A Quantum-Mechanical Study of Antiphase Boundaries in Ferromagnetic B2-Phase Fe2CoAl Alloy
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heusler, F.; Starck, W.; Haupt, E. Über magnetische Manganlegierungen. Verh. Dtsch. Phys. Ges. 1903, 5, 219. [Google Scholar]
- Webster, P. Heusler Alloys. Contemp. Phys. 1969, 10, 559–577. [Google Scholar] [CrossRef]
- Graf, T.; Felser, C.; Parkin, S.S.P. Simple rules for the understanding of Heusler compounds. Prog. Solid State Chem. 2011, 39, 1–50. [Google Scholar] [CrossRef]
- Picozzi, S.; Continenza, A.; Freeman, A. Co2MnX (X=Si, Ge, Sn) Heusler compounds: An ab initio study of their structural, electronic, and magnetic properties at zero and elevated pressure. Phys. Rev. B 2002, 66, 094421. [Google Scholar] [CrossRef]
- Webster, P. Magnetic and chemical order in Heusler alloys containing cobalt and manganese. J. Phys. Chem. Solids 1971, 32, 1221. [Google Scholar] [CrossRef]
- Kübler, J.; Williams, A.; Sommers, C. Formation and coupling of magnetic-moments in Heusler alloys. Phys. Rev. B 1983, 28, 1745–1755. [Google Scholar] [CrossRef]
- Galanakis, I.; Dederichs, P.H.; Papanikolaou, N. Slater-Pauling behavior and origin of the half-metallicity of the full-Heusler alloys. Phys. Rev. B 2002, 66, 174429. [Google Scholar] [CrossRef]
- Miura, Y.; Nagao, K.; Shirai, M. Atomic disorder effects on half-metallicity of the full-Heusler alloys Co2(Cr1-xFex)Al: A first-principles study. Phys. Rev. B 2004, 69, 144413. [Google Scholar] [CrossRef]
- Galanakis, I.; Dederichs, P.H.; Papanikolaou, N. Origin and properties of the gap in the half-ferromagnetic Heusler alloys. Phys. Rev. B 2002, 66, 134428. [Google Scholar] [CrossRef]
- Kandpal, H.C.; Fecher, G.H.; Felser, C. Calculated electronic and magnetic properties of the half-metallic, transition metal based Heusler compounds. J. Phys. D Appl. Phys. 2007, 40, 1507–1523. [Google Scholar] [CrossRef]
- Galanakis, I.; Mavropoulos, P.; Dederichs, P.H. Electronic structure and Slater-Pauling behaviour in half-metallic Heusler alloys calculated from first principles. J. Phys. D Appl. Phys. 2006, 39, 765–775. [Google Scholar] [CrossRef]
- Buschow, K.; Van Engen, P. Magnetic and magneto-optical properties of Heusler alloys based on aluminum and gallium. J. Magn. Magn. Mater. 1981, 25, 90–96. [Google Scholar] [CrossRef]
- Chadov, S.; Qi, X.; Kübler, J.; Fecher, G.H.; Felser, C.; Zhang, S.C. Tunable multifunctional topological insulators in ternary Heusler compounds. Nat. Mater. 2010, 9, 541–545. [Google Scholar] [CrossRef]
- Lin, H.; Wray, L.A.; Xia, Y.; Xu, S.; Jia, S.; Cava, R.J.; Bansil, A.; Hasan, M.Z. Half-Heusler ternary compounds as new multifunctional experimental platforms for topological quantum phenomena. Nat. Mater. 2010, 9, 546–549. [Google Scholar] [CrossRef]
- Planes, A.; Manosa, L.; Acet, M. Magnetocaloric effect and its relation to shape-memory properties in ferromagnetic Heusler alloys. J. Phys. Condens. Matter 2009, 21, 233201. [Google Scholar] [CrossRef]
- Entel, P.; Buchelnikov, V.; Khovailo, V.; Zayak, A.; Adeagbo, W.; Gruner, M.; Herper, H.; Wassermann, E. Modelling the phase diagram of magnetic shape memory Heusler alloys. J. Phys. D Appl. Phys. 2006, 39, 865–889. [Google Scholar] [CrossRef]
- Gilleßen, M.; Dronskowski, R. A combinatorial study of full Heusler alloys by first-principles computational methods. J. Comput. Chem. 2009, 30, 1290. [Google Scholar] [CrossRef]
- Gilleßen, M.; Dronskowski, R. A combinatorial study of inverse Heusler alloys by first-principles computational methods. J. Comput. Chem. 2010, 31, 612. [Google Scholar] [CrossRef]
- Sauthoff, G. Intermetallics; VCH Verlagsgesellschaft: Weinheim, Germany, 1995. [Google Scholar]
- Liu, C.T.; Stringer, J.; Mundy, J.N.; Horton, L.L.; Angelini, P. Ordered intermetallic alloys: An assessment. Intermetallics 1997, 5, 579–596. [Google Scholar] [CrossRef]
- Stoloff, N.S. Iron aluminides: Present status and future prospects. Mater. Sci. Eng. A 1998, 258, 1–14. [Google Scholar] [CrossRef]
- Liu, C.T.; Lee, E.H.; McKamey, C.G. An environmental-effect as the major cause for room-temperature embrittlement in FeAl. Scr. Metall. Mater. 1989, 23, 875–880. [Google Scholar] [CrossRef]
- Lynch, R.J.; Heldt, L.A.; Milligan, W.W. Effects of alloy composition on environmental embrittlement of B2 ordered iron aluminides. Scr. Metall. Mater. 1991, 25, 2147–2151. [Google Scholar] [CrossRef]
- Liu, C.T.; McKamey, C.G.; Lee, E.H. Environmental-effects on room-temperature ductility and fracture in Fe3Al. Scr. Metall. Mater. 1990, 24, 385–389. [Google Scholar] [CrossRef]
- Lynch, R.J.; Gee, K.A.; Heldt, L.A. Environmental embrittlement of single-crystal and thermomechanically processed B2-ordered iron aluminides. Scr. Metall. Mater. 1994, 30, 945–950. [Google Scholar] [CrossRef]
- Zamanzade, M.; Barnoush, A.; Motz, C. A review on the properties of iron aluminide intermetallics. Crystals 2016, 6, 10. [Google Scholar] [CrossRef]
- Kattner, U.; Burton, B. Al-Fe (Aluminium-Iron). In Phase Diagrams of Binary Iron Alloys; Okamoto, H., Ed.; ASM International: Materials Park, OH, USA, 1993; pp. 12–28. [Google Scholar]
- Palm, M.; Inden, G.; Thomas, N. The Fe-Al-Ti system. J. Phase Equilibria 1995, 16, 209–222. [Google Scholar] [CrossRef]
- Vernieres, J.; Benelmekki, M.; Kim, J.H.; Grammatikopoulos, P.; Bobo, J.F.; Diaz, R.E.; Sowwan, M. Single-step gas phase synthesis of stable iron aluminide nanoparticles with soft magnetic properties. APL Mater. 2014, 2, 116105. [Google Scholar] [CrossRef]
- Jirásková, Y.; Pizúrová, N.; Titov, A.; Janičkovič, D.; Friák, M. Phase separation in Fe-Ti-Al alloy—Structural, magnetic, and Mössbauer study. J. Magn. Magn. Mater. 2018, 468, 91–99. [Google Scholar] [CrossRef]
- Palm, M.; Lacaze, J. Assessment of the Al-Fe-Ti system. Intermetallics 2006, 14, 1291–1303. [Google Scholar] [CrossRef]
- Dobeš, F.; Dymáček, P.; Friák, M. Force-to-stress conversion methods in small punch testing exemplified by creep results of Fe-Al alloy with chromium and cerium additions. IOP Conf. Ser. Mater. Sci. Eng. 2018, 461, 012017. [Google Scholar] [CrossRef]
- Dobeš, F.; Dymáček, P.; Friák, M. Small punch creep of Fe-Al-Cr alloy with Ce addition and its relation to uniaxial creep tests. Met. Mater. Kovové Mater. 2018, 56, 205. [Google Scholar] [CrossRef]
- Palm, M.; Sauthoff, G. Deformation behaviour and oxidation resistance of single-phase and two-phase L21-ordered Fe-Al-Ti alloys. Intermetallics 2004, 12, 1345–1359. [Google Scholar] [CrossRef]
- Sundman, B.; Ohnuma, I.; Dupin, N.; Kattner, U.R.; Fries, S.G. An assessment of the entire Al-Fe system including D03 ordering. Acta Mater. 2009, 57, 2896–2908. [Google Scholar] [CrossRef]
- Dymáček, P.; Dobeš, F.; Jirásková, Y.; Pizúrová, N.; Friák, M. Tensile, creep and fracture testing of prospective Fe-Al-based alloys using miniature specimens. Theor. Appl. Fract. Mech. 2019, 99, 18–26. [Google Scholar] [CrossRef]
- Dobeš, F.; Dymáček, P.; Friák, M. The influence of niobium additions on creep resistance of Fe-27 at. % Al alloys. Metals 2019, 9, 739. [Google Scholar] [CrossRef]
- Grigorchik, A.N.; Astrashab, V.E.; Kukareko, V.A.; Belotserkovsky, M.A.; Sosnovsky, V.A. High-temperature heat treatment of hypersonic metallization coatings from pseudoalloy “Fe-Al”. Lett. Mater. 2021, 11, 198–203. [Google Scholar] [CrossRef]
- Deevi, S.C. Advanced intermetallic iron aluminide coatings for high temperature applications. Prog. Mater. Sci. 2021, 118, 100769. [Google Scholar] [CrossRef]
- Tolochyn, O.I.; Baglyuk, G.A.; Tolochyna, O.V.; Evych, Y.I.; Podrezov, Y.M.; Molchanovska, H.M. Structure and physicomechanical properties of the Fe3Al intermetallic compound obtained by impact hot compaction. Mater. Sci. 2021, 56, 499–508. [Google Scholar] [CrossRef]
- Komarov, O.N.; Zhilin, S.G.; Predein, V.V.; Popov, A.V. Mechanisms for forming iron-containing intermetallics prepared by aluminothermy and the effect of special treatment methods on their properties. Metallurgist 2020, 64, 810–821. [Google Scholar] [CrossRef]
- Vodičková, V.; Švec, M.; Hanus, P.; Novák, P.; Záděra, A.; Keller, V.; Prokopčáková, P.P. The effect of simultaneous Si and Ti/Mo alloying on high-temperature strength of Fe3Al-based iron aluminides. Molecules 2020, 25, 4268. [Google Scholar] [CrossRef]
- Luo, X.; Cao, J.; Meng, G.; Chuan, Y.; Yao, Z.; Xie, H. Systematical investigation on the microstructures and tribological properties of Fe-Al laser cladding coatings. Appl. Surf. Sci. 2020, 516, 146121. [Google Scholar] [CrossRef]
- Luo, X.; Cao, J.; Meng, G.; Yu, F.; Jiang, Q.; Zhang, P.; Xie, H. Double glow plasma surface metallurgy technology fabricated Fe-Al-Cr coatings with excellent corrosion resistance. Coatings 2020, 10, 575. [Google Scholar] [CrossRef]
- Teker, T.; Yilmaz, S.O. Synthesis and structural characterization of Fe based Ti+Ni3Al+Al2O3 reinforcement composite produced by mechanical alloying. Rev. Metal. 2020, 56, 4. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, Y.; Niu, M.; Shao, M.; Geng, X. Microstructure and mechanical behavior of in situ TiC reinforced Fe3Al (Fe-23Al-3Cr) matrix composites by mechanical alloying and vacuum hot-pressing sintering technology. Vacuum 2020, 180, 109544. [Google Scholar] [CrossRef]
- Ghazanfari, H.; Blais, C.; Gariepy, M.; Savoie, S.; Schulz, R.; Alamdari, H. Improving wear resistance of metal matrix composites using reinforcing particles in two length-scales: Fe3Al/TiC composites. Surf. Coatigs Technol. 2020, 386, 125502. [Google Scholar] [CrossRef]
- Khodaei, M. Characterization of Al2O3 in Fe3Al-30 vol.% Al2O3 nanocomposite powder synthesized by mechanochemical process. J. Nanostruct. 2020, 10, 456–462. [Google Scholar] [CrossRef]
- Altunin, R.R.; Moiseenko, E.T.; Zharkov, S.M. Structural phase transformations during a solid-state reaction in a bilayer Al/Fe thin-film nanosystem. Phys. Solid State 2020, 62, 200–205. [Google Scholar] [CrossRef]
- Tolochyn, O.I.; Tolochyna, O.V.; Bagliuk, H.A.; Yevych, Y.I.; Podrezov, Y.M.; Mamonova, A.A. Influence of sintering temperature on the structure and properties of powder iron aluminide Fe3Al. Powder Metall. Met. Ceram. 2020, 59, 150–159. [Google Scholar] [CrossRef]
- Adler, L.; Fu, Z.; Koerner, C. Electron beam based additive manufacturing of Fe3Al based iron aluminides—Processing window, microstructure and properties. Mater. Sci. Eng. A 2020, 785, 139369. [Google Scholar] [CrossRef]
- Michalcová, A.; Ozkan, M.; Mikula, P.; Marek, I.; Knaislová, A.; Kopeček, J.; Vojtěch, D. The influence of powder milling on properties of SPS compacted FeAl. Molecules 2020, 25, 2263. [Google Scholar] [CrossRef]
- Peska, M.; Karczewski, K.; Rzeszotarska, M.; Polanski, M. Direct synthesis of Fe-Al alloys from elemental powders ssing laser engineered net shaping. Materials 2020, 13, 531. [Google Scholar] [CrossRef]
- Luo, X.; Cao, J.; Meng, G.; Zhou, Y.; Xie, H. Long-range-ordered Fe3Al with excellent electromagnetic wave absorption. J. Mater. Sci. Mater. Electron. 2020, 31, 15608–15615. [Google Scholar] [CrossRef]
- Ismail, A.; Bahanan, W.; Bin Hussain, P.; Saat, A.M.; Shaik, N.B. Diffusion bonding of Al-Fe enhanced by gallium. Processes 2020, 8, 824. [Google Scholar] [CrossRef]
- Watson, R.E.; Weinert, M. Transition-metal aluminide formation: Ti, V, Fe, and Ni aluminides. Phys. Rev. B 1998, 58, 5981–5988. [Google Scholar] [CrossRef]
- Gonzales-Ormeno, P.; Petrilli, H.; Schön, C. Ab-initio calculations of the formation energies of BCC-based superlattices in the Fe-Al system. Calphad 2002, 26, 573–582. [Google Scholar] [CrossRef]
- Connetable, D.; Maugis, P. First principle calculations of the kappa-Fe3AlC perovskite and iron-aluminium intermetallics. Intermetallics 2008, 16, 345–352. [Google Scholar] [CrossRef]
- Kellou, A.; Grosdidier, T.; Raulot, J.M.; Aourag, H. Atomistic study of magnetism effect on structural stability in Fe3Al and Fe3AlX (X = H, B, C, N, O) alloys. Phys. Status Solidi B Basic Solid State Phys. 2008, 245, 750–755. [Google Scholar] [CrossRef]
- Šesták, P.; Friák, M.; Holec, D.; Všianská, M.; Šob, M. Strength and brittleness of interfaces in Fe-Al superalloy nanocomposites under multiaxial loading: An ab initio and atomistic study. Nanomaterials 2018, 8, 873. [Google Scholar] [CrossRef]
- Lechermann, F.; Fähnle, M.; Meyer, B.; Elsässer, C. Electronic correlations, magnetism, and structure of Fe-Al subsystems: An LDA + U study. Phys. Rev. B 2004, 69, 165116. [Google Scholar] [CrossRef]
- Airiskallio, E.; Nurmi, E.; Heinonen, M.H.; Vayrynen, I.J.; Kokko, K.; Ropo, M.; Punkkinen, M.P.J.; Pitkanen, H.; Alatalo, M.; Kollar, J.; et al. High temperature oxidation of Fe-Al and Fe-Cr-Al alloys: The role of Cr as a chemically active element. Corros. Sci. 2010, 52, 3394–3404. [Google Scholar] [CrossRef]
- Lechermann, F.; Welsch, F.; Elsässer, C.; Ederer, C.; Fähnle, M.; Sanchez, J.; Meyer, B. Density-functional study of Fe3Al: LSDA versus GGA. Phys. Rev. B 2002, 65, 132104. [Google Scholar] [CrossRef]
- Friák, M.; Slávik, A.; Miháliková, I.; Holec, D.; Všianská, M.; Šob, M.; Palm, M.; Neugebauer, J. Origin of the low magnetic moment in Fe2AlTi: An Ab initio study. Materials 2018, 11, 1732. [Google Scholar] [CrossRef]
- Ju, J.; Kang, M.; Zhou, Y.; Yang, C.; Wang, K.; Li, J.; Wang, R.; Fu, H.; Wang, J. First-principles investigations of the stability, electronic structures, mechanical properties and thermodynamic properties of FexAlyCz compounds in Fe-Cr-B-Al-C alloy. J. Phys. Chem. Solids 2020, 143, 109366. [Google Scholar] [CrossRef]
- Miháliková, I.; Friák, M.; Jirásková, Y.; Holec, D.; Koutná, N.; Šob, M. Impact of nano-scale distribution of atoms on electronic and magnetic properties of phases in Fe-Al nanocomposites: An ab initio study. Nanomaterials 2018, 8, 1059. [Google Scholar] [CrossRef] [PubMed]
- Friák, M.; Holec, D.; Šob, M. Quantum-mechanical study of nanocomposites with low and ultra-low interface energies. Nanomaterials 2018, 8, 1057. [Google Scholar] [CrossRef] [PubMed]
- Kulikov, N.I.; Postnikov, A.V.; Borstel, G.; Braun, J. Onset of magnetism in B2 transition-metal aluminides. Phys. Rev. B 1999, 59, 6824–6833. [Google Scholar] [CrossRef]
- Friák, M.; Neugebauer, J. Ab initio study of the anomalous volume-composition dependence in Fe-Al alloys. Intermetallics 2010, 18, 1316–1321. [Google Scholar] [CrossRef]
- Ipser, H.; Semenova, O.; Krachler, R. Intermetallic phases with D03-structure: A statistical-thermodynamic model. J. Alloy. Compd. 2002, 338, 20–25. [Google Scholar] [CrossRef]
- Fähnle, M.; Drautz, R.; Lechermann, F.; Singer, R.; Diaz-Ortiz, A.; Dosch, H. Thermodynamic properties from ab-initio calculations: New theoretical developments, and applications to various materials systems. Phys. Status Solidi B Basic Solid State Phys. 2005, 242, 1159–1173. [Google Scholar] [CrossRef]
- Kirklin, S.; Saal, J.E.; Hegde, V.I.; Wolverton, C. High-throughput computational search for strengthening precipitates in alloys. Acta Mater. 2016, 102, 125–135. [Google Scholar] [CrossRef]
- Liu, S.; Duan, S.; Ma, B. First-principles calculation of vibrational entropy for Fe-Al compounds. Phys. Rev. B 1998, 58, 9705–9709. [Google Scholar]
- Čížek, J.; Lukáč, F.; Procházka, I.; Kužel, R.; Jirásková, Y.; Janičkovič, D.; Anwand, W.; Brauer, G. Characterization of quenched-in vacancies in Fe-Al alloys. Physica B 2012, 407, 2659–2664. [Google Scholar] [CrossRef]
- Miháliková, I.; Friák, M.; Koutná, N.; Holec, D.; Šob, M. An ab initio study of vacancies in disordered magnetic systems: A case study of Fe-rich Fe-Al phases. Materials 2019, 12, 1430. [Google Scholar] [CrossRef]
- Amara, H.; Fu, C.C.; Soisson, F.; Maugis, P. Aluminum and vacancies in α-iron: Dissolution, diffusion, and clustering. Phys. Rev. B 2010, 81, 174101. [Google Scholar] [CrossRef]
- Friák, M.; Černý, M.; Všianská, M.; Šob, M. Impact of antiphase boundaries on structural, magnetic and vibrational properties of Fe3Al. Materials 2020, 13, 4884. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Yang, J. First principle calculations and mechanical properties of the intermetallic compounds in a laser welded steel/aluminum joint. Opt. Laser Technol. 2020, 122, 105875. [Google Scholar] [CrossRef]
- Friák, M.; Černý, M.; Šob, M. The effect of hydrogen on the stress-strain response in Fe3Al: An ab initio molecular-dynamics study. Materials 2021, 14, 4155. [Google Scholar] [CrossRef]
- Grover, A.K.; Pillay, R.G.; Nagarajan, V.; Tandon, P.N. Site preference and local environment effects in ferromagnetic ternary alloys. J. Magn. Magn. Mater. 1980, 15, 699–700. [Google Scholar] [CrossRef]
- Marcinkowski, M.; Brown, N. Theory and direct observation of dislocations in the Fe3Al superlattices. Acta Metall. 1961, 9, 764–786. [Google Scholar] [CrossRef]
- Marcinkowski, M.J.; Brown, N. Direct observation of antiphase boundaries in the Fe3Al superlattice. J. Appl. Phys. 1962, 33, 537–552. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Y. The partitioning behavior of dual solutes at the antiphase domain boundary in the B2 intermetallic: A microscopic phase-field study. J. Alloy. Compd. 2020, 824, 153923. [Google Scholar] [CrossRef]
- Koizumi, Y.; Allen, S.M.; Ouchi, M.; Minamino, Y. Effects of solute and vacancy segregation on antiphase boundary migration in stoichiometric and Al-rich Fe3Al: A phase-field simulation study. Intermetallics 2010, 18, 1297–1302. [Google Scholar] [CrossRef][Green Version]
- Koizumi, Y.; Allen, S.M.; Minamino, Y. Effects of solute and vacancy segregation on migration of a/4〈111〉 and a/2〈100〉 antiphase boundaries in Fe3Al. Acta Mater. 2009, 57, 3039–3051. [Google Scholar] [CrossRef][Green Version]
- Koizumi, Y.; Allen, S.M.; Ouchi, M.; Minamino, Y.; Chiba, A. Phase-field simulation of D03-type antiphase boundary migration in Fe3Al with vacancy and solute segregation. Solid State Phenom. 2011, 172, 1313–1319. [Google Scholar] [CrossRef]
- Allen, S.; Cahn, J. Microscopic theory for antiphase boundary motion and its application to antiphase domain coarsening. Acta Metall. 1979, 27, 1085–1095. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Y.; Cheng, Y. The formation and dynamic evolution of antiphase domain boundary in FeAl alloy: Computational simulation in atomic scale. Mater. Res. Ibero-Am. J. Mater. 2018, 21, e20171048. [Google Scholar] [CrossRef]
- Balagurov, A.M.; Bobrikov, I.A.; Sumnikov, V.S.; Golovin, I.S. Antiphase domains or dispersed clusters? Neutron diffraction study of coherent atomic ordering in Fe3Al-type alloys. Acta Mater. 2018, 153, 45–52. [Google Scholar] [CrossRef]
- Murakami, Y.; Niitsu, K.; Tanigaki, T.; Kainuma, R.; Park, H.S.; Shindo, D. Magnetization amplified by structural disorder within nanometre-scale interface region. Nat. Commun. 2014, 5, 4133. [Google Scholar] [CrossRef] [PubMed]
- Oguma, R.; Matsumura, S.; Eguchi, T. Kinetics of B2-and D03 type ordering and formation of domain structures in Fe-Al alloys. J. Phys. Condens. Matter 2008, 20, 275225. [Google Scholar] [CrossRef] [PubMed]
- Friák, M.; Všianská, M.; Šob, M. A quantum-mechanical study of clean and Cr-segregated antiphase boundaries in Fe3Al. Materials 2019, 12, 3954. [Google Scholar] [CrossRef]
- Friák, M.; Buršíková, V.; Pizúrová, N.; Pavlů, J.; Jirásková, Y.; Homola, V.; Miháliková, I.; Slávik, A.; Holec, D.; Všianská, M.; et al. Elasticity of phases in Fe-Al-Ti superalloys: Impact of atomic order and anti-phase boundaries. Crystals 2019, 9, 299. [Google Scholar] [CrossRef]
- Friák, M.; Golian, M.; Holec, D.; Koutná, N.; Šob, M. An ab initio study of magnetism in disordered Fe-Al alloys with thermal antiphase boundaries. Nanomaterials 2020, 10, 44. [Google Scholar] [CrossRef]
- Friák, M.; Černý, M.; Šob, M. The impact of vibrational entropy on the segregation of Cu to antiphase boundaries in Fe3Al. Magnetochemistry 2021, 7, 108. [Google Scholar] [CrossRef]
- Zunger, A.; Wei, S.; Ferreira, L.; Bernard, J. Special quasirandom structures. Phys. Rev. Lett. 1990, 65, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Oganov, A.R.; Glass, C.W. Crystal structure prediction using ab initio evolutionary techniques: Principles and applications. J. Chem. Phys. 2006, 124, 244704. [Google Scholar] [CrossRef] [PubMed]
- Lyakhov, A.O.; Oganov, A.R.; Stokes, H.T.; Zhu, Q. New developments in evolutionary structure prediction algorithm USPEX. Comput. Phys. Commun. 2013, 184, 1172–1182. [Google Scholar] [CrossRef]
- Oganov, A.R.; Lyakhov, A.O.; Valle, M. How evolutionary crystal structure prediction works—and why. Accounts Chem. Res. 2011, 44, 227–237. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. B 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. A 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef] [PubMed]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: A critical analysis. Can. J. Phys. 1980, 58, 1200. [Google Scholar] [CrossRef]
- Všianská, M.; Friák, M.; Šob, M. An ab initio study of Fe3Al: A critical review of generalized gradient approximation. Comp. Phys. Comm. 2022. to be published. [Google Scholar]
- Friák, M.; Oweisová, S.; Pavlů, J.; Holec, D.; Šob, M. An ab initio study of thermodynamic and mechanical stability of Heusler-based Fe2AlCo polymorphs. Materials 2018, 11, 1543. [Google Scholar] [CrossRef]
- Gracias, J. Ab Initio Modelling of Properties of Al3Fe13 and Al4Co4Fe8 Phase. Bachelor’s Thesis, Masaryk University in Brno, Brno, Czech Republic, 2020. (In Czech). [Google Scholar]
- von Pezold, J.; Dick, A.; Friak, M.; Neugebauer, J. Generation and performance of special quasirandom structures for studying the elastic properties of random alloys: Application to Al-Ti. Phys. Rev. B 2010, 81, 094203. [Google Scholar] [CrossRef]
- Jain, V.; Nehra, J.; Sudheesh, V.D.; Nambakkat, L.; Venugopalan, K. Comparative study of the structural and magnetic properties of bulk and nano-sized Fe2CoAl. AIP Conf. Proc. 2013, 1536, 935. [Google Scholar] [CrossRef]
- Moakher, M.; Norris, A.N. The closest elastic tensor of arbitrary symmetry to an elasticity tensor of lower symmetry. J. Elast. 2006, 85, 215–263. [Google Scholar] [CrossRef]
- Gaillac, R.; Pullumbi, P.; Coudert, F.X. ELATE: An open-source online application for analysis and visualization of elastic tensors. J. Phys. Condens. Matter 2016, 28, 275201. [Google Scholar] [CrossRef] [PubMed]
- Voigt, W. Lehrbuch der Kristallphysik; Teubner: Stuttgart, Germany, 1928. [Google Scholar]
- Reuss, A. Account of the liquid limit of mixed crystals on the basis of the plasticity condition for single crystal. Z. Angew. Math. Mech. 1929, 9, 49–58. [Google Scholar] [CrossRef]
- Hill, R. The elastic behaviour of a Ccystalline aggregate. Proc. Phys. Soc. Sect. A 1952, 65, 349–354. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
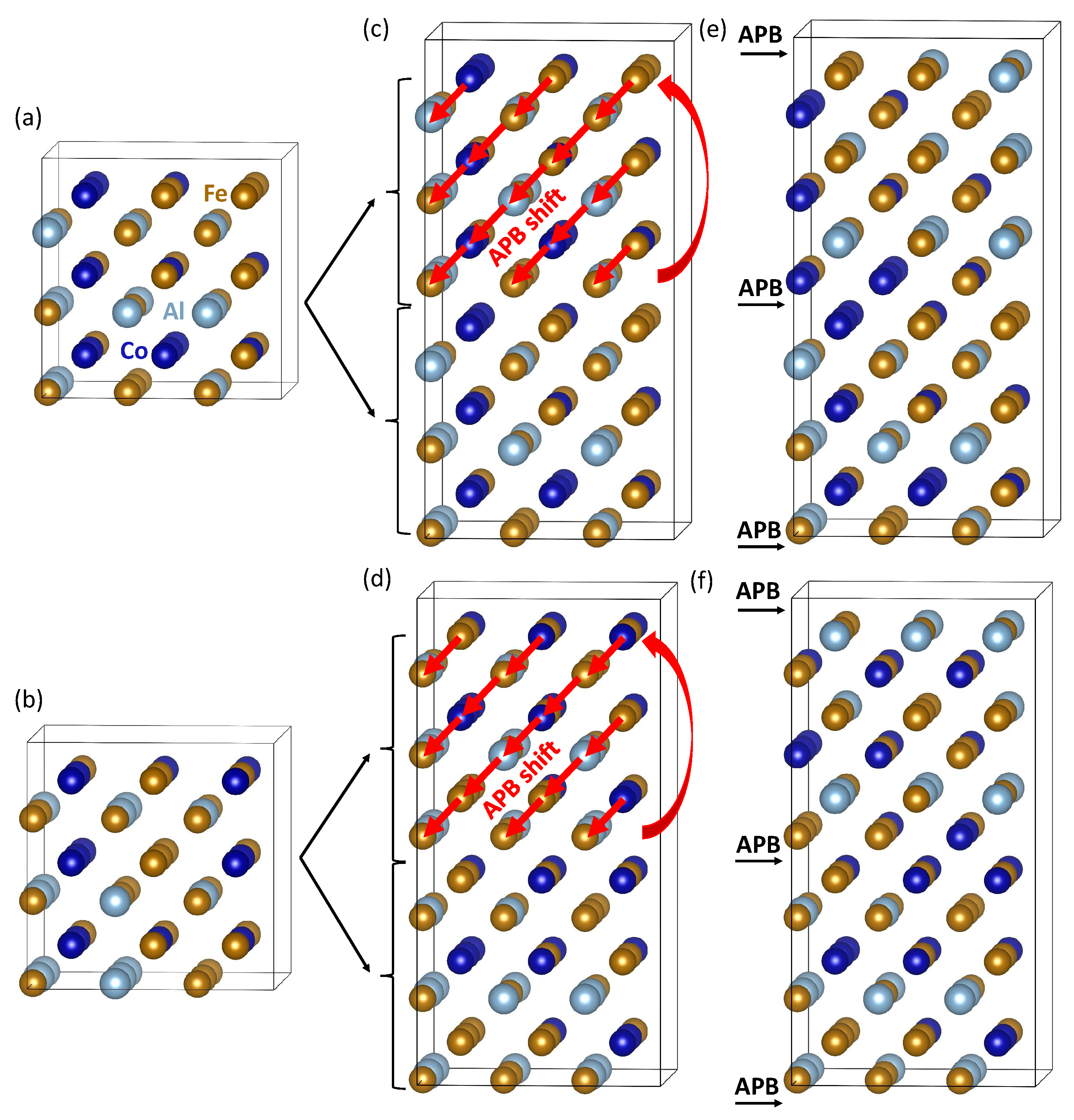
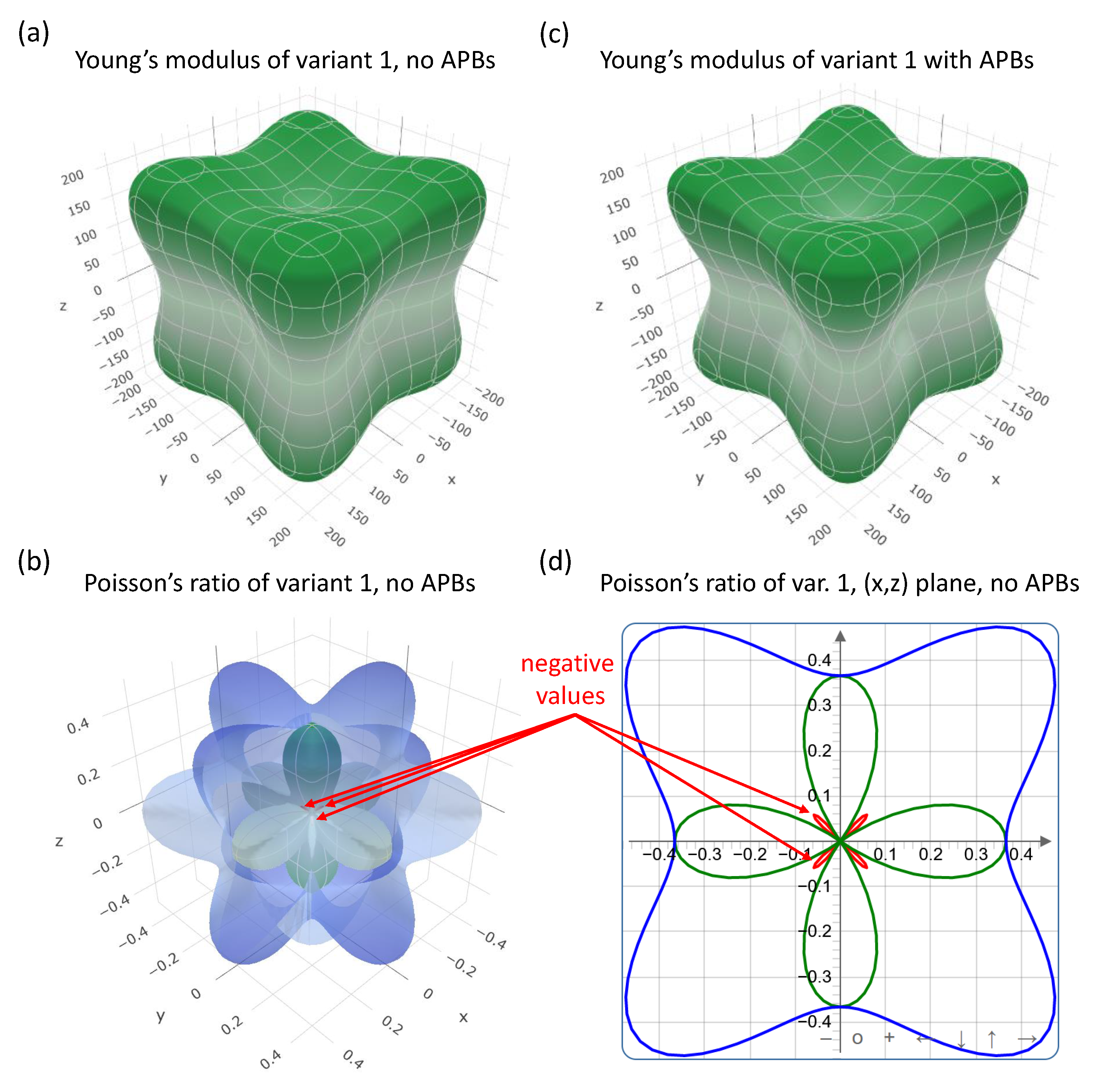
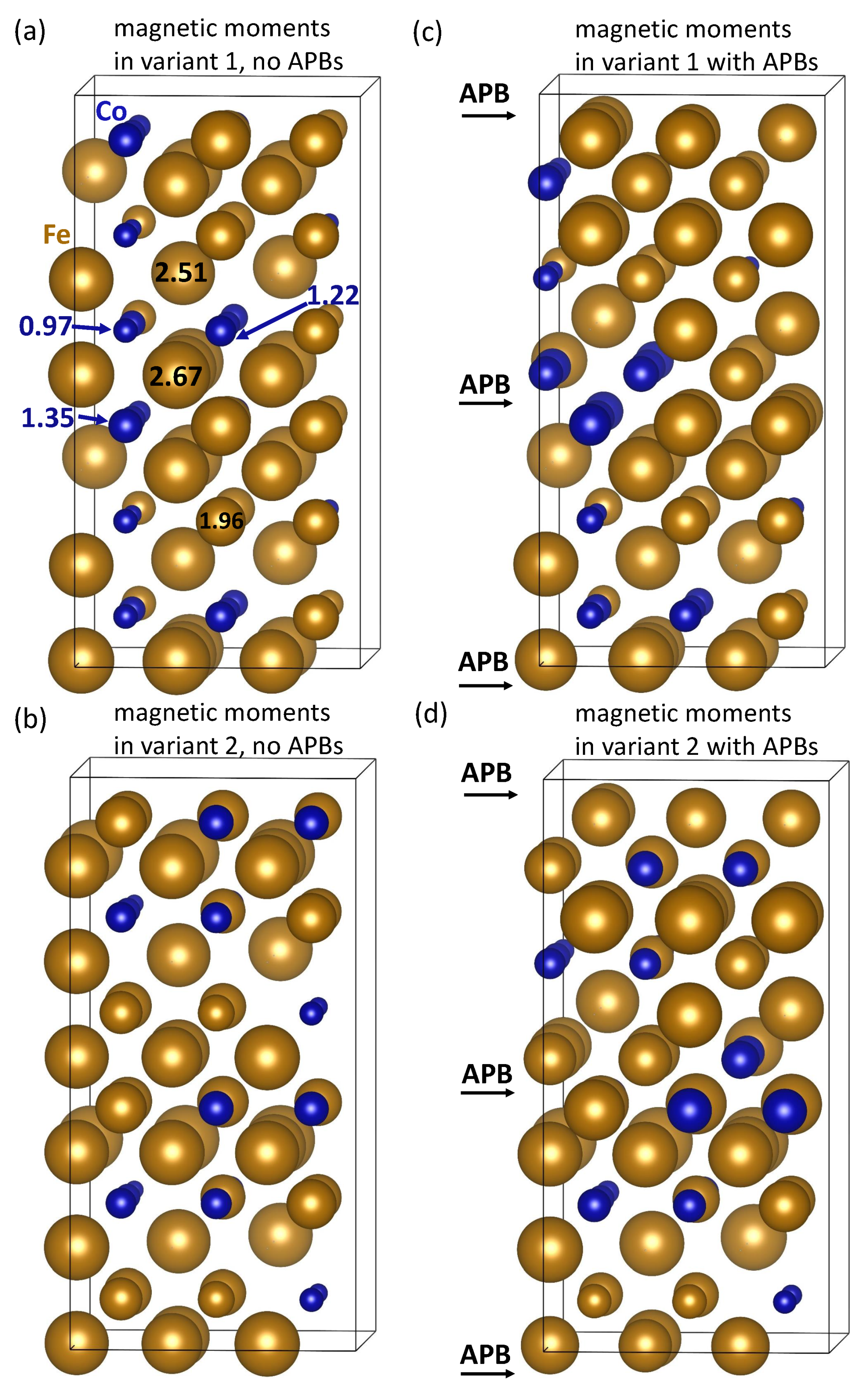
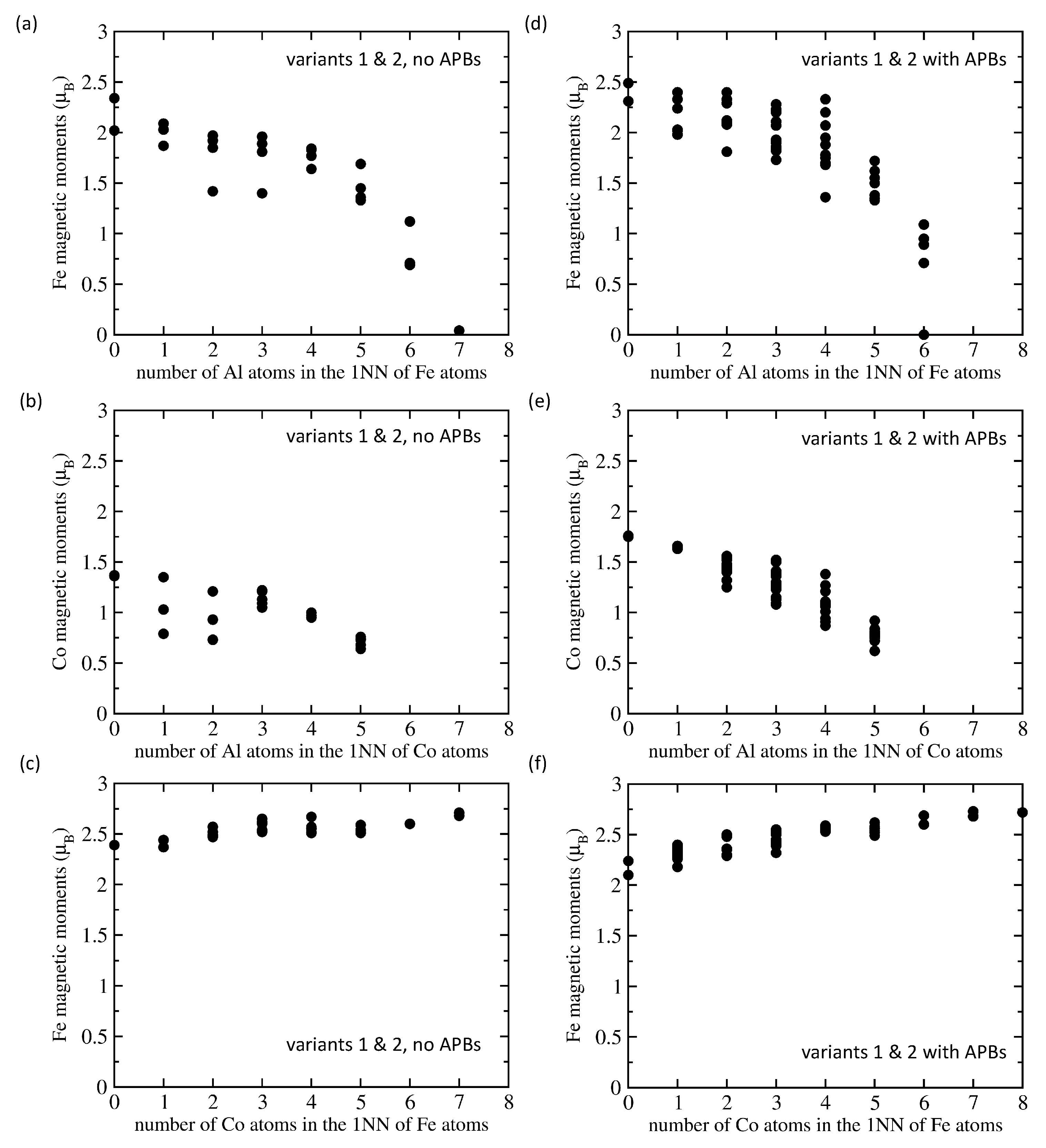
| Structure | V | |||||
|---|---|---|---|---|---|---|
| eV/atom | Å/atom | Å | /atom | mJ/m | ||
| var. 1 no APBs | −0.243 | 11.56 | 2.849 | 1.000 | 1.266 | — |
| var. 1 with APBs | −0.226 | 11.65 | — | 1.011 | 1.344 | 199 |
| exp. A2-phase [112] | — | 11.77 | 2.866 | 1.000 | 1.18–1.23 | — |
| var. 2 no APBs | −0.244 | 11.58 | 2.850 | 1.000 | 1.274 | — |
| var. 2 with APBs | −0.218 | 11.72 | — | 1.006 | 1.380 | 310 |
| Single-Crystal | ||||||
| Elastic Constants | (GPa) | (GPa) | (GPa) | (GPa) | (GPa) | (GPa) |
| var. 1 without APBs | 244 | 244 | 141 | 141 | 131 | 131 |
| var. 1 with APBs | 232 | 228 | 138 | 143 | 134 | 132 |
| var. 2 without APBs | 247 | 247 | 141 | 141 | 132 | 132 |
| var. 2 with APBs | 234 | 227 | 136 | 141 | 133 | 132 |
| (GPa) | (GPa) | (GPa) | (GPa) | |||
| var. 1 without APBs | 141 | 315 | 52 | 131 | −0.083 | 0.626 |
| var. 1 with APBs | 117 | 318 | 43 | 134 | −0.139 | 0.728 |
| var. 2 without APBs | 145 | 317 | 53 | 132 | −0.075 | 0.614 |
| var. 2 with APBs | 120 | 316 | 45 | 133 | −0.125 | 0.715 |
| Polycrystal | B | Y | G | |
|---|---|---|---|---|
| Elasticity | Voigt/Reuss/Hill Values in GPa | Voigt/Reuss/Hill | ||
| APB-free var. 1 | 175/175/175 | 250/211/231 | 99/81/90 | 0.262/0.300/0281 |
| var. 1 with APBs | 171/171/171 | 247/194/221 | 98/74/86 | 0.260/0.311/0.285 |
| APB-free var. 2 | 176/176/176 | 253/215/234 | 100/83/92 | 0.261/0.297/0.279 |
| var. 2 with APBs | 170/170/170 | 247/198/223 | 98/76/87 | 0.258/0.306/0.282 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Friák, M.; Gracias, J.; Pavlů, J.; Šob, M. A Quantum-Mechanical Study of Antiphase Boundaries in Ferromagnetic B2-Phase Fe2CoAl Alloy. Magnetochemistry 2021, 7, 137. https://doi.org/10.3390/magnetochemistry7100137
Friák M, Gracias J, Pavlů J, Šob M. A Quantum-Mechanical Study of Antiphase Boundaries in Ferromagnetic B2-Phase Fe2CoAl Alloy. Magnetochemistry. 2021; 7(10):137. https://doi.org/10.3390/magnetochemistry7100137
Chicago/Turabian StyleFriák, Martin, Josef Gracias, Jana Pavlů, and Mojmír Šob. 2021. "A Quantum-Mechanical Study of Antiphase Boundaries in Ferromagnetic B2-Phase Fe2CoAl Alloy" Magnetochemistry 7, no. 10: 137. https://doi.org/10.3390/magnetochemistry7100137
APA StyleFriák, M., Gracias, J., Pavlů, J., & Šob, M. (2021). A Quantum-Mechanical Study of Antiphase Boundaries in Ferromagnetic B2-Phase Fe2CoAl Alloy. Magnetochemistry, 7(10), 137. https://doi.org/10.3390/magnetochemistry7100137








