Abstract
Despite their high potential, most of the clinically approved iron oxide (IO)-based contrast agents for magnetic resonance imaging (MRI) have been withdrawn from the market either due to safety issues or lack of sales. To address this challenge, erythrocyte membranes have been used to prepare IO-based T2 contrast agents with superior MRI properties and higher safety margin. A simple formulation procedure has been proposed, and the nanostructures’ morphology and physicochemical properties have been evaluated. We compared their performance in terms of contrast ability in MRI to the more clinically established magneto-liposomes and non-encapsulated nanoparticles (NPs). The encapsulation of 5-nm iron oxide nanoparticles (IO NPs) in the liposomes and erythrocyte membrane vesicles (EMVs) led to a significant improvement in their r2 relaxivity. r2 values increased to r2 = 188 ± 2 mM−1s−1 for magneto-liposomes and r2 = 269 ± 3 mM−1s−1 for magneto-erythrocyte membranes, compared to “free” IO NPs with (r2 = 12 ± 1 mM−1 s−1), measured at a 9.4 T MRI scanner. The superiority of magneto-erythrocyte membranes in terms of MRI contrast efficacy is clearly shown on T2-weighted MR images. Our study revealed the hemocompatibility of the developed contrast agents in the MRI-relevant concentration range.
1. Introduction
Magnetic resonance imaging (MRI) is recognized as one of the most important diagnostic tools in medicine because of its excellent soft tissue contrast and the absence of radiation risk [1,2]. Worldwide, more than 20,000 clinical MRI scanners exist, with more than 50 million examinations per year [3]. Contrast agents are applied to enhance the contrast between normal and diseased tissues and therefore improve diagnostics. In clinics, the most commonly used are gadolinium (Gd) chelates [4] (T1 contrast agents) and iron oxide nanoparticles (IO NPs) [5] (T2 contrast agents). Since IO NPs are not associated with the risk of nephrogenic sclerosis, they can serve as a safer contrast agent than open-chain chelated Gd contrast agents [6]. Moreover, IO NPs can better show differences in the microvascular permeability of malignant and benign breast tumors [7]. Up to now, several iron-based MRI contrast agents have been developed [8], but only five have been clinically approved as contrast agents for MRI [4,8]. At the moment, only Resovist® (carboxydextran-coated IO NPs) and Feraheme® (carbohydrate-coated IO NPs) are available [5]. By definition, contrast agents with high relaxivity values can provide an equivalent contrast effect at low concentration. Among the clinically approved IO NP-based contrast agents, the highest relaxivity r2 value is shown by Resovist (189 mM−1 s−1), available only in Japan. Therefore, there is a clear market space and clinical need for the development of novel IO-based MRI contrast agents with a high safety margin and superior MRI contrast efficacy.
The relaxivity r2 of superparamagnetic NPs can be enhanced via the optimization of NPs’ magnetic properties or coating. Several publications report the optimal IO NP composition, size, shape and crystallinity, which are all important for achieving good magnetic properties, namely high saturation magnetization (ms), and thus high r2 values [7,9,10,11]. The proton relaxation occurs mainly at the interface between the magnetic NP and the surrounding aqueous environment, and therefore the NP coating has an important influence on the T1- and T2-relaxation processes [8]. However, this aspect has been largely understudied. We have shown recently that embedding IO NPs in the bilayer of the liposomes can boost their MRI contrast ability compared to “free” IO NPs and demonstrated a selective uptake by cancer cells, while the accumulation in normal cells was minimal [12]. Among different drug delivery systems, liposomes are assumed to be one of the safest developed until now. However, they are built from synthetic phospholipids. Most of the synthetic NPs can be recognized and eliminated as a foreign substance by the immune system. PEGylation of NPs (coating with polyethylene glycol-based molecules) is often applied to decrease the fast elimination by the reticuloendothelial system and thus increase the circulation time. PEG (polyethylene glycol) is widely used in food and drugs, but the development of anti-PEG antibodies, as a consequence of the repetitive exposure to PEG, can lead to the fast elimination of PEG-coated NPs [13]. Furthermore, all clinically approved IO-based MRI contrast agents have dextran-based coatings, which can cause an immune response that involves a dextran reactive antibody reaction [6]. Consequently, different preventive strategies have to be applied to reduce the occurrence of unwanted complications [6]. Therefore, the development of safer coatings is urgently needed.
To achieve this, we propose the use of the body’s own cells as contrast agent carriers. Disguised with cell membranes, therapeutic/diagnostic moieties can act as autogenous cells and thus exhibit inherent biocompatibility. Among different circulatory cells, erythrocytes are the most abundant and can be easily isolated in large quantities to decrease the cost and complexity of the treatment compared to other cell-based therapies [14]. To date, only three articles [15,16,17] reported the encapsulation of IO NPs into erythrocytes. Commercially available (dextran- or carbodextran-coated) IO NPs were encapsulated in erythrocytes [15]. A biodistribution study showed a prolonged circulation time compared to PEG-coated IO NPs and a lower accumulation in the major organs of the reticuloendothelial system (liver and spleen) [16]. Finally, the report from 2018 showed that IO NPs (Resovist) encapsulated into erythrocytes can be detected with MRI in vivo [17]. They produced magneto-erythrocytes with a size of a few μm, which can hinder their accessibility to target sites. Herein, we propose to prepare erythrocyte membrane vesicles (EMVs) in the range of 100–200 nm, which is assumed to be the optimal size range of NPs for systemic delivery [18]. For example, it was demonstrated recently that magneto-liposomes with a size of about 150 nm can efficiently accumulate in tumors in vivo and thus strongly enhance the MRI contrast at the target site [19]. Thus, we postulate that magneto-EMVs in this size range should reach tumor tissues too with even improved tumor accumulation, due to the longer expected circulation time. A simple protocol that can be easily scaled-up is proposed. While research exists into synthetic magneto-liposomes, our nanosystem with biomimetic magneto-EMVs is a complete novelty and represents a huge step forward in the newly evolving field of biomimetic nanomedicine. In parallel, our magneto-EMVs were compared to a more established and clinically approved liposomal system, both containing the same IO NPs in terms of MRI contract efficacy and hemocompatibility.
2. Materials and Methods
2.1. Chemicals
For the synthesis of IO NPs, iron acetylacetonate Fe(acac)3 (>99.9% Sigma-Aldrich, Munich, Germany), benzyl ether (>98%, Merck, Kenilworth, NJ, USA), oleic acid (OA, >99%, Sigma-Aldrich), oleylamine (OLA, 70%, Sigma-Aldrich), 1,2-hexadecanediol (90%, Sigma-Aldrich), hexane (>95%, Sigma Aldrich), chloroform (>99.5%, Sigma Aldrich) and ethanol absolute anhydrous (>99.9%, Carlo Erba Reagents, Barcelona, Spain) were used. For the ligand-exchange reaction and the functionalization of the IO surface with the HCA ligand, the following reagents were used: tetrahydrofuran (THF, anhydrous, >99.9%, Sigma Aldrich), hydrocaffeic acid (HCA, 3-(3,4-Dihydroxyphenyl)propionic acid, >98%, Sigma Aldrich) and NaOH (anhydrous, >98%, Sigma Aldrich). Liposomes were prepared using 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine (DPPC), 1-myristoyl-2-stearoyl-sn-glycero-3-phosphocholine (MSPC) and N-[carbonyl-methoxy(polyethylene glycol)-2000]-1,2-distearoyl-sn-glycero-3-phosphoethanolamine sodium salt (DSPE-PEG2000), which were a kind gift from Lipoid GmbH (Ludwigshafen, Germany). To prepare the EMVs, the following chemicals were used: phosphate-buffered saline buffer (PBS, Sigma Aldrich, tablets), Alsever’s medium (TCS Biosciences Ltd., Buckingham, UK) and NaCl (Sigma Aldrich, >99.5%). For the ICP-MS analysis, nitric acid (65% HNO3), hydrochloric acid (30% HCl) and hydrogen peroxide (30% H2O2) were obtained from Merck Millipore (Milford, MA, USA). A standard solution of Fe (1000 µg Fe/mL in 2–3% HNO3) was obtained from Merck (Darmstadt, Germany). For the determination of the phospholipid content in the samples, a colorimetric phospholipid quantification assay kit (Sigma Aldrich) was used.
2.2. Synthesis of IO NPs
IO NPs were synthesized using thermal decomposition [20]. Oleic acid (OA, 6 mmol), oleylamine (OLA, 6 mmol), Fe(acac)3 (2 mmol) and 1,2-hexadecanediol (10 mmol) were dissolved in 20 mL of benzyl ether under a nitrogen atmosphere. The mixture was magnetically stirred and heated first at 200 °C for 2 h and then at 260 °C for 1 h. Then, the solution was cooled to room temperature and purified by adding ethanol followed by centrifugation (6500 rpm/10 min). This process was repeated three times. The formed NPs were dispersed in CHCl3 and stored at 4 °C.
2.3. Preparation of HCA-Coated IO NPs
Hydrophilic NPs were obtained via a ligand exchange reaction, where oleic acid (OA) and oleylamine (OLA) ligands were replaced with hydrocaffeic acid (HCA). Briefly, hydrophobic IO NPs (20 mg) were dispersed in 1 mL of tetrahydrofuran (THF) [21], and a solution of the ligand was prepared by dissolving 50 mg of HCA in 5 mL of THF. Hydrophobic NPs were added dropwise to the ligand solution, and the mixture was then stirred at 50 °C for 3 h to complete the reaction. Upon cooling the reaction mixture to room temperature, 0.5 mL of 0.5 M NaOH was added to precipitate the NPs, which were collected by centrifugation, redispersed in a PBS buffer and stored at 4 °C.
2.4. Preparation of Magneto-Liposomes
Magneto-liposomes were prepared by a thin-film hydration method followed by extrusion [12]. Briefly, 0.1 mL of hydrophobic IO NPs (1 mg/mL), 8.6 µmol of DPPC (10 mg/mL), 1 µmol of MSPC (5 mg/mL) and 0.4 µmol of DSPE-PEG2000 (10 mg/mL) were dissolved in CHCl3. The organic mixture was transferred to a round bottom flask (25 mL), and then CHCl3 was removed at 40 °C using a rotary evaporator (Rotavapor R-100 equipped with heating bath, Interface I-100 and vacuum pump V-100, Bunchi, Switzerland). Once the lipid film was formed, the pressure in the flask was reduced to 10 mbar for 1 h. The dried lipid film was then hydrated with 2 mL of 1 × PBS (pH 7.4) at 60 °C for 30 min to achieve a final phospholipid concentration of 5 mM. To ensure an efficient hydration, the lipid-film-containing flasks were briefly warmed in a circulating water bath to the desired temperature, followed by the addition of the pre-warmed buffer solution. The liposomes’ size was reduced using the mini-extruder (Avanti Polar lipid, Alabaster, AL, USA). The samples were extruded at 60 °C through 800 nm (5 cycles) and 200 nm (15 cycles) membrane filters (PC membranes, Avanti Polar lipid). Due to the hydrophobicity, non-embedded NPs remained on the membrane filter and were therefore removed during the extrusion process. The liposomes and magneto-liposomes were left to anneal for 2 h at room temperature and stored at 4 °C for further experiments.
2.5. Preparation of Magneto-EMVs
Erythrocytes were isolated from the fresh whole sheep blood supplied by the Veterinary Faculty (University of Ljubljana, Slovenia) in Alsever’s medium and used within two days. Before use, the erythrocytes were washed with a physiological 0.9% NaCl solution and centrifuged (at 600× g for 15 min) and then the supernatant was discarded. Two more NaCl washes were performed before use. A sample containing 3 mL of 20 vol.% of erythrocytes in a PBS buffer was centrifugated (model 5804 Eppendorf) at 8000 rpm for 10 min. The supernatant was removed, and the pellet was redispersed with 2 mL of 0.1 × PBS using an ultrasonicator for 30 s (ultrasonic processor Sonics Vibra cell VC-505, pulses: 1s on, 1s off, 20% amplitude). Then, the sample was incubated at 4 °C for 15 min to release the hemoglobin. The incubation-centrifugation step was repeated until the supernatant was clear and only white pellets of cell membranes remain. The prepared empty EMVs were then redispersed in 2 mL of 1 × PBS. For the preparation of the magneto-EMVs, 25 µL of IO NPs in chloroform (1 mg/mL) were added and sonicated for 150 s (ultrasonic processor Sonics Vibra cell VC-505, pulses: 1 s on, 1 s off, 20% amplitude). With this step, a stable emulsion was formed. Chloroform was removed using a rotary evaporator (10 min at 400 mbar). Particle size was reduced using the mini-extruder. The samples were extruded at room temperature through 800 nm (5 cycles) and 200 nm (15 cycles) membrane filters. For the empty EMVs, i.e., containing no IO NPs, the same extrusion protocol was used, but no NPs were added. The samples were left to anneal for 2 h at room temperature and stored at 4 °C for further experiments.
2.6. Hydrodynamic Diameter and Zeta Potential Measurements
The hydrodynamic diameter, polydispersity index (PDI) and zeta potential (ζ-potential) of empty and IO-containing liposomes and EMVs were determined using a zeta potential and particle size analyzer ZetaPALS (Brookhaven Instruments Corporation, Holtsville, NY, USA) at 25 °C. Disposable polystyrene cells and folded capillary cells (Malvern, UK) were used for dynamic light scattering (DLS) and zeta potential measurements, respectively.
2.7. Magnetic Measurements
For the magnetic characterization, IO NPs were prepared in the form of a dry powder (10 mg). Magnetic measurements were performed with a vibrating-sample magnetometer (VSM, model FCM 10, MicroSense, Lowell, MA, USA) at room temperature.
2.8. Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
The total concentrations of Fe in the analyzed samples were determined using an Agilent 7700 ICP-MS instrument (Agilent Technologies, Tokyo, Japan). A standard solution of Fe was diluted with water for the preparation of fresh calibration standard solutions. For the determination of Fe in aqueous suspensions of IO NPs, magneto-liposomes and magneto-EMVs, 0.1 mL of the initial sample was mixed with 1 mL of nitric acid (65% HNO3, suprapure) and heated in an oil bath at 80 °C for 1 h. After the digestion, all samples were diluted with MilliQ water to a final volume of 10 mL, and appropriately diluted prior to the ICP-MS measurements. The digestion was performed in triplicates. The Fe content in each sample was determined using an Fe standard curve.
2.9. Phospholipid Assay
A phospholipid quantification assay kit (colorimetric detection at 570 nm) was used according to the supplier’s instructions. With this assay, phospholipids are enzymatically hydrolyzed to release choline, which is then determined using choline oxidase and a hydrogen peroxide dye. Liposomes contain only choline-based phospholipids, and therefore a good match between the input chemicals (5 mM, 3.7 mg/mL) and the obtained results is expected. In the case of EMVs, their complex structure has to be considered in the interpretation of the results. In erythrocyte membranes, choline-containing phospholipids represent approx. 55% of the membrane mass (for sheep erythrocytes used in this study) [22], and the rest is cholesterol (approx. 30%), glycolipids (10%), proteins and other moieties [22,23].
2.10. Transmission Electron Microscopy (TEM) and Cryogenic Transmission Electron Microscopy (cryo-TEM)
Samples for the TEM analysis were prepared by adding 10 µL of suspension on a carbon-coated 400 mesh copper grid (Ted Pella, Inc., Redding, CA, USA) and air-dried. Sample grids were examined with a JEOL JEM-2100 operating at an accelerating voltage of 200 kV. The samples for the cryo-TEM were prepared using the Vitrobot Mark IV (Thermo Fischer Scientific, Waltham, MA, USA). Quantifoil® R 2/2, 200 mesh holey carbon grids (Quantifoil Micro Tools GmbH, Großlöbichau, Germany) were glow-discharged for 60 s at 20 mA and positive polarity in an air atmosphere (GloQube® Plus, Quorum, Laughton, UK). Vitrobot conditions were set to 4 °C, 95% relative humidity, Blot time 5.5 s and Blot force 2. 3 µL of the suspension was applied to the grid, blotted and vitrified in liquid ethane. The samples were visualized with a 200 kV Glacios microscope (Thermo Fischer Scientific, Waltham, MA, USA).
2.11. NMR Relaxivity Measurements
An aqueous suspension of HCA-IO NPs, magneto-liposomes and magneto-EMVs was prepared with the following concentrations: 0 (only water), 0.25, 0.5, 1, 2.5 and 5 µg/mL (=0.0045–0.0895 mM) with respect to the Fe content (determined via ICP-MS analysis). Relaxation time measurements of suspensions were carried out using an NMR/MRI system consisting of a high-resolution 9.4-T superconducting magnet (Jastec, Kobe, Hyogo, Japan) and a Redstone NMR spectrometer (Tecmag, Houston, TX, USA). The T1 relaxation times were measured using an inversion-recovery sequence with 14 different inversion times, ranging from 50 µs to 10 s, while the T2 relaxation times were measured using the Carr Purcell Meiboom Gill (CPMG) sequence with multiple spin-echoes. The T1 and T2 relaxation times were calculated from the best fits between the measurements and the corresponding model for either T2 relaxation (exponential dependency of the echo-signal on the echo number) or T1 relaxation (dependency of the inversion recovery signal on the inversion time). The dependencies of the longitudinal (1/T1) and transverse (1/T2) relaxation rates on the Fe concentration in the sample were used to extract the relaxivities r1 and r2. These are defined as proportionality constants between the contrast-agent-induced increase of the corresponding relaxation rate and the MR contrast agent’s concentration.
Here, C denotes the contrast agent’s concentration, while the argument (0) denotes the relaxation rates of pure water (no contrast agent present).
2.12. MRI Phantom Experiments
For the acquisition of images for each sample (HCA-IO NPs, magneto-liposomes or magneto-EMVs), the tubes with suspensions containing all six different Fe concentrations (0–5 µg/mL) were arranged in a rosette-like stack, and then MRI-scanned together in the transverse orientation to the tubes in the spin-echo sequences to obtain T2-weighted images of the stack of tubes. The geometrical and resolution parameters were the following: field of view 20 mm, imaging matrix 256 by 256, slice thickness 2 mm; while the contrast parameters were: TE/TR = 40/3000 ms (echo time/repetition time). MR scanning was performed at a room temperature of 25 °C. Images were analyzed using ImageJ software. For a more clear presentation, the images were aligned in a horizontal arrangement with an increasing concentration of Fe from left to right.
2.13. Hemotoxicity Study
For the hemolysis study, the erythrocytes were isolated from the whole sheep blood, as described in the section for the preparation of EMVs. Control (PBS only), HCA-IO NPs, empty liposomes (0.5 mM phospholipids), magneto-liposomes, empty EMVs (0.5 mM phospholipids) and magneto-EMVs were incubated with 5 vol.% of erythrocytes in PBS (pH 7.4) for 3 h at 37 °C with constant orbital shaking in 1.5 mL tubes (Eppendrof, Germany, volume of samples 1 mL, all samples in triplicates). The concentration of Fe in samples containing IO NPs was 5 or 50 μg/mL. After incubation, the tubes were centrifuged (1500 rpm/4 min) to sediment the cells, and the supernatant was analyzed in triplicates. Hemolysis was valuated by measuring the released hemoglobin absorbance (A) at 541 nm using a spectrophotometer (Synergy H4 Hybrid microplate reader, BioTek, Winooski, VT, USA). Samples representing “100% dead” were prepared by lysing the control samples with deionized water via hypotonic osmotic shock. The Percentage of hemolysis was then calculated as follows: Hemolysis (%) = 100∙(Asample − Acontrol)/(A100% dead − Acontrol).
3. Results and Discussion
3.1. Preparation of Hydrophobic, OA/OLA-Coated IO NPs
The thermal decomposition method was used, as it offers the best control over the particle size and yields monodispersed NPs [16]. The synthesized OA/OLA-coated IO NPs were 4.5 ± 1 nm in diameter, as confirmed by the transmission electron microscopy (TEM) analysis (Figure 1a). The acquired selected area diffraction pattern (SAED) was compared to the calculated diffraction pattern (ICSD 65340), which confirmed the cubic inverse spinel crystal structure of IO NPs (Figure 1a, inset). The superparamagnetic nature of the OA/OLA-coated IO NPs was confirmed with the magnetic measurement (Figure 1b). The saturation magnetization of 32 emu/g is the expected value for 5 nm γ-Fe2O3 NPs [24].
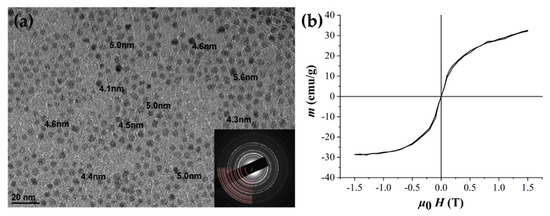
Figure 1.
Characterization of iron oxide nanoparticles (IO NPs). (a) TEM image of oleic acid (OA)/oleylamine (OLA)-coated IO NPs with measured particle size and corresponding selected area diffraction pattern (SAED) pattern indicating an inverse spinel crystal structure (inset, good match between simulated and recorded pattern). (b) Magnetic characterization at 25 °C shows the superparamagnetic nature of NPs.
3.2. Characterization of Magneto-Liposomes and Magneto-EMVs
For the preparation of the magneto-liposomes, IO NPs were encapsulated in the liposomes composed of three different phospholipids with DPPC/MSPC/DSPE-mPEG2000 = 90:10:4 molar ratio, i.e., low temperature-sensitive formulation (ThermoDox®, Celsion Corporation, Lawrenceville, NJ, USA). This formulation is stable at 37 °C and undergoes a gel-to-liquid crystalline phase transition under mild hyperthermia conditions (41–42 °C), resulting in a fast drug release, which is of vital importance in, for example, hyperthermia cancer treatments. Such formulation is of interest for the preparation of so-called theranostic nanocarriers, where imaging and treatment can be done with a single nanostructure. In this article, we explored only the imaging part, including IO NPs for MRI. Liposomes were prepared via the thin-film hydration method [12], with a mixture of phospholipids and hydrophobic NPs in chloroform. For the preparation of empty liposomes, the addition of NPs was omitted. Dry thin film was hydrated using a PBS (phosphate-buffered saline) buffer with pH 7.4. To reduce the particle size and prepare monodisperse samples, magneto-liposomes were extruded through the membranes with pore sizes of 800 and 200 nm.
For the preparation of magneto-EMVs, we proposed a simple formulation procedure that avoids cycles of incubation and centrifugation, which is applied to remove the non-encapsulated particles/molecules (used, for example, for fluorescent dye loading [25] into EMVs) that we found to cause the instability of the final samples. Therefore, our procedure for the preparation of magneto-EMVs omits the use of the centrifugation step, that causes severe aggregation and materials loss. Instead, we applied the oil-in-water micro-emulsion method (IO NPs in chloroform as an oil phase and empty-EMVs in PBS as an aqueous phase) with short sonication. To the best of our knowledge, such approach was used for the first time for the preparation of magneto-EMVs. Briefly, purified EMVs in PBS were mixed with the hydrophobic IO NPs in chloroform, and the magneto-EMVs were formed using the microemulsion method via short sonication (150 s). Chloroform was removed with rotary evaporation, which resulted in a stable suspension. As in the case of liposomes, particle size was reduced using the extrusion step. For the preparation of empty EMVs, the addition of NPs was omitted, and isolated EMVs were extruded using the same extrusion protocol.
The physicochemical properties—i.e., hydrodynamic size, polydispersity index (PDI) and zeta potential as determined using DLS—of the empty liposomes, magneto-liposomes, empty EMVs and magneto-EMVs prepared in this study are shown in Table 1. As expected, the addition of NPs increases the particles’ hydrodynamic size in both cases for about 50–60 nm. The zeta potential close to zero observed with liposomes is due to the PEG group that provides steric stabilization [26]. In the case of EMVs, a negative zeta potential was observed due to the presence of the carboxyl group of sialic acids in the cell membrane [27], anionic phospholipids and anionic membrane proteins [28].

Table 1.
List of samples with corresponding physicochemical properties. Hydrodynamic size, polydispersity index (PDI) and zeta potential as determined using DLS (n = 3). Phospholipid content was determined using a colorimetric phospholipid assay kit (n = 3). Fe content in samples as measured using ICP-MS (n = 3). N/A, not applicable.
The determined phospholipid concentration was 4.5 ± 0.3 mM and 0.5 ± 0.2 mM for empty liposomes and empty EMVs, respectively. The initial total phospholipid concentration used for the preparation of liposomes was 5 mM, and, therefore, a decrease in the final sample can be attributed to the losses during the sample preparation, especially during extrusion, where some material can be lost on the filter membranes. The phospholipid concentration in the empty EMVs was found to be 0.5 ± 0.2 mM, which agrees with the literature, where, for 0.5 mL of erythrocytes, the phospholipid content was determined to be 0.31 ± 0.03 mM [29]. In our case, we used 0.6 mL of erythrocytes (3 mL containing 20 vol.% of erythrocytes) for the preparation of EMVs; therefore, the measured values are accordingly higher. Importantly, the phospholipids represent only approx. 55% of the membrane structure, and therefore the total membrane mass in the sample is higher; the rest are cholesterol (30%), glycolipids (10%) and proteins [22,23]. Furthermore, the ICP-MS analysis showed that the Fe content in the magneto-liposomes was 8.2 times higher (5.32 ± 0.08 μg/mL) than in the magneto-EMVs (0.65 ± 0.05 μg/mL) due to the higher initial IO NPs used (phospholipid content in liposomes was 9 times higher than in the used EMVs). Therefore, the Fe-phospholipid ratio is comparable in both samples, which is of vital importance for the following tests: hemolysis assay and MRI experiments. All samples there were normalized to the same Fe content.
The morphological investigation of the samples was conducted using TEM. Cryo-TEM images of empty liposomes and empty EMVs are shown in Figure 2a,b, respectively. Empty liposomes are unilamellar with a spherical shape [30]. Interestingly, the size distribution is wider (50–200 nm) than expected from the DLS measurements. The empty EMVs consist of intact unilamellar spheres/discs (red arrows) and fragments of membranes. The observed complex nature of the sample is reflected in the large particle size determined with DLS analysis (>300 nm). DLS yields the particles size (hydrodynamic diameter) assuming discrete and noninteracting particles, while TEM sample preparation can induce artefacts; therefore, combining different methods to elucidate the nanoparticles’ size and morphology is crucial. In the case of magneto-liposomes (Figure 2c) and magneto-EMVs (Figure 2d), clusters of IO NPs are coated with approximately 5 nm layer of phospholipids or erythrocyte membranes, respectively, with a size range from 30 nm up to 80 nm for the individual magnetic nanostructure. Again, particle size determined via TEM analysis differs from the DLS values due to the above-mentioned reasons. Hydrophobic NPs are expected to be embedded in the bilayer/membrane and not in the nanocarriers’ core, as was observed in our case. However, for this to occur, the choice of the surface ligands that facilitate the inclusion in the bilayer must be considered along with the NPs size limitation (approx. 5 nm). Our observation can be attributed to the fact that OLA/OA ligands hinder the inclusion of NPs into the bilayer due to the kink in the chain at the site of the double bond, thus forming micelle-like structures with aggregates that are coated with a lipid layer [31] (Figure 2c). On the contrary, when OA/OLA are replaced with a ligand such as nitrodopamine palmitate (no double bond in the chain and higher affinity toward the IO surface, which improves stabilization and maintains the spacing between individual NPs), liposomes with IO NPs, embedded in the bilayer, can be prepared [12,31]. Importantly, the size of the magnetic clusters coated with a lipid bilayer/membrane (Figure 2c,d, respectively) is in both cases comparable, which is crucial for the reliable comparison of MRI contrast efficacy.
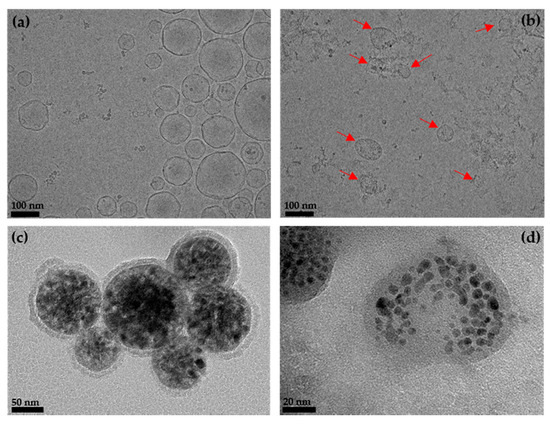
Figure 2.
Cryo-TEM images of (a) empty liposomes and (b) empty erythrocyte membrane vesicles (EMVs) (red arrows indicate unilamellar spheres/discs). TEM images of (c) magneto-liposomes and (d) magneto-EMVs.
3.3. NMR Relaxivity and MRI Measurements
As the synthesized IO NPs were hydrophobic, hydrophilic NPs were prepared via the ligand exchange reaction, where OA and OLA were replaced by hydrocaffeic acid (HCA), using a protocol described in our previous study [21], and used for comparison as a non-phospholipid coating. The successful ligand-exchange reaction is reflected in the highly negative zeta potential (−42 ± 1.1 mV at pH 7) of the HCA-coated IO NPs, which arises from the deprotonated carboxylic group of the HCA ligand [21]. Therefore, a strong electrostatic repulsion between the NPs results in a long-term suspension stability (several months). Importantly, the colloidal stability of the magneto-liposomes and magneto-EMVs after exposure to the magnetic field remains unchanged. On the contrary, in the case of HCA-coated IO NPs, despite the good colloidal stability, NP agglomeration after the relaxivity measurements was observed. This further indicates the suitability of liposomal/membrane coating for MRI. The effectiveness of our magneto-liposomes and magneto-EMVs as an MRI contrast agent was investigated by measuring the dependence of the longitudinal (T1) and transverse (T2) relaxation times on the Fe concentration (0.5–5 µg/mL = 0.009–0.9 mM) in the samples (Figure 3a,b, respectively). Superparamagnetic IO NPs were considered as T2 contrast agents [5], and therefore all samples had a weaker effect on the shortening of the T1 rather than the T2 relaxation time in the entire tested concentration range. Thus, T2 values dropped significantly already at an Fe concentration as low as 0.5 µg/mL = 0.009 mM. The T1 and T2 values were then used to calculate the longitudinal (r1) and transversal relaxivities (r2) (Figure 3c,d, respectively), and are listed in Table 2. The relaxivity values for HCA-coated IO NPs were r1 = 1.16 ± 0.08 mM−1 s−1 and r2 = 12 ± 1 mM−1 s−1. Ultra-small IO NPs usually have a low r2 value due to a low saturation magnetization (Figure 1b). For example, the highest reported r2 for small IO NPs (4–5 nm) ranged from 24 to 44 mM−1 s−1 [32,33]. In comparison, the encapsulation of IO NPs in the liposomes significantly increased the r2 values (r2 = 188 ± 2 mM−1s−1) as a consequence of the enhanced surface hydration and water exchange rate (discussed in detail elsewhere [12,34]), showing that an appropriate coating can boost the MRI contrast efficacy of magnetic NPs [8]. In the previous study [8], we compared different formulations (DOPC, DPPC and DSPC-based ones, with/without cholesterol addition) to HCA-coated IO NPs. Here we focused on a more clinically established formulation (DPPC/MSPC/DSPE-mPEG2000 = 90:10:4 molar ratio), and therefore slightly different r2 values were obtained. However, the erythrocyte membrane coating made this value even higher (r2 = 269 ± 3 mM−1s−1), indicating the superiority of these nanostructures in terms of contrast efficacy. Due to the complex structure of the erythrocyte membrane, it is difficult to define precisely which moieties are responsible for the enhanced surface hydrophilicity and water exchange rate, which are both important for magnetic NP-water proton interactions. We have shown, with the example of the liposomes with different phospholipid compositions [12], that a more fluid bilayer yields higher r2 values. Erythrocyte membrane fluidity is a crucial factor for the red cell functions. In general, the membrane lipid fluidity depends on several factors, such as the type of phospholipids, the level of saturation of the fatty acids, the length of the acyl chains, the cholesterol type (free or esterified) and also the presence/absence of amphipathic compounds such as lysophosphatides [35]. In the case of our liposomes (composition DPPC/MSPC/DSPE-mPEG2000 = 90:10:4 molar ratio), only saturated lipids and lysoslipid were used, which reduced the bilayer fluidity—and consequently the r2 value, compared to, for example, liposomes made of unsaturated phospholipids (DOPC) [12]. In the erythrocyte membranes, unsaturated lipids, glycolipids and proteins importantly influence the membrane fluidity, which facilitates the interactions between magnetic NPs and the surrounding water protons, and therefore a higher r2 value can be observed.
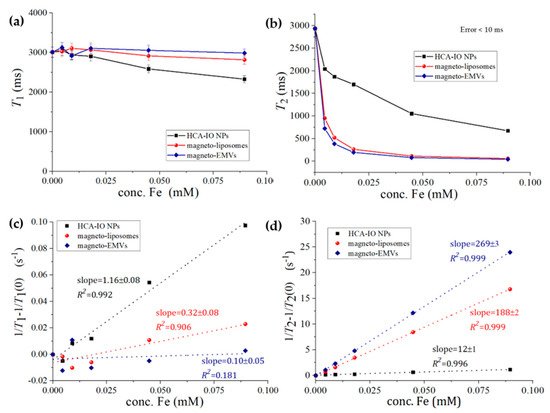
Figure 3.
(a) T1 and (b) T2 relaxation times of hydrophilic HCA-coated IO NPs, magneto-liposomes and magneto-EMVs with the corresponding (c) inverse longitudinal 1/T1–1/T1(0) and (d) inverse transverse 1/T2–1/T2(0) relaxation rate increase, where T1(0) and T2(0) represent the T1 and T1 relaxation times for water containing no particles.

Table 2.
Calculated longitudinal (r1) and transverse (r2) relaxivities of hydrocaffeic acid (HCA)-IO NPs, magneto-liposomes and magneto-EMVs, with the corresponding r2/r1 ratios.
Moreover, r1 was lowered to 0.32 mM−1 s−1 (magneto-liposomes) and to approximately 0.1 mM−1 s−1 or less (magneto-EMVs) compared to HCA-IO NPs (1.16 mM−1 s−1). Since magneto-EMVs have a very weak influence on T1 values in this concentration range, only the approximate r1 value can be provided (due to the fluctuation of T1 values around the value for pure water, data cannot be used for accurate calculation). Consequently, the r2/r1 ratio significantly increased (up to approx. 2690 for magneto-EMVs), which indicates that these magneto-nanostructures are much more efficient T2 MRI contrast agents than the “free” HCA-coated IO NPs (r2/r1 = 10). This may indicate that our magneto-EMVs are a very promising MRI contrast agent (even more than magneto-liposomes). A high r2 value is a prerequisite for the material to be an effective T2 agent, with r2/r1 > 10 [36]. Currently, among the already clinically approved IO-based contrast agents, only Resovist® (60 nm, carboxydextran-coated IO NPs, available only in Japan) and Feraheme® (30 nm, carbohydrate-coated IO NPs, withdrawn from the EU market and available in the US to treat iron-deficiency anemia in adults with chronic kidney disease (off-label use as MRI contrast agent)) are available on the market, with r2 values of 189 and 89 mM−1s−1, respectively [12]. Importantly, despite their large size (30–60 nm, compared to our 5 nm IO NPs), relaxivities are low, which further proves the importance of coating optimization that enables stability, biocompatibility and improved MRI contrast efficacy, as was achieved with our magneto-EMVs. Boni et al. [37] loaded erythrocytes with maghemite (γ-Fe2O3) NPs with an average size of 8.9 ± 2.2 nm via hypotonic dialysis, isotonic resealing and reannealing, and obtained r2 values of 57 mM−1s−1, far below ours. This can be attributed to the fact that they used entire cells as carriers, while we used only erythrocyte membranes to coat IO NPs, which facilitates the interaction between water molecules and magnetic centers and, consequently, improves MRI contrast performance.
To further support these results, T2-weighted MRI images were recorded for all three samples (Figure 4). As expected, magneto-liposomes and even more magneto-EMVs provided a significantly better negative contrast than HCA-coated IO NPs at lower Fe concentrations, proving the superiority of magneto-EMVs as T2 MRI contrast agents.
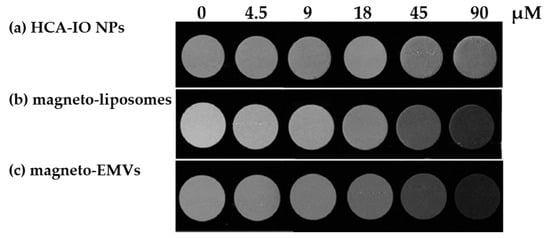
Figure 4.
T2–weighted MRI images of (a) hydrophilic HCA-coated IO NPs, (b) magneto-liposomes and (c) magneto-EMVs, containing different concentrations of Fe (0–90 μM = 0–5 μg/mL).
3.4. Hemotoxicity Study
For the hemolysis study, controls (PBS only), HCA-IO NPs, empty liposomes, magneto-liposomes, empty EMVs and magneto-EMVs were incubated with 5 vol.% of erythrocytes in PBS for 3 h at 37 °C. Based on the MRI experiments, an Fe concentration much lower than 5 μg/mL is required to induce a strong contrast on the T2-weighted image. Therefore, the testing concentration of Fe in IO-containing samples was selected accordingly (5 μg Fe/mL). Additionally, we tested a 10-times higher concentration as well (50 μg Fe/mL) (both determined via ICP-MS analysis). The results shown in Figure 5 revealed that at very high Fe concentration (50 μg Fe/mL), a slight increase in the hemolysis can be observed in all three samples (from 2.4 to 5.8%), while empty carriers or samples containing 5 μg Fe/mL induced no hemotoxicity in the MRI-relevant concentration range. The higher hemotoxicity in magneto-liposomes can be attributed to the presence of a higher phospholipid concentration, and not only to the higher IO NPs (the Fe-phospholipid ratio was kept constant in all cases). This was observed in our previous experiments, where only empty liposomes with different phospholipid concentrations were tested (0.5, 1 and 5 mM), and where, at 5 mM, a hemolysis of about 5% was measured, while at lower phospholipid concentrations the hemolysis was at the same level as that of the control (data not shown). No such trend was observed with empty EMVs (all concentrations were comparable to that of the control), indicating their good hemocompatibility.
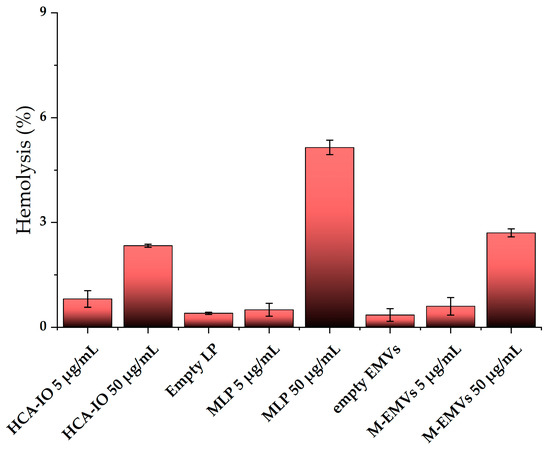
Figure 5.
Hemolysis of red blood cells after 3 h incubation at 3 h at 37° C with samples containing 5 or 50 μg Fe/mL (concentration determined with ICP-MS analysis) (n = 3). Hemolysis values are normalized to the control values (PBS only). LP = liposomes, MLP = magneto-liposomes, M-EMVs = magneto-EMVs.
4. Conclusions
In conclusion, we investigated the encapsulation of IO NPs in erythrocyte membrane vesicles (EMVs) and compared their performance in terms of contrast ability in MRI to the more clinically established magneto-liposomes and non-phospholipid-based coating (HCA-IO NPs). A facile synthesis approach for the preparation of magneto-EMVs has been proposed using short ultrasonication to avoid incubation/high-speed centrifugation cycles, that were found to cause suspension instability and materials loss. The MRI experiments showed that the encapsulation of small IO NPs (5 nm in size) into erythrocyte membrane vesicles significantly improved the r2 values and thus the T2 contrast efficacy, which was demonstrated on the MR images. Finally, the hemotoxicity assay revealed that magneto-EMVs induce negligible hemotoxicity in the MRI-relevant Fe concentration range, which further proves their potential as safer and more efficient MRI contrast agents that the ones currently on the market, and even superior to the lipid formulation that is clinically approved and was used in our study for comparison purposes. However, to fully support this, further detailed in vitro and in vivo studies are warranted. Furthermore, magneto-EMVs (as well as liposomes) could encapsulate a broad spectrum of therapeutics and diagnostics in their aqueous core or bilayer, thus offering a versatile platform for the preparation of multifunctional (theranostic) NPs. The preparation of EMVs involves the use of biological materials. Therefore, strict handling protocols and blood group matching are required to avoid the risk of contamination and immunogenicity. Despite these challenges, cell membrane-based biomimetic nanocarriers are an exciting and unique strategy for the development of safer contrast agents, and represent a new research paradigm.
Author Contributions
Conceptualization, N.K.; methodology, N.K., I.S., M.K. and M.P.; formal analysis, N.K., P.M., M.K. and I.S.; funding acquisition, N.K.; investigation, N.K., P.M., I.S.; project writing—original draft, N.K.; writing—review & editing, N.K., I.S., M.K., M.P. and W.A.-J., administration, N.K.; supervision, N.K.; visualization, N.K. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the Slovenian Research Agency ARRS (project numbers Z2-9218, P2-0084, P1-0060 and P1-0391) and the Engineering and Physical Sciences Research Council (EPSRC) (EP/M008657/1).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shin, T.; Choi, Y.; Kim, S.; Cheon, J. Recent advances in magnetic nanoparticle-based multi-modal imaging. Chem. Soc. Rev. 2015, 44, 4501–4516. [Google Scholar] [CrossRef]
- Slichter, C.P. Principles of Magnetic Resonance; Springer Series in Solid-State Sciences; Springer: Berlin/Heidelberg, Germany, 1990; Volume 1, ISBN 978-3-642-08069-2. [Google Scholar]
- Merbach, A.; Helm, L.; Tóth, É. The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging: Second Edition; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 9781119991762. [Google Scholar]
- Boros, E.; Gale, E.M.; Caravan, P. MR imaging probes: Design and applications. Dalt. Trans. 2015, 44, 4804. [Google Scholar] [CrossRef]
- Wang, Y.-X.J. Superparamagnetic iron oxide based MRI contrast agents: Current status of clinical application. Quant. Imaging Med. Surg. 2011, 1, 35–40. [Google Scholar] [PubMed]
- Daldrup-Link, H.E. Ten things you might not know about iron oxide nanoparticles. Radiology 2017, 284, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Daldrup-Link, H.E.; Rydland, J.; Helbich, T.H.; Bjørnerud, A.; Turetschek, K.; Kvistad, K.A.; Kaindl, E.; Link, T.M.; Staudacher, K.; Shames, D.; et al. Quantification of Breast Tumor Microvascular Permeability with Feruglose-enhanced MR Imaging: Initial Phase II Multicenter Trial. Radiology 2003, 229, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, L.; Chen, H.; Hu, K.; Delahunty, I.; Gao, S.; Xie, J. Surface impact on nanoparticle-based magnetic resonance imaging contrast agents. Theranostics 2018, 8, 2521–2548. [Google Scholar] [CrossRef]
- Avasthi, A.; Caro, C.; Pozo-Torres, E.; Leal, M.P.; García-Martín, M.L. Magnetic Nanoparticles as MRI Contrast Agents. Top. Curr. Chem. 2020, 378, 40. [Google Scholar] [CrossRef]
- Caspani, S.; Magalhães, R.; Araújo, J.P.; Sousa, C.T. Magnetic nanomaterials as contrast agents for MRI. Materials 2020, 13, 2586. [Google Scholar] [CrossRef]
- Rahim, S.; Jan Iftikhar, F.; Malik, M.I. Biomedical applications of magnetic nanoparticles. In Metal Nanoparticles for Drug Delivery and Diagnostic Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 301–328. ISBN 9780128169605. [Google Scholar]
- Kostevšek, N.; Cheung, C.C.L.; Serša, I.; Kreft, M.E.; Monaco, I.; Franchini, M.C.; Vidmar, J.; Al-Jamal, W.T. Magneto-liposomes as MRI contrast agents: A systematic study of different liposomal formulations. Nanomaterials 2020, 10, 889. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; He, Y.; Zhang, S.; Qin, J.; Wang, J. Cell membrane-based nanoparticles: A new biomimetic platform for tumor diagnosis and treatment. Acta Pharm. Sin. B 2018, 8, 14–22. [Google Scholar] [CrossRef]
- Phua, K.K.L.; Boczkowski, D.; Dannull, J.; Pruitt, S.; Leong, K.W.; Nair, S.K. Whole Blood Cells Loaded with Messenger RNA as an Anti-Tumor Vaccine. Adv. Healthc. Mater. 2014, 3, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Sfara, C.; Manuali, E.; Bruce, I.J.; Magnani, M. Encapsulation of superparamagnetic nanoparticles into red blood cells as new carriers of MRI contrast agents. Nanomedicine 2011, 6, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Bu, L.L.; Xu, J.H.; Cai, B.; Yu, G.T.; Yu, X.; He, Z.; Huang, Q.; Li, A.; Guo, S.S.; et al. Red Blood Cell Membrane as a Biomimetic Nanocoating for Prolonged Circulation Time and Reduced Accelerated Blood Clearance. Small 2015, 11, 6225–6236. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Pacifico, S.; Sfara, C.; Tamma, M.; Magnani, M. Ferucarbotran-loaded red blood cells as long circulating MRI contrast agents: First in vivo results in mice. Nanomedicine 2018, 13, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Perrault, S.D.; Walkey, C.; Jennings, T.; Fischer, H.C.; Chan, W.C.W. Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. 2009, 9, 1909–1915. [Google Scholar] [CrossRef]
- Ma, G.; Kostevšek, N.; Monaco, I.; Ruiz, A.; Markelc, B.; Cheung, C.C.L.; Hudoklin, S.; Kreft, M.E.; Hassan, H.A.F.M.; Barker, M.; et al. PD1 blockade potentiates the therapeutic efficacy of photothermally-activated and MRI-guided low temperature-sensitive magnetoliposomes. J. Control. Release 2021, 332, 419–433. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H.; Robinson, D.B.; Raoux, S.; Rice, P.M.; Wang, S.X.; Li, G. Monodisperse MFe2O4 (M = Fe, Co, Mn) nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279. [Google Scholar] [CrossRef]
- Kostevšek, N.; Hudoklin, S.; Kreft, M.E.; Serša, I.; Sepe, A.; Jagličić, Z.; Vidmar, J.; Ščančar, J.; Šturm, S.; Kobe, S.; et al. Magnetic interactions and: In vitro study of biocompatible hydrocaffeic acid-stabilized Fe-Pt clusters as MRI contrast agents. RSC Adv. 2018, 8, 14694–14704. [Google Scholar] [CrossRef]
- Virtanen, J.A.; Cheng, K.H.; Somerharju, P. Phospholipid composition of the mammalian red cell membrane can be rationalized by a superlattice model. Proc. Natl. Acad. Sci. USA 1998, 95, 4964–4969. [Google Scholar] [CrossRef] [PubMed]
- Nelson, G.J. Lipid composition of erythrocytes in various mammalian species. Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab. 1967, 144, 221–232. [Google Scholar] [CrossRef]
- Morales, M.P.; Veintemillas-Verdaguer, S.; Serna, C.J. Magnetic properties of uniform g-Fe2O3 nanoparticles smaller than 5 nm prepared by laser pyrolysis. J. Mater. Res. 1999, 14, 3066–3072. [Google Scholar] [CrossRef]
- Bahmani, B.; Bacon, D.; Anvari, B. Erythrocyte-derived photo-theranostic agents: Hybrid nano-vesicles containing indocyanine green for near infrared imaging and therapeutic applications. Sci. Rep. 2013, 3, 2180. [Google Scholar] [CrossRef]
- Nakamura, K.; Yamashita, K.; Itoh, Y.; Yoshino, K.; Nozawa, S.; Kasukawa, H. Comparative studies of polyethylene glycol-modified liposomes prepared using different PEG-modification methods. Biochim. Biophys. Acta Biomembr. 2012, 1818, 2801–2807. [Google Scholar] [CrossRef]
- Fernandes, H.P.; Cesar, C.L.; Barjas-Castro, M. de L. Electrical properties of the red blood cell membrane and immunohematological investigation. Rev. Bras. Hematol. Hemoter. 2011, 33, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.C.; Wu, H.C.; Hoang, D.; Bentley, W.E.; D’Souza, W.D.; Raghavan, S.R. Colloidal Properties of Nanoerythrosomes Derived from Bovine Red Blood Cells. Langmuir 2016, 32, 171–179. [Google Scholar] [CrossRef]
- Chhabria, V. Development of Nanosponges from Erythrocyte Ghosts for Removal of Streptolysin-O and α Haemolysin from Mammalian Blood. Ph.D. Thesis, University of Central Lancashire, Preston, UK, 2017. [Google Scholar]
- Almgren, M.; Edwards, K.; Karlsson, G. Cryo transmission electron microscopy of liposomes and related structures. Colloids Surf. A Physicochem. Eng. Asp. 2000, 174, 3–21. [Google Scholar] [CrossRef]
- Amstad, E.; Kohlbrecher, J.; Müller, E.; Schweizer, T.; Textor, M.; Reimhult, E. Triggered release from liposomes through magnetic actuation of iron oxide nanoparticle containing membranes. Nano Lett. 2011, 11, 1664–1670. [Google Scholar] [CrossRef]
- Li, J.; Shi, X.; Shen, M. Hydrothermal Synthesis and Functionalization of Iron Oxide Nanoparticles for MR Imaging Applications. Part. Part. Syst. Charact. 2014, 31, 1223–1237. [Google Scholar] [CrossRef]
- Li, Z.; Yi, P.W.; Sun, Q.; Lei, H.; Li Zhao, H.; Zhu, Z.H.; Smith, S.C.; Lan, M.B.; Lu, G.Q. Ultrasmall water-soluble and biocompatible magnetic iron oxide nanoparticles as positive and negative dual contrast agents. Adv. Funct. Mater. 2012, 22, 2387–2393. [Google Scholar] [CrossRef]
- Kostevšek, N. A Review on the Optimal Design of Magnetic Nanoparticle-Based T2 MRI Contrast Agents. Magnetochemistry 2020, 6, 11. [Google Scholar] [CrossRef]
- De Oliveira, S.; Saldanha, C. An overview about erythrocyte membrane. Clin. Hemorheol. Microcirc. 2010, 44, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Caravan, P.; Ellison, J.J.; McMurry, T.J.; Lauffer, R.B. Gadolinium(III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications. Chem. Rev. 1999, 99, 2293–2352. [Google Scholar] [CrossRef] [PubMed]
- Boni, A.; Ceratti, D.; Antonelli, A.; Sfara, C.; Magnani, M.; Manuali, E.; Salamida, S.; Gozzi, A.; Bifone, A. USPIO-loaded red blood cells as a biomimetic MR contrast agent: A relaxometric study. Contrast Media Mol. Imaging 2014, 9, 229–236. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).