New Radical Cation Salts Based on BDH-TTP Donor: Two Stable Molecular Metals with a Magnetic [ReF6]2− Anion and a Semiconductor with a [ReO4]− Anion
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Crystal Structure
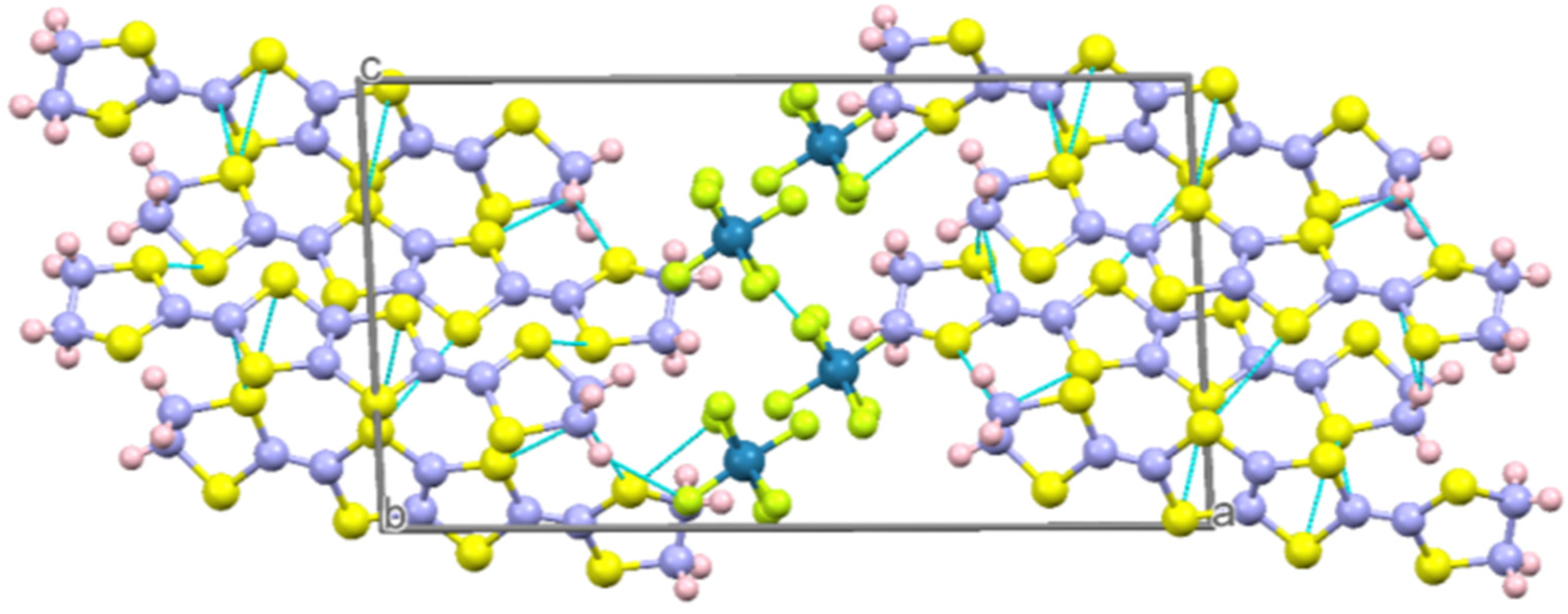
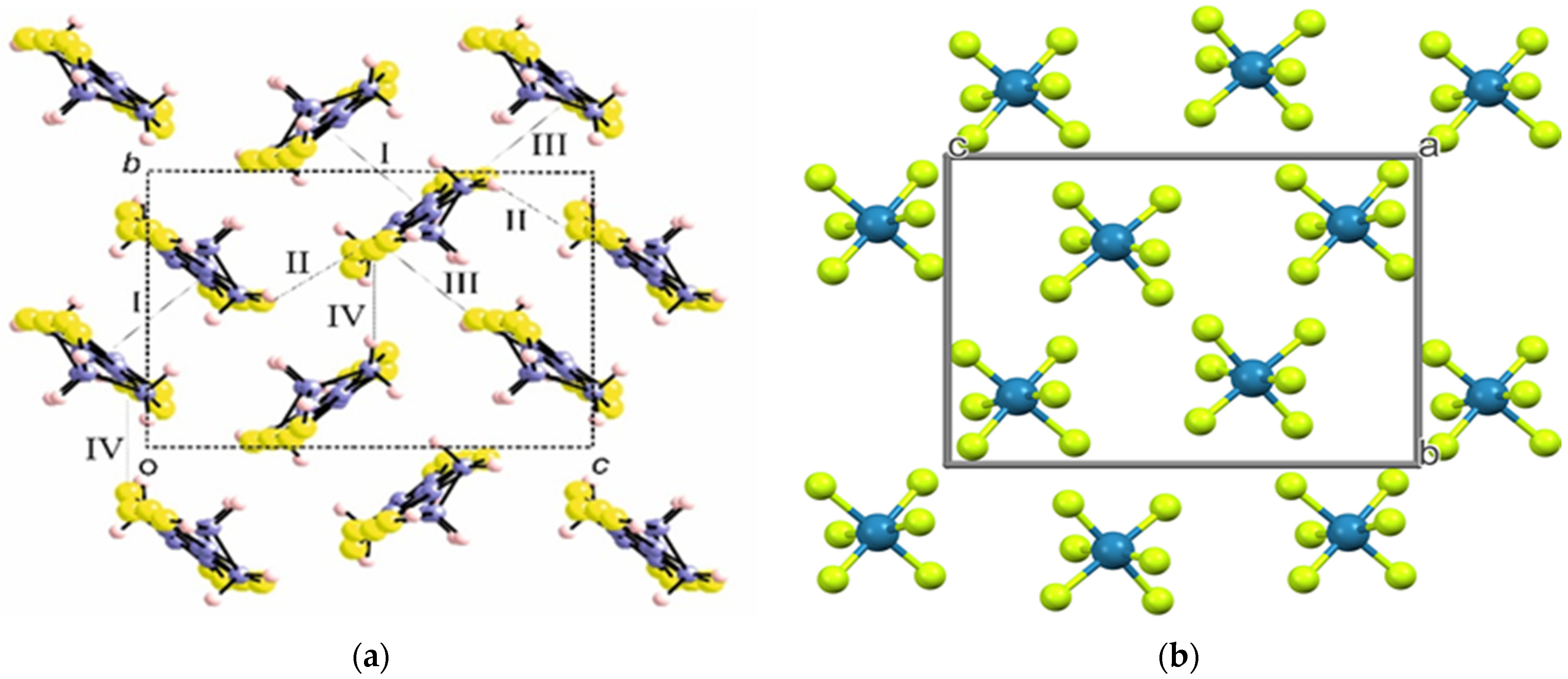
2.2.1. Crystal Structure of the Salts κ-(BDH-TTP)4ReF6 (1) and κ-(BDH-TTP)4ReF6∙4.8H2O (2)
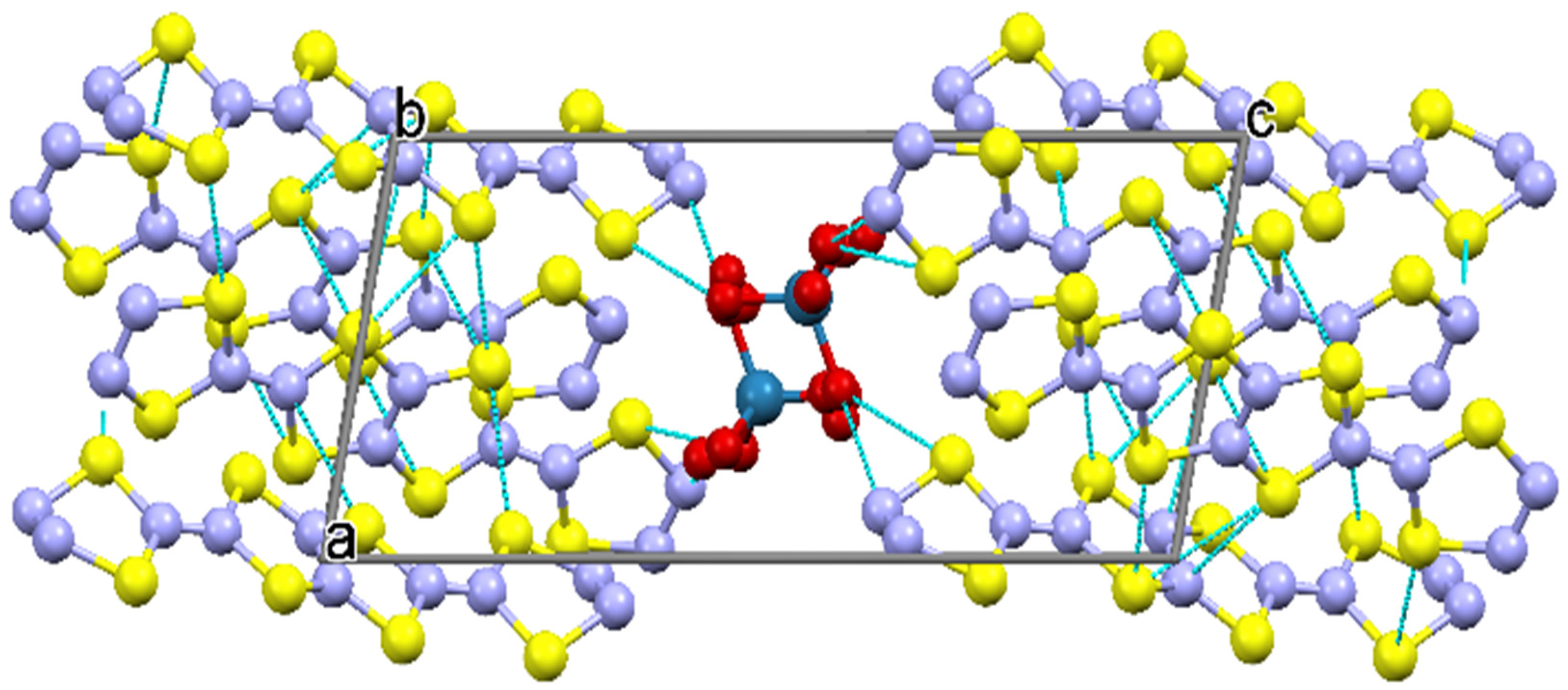
2.2.2. Crystal Structure of the Pseudo-κ″-(BDH-TTP)3(ReO4)2 (3) Salt
2.3. Electronic Structure of the BDH-TTP Salts
2.3.1. Electronic Structure of κ-(BDH-TTP)4ReF6 (1) and κ-(BDH-TTP)4ReF6∙4.8H2O (2)
2.3.2. Electronic Structure of the Pseudo-κ″-(BDH-TTP)3(ReO4)2 (3) Salt
2.4. Conductivity and Magnetic Properties
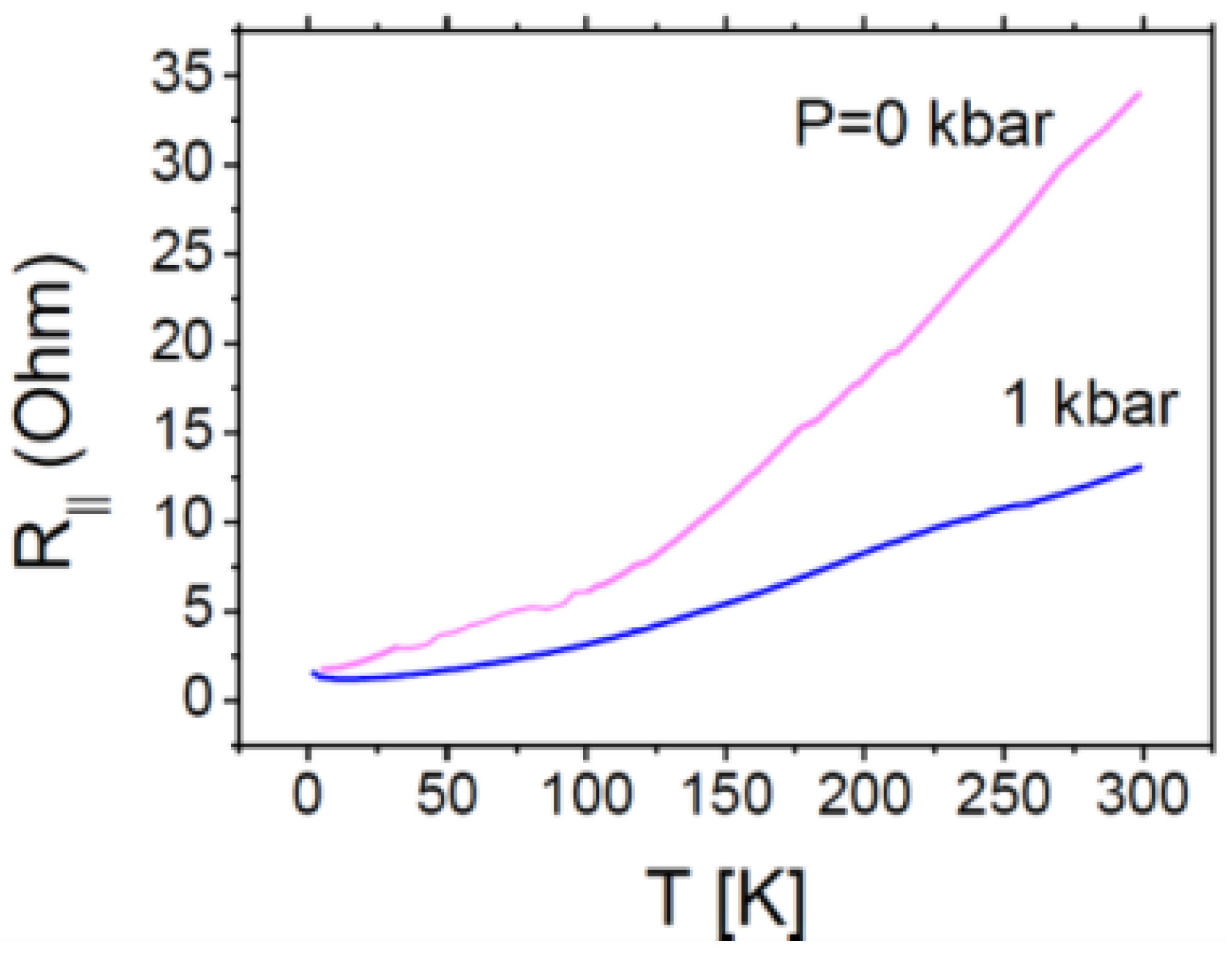
2.4.1. Conducting Properties of the κ-(BDH-TTP)4ReF6 (1) and κ-(BDH-TTP)4ReF6∙4.8H2O (2) Crystals
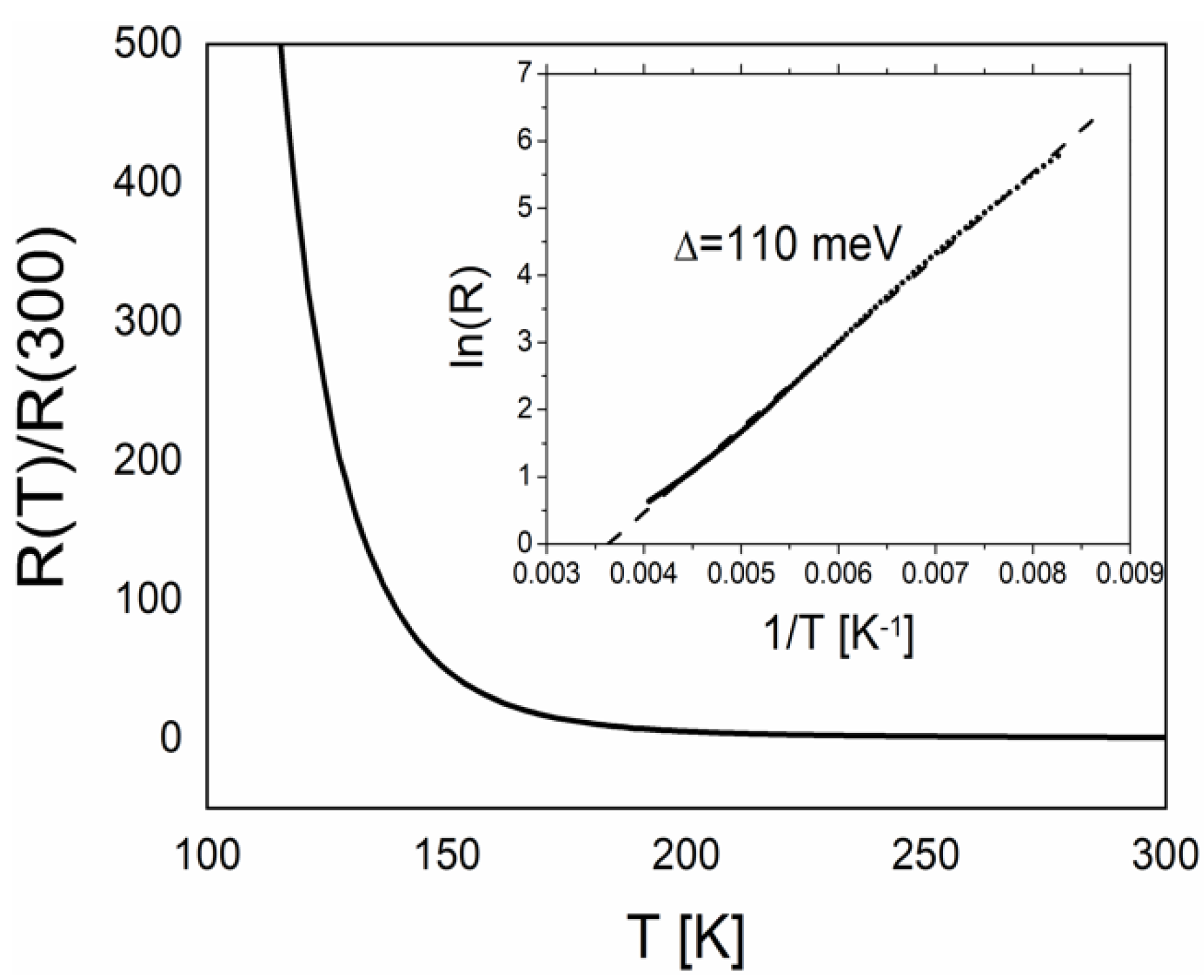
2.4.2. Conductivity of the Pseudo-κ″-(BDH-TTP)3(ReO4)2 (3) Crystals
2.4.3. ac- Magnetic Properties of the κ-(BDH-TTP)4[ReF6] Salt (1)
3. Materials and Methods
3.1. Synthesis of the Salts
3.1.1. Synthesis of the Crystals κ-(BDH-TTP)4ReF6 (1)
3.1.2. Synthesis of the Crystals κ-(BDH-TTP)4ReF6∙4.8H2O (2) and Pseudo-κ″-(BDH-TTP)3(ReO4)2 (3)
3.2. Electron-Probe X-ray Microanalysis
3.3. Single Crystal X-ray Analysis
3.4. Band Structure Analysis
3.5. Conducting Properties
3.6. Magnetic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ouahab, L. Multifunctional Molecular Materials, 1st ed.; Pan Stanford Publishing Pte. Ltd.: Singapore, 2013. [Google Scholar]
- Coronado, E.; Day, P. Magnetic Molecular Conductors. Chem. Rev. 2004, 104, 5419–5449. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Cui, H.; Kobayashi, A. Organic Metals and Superconductors Based on BETS (BETS = Bis(ethylenedithio)-tetraselenafulvalene. Chem. Rev. 2004, 104, 5265–5288. [Google Scholar] [CrossRef] [PubMed]
- Kushch, N.D.; Yagubskii, E.B.; Kartsovnik, M.V.; Buravov, L.I.; Dubrovskii, A.D.; Chekhlov, A.N.; Biberacher, W. π-donor BETS Based Bifunctional Superconductor with Polymeric Dicyanamidomanganate(II) Anion Layer: κ-(BETS)2Mn[N(CN)2]3. J. Am. Chem. Soc. 2008, 130, 7238–7240. [Google Scholar] [CrossRef] [PubMed]
- Prokhorova, T.G.; Yagubskii, E.B. Organic Conductors and Superconductors Based on Bis(ethylenedithio)tetrathia-fulvalene Radical Cation Salts with Supramolecular Tris(oxalato)metallate Anions. Russ. Chem. Rev. 2017, 86, 164–180. [Google Scholar] [CrossRef]
- Cosquer, G.; Shen, Y.; Almeida, M.; Yamashita, M. Conducting Single-molecule Magnet Materials. Dalton Trans. 2018, 47, 7616–7627. [Google Scholar] [CrossRef]
- Gatteschi, D.; Sessoli, R.; Villain, J. Molecular Nanomagnets, Mesoscopic Physics and Nanotechnology; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Milios, C.; Winpenny, R.E.P. Cluster-Based Single-Molecule Magnets. In Structure and Bonding; Gao, S., Ed.; Springer: Berlin/Heildelberg, Germany, 2015; Volume 164, pp. 1–109. [Google Scholar]
- Bogani, L.; Wernsdorfer, W. Molecular Spintronics Using Single-molecule Magnets. Nature Mater. 2008, 7, 179–186. [Google Scholar] [CrossRef]
- Aromi, G.; Aguilà, D.; Gamez, P.; Luis, F.; Roubeau, O. Molecules as Prototypes for Spin-Based CNOT and SWAP Quantum Gates. Chem. Soc. Rev. 2012, 41, 537–546. [Google Scholar]
- Bartolomé, J.; Luis, F.; Fernandez, J. Molecular Magnets–Physics and Applications; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Kubo, K.; Hiraga, H.; Miyasaka, H.; Yamashita, M. Multifunctional Single-molecule Magnets with Electrical Conductivity. In Multifunctional Molecular Materials; Ouahab, L., Ed.; Pan Stanford Publishing Pte. Ltd.: Singapore, 2013; pp. 61–103. [Google Scholar]
- Zhang, X.; Xie, H.; Ballesteros-Rivas, M.; Woods, T.J.; Dunbar, K.R. Conducting Molecular Nanomagnet of Dy(III) with Partially Charged TCNQ Radicals. Chem. Eur. J. 2017, 23, 7448–7452. [Google Scholar] [CrossRef]
- Shen, Y.; Cosquer, G.B.; Breedlove, K.; Yamashita, M. Hybrid Molecular Compound Exhibiting Slow Magnetic Relaxation and Electrical Conductivity. Magnetochemistry 2016, 2, 44. [Google Scholar] [CrossRef]
- Pedersen, K.S.; Sigrist, M.; Sorensen, M.A.; Barra, A.-L.; Weyhermuller, T.; Piligkos, S.; Thuesen, C.A.; Vinum, M.G.; Mutka, H.; Weihe, H.; et al. [ReF6]2−: A Robust Module for the Design of Molecule-Based Magnetic Materials. Angew. Chem. Int. Ed. 2014, 53, 1351–1354. [Google Scholar] [CrossRef]
- Kushch, N.D.; Buravov, L.I.; Kushch, P.P.; Shilov, G.V.; Yamochi, H.; Ishikawa, M.; Otsuka, A.; Shakin, A.; Maximova, O.V.; Volkova, O.S.; et al. The Multifunctional Compound Combining Conductivity and Single-Molecule Magnetism in the Same Temperature Range. Inorg. Chem. 2018, 57, 2386–2389. [Google Scholar] [CrossRef]
- Shen, Y.; Ito, H.; Zhang, H.; Yamochi, H.; Katagiri, S.; Yoshina, S.K.; Otsuka, A.; Ishikawa, M.; Cosquer, G.; Uchida, K.; et al. Simultaneous Manifestations of Metallic Conductivity and Single-Molecule Magnetism in a Layered Molecule-based Compound. Chem. Sci. 2020, 11, 11154–11161. [Google Scholar] [CrossRef]
- Yamada, J.-I.; Akutsu, H.; Nishikawa, H.; Kikuchi, K. New Trends in the Synthesis of π-Electron Donors for Molecular Conductors and Supercondutors. Chem. Rev. 2004, 104, 5057–5083. [Google Scholar] [CrossRef] [PubMed]
- Bardin, A.; Akutsu, H.; Yamada, J.-I. New Family of Six Stable Metals with a Nearly Isotropic Triangular Lattice of Organic Radical Cations and Diluted Paramagnetic System of Anions: κ(κ⊥)-(BDH-TTP)4MX4·Solv, Where M = CoII, MnII.; X = Cl, Br and Solv = (H2O)5, (CH2X). Cryst. Growth Des. 2016, 16, 1228–1246. [Google Scholar] [CrossRef]
- Williams, J.M.; Ferraro, R.J.; Thorn, R.J.; Carlson, K.D.; Geiser, U.; Wang, H.H.; Kini, A.M.; Whangbo, M.-H. Organic Superconductors (Including Fullerenes); Prentice Hall, Englewood Cliffs: New Jersey, NJ, USA, 1992. [Google Scholar]
- Yamada, J.; Watanabe, M.; Anzai, H.; Nishikawa, H.; Ikemoto, I.; Kikuchi, K. BDH-TTP as a Structural Isomer of BEDT-TTF, and Its Two-Dimensional Hexafluorophosphate Salt. Angew. Chem. Int. Ed. 1999, 38, 810–813. [Google Scholar] [CrossRef]
- Kurmoo, M.; Graham, A.W.; Day, P.; Coles, S.J.; Hursthouse, M.B.; Caulfield, J.L.; Singleton, J.; Pratt, F.L.; Hayes, W.; Ducasse, L.; et al. Superconducting and Semiconducting Magnetic Charge Transfer Salts: (BEDT-TTF)4AFe(C2O4)3⋅C6H5CN (A = H2O, K, NH4). J. Am. Chem. Soc. 1995, 49, 12209–12217. [Google Scholar] [CrossRef]
- Whangbo, M.H.; Williams, J.M.; Leung, P.C.W.; Beno, M.A.; Emge, T.J.; Wang, H.H. Role of the Intermolecular Interactions in the Two-Dimensional Ambient-Pressure Organic Superconductors β-(ET)2I3 and β -(ET)2IBr2. Inorg. Chem. 1985, 24, 3500–3502. [Google Scholar] [CrossRef]
- Shevyakova, I.; Buravov, L.; Tkacheva, V.; Zorina, L.; Khasanov, S.; Simonov, S.; Yamada, J.; Canadell, E.; Shibaeva, R.; Yagubskii, E. New Organic Metals Based on BDH-TTP Radical Cation Salts with the Photochromic Nitroprusside Anion [FeNO(CN)5]2-. Adv. Funct. Mater. 2004, 14, 660–668. [Google Scholar] [CrossRef]
- Kushch, N.D.; Kazakova, A.V.; Buravov, L.I.; Yagubskii, E.B.; Simonov, S.V.; Zorina, L.V.; Khasanov, S.S.; Shibaeva, R.P.; Canadell, E.; Son, H.; et al. The First BDH-TTP Radical Cation Salts with Mercuric Counterions, κ-(BDH-TTP)4[Hg(SCN)4]C6H5NO2and κ- (BDH-TTP)6[Hg(SCN)3][Hg(SCN)4] . Synth. Met. 2005, 155, 588–594. [Google Scholar] [CrossRef]
- Agilent. CrysAlis PRO Version171.35.19; Agilent Technologies UK Ltd.: Yarnton, Oxfordshire, UK, 2011. [Google Scholar]
- Sheldrick, G.M. SHELXL-97. Program for Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Whangbo, M.H.; Hoffmann, R. The Band Structure of the Tetracyanoplatinate Chain. J. Am. Chem. Soc. 1978, 100, 6093–6098. [Google Scholar] [CrossRef]
- Ammeter, J.H.; Buergi, H.B.; Thibeault, J.C.; Hoffmann, R. Counterintuitive Orbital Mixing in Semiempirical and ab initio Molecular Orbital Calculations. J. Am. Chem. Soc. 1978, 100, 3686–3692. [Google Scholar] [CrossRef]
- Penicaud, A.; Boubekeur, K.; Batail, P.; Canadell, E.; Auban-Senzier, P.; Jerome, D. Hydrogen-bond Tuning of Macroscopic Transport Properties from the Neutral Molecular Component Site along the Series of Metallic Organic-inorganic Solvates (BEDT-TTF)4Re6Se5Cl9∙[G], [Gt = DMF, THF, dioxane]. J. Am. Chem. Soc. 1993, 115, 4101–4112. [Google Scholar] [CrossRef]
- Buravov, L.I. Calculation of Resistance Anisotropy with Allowance for the Ends of the Sample with the Help of a Conformal Transformation. Sov. Phys. Tech. Phys. 1989, 34, 464–469. [Google Scholar]


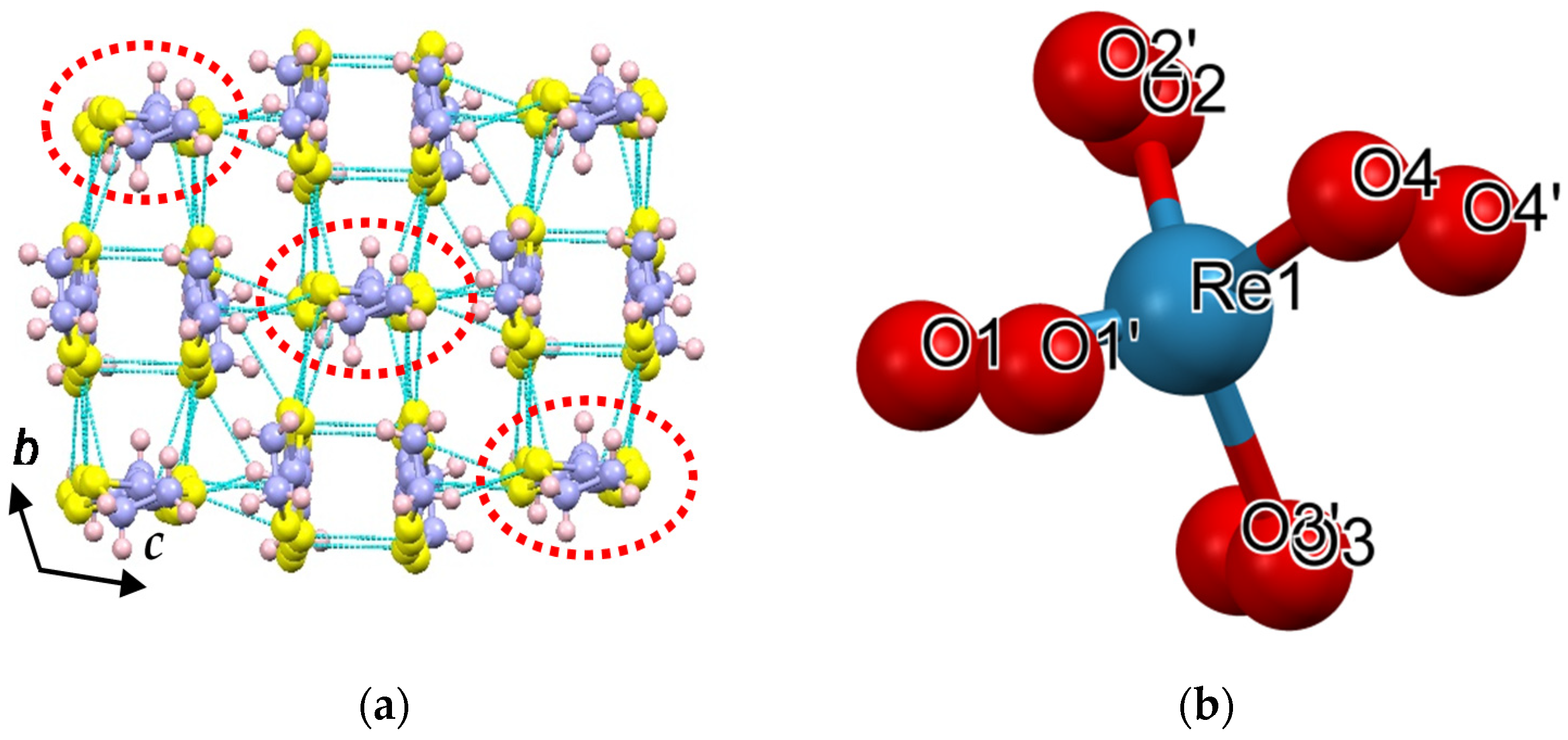

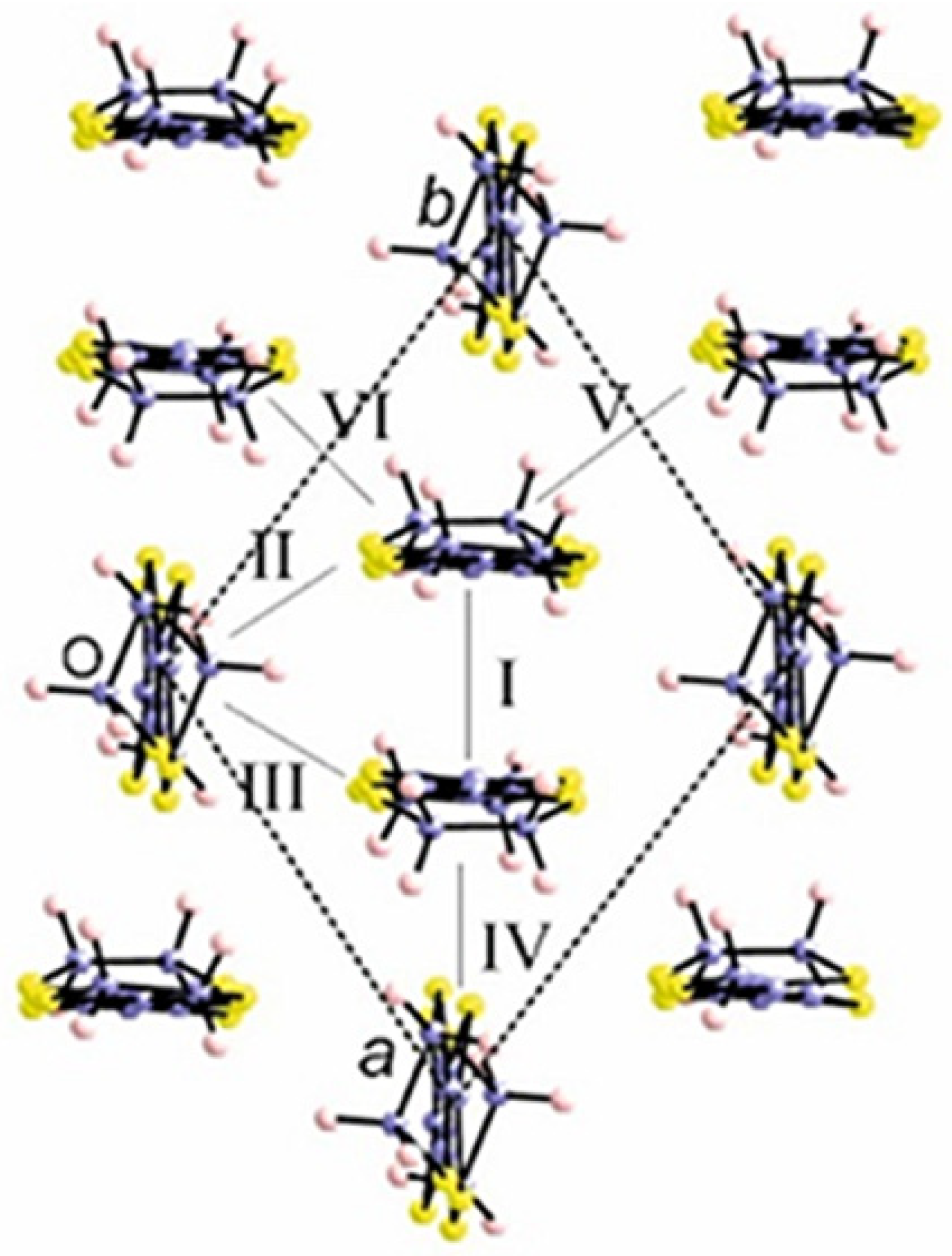
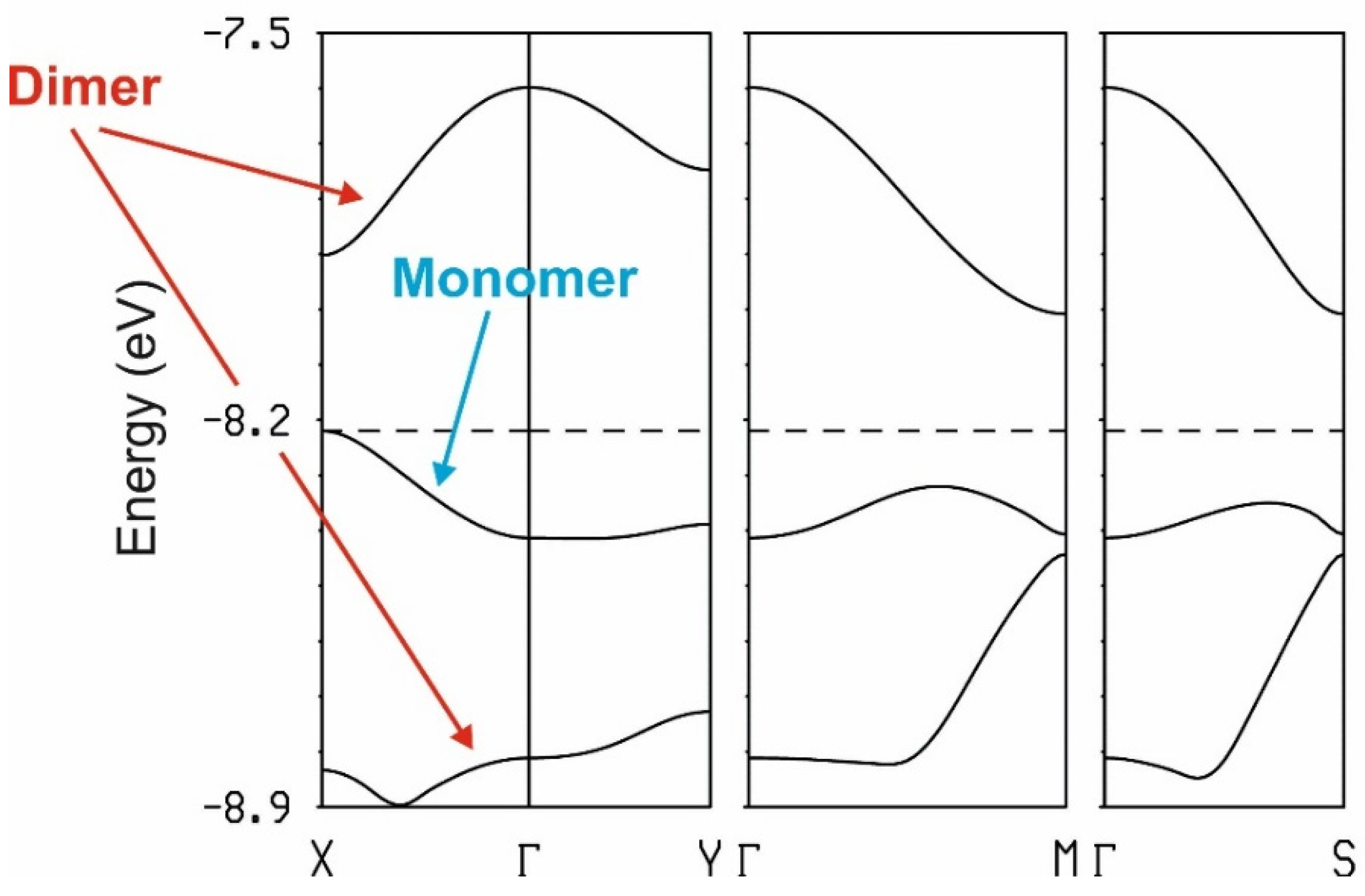
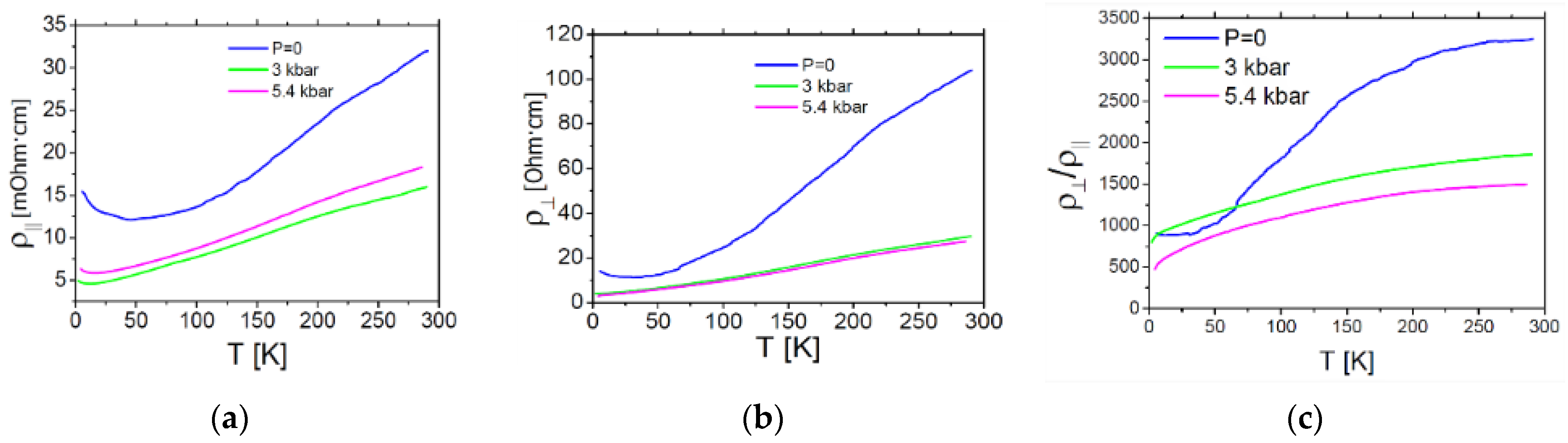
| Short Contact | Contact Length, Å | Symmetry Operation for the 1st Atom in Contact |
|---|---|---|
| S1…S2 | 3.584 | x, y, z |
| S3…S1 | 3.531 | x, y, z |
| S8…S3 | 3.549 | x, y, z |
| S7…S5 | 3.516 | x, y, z |
| S8…C5 | 3.434 | x, y, z |
| S4…H10A | 2.977 | x, y, z |
| S6…H10A | 2.942 | x, y, z |
| S5…H9A | 2.858 | x, y, z |
| C6…H9A | 2.822 | x, y, z |
| Short Contact | Contact Length, Å | Symmetry Operation for the 1st Atom in Contact |
|---|---|---|
| S3…S1 | 3.569 | x, y, z |
| S2…S5 | 3.581 | x, y, z |
| S4…S6 | 3.537 | x, y, z |
| S4…S1 | 3.531 | x, y, z |
| S3…S6 | 3.593 | x, y, z |
| S5…S8 | 3.490 | x, y, z |
| C5…S6 | 3.416 | x, y, z |
| C7H…S2 | 2.057 | x, y, z |
| C7H…S7 | 2.878 | x, y, z |
| S8…H8B | 2.957 | x, y, z |
| C6…H8B | 2.782 | x, y, z |
| Short Cotact | Length, Å | Sym. Operation for the 1st Atom | Short Contact | Length, Å | Sym. Operation for the 1st Atom |
|---|---|---|---|---|---|
| O3…S10 | 3.045 | x, y, z | S12… 3 | 3.498 | x, y, z |
| O4…S5 | 3.045 | x, y, z | S12…S2 | 3.260 | x, y, z |
| O1…C5 | 3.126 | x, y, z | S10…S4 | 3.579 | x, y, z |
| O3…C14 | 3.160 | x, y, z | C15…S3 | 3.466 | x, y, z |
| O3…C6 | 3.204 | x, y, z | S9…S6 | 3.482 | x, y, z |
| O4…C10 | 3.160 | x, y, z | S9…C7 | 3.296 | x, y, z |
| O3…C6 | 3.204 | x, y, z | S6…S2 | 3.423 | x, y, z |
| O2…C6 | 3.059 | x, y, z | S11…S7 | 3.244 | x, y, z |
| O2…H6A | 2.246 | x, y, z | S11…S6 | 3.531 | x, y, z |
| O1…H6A | 2.707 | x, y, z | C15…S7 | 3.383 | x, y, z |
| O4…H14B | 2.533 | x, y, z | S9…S8 | 3.457 | x, y, z |
| O2…H13A | 2.496 | x, y, z | S3…S7 | 3.466 | x, y, z |
| O1…H5B | 2.559 | x, y, z | S10…H6B | 2.943 | −1 + x, 1 + y, z |
| O1…H9A | 2.612 | x, y, z | S4…H6B | 2.873 | x, y, z |
| C=C Bond in the Dimer Radical Cation | Length of C=C Bond, Å | C=C Bond in the Single Radical Cation | Length of C=C Bond, Å |
|---|---|---|---|
| central C1=C2 | 1.374 | central C15=C15 | 1.362 |
| terminal C3=C4 | 1.358 | terminal C11=C12 | 1.345 |
| terminal C7=C8 | 1.365 | terminal C11=C12 | 1.345 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kushch, N.D.; Shilov, G.V.; Buravov, L.I.; Yagubskii, E.B.; Zverev, V.N.; Canadell, E.; Yamada, J.-i. New Radical Cation Salts Based on BDH-TTP Donor: Two Stable Molecular Metals with a Magnetic [ReF6]2− Anion and a Semiconductor with a [ReO4]− Anion. Magnetochemistry 2021, 7, 54. https://doi.org/10.3390/magnetochemistry7040054
Kushch ND, Shilov GV, Buravov LI, Yagubskii EB, Zverev VN, Canadell E, Yamada J-i. New Radical Cation Salts Based on BDH-TTP Donor: Two Stable Molecular Metals with a Magnetic [ReF6]2− Anion and a Semiconductor with a [ReO4]− Anion. Magnetochemistry. 2021; 7(4):54. https://doi.org/10.3390/magnetochemistry7040054
Chicago/Turabian StyleKushch, Nataliya D., Gennady V. Shilov, Lev I. Buravov, Eduard B. Yagubskii, Vladimir N. Zverev, Enric Canadell, and Jun-ichi Yamada. 2021. "New Radical Cation Salts Based on BDH-TTP Donor: Two Stable Molecular Metals with a Magnetic [ReF6]2− Anion and a Semiconductor with a [ReO4]− Anion" Magnetochemistry 7, no. 4: 54. https://doi.org/10.3390/magnetochemistry7040054
APA StyleKushch, N. D., Shilov, G. V., Buravov, L. I., Yagubskii, E. B., Zverev, V. N., Canadell, E., & Yamada, J.-i. (2021). New Radical Cation Salts Based on BDH-TTP Donor: Two Stable Molecular Metals with a Magnetic [ReF6]2− Anion and a Semiconductor with a [ReO4]− Anion. Magnetochemistry, 7(4), 54. https://doi.org/10.3390/magnetochemistry7040054






