Coexistence of Spin Canting and Metamagnetism in a One-Dimensional Mn(II) Compound Bridged by Alternating Double End-to-End and Double End-On Azido Ligands and the Analog Co(II) Compound †
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthetic Efforts
2.2. Characterization of the Compounds by IR and Elemental Analysis
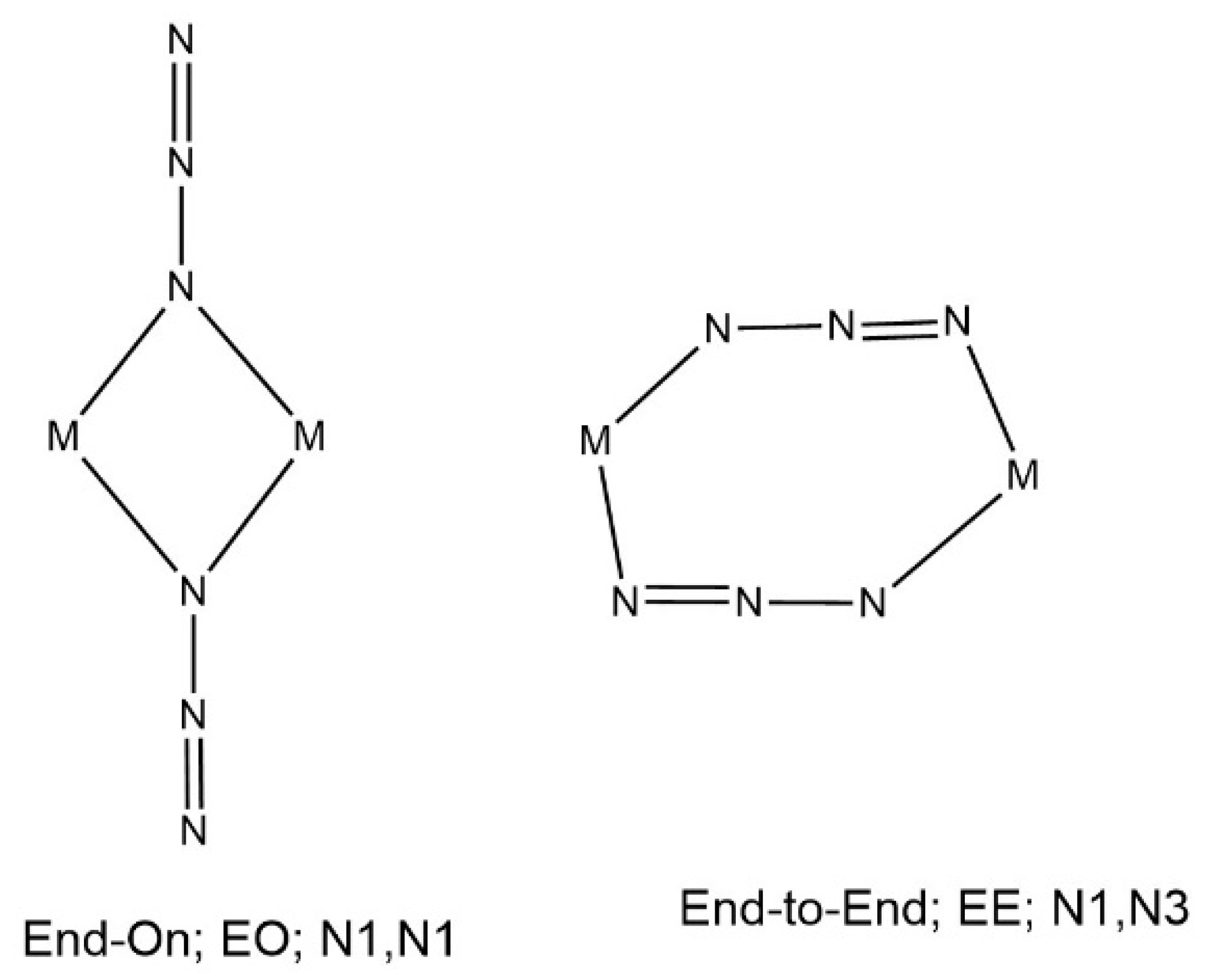
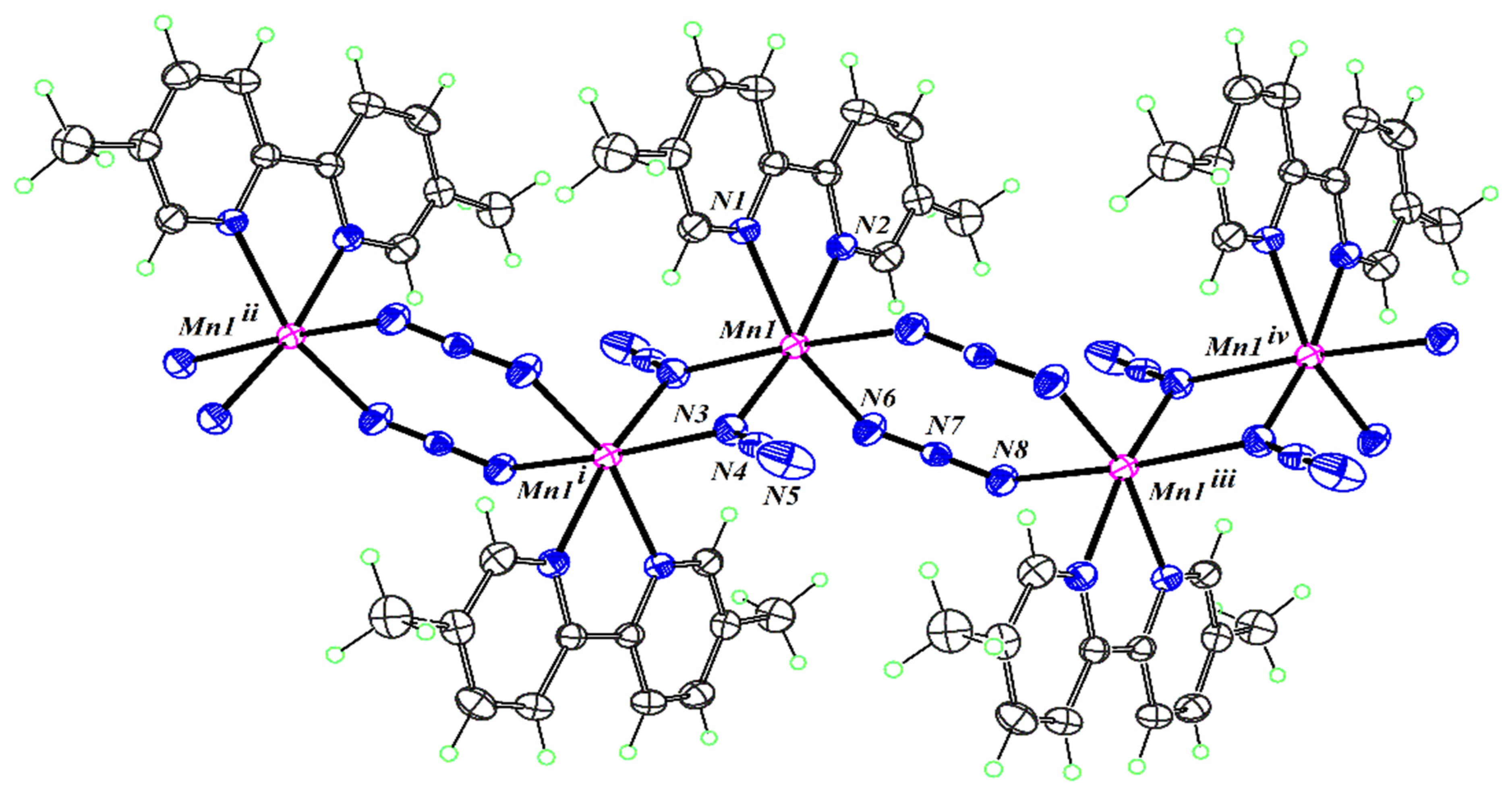
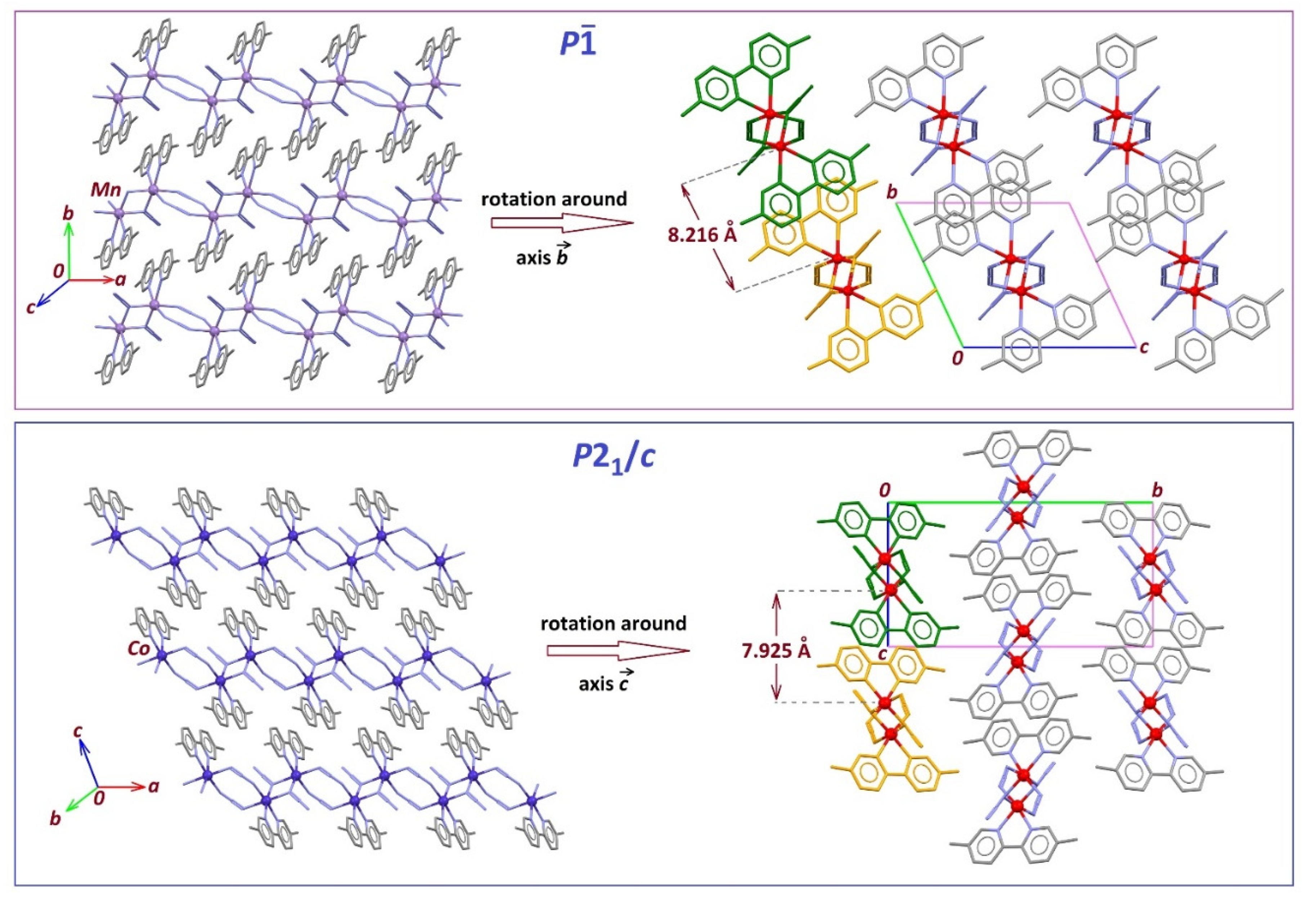
2.3. Structure Description of the Compounds
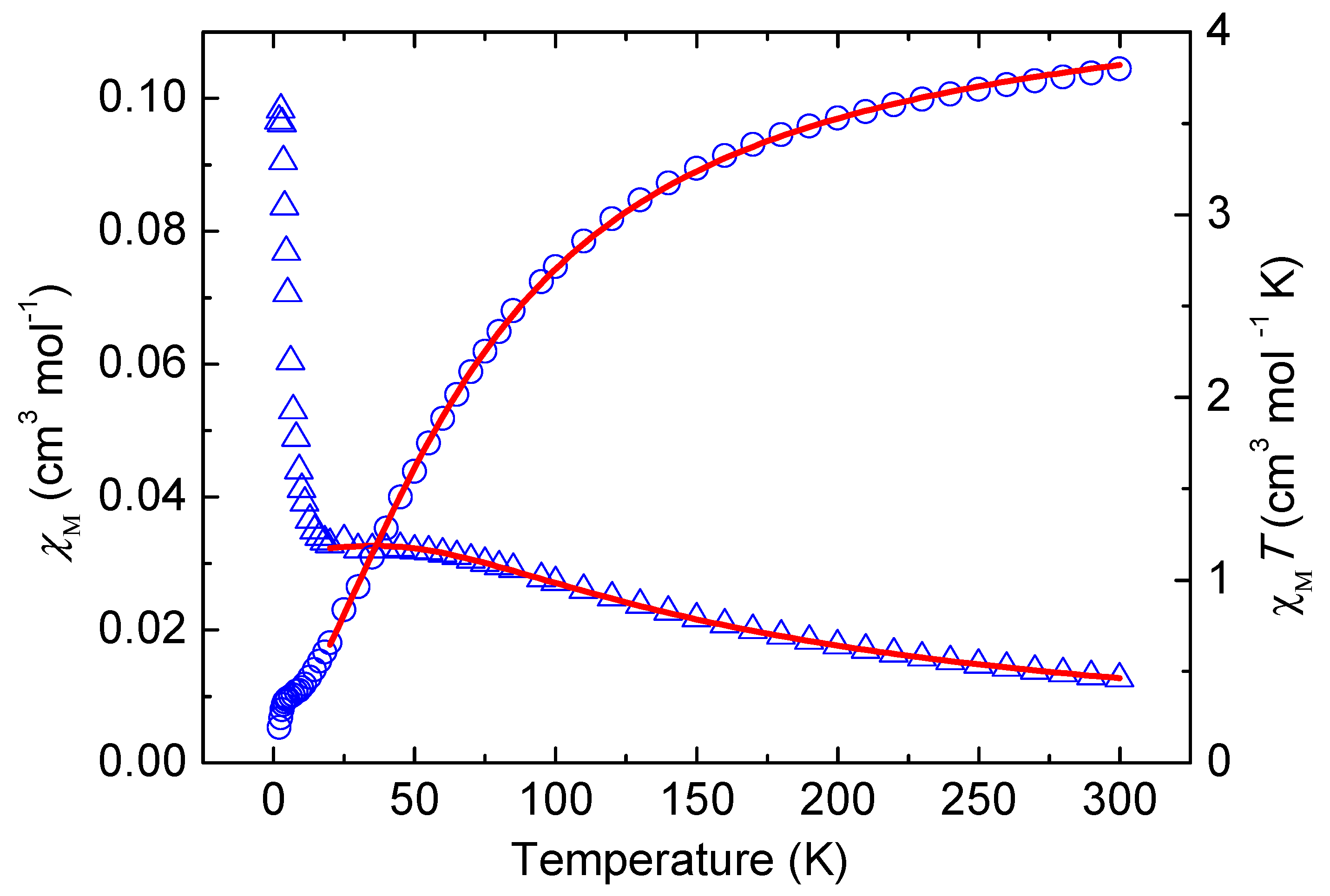
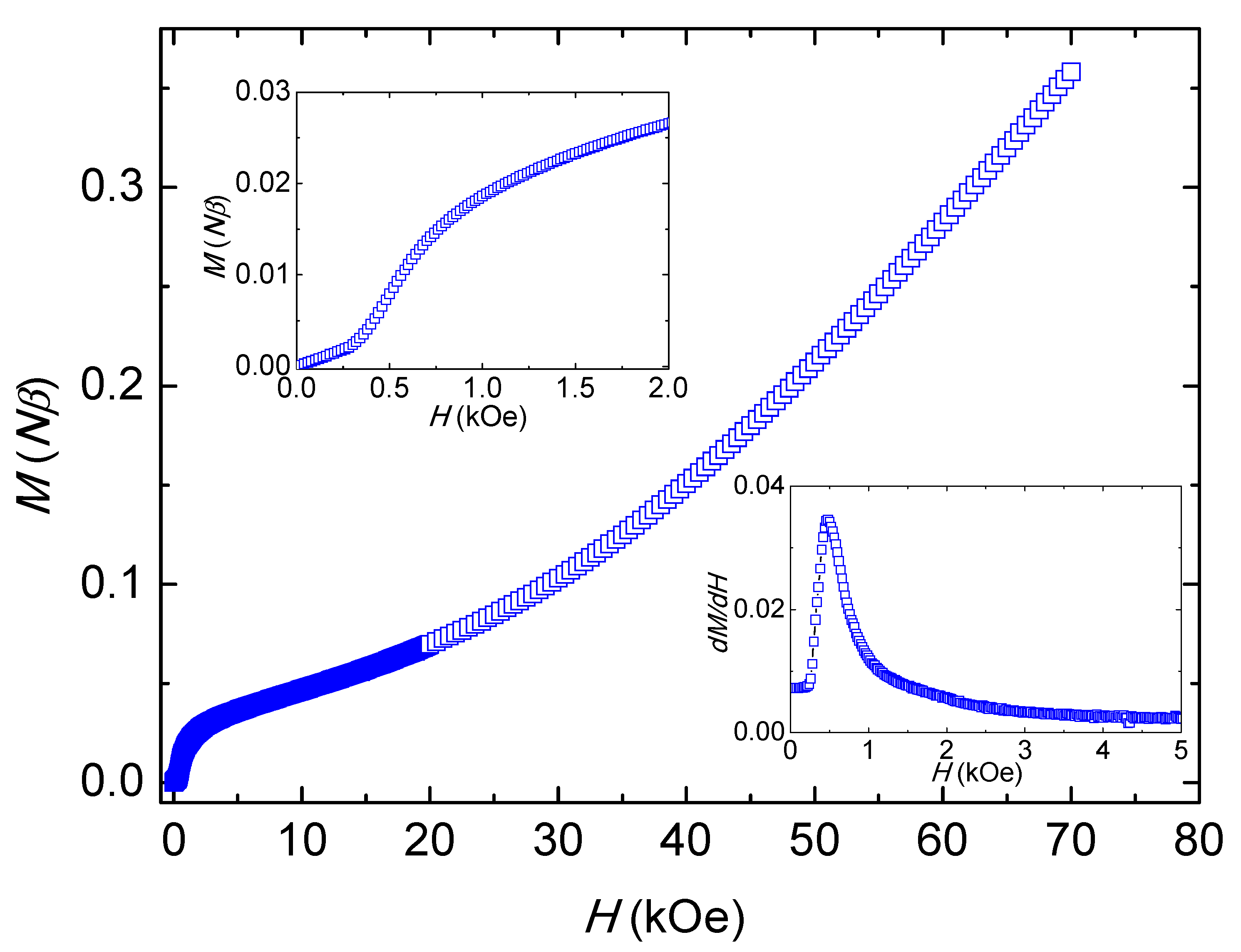
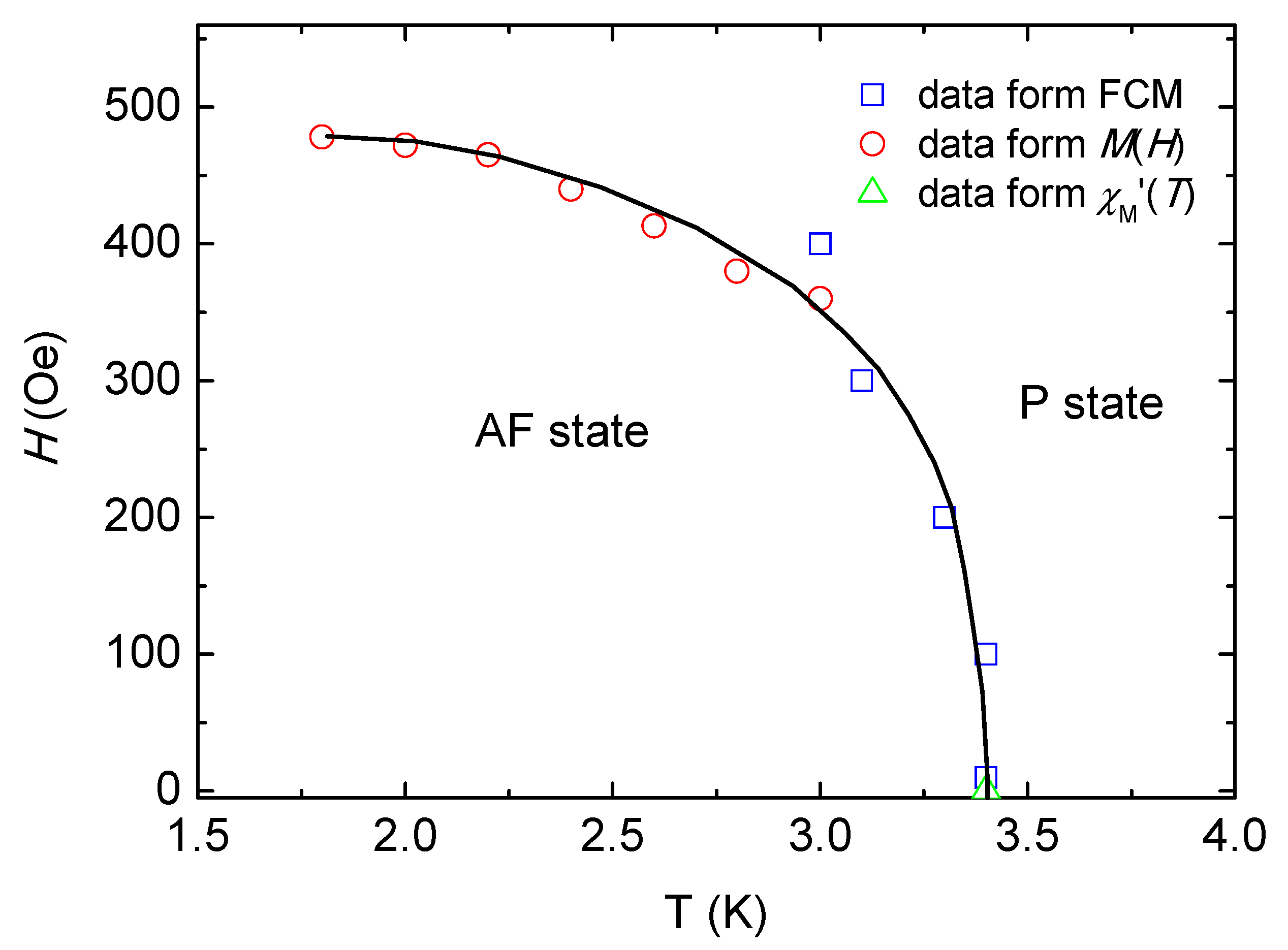
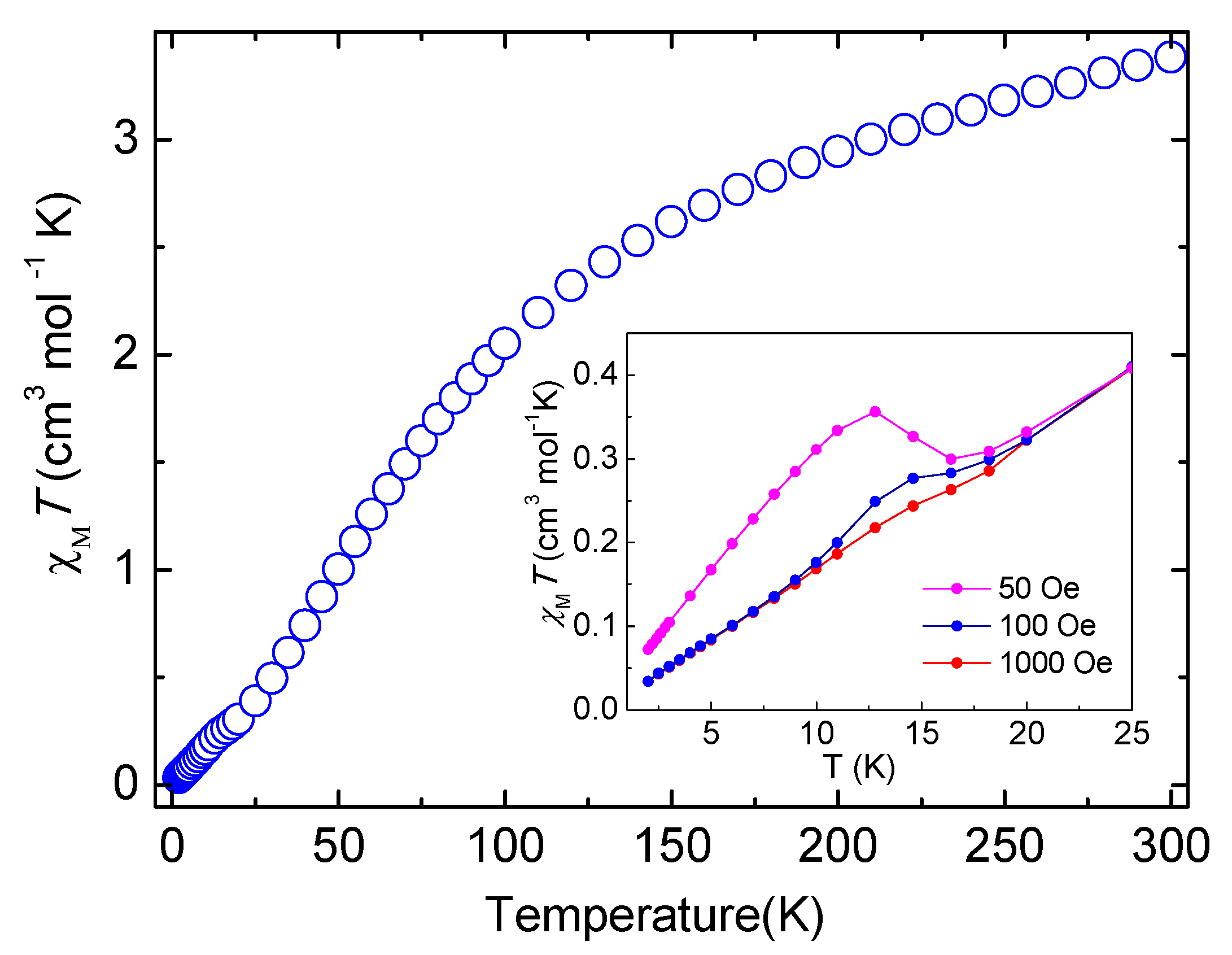
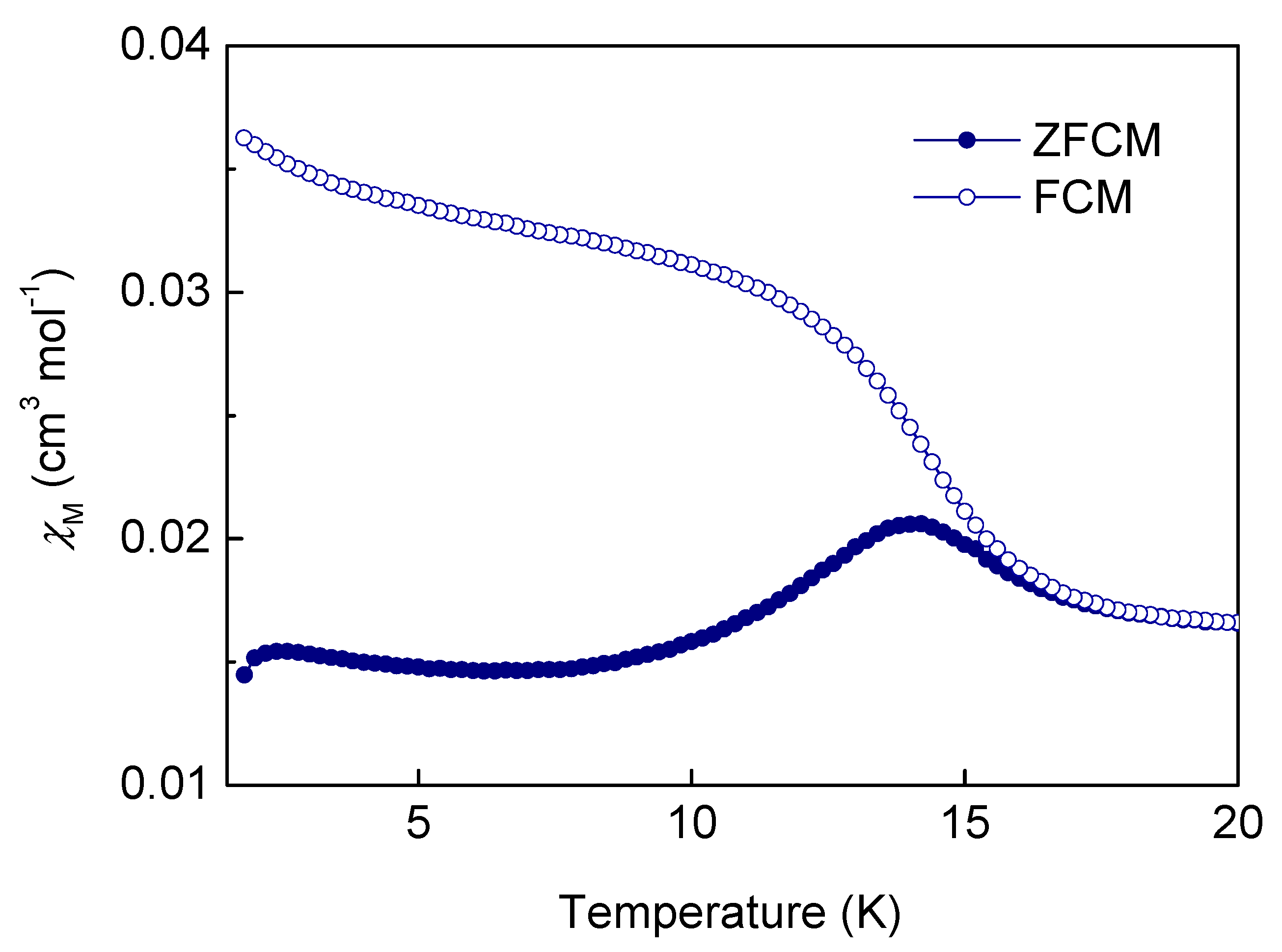
2.4. Magnetic Properties
3. Concluding Remarks
4. Material and Methods
4.1. General Remarks
4.2. Synthesis
4.3. X-ray Crystallography
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khasainov, B.; Comet, M.; Veyssiere, B.; Spitzer, D. On the Mechanism of Efficiency of Lead Azide. Propellants Explos. Pyrotech. 2016, 42, 547–557. [Google Scholar] [CrossRef]
- Xu, J.-G.; Sun, C.; Zhang, M.-J.; Liu, B.-W.; Li, X.-Z.; Lu, J.; Wang, S.-H.; Zheng, F.-K.; Guo, G.-C. Coordination Polymerization of Metal Azides and Powerful Nitrogen-Rich Ligand toward Primary Explosives with Excellent Energetic Performances. Chem. Mater. 2017, 29, 9725–9733. [Google Scholar] [CrossRef]
- Ma, X.; Liu, Y.; Song, W.; Wang, Z.; Liu, X.; Xie, G.; Chen, S.; Gao, S. A difunctional azido-cobalt(ii) coordination polymer exhibiting slow magnetic relaxation behaviour and high-energy characteristics with good thermostability and insensitivity. Dalton Trans. 2018, 47, 12092–12104. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-G.; Li, X.-Z.; Wu, H.-F.; Zheng, F.-K.; Chen, J.; Guo, G.-C. Substitution of Nitrogen-Rich Linkers with Insensitive Linkers in Azide-Based Energetic Coordination Polymers toward Safe Energetic Materials. Cryst. Growth Des. 2019, 19, 3934–3944. [Google Scholar] [CrossRef]
- Zhang, W.; Li, T.; Zhang, B.; Wang, L.; Zhang, T.; Zhang, J. Planar, Energetic, π–π-Stacked Compound with Weak Interactions Resulting in a High-Impact- and Low-Friction-Sensitive, Safer, Primary Explosive. Inorg. Chem. 2019, 58, 7653–7656. [Google Scholar] [CrossRef]
- Beck, W.; Werner, K.V. Reaktionen der Azido- und Isocyanatorhodium(I)-Komplexetrans-(Ph3P)2Rh(CO)X mit dem Nitrosyl-Kation in Gegenwart von Alkoholen. Eur. J. Inorg. Chem. 1973, 106, 868–873. [Google Scholar] [CrossRef]
- Hu, B.-W.; Zhao, J.-P.; Yang, Q.; Liu, F.-C. Dzyaloshinski–Moriya (D–M) oriented weak ferromagnets in isomorphic coordination architectures constructed by flexible 1,2,4-triazole-1-acetate ligands with the assistance of halogen or pseudohalogen anions. Inorg. Chem. Commun. 2013, 35, 290–294. [Google Scholar] [CrossRef]
- Ribas, J.; Escuer, A.; Monfort, M.; Vicente, R.; Cortés, R.; Lezama, L.; Rojo, T. Polynuclear NiII and MnII azido bridging complexes. Structural trends and magnetic behavior. Coord. Chem. Rev. 1999, 193–195, 1027–1068. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Ramírez, C.P.; Bernès, S.; Anzaldo, S.H.; Ortega, Y.R. Structure and NMR properties of the dinuclear complex di-μ-azido-κ4 N 1:N 1-bis[(azido-κN)(pyridine-2-carboxamide-κ2 N 1,O)zinc(II)]. Acta Crystallogr. Sect. E Crystallogr. Commun. 2021, 77, 111–116. [Google Scholar] [CrossRef]
- Monfort, M.; Resino, I.; Ribas, J.; Solans, X.; Font-Bardia, M.; Rabu, P.; Drillon, M. Synthesis, structure, and magnetic properties of two new ferromagnetic/antiferromagnetic one-dimensional nickel(II) complexes. Magnetostructural correlations. Inorg. Chem. 2000, 39, 2572–2576. [Google Scholar] [CrossRef] [PubMed]
- Monfort, M.; Resino, I.; Ribas, J.; Stoeckli-Evans, H. A Metamagnetic Two-Dimensional Molecular Material with Nickel(II) and Azide. Angew. Chem. Int. Ed. 2000, 39, 191–193. [Google Scholar] [CrossRef]
- Cortés, R.; Drillon, M.; Solans, X.; Lezama, A.L.; Rojo, T. Alternating Ferromagnetic–Antiferromagnetic Interactions in a Manganese(II)–Azido One-Dimensional Compound: [Mn(bipy)(N3)2]. Inorg. Chem. 1997, 36, 677–683. [Google Scholar] [CrossRef]
- Lu, Z.; Gamez, P.; Kou, H.-Z.; Fan, C.; Zhang, H.; Sun, G. Spin canting and metamagnetism in the two azido-bridged 1D complexes [Ni(3,5-dmpy)2(N3)2]n and [Co1.5(3,5-dmpy)3(N3)3]n. CrystEngComm 2012, 14, 5035–5041. [Google Scholar] [CrossRef]
- Van Albada, G.A.; Van Der Horst, M.G.; Mutikainen, I.; Turpeinen, U.; Reedijk, J. Synthesis, Crystal Structure and Spectroscopy of catena-poly-bis(azido-N1,N1)(2-Aminopyrimidine)Copper(II). J. Chem. Crystallogr. 2008, 38, 413–417. [Google Scholar] [CrossRef]
- Van Albada, G.A.; Van Der Horst, M.G.; Mutikainen, I.; Turpeinen, U.; Reedijk, J. Dinuclear and polynuclear Cu(II) azido-bridged compounds with 7-azaindole as a ligand. Synthesis, characterization and 3D structures. Inorg. Chim. Acta 2011, 367, 15–20. [Google Scholar] [CrossRef]
- Van Albada, G.A.; Mutikainen, I.; Roubeau, O.; Reedijk, J. A dinuclear end-on azide-bridged copper(II) compound with weak antiferromagnetic interaction—Synthesis, characterization, magnetism and X-ray structure of bis[(μ-azido-κN1)-(azido-κN1)(1,3-bis(benzimidazol-2-yl)-2-methylpropane)copper(II)]. J. Mol. Struct. 2013, 1036, 252–256. [Google Scholar] [CrossRef]
- Setifi, Z.; Ghazzali, M.; Glidewell, C.; Pérez, O.; Setifi, F.; Gómez-García, C.J.; Reedijk, J. Azide, water and adipate as bridging ligands for Cu(II): Synthesis, structure and magnetism of (μ4-adipato-κ-O)(μ-aqua)(μ-azido-κN1,N1)copper(II) monohydrate. Polyhedron 2016, 117, 244–248. [Google Scholar] [CrossRef]
- Van Albada, G.A.; Mohamadou, A.; Mutikainen, I.; Turpeinen, U.; Reedijk, J. Diazidobis(2,2′-biimidazoline)nickel(II). Acta Crystallogr. Sect. E Struct. Rep. Online 2004, 60, m237–m238. [Google Scholar] [CrossRef]
- Van Albada, G.A.; Smeets, W.J.J.; Spek, A.L.; Reedijk, J. The crystal structure and IR spectra of μ-(bipyrimidine-N1,N1′,N5,N5′)-bis[(azido-N1)(methanol)(bipyrimidine-N1,N1′)copper(II)] bis(triflate) bis(methanol). J. Chem. Crystallogr. 1998, 28, 427–432. [Google Scholar] [CrossRef]
- Mautner, F.A.; Sudy, B.; Massoud, A.A.; Abu-Youssef, M.A.M. Synthesis and characterization of Mn(II) and Zn(II) azido complexes with halo-substituted pyridine derivative ligands. Transit. Met. Chem. 2013, 38, 319–325. [Google Scholar] [CrossRef]
- Yue, Y.-F.; Gao, E.-Q.; Fang, C.-J.; Zheng, T.; Liang, J.; Yan, C.-H. Three Azido-Bridged Mn(II) Complexes Based on Open-Chain Diazine Schiff-base Ligands: Crystal Structures and Magnetic Properties. Cryst. Growth Des. 2008, 8, 3295–3301. [Google Scholar] [CrossRef]
- Gao, E.-Q.; Bai, S.-Q.; Yue, Y.-F.; Wang, Z.-M.; Yan, C.-H. New One-Dimensional Azido-Bridged Manganese(II) Coordination Polymers Exhibiting Alternating Ferromagnetic–Antiferromagnetic Interactions: Structural and Magnetic Studies. Inorg. Chem. 2003, 42, 3642–3649. [Google Scholar] [CrossRef] [PubMed]
- Abu-Youssef, M.A.M.; Drillon, M.; Escuer, A.; Goher, M.A.S.; Mautner, F.A.; Vicente, R. Topological Ferrimagnetic Behavior of Two New [Mn(L)2(N3)2]nChains with the New AF/AF/F Alternating Sequence (L = 3-Methylpyridine or 3,4-Dimethylpyridine). Inorg. Chem. 2000, 39, 5022–5027. [Google Scholar] [CrossRef] [PubMed]
- Abu-Youssef, M.A.M.; Escuer, A.; Goher, M.A.S.; Mautner, F.A.; Reiss, G.J.; Vicente, R. Can a homometallic chain be ferromagnetic? Angew. Chem. Int. Ed. 2000, 39, 1624–1626. [Google Scholar] [CrossRef]
- Ribas, J.; Monfort, M.; Ghosh, B.K.; Solans, X.; Font-Bardía, M. Versatility of the azido bridging ligand in the first two examples of ferro–antiferromagnetic alternating nickel(II) chains. J. Chem. Soc. Chem. Commun. 1995, 23, 2375–2376. [Google Scholar] [CrossRef]
- Ribas, J.; Monfort, M.; Resino, I.; Solans, X.; Rabu, P.; Maingot, F.; Drillon, M. A Unique NiII Complex with Three Different Azido Bridges: Magneto-Structural Correlations in the First Triply Alternating S = 1 Chain. Angew. Chem. Int. Ed. 1996, 35, 2520–2522. [Google Scholar] [CrossRef]
- Cortes, R.; Lezama, L.; Pizarro, J.L.; Arriortua, M.I.; Solans, X.; Rojo, T. Alternating Ferromagnetic and Antiferromagnetic Interactions In a MnII Chain With Alternating End-On and End-To-End Bridging Azido Ligands. Angew. Chem. Int. Ed. 1994, 33, 2488–2489. [Google Scholar] [CrossRef]
- Viau, G.; Lombardi, M.G.; De Munno, G.; Julve, M.; Lloret, F.; Faus, J.; Caneschi, A.; Clemente-Juan, J.M. The azido ligand: A useful tool in designing chain compounds exhibiting alternating ferro- and antiferro-magnetic interactions. Chem. Commun. 1997, 13, 1195–1196. [Google Scholar] [CrossRef]
- Alvarez, S.; Avnir, D.; Llunell, M.; Pinsky, M. Continuous symmetry maps and shape classification. The case of six-coordinated metal compounds. New J. Chem. 2002, 26, 996–1009. [Google Scholar] [CrossRef]
- Bhowmik, P.; Biswas, S.; Chattopadhyay, S.; Diaz, C.; Gómez-García, C.J.; Ghosh, A. Synthesis, crystal structure and magnetic properties of two alternating double μ1,1 and μ1,3 azido bridged Cu(ii) and Ni(ii) chains. Dalton Trans. 2014, 43, 12414–12421. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Q.; Tan, Q.-H.; Guo, X.-Y.; Liu, H.-T.; Liu, Z.-L.; Gao, E.-Q. Novel manganese(ii) and cobalt(ii) 2D polymers containing alternating chains with mixed azide and carboxylate bridges: Crystal structure and magnetic properties. RSC Adv. 2016, 6, 72326–72332. [Google Scholar] [CrossRef]
- Fu, A.; Huang, X.; Li, J.; Yuen, T.; Lin, C.L. Controlled synthesis and magnetic properties of 2D and 3D iron azide networks infinity 2[Fe(N3)2(4,4′-bpy)] and infinity 3[Fe(N3)2(4,4′-bpy)]. Chemistry 2002, 8, 2239–2247. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Liu, C.-M.; Zhang, D.-Q.; Gao, S.; Zhu, D.-B. Spin-canting in a 1D chain Mn(II) complex with alternating double end-on and double end-to-end azido bridging ligands. Inorg. Chem. Commun. 2007, 10, 897–901. [Google Scholar] [CrossRef]
- Kar, P.; Drew, M.G.B.; Gómez-García, C.J.; Ghosh, A. Coordination Polymers Containing Manganese(II)-Azido Layers Connected by Dipyridyl-tetrazine and 4,4′-Azobis(pyridine) Linkers. Inorg. Chem. 2013, 52, 1640–1649. [Google Scholar] [CrossRef] [PubMed]
- Mautner, F.A.; Berger, C.; Scherzer, M.; Fischer, R.C.; Maxwell, L.; Ruiz, E.; Vicente, R. Different topologies in three manganese-μ-azido 1D compounds: Magnetic behavior and DFT-quantum Monte Carlo calculations. Dalton Trans. 2015, 44, 18632–18642. [Google Scholar] [CrossRef] [PubMed]
- Abu-Youssef, M.A.M.; Escuer, A.; Gatteschi, D.; Goher, M.A.S.; Mautner, F.A.; Vicente, R. Synthesis, Structural Characterization, Magnetic Behavior, and Single Crystal EPR Spectra of Three New One-Dimensional Manganese Azido Systems with FM, Alternating FM-AF, and AF Coupling. Inorg. Chem. 1999, 38, 5716–5723. [Google Scholar] [CrossRef]
- Gao, E.-Q.; Cheng, A.-L.; Xu, Y.-X.; He, M.-Y.; Yan, C.-H. From Low-Dimensional Manganese(II) Azido Motifs to Higher-Dimensional Materials: Structure and Magnetic Properties. Inorg. Chem. 2005, 44, 8822–8835. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Wang, L.; Wang, Z.-M.; Su, G.; Gao, S. Coexistence of Spin-Canting, Metamagnetism, and Spin-Flop in a (4,4) Layered Manganese Azide Polymer. Chem. Mater. 2005, 17, 6369–6380. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Hao, Z.-M.; Zhang, W.-X.; Chen, X.-M. Dehydration-Induced Conversion from a Single-Chain Magnet into a Metamagnet in a Homometallic Nanoporous Metal–Organic Framework. Angew. Chem. Int. Ed. 2007, 46, 3456–3459. [Google Scholar] [CrossRef]
- Cheng, X.-N.; Xue, W.; Huang, J.-H.; Chen, X.-M. Spin canting and/or metamagnetic behaviours of four isostructural grid-type coordination networks. Dalton Trans. 2009, 29, 5701–5707. [Google Scholar] [CrossRef] [PubMed]
- Bellitto, C.; Federici, F.; Colapietro, M.; Portalone, G.; Caschera, D. X-ray Single-Crystal Structure and Magnetic Properties of Fe[CH3PO3)]·H2O: A Layered Weak Ferromagnet. Inorg. Chem. 2002, 41, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Shang, R.; Hu, K.-L.; Wang, Z.-M.; Gao, S. A New Series of Chiral Metal Formate Frameworks of [HONH3][MII(HCOO)3] (M = Mn, Co, Ni, Zn, and Mg): Synthesis, Structures, and Properties. Inorg. Chem. 2012, 51, 13363–13372. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.-N.; Xue, W.; Zhang, W.-X.; Chen, X.-M. Weak Ferromagnetism and Dynamic Magnetic Behavior of Two 2D Compounds with Hydroxy/Carboxylate-Bridged Co(II) Chains. Chem. Mater. 2008, 20, 5345–5350. [Google Scholar] [CrossRef]
- Wang, T.-T.; Ren, M.; Bao, S.-S.; Liu, B.; Pi, L.; Cai, Z.-S.; Zheng, Z.-H.; Xu, Z.-L.; Zheng, L.-M. Effect of Structural Isomerism on Magnetic Dynamics: From Single-Molecule Magnet to Single-Chain Magnet. Inorg. Chem. 2014, 53, 3117–3125. [Google Scholar] [CrossRef]
- Boca, R.; Herchel, R. Antisymmetric exchange in polynuclear metal complexes. Coord. Chem. Rev. 2010, 254, 2973–3025. [Google Scholar] [CrossRef]
- Verdaguer, M.; Bleuzen, A.; Marvaud, V.; Vaissermann, J.; Seuleiman, M.; Desplanches, C.; Scuiller, A.; Train, C.; Garde, R.; Gelly, G.; et al. Molecules to build solids: High TC molecule-based magnets by design and recent revival of cyano complexes chemistry. Coord. Chem. Rev. 1999, 190–192, 1023–1047. [Google Scholar] [CrossRef]
- Rodríguez, A.; Kivekäs, R.; Colacio, E. Unique self-assembled 2D metal-tetrazolate networks: Crystal structure and magnetic properties of [M(pmtz)2](M = Co(ii) and Fe(ii); Hpmtz = 5-(pyrimidyl)tetrazole). Chem. Commun. 2005, 41, 5228–5230. [Google Scholar] [CrossRef]
- Li, J.-R.; Yu, Q.; Sañudo, E.C.; Tao, Y.; Bu, X.-H. An azido–CuII–triazolate complex with utp-type topological network, showing spin-canted antiferromagnetism. Chem. Commun. 2007, 25, 2602–2604. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Wei, H.-Y.; Wang, Z.-M.; Chen, Z.-D.; Gao, S. FormateThe Analogue of Azide: Structural and Magnetic Properties of M(HCOO)2(4,4′-bpy)·nH2O (M = Mn, Co, Ni; n = 0, 5). Inorg. Chem. 2005, 44, 572–583. [Google Scholar] [CrossRef]
- Escuer, A.; Cano, J.; Goher, M.A.; Journaux, Y.; Lloret, F.; Mautner, F.A.; Vicente, R. Synthesis, Structural Characterization, and Monte Carlo Simulation of the Magnetic Properties of Two New Alternating MnIIAzide 2-D Honeycombs. Study of the Ferromagnetic Ordered Phase below 20 K. Inorg. Chem. 2000, 39, 4688–4695. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhang, W.-X.; Ye, B.-H.; Lin, J.-B.; Chen, X.-M. Spin Canting and Topological Ferrimagnetism in Two Manganese(II) Coordination Polymers Generated by In Situ Solvothermal Ligand Reactions. Eur. J. Inorg. Chem. 2007, 2007, 2668–2676. [Google Scholar] [CrossRef]
- Carlin, R.L.; Van Duyneveldt, A.J. Field-dependent magnetic phenomena. Acc. Chem. Res. 1980, 13, 231–236. [Google Scholar] [CrossRef]
- Manson, J.L.; Kmety, C.R.; Palacio, F.; Epstein, A.J.; Miller, J.S. Low-Field Remanent Magnetization in the Weak Ferromagnet Mn[N(CN)2]2. Evidence for Spin-Flop Behavior. Chem. Mater. 2001, 13, 1068–1073. [Google Scholar] [CrossRef]
- Pinkowicz, D.; Rams, M.; Nitek, W.; Czarnecki, B.; Sieklucka, B. Evidence for magnetic anisotropy of [NbIV(CN)8]4– in a pillared-layered Mn2Nb framework showing spin-flop transition. Chem. Commun. 2012, 48, 8323–8325. [Google Scholar] [CrossRef]
- Lu, Y.-B.; Wang, M.-S.; Zhou, W.-W.; Xu, G.; Guo, G.-C.; Huang, J.-S. Novel 3-D PtS-like Tetrazolate-Bridged Manganese(II) Complex Exhibiting Spin-Canted Antiferromagnetism and Field-Induced Spin-Flop Transition. Inorg. Chem. 2008, 47, 8935–8942. [Google Scholar] [CrossRef] [PubMed]
- Schlueter, J.A.; Manson, J.L.; Hyzer, K.A.; Geiser, U. Spin Canting in the 3D Anionic Dicyanamide Structure (SPh3)Mn(dca)3(Ph = Phenyl, dca = Dicyanamide). Inorg. Chem. 2004, 43, 4100–4102. [Google Scholar] [CrossRef]
- Ray, U.; Jasimuddin, S.; Ghosh, B.K.; Monfort, M.; Ribas, J.; Mostafa, G.; Lu, T.-H.; Sinha, C. A New Alternating Ferro-Antiferromagnetic One-Dimensional Azido-Bridged (Arylazoimidazole)manganese(II), [Mn(TaiEt)(N3)2]n [TaiEt = 1-Ethyl-2-(p-tolylazo)imidazole], Exhibiting Bulk Weak Ferromagnetic Long-Range Ordering. Eur. J. Inorg. Chem. 2004, 2004, 250–259. [Google Scholar] [CrossRef]
- Boonmak, J.; Nakano, M.; Youngme, S. Structural diversity and magnetic properties in 1D and 2D azido-bridged cobalt(ii) complexes with 1,2-bis(2-pyridyl)ethylene. Dalton Trans. 2011, 40, 1254–1260. [Google Scholar] [CrossRef]
- Boonmak, J.; Nakano, M.; Chaichit, N.; Pakawatchai, C.; Youngme, S. Spin Canting and Metamagnetism in 2D and 3D Cobalt(II) Coordination Networks with Alternating Double End-On and Double End-to-End Azido Bridges. Inorg. Chem. 2011, 50, 7324–7333. [Google Scholar] [CrossRef]
- Wang, X.-T.; Wang, X.-H.; Wang, Z.-M.; Gao, S. Diversity of Azido Magnetic Chains Constructed with Flexible Ligand 2,2′-Dipyridylamine. Inorg. Chem. 2009, 48, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-Y.; Wang, X.-Y.; Liu, T.; Xu, H.-B.; Zhao, F.; Wang, Z.-M.; Gao, S. Synthesis, Structure, and Magnetism of Three Azido-Bridged Co2+Compounds with a Flexible Coligand 1,2-(Tetrazole-1-yl)ethane. Inorg. Chem. 2008, 47, 8134–8142. [Google Scholar] [CrossRef] [PubMed]
- Bałanda, M. AC Susceptibility Studies of Phase Transitions and Magnetic Relaxation: Conventional, Molecular and Low-Dimensional Magnets. Acta Phys. Pol. A 2013, 124, 964–976. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532–533. [Google Scholar] [CrossRef]
- Eccles, K.S.; Stokes, S.P.; Daly, C.A.; Barry, N.M.; McSweeney, S.P.; O’Neill, D.J.; Kelly, D.M.; Jennings, W.B.; Dhubhghaill, O.M.N.; Moynihan, H.A.; et al. Evaluation of the Bruker SMART X2S: Crystallography for the nonspecialist? J. Appl. Crystallogr. 2010, 44, 213–215. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef]
| Compounds | [Mn(N3)2(dmbpy)] | [Co(N3)2(dmbpy)] |
|---|---|---|
| M–N1 | 2.272(3) | 2.115(5) |
| M–N2 | 2.256(4) | 2.133(5) |
| M–N3 | 2.215(4) | 2.144(5) |
| M–N3 | 2.222(3) (a) | 2.198(6) (c) |
| M–N6 | 2.208(3) | 2.140(6) |
| M–N8 | 2.263(3) (b) | 2.204(6) (d) |
| N1–M–N2 | 72.26(11) | 76.7(2) |
| N3–M–N6 | 99.50(15) | 95.2(2) |
| N1–M–N3 | 95.59(12) (a) | 94.1(2) |
| N1–M–N6 | 162.97(15) | 170.6(2) |
| N2–M–N6 | 91.83(14) | 94.3(2) |
| N3–M–N3 | 79.08(12) (a) | 78.6(2) (c) |
| N6–M–N8 | 90.56(12) (b) | 88.8(2) (d) |
| N2–M–N3 | 168.67(12) | 167.5(2) |
| Compound/Deposition in CCDC | 1/1954571 | 2/1954572 |
|---|---|---|
| Formula | C12H12MnN8 | C12H12CoN8 |
| Fw | 323.24 | 327.23 |
| Crystal size (mm3) | 0.5 × 0.3 × 0.2 | 0.30 × 0.30 × 0.18 |
| Space group | P-1 | P21/c |
| a (Å) | 7.8058(18) | 7.397(2) |
| b (Å) | 9.538(3) | 18.373(4) |
| c (Å) | 10.601(3) | 10.661(3) |
| α (°) | 113.630(8) | - |
| β (°) | 102.610(8) | 110.571(9) |
| γ (°) | 93.223(8) | - |
| V (Å3) | 696.4(3) | 1356.5(6) |
| Z, Z’ | 2, 1 | 4, 1 |
| Diffractometer | Bruker X2S | Bruker X2S |
| Radiation | Mo-Kα | Mo-Kα |
| T (K) | 200 | 200 |
| Abs. coef. (mm−1) | 0.954 | 1.272 |
| Transmission fact. | 0.56–0.83 | 0.51–0.80 |
| Refl. collected | 6450 | 8922 |
| Sinθ/λ (Å−1) | 0.62 | 0.61 |
| Rint (%) | 4.26 | 8.68 |
| Completeness (%) | 97.4 | 99.2 |
| Data/parameters | 2658/192 | 2575/194 |
| Restraints | 0 | 0 |
| R1, wR2 [I > 2σ(I)] | 5.87, 16.64 | 6.53, 16.48 |
| R1, wR2 [all data] | 7.07, 17.60 | 8.26, 17.88 |
| GOF on F2 | 1.035 | 1.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benamara, N.; Setifi, Z.; Yang, C.-I.; Bernès, S.; Geiger, D.K.; Kürkçüoğlu, G.S.; Setifi, F.; Reedijk, J. Coexistence of Spin Canting and Metamagnetism in a One-Dimensional Mn(II) Compound Bridged by Alternating Double End-to-End and Double End-On Azido Ligands and the Analog Co(II) Compound. Magnetochemistry 2021, 7, 50. https://doi.org/10.3390/magnetochemistry7040050
Benamara N, Setifi Z, Yang C-I, Bernès S, Geiger DK, Kürkçüoğlu GS, Setifi F, Reedijk J. Coexistence of Spin Canting and Metamagnetism in a One-Dimensional Mn(II) Compound Bridged by Alternating Double End-to-End and Double End-On Azido Ligands and the Analog Co(II) Compound. Magnetochemistry. 2021; 7(4):50. https://doi.org/10.3390/magnetochemistry7040050
Chicago/Turabian StyleBenamara, Nesrine, Zouaoui Setifi, Chen-I Yang, Sylvain Bernès, David K. Geiger, Güneş Süheyla Kürkçüoğlu, Fatima Setifi, and Jan Reedijk. 2021. "Coexistence of Spin Canting and Metamagnetism in a One-Dimensional Mn(II) Compound Bridged by Alternating Double End-to-End and Double End-On Azido Ligands and the Analog Co(II) Compound" Magnetochemistry 7, no. 4: 50. https://doi.org/10.3390/magnetochemistry7040050
APA StyleBenamara, N., Setifi, Z., Yang, C.-I., Bernès, S., Geiger, D. K., Kürkçüoğlu, G. S., Setifi, F., & Reedijk, J. (2021). Coexistence of Spin Canting and Metamagnetism in a One-Dimensional Mn(II) Compound Bridged by Alternating Double End-to-End and Double End-On Azido Ligands and the Analog Co(II) Compound. Magnetochemistry, 7(4), 50. https://doi.org/10.3390/magnetochemistry7040050