chloride tetramethylsulfoxide [CuCl2 (TMSO)] and copper (II) chloride monodimethylsulfoxide [CuCl2 (DMSO)]: Ferromagnetic spin-1/2 Heisenberg linear chains. Phys. Rev. B 1979, 20, 2154–2162. [Google Scholar] [CrossRef]
- Willett, R.D.; Landee, C.P. Ferromagnetism in one dimensional systems: Synthesis and structural characterization. J. Appl. Phys. 1981, 52, 2004–2009. [Google Scholar] [CrossRef]
- Takeda, K.; Konishi, K.; Nedachi, K.; Mukai, K. Experimental Study of Quantum Statistics for the S = 1/2 Quasi-One-Dimensional Organic Ferromagnet. Phys. Rev. Lett. 1995, 74, 1673–1676. [Google Scholar] [CrossRef]
- Willett, R.D.; Landee, C.P.; Gaura, R.M.; Swank, D.D.; Groenendijk, H.A.; van Duyneveldt, A.J. Magnetic properties of one-dimensional spin 1/2 ferromagnets: Metamagnetic behavior of (C6H11NH3)CuCl3. J. Magn. Magn. Mat. 1980, 15–18, 1055–1056. [Google Scholar] [CrossRef]
- Schouten, J.C.; van der Geest, G.J.; de Jonge, W.J.M.; Kopinga, K. Specific heat of (C6H11NH3) CuCl3 (CHAC), a system of ferromagnetic chains. Phys. Lett. A 1980, 78, 398–400. [Google Scholar] [CrossRef]
- Takeda, K.; Hamano, T.; Kawae, M.; Hidaka, M.; Takahashi, M.; Kawasaki, S.; Mukai, K. Experimental Check of Heisenberg Chain Quantum Statistics for a Ferromagnetic OrganicRadical Crystal. J. Phys. Soc. Jpn. 1995, 64, 2343–2346. [Google Scholar] [CrossRef]
- Landee, C.P.; Willett, R.D. Tetramethylammonium Copper Chloride and tris (Trimethylammonium) Copper Chloride: S = 1/2 Heisenberg One-Dimensional Ferromagnets. Phys. Rev. Lett. 1979, 43, 463–466. [Google Scholar] [CrossRef]
- Dupas, C.; Renard, J.P.; Seiden, J.; Cheikh-Rouhou, A. Static magnetic properties of (CH3)4NMnxCu1–xCl3, a quantum ferromagnetic chain with classical impurities: Experiment and theory. Phys. Rev. B 1982, 25, 3261–3272. [Google Scholar] [CrossRef]
- Kopinga, K.; Tinus, A.M.C.; de Jonge, W.J.M. Magnetic behavior of the ferromagnetic quantum chain systems (C6H11NH3)CuCl3 (CHAC) and (C6H11NH3)CuBr3 (CHAB). Phys. Rev. B 1982, 25, 4685–4690. [Google Scholar] [CrossRef]
- Sugano, T.; Blundell, S.J.; Lancaster, T.; Pratt, F.L.; Mori, H. Magnetic order in the purely organic quasi-one-dimensional ferromagnet 2-benzimidazolyl nitronyl nitroxide. Phys. Rev. B 2010, 82, 180401(R). [Google Scholar] [CrossRef]
- Blundell, S.J.; Möller, J.S.; Lancaster, T.; Baker, P.J.; Pratt, F.L.; Seber, G.; Lahti, P.M. μSR study of magnetic order in the organic quasi-one-dimensional ferromagnet F4BImNN. Phys. Rev. B 2013, 88, 064423. [Google Scholar] [CrossRef]
- Clemente-León, M.; Coronado, E.; Galán-Mascarós, J.R.; Gómez-García, C.J. Intercalation of decamethylferrocenium cations in bimetallic oxalate-bridged two-dimensional magnets. Chem. Commun. 1997, 1727–1728. [Google Scholar] [CrossRef]
- Coronado, E.; Galan-Mascaros, J.R.; Gomez-Garcia, C.J.; Burriel, R. A molecular chemical approach to the magnetic multilayers. J. Magn. Magn. Mater. 1999, 197, 558–560. [Google Scholar] [CrossRef]
- Lancaster, T.; Blundell, S.J.; Pratt, F.L.; Coronado, E.; Galan-Mascaros, J.R. Magnetic order and local field distribution in the hybrid magnets [Fe][MnCr(ox)3] and [Co][FeFe(ox)3]: A muon spin relaxation study. J. Mater. Chem. 2004, 14, 1518–1520. [Google Scholar] [CrossRef]
- Zheludev, A.; Barone, V.; Bonnet, M.; Delley, B.; Grand, A.; Ressouche, E.; Rey, P.; Subra, R.; Schweizer, J. Spin density in a nitronyl nitroxide free radical. Polarized neutron diffraction investigation and ab initio calculations. J. Am. Chem. Soc. 1994, 116, 2019–2027. [Google Scholar] [CrossRef]
- Heise, H.; Kohler, F.H.; Mota, F.; Novoa, J.J.; Veciana, J. Determination of the Spin Distribution in Nitronylnitroxides by Solid-State 1H, 2H, and 13C NMR Spectroscopy. J. Am. Chem. Soc. 1999, 121, 9659–9667. [Google Scholar] [CrossRef]
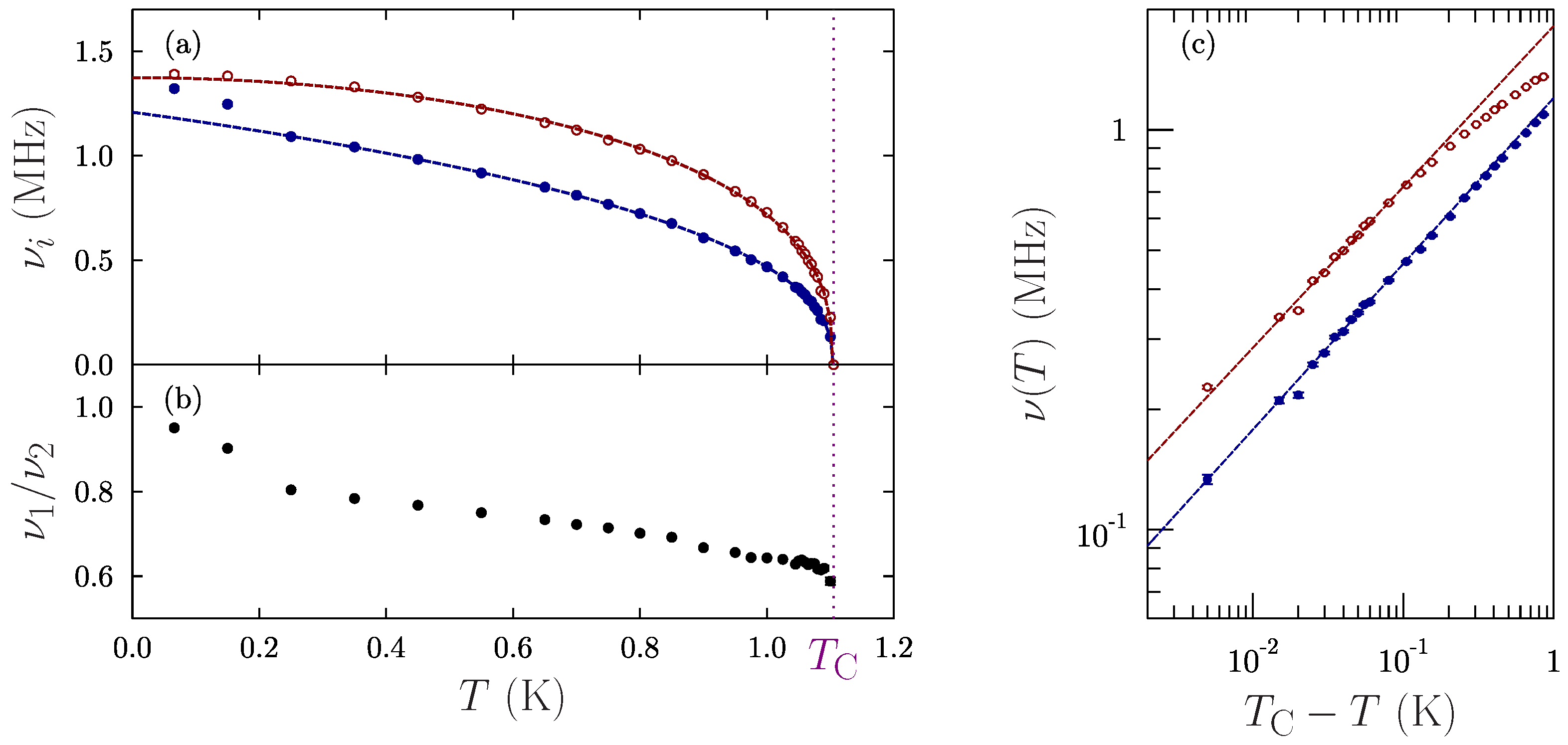
| Parameter | Frequency 1 | Frequency 2 |
|---|---|---|
| (K) | 1.105(1) | 1.104(1) |
| (MHz) | 1.21(2) | 1.37(2) |
| 1.03(6) | 1.96(14) | |
| 0.41(1) | 0.37(1) |
| Compound | Reference | (K) | (K) | |
|---|---|---|---|---|
| -NPNN | [6] | 2.15 | 0.65 | 0.30 |
| MeNHCuCl2HO | [39] | 0.85 | 0.165 | 0.19 |
| CuCl(DMSO) | [40,41] | 45 | 4.8 | 0.11 |
| p-CDTV | [42] | 6.0 | 0.67 | 0.11 |
| CuCl(TMSO) | [40,41] | 39 | 3 | 0.08 |
| CHAC | [43,44] | 45–53 | 2.18 | 0.04–0.05 |
| p-CDpOV | [45] | 5.5 | 0.21 | 0.038 |
| TMCuC | [41,46,47] | 30, 45 | 1.24 | 0.03–0.04 |
| CHAB | [48] | 55 | 1.50 | 0.027 |
| 2-BImNN | [49] | 22 | 1.0 | 0.045 |
| F4BImNN | [50] | 22 | 0.72 | 0.033 |
| DNPNN | [21], this work | 5.6 | 1.015 | 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blundell, S.J.; Lancaster, T.; Baker, P.J.; Pratt, F.L.; Shiomi, D.; Sato, K.; Takui, T. The Internal Field in a Ferromagnetic Crystal with Chiral Molecular Packing of Achiral Organic Radicals. Magnetochemistry 2021, 7, 71. https://doi.org/10.3390/magnetochemistry7050071
Blundell SJ, Lancaster T, Baker PJ, Pratt FL, Shiomi D, Sato K, Takui T. The Internal Field in a Ferromagnetic Crystal with Chiral Molecular Packing of Achiral Organic Radicals. Magnetochemistry. 2021; 7(5):71. https://doi.org/10.3390/magnetochemistry7050071
Chicago/Turabian StyleBlundell, Stephen J., Tom Lancaster, Peter J. Baker, Francis L. Pratt, Daisuke Shiomi, Kazunobu Sato, and Takeji Takui. 2021. "The Internal Field in a Ferromagnetic Crystal with Chiral Molecular Packing of Achiral Organic Radicals" Magnetochemistry 7, no. 5: 71. https://doi.org/10.3390/magnetochemistry7050071
APA StyleBlundell, S. J., Lancaster, T., Baker, P. J., Pratt, F. L., Shiomi, D., Sato, K., & Takui, T. (2021). The Internal Field in a Ferromagnetic Crystal with Chiral Molecular Packing of Achiral Organic Radicals. Magnetochemistry, 7(5), 71. https://doi.org/10.3390/magnetochemistry7050071






