Effect of Magnetic Field and Aggregation on Electrical Characteristics of Magnetically Responsive Suspensions for Novel Hybrid Liquid Capacitor
Abstract
1. Introduction
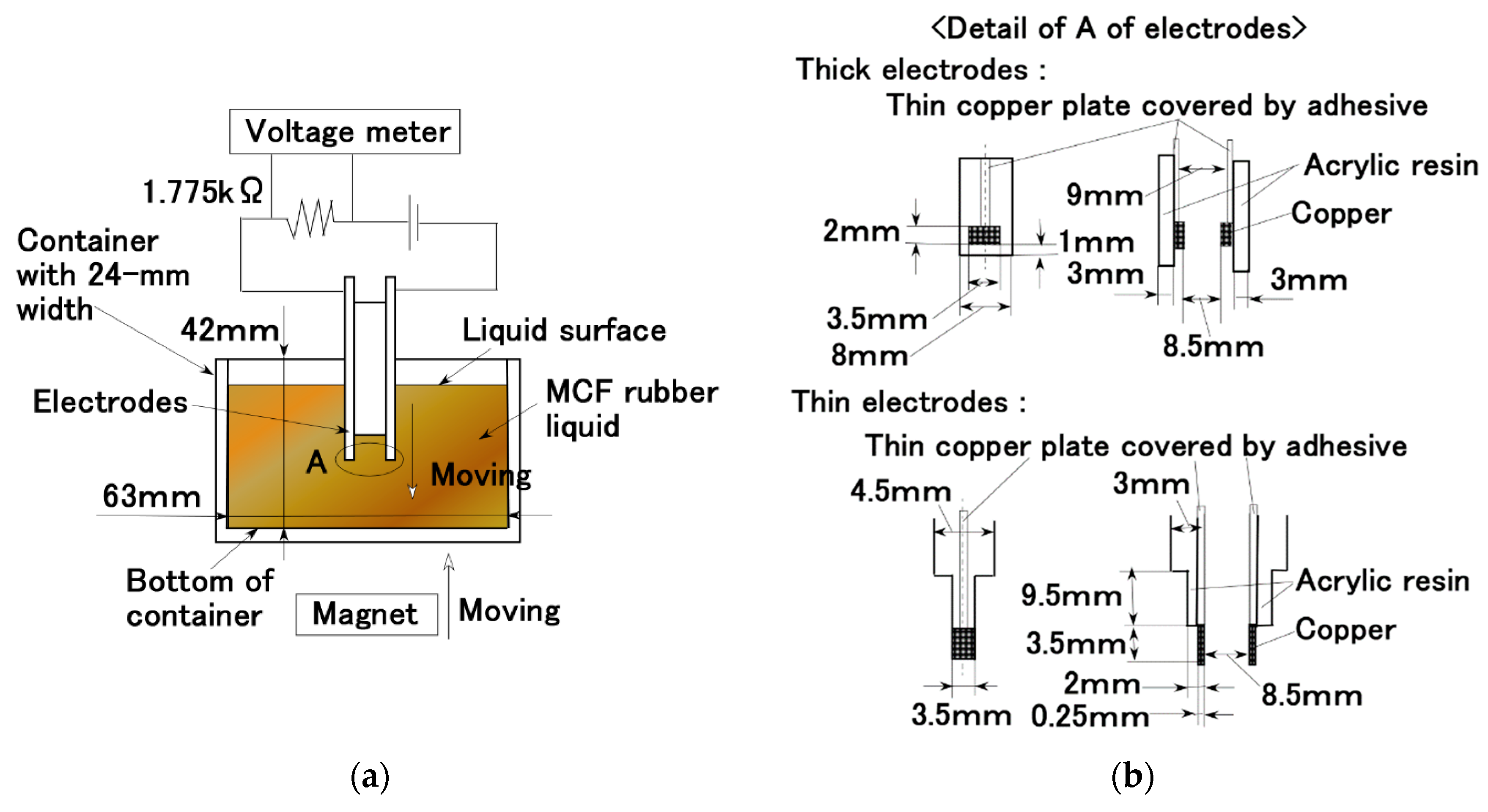
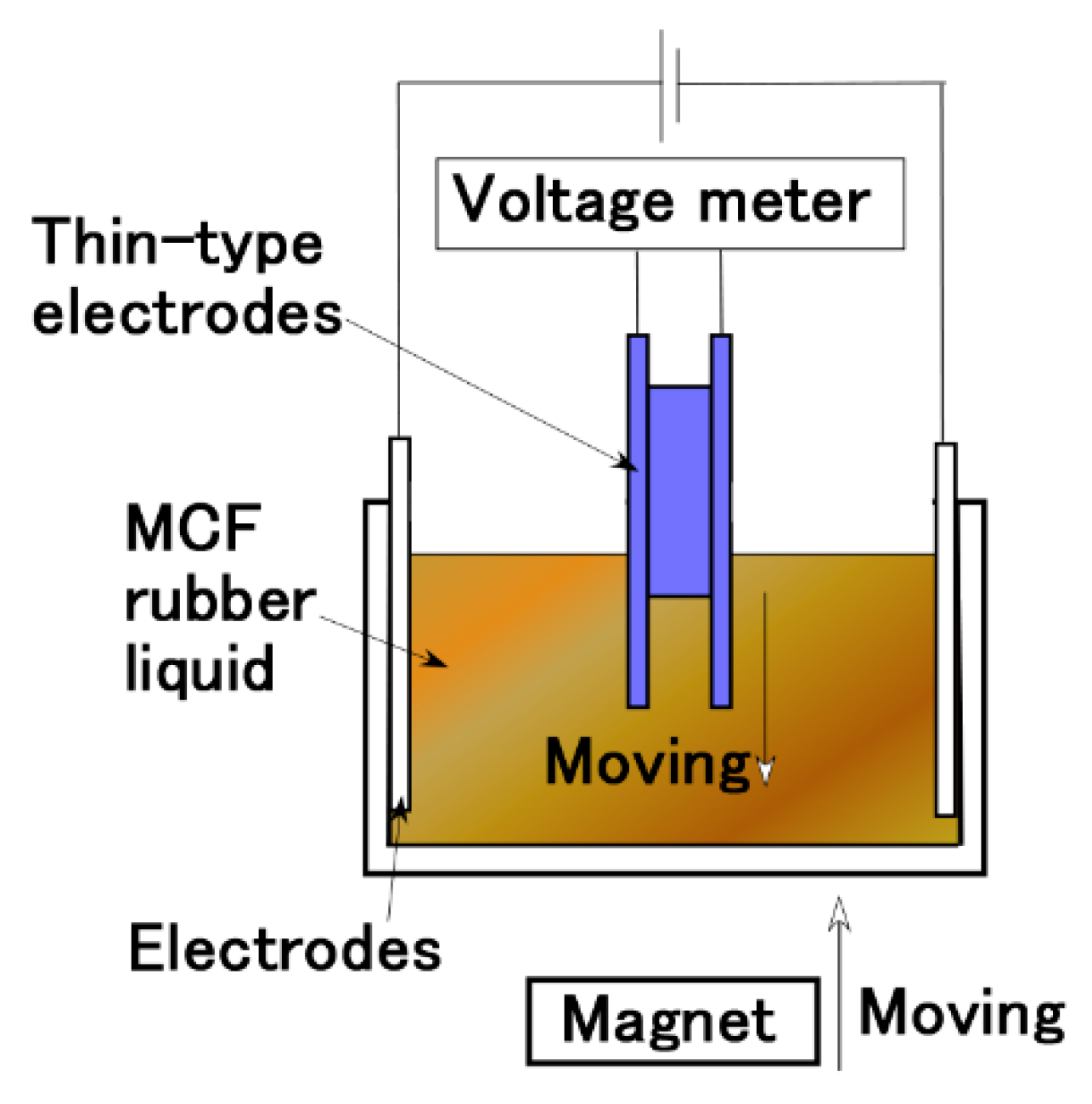
2. Experimental Procedure
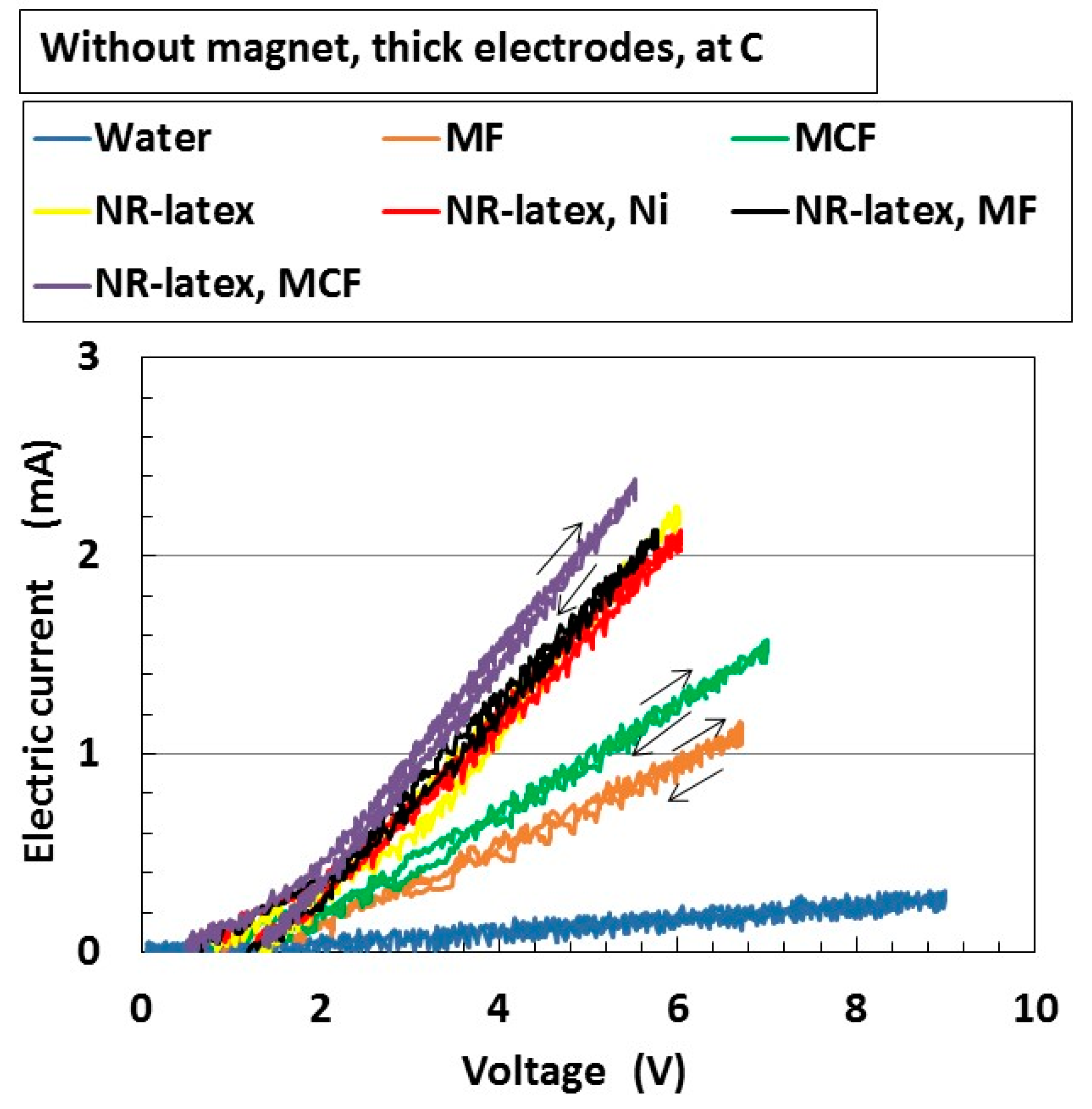
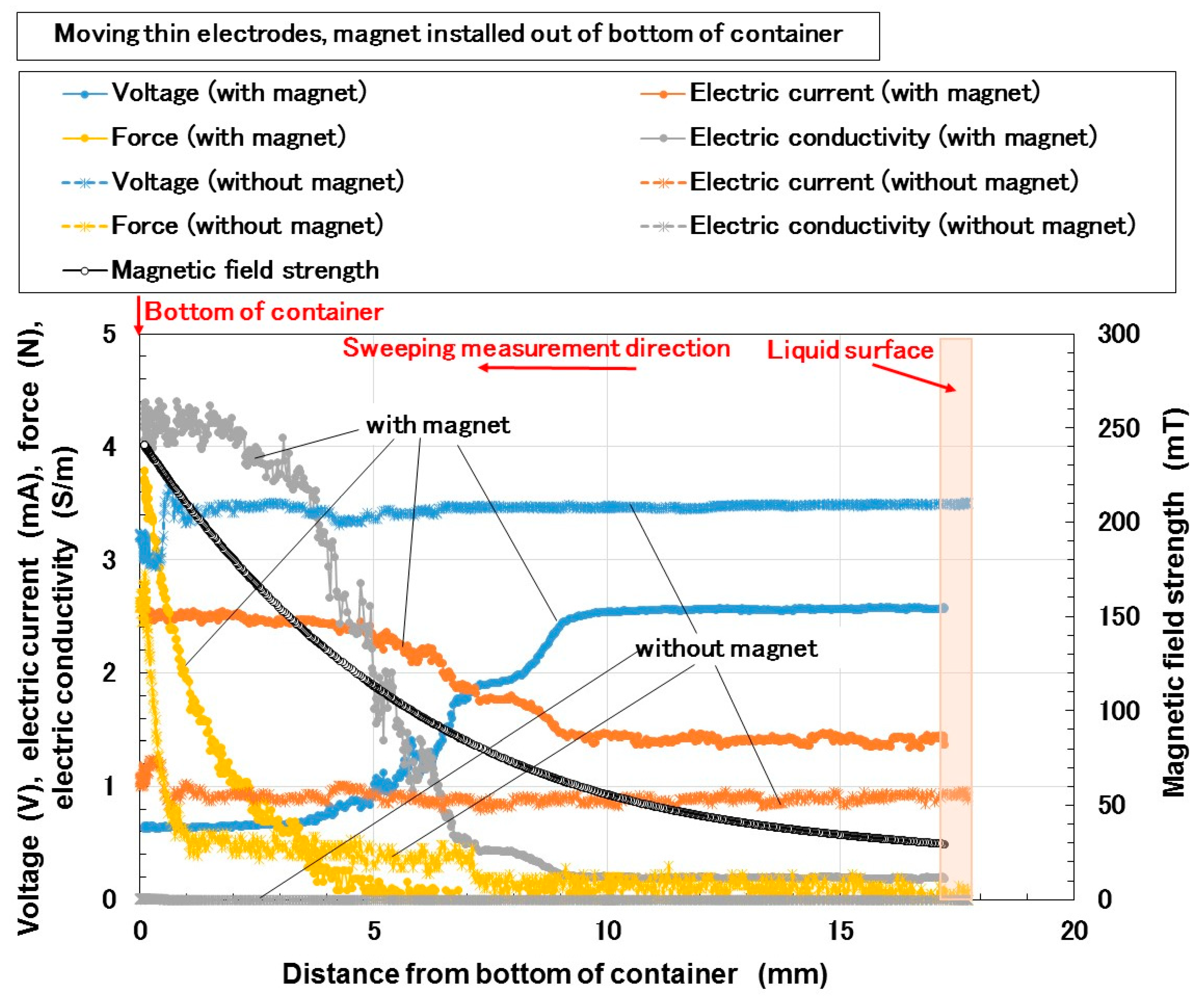
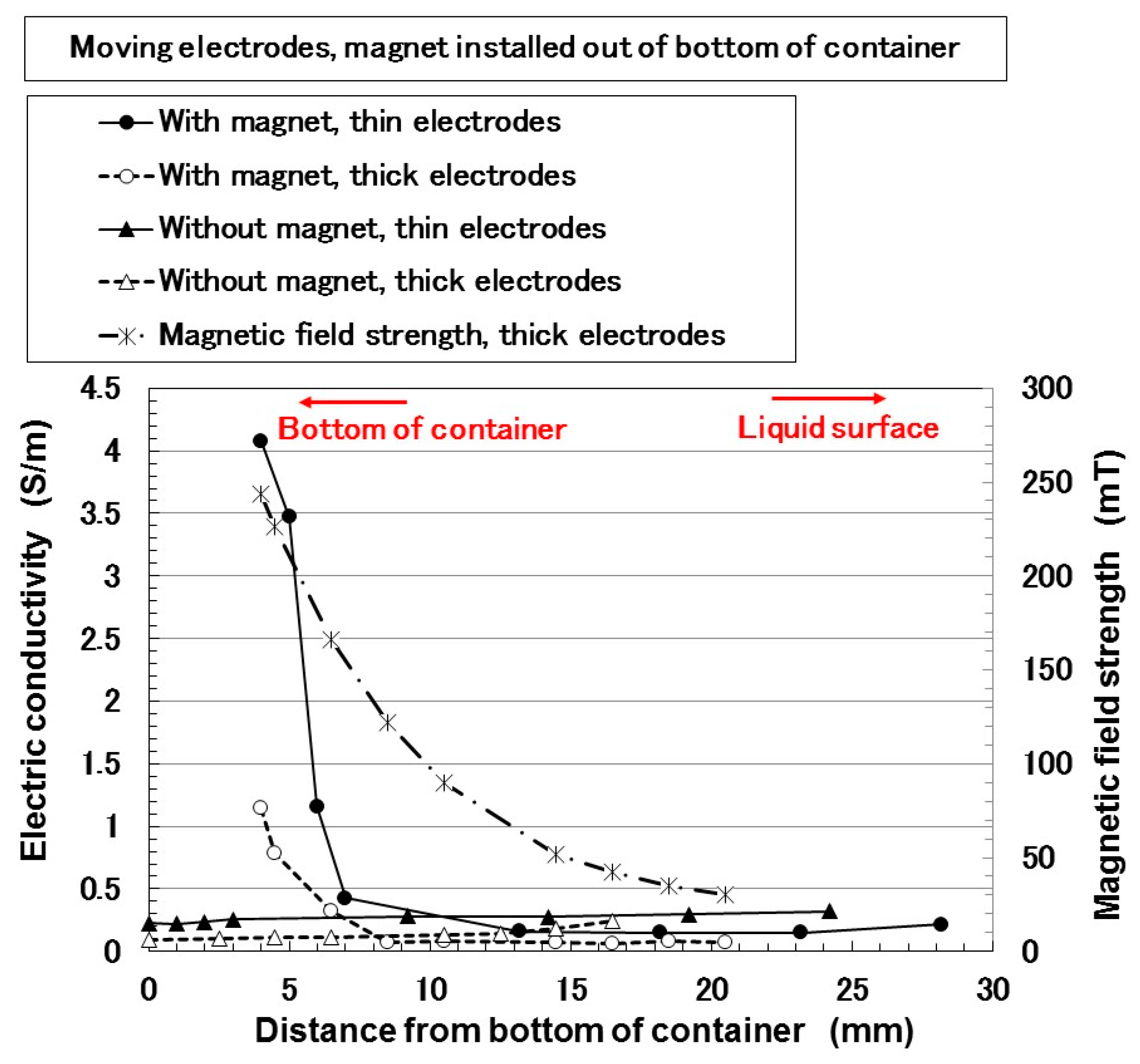
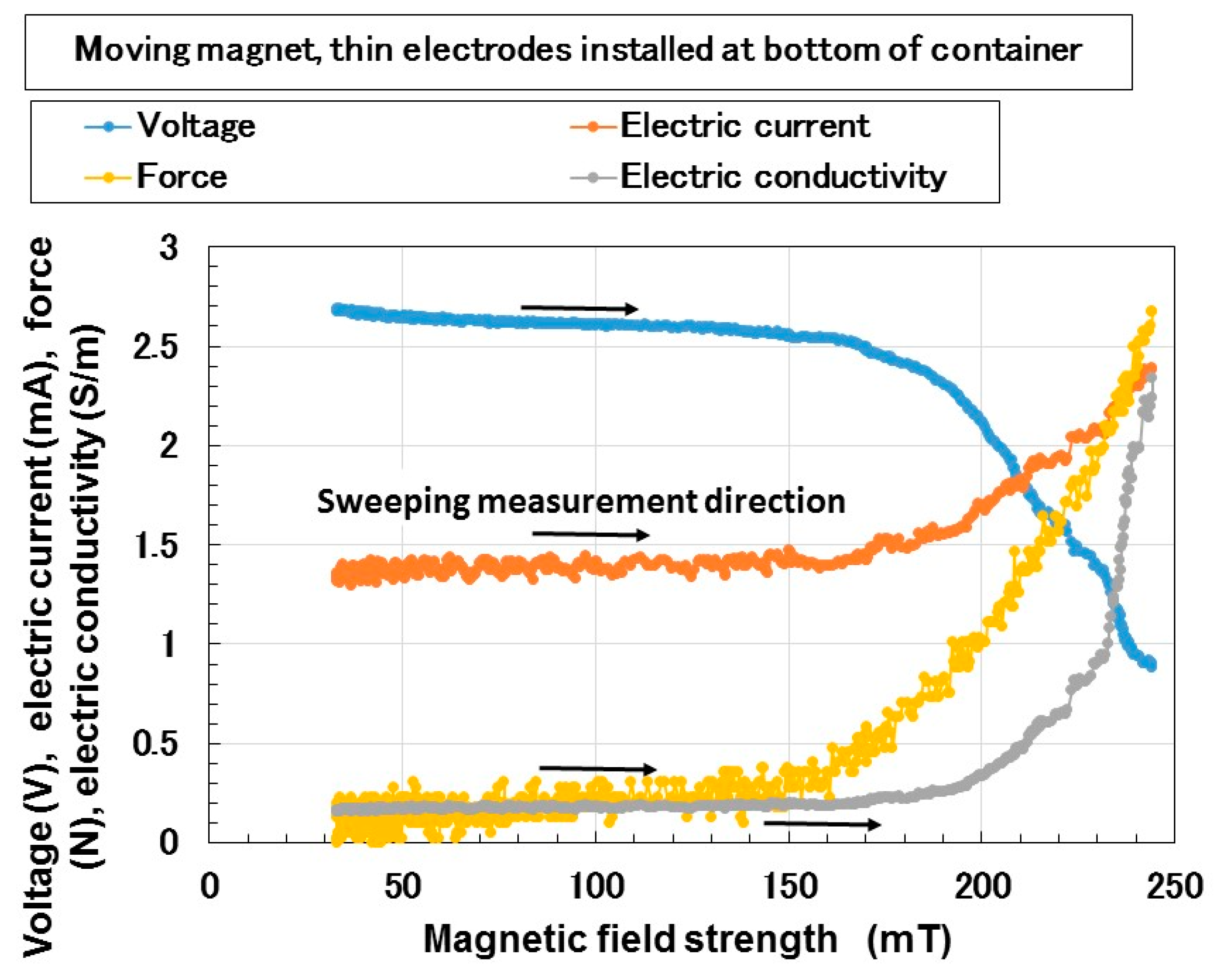
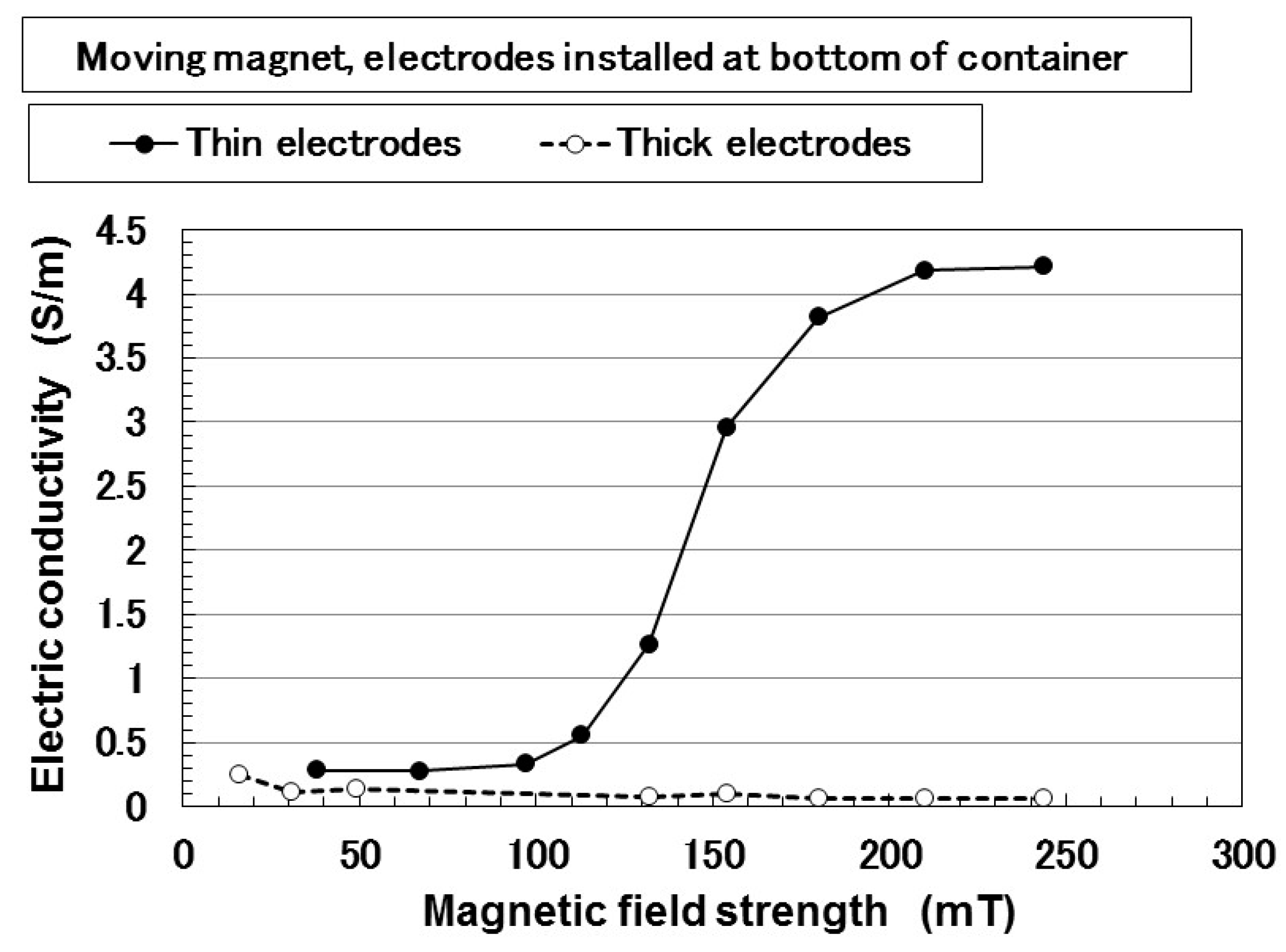
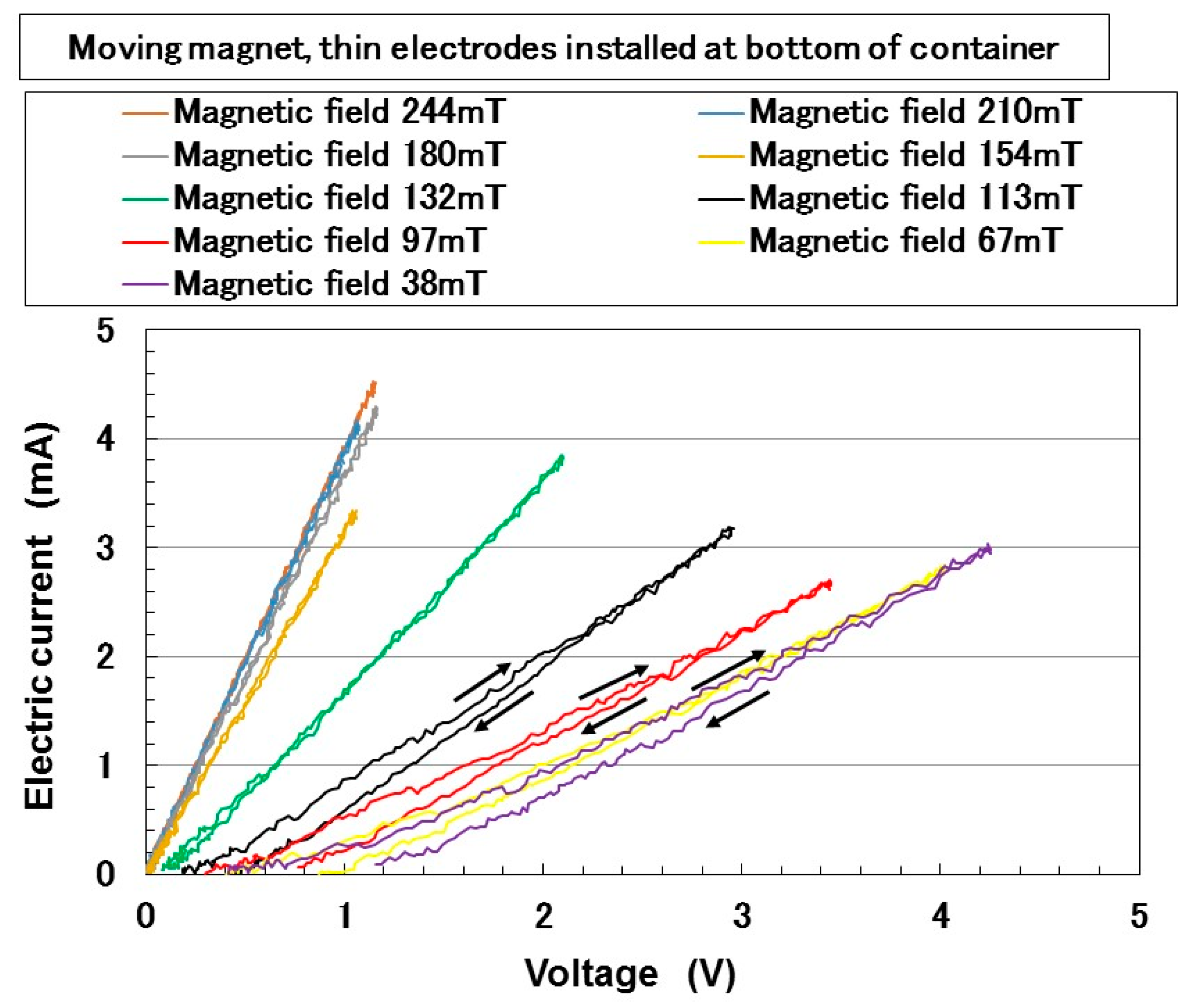
3. Electrical Properties of MCF Rubber Liquid
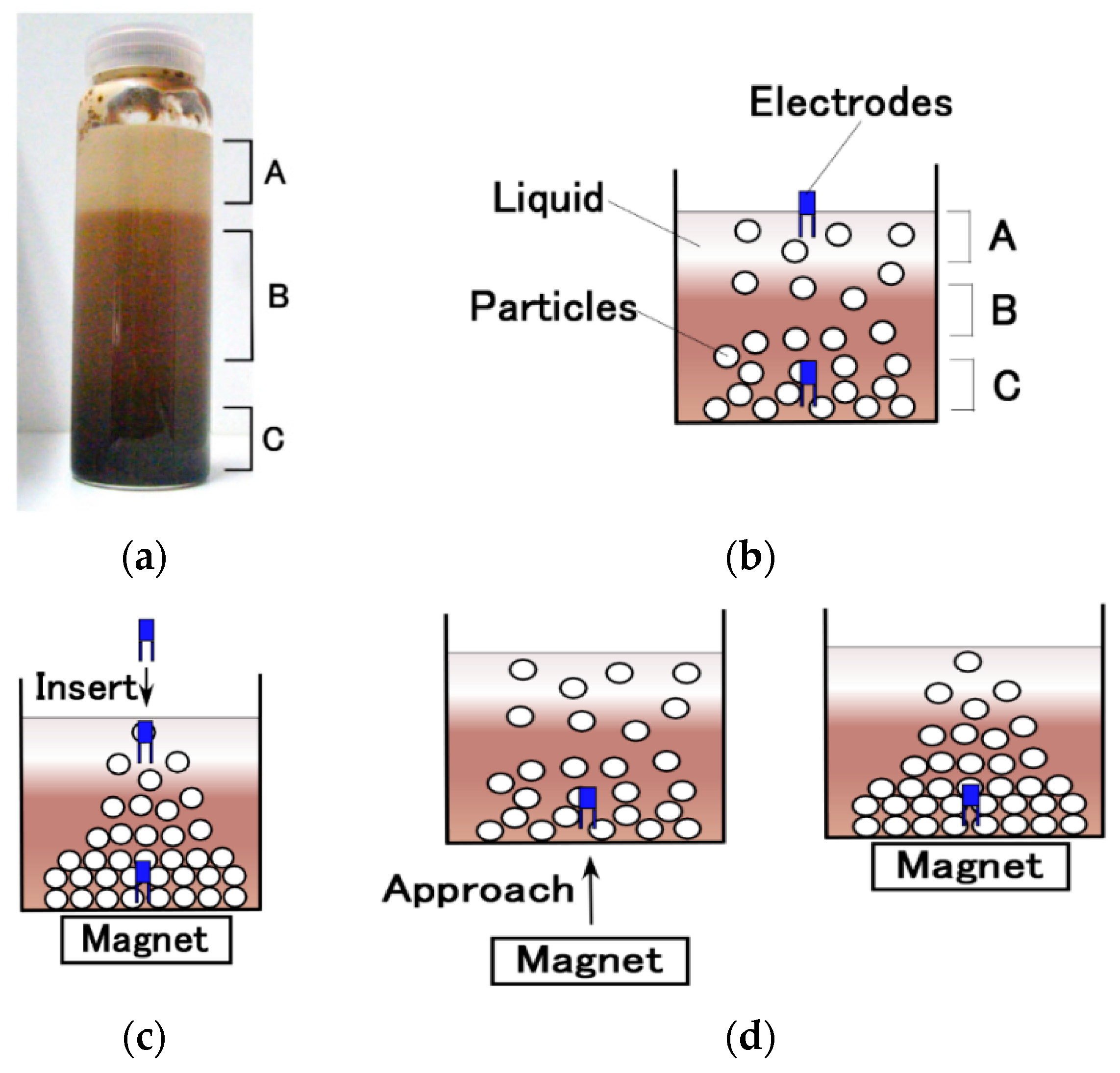
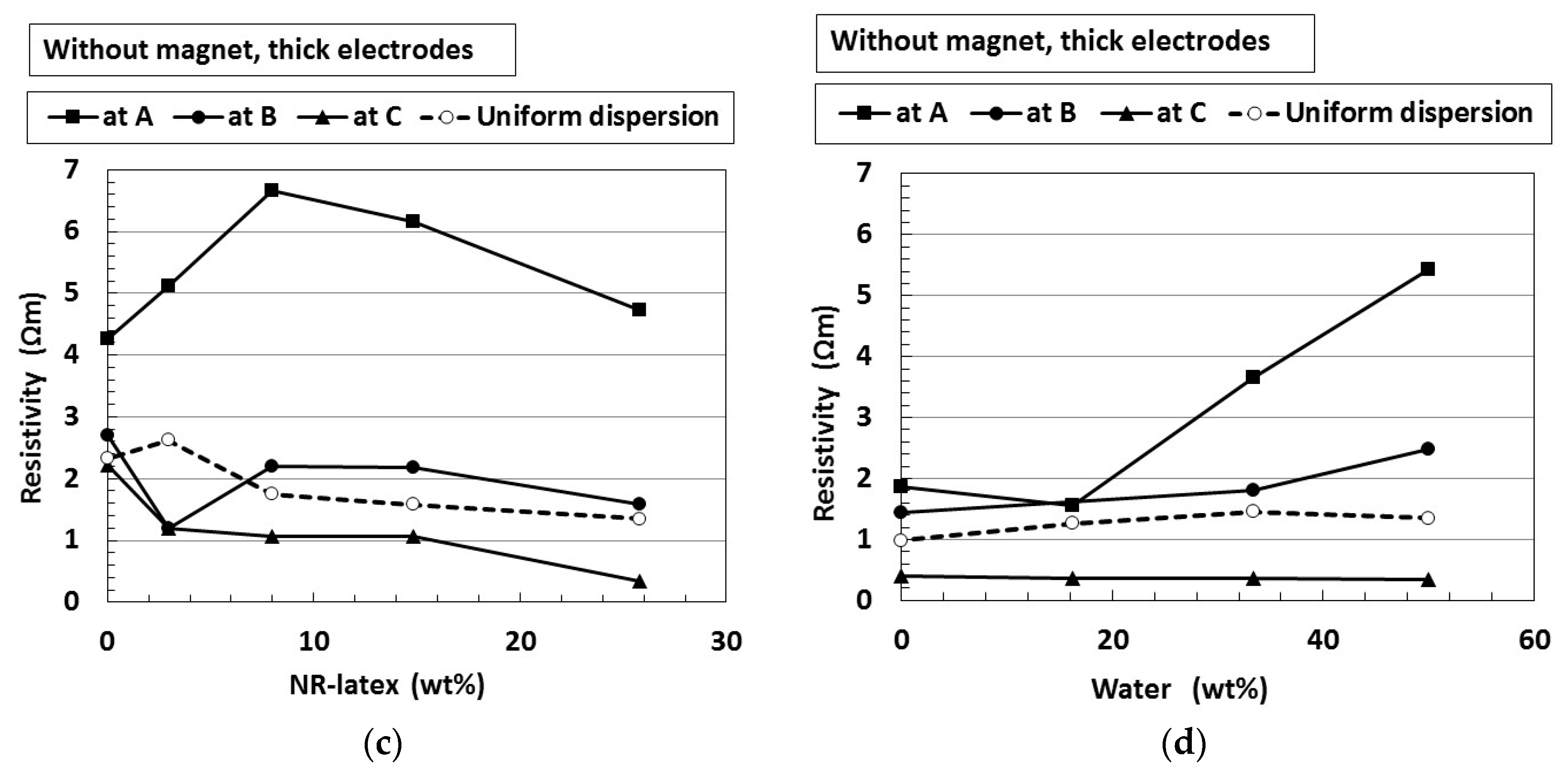
4. Effect of Particles Aggregation
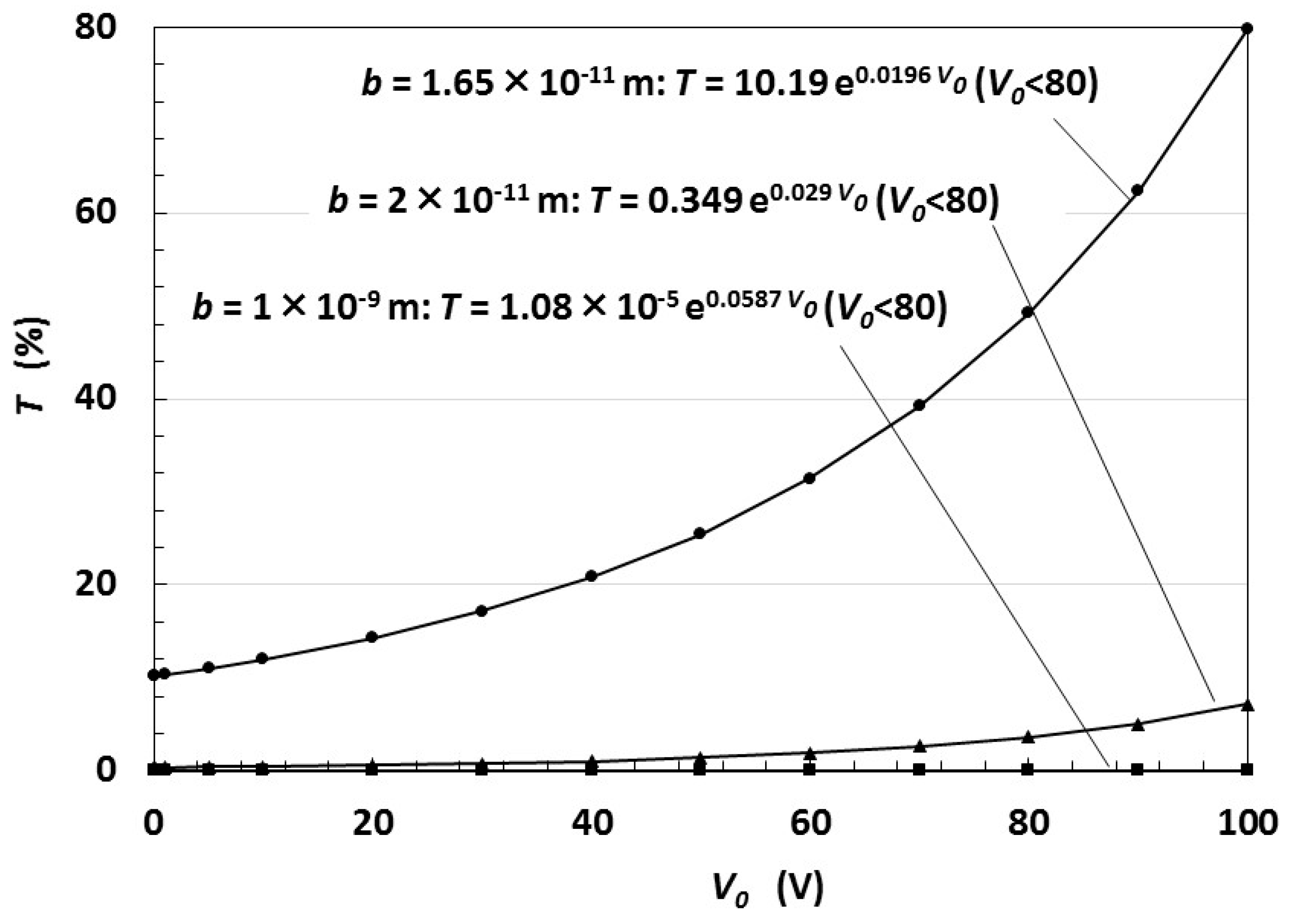
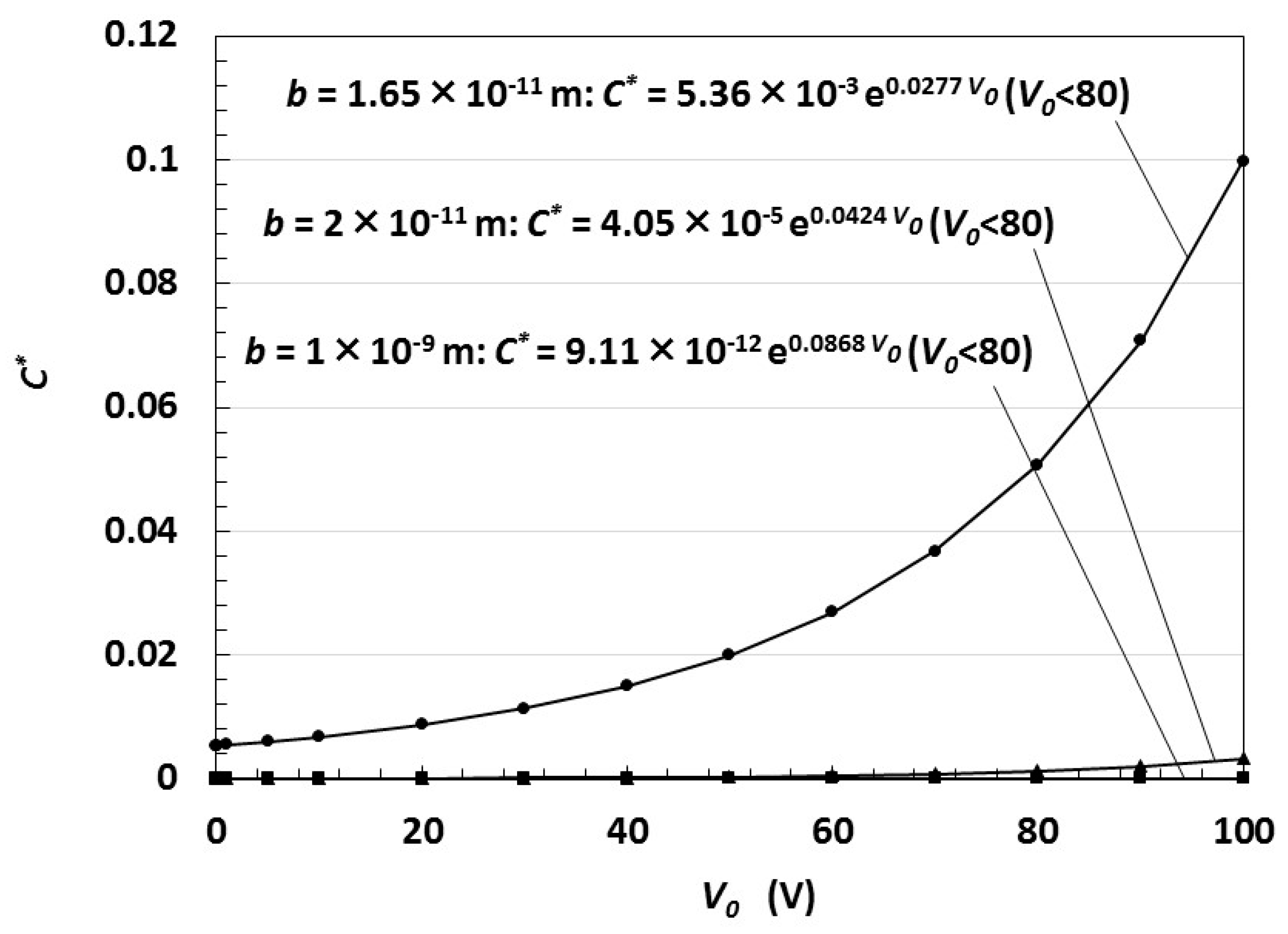
5. Electric Charge
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, G.X.; Cai, J.; Xu, H.F. Enhanced capacitance of a NiO electrode prepared in the magnetic field. J. Appl. Electrochem. 2014, 44, 391–398. [Google Scholar] [CrossRef]
- Shimada, K.; Wu, Y.; Masuo, Y.; Yamamoto, K. Float polishing technique using new tool consisting of micro magnetic clusters. J. Mater. Process. Technol. 2005, 162–163, 690–695. [Google Scholar] [CrossRef]
- Svasand, E.; Kristiansen, K.D.L.; Martinsen, O.G.; Helgesen, G.; Grimnes, S.; Skjeltorp, A.T. Behavior of carbon cone particle dispersions in electric and magnetic fields. Colloids Surf. A Physicochem. Eng. Asp. 2009, 339, 211–216. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Fattah, A.R.A.; Ghosh, S.; Puri, I.K. Magnetoresponsive conductive colloidal suspensions with magnetized carbon nanotubes. J. Magn. Magn. Mater. 2017, 421, 292–299. [Google Scholar] [CrossRef]
- Yu, M.; Yang, P.; Fu, J.; Liu, S.; Choi, S.B. A theoretical model for the field-dependent conductivity of magneto-rheological gels and experimental verification. Sens. Actuators A 2016, 245, 127–134. [Google Scholar] [CrossRef]
- Hao, T. Electrorheological fluids. Adv. Mater. 2001, 13, 1847–1857. [Google Scholar] [CrossRef]
- Klingenberg, D.J. Magnetorheology: Applications and challenges. AIChE J. 2001, 47, 246–249. [Google Scholar] [CrossRef]
- Jang, S.H.; Park, Y.L.; Yin, H. Influence of coalescence on the anisotropic mechanical and electrical properties of nickel powder/polydimethylsiloxane composites. Materials 2016, 9, 239. [Google Scholar] [CrossRef]
- Laflamme, S.; Kollosche, M.; Connor, J.J.; Kofod, G. Robust flexible capacitive surface sensor for structural health monitoring applications. J. Eng. Mech. 2013, 139, 879–885. [Google Scholar] [CrossRef]
- Wu, X.; Lu, C.; Han, Y.; Zhou, Z.; Yuan, G.; Zhang, X. Cellulose nanowhisker modulated 3D hierarchical conductive structure of carbon black/natural rubber nanocomposites for liquid and strain sensing application. Compos. Sci. Technol. 2016, 124, 44–51. [Google Scholar] [CrossRef]
- Pang, H.; Xu, L.; Yan, D.X.; Li, Z.M. Conductive polymer composites with segregated structures. Prog. Polym. Sci. 2014, 39, 1908–1933. [Google Scholar] [CrossRef]
- Taherian, R. Experimental and analytical model for the electrical conductivity of polymer-based nanocomposites. Compos. Sci. Technol. 2016, 123, 17–31. [Google Scholar] [CrossRef]
- Dai, X.; Qiang, X.; Li, J.; Yao, T.; Wang, Z.; Song, H. Design and functionalization of magnetic ionic liquids surfactants (MILSs) containing alkyltrimethylammonium fragment. J. Mol. Liq. 2019, 277, 170–174. [Google Scholar] [CrossRef]
- Lee, K.B.; Kim, J.R.; Park, G.C.; Cho, H.K. Feasibility test of a liquid film thickness sensor on a flexible printed circuit board using a three-electrode conductance method. Sensors 2017, 17, 42. [Google Scholar] [CrossRef] [PubMed]
- Danisman, M.; Tuncol, G.; Kaynar, A.; Sozer, E.M. Monitoring of resin flow in the resin transfer molding (RTM) process using point-voltage sensors. Compos. Sci. Technol. 2007, 67, 367–379. [Google Scholar] [CrossRef]
- Altava, B.; Compan, V.; Andrio, A.; Castillo, L.F.D.; Molla, S.; Burgnuete, M.I.; Verdugo, E.G.; Luis, S.V. Conductive films based on composite polymers containing ionic liquids absorbed on crosslinked polymeric ionic-like liquids (SILLPs). Polymer 2015, 72, 69–81. [Google Scholar] [CrossRef]
- Ge, X.; Gu, C.D.; Wang, X.L.; Tu, J.P. Endowing manganese oxide with fast adsorption ability through controlling the manganese carbonate precursor assembled in ionic liquid. J. Colloid Interface Sci. 2015, 438, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Le, H.H.; Das, A.; Basak, S.; Tahir, M.; Wieβner, S.; Fischer, D.; Reuter, U.; Stockelhuber, K.W.; Bhowmick, A.K.; Do, Q.K.; et al. Effect of different ionic liquids on the dispersion and phase selective wetting of carbon nanotubes in rubber blends. Polymer 2016, 105, 284–297. [Google Scholar] [CrossRef]
- Matchawet, S.; Kaesaman, A.; Vennemann, N.; Kumerlowe, C.; Nakason, C. Effectes of imidazolium ionic liquid on cure characteristics, electrical conductivity and other related properties of epoxidized natural rubber vulcanizates. Eur. Polym. J. 2017, 87, 344–359. [Google Scholar] [CrossRef]
- Genovese, M.; Lian, K. Polyoxometalate modified inorganic-organic nanocomposite materials for energy storage applications: A review. Curr. Opin. Solid State Mater. Sci. 2015, 19, 126–137. [Google Scholar] [CrossRef]
- Boaretto, N.; Joost, C.; Seyfried, M.; Vezzu, K.; Noto, V.D. Conductivity and properties of polysiloxane-polyether cluster-LiTFSI networks as hybrid polymer electrolytes. J. Power Sources 2016, 325, 427–437. [Google Scholar] [CrossRef]
- Shimada, K.; Saga, N. Mechanical enhancement of sensitivity in natural rubber using electrolytic polymerization aided by a magnetic field and MCF for application in haptic sensors. Sensors 2016, 16, 1521. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Saga, N. Detailed mechanism and engineering applicability of electrolytic polymerization aided by a magnetic field in natural rubber by mechanical approach for sensing (Part 1): The effect of experimental conditions on electrolytic polymerization. World J. Mech. 2016, 6, 357–378. [Google Scholar] [CrossRef]
- Shimada, K.; Saga, N. Detailed mechanism and engineering applicability of electrolytic polymerization aided by a magnetic field in natural rubber by mechanical approach for sensing (Part 2): Other and intrinsic effects on MCF rubber property. World J. Mech. 2016, 6, 379–395. [Google Scholar] [CrossRef]
- Shimada, K.; Saga, N. Development of a hybrid piezo natural rubber piezoelectricity and piezoresistivity sensor with magnetic clusters made by electric and magnetic field assistance and filling with magnetic compound fluid. Sensors 2017, 17, 1521. [Google Scholar] [CrossRef]
- Shimada, K. Enhancement of MCF rubber utilizing electric and magnetic fields, and clarification of electrolytic polymerization. Sensors 2017, 17, 767. [Google Scholar] [CrossRef]
- Shimada, K.; Michizuki, O.; Kubota, Y. The effect of particles on electrolytically polymerized thin natural MCF rubber for soft sensors installed in artificial skin. Sensors 2017, 17, 896. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K. Elastic MCF rubber with photovoltaics and sensing for use as artificial or hybrid skin (H-Skin): 1st report on dry-type solar cell rubber with piezoelectricity for compressive sensing. Sensors 2018, 18, 1841. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K. Elastic MCF rubber with photovoltaics and sensing on hybrid skin (H-Skin) for artificial skin by utilizing natural rubber: 2nd report on effect of tension and compression on properties of hybrid photo- and piezo-electricity in wet-type solar cell rubber. Sensors 2018, 18, 1848. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K. MCF rubber with photovoltaics and sensing for use as artificial or hybrid skin (H-Skin): Third report on electric charge and storage under tension and compression. Sensors 2018, 18, 1853. [Google Scholar] [CrossRef]
- Shimada, K.; Fujita, T.; Oka, H.; Akagami, Y.; Kamiyama, S. Hydrodynamic and magnetized characteristics of MCF (magnetic compound fluid). Trans. Jpn. Soc. Mech. Eng. 2001, 664, 3034–3040. [Google Scholar] [CrossRef]
- Shimada, K.; Wu, Y.; Wong, Y.C. Effect of magnetic cluster and magnetic field on polishing using magnetic compound fluid (MCF). J. Magn. Magn. Mater. 2003, 262, 242–247. [Google Scholar] [CrossRef]
- Shimada, K.; Miyazaki, T.; Shibayama, A.; Fujita, T. Extraction of magnetic clusters self-assembled by a magnetic field. Smart Mater. Struct. 2003, 12, 297–303. [Google Scholar] [CrossRef]
- Xie, Z.; Chang, K.; Li, B.; Tang, H.; Fu, X.; Chang, Z.; Yuan, X.Z.; Wang, H. Glucose-assisted synthesis of highly dispersed LiMnPO4 nanoparticles at a low temperature for lithium ion batteries. Electrochim. Acta 2016, 189, 205–214. [Google Scholar] [CrossRef]
- Kwon, N.H. The effect of carbon morphology of the LiCoO2 cathode of lithium ion batteries. Solid State Sci. 2013, 21, 59–65. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, X.; Hu, H.; Wei, S.; Fu, Y.; Shen, Y. In situ growth and performance of spherical Fe2F5/H2O nanoparticles multi-walled carbon nanotube network matrix as cathode material for sodium ion batteries. J. Power Sources 2016, 316, 170–175. [Google Scholar] [CrossRef]
- Zhang, Y.; Narayanan, A.; Mugele, F.; Stuart, M.A.C.; Duits, M.H.G. Charge inversion and colloidal stability of carbon black in battery electrolyte solutions. Colloids Surf. A Physicochem. Eng. Asp. 2016, 489, 461–468. [Google Scholar] [CrossRef]
- Song, H.J.; Kim, J.C.; Roh, H.S.; Lee, C.W.; Park, S.; Kim, D.W.; Hong, K.S. Anion-controlled synthesis of TiO2 nano-aggregates for Li ion battery electrodes. Mater. Charact. 2014, 96, 13–20. [Google Scholar] [CrossRef]
- Yang, L.; Yan, Q.; Xi, G.; Niu, L.; Lou, T.; Wang, T.; Wang, X. Preparation of cobalt ferrite nanoparticles by using spent Li-ion batteries. J. Mater. Sci. 2011, 46, 6106–6110. [Google Scholar] [CrossRef]
- Wang, S.; Hu, X.; Dai, Y. Preparation and electrochemical performance of polymer-derived SiBCN-graphene composite as anode material for lithium ion batteries. Ceram. Int. 2017, 43, 1210–1216. [Google Scholar] [CrossRef]
- Wang, M.; Shan, Z.; Tian, J.; Yang, K.; Liu, X.; Liu, H.; Zhu, K. Mixtures of unsaturated imidazolium based ionic liquid and organic carbonate as electrolyte for Li-ion batteries. Electrochim. Acta 2013, 95, 301–307. [Google Scholar] [CrossRef]
- Zhao, Y.; Bostrom, T. Ionic liquid and nanoparticle based magnetic electrolytes: Design, preparation, and electrochemical stability characterization. J. Mol. Liq. 2016, 213, 268–272. [Google Scholar] [CrossRef]
- Ito, T.; Otobe, S.; Oda, T.; Kojima, T.; Ono, S.; Watanabe, M.; Kiyota, Y.; Misawa, T.; Koguchi, S.; Higuchi, M.; et al. Polymerizable ionic liquid crystals comprising polyoxometalate clusters toward inorganic-organic hybrid solid electrolytes. Polymers 2017, 9, 290. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Li, K.; Xie, L.; Wang, J.; Li, Y. Preparation of mesoporous carbon spheres with a bimodal pore size distribution and its application for electrochemical double layer capacitors based on ionic liquid as the electrolyte. Microporous Mesoporous Mater. 2012, 151, 282–286. [Google Scholar] [CrossRef]


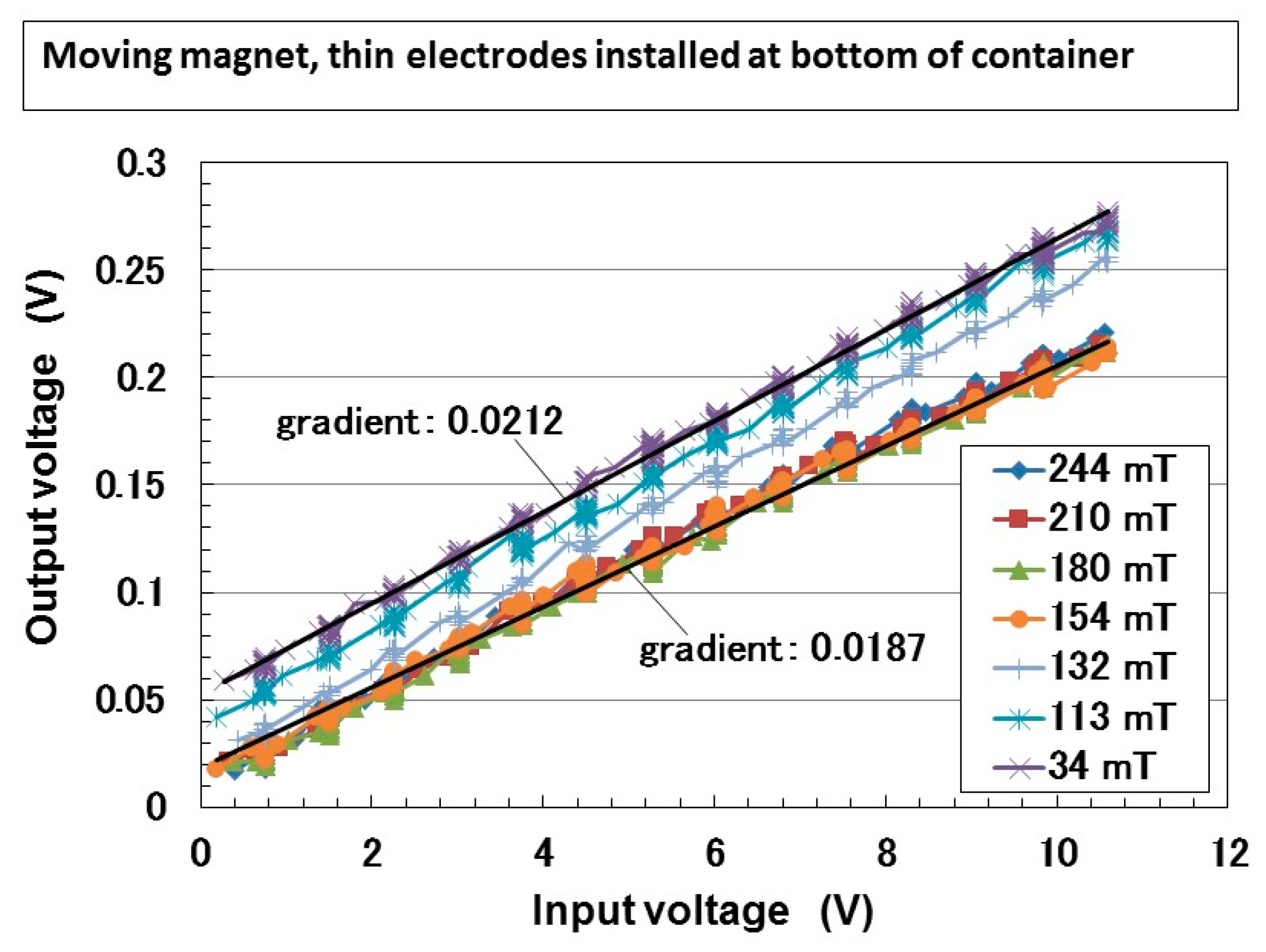
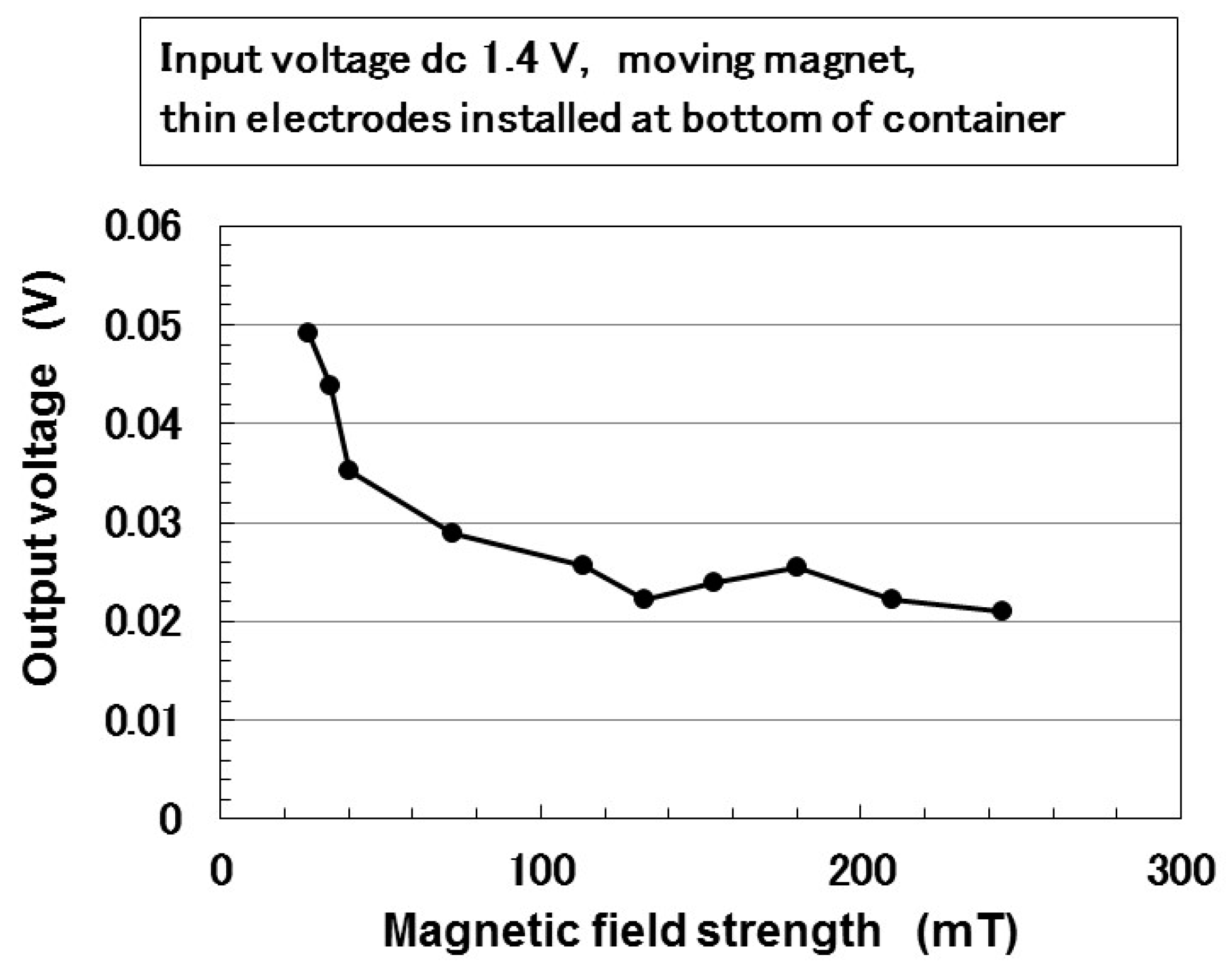
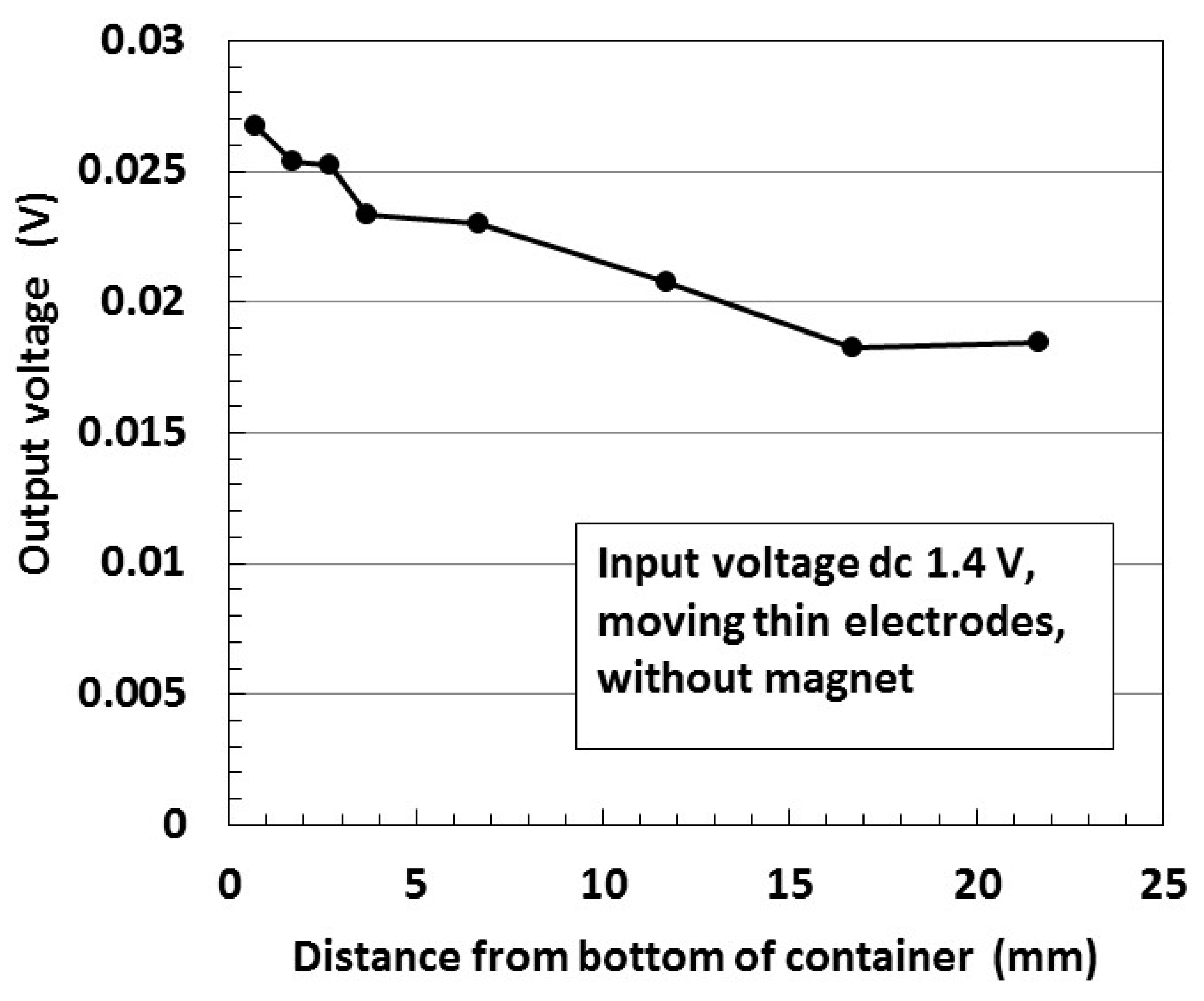
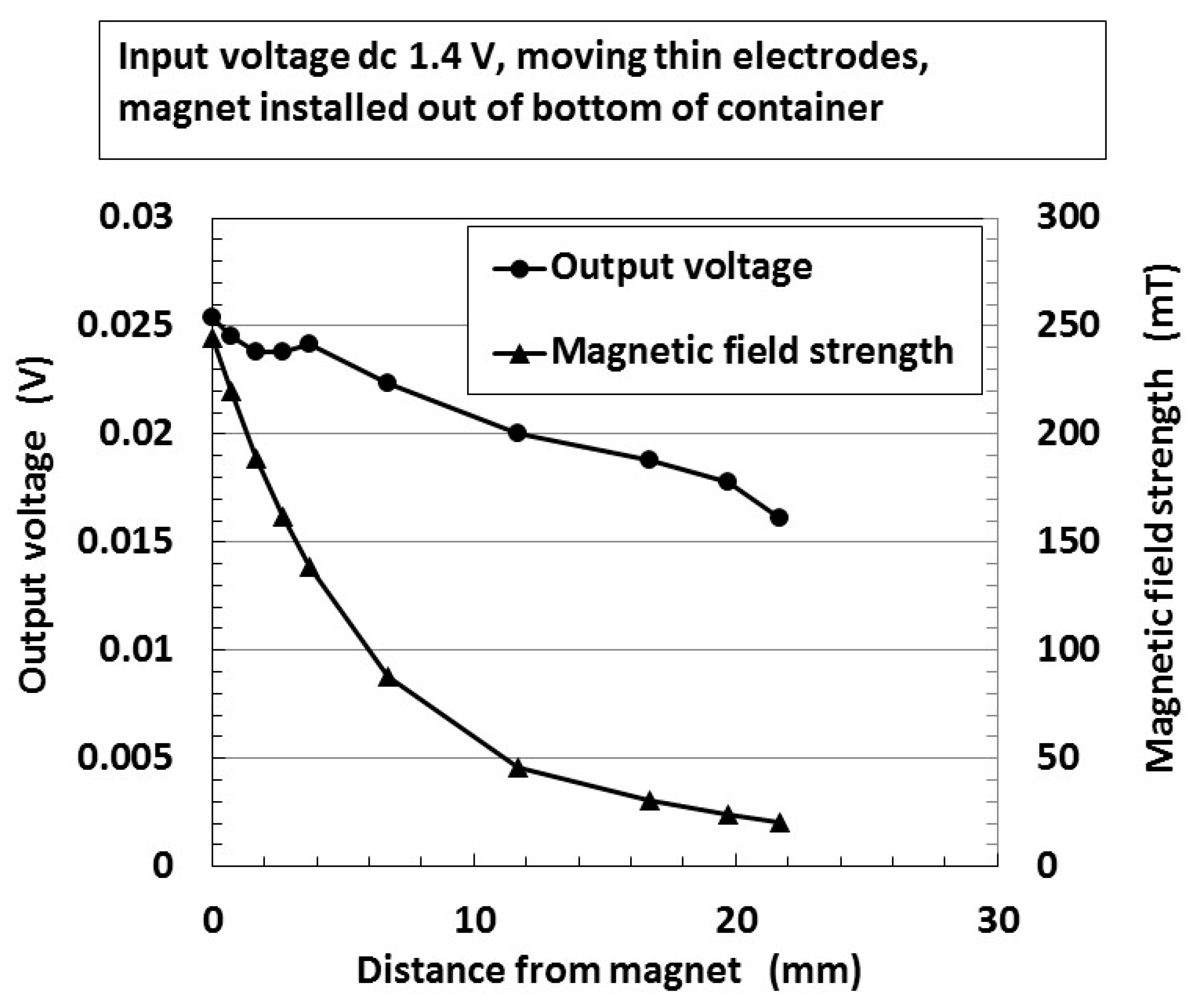
| Ni [g] | MF [g] | NR-Latex [g] | Water [g] | Electric Conductivity [S/m × 10−2] | |
|---|---|---|---|---|---|
| Water | 1.69 | ||||
| MF | 1.5 | 15.5 | 11.3 | ||
| MCF | 6 | 1.5 | 15.5 | 14.8 | |
| NR-latex | 8 | 15.5 | 25.7 | ||
| NR-latex, Ni | 6 | 8 | 15.5 | 23.5 | |
| NR-latex, MF | 1.5 | 5 | 15.5 | 25.5 | |
| NR-latex, MCF | 6 | 1.5 | 8 | 15.5 | 29.8 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimada, K. Effect of Magnetic Field and Aggregation on Electrical Characteristics of Magnetically Responsive Suspensions for Novel Hybrid Liquid Capacitor. Magnetochemistry 2019, 5, 38. https://doi.org/10.3390/magnetochemistry5020038
Shimada K. Effect of Magnetic Field and Aggregation on Electrical Characteristics of Magnetically Responsive Suspensions for Novel Hybrid Liquid Capacitor. Magnetochemistry. 2019; 5(2):38. https://doi.org/10.3390/magnetochemistry5020038
Chicago/Turabian StyleShimada, Kunio. 2019. "Effect of Magnetic Field and Aggregation on Electrical Characteristics of Magnetically Responsive Suspensions for Novel Hybrid Liquid Capacitor" Magnetochemistry 5, no. 2: 38. https://doi.org/10.3390/magnetochemistry5020038
APA StyleShimada, K. (2019). Effect of Magnetic Field and Aggregation on Electrical Characteristics of Magnetically Responsive Suspensions for Novel Hybrid Liquid Capacitor. Magnetochemistry, 5(2), 38. https://doi.org/10.3390/magnetochemistry5020038





