Microstructure and Magnetic Properties of Ce14Fe78Co2B6 Nanopowders Prepared by Ball Milling at Low Temperature
Abstract
:1. Introduction
2. Materials and Methods Experimental
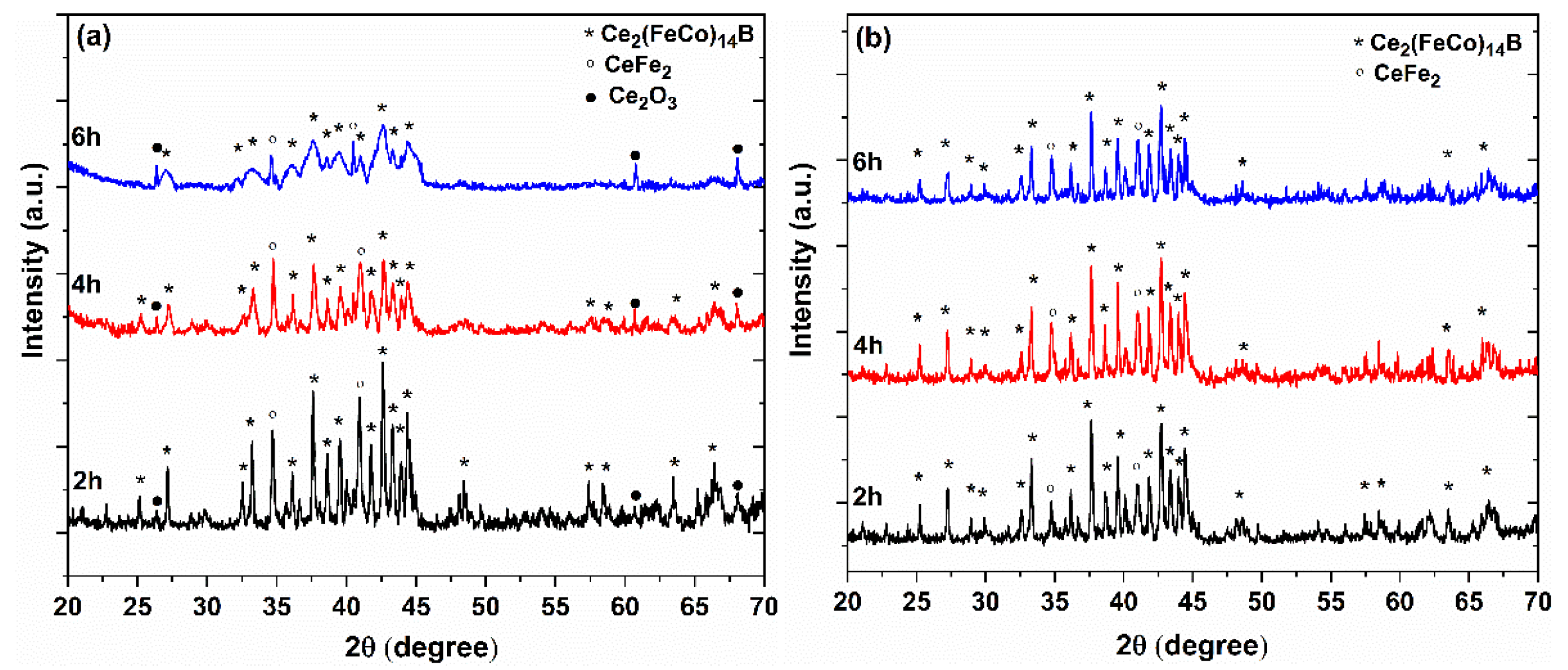
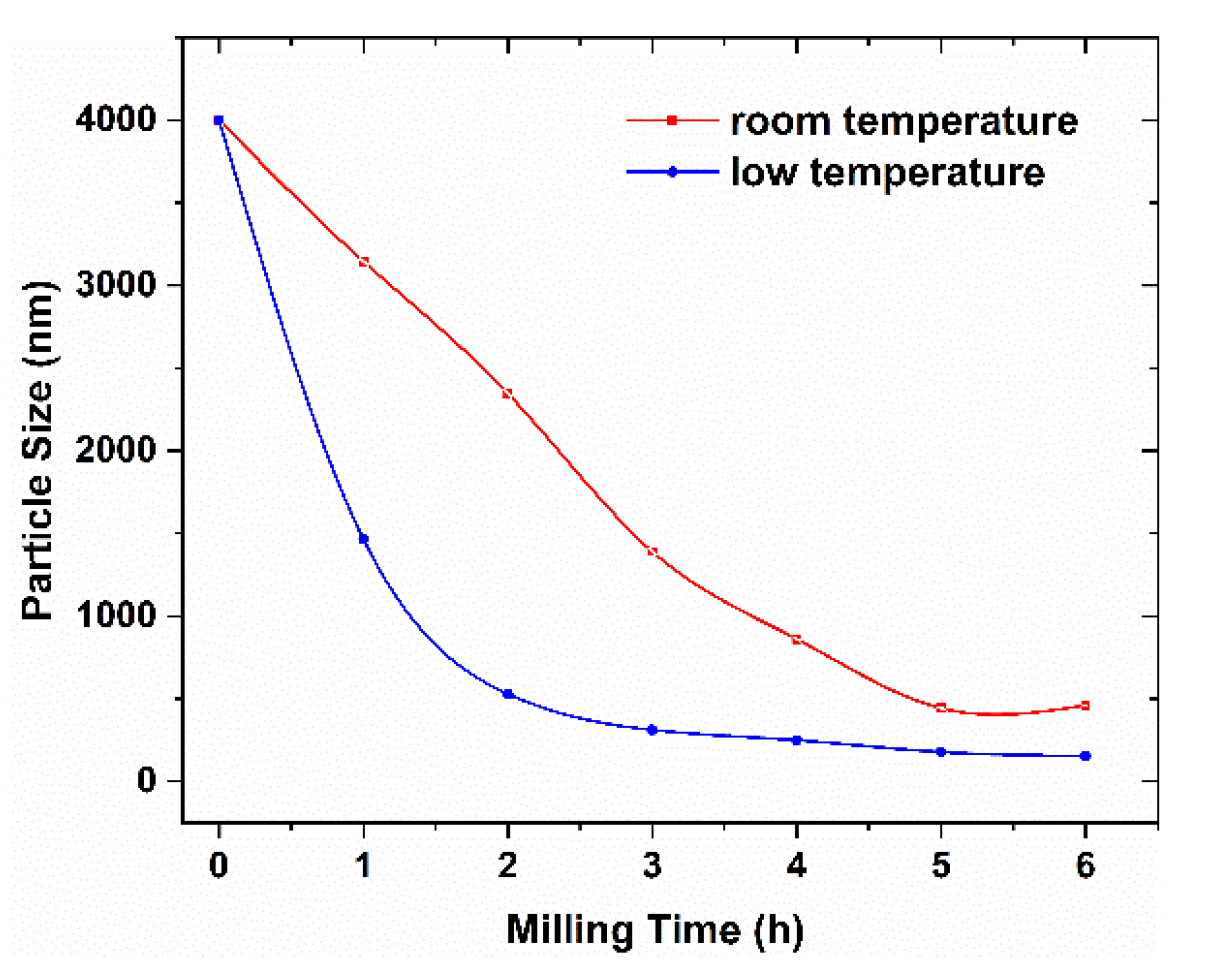
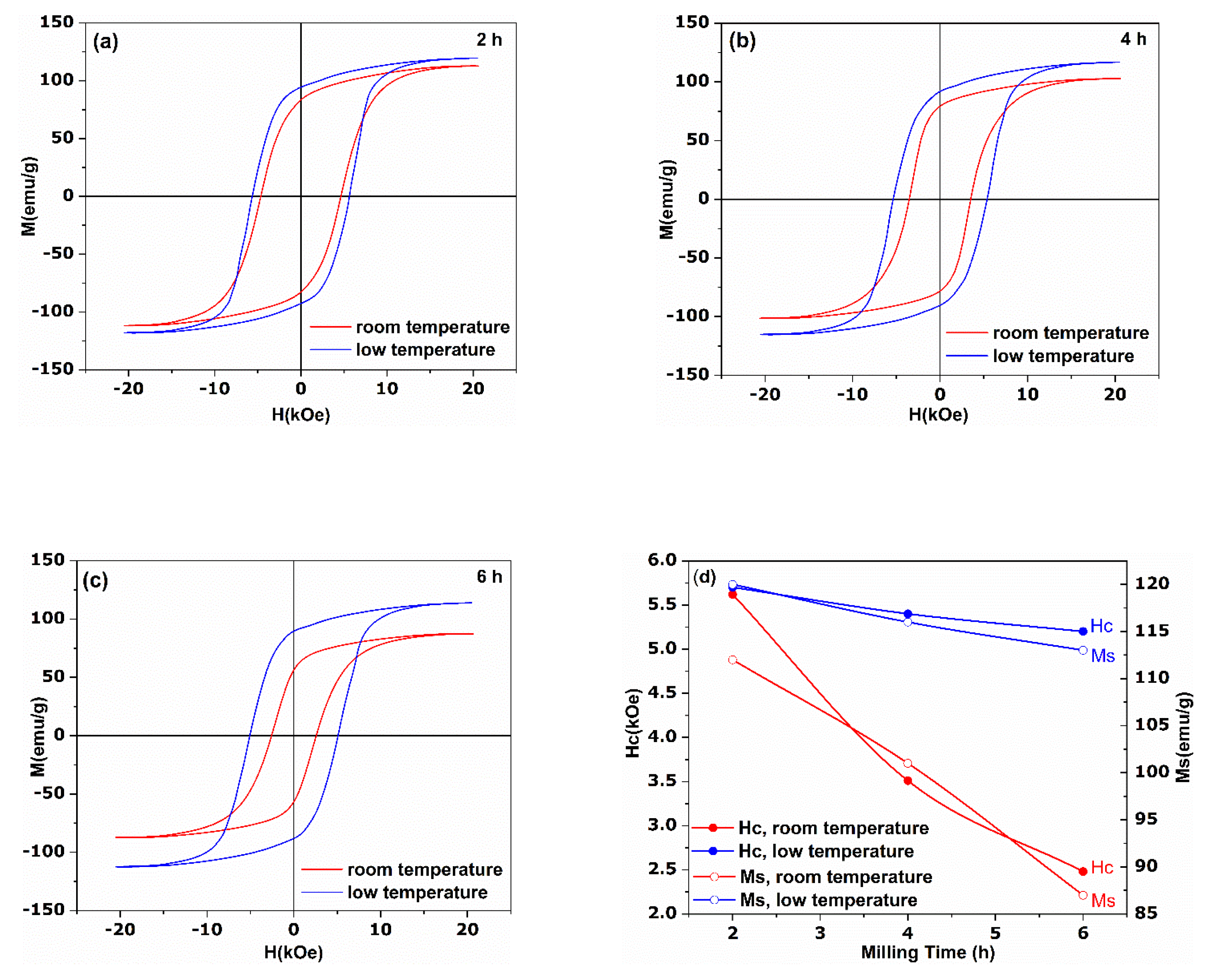
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gutfleisch, O.; Willard, M.A.; Brück, E.; Chen, C.H.; Sankar, S.G.; Liu, J.P. Magnetic Materials and Devices for the 21st Century: Stronger, Lighter, and More Energy Efficient. Adv. Mater. 2011, 23, 821–842. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, Y. Recent development of Nd–Fe–B sintered magnets and their applications. J. Magn. Magn. Mater. 2006, 303, 344–347. [Google Scholar] [CrossRef]
- Coey, J.M.D. Permanent magnets: Plugging the gap. Scr. Mater. 2012, 67, 524–529. [Google Scholar] [CrossRef]
- Marinescu, M.; Chiriac, H.; Grigoras, M. Magnetic properties of bulk nanocomposite permanent magnets based on NdDyFeB alloys with additions. J. Magn. Magn Mater. 2005, 290–291, 1267–1269. [Google Scholar] [CrossRef]
- Schreiber, A.; Marx, J.; Zapp, P.; Hake, J.F.; Voßenkaul, D.; Friedrich, B. Environmental Impacts of Rare Earth Mining and Separation Based on Eudialyte: A New European Way. Resources 2016, 5, 32. [Google Scholar] [CrossRef] [Green Version]
- Rare Earth Elements-Purification, Separation and Recycling. Available online: https://www.ivl.se/download/18.76c6e08e1573302315f3b85/1480415049402/C211.pdf (accessed on 9 December 2021).
- How Rare-Earth Mining Has Devastated China’s Environment. Available online: https://earth.org/rare-earth-mining-has-devastated-chinas-environment/ (accessed on 9 December 2021).
- Pathak, A.K.; Gschneidner, K.A., Jr.; Khan, M.; McCallum, R.W.; Pecharsky, V.K. High performance Nd-Fe-B permanent magnets without critical elements. J. Alloy. Compd. 2016, 668, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.G.; Li, W.; Wang, J.D.; Zheng, L.Y.; Li, Y.F.; Zhang, K.; Feng, H.B.; Liu, T. Influence of Ce Content on the Rectangularity of Demagnetization Curves and Magnetic Properties of Re-Fe-B Magnets Sintered by Double Main Phase Alloy Method. IEEE Trans. Magn. 2014, 50, 1000104. [Google Scholar] [CrossRef]
- Grigoras, M.; Lostun, M.; Stoian, G.; Herea, D.D.; Chiriac, H.; Lupu, N. Microstructure and magnetic properties of Ce10+xFe84−xB6 nanocrystalline ribbons versus preparation conditions. J. Magn. Magn. Mater. 2017, 432, 119–123. [Google Scholar] [CrossRef]
- Gabay, A.M.; Martín-Cid, A.; Barandiaran, J.M.; Salazar, D.; Hadjipanayis, G.C. Low-cost Ce1-xSmx(Fe, Co, Ti)12 alloys for permanent magnets. AIP Adv. 2016, 6, 056015. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Liu, Y.; Li, J.; Shi, Q. Phase and microstructure formation of strip-cast Ce–Fe–B alloys for multi-phases RE-Fe-B magnets. Intermetallics 2019, 114, 106606. [Google Scholar] [CrossRef]
- Skoug, E.J.; Meyer, M.S.; Pinkerton, F.E.; Tessema, M.M.; Haddad, D.; Herbst, J.F. Crystal structure and magnetic properties of Ce2Fe14−xCoxB alloys. J. Alloy. Compd. 2013, 574, 552–555. [Google Scholar] [CrossRef]
- Skomski, R.; Coey, J.M.D. Giant energy product in nanostructured two-phase magnets. Phys. Rev. B 1993, 48, 15812–15816. [Google Scholar] [CrossRef] [Green Version]
- Fukada, T.; Matsuura, M.; Goto, R.; Tezuka, N.; Sugimoto, S.; Une, Y.; Sagawa, M. Evaluation of the Microstructural Contribution to the Coercivity of Fine-Grained Nd–Fe–B Sintered Magnets. Mater. Trans. 2012, 53, 1967–1971. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, M.; Matsuura, M.; Tezuka, N.; Sugimoto, S.; Une, Y.; Kubo, H.; Sagawa, M. Preparation of ultrafine jet-milled powders for Nd-Fe-B sintered magnets using hydrogenation–disproportionation–desorption–recombination and hydrogen decrepitation processes. Appl. Phys. Lett. 2013, 103, 022404. [Google Scholar] [CrossRef]
- McGuiness, P.J.; Zhang, X.J.; Yin, X.J.; Harris, I.R. Hydrogenation, disproportionation and desorption (HDD): An effective processing route for Nd2Fe14B-type magnets. J. Less. Common. Met. 1990, 158, 359–365. [Google Scholar] [CrossRef]
- Akdogan, N.G.; George, C.H.; David, J.S. Novel Nd2Fe14B nanoflakes and nanoparticles for the development of high energy nanocomposite magnets. Nanotechnology 2010, 21, 295705. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Zheng, L.; Li, W.; Liu, J.; Hadjipanayis, G. Single-crystal and textured polycrystalline Nd2Fe14B flakes with a submicron or nanosize thickness. Acta Mater. 2012, 60, 1721–1730. [Google Scholar] [CrossRef]
- Chakka, V.M.; Altuncevahir, B.; Jin, Z.Q.; Li, Y.; Liua, J.P. Magnetic nanoparticles produced by surfactant-assisted ball milling. J. Appl. Phys. 2006, 99, 08E912. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, S.; Zhang, J.; Liu, J.P.; Xia, W.; Du, J.; Yan, A.; Yi, J.; Li, W.; Guo, Z. Highly anisotropic SmCo5 nanoflakes by surfactant-assisted ball milling at low temperature. J. Magn. Magn. Mater. 2015, 374, 108–115. [Google Scholar] [CrossRef]
- Fang, Q.; An, X.; Wang, F.; Li, Y.; Du, J.; Xia, W.; Yan, A.; Liu, J.P.; Zhang, J. The structure and magnetic properties of Sm–Fe–N powders prepared by ball milling at low temperature. J. Magn. Magn. Mater. 2016, 410, 116–122. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Y.; Li, J.; Wang, R. Ultrafine nanocrystalline NdFeB prepared by cryomilling with HDDR process. J. Alloy. Compd. 2018, 750, 401–408. [Google Scholar] [CrossRef]
- Külah, E.; Marot, L.; Steiner, R.; Romanyuk, A.; Jung, T.A.; Wäckerlin, A.; Meyer, E. Surface chemistry of rare-earth oxide surfaces at ambient conditions: Reactions with water and hydrocarbons. Sci. Rep. 2017, 7, 43369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Evans, H.; Harris, I.; Jones, I. The Oxidation of NdFeB Magnets. Oxid. Met. 2003, 59, 167–182. [Google Scholar] [CrossRef]
- Pragnell, W.M.; Williams, A.J.; Evans, H.E. The oxidation of SmCo magnets. J. Appl. Phys. 2008, 103, 07E127. [Google Scholar] [CrossRef]
- Tiwary, C.S.; Kashyap, S.; Biswas, K.; Chattopadhyay, K. Synthesis of pure iron magnetic nanoparticles in large quantity. J. Phys. D Appl. Phys. 2013, 46, 385001. [Google Scholar] [CrossRef]
- Katiyar, N.K.; Biswas, K.; Tiwary, C.S. Cryomilling as environmentally friendly synthesis route to prepare nanomaterials. Int. Mater. Rev. 2021, 66, 493–532. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Li, Z.; Peng, L.; Shen, B.; Hu, F.; Sun, J. Magnetization process of nanocrystalline mischmetal-Fe-B ribbons. J. Alloy. Compd. 2016, 688, 1053–1057. [Google Scholar] [CrossRef]
- Poudyal, N.; Rong, C.-B.; Liu, J.P. Effects of particle size and composition on coercivity of Sm–Co nanoparticles prepared by surfactant-assisted ball milling. J. Appl. Phys. 2010, 107, 09A703. [Google Scholar] [CrossRef]
- Li, W.; Hu, X.; Cui, B.; Yang, J.; Han, J.; Hadjipanayis, G. Magnetic property and microstructure of single crystalline Nd2Fe14B ultrafine particles ball milled from HDDR powders. J. Magn. Magn. Mater. 2013, 339, 71–74. [Google Scholar] [CrossRef]
- Yi, M.; Zhang, H.; Gutfleisch, O.; Xu, B.-X. Multiscale Examination of Strain Effects in Nd-Fe-B Permanent Magnets. Phys. Rev. Appl. 2017, 8, 014011. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigoras, M.; Lostun, M.; Borza, F.; Porcescu, M.; Stoian, G.; Lupu, N. Microstructure and Magnetic Properties of Ce14Fe78Co2B6 Nanopowders Prepared by Ball Milling at Low Temperature. Magnetochemistry 2021, 7, 160. https://doi.org/10.3390/magnetochemistry7120160
Grigoras M, Lostun M, Borza F, Porcescu M, Stoian G, Lupu N. Microstructure and Magnetic Properties of Ce14Fe78Co2B6 Nanopowders Prepared by Ball Milling at Low Temperature. Magnetochemistry. 2021; 7(12):160. https://doi.org/10.3390/magnetochemistry7120160
Chicago/Turabian StyleGrigoras, Marian, Mihaela Lostun, Firuta Borza, Marieta Porcescu, George Stoian, and Nicoleta Lupu. 2021. "Microstructure and Magnetic Properties of Ce14Fe78Co2B6 Nanopowders Prepared by Ball Milling at Low Temperature" Magnetochemistry 7, no. 12: 160. https://doi.org/10.3390/magnetochemistry7120160
APA StyleGrigoras, M., Lostun, M., Borza, F., Porcescu, M., Stoian, G., & Lupu, N. (2021). Microstructure and Magnetic Properties of Ce14Fe78Co2B6 Nanopowders Prepared by Ball Milling at Low Temperature. Magnetochemistry, 7(12), 160. https://doi.org/10.3390/magnetochemistry7120160








