Interaction of Nanocomposites Based on the FemOn–SiO2 System with an Electromagnetic Field in an Ultra-Wide Frequency Range
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Physicochemical Properties of the Nanocomposites
3.2. Physical Effects Observed in the Interaction of FemOn–SiO2 Nanocomposites with EMF in an Ultra-Wide Frequency Range
3.2.1. Effects of Exposure to QSMF
3.2.2. Effects of Exposure to AMF
3.2.3. Effects of Exposure to MW
3.2.4. Effects of Exposure to THz
3.2.5. Effects of Exposure to FIR and MIR
3.2.6. Effects of Exposure to NIR, Vis and UV
3.2.7. Effects of Exposure to X-ray
3.2.8. Effects of Exposure to γ-Radiation
4. Discussion
5. Conclusions
Funding
Conflicts of Interest
References
- Klein, L.; Jitianu, A.; Aparicio, M. (Eds.) Handbook of Sol-Gel Science and Technology, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 9–15. ISBN 978-3-319-32101-1. [Google Scholar]
- Owens, G.J.; Singh, R.K.; Foroutan, F.; Alqaysi, M.; Han, C.-M.; Mahapatra, C.; Kim, H.-W.; Knowles, J.C. Sol-gel based materials for biomedical applications. Prog. Mater. Sci. 2016, 77, 1–79. [Google Scholar] [CrossRef]
- Katz, E. Synthesis, properties and applications of magnetic nanoparticles and nanowires—A brief introduction. Magnetochemistry 2019, 5, 61. [Google Scholar] [CrossRef]
- Barrera, G.; Tiberto, P.; Allia, P.; Bonelli, B.; Esposito, S.; Marocco, A.; Pansini, M.; Leterrier, Y. Magnetic properties of nanocomposites. Appl. Sci. 2019, 9, 212. [Google Scholar] [CrossRef]
- Gul, S.; Khan, S.B.; Rehman, I.U.; Khan, M.A.; Khan, M.I. A Comprehensive review of magnetic nanomaterials modern day theranostics. Front. Mater. 2019, 6, 179. [Google Scholar] [CrossRef]
- Kalia, S.; Kango, S.; Kumar, A.; Haldorai, Y.; Kumari, B.; Kumar, R. Magnetic polymer nanocomposites for environmental and biomedical applications. Colloid. Polym. Sci. 2014, 292, 2025–2052. [Google Scholar] [CrossRef]
- Hauser, A.K.; Wydra, R.J.; Stocke, N.A.; Anderson, K.W.; Hilt, J.Z. Magnetic nanoparticles and nanocomposites for remote controlled therapies. J. Control. Release 2015, 219, 79–94. [Google Scholar] [CrossRef]
- Toropova, Y.G.; Golovkin, A.S.; Malashicheva, A.B.; Korolev, D.V.; Gorshkov, A.N.; Gareev, K.G.; Afonin, M.V.; Galagudza, M.M. In vitro toxicity of FemOn, FemOn-SiO2 composite, and SiO2-FemOn core-shell magnetic nanoparticles. Int. J. Nanomed. 2017, 12, 593–603. [Google Scholar] [CrossRef]
- Toropova, Y.G.; Pechnikova, N.A.; Zelinskaya, I.A.; Korolev, D.V.; Gareev, K.G.; Markitantova, A.S.; Bogushevskaya, V.D.; Povolotskaya, A.V.; Manshina, A.A. Hemocompatibility of magnetic magnetite nanoparticles and magnetite-silica composites in vitro. Bull. Sib. Med. 2018, 17, 157–167. [Google Scholar] [CrossRef]
- Gareev, K.G.; Babikova, К.Y.; Postnov, V.N.; Naumisheva, E.B.; Korolev, D.V. Fluorescence imaging of the nanoparticles modified with indocyanine green. J. Phys. Conf. Ser. 2017, 917, 042008. [Google Scholar] [CrossRef]
- Korolev, D.V.; Postnov, V.N.; Evreinova, N.V.; Babikova, K.Y.; Naumysheva, E.B.; Shulmeister, G.A.; Magruk, M.A.; Mishanin, V.I.; Toropova, Y.G.; Gareev, K.G.; et al. Synthesis of magnetic nanoparticles with radiopaque marker. Russ. J. Gen. Chem. 2018, 88, 2698–2701. [Google Scholar] [CrossRef]
- Korolev, D.V.; Evreinova, N.V.; Zakharova, E.V.; Gareev, K.G.; Naumysheva, E.B.; Postnov, D.V.; Postnov, V.N.; Galagudza, M.M. Phosphocreatine immobilization of the surface of silica and magnetite nanoparticles for targeted drug delivery. Russ. Chem. Bull. 2019, 68, 1096–1101. [Google Scholar] [CrossRef]
- Korolev, D.V.; Gareev, K.G.; Zorin, V.N.; Evreinova, N.V.; Postnov, V.N.; Romanova, T.N. Synthesis of glycidoxy spacer on the surface of magnetic nanoparticles and immobilization of albumin. J. Phys. Conf. Ser. 2019, 1410, 012069. [Google Scholar] [CrossRef]
- Bogachev, Y.V.; Chernenco, J.S.; Gareev, K.G.; Kononova, I.E.; Matyushkin, L.B.; Moshnikov, V.A.; Nalimova, S.S. The study of aggregation processes in colloidal solutions of magnetite–silica nanoparticles by NMR relaxometry, AFM, and UV–Vis-Spectroscopy. Appl. Magn. Reson. 2014, 45, 329–337. [Google Scholar] [CrossRef]
- Bogachev, Y.V.; Gareev, K.G.; Matyushkin, L.B.; Moshnikov, V.A.; Naumova, A.N. Study of magnetite nanoparticle suspensions by photometry and NMR relaxometry. Phys. Solid State 2013, 55, 2313–2317. [Google Scholar] [CrossRef]
- Gareev, K.G.; Luchinin, V.V.; Sevost’yanov, E.N.; Testov, I.O.; Testov, O.A. Frequency dependence of an electromagnetic absorption coefficient in magnetic fluid. Tech. Phys. 2019, 64, 893–896. [Google Scholar] [CrossRef]
- Kharitonskii, P.V.; Gareev, K.G.; Ionin, S.A.; Ryzhov, V.A.; Bogachev, Y.V.; Klimenkov, B.D.; Kononova, I.E.; Moshnikov, V.A. Microstructure and magnetic state of Fe3O4-SiO2 colloidal particles. J. Magn. 2015, 20, 221–228. [Google Scholar] [CrossRef]
- Kononova, I.E.; Gareev, K.G.; Moshnikov, V.A.; Al’myashev, V.I.; Kucherova, O.V. Self-assembly of fractal magnetite–silica aggregates in a static magnetic field. Inorg. Mater. 2014, 50, 68–74. [Google Scholar] [CrossRef]
- Gareev, K.G.; Kononova, I.E.; Levitckii, V.S.; Moshnikov, V.A.; Nalimova, S.S. Influence of constant magnetic field on aggregation processes in magnetite colloids. J. Phys. Conf. Ser. 2014, 572, 012027. [Google Scholar] [CrossRef]
- Vezo, O.S.; Gareev, K.G.; Korolev, D.V.; Kuryshev, I.A.; Lebedev, S.V.; Moshnikov, V.A.; Sergienko, E.S.; Kharitonskii, P.V. Aggregate stability and magnetic characteristics of colloidal FemOn–SiO2 particles obtained by Sol–Gel method. Phys. Solid State 2017, 59, 1008–1013. [Google Scholar] [CrossRef]
- Kharitonskii, P.V.; Gareev, K.G.; Frolov, A.M.; Lebedev, S.V.; Velikorussov, P.V. The investigation of superparamagnetic colloidal particles FemOn-SiO2. Solid State Phenom. 2016, 249, 138–141. [Google Scholar] [CrossRef]
- Gareev, K.G.; Ionin, S.A.; Korolev, D.V.; Luchinin, V.V.; Moshnikov, V.A.; Panov, M.F.; Permyakov, N.V. Study of colloidal particles FemOn-SiO2 synthesized by two different techniques. J. Phys. Conf. Ser. 2015, 643, 012088. [Google Scholar] [CrossRef]
- Kharitonskii, P.; Kamzin, A.; Gareev, K.; Valiullin, A.; Vezo, O.; Sergienko, E.; Korolev, D.; Kosterov, A.; Lebedev, S.; Gurylev, A.; et al. Magnetic granulometry and Mössbauer spectroscopy of FemOn-SiO2 colloidal nanoparticles. J. Magn. Magn. Mater. 2018, 461, 30–36. [Google Scholar] [CrossRef]
- Kharitonskii, P.; Gareev, K.; Korolev, D.; Sergienko, E. Magnetic properties of FemOn-SiO2 colloidal nanoparticles: Theoretical and experimental aspects. AIP Conf. Proc. 2016, 1748, 050009. [Google Scholar] [CrossRef]
- Kharitonskii, P.; Frolov, A.; Gareev, K.; Korolev, D.; Sergienko, E.; Ivanova, E.; Vlasenko, S. The anhysteretic remanent magnetization of magnetite-silica composite nanoparticles. AIP Conf. Proc. 2017, 1874, 040010. [Google Scholar] [CrossRef]
- Smerdov, R.S.; Bocharova, T.V.; Gareev, K.G. Spectroscopic properties of superparamagnetic FemOn-SiO2 nanoparticle colloidal solutions. J. Phys. Conf. Ser. 2016, 769, 012037. [Google Scholar] [CrossRef]
- Gracheva, I.E.; Olchowik, G.; Gareev, K.G.; Moshnikov, V.A.; Kuznetsov, V.V.; Olchowik, J.M. Investigations of nanocomposite magnetic materials based on the oxides of iron, nickel, cobalt and silicon dioxide. J. Phys. Chem. Sol. 2013, 74, 656–663. [Google Scholar] [CrossRef]
- Gareev, K.G.; Gracheva, I.E.; Moshnikov, V.A. The Sol-Gel method and study of Fe2O3–NiO–Co3O4–SiO2 magnetic nanocomposites. Glass Phys. Chem. 2013, 39, 548–554. [Google Scholar] [CrossRef]
- Tarasov, S.A.; Gracheva, I.E.; Gareev, K.G.; Gordyushenkov, O.E.; Lamkin, I.A.; Men’kovich, E.A.; Moshnikov, V.A.; Presnyakova, A.V. Atomic force microscopy and photoluminescence analysis of porous metal-oxide materials. Semiconductors 2012, 46, 1584–1588. [Google Scholar] [CrossRef]
- Afonin, V.; Balbekin, N.S.; Gareev, G.Z.; Gareev, K.G.; Gorshkov, A.N.; Korolev, D.V.; Luchinin, V.V.; Smolyanskaya, O.A. Features of the terahertz spectra of iron oxide nanoparticles in a silicon dioxide shell and of iron oxide and hydroxide nanoparticles. J. Opt. Technol. 2017, 84, 515–520. [Google Scholar] [CrossRef]
- Smerdov, R.S.; Bocharova, T.V.; Levitskii, V.S.; Gareev, K.G.; Moshnikov, V.A.; Terukov, E.I. Spectroscopic properties of γ-Irradiated FemOn-SiO2 composite nanoparticles. Phys. Solid State 2016, 58, 919–923. [Google Scholar] [CrossRef]
- Al’myashev, V.I.; Gareev, K.G.; Ionin, S.A.; Levitskii, V.S.; Moshnikov, V.A.; Terukov, E.I. Investigation of the structure, elemental and phase compositions of Fe3O4-SiO2 composite layers by scanning electron microscopy, X-ray spectroscopy, and thermal nitrogen desorption methods. Phys. Solid State 2014, 56, 2155–2159. [Google Scholar] [CrossRef]
- Gareev, K.G.; Nepomnyashchaya, E.K. Obtaining and characterizing a water-based magnetic fluid. Bull. Russ. Acad. Sci. Phys. 2019, 83, 904–905. [Google Scholar] [CrossRef]
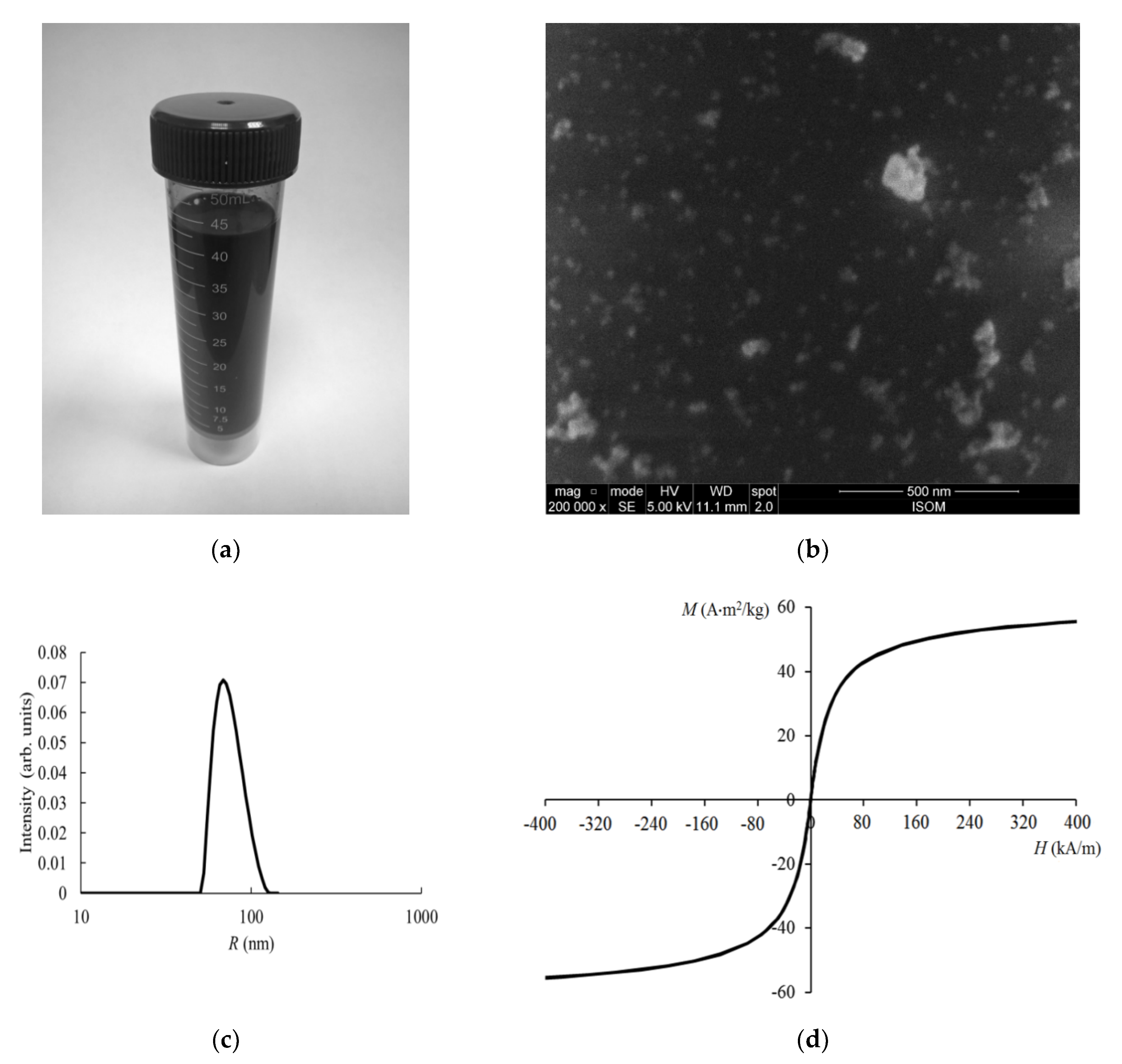
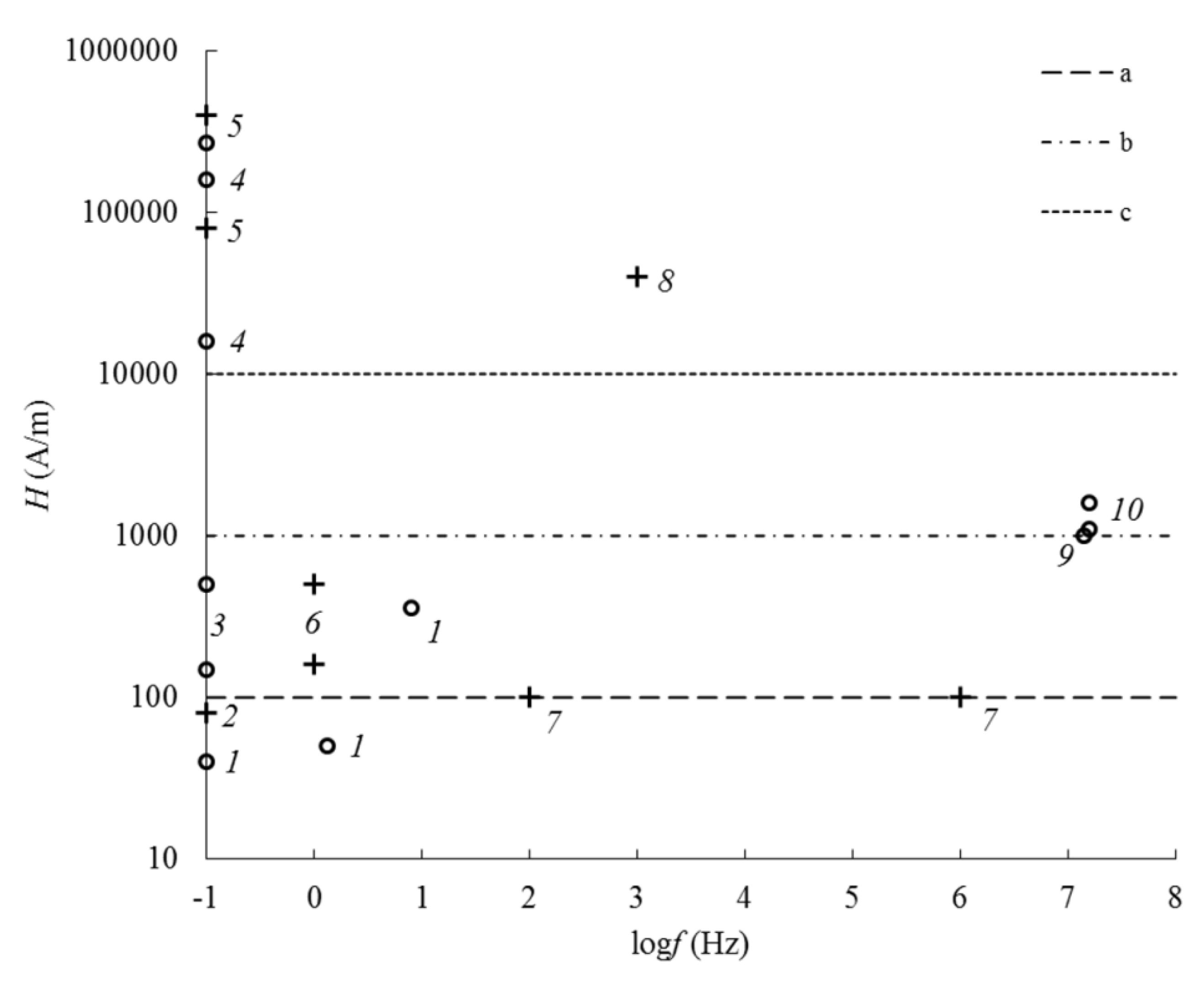
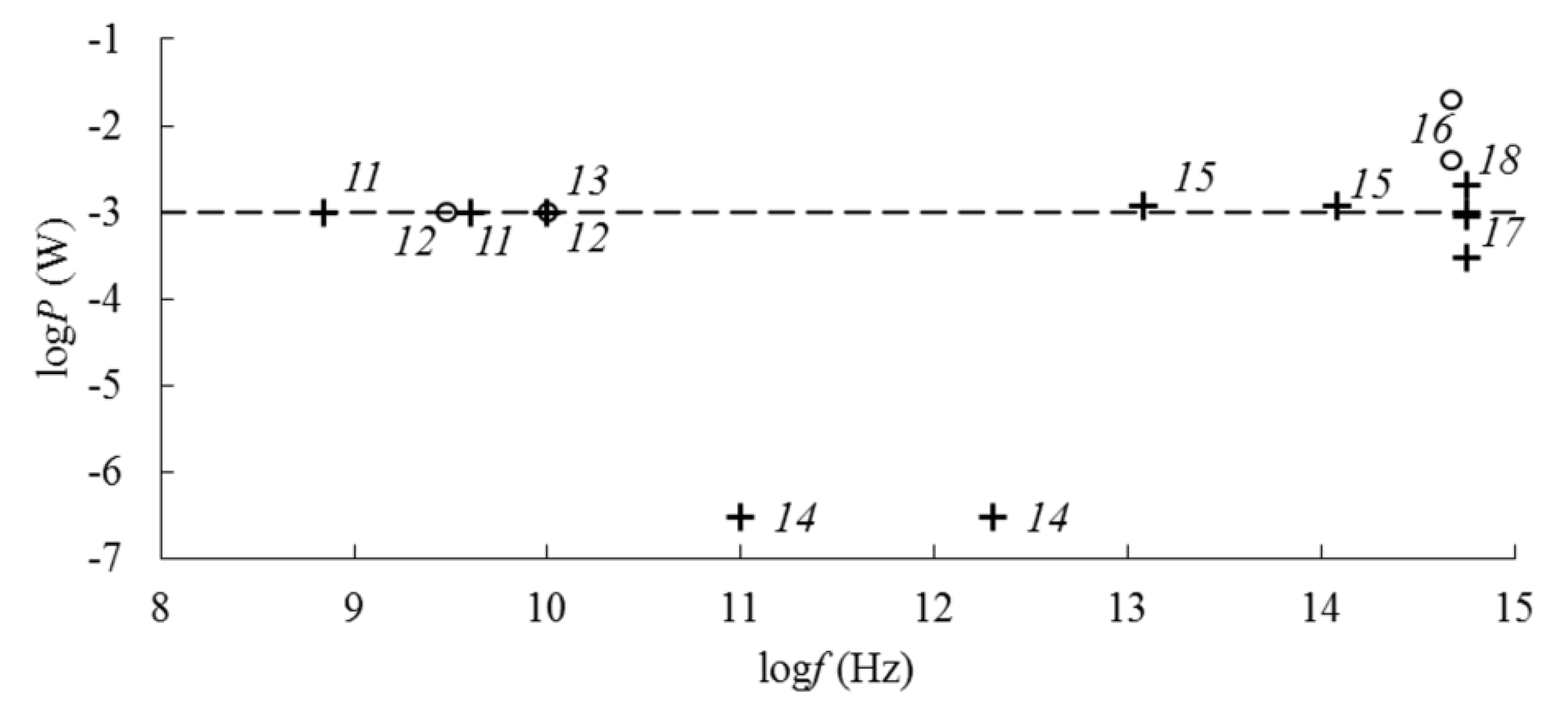
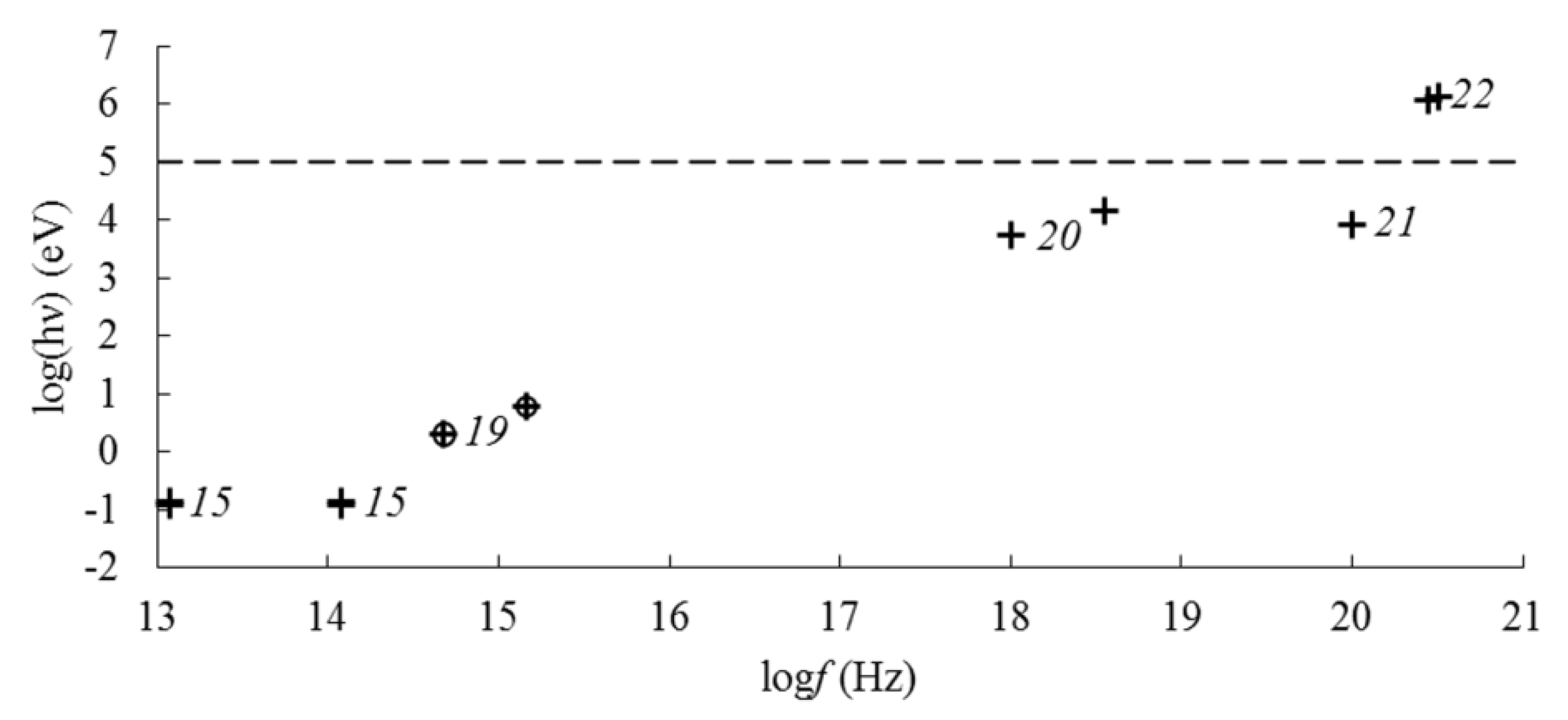
| Frequency | EMF Type | Aggregate State | Type of Iron Oxide | Exposure Level | Observed Effects | Refs. |
|---|---|---|---|---|---|---|
| 0–10 Hz | QSMF | solid | Fe3O4, γ-Fe2O3 | 160–500 A/m | magnetization reversal | [8,20,21,22,23,24] |
| 80–400 kA/m | Zeeman effect (for observing electron paramagnetic resonance (EPR)) | [26] | ||||
| 80 A/m | creation of anhysteretic remanent magnetization (ARM) | [24,25] | ||||
| liquid | Fe3O4, γ-Fe2O3 | 150–500 A/m | reversible aggregation of nanoparticles (NPs) | [19,20] | ||
| 16–160 kA/m | irreversible formation of linear aggregates of NPs | [15,18] | ||||
| 40–360 A/m | magnetization reversal | [17] | ||||
| 267 kA/m | Zeeman effect (for observing nuclear magnetic resonance (NMR)) | [14,15] | ||||
| 1 kHz | AMF | solid | Fe3O4, γ-Fe2O3 | 40 kA/m | destruction of ARM | [23,25] |
| 102–106 Hz | liquid | α-Fe2O3 | 100 A/m | dispersion of initial magnetic permeability | [27,28] | |
| 14 MHz | liquid | Fe3O4, γ-Fe2O3 | 1 kA/m | NMR | [14,15] | |
| 15.7 MHz | liquid | Fe3O4, γ-Fe2O3 | 1.1–1.6 kA/m | nonlinearity of the magnetization in a longitudinal field | [17] | |
| 10 GHz | MW | solid | Fe3O4, γ-Fe2O3 | 1 mW | EPR | [26] |
| 0.1–18 GHz | liquid | Fe3O4, γ-Fe2O3 | permeability dispersion | [16] | ||
| 0.2–1.8 THz | THz | solid | Fe3O4, γ-Fe2O3 | 0.3 μW | dispersion of refractive index and absorption coefficient | [30] |
| 12–120 THz | FIR and MIR | solid | Fe3O4, γ-Fe2O3 | 0.12–0.14 eV, 1.2 mW | vibration absorption of Si–O–Si groups | [8,22,24] |
| 1014–1015 Hz | UV, Vis, NIR | solid | Fe3O4, α-Fe2O3, γ-Fe2O3 | 0.3–0.9 mw (5–15 kW/cm2) | Raman scattering | [31,32] |
| 1–2 mW (17–35 kW/cm2) | phase transition magnetite → maghemite → hematite | [29,31,32] | ||||
| liquid | Fe3O4, γ-Fe2O3 | 2–6 eV | intrinsic absorption in iron oxide and in silica | [14,15] | ||
| Fe3O4, γ-Fe2O3 | 4–20 mW | Dynamic light scattering | [8,20,23,33] | |||
| 1018–1021 Hz | X-ray | solid | Fe3O4, α-Fe2O3, γ-Fe2O3 | 5.4–8.0 keV (0.154–0.229 nm) | X-ray diffraction on the crystal lattice | [17,23,27,28,31] |
| γ | Fe3O4, γ-Fe2O3 | 14.4 keV, 20 mCi | Mössbauer effect | [23] | ||
| Fe3O4, γ-Fe2O3 | 1.17–1.33 MeV, 106 rad | radiation-induced phase transition magnetite → maghemite | [32] |
| Modified Structure | State | Modification Method | Threshold Level | Refs. |
|---|---|---|---|---|
| Microstructure | Liquid | Influence of quasistatic magnetic field | 104 A/m | [14,18] |
| Magnetic structure | Liquid | 102 A/m | [17] | |
| Solid | 103 A/m | [8,20,21,22,23,24] | ||
| Crystalline structure | Liquid | Local laser heating | 10−3 W | [19,31,32] |
| γ-irradiation | 105 eV | [26,32] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gareev, K.G. Interaction of Nanocomposites Based on the FemOn–SiO2 System with an Electromagnetic Field in an Ultra-Wide Frequency Range. Magnetochemistry 2020, 6, 24. https://doi.org/10.3390/magnetochemistry6020024
Gareev KG. Interaction of Nanocomposites Based on the FemOn–SiO2 System with an Electromagnetic Field in an Ultra-Wide Frequency Range. Magnetochemistry. 2020; 6(2):24. https://doi.org/10.3390/magnetochemistry6020024
Chicago/Turabian StyleGareev, Kamil G. 2020. "Interaction of Nanocomposites Based on the FemOn–SiO2 System with an Electromagnetic Field in an Ultra-Wide Frequency Range" Magnetochemistry 6, no. 2: 24. https://doi.org/10.3390/magnetochemistry6020024
APA StyleGareev, K. G. (2020). Interaction of Nanocomposites Based on the FemOn–SiO2 System with an Electromagnetic Field in an Ultra-Wide Frequency Range. Magnetochemistry, 6(2), 24. https://doi.org/10.3390/magnetochemistry6020024





