Copper(II) Carboxylates with 2,3,4-Trimethoxybenzoate and 2,4,6-Trimethoxybenzoate: Dinuclear Cu(II) Cluster and µ-Aqua-Bridged Cu(II) Chain Molecule
Abstract
1. Introduction
2. Results and Discussion
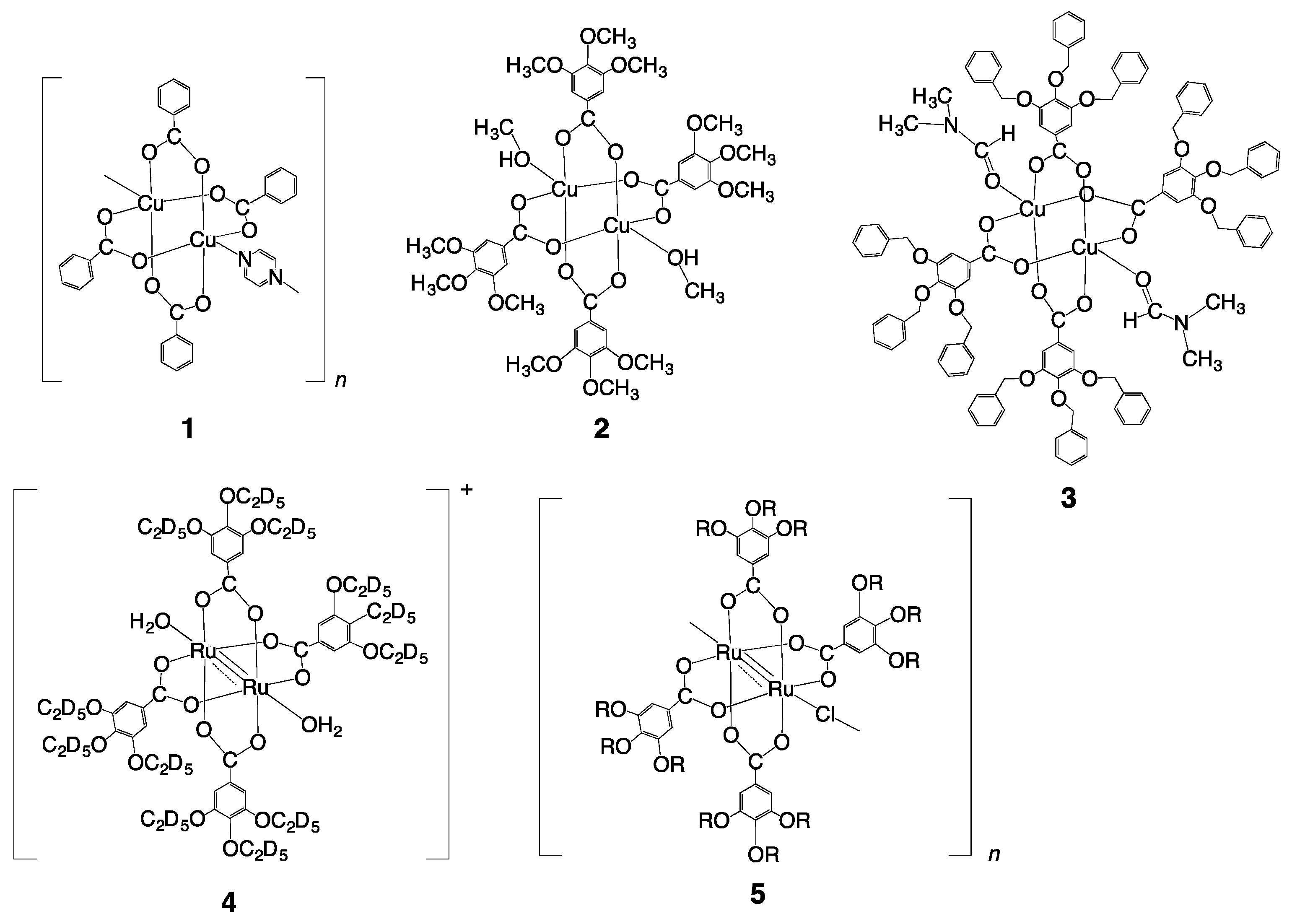
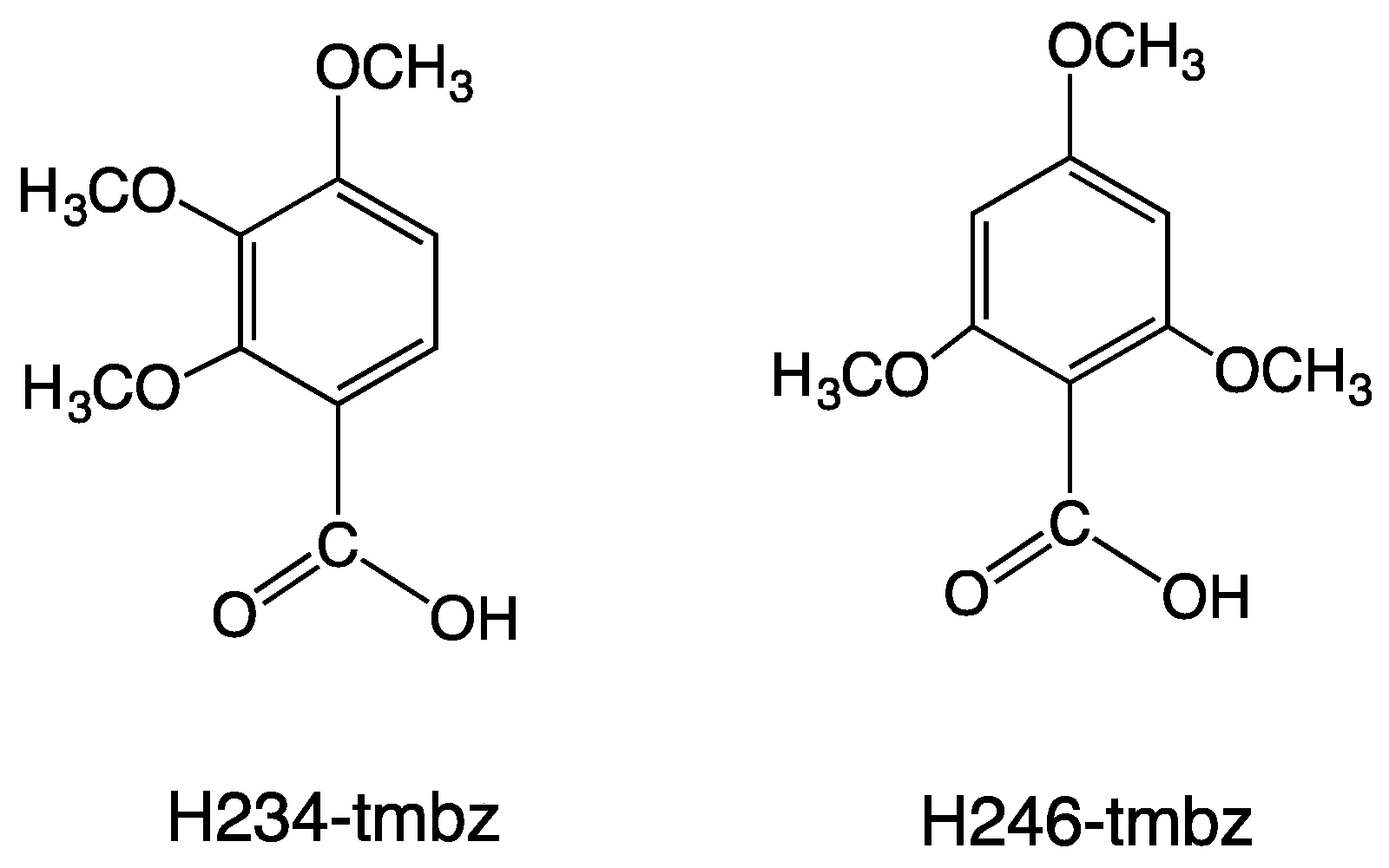
2.1. Synthesis of Copper(II) Carboxylates
2.2. Infrared Spectra of Copper(II) Carboxylates
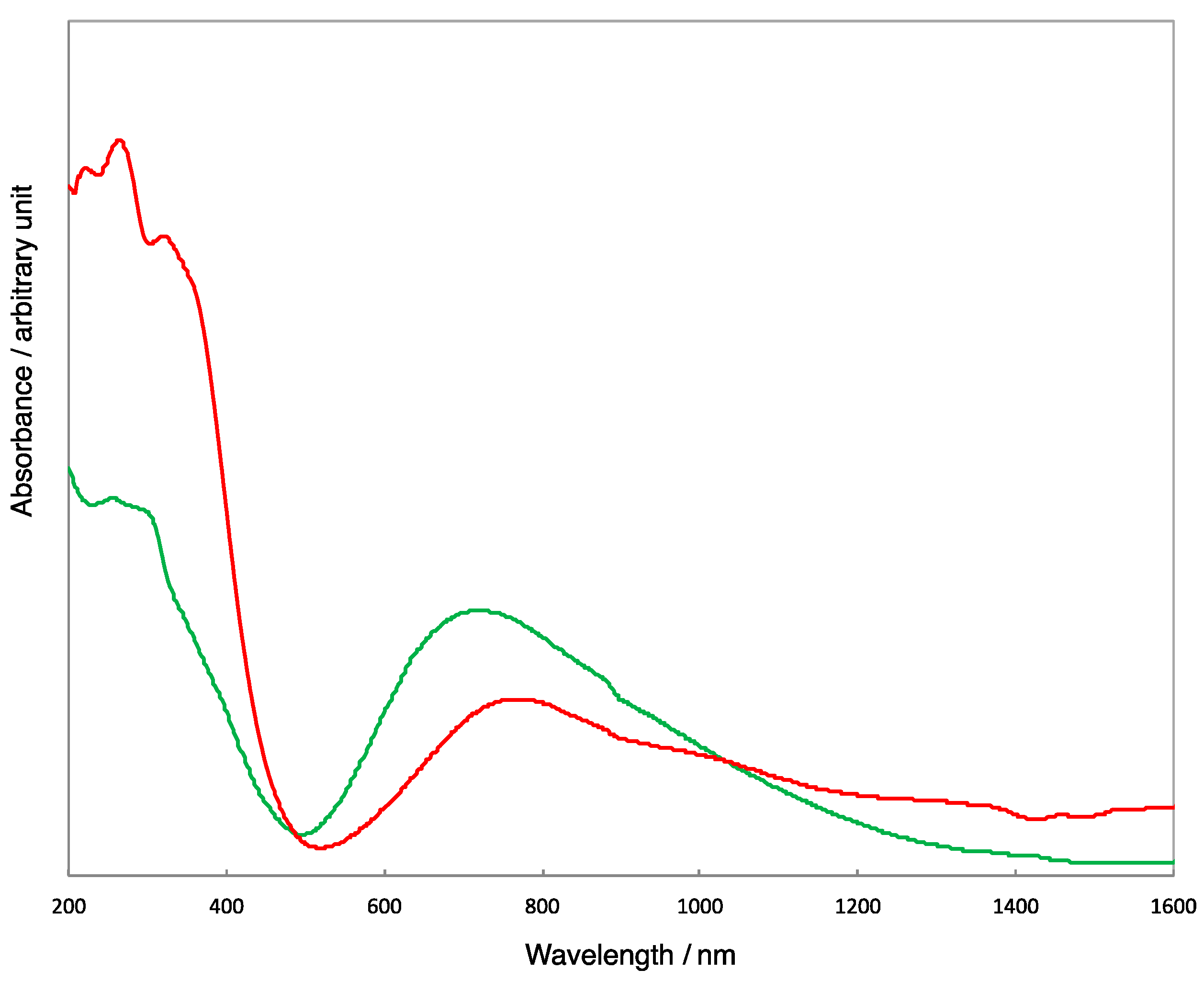
2.3. Electronic Spectra of Copper(II) Carboxylates
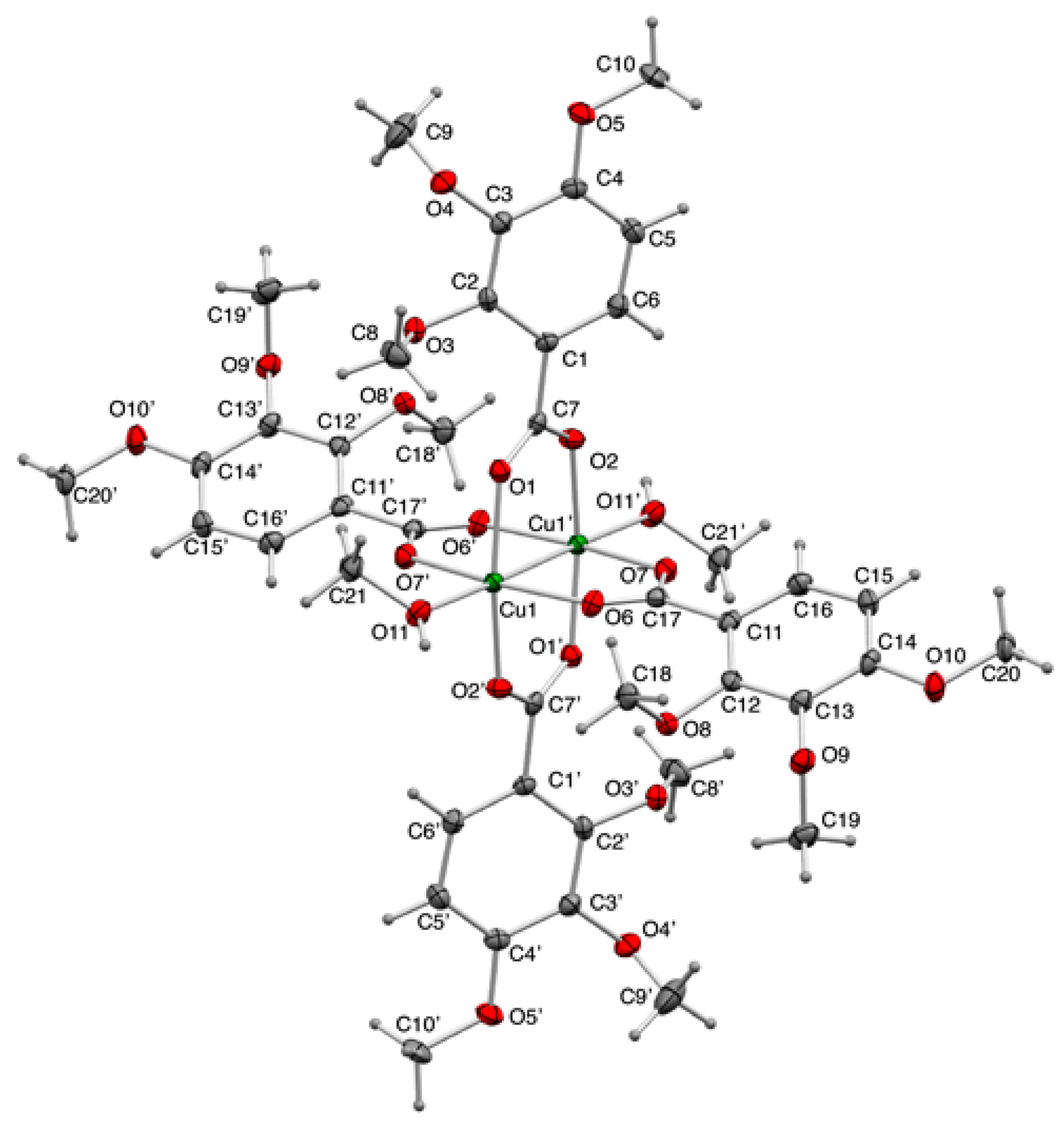
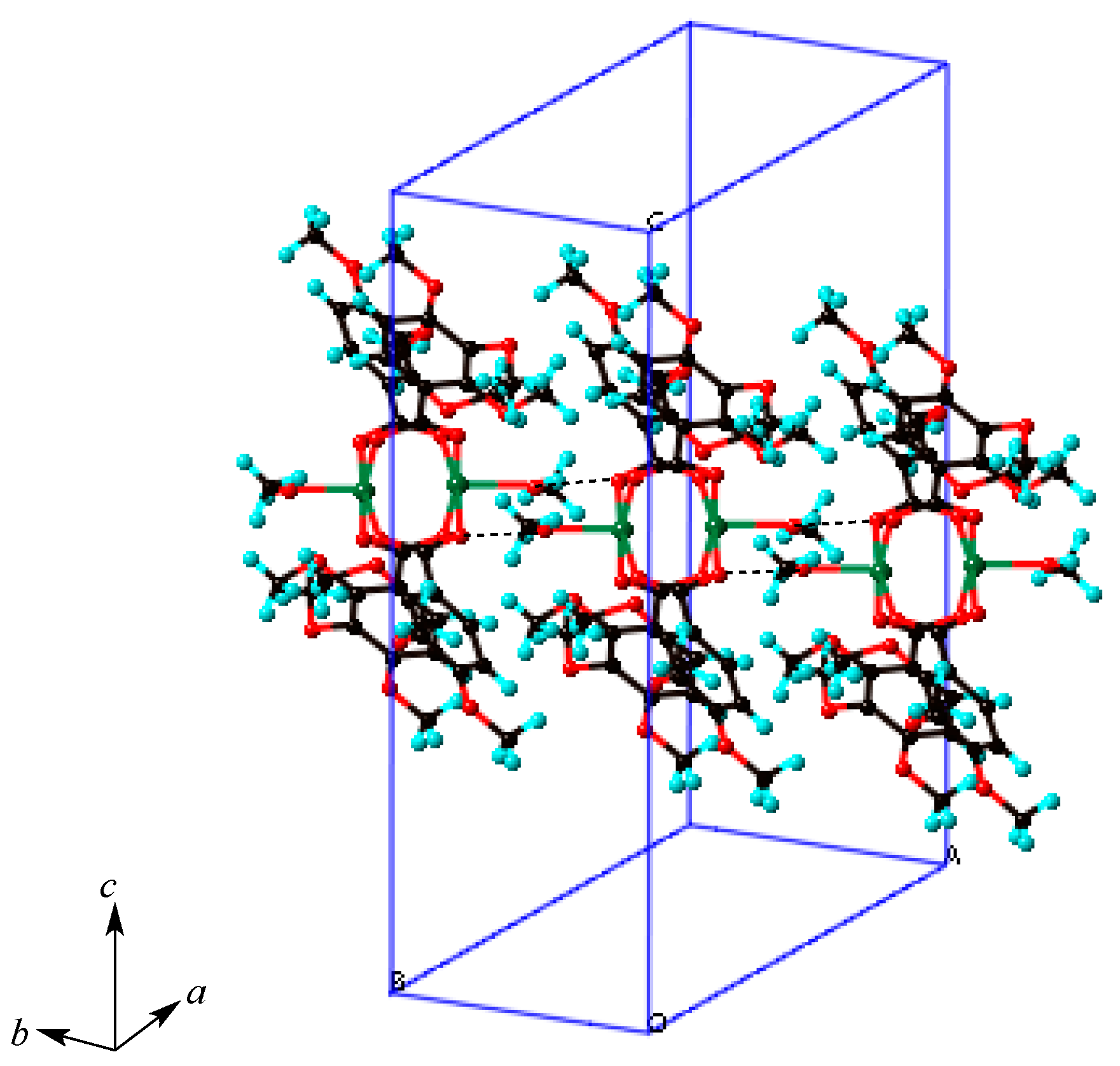
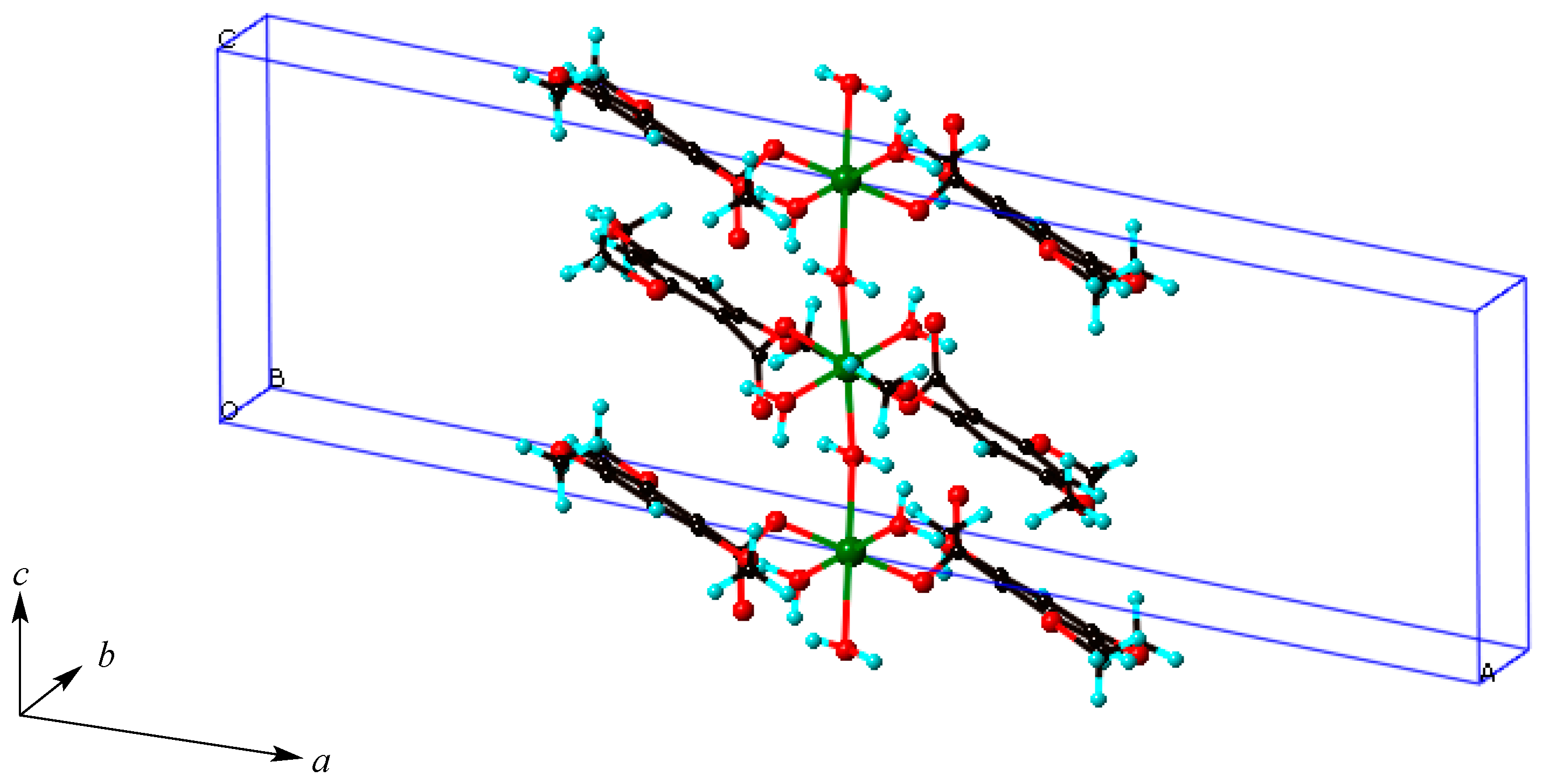
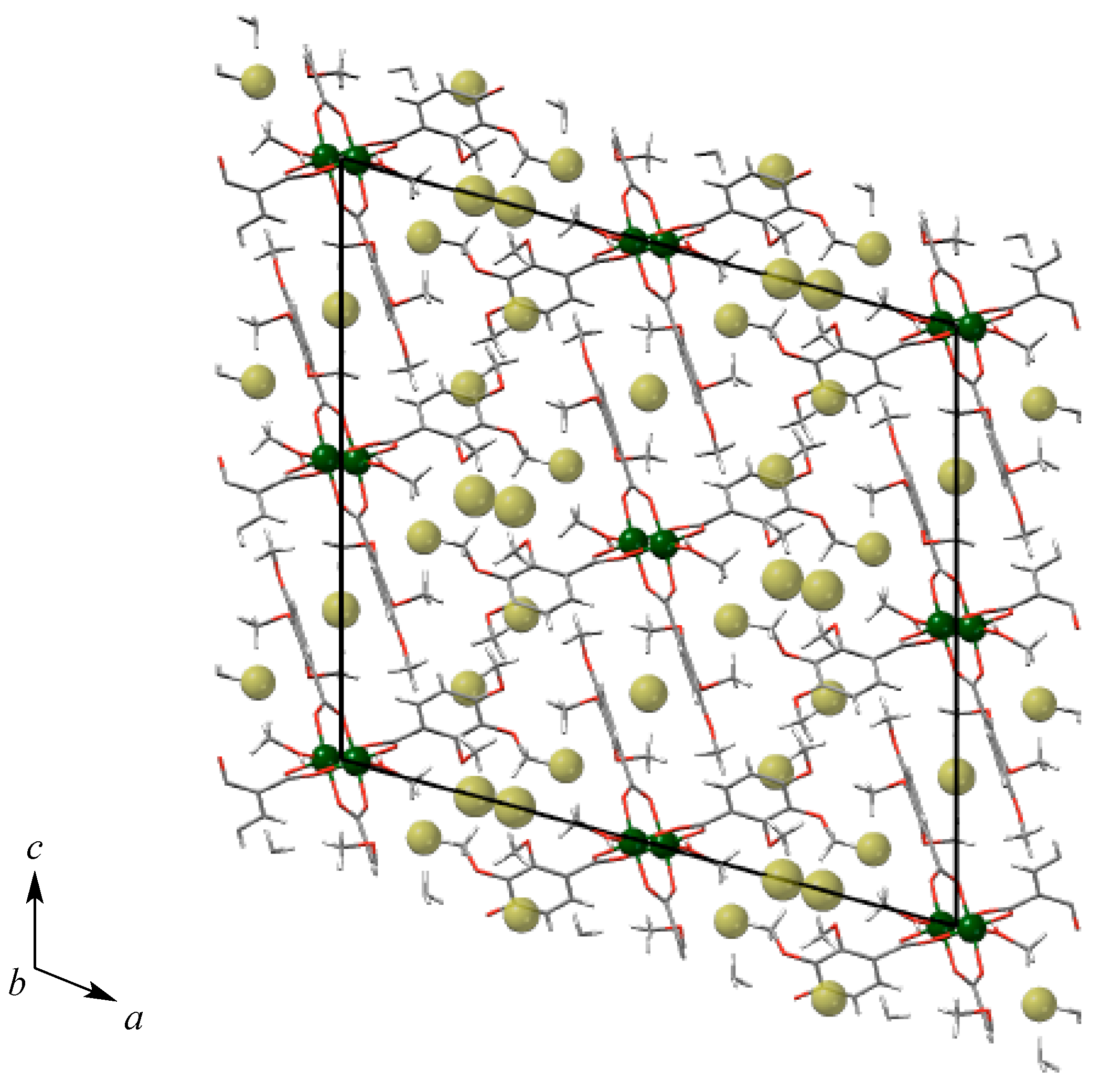
2.4. Crystal Structures of Copper(II) Carboxylates
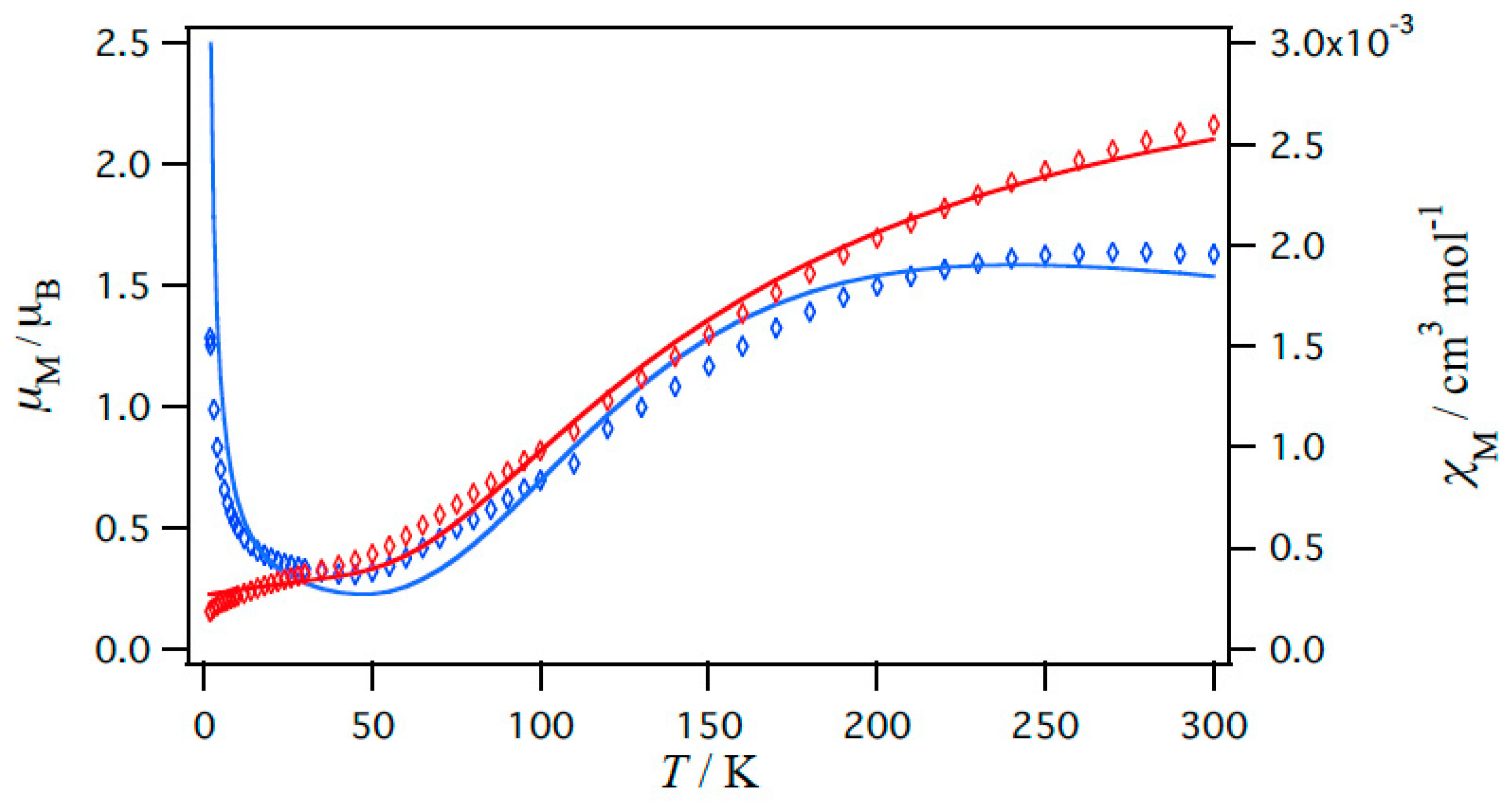
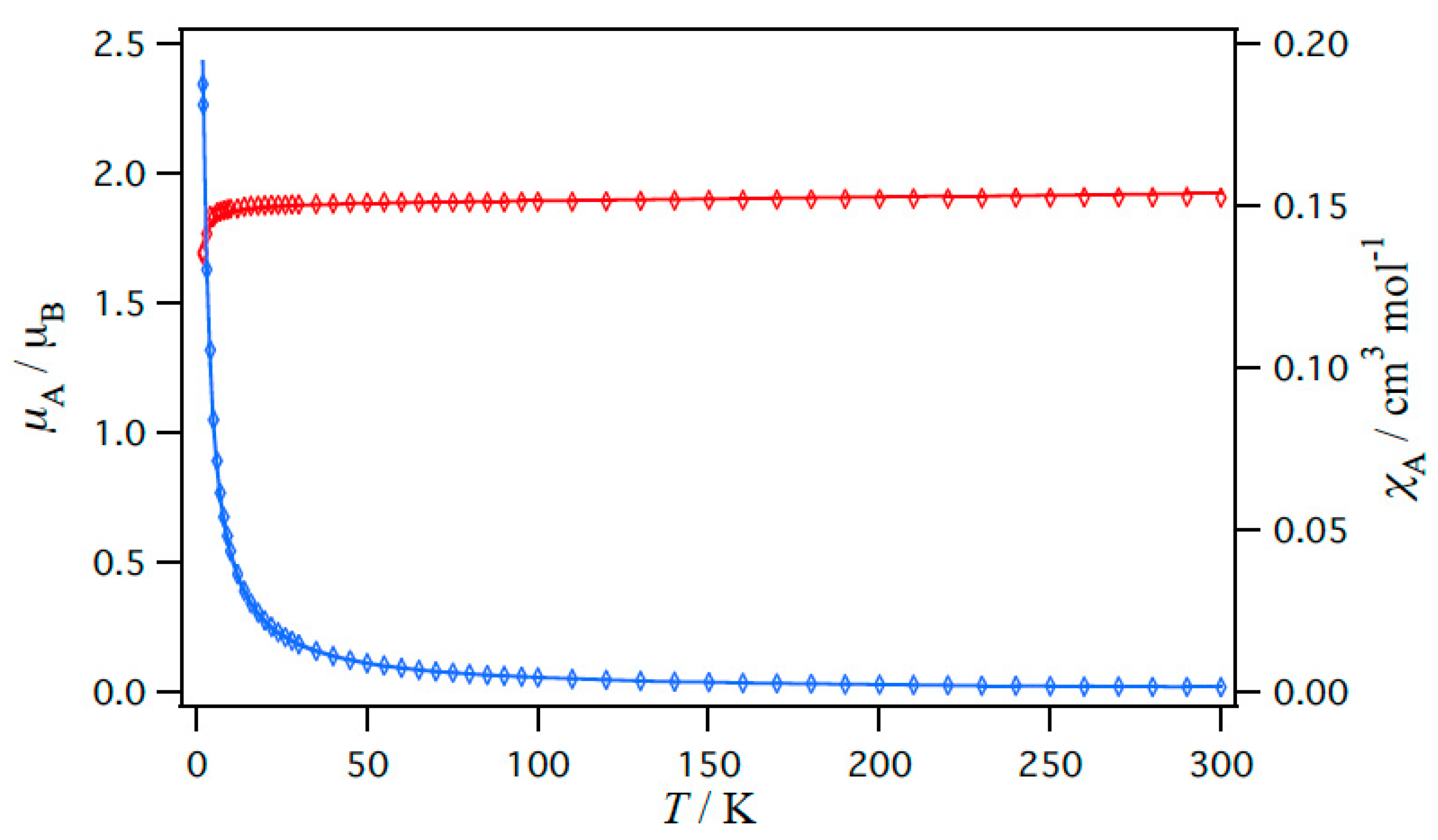
2.5. Magnetic Properties of Copper(II) Carboxylates
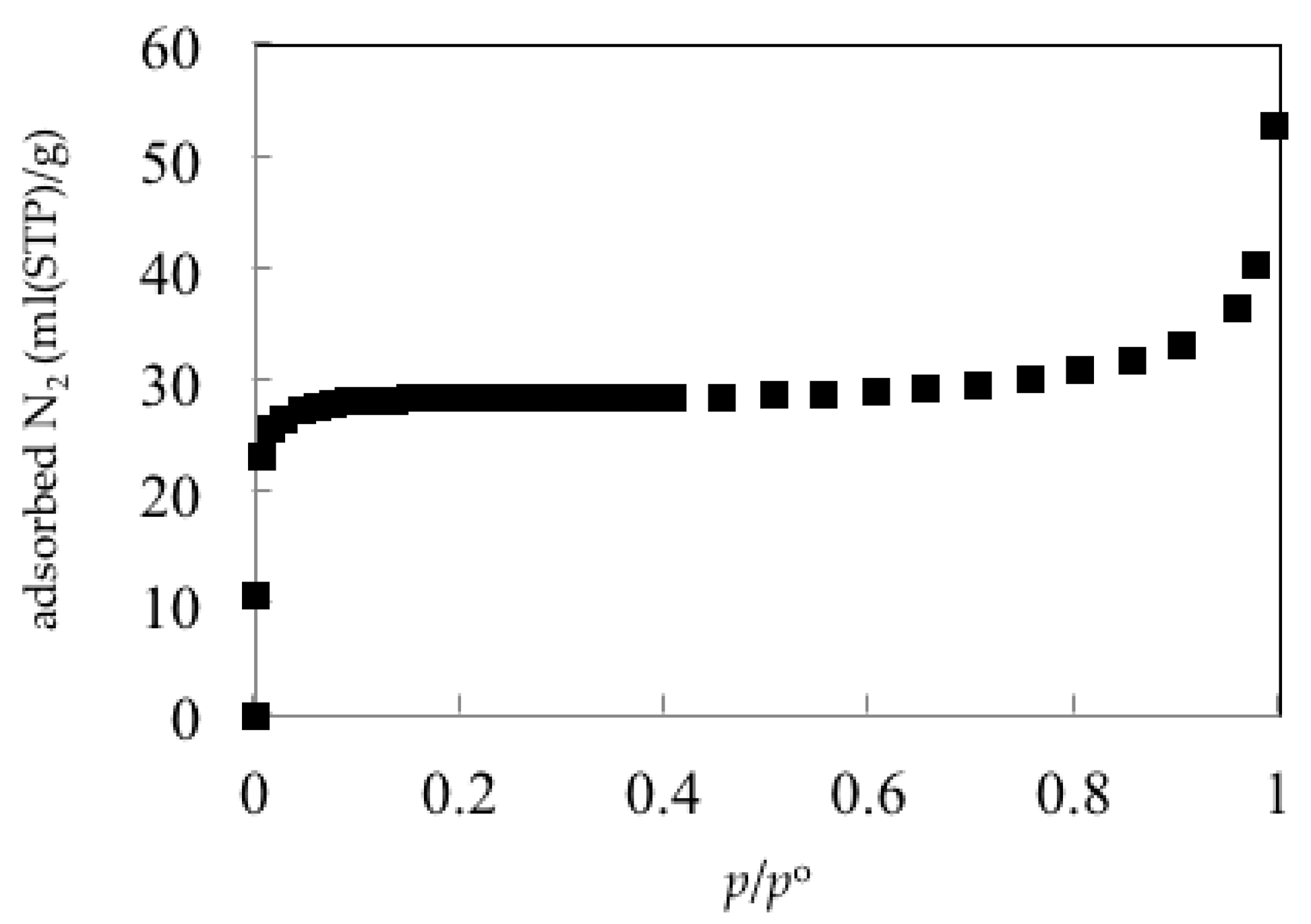
2.6. Adsorption Properties of Copper(II) Carboxylate
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Van Niekerk, J.N.; Schoening, F.R. A New Type of Copper Complex as found in the Crystal Structure of Cupric Acetate, Cu2(CH3COO)4.2H2O. Acta Crystallogr. 1953, 6, 227–232. [Google Scholar] [CrossRef]
- Bleaney, B.; Bowers, K.D. Anomalous paramagnetism of copper acetate. Proc. Roy. Soc. Lond. 1952, A214, 451–465. [Google Scholar] [CrossRef]
- Kato, N.; Jonassen, H.B.; Fanning, J.C. Copper(II) Complexes with Subnormal Magnetic Moments. Chem. Rev. 1964, 64, 99–128. [Google Scholar] [CrossRef]
- Doedens, R.J. Structure and Metal-Metal Interactions in Copper(II) Carboxylate Complexes. Prog. Inorg. Chem. 1976, 21, 209–231. [Google Scholar]
- Catterick, J.; Thornton, P. Structures and Physical Properties of Polynuclear Carboxylates. Adv. Inorg. Chem. Radiochem. 1977, 20, 291–362. [Google Scholar] [CrossRef]
- Melnik, M. Study of the relation between the structural data and magnetic interaction in oxo-bridged binuclear copper(II) compounds. Coord. Chem. Rev. 1982, 42, 259–293. [Google Scholar] [CrossRef]
- Kato, M.; Muto, Y. Factors affecting the magnetic properties of dimeric copper(II) complexes. Coord. Chem. Rev. 1988, 92, 45–83. [Google Scholar] [CrossRef]
- Kawata, T.; Uekusa, H.; Ohba, S.; Furukawa, T.; Tokii, T.; Muto, Y. Magneto-Structural Correlation in Dimeric Copper(II) Benzoates. Acta Crystallogr. Part. B 1992, 48, 253–261. [Google Scholar] [CrossRef]
- Sundberg, M.R.; Uggla, R.; Melnik, M. Comparison of the structural parameters in copper(II) acetate-type dimers containing distorted square pyramidal Cu4O and CuO4N chromophores. Polyhedron 1996, 15, 1157–1163. [Google Scholar] [CrossRef]
- Valach, F.; Melnik, M.; Bernardinelli, G.; Fromm, K.M. A structural study of copper(II) carboxylates: Crystal structure and physical characterization of [Cu2(2-bromopropionato)4(caffeine)2]. J. Chem. Crystallogr. 2006, 36, 571–580. [Google Scholar] [CrossRef]
- Mikuriya, M. Copper(II) Acetate as a Motif of Metal-Assembled complexes. Bull. Jpn. Soc. Coord. Chem. 2008, 52, 17–28. [Google Scholar] [CrossRef][Green Version]
- Nukada, R.; Mori, W.; Takamizawa, S.; Mikuriya, M.; Handa, M.; Naono, H. Microporous Structure of a Chain Compound of Copper(II) Benzoate Bridged by Pyrazine. Chem. Lett. 1999, 28, 367–368. [Google Scholar] [CrossRef]
- Nukada, R.; Mikuriya, M.; Handa, M.; Naono, H. Hydrophobic micropore in a chain compound of dinuclear copper(II) benzoate with pyrazine—Adsorption properties for N2. CCl4, H2O, CO2, and CH3CN. In Proceedings of the 2nd International Porous and Powder Materials Symposium and Exhibition PPM 2015, Izmir, Turkey, 15–18 September 2015; Ozdemir, S.K., Polat, M., Tanoglu, M., Eds.; The Organizing Committee of The International Porous and Power Materials Symposium and Exhibition, Uc Adim Printing House: Izmir, Turkey, 2015; pp. 77–81. ISBN 978-975-6590-07-2. [Google Scholar]
- Horikoshi, R.; Mikuriya, M. One-Dimensional Coordination Polymers from the Self-Assembly of Copper(II) Carboxylates and 4,4′-Dithiobis(pyridine). Bull. Chem. Soc. Jpn. 2005, 78, 827–834. [Google Scholar] [CrossRef]
- Wada, S.; Yoshioka, D.; Mikuriya, M. Synthesis, Crystal Structures, and Magnetic Properties of Dinuclear and Hexanuclear Copper(II) Complexes with Cyclam-based Macrocyclic Ligands Having Four Schiff-Base Pendant Arms. Bull. Chem. Soc. Jpn. 2010, 83, 364–374. [Google Scholar] [CrossRef]
- Mikuriya, M.; Yamakawa, C.; Tanabe, K.; Yoshioka, D.; Mitsuhashi, R.; Tanaka, H.; Handa, M. Synthesis, crystal structure, magnetic property, and N2-gas-adsorption property of dinuclear copper(II) 3,4,5-trimethoxybenzoate. J. Turk. Chem. Soc. Sect. A 2018, 5, 103–110. [Google Scholar] [CrossRef]
- Mikuriya, M.; Indrawati, R.; Hashido, R.; Matsubara, S.; Nakamura, C.; Yoshioka, D.; Yokota, K.; Fukuzaki, M.; Handa, M. Chain Compounds Based on Paddle-wheel Copper(II) Carboxylate Bearing Four Nitroxide Radicals. Magnetochemistry 2018, 4, 22. [Google Scholar] [CrossRef]
- Mikuriya, M.; Yamakawa, C.; Masuda, N.; Yoshioka, D.; Yamaguchi, S.; Yamada, H.; Mizuta, T.; Kawata, N.; Tanaka, H.; Handa, M. Dinuclear Copper(II) 3,4,5-Tri-O-benzylgallate. Open Chem. J. 2019, 6, 19–26. [Google Scholar] [CrossRef]
- Ishida, H.; Handa, M.; Mikuriya, M. Synthesis and Crystal Structure of Aqua Adduct of Dinuclear Ruthenium(II,III) 3,4,5-Tri(ethoxy-d5)benzoate Tetrafluoroborate. X-ray Struct. Anal. Online 2014, 30, 9–10. [Google Scholar] [CrossRef]
- Ishida, H.; Handa, M.; Ikeue, T.; Taguchi, J.; Mikuriya, M. Synthesis, crystal structure, and 1H NMR spectra of chlorido-bridged chain complex of dinuclear ruthenium(II,III) 3,4,5-tri(ethoxy-d5)benzoate. Chem. Pap. 2010, 64, 767–775. [Google Scholar] [CrossRef]
- Ishida, H.; Handa, M.; Hiromitsu, I.; Ujiie, S.; Yoshioka, D.; Mitsuhashi, R.; Mikuriya, M. Magnetic and liquid-crystalline properties of chlorido- and cyanato-bridged chain complexes of mixed-valent dinuclear ruthenium(II,III) 3,4,5-trialkoxybenzoates. New J. Chem. 2019, 43, 1134–1145. [Google Scholar] [CrossRef]
- Ishida, H.; Handa, M.; Hiromitsu, I.; Mikuriya, M. Fastener effect on magnetic properties of chain compounds of dinuclear ruthenium carboxylates. Chem. Pap. 2013, 67, 743–750. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 64–67. ISBN 978-0-471-74493-1. [Google Scholar]
- Ferraro, J.R.; Walker, W.R. Infrared Spectra of Hydroxy-Bridged Copper(II) Compounds. Inorg. Chem. 1965, 4, 1382–1386. [Google Scholar] [CrossRef]
- Chaudhuri, P.; Ventur, D.; Wieghardt, K.; Peters, E.-M.; Peters, K.; Simon, A. Preparation, Magnetism, and Crystal Structures of the Tautomers [L(Cu(µ2-OH)2CuL)(ClO4)2 (Blue) and [LCu(µ2-OH2)(µ2-O)CuL](ClO4)2 (Green): µ-Aqua-µ-oxo vs. Di-µ-hydroxo Linkage. Angew. Chem. Int. Ed. Engl. 1983, 24, 57–59. [Google Scholar] [CrossRef]
- Nakanishi, K.; Solomon, P.H.; Furutachi, N. Infrared Absorption Spectroscopy; Nankodo: Tokyo, Japan, 1978. (In Japanese) [Google Scholar]
- Murakami, Y.; Sakata, K. Kireto Kagaku; Ueno, K., Ed.; Nankodo: Tokyo, Japan, 1976; Volume 1, pp. 91–396. (In Japanese) [Google Scholar]
- Todaro, M.; Sciortino, L.; Gelardi, F.M.; Buscarino, G. Determination of Geometry Arrangement of Copper Ions in HKUST-1 by XAFS During a Prolonged Exposure to Air. J. Phys. Chem. C 2017, 121, 24853–24860. [Google Scholar] [CrossRef]
- Scatena, R.; Guntern, Y.T.; Macchi, P. Electron Density and Dielectric Properties of Highly Porous MOFs: Binding and Mobility of Guest Molecules in Cu3(BTC)2 and Zn3(BTC)2. J. Am. Chem. Soc. 2019, 141, 9382–9390. [Google Scholar] [CrossRef] [PubMed]
- Erre, L.S.; Micera, G.; Piu, P.; Cariati, F.; Ciani, G. Interaction of metal ions with humic acid-like models. Part 4. Synthesis, spectral properties, and crystal and molecular structure of tetrakis(.mu.-2,6-dimethoxybenzoato)diaquodicopper(II) and of bis(.mu.-2,6-dimethoxybenzoato)bis(.mu.-acetato)diaquodicopper(II), a case of a dimeric copper(II) carboxylate complex with mixed bridges. Inorg. Chem. 1985, 24, 2297–2300. [Google Scholar] [CrossRef]
- Harrison, W.; Rettig, S.; Trotter, J. Crystal and molecular structure of tetra-µ-O-bromobenzoato-bis[aquocopper(II)]. J. Chem. Soc. Dalton Trans. 1972, 1852–1856. [Google Scholar] [CrossRef]
- O’Connor, C.J. Magnetochemistry—Advances in Theory and Experimentation. Prog. Inorg. Chem. 1982, 29, 205–283. [Google Scholar] [CrossRef]
- Lewis, J.; Mabbs, F.E.; Royston, L.K.; Smail, W.R. The preparation, magnetic susceptibilities, and electron resonance of some copper(II) carboxylate compounds. J. Chem. Soc. A 1969, 291–296. [Google Scholar] [CrossRef]
- Zhang, X.X.; Chui, S.S.-Y.; Williams, I.D. Cooperative magnetic behavior in the coordination polymers [Cu3(TMA)2L3] (L = H2O, pyridine). J. Appl. Phys. 2000, 87, 6007–6009. [Google Scholar] [CrossRef]
- Da Silva, G.G.; Machado, F.L.A.; Junior, S.A.; Padron-Hernandez, E. Metal-organic framework: Structure and magnetic properties of [Cu3(BTC)2(L)x·(CuO)y]n (L = H2O, DMF). J. Solid State Chem. 2017, 253, 1–5. [Google Scholar] [CrossRef]
- Bonner, J.C.; Fisher, M.E. Linear Magnetic Chains with Anisotropic Coupling. Phys. Rev. 1964, 135, A640–A658. [Google Scholar] [CrossRef]
- Mautner, F.A.; Mikuriya, M.; Ishida, H.; Sakiyama, H.; Louka, F.R.; Humphrey, J.W.; Massoud, S.S. Dicyanamido-metal(II) complexes. Part 4: Synthesis, structure and magnetic characterization of polynuclear Cu(II) and Ni(II) complexes bridged by µ-1,5-dicyanamide. Inorg. Chim. Acta 2009, 362, 4073–4080. [Google Scholar] [CrossRef]
- Massoud, S.S.; Vicente, R.; Fontenot, P.R.; Gallo, A.A.; Mikuriya, M.; Albering, J.H.; Mautner, F.A. Polynuclear croconato-bridged-copper(II) complexes derived from tri- and tetra-dentate amines. Polyhedron 2012, 46, 66–73. [Google Scholar] [CrossRef]
- Mautner, F.A.; Koikawa, M.; Mikuriya, M.; Harrelson, E.V.; Massoud, S.S. Copper(II)-azido complexes constructed from polypyridyl amine ligands. Polyhedron 2013, 59, 17–22. [Google Scholar] [CrossRef]
- Louka, F.R.; Massoud, S.S.; Haq, T.K.; Koikawa, M.; Mikuriya, M.; Omote, M.; Fischer, R.C.; Mautner, F.A. Synthesis, structural characterization and magnetic properties of one-dimensional Cu(II)-azido coordination polymers. Polyhedron 2017, 138, 177–184. [Google Scholar] [CrossRef]
- Mikuriya, M.; Higashiguchi, M.; Sakai, T.; Yoshioka, D.; Handa, M. Chain compounds of rhodium(II) benzoate bridged by N,N’-didentate ligands. In Progress in Coordination and Bioinorganic Chemistry; Melnik, M., Sirota, A., Eds.; Slovak Technical University Press: Bratislava, Slovakia, 2003; pp. 213–218. [Google Scholar]
- Takamizawa, S.; Hiroki, T.; Nakata, E.; Mochizuki, K.; Mori, W. Crystal Structure and Gas Adsorption Property of Rhodium(II) Benzoate Pyrazine. Chem. Lett. 2002, 31, 1208–1209. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; VCH Publications: New York, NY, USA, 1993; pp. 3–4. ISBN 1-56081-566-3. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]

| 6′ | 7 | |
|---|---|---|
| Empirical formula | C42H52Cu2O22 | C20H32CuO15 |
| Formula weight | 1035.91 | 575.99 |
| Temperature/K | 90 | 90 |
| Crystal dimensions/mm | 0.30 × 0.22 × 0.11 | 0.40 × 0.22 × 0.06 |
| Crystal system | monoclinic | monoclinic |
| Space group | C2/c | C2/c |
| a/Å | 26.014(4) | 28.686(3) |
| b/Å | 7.4409(12) | 10.4617(11) |
| c/Å | 24.510(4) | 8.2841(9) |
| β/o | 105.174(3) | 101.792(2) |
| V/Å3 | 4579.0(13) | 2433.6(5) |
| Z | 4 | 4 |
| dcalcd./gcm−3 | 1.503 | 1.572 |
| μ/mm−1 | 1.012 | 0.973 |
| F(000) | 2152 | 1204 |
| Reflections collected | 14670 | 7502 |
| Independent reflections (Rint) | 5524 (0.0441) | 2947 (0.0385) |
| θ range for data collection | 1.722 to 28.490° | 2.902 to 28.435 |
| Data/Restraints/Parameters | 5524/0/309 | 2947/2/188 |
| R1, wR2 [I > 2σ(I)] [a] | 0.0430, 0.1124 | 0.0458, 0.1023 |
| R1, wR2 (all data) [a] | 0.0622, 0.1282 | 0.0658, 0.1131 |
| Goodness-of-fit on F2 | 0.820 | 1.038 |
| CCDC number | 1570723 | 2060931 |
| 6′ | |||
|---|---|---|---|
| Cu1···Cu1′ | 2.6009(7) | Cu1-O6 | 1.9504(18) |
| Cu1-O1 | 1.9827(17) | Cu1-O7′ | 1.9574(17) |
| Cu1-O2′ | 1.9802(17) | Cu1-O11 | 2.1309(19) |
| O1-Cu1-O2′ | 169.71(7) | O2′-Cu1-O7′ | 90.54(7) |
| O1-Cu1-O6 | 90.72(7) | O2′-Cu1-O11 | 96.23(7) |
| O1-Cu1-O7′ | 88.75(7) | O6-Cu1-O7′ | 169.47(7) |
| O1-Cu1-O11 | 94.05(7) | O6-Cu1-O11 | 98.36(8) |
| O2′-Cu1-O6 | 88.11(8) | O7′-Cu1-O11 | 92.16(7) |
| 7 | |||
| Cu1-Cu1” | 4.1420(5) | Cu1-O6 | 1.9461(18) |
| Cu1-O1 | 2.0583(18) | Cu1-O7 | 2.3018(12) |
| O1-Cu1-O1′’ | 180.0 | O1-Cu1-O7 | 85.61(6) |
| O1-Cu1-O6 | 87.65(7) | O1-Cu1-O7′ | 180.0 |
| O1-Cu1-O6′ | 92.35(8) | O7-Cu1-O7′ | 180.0 |
| Cu1-O7-Cu1” | 128.24(12) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikuriya, M.; Yamakawa, C.; Tanabe, K.; Nukita, R.; Amabe, Y.; Yoshioka, D.; Mitsuhashi, R.; Tatehata, R.; Tanaka, H.; Handa, M.; et al. Copper(II) Carboxylates with 2,3,4-Trimethoxybenzoate and 2,4,6-Trimethoxybenzoate: Dinuclear Cu(II) Cluster and µ-Aqua-Bridged Cu(II) Chain Molecule. Magnetochemistry 2021, 7, 35. https://doi.org/10.3390/magnetochemistry7030035
Mikuriya M, Yamakawa C, Tanabe K, Nukita R, Amabe Y, Yoshioka D, Mitsuhashi R, Tatehata R, Tanaka H, Handa M, et al. Copper(II) Carboxylates with 2,3,4-Trimethoxybenzoate and 2,4,6-Trimethoxybenzoate: Dinuclear Cu(II) Cluster and µ-Aqua-Bridged Cu(II) Chain Molecule. Magnetochemistry. 2021; 7(3):35. https://doi.org/10.3390/magnetochemistry7030035
Chicago/Turabian StyleMikuriya, Masahiro, Chihiro Yamakawa, Kensuke Tanabe, Raigo Nukita, Yuki Amabe, Daisuke Yoshioka, Ryoji Mitsuhashi, Ryota Tatehata, Hidekazu Tanaka, Makoto Handa, and et al. 2021. "Copper(II) Carboxylates with 2,3,4-Trimethoxybenzoate and 2,4,6-Trimethoxybenzoate: Dinuclear Cu(II) Cluster and µ-Aqua-Bridged Cu(II) Chain Molecule" Magnetochemistry 7, no. 3: 35. https://doi.org/10.3390/magnetochemistry7030035
APA StyleMikuriya, M., Yamakawa, C., Tanabe, K., Nukita, R., Amabe, Y., Yoshioka, D., Mitsuhashi, R., Tatehata, R., Tanaka, H., Handa, M., & Tsuboi, M. (2021). Copper(II) Carboxylates with 2,3,4-Trimethoxybenzoate and 2,4,6-Trimethoxybenzoate: Dinuclear Cu(II) Cluster and µ-Aqua-Bridged Cu(II) Chain Molecule. Magnetochemistry, 7(3), 35. https://doi.org/10.3390/magnetochemistry7030035









