Biomolecular EPR Meets NMR at High Magnetic Fields
Abstract
:1. Introduction
2. NMR versus EPR Spectroscopy at High Magnetic Fields
- (1)
- enhanced spectral resolution,
- (2)
- enhanced orientational selectivity in disordered samples,
- (3)
- enhanced detection sensitivity for restricted-volume samples,
- (4)
- enhanced “snapshot” sensitivity for probing fast motional dynamics.
3. High-Field EPR Instrumentation
- -
- Centre Européen de Résonance Magnétique Nucléaire in Lyon, France (CRMN—CNRS/École Normale Supérieure Lyon/Université Claude Bernard Lyon). This is a large-scale European facility for ultrahigh-field NMR operated by the CNRS (Centre National de la Recherche Scientifique). Since 2009 an NMR spectrometer is working there at a proton frequency of 1 GHz. By acquiring this new NMR tool, the facility offers unique analytical capacity to the international scientific community. Dozens of national and international research projects have been running so far; they involve researchers from throughout the world. In 2015, BRUKER Corp. had announced that a 1.2 GHz (28.2 T) NMR instrument has been ordered by the CNRS to be placed at the University of Lille (France). The acquisition of this 1.2 GHz spectrometer will keep France at the leading edge of NMR technology. This national instrument will be installed in Lille for a broad panel of interdisciplinary research areas ranging from structural biology to catalysis, from sustainable energy development to bio-medical applications. It will be available to the international scientific community for cooperation projects.
- -
- The Danish Center for Ultrahigh-Field NMR Spectroscopy (localized at the Department of Chemistry, Aarhus University) hosts a number of state-of-the-art NMR spectrometers operating at a multitude of frequencies up to 950 MHz and equipped for solid- and liquid-state NMR experiments of inorganic, organic, and biological molecules. All equipment is available for Danish and external users through cooperation projects with the Danish Center.
- -
- CERM (Centro di Ricerca di Risonanze Magnetiche) is a center for research, knowledge transfer, and higher education of the University of Florence, located at the Polo Scientifico (Scientific Campus) in Sesto Fiorentino. CERM applies nuclear magnetic resonance (NMR) to fundamental questions in the Life Sciences. The collection of instrumentation at CERM is among the most advanced in the world. NMR Instruments include magnets from 400 MHz to 950 MHz, many equipped with unique probes for disparate experimental needs. The Consorzio Interuniversitario Risonanze Magnetiche di Metallo Proteine (CIRMMP) provides access to equipment at a national and European level.
- -
- The National Ultrahigh-Field NMR Facility for Solids (Ottawa, Canada, managed by the University of Ottawa) is a national scientific user facility funded by the Canada Foundation for Innovation (CFI). The equipment consists of a Bruker 900 MHz NMR spectrometer with ancillary equipment to acquire ultrahigh field static and fast spinning NMR spectra of solid materials. The uniqueness of the Facility is that it is dedicated to solid-state NMR research, where the highest magnetic fields are beneficial for quadrupolar and low-gamma nuclei such as oxygen-17, magnesium-25, and chlorine-35 among others. This type of instrument is not available elsewhere in Canada.
- -
- The Netherlands facility for ultrahigh NMR spectroscopy (uNMR-NL, managed by the University of Utrecht). Distributed over four sites (Utrecht University, Leiden University, Wageningen University, Radboud University Nijmegen), the uNMR-NL facility offers expertise and measurement time on spectrometers currently ranging from 600–950 MHz for solid- and liquid-state NMR as well as micro-imaging. The four major Dutch centers for magnetic resonance research in structural biology (Utrecht), paramagnetic bio-NMR and instrumentation development (Leiden), new materials (Nijmegen) and micro-imaging for plant, food and bio-nanotechnology (Wageningen) had formed such a consortium for implementation of this national facility that aims at providing open access to a new generation of NMR instruments operating at highest existing field strength across scientific disciplines and industrial research. As an important step in this direction, the uNMR-NL consortium received funding in the The Netherlands Organisation for Scientific Research (NWO) National Roadmap for Research Infrastructures to accqire the first 1.2 GHz (28.2 T) NMR spectrometer in the Netherlands. The installation of the 1.2 GHz NMR system (BRUKER) is expected in 2019.
- -
- The Center for Biomolecular Magnetic Resonance (BMRZ) at the Goethe University in Frankfurt (M), Germany. BMRZ is part of the European Large Scale Facilities and incorporates various high-field liquid and solid-state NMR spectrometers, as well as DNP-NMR and advanced EPR instrumentation. The 1.2 GHz (28.2 T) NMR spectrometer (BRUKER), ordered in 2015, is expected to be soon available to the scientific community in Germany and Europe. Research at the BMRZ center is dedicated to the elucidation of structure and functional mechanisms of biomolecules ranging from RNA and RNA-protein complexes, via large protein complexes to membrane proteins. Former Managing Director of the BMRZ center, Professor Harald Schwalbe, Goethe University in Frankfurt (M), happily remarked in relation to the new generation of ultrahigh-field NMR spectrometers: “The 1.2 GHz NMR system will allow us to investigate structure, dynamics and biological function of increasingly large and challenging biomolecular complexes. We will also be able to provide access for European researchers”. In 2017, Harald Schwalbe wrote an illumination Guest Editorial in Angewandte Chemie with the title “New 1.2 GHz NMR Spectrometers—New Horizons?” in which he gives an expert account of the key steps in magnet science and technology developments that were necessary to ultimately enable fabrication of such a revolutionary NMR instrument [121]. From this article we quote the last paragraph:“NMR is not the only structural biology technique undergoing revolutionary changes. Findings triggered by the development of free-electron laser crystallography (XFEL) and by new detectors for cryo-EM single particle and tomography analyses are impressive. Germany reacted by providing funding for the European XFEL installation in Hamburg, including four 1.2 GHz NMR spectrometers and for several cryo-EM machines. These funding decisions came at the right moment. It is important to note that all of the initiatives in structural biology have a national as well as a European dimension. NMR centers in Florence, Utrecht and Frankfurt will provide access to the new 1.2 GHz spectrometers for researchers from all over the European Union. Given the current isolationist movements, it will always be important to link national and European, if not global research endeavors, for the benefit of fundamental and applied research alike.”
4. Advanced Multifrequency EPR Techniques, a Brief Chronological Account
4.1. CW TREPR
4.2. Pulse EPR
4.3. ENDOR
4.3.1. Solid-State ENDOR
4.3.2. Solution ENDOR and TRIPLE Resonance
4.3.3. Pulse ENDOR
“In most practical situations, cw ENDOR is the method of choice for the measurement of small hyperfine couplings in liquid solution, whereas in solids pulse ENDOR is often superior.”
4.4. ESEEM
4.5. HYSCORE
4.6. ELDOR-Detected NMR (EDNMR)
4.7. PELDOR (DEER)
4.8. Terahertz High-Field EPR Spectroscopy
5. Site-Directed Spin Labeling (SDSL) in High-Field EPR Spectroscopy
5.1. Overview of Studies on Nitroxide Spin-Labeled Proteins and DNA Complexes
5.2. EPR Triangulation
5.3. An Illustrative Example: High-Field EPR on Nitroxide Spin-Labeled Bacteriorhodopsin
“X-ray free-electron lasers have opened up the possibility of structure determination of protein crystals at room temperature, free of radiation damage. The femtosecond-duration pulses of these sources enable diffraction signals to be collected from samples at doses of 1000 MGy or higher. The sample is vaporized by the intense pulse, but not before the scattering that gives rise to the diffraction pattern takes place. Consequently, only a single flash diffraction pattern can be recorded from a crystal, giving rise to the method of serial crystallography where tens of thousands of patterns are collected from individual crystals that flow across the beam and the patterns are indexed and aggregated into a set of structure factors. The high-dose tolerance and the many-crystal averaging approach allow data to be collected from much smaller crystals than have been examined at synchrotron radiation facilities, even from radiation-sensitive samples.”
- -
- For the RC complex from the non-sulfur purple bacterium Blastochloris viridis femtosecond X-ray diffraction work was done in 2013 by a consortium of 45 authors from 12 institutions [524]. The X-ray diffraction data were recorded from microcrystals of the RC to 2.8 Å resolution, and its serial femtosecond crystallography structures were determined to 3.5 Å resolution. Remarkably, although every microcrystal is exposed to a radiation dose of 33 MGy, no signs of X-ray-induced radiation damage are visible in this integral membrane protein structure. This reveals an exytremely important advantage of the femtosecond crystallography based on an ultrafast pulsed X-ray free-electron-laser method: It avoids radiation damages of protein complexes. Clearly, this crystallgraphic method has considerable potential for solving many challenging problems in current structural biology.
- -
- The Photosystem II (PS II) complexes of the cyanobacterium Thermosynechococcus (T.) elongatus [525,526,527,528,529] and T. vulcanus [530,531] have also been studied by serial femtosecond crystallography using X-ray lasers provided by the facilities at Stanford, CA, USA (LCLS) and Hyogo, Japan (SACLA). In this ground breaking work the advantages of the technique could be clearly demonstrated, namely that diffraction data can now be obtained for very small PS II crystals without radiation damage and that transient—even short-lived—states of the enzyme can be accessed in real time at room temperature [526]. The technique is of particular importance for the investigation of the water oxidizing/oxygen evolving enzyme located in PS II that undergoes significant structural changes within its catalytic cycle (for a detailed EPR story of the OEC, see Section 8, Case Study II).
6. Selected Topics of Current High-Field EPR Spectroscopy on Biosystems
6.1. Extending the Distance Range between Molecular Spin Centers by High-Field Dipolar EPR with Gd3+ Spin Probes
6.2. Exploring by Ultrahigh-Field EPR the Molecular Basis of Radiation Resistance of Certain Bacterial Cells Containg Small High-Symmetry Antioxidant Complexes of Manganous Ions
6.3. The Effect of Protein-Solvent Interactions for Biological Function and the Survival of Organisms under Extreme Stress Situations of Heat and Dryness
6.4. Structure and Function of Transition Metal Centers in Metalloproteins
7. Case Study I: High-Field ELDOR-Detected NMR (EDNMR) as a General Method for Electron–Nuclear Hyperfine Spectroscopy with an Application on Nitroxide Radical and Transition Metal Containing Systems
7.1. Introduction
- (i)
- Enhanced spectral resolution: the frequency at which the NMR transition of a particular nucleus is observed is linearly dependent on the applied magnetic field (Figure 7c). As such, moving to higher magnetic fields allows different nuclei to be more readily discriminated, as is the case in NMR spectroscopy.
- (ii)
- (iii)
- (iv)
7.2. Nitrogen EDNMR on Nitroxide Radicals in Organic Solvents
7.3. EDNMR on Nitroxide Labeled Bacterial Reaction Centers Embedded in a Trehalose Glass
7.4. EDNMR on Transition Metal Containing Systems
7.5. Conclusion
8. Case Study II: High-Field ENDOR and EDNMR Studies of the Oxygen Evolving Complex (OEC) in PS II
8.1. Introduction
8.2. Structure of Photosystem II, Primary Events and the Water Oxidation Cycle
8.3. Electronic Structure of the OEC
8.4. Substrate Binding to the OEC
8.5. Conclusion and Future Challenges in Biological Water Oxidation
9. Conclusions
- -
- New developments in pulsed microwave and sweepable cryomagnet technology as well as ultrafast electronics for signal data handling and processing have pushed the limits of modern EPR spectroscopy to the mm- and sub-mm wavelength and 15 T Zeeman field regions. Sub-micromolar concentrations of paramagnetic molecules such as nitroxide spin-labeled protein complexes have become sufficient to characterize reaction intermediates—offering new application possibilities in biochemistry and molecular biology [17,28,29]. Moreover, for multifrequency EPR experiments on frozen solutions typical sample volumes are 250 μL (S-band), 150 μL (X-band), 10 μL (Q-band) and 1 μL (W-band), see [649,650,651,652,653]. This is orders of magnitude better than the sample volume requirements for modern NMR spectroscopy.
- -
- Modern multifrequency EPR methods, in particular at high magnetic fields and microwave frequencies, provide unique information on structure and dynamics of stable and transient radicals and radical pairs occurring in chemical and biological processes. Optimized time windows can be selected by the multifrequency EPR approach to disentangle different modes of motion at biologically relevant timescales.
- -
- Compared to optical spectroscopy, both NMR and EPR, notoriously suffer from low detection sensitivity, in fact NMR much more than EPR (by a factor of 106). Hence, both techniques strive for “quantum transformation” in a double-resonance experiment by which the radiofrequency or microwave quanta absorbed at resonance are not detected directly but rather by their effect on a coupled quantum system of higher energy [653,654,655]. For this strategy, ENDOR is a paradigmatic example.
- -
- A different strategy for sensitivity enhancement is taking advantage of spin-polarization effects on a nuclear-spin system by a coupled electron-spin system. Powerful polarizing mechanisms are, for instance, CIDNP, CIDEP; they cover a wide field of research and applications. The key references of original and overview articles and books in the literature of this field are just too many to be cited here. From the numerous review articles and monographs published over recent decades we give only a few examples which we found useful, such as [132,656,657,658,659,660,661]. A recent illuminating research article by Michael Wasielewski and coworkers on spin polarization transfer by the radical pair mechanism [661] contains a representative list of refrences with leading citations included.
- -
- Many organic cofactors in electron-transfer proteins have only small g anisotropies and, hence, to resolve the canonical g-tensor orientations in disordered samples such as frozen solutions much higher magnetic fields are required than available in X-band EPR. Thereby, orientation-selective hydrogen bonding and polar interactions in the protein binding sites can be traced by high-field EPR, which provides information complementary to what is available from high-resolution X-ray crystallography.
- -
- Organic radicals are often generated as transient intermediates during photoinitiated processes in proteins. To identify and distinguish them by the small differences in their g-factors and hyperfine interactions, time-resolved high-field EPR becomes the method of choice for elucidating reaction pathways.
- -
- Pulse EPR techniques for solid-state applications have come to the fore and can provide details of weak electron–electron or electron–nuclear couplings and their orientational dependence—even in disordered powder samples or glasses. State-of-the-art pulse high-field EPR spectrometers at 94 GHz offer single nanoseconds length π/2 pulses and short dead-times with GHz detection bandwidths permitting multi-dimensional, multi-resonance experiments at the 1 μM concentration level.
- -
- PELDOR, ENDOR, ESEEM or EDNMR at high Zeeman fields take additional advantage of the orientation selection of molecular sub-ensembles in powder or frozen-solution samples. Thereby, even in the case of small g anisotropies, these techniques can provide single-crystal-like information about electron dipolar and electron–nuclear hyperfine interactions, including the directions of interspin dipolar axes in coupled radical pairs and hydrogen bonds to cofactors in the protein.
- -
- In metallo-protein high-spin systems, such as the Mn2+ or Co2+ proteins, the EPR spectrum analysis can be drastically simplified at high Zeeman fields due to the suppression of second-order effects. This is also accompanied by a considerable increase in sensitivity. In case of large zero-field splittings, EPR transitions might not be observable at all at X-band, but become accessible at higher quantum energies of mm or sub-mm microwaves.
- -
- High-field EPR noticeably extends the applicability of the site-directed spin-labeling technique, which was originally combined with X-band EPR. Owing to the spectral resolution of both the g-tensor and hyperfine-tensor components of nitroxide spin probes, polarity and proticity profiles of the protein microenvironment can be identified, for example in transmembrane channel proteins.
- -
- High-field EPR/high-frequency instrumentation development will remain a challenging task. This specifically refers to sufficient output power of mm and sub-mm microwave sources for fast ns pulsing. Ultrahigh-field magnet technology improvements will continue to play a decisive role in this endeavor. We concur with the assessment of a reviewer of this paper stating that “EPR is on a journey and the next ten years will see a push to the very highest fields and frequencies, partly led by advances in mm-wave/THz technology”.
- -
- Both the NMR and EPR communities are driven by the same motivation—to understand spin interactions in complex systems for revealing their structure and dynamics—be it from the viewpoint of materials science, or biological or medical sciences. The big issues in the natural and life sciences—environment, sustainable energy, health and disease, food and water—ask for the best of all methodologies to apply, including EPR and NMR. Success of contributions to such big issues will rely on the design of new magnetic-resonance experiments “off the beaten track” as well as on in-depth theoretical analyses of the experimental results on the basis of modern quantum-chemical methodologies.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Möbius, K.; Lubitz, W.; Savitsky, A. High-field EPR on membrane proteins—Crossing the gap to NMR. Prog. Nucl. Magn. Reson. Spectrosc. 2013, 75, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, A.; Jeschke, G. Principles of Pulse Electron Paramagnetic Resonance; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Neuhaus, D.; Bodenhausen, G.; Gadian, D.; Meier, B.; Morris, G. Fifty years of “progress in NMR spectroscopy”—An editorial from the present editorial board. Prog. Nucl. Magn. Reson. Spectrosc. 2016, 94–95, A1–A2. [Google Scholar] [CrossRef] [PubMed]
- Feeney, J.; Emsley, J.W. Forty years of Progress in Nuclear Magnetic Resonance Spectroscopy. Prog. Nucl. Magn. Reson. Spectrosc. 2007, 50, 179–198. [Google Scholar]
- Meirovitch, E.; Shapiro, Y.E.; Pohmeno, A.; Freed, J.H. Structural dynamics of bio-macromolecules by NMR: The slowly relaxing local structure approach. Prog. Nucl. Magn. Reson. Spectrosc. 2010, 56, 360–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeschke, G. Conformational dynamics and distribution of nitroxide spin labels. Prog. Nucl. Magn. Reson. Spectrosc. 2013, 72, 42–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griesinger, C.; Bennati, M.; Vieth, H.M.; Luchinat, C.; Parigi, G.; Hoefer, P.; Engelke, F.; Glaser, S.J.; Denysenkov, V.; Prisner, T.F. Dynamic nuclear polarization at high magnetic fields in liquids. Prog. Nucl. Magn. Reson. Spectrosc. 2012, 64, 4–28. [Google Scholar] [CrossRef] [PubMed]
- Webb, A. Cavity- and waveguide-resonators in electron paramagnetic resonance, nuclear magnetic resonance, and magnetic resonance imaging. Prog. Nucl. Magn. Reson. Spectrosc. 2014, 83, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, K.L.; Pravdivtsev, A.N.; Yurkovskaya, A.V.; Vieth, H.M.; Kaptein, R. The role of level anti-crossings in nuclear spin hyperpolarization. Prog. Nucl. Magn. Reson. Spectrosc. 2014, 81, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Suter, D.; Jelezko, F. Single-spin magnetic resonance in the nitrogen-vacancy center of diamond. Prog. Nucl. Magn. Reson. Spectrosc. 2017, 98–99, 50–62. [Google Scholar] [CrossRef] [PubMed]
- De Boer, E.; Van Willigen, H. Nuclear magnetic resonance of paramagnetic systems. Prog. Nucl. Magn. Reson. Spectrosc. 1967, 2, 111–161. [Google Scholar] [CrossRef]
- Mar, G.N.L. Nuclear Magnetic Resonance of Paramagnetic Macromolecules; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1995. [Google Scholar]
- Ubbink, M.; Worrall, J.A.R.; Canters, G.W.; Groenen, E.J.J.; Huber, M. Paramagnetic resonance of biological metal centers. Annu. Rev. Biophys. Biomol. Struct. 2002, 31, 393–422. [Google Scholar] [CrossRef] [PubMed]
- Bertini, I.; Luchinat, C.; Parigi, G.; Pierattelli, R. Perspectives in paramagnetic NMR of metalloproteins. Dalton Trans. 2008, 3782–3790. [Google Scholar] [CrossRef] [PubMed]
- Otting, G. Protein NMR using paramagnetic ions. Annu. Rev. Biophys. 2010, 39, 387–405. [Google Scholar] [CrossRef] [PubMed]
- Su, X.-C.; Otting, G. Paramagnetic labelling of proteins and oligonucleotides for NMR. J. Biomol. NMR 2009, 46, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Möbius, K.; Savitsky, A. High-Field EPR Spectroscopy on Proteins and Their Model Systems: Characterization of Transient Paramagnetic States; RSC Publishing: London, UK, 2009; p. 385. [Google Scholar]
- Bertini, I.; Luchinat, C.; Parigi, G.; Ravera, E. NMR of Paramagnetic Molecules, 2nd ed.; Elsevier: Boston, MA, USA, 2017. [Google Scholar]
- Berliner, L.J.; Eaton, S.S.; Eaton, G.R. Distance Measurements in Biological Systems by EPR, Biological Magnetic Resonance; Kluwer: New York, NY, USA, 2002; Volume 19. [Google Scholar]
- Song, Y.; Meade, T.J.; Astashkin, A.V.; Klein, E.L.; Enemark, J.H.; Raitsimring, A. Pulsed dipolar spectroscopy distance measurements in biomacromolecules labeled with Gd(III) markers. J. Magn. Reson. 2011, 210, 59–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaminker, I.; Yagi, H.; Huber, T.; Feintuch, A.; Otting, G.; Goldfarb, D. Spectroscopic selection of distance measurements in a protein dimer with mixed nitroxide and Gd3+ spin labels. Phys. Chem. Chem. Phys. 2012, 14, 4355–4358. [Google Scholar] [CrossRef] [PubMed]
- Yagi, H.; Banerjee, D.; Graham, B.; Huber, T.; Goldfarb, D.; Otting, G. Gadolinium tagging for high-precision measurements of 6 nm distances in protein assemblies by EPR. J. Am. Chem. Soc. 2011, 133, 10418–10421. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.K. Multifrequency Electron Paramagnetic Resonance: Theory and Applications; Wiley-VCH: Berlin, Germany, 2011. [Google Scholar]
- Rist, G.; Hyde, J.S. Ligand ENDOR of Cu-8-hydroxyquinolinate substituted into a single crystal and a powder of phthalimide. J. Chem. Phys. 1968, 49, 2449–2451. [Google Scholar] [CrossRef]
- Rist, G.; Hyde, J.S. Ligand ENDOR of Cu-8-hydroxyquinolinate substituted into organic single crystals. J. Chem. Phys. 1969, 50, 4532–4542. [Google Scholar] [CrossRef]
- Rist, G.; Hyde, J.S. Ligand ENDOR of metal complexes in powders. J. Chem. Phys. 1970, 52, 4633–4643. [Google Scholar] [CrossRef]
- Hoffman, B.M.; DeRose, V.J.; Doan, P.E.; Gurbiel, R.J.; Houseman, A.L.P.; Telser, J. Metalloenzyme active-site structure and function through multifrequency cw and pulsed ENDOR. In EMR of Paramagnetic Molecules; Berliner, L.J., Reuben, J., Eds.; Springer: Boston, MA, USA, 1993; pp. 151–218. [Google Scholar]
- Song, L.; Liu, Z.; Kaur, P.; Esquiaqui, J.M.; Hunter, R.I.; Hill, S.; Smith, G.M.; Fanucci, G.E. Toward increased concentration sensitivity for continuous wave EPR investigations of spin-labeled biological macromolecules at high fields. J. Magn. Reson. 2016, 265, 188–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruickshank, P.A.S.; Bolton, D.R.; Robertson, D.A.; Hunter, R.I.; Wylde, R.J.; Smith, G.M. A kilowatt pulsed 94 GHz electron paramagnetic resonance spectrometer with high concentration sensitivity, high instantaneous bandwidth, and low dead time. Rev. Sci. Instrum. 2009, 80, 103102. [Google Scholar] [CrossRef] [PubMed]
- Borbat, P.P.; Costa-Filho, A.J.; Earle, K.A.; Moscicki, J.K.; Freed, J.H. Electron spin resonance in studies of membranes and proteins. Science 2001, 291, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, Y.D.; Grishin, Y.A. Techniques for EPR spectroscopy of pulsed electron double resonance (PELDOR): A review. Instrum. Exp. Technnol. 2009, 52, 615–636. [Google Scholar] [CrossRef]
- Spindler, P.E.; Glaser, S.J.; Skinner, T.E.; Prisner, T.F. Broadband inversion PELDOR spectroscopy with partially adiabatic shaped pulses. Angew. Chem. Int. Ed. 2013, 52, 3425–3429. [Google Scholar] [CrossRef] [PubMed]
- Schiemann, O.; Prisner, T.F. Long-range distance determinations in biomacromolecules by EPR spectroscopy. Q. Rev. Biophys. 2007, 40, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, G. Determination of the nanostructure of polymer materials by electron paramagnetic resonance spectroscopy. Macromol. Rapid Commun. 2002, 23, 227–246. [Google Scholar] [CrossRef]
- Jeschke, G.; Polyhach, Y. Distance measurements on spin-labelled biomacromolecules by pulsed electron paramagnetic resonance. Phys. Chem. Chem. Phys. 2007, 9, 1895–1910. [Google Scholar] [CrossRef] [PubMed]
- Wegener, A.A.; Klare, J.P.; Engelhard, M.; Steinhoff, H.J. Structural insights into the early steps of receptor-transducer signal transfer in archaeal phototaxis. EMBO J. 2001, 20, 5312–5319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borbat, P.P.; Freed, J.H. Pros and cons of pulse dipolar ESR. EPR Newsl. 2007, 17, 21–33. [Google Scholar]
- Altenbach, C.; Kusnetzow, A.K.; Ernst, O.P.; Hofmann, K.P.; Hubbell, W.L. High-resolution distance mapping in rhodopsin reveals the pattern of helix movement due to activation. Proc. Natl. Acad. Sci. USA 2008, 105, 7439–7444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgieva, E.R.; Ramlall, T.F.; Borbat, P.P.; Freed, J.H.; Eliezer, D. Membrane-bound alpha-synuclein forms an extended helix: Long-distance pulsed ESR measurements using vesicles, bicelles, and rodlike micelles. J. Am. Chem. Soc. 2008, 130, 12856–12857. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Bortolus, M.; McHaourab, H.S. Conformational cycle of the ABC transporter msba in liposomes: Detailed analysis using double electron-electron resonance spectroscopy. J. Mol. Biol. 2009, 393, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Grinberg, O.; Dubinskii, A.A. The early years. In Very High Frequency (VHF) ESR/EPR, Biological Magnetic Resonance; Grinberg, O., Berliner, L.J., Eds.; Kluwer/Plenum Publishers: New York, NY, USA, 2004; Volume 22, pp. 1–15. [Google Scholar]
- Lebedev, Y.S. Very high-field EPR. In Foundations of Modern EPR; Eaton, S.S., Salikhov, K.M., Eds.; World Scientific: Singapore, 1998; p. 731. [Google Scholar]
- Lebedev, Y.S. High-frequency continuous-wave electron spin resonance. In Modern Pulsed and Continuous-Wave Electron Spin Resonance; Kevan, L., Bowman, M.K., Eds.; John Wiley: New York, NY, USA, 1990; pp. 365–404. [Google Scholar]
- Haindl, E.; Möbius, K.; Oloff, H. A 94 GHz electron-paramagnetic-res spectrometer with fabry-perot resonator. Zeitschrift für Naturforschung A 1985, 40, 169–172. [Google Scholar] [CrossRef]
- Burghaus, O.; Rohrer, M.; Götzinger, T.; Plato, M.; Möbius, K. A novel high-field high-frequency EPR and ENDOR spectrometer operating at 3 mm wavelength. Meas. Sci. Technol. 1992, 3, 765–774. [Google Scholar] [CrossRef]
- Weber, R.T.; Disselhorst, J.; Prevo, L.J.; Schmidt, J.; Wenckebach, W.T. Electron spin-echo spectroscopy at 95 GHz. J. Magn. Reson. 1989, 81, 129–144. [Google Scholar] [CrossRef]
- Allgeier, J.; Disselhorst, J.A.J.M.; Weber, R.T.; Wenckebach, W.T.; Schmidt, J. High-frequency pulsed electron spin resonance. In Modern Pulsed and Continuous-Wave Electron Spin Resonance; Kevan, L., Bowman, M.K., Eds.; John Wiley: New York, NY, USA, 1990; pp. 267–283. [Google Scholar]
- Burghaus, O.; Toth-Kischkat, A.; Klette, R.; Möbius, K. Proton ENDOR at a microwave frequency of 97 GHz. J. Magn. Reson. 1988, 80, 383–388. [Google Scholar] [CrossRef]
- Prisner, T.F.; Rohrer, M.; Möbius, K. Pulsed 95-GHz, high-field EPR heterodyne spectrometer with high spectral and time resolution. Appl. Magn. Reson. 1994, 7, 167–183. [Google Scholar] [CrossRef]
- Disselhorst, J.A.J.M.; van der Meer, H.; Poluektov, O.G.; Schmidt, J. A pulsed EPR and ENDOR spectrometer operating at 95 GHz. J. Magn. Reson. A 1995, 115, 183–188. [Google Scholar] [CrossRef]
- Prisner, T.F.; Un, S.; Griffin, R.G. Pulsed ESR at 140 GHz. Isr. J. Chem. 1992, 32, 357–363. [Google Scholar] [CrossRef]
- Prisner, T.F. Pulsed high frequency/high-field EPR. Adv. Magn. Opt. Reson. 1997, 20, 245–299. [Google Scholar]
- Rohrer, M.; Brugmann, O.; Kinzer, B.; Prisner, T.F. High-field/high-frequency EPR spectrometer operating in pulsed and continuous-wave mode at 180 GHz. Appl. Magn. Reson. 2001, 21, 257–274. [Google Scholar] [CrossRef]
- Smith, G.M.; Lesurf, J.C.G.; Mitchell, R.H.; Riedi, P.C. Quasi-optical cw mm-wave electron spin resonance spectrometer. Rev. Sci. Instrum. 1998, 69, 3924–3937. [Google Scholar] [CrossRef]
- Lynch, W.B.; Earle, K.A.; Freed, J.H. 1-mm wave electron-spin-resonance spectrometer. Rev. Sci. Instrum. 1988, 59, 1345–1351. [Google Scholar] [CrossRef]
- Fuchs, M.R.; Prisner, T.F.; Möbius, K. A high-field/high-frequency heterodyne induction-mode electron paramagnetic resonance spectrometer operating at 360 GHz. Rev. Sci. Instrum. 1999, 70, 3681–3683. [Google Scholar] [CrossRef]
- Muller, F.; Hopkins, M.A.; Coron, N.; Grynberg, M.; Brunel, L.C.; Martinez, G. A high magnetic-field EPR spectrometer. Rev. Sci. Instrum. 1989, 60, 3681–3684. [Google Scholar] [CrossRef]
- Reijerse, E.; Schmidt, P.P.; Klihm, G.; Lubitz, W. A cw and pulse EPR spectrometer operating at 122 and 244 GHz using a quasi-optical bridge and a cryogen-free 12 T superconducting magnet. Appl. Magn. Reson. 2007, 31, 611–626. [Google Scholar] [CrossRef]
- Fuchs, M. A High-Field/High-Frequency Electron Paramagnetic Resonance Spectrometer (360 GHz/14 T). Ph.D. Thesis, Free Universty Berlin, Berlin, Germany, 1999. [Google Scholar]
- Schnegg, A.; Dubinskii, A.A.; Fuchs, M.R.; Grishin, Y.A.; Kirilina, E.P.; Lubitz, W.; Plato, M.; Savitsky, A.; Möbius, K. High-field EPR, ENDOR and eldor on bacterial photosynthetic reaction centers. Appl. Magn. Reson. 2007, 31, 59–98. [Google Scholar] [CrossRef]
- Fuhs, M.; Möbius, K. Pulsed-high field/high-frequency EPR spectroscopy. In High Magnetic Fields: Applications in Condensed Matter Physics and Spectroscopy; Berthier, C., Lévy, L.P., Martinez, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 476–493. [Google Scholar]
- Schnegg, A. High-Field EPR on Electrontransfer Proteins. Ph.D. Thesis, Free Universty Berlin, Berlin, Germany, 2003. [Google Scholar]
- Grishin, Y.A.; Fuchs, M.R.; Schnegg, A.; Dubinskii, A.A.; Dumesh, B.S.; Rusin, F.S.; Bratman, V.L.; Möbius, K. Pulsed orotron—A new microwave source for submillimeter pulse high-field electron paramagnetic resonance spectroscopy. Rev. Sci. Instrum. 2004, 75, 2926–2936. [Google Scholar] [CrossRef]
- Fuchs, M.R.; Schleicher, E.; Schnegg, A.; Kay, C.W.M.; Törring, J.T.; Bittl, R.; Bacher, A.; Richter, G.; Möbius, K.; Weber, S. G-tensor of the neutral flavin radical cofactor of DNA photolyase revealed by 360-GHz electron paramagnetic resonance spectroscopy. J. Phys. Chem. B 2002, 106, 8885–8890. [Google Scholar] [CrossRef]
- Weber, S.; Kay, C.W.M.; Mögling, H.; Möbius, K.; Hitomi, K.; Todo, T. Photoactivation of the flavin cofactor in xenopus laevis (6–4) photolyase: Observation of a transient tyrosyl radical by time-resolved electron paramagnetic resonance. Proc. Natl. Acad. Sci. USA 2002, 99, 1319–1322. [Google Scholar] [CrossRef] [PubMed]
- Schnegg, A.; Kay, C.W.M.; Schleicher, E.; Hitomi, K.; Todo, T.; Möbius, K.; Weber, S. The G-tensor of the flavin cofactor in (6–4) photolyase: A 360 GHz/12.8 T electron paramagnetic resonance study. Mol. Phys. 2006, 104, 1627–1633. [Google Scholar] [CrossRef]
- Okafuji, A.; Schnegg, A.; Schleicher, E.; Möbius, K.; Weber, S. G-tensors of the flavin adenine dinucleotide radicals in glucose oxidase: A comparative multifrequency electron paramagnetic resonance and electron-nuclear double resonance study. J. Phys. Chem. B 2008, 112, 3568–3574. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, M.R.; Schnegg, A.; Plato, M.; Schulz, C.; Müh, F.; Lubitz, W.; Möbius, K. The primary donor cation p+· in potosynthetic reaction centers of site-directed mutants of Rhodobacter sphaeroides: G-tensor shifts revealed by high-field EPR at 360 GHz/12.8 T. Chem. Phys. 2003, 294, 371–384. [Google Scholar] [CrossRef]
- Möbius, K.; Goldfarb, D. High-field/high-frequency electron paramagnetic resonance involving single- and multiple-transition schemes. In Biophysical Techniques in Photosynthesis, Vol. II; Aartsma, T.J., Matysik, J., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 267–304. [Google Scholar]
- Misra, S.K.; Möbius, K.; Savitsky, A. Multifrequency EPR on photosynthetic systems. In Multifrequency Electron Paramagnetic Resonance; Misra, S.K., Ed.; Wiley-VCH: Berlin, Germany, 2011; pp. 875–911. [Google Scholar]
- Möbius, K.; Savitsky, A.; Fuchs, M. Primary processes in photosynthesis: What do we learn from high-field EPR spectroscopy? In Very High Frequency (VHF) ESR/EPR, Biological Magnetic Resonance Vol. 22; Grinberg, O.Y., Berliner, L.J., Eds.; Springer: Boston, MA, USA, 2004; pp. 45–93. [Google Scholar]
- Möbius, K.; Schnegg, A.; Plato, M.; Fuchs, M.R.; Savitsky, A. High-field EPR spectroscopy on transfer proteins in biological action. Acta Phys. Pol. A 2005, 108, 215–234. [Google Scholar] [CrossRef]
- Galkin, A.A.; Grinberg, O.Y.; Dubinskii, A.A.; Kabdin, N.N.; Krymov, V.N.; Kurochkin, V.I.; Lebedev, Y.S.; Oranskii, L.F.; Shuvalov, V.F. EPR spectrometer in 2-mm range for chemical-research. Instrum. Exp. Tech. 1977, 20, 1229. [Google Scholar]
- Bresgunov, A.Y.; Dubinskii, A.A.; Krimov, V.N.; Petrov, Y.G.; Poluektov, O.G.; Lebedev, Y.S. Pulsed EPR in 2-mm band. Appl. Magn. Reson. 1991, 2, 715–728. [Google Scholar] [CrossRef]
- van der Meer, H.J.; Disselhorst, J.A.J.M.; Allgeier, J.; Schmidt, J.; Wenckebach, W.T. A low-temperature insert for a 95 GHz electron-spin-echo spectrometer. Meas. Sci. Technol. 1990, 1, 396–400. [Google Scholar] [CrossRef]
- Schmalbein, D.; Maresch, G.G.; Kamlowski, A.; Höfer, P. The bruker high-frequency-EPR system. Appl. Magn. Reson. 1999, 16, 185–205. [Google Scholar] [CrossRef]
- Wang, W.; Belford, R.L.; Clarkson, R.B.; Davis, P.H.; Forrer, J.; Nilges, M.J.; Timken, M.D.; Walczak, T.; Thurnauer, M.C.; Norris, J.R.; et al. Very high-frequency EPR-94 GHz instrument and applications to primary reaction centers from photosynthetic red bacteria and to other disordered-systems. Appl. Magn. Reson. 1994, 6, 195–215. [Google Scholar] [CrossRef]
- Nilges, M.J.; Smirnov, A.I.; Clarkson, R.B.; Belford, R.L. Electron paramagnetic resonance w-band spectrometer with a low-noise amplifier. Appl. Magn. Reson. 1999, 16, 167–183. [Google Scholar] [CrossRef]
- Gromov, I.; Krymov, V.; Manikandan, P.; Arieli, D.; Goldfarb, D. A W-band pulsed ENDOR spectrometer: Setup and application to transition metal centers. J. Magn. Reson. 1999, 139, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, W.; Earle, K.A.; Dunnam, C.R.; Moscicki, J.K.; Freed, J.H. High-power 95 GHz pulsed electron spin resonance spectrometer. Rev. Sci. Instrum. 2004, 75, 1194–1208. [Google Scholar] [CrossRef]
- Brutlach, H.; Bordignon, E.; Urban, L.; Klare, J.P.; Reyher, H.J.; Engelhard, M.; Steinhoff, H.J. High-field EPR and site-directed spin labeling reveal a periodical polarity profile: The sequence 88 to 94 of the phototransducer NpHtrII in complex with sensory rhodopsin, NpSRII. Appl. Magn. Reson. 2006, 30, 359–372. [Google Scholar] [CrossRef]
- Hyde, J.S.; Froncisz, W.; Sidabras, J.W.; Camenisch, T.G.; Anderson, J.R.; Strangeway, R.A. Microwave frequency modulation in cw EPR at W-band using a loop-gap resonator. J. Magn. Reson. 2007, 185, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.; Dalal, N.S.; Brooks, J.S. A multifrequency-resonator-based system for high-sensitivity high-field EPR investigations of small single crystals. Appl. Magn. Reson. 1999, 16, 237–245. [Google Scholar] [CrossRef]
- Reijerse, E.J.; van Dam, P.J.; Klaassen, A.A.K.; Hagen, W.R.; van Bentum, P.J.M.; Smith, G.M. Concepts in high-frequency EPR—Applications to bio-inorganic systems. Appl. Magn. Reson. 1998, 14, 153–167. [Google Scholar] [CrossRef]
- Dorlet, P.; Seibold, S.A.; Babcock, G.T.; Gerfen, G.J.; Smith, W.L.; Tsai, A.L.; Un, S. High-field EPR study of tyrosyl radicals in prostaglandin H-2 synthase-1. Biochemistry 2002, 41, 6107–6114. [Google Scholar] [CrossRef] [PubMed]
- Gunn, A.; Brynda, M.; Britt, R.D. Construction of laboratory built D-band (130 GHz) pulsed and cw EPR spectrometer. Abstr. Pap. Am. Chem. Soc. 2005, 229, U751. [Google Scholar]
- Stich, T.A.; Lahiri, S.; Yeagle, G.; Dicus, M.; Brynda, M.; Gunn, A.; Aznar, C.; DeRose, V.J.; Britt, R.D. Multifrequency pulsed EPR studies of biologically relevant manganese(II) complexes. Appl. Magn. Reson. 2007, 31, 321–341. [Google Scholar] [CrossRef] [PubMed]
- Bennati, M.; Farrar, C.T.; Bryant, J.A.; Inati, S.J.; Weis, V.; Gerfen, G.J.; Riggs-Gelasco, P.; Stubbe, J.; Griffin, R.G. Pulsed electron-nuclear double resonance (ENDOR) at 140 GHz. J. Magn. Reson. 1999, 138, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Becerra, L.R.; Gerfen, G.J.; Bellew, B.F.; Bryant, J.A.; Hall, D.A.; Inati, S.J.; Weber, R.T.; Un, S.; Prisner, T.F.; McDermott, A.E.; et al. A spectrometer for dynamic nuclear-polarization and electron-paramagnetic-resonance at high-frequencies. J. Magn. Reson. A 1995, 117, 28–40. [Google Scholar] [CrossRef]
- Prisner, T.; Rohrer, M.; MacMillan, F. Pulsed EPR spectroscopy: Biological applications. Annu. Rev. Phys. Chem. 2001, 52, 279–313. [Google Scholar] [CrossRef] [PubMed]
- Cardin, J.T.; Kolaczkowski, S.V.; Anderson, J.R.; Budil, D.E. Quasioptical design for an EPR spectrometer based on a horizontal-bore superconducting solenoid. Appl. Magn. Reson. 1999, 16, 273–292. [Google Scholar] [CrossRef]
- Van Tol, J.; Brunel, L.C.; Wylde, R.J. A quasioptical transient electron spin resonance spectrometer operating at 120 and 240 GHz. Rev. Sci. Instrum. 2005, 76, 074101. [Google Scholar] [CrossRef]
- Blok, H.; Disselhorst, J.; Orlinskii, S.B.; Schmidt, J. A continuous-wave and pulsed electron spin resonance spectrometer operating at 275 GHz. J. Magn. Reson. 2004, 166, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Blok, H.; Disselhorst, J.; van der Meer, H.; Orlinskii, S.B.; Schmidt, J. ENDOR spectroscopy at 275 GHz. J. Magn. Reson. 2005, 173, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Ivancich, A.; Mattioli, T.A.; Un, S. Effect of protein microenvironment on tyrosyl radicals. A high-field (285 GHz) EPR, resonance raman, and hybrid density functional study. J. Am. Chem. Soc. 1999, 121, 5743–5753. [Google Scholar] [CrossRef]
- Un, S.; Dorlet, P.; Rutherford, A.W. A high-field EPR tour of radicals in photosystems I and II. Appl. Magn. Reson. 2001, 21, 341–361. [Google Scholar] [CrossRef]
- Annino, G.; Cassettari, M.; Fittipaldi, M.; Longo, I.; Martinelli, M.; Massa, C.A.; Pardi, L.A. High-field, multifrequency EPR spectroscopy using whispering gallery dielectric resonators. J. Magn. Reson. 2000, 143, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.K.; Pardi, L.A.; Krzystek, J.; Sienkiewicz, A.; Goy, P.; Rohrer, M.; Brunel, L.C. Ultrawide band multifrequency high-field emr technique: A methodology for increasing spectroscopic information. J. Magn. Reson. 2000, 142, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.K.; Maniero, A.L.; van Tol, H.; Saylor, C.; Brunel, L.C. High-field emr: Recent cw developments at 25 tesla, and next-millennium challenges. Appl. Magn. Reson. 1999, 16, 299–308. [Google Scholar] [CrossRef]
- Rohrer, M.; Krzystek, J.; Williams, V.; Brunel, L.C. Fabry-perot resonator for high-field multi-frequency ESR at millimetre and submillimetre wavelengths. Meas. Sci. Technol. 1999, 10, 275–284. [Google Scholar] [CrossRef]
- Moll, H.P.; Kutter, C.; van Tol, J.; Zuckerman, H.; Wyder, P. Principles and performance of an electron spin echo spectrometer using far infrared lasers as excitation sources. J. Magn. Reson. 1999, 137, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Earle, K.A.; Tipikin, D.S.; Freed, J.H. Far-infrared electron-paramagnetic-resonance spectrometer utilizing a quasioptical reflection bridge. Rev. Sci. Instrum. 1996, 67, 2502–2513. [Google Scholar] [CrossRef]
- Earle, K.A.; Freed, J.H. Quasioptical hardware for a flexible fir-EPR spectrometer. Appl. Magn. Reson. 1999, 16, 247–272. [Google Scholar] [CrossRef]
- Nojiri, H.; Motokawa, M.; Okuda, K.; Kageyama, H.; Ueda, Y.; Tanaka, H. Thz-ESR system by using single shot and repeating pulsed magnetic fields. J. Phys. Soc. Jpn. 2003, 72 (Suppl. B), 109–116. [Google Scholar] [CrossRef]
- Tatsukawa, T.; Maeda, T.; Sasai, H.; Idehara, T.; Mekata, I.; Saito, T.; Kanemaki, T. ESR spectrometer with a wide frequency-range using a gyrotron as a radiation power source. Int. J. Infrared Millim. Waves 1995, 16, 293–305. [Google Scholar] [CrossRef]
- Tatsukawa, T.; Shirai, T.; Imaizumi, T.; Idehara, T.; Ogawa, I.; Kanemaki, T. Ruby ESR over a wide frequency range in the millimeter wave region. Int. J. Infrared Millim. Waves 1998, 19, 859–874. [Google Scholar] [CrossRef]
- Mitsudo, S.; Higuchi, T.; Kanazawa, K.; Idehara, T.; Ogawa, I.; Chiba, M. High field ESR measurements using gyrotron fu series as radiation sources. J. Phys. Soc. Jpn. 2003, 72 (Suppl. B), 172–176. [Google Scholar] [CrossRef]
- Morley, G.W.; Brunel, L.C.; van Tol, J. A multifrequency high-field pulsed electron paramagnetic resonance/electron-nuclear double resonance spectrometer. Rev. Sci. Instrum. 2008, 79, 064703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujii, Y.; Ishikawa, Y.; Ohya, K.; Miura, S.; Koizumi, Y.; Fukuda, A.; Omija, T.; Mitsudo, S.; Mizusaki, T.; Matsubara, A.; et al. Development of very-low-temperature millimeter-wave electron-spin-resonance measurement system. Appl. Magn. Reson. 2018, 49, 783–801. [Google Scholar] [CrossRef]
- Cho, F.H.; Stepanov, V.; Takahashi, S. A high-frequency electron paramagnetic resonance spectrometer for multi-dimensional, multi-frequency, and multi-phase pulsed measurements. Rev. Sci. Instrum. 2014, 85, 075110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, F.H.; Stepanov, V.; Abeywardana, C.; Takahashi, S. 230/115 GHz electron paramagnetic resonance/double electron-electron resonance spectroscopy. In Methods in Enzymology; Qin, P.Z., Warncke, K., Eds.; Elsevier Academic Press Inc.: San Diego, CA, USA, 2015; Volume 563, pp. 95–118. [Google Scholar]
- Takahashi, S.; Brunel, L.C.; Edwards, D.T.; van Tol, J.; Ramian, G.; Han, S.; Sherwin, M.S. Pulsed electron paramagnetic resonance spectroscopy powered by a free-electron laser. Nature 2012, 489, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Allen, D.G.; Seifter, J.; Ramian, G.; Sherwin, M.S.; Brunel, L.-C.; van Tol, J. Pulsed EPR spectrometer with injection-locked ucsb free-electron laser. Infrared Phys. Technol. 2008, 51, 426–428. [Google Scholar] [CrossRef]
- Nafradi, B.; Gaal, R.; Feher, T.; Forro, L. Microwave frequency modulation in continuous-wave far-infrared ESR utilizing a quasi-optical reflection bridge. J. Magn. Reson. 2008, 192, 265–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neugebauer, P.; Bloos, D.; Marx, R.; Lutz, P.; Kern, M.; Aguilà, D.; Vaverka, J.; Laguta, O.; Dietrich, C.; Clérac, R.; et al. Ultra-broadband EPR spectroscopy in field and frequency domains. Phys. Chem. Chem. Phys. 2018, 20, 15528–15534. [Google Scholar] [CrossRef] [PubMed]
- Schnegg, A.; Behrends, J.; Lips, K.; Bittl, R.; Holldack, K. Frequency domain fourier transform THz-EPR on single molecule magnets using coherent synchrotron radiation. Phys. Chem. Chem. Phys. 2009, 11, 6820–6825. [Google Scholar] [CrossRef] [PubMed]
- Nehrkorn, J.; Holldack, K.; Bittl, R.; Schnegg, A. Recent progress in synchrotron-based frequency-domain fourier-transform THz-EPR. J. Magn. Reson. 2017, 280, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Ozerov, M.; Bernath, B.; Kamenskyi, D.; Redlich, B.; van der Meer, A.F.G.; Christianen, P.C.M.; Engelkamp, H.; Maan, J.C. A THz spectrometer combining the free electron laser flare with 33 T magnetic fields. Appl. Phys. Lett. 2017, 110, 094106. [Google Scholar] [CrossRef]
- Sakurai, T.; Matsui, R.; Kawasaki, K.; Okubo, S.; Ohta, H.; Matsubayashi, K.; Uwatoko, Y.; Kudo, K.; Koike, Y. Development of high-pressure and multi-frequency ESR system and its application to quantum spin system. Appl. Magn. Reson. 2015, 46, 1007–1012. [Google Scholar] [CrossRef]
- Siaw, T.A.; Leavesley, A.; Lund, A.; Kaminker, I.; Han, S. A versatile and modular quasi optics-based 200 GHz dual dynamic nuclear polarization and electron paramagnetic resonance instrument. J. Magn. Reson. 2016, 264, 131–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwalbe, H. New 1.2 GHz NMR spectrometers—New horizons? Angew. Chem. Int. Ed. 2017, 56, 10252–10253. [Google Scholar] [CrossRef] [PubMed]
- Zavoisky, E. Relaxation of liquid solutions for perpendicular fields. J. Phys. USSR 1945, 9, 211–216. [Google Scholar]
- Zavoisky, E. Paramagnetic absorption in some salts in perpendicular magnetic fields. Zh. Eksper. Teor. Fiz. 1946, 16, 603–606. [Google Scholar]
- Purcell, E.M.; Torrey, H.C.; Pound, R.V. Resonance absorption by nuclear magnetic moments in a solid. Phys. Rev. 1946, 69, 37–38. [Google Scholar] [CrossRef]
- Bloch, F.; Hansen, W.W.; Packard, M. Nuclear induction. Phys. Rev. 1946, 69, 127. [Google Scholar] [CrossRef]
- Hausser, K. Award address for professor E. K. Zavoisky. J. Magn. Reson. 1978, 29, 179–181. [Google Scholar] [CrossRef]
- Kim, S.S.; Weissman, S.I. Detection of transient electron-paramagnetic resonance. J. Magn. Reson. 1976, 24, 167–169. [Google Scholar] [CrossRef]
- McLauchlan, K.A.; Yeung, M.T. Time-resolved ESR studies of free radicals. In Electron Spin Resonance; Royal Society of Chemistry: Cambridge, UK, 1994; Volume 14, pp. 32–62. [Google Scholar]
- Stehlik, D.; Möbius, K. New EPR methods for investigating photoprocesses with paramagnetic intermediates. Annu. Rev. Phys. Chem. 1997, 48, 745–784. [Google Scholar] [CrossRef] [PubMed]
- Hoff, A.J. Advanced EPR: Applications in Biology and Biochemistry; Elsevier: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Savitsky, A.; Kühn, M.; Duche, D.; Möbius, K.; Steinhoff, H.J. Spontaneous refolding of the pore-forming colicin a toxin upon membrane association as studied by X-band and W-band high-field electron paramagnetic resonance spectroscopy. J. Phys. Chem. B 2004, 108, 9541–9548. [Google Scholar] [CrossRef]
- Savitsky, A.; Möbius, K. Photochemical reactions and photoinduced electron-transfer processes in liquids, frozen solutions, and proteins as studied by multifrequency time-resolved EPR spectroscopy. Helv. Chim. Acta 2006, 89, 2544–2589. [Google Scholar] [CrossRef]
- Lubitz, W.; Lendzian, F.; Bittl, R. Radicals, radical pairs and triplet states in photosynthesis. Acc. Chem. Res. 2002, 35, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Savitsky, A.N.; Galander, M.; Möbius, K. W-band time-resolved electron paramagnetic resonance spectroscopy on transient organic radicals in solution. Chem. Phys. Lett. 2001, 340, 458–466. [Google Scholar] [CrossRef]
- Hahn, E.L. Spin echos. Phys. Rev. 1950, 80, 580–594. [Google Scholar] [CrossRef]
- Ernst, R.R. Sensitivity enhancement in magnetic resonance. I. Analysis of method of time averaging. Rev. Sci. Instrum. 1965, 36, 1689. [Google Scholar] [CrossRef]
- Ernst, R.R.; Anderson, W.A. Application of fourier transform spectroscopy to magnetic resonance. Rev. Sci. Instrum. 1966, 37, 93–102. [Google Scholar] [CrossRef]
- Blume, R.J. Electron spin relaxation times in sodium-ammonia solutions. Phys. Rev. 1958, 109, 1867–1873. [Google Scholar] [CrossRef]
- Keijzers, C.P.; Reijerse, E.J.; Schmidt, J. Pulsed EPR: A New Field of Applications; North Holland: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Salikhov, K.M.; Semenov, A.G.; Tsvetkov, Y.D. Electron Spin Echo and Its Applications; Nauka: Moscow, Russia, 1976. [Google Scholar]
- Milov, A.D.; Salikhov, K.M.; Tsvetkov, Y.D. Effect of spin dipole-dipole interaction on phase relaxation in magnetically dilute solid bodies. Zh. Eksper. Teor. Fiz. 1972, 63, 2329–2335. [Google Scholar]
- Milov, A.D.; Salikhov, K.M.; Shirov, M.D. Application of eldor in electron-spin echo for paramagnetic center space distribution in solids. Fiz. Tverd. Tela 1981, 23, 975–982. [Google Scholar]
- Emshwiller, M.; Hahn, E.L.; Kaplan, D. Pulsed nuclear resonance spectroscopy. Phys. Rev. 1960, 118, 414–424. [Google Scholar] [CrossRef]
- Salikhov, K.M.; Bock, C.H.; Stehlik, D. Time development of electron spin polarization in magnetically coupled, spin correlated radical pairs. Appl. Magn. Reson. 1990, 1, 195–211. [Google Scholar] [CrossRef]
- Salikhov, K.M.; Kandrashkin, Y.; Salikhov, A.K. Peculiarities of free induction and primary spin echo signals for spin-correlated radical pairs. Appl. Magn. Reson. 1992, 3, 199–216. [Google Scholar] [CrossRef]
- Goldfarb, D.; Stoll, S. EPR Spectroscopy: Fundamentals and Methods; Wilez: New York, NY, USA, 2018; p. 648. [Google Scholar]
- Goldfarb, D.; Krymov, V. W-band pulsed ENDOR of transition metal centers, biological magnetic resonance. In Very High Frequency (VHF) ESR/EPR; Grinberg, O., Berliner, L.J., Eds.; Kluwer/Plenum: New York, NY, USA, 2004; Volume 22, pp. 305–351. [Google Scholar]
- Maniero, A.L. High frequency ENDOR spectroscopy. In Very High Frequency (VHF) ESR/EPR, Biological Magnetic Resonance; Grinberg, O., Berliner, L.J., Eds.; Kluwer/Plenum: New York, NY, USA, 2004; Volume 22, pp. 478–494. [Google Scholar]
- Feher, G. Method of polarizing nuclei in paramagnetic substances. Phys. Rev. 1956, 103, 500–501. [Google Scholar] [CrossRef]
- Feher, G. Observation of nuclear magnetic resonances via the electron spin resonance line. Phys. Rev. 1956, 103, 834–835. [Google Scholar] [CrossRef]
- Freed, J.H. Theory of multiple resonance and ESR saturation in liquids and related media. In Mulitiple Electron Resonance Spectroscopy; Dorio, M.M., Freed, J.H., Eds.; Plenum: New York, NY, USA, 1979; pp. 73–142. [Google Scholar]
- Möbius, K.; Plato, M.; Lubitz, W. Radicals in solution studied by ENDOR and triple resonance spectroscopy. Phys. Rep. 1982, 87, 171–208. [Google Scholar] [CrossRef]
- Plato, M.; Lubitz, W.; Möbius, K. A solution ENDOR sensitivity study of various nuclei in organic radicals. J. Phys. Chem. 1981, 85, 1202–1219. [Google Scholar] [CrossRef]
- Kurreck, H.; Kirste, B.; Lubitz, W. Electron Nuclear Double Resonance Spectroscopy of Radicals in Solution—Applications to Organic and Biological Chemistry; VCH Publishers, Inc.: Deerfield Beach, FL, USA, 1988; pp. 1–374. [Google Scholar]
- Kevan, L.; Kispert, L.D. Electron Spin Double Resonance Spectroscopy; John Wiley: New York, NY, USA, 1976. [Google Scholar]
- Cederquist, A. Electron-Nuclear Double Resonance in Homogeneously Broadened Systems: Metal Ammonia Solutions. Ph.D. Thesis, Washington University, St. Louis, MO, USA, 1963. [Google Scholar]
- Hyde, J.S.; Maki, A.H. ENDOR of free radical in solution. J. Chem. Phys. 1964, 40, 3117–3118. [Google Scholar] [CrossRef]
- Möbius, K.; Biehl, R. Electron-nuclear-nuclear triple resonance of radicals in solution. In Multiple Electron Resonance Spectroscopy; Dorio, M.M., Freed, J.H., Eds.; Plenum Press: New York, NY, USA, 1979; pp. 475–508. [Google Scholar]
- Feher, G. Electron nuclear double resonance (ENDOR) experiments. Physica 1958, 24, S80–S87. [Google Scholar] [CrossRef]
- Freed, J.H. Theory of saturation and double resonance effects in ESR spectra. 4. Electron-nuclear triple resonance. J. Chem. Phys. 1969, 50, 2271–2272. [Google Scholar] [CrossRef]
- Dinse, K.P.; Biehl, R.; Möbius, K. Electron nuclear triple resonance of free-radicals in solution. J. Chem. Phys. 1974, 61, 4335–4341. [Google Scholar] [CrossRef]
- Gerson, F.; Huber, W. Electron Spin Resonance Spectroscopy of Organic Radicals; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Biehl, R.; Plato, M.; Möbius, K. General triple resonance on free-radicals in solution—Determination of relative signs of isotropic hyperfine coupling-constants. J. Chem. Phys. 1975, 63, 3515–3522. [Google Scholar] [CrossRef]
- Cook, R.J.; Whiffen, D.H. Relative signs of hyperfine coupling constants by double ENDOR experiment. Proc. Phys. Soc. Lond. 1964, 84, 845–848. [Google Scholar] [CrossRef]
- Hoff, A.J.; Möbius, K. Nitrogen electron nuclear double resonance and proton triple resonance experiments on the bacteriochlorophyll cation in solution. Proc. Natl. Acad. Sci. USA 1978, 75, 2296–2300. [Google Scholar] [CrossRef] [PubMed]
- Lendzian, F.; Bönigk, B.; Plato, M.; Möbius, K.; Lubitz, W. 15N ENDOR experiments on the primary donor cation radical d+ in bacterial reaction center single crystals of RB. Shaeroides R-26. In The Photosynthetic Bacterial Reaction Center II; Breton, J., Vermeglio, A., Eds.; Plenum: New York, NY, USA, 1992; Volume 237, pp. 89–97. [Google Scholar]
- Lubitz, W.; Lendzian, F. ENDOR spectroscopy. In Biophysical Techniques in Photosynthesis; Amesz, J., Hoff, A.J., Eds.; Kluwer: Dordrecht, The Netherlands, 1996; pp. 255–275. [Google Scholar]
- Levanon, H.; Möbius, K. Advanced EPR spectroscopy on electron transfer processes in photosynthesis and biomimetic model systems. Annu. Rev. Biophys. Biomol. Struct. 1997, 26, 495–540. [Google Scholar] [CrossRef] [PubMed]
- Allendoerfer, R.D.; Maki, A.H. A phenomenological description of ENDOR in solution; example: The tri-t-butyl phenoxyl radical. J. Magn. Reson. 1970, 3, 396–410. [Google Scholar] [CrossRef]
- Leniart, D.S.; Connor, H.D.; Freed, J.H. Esr and ENDOR study of spin relaxation of semiquinones in liquid solution. J. Chem. Phys. 1975, 63, 165–199. [Google Scholar] [CrossRef]
- Freed, J.H. Theory of saturation and double-resonance effects in ESR spectra. J. Chem. Phys. 1965, 43, 2312–2332. [Google Scholar] [CrossRef]
- Freed, J.H. Theory of saturation and double resonance effects in electron spin resonance spectra. 2. Exchange vs. dipolar mechanisms. J. Phys. Chem. 1967, 71, 38–51. [Google Scholar] [CrossRef]
- Freed, J.H.; Leniart, D.S.; Hyde, J.S. Theory of saturation and double resonance effects in ESR spectra. 3. Rf coherence and line shapes. J. Chem. Phys. 1967, 47, 2762–2773. [Google Scholar] [CrossRef]
- Freed, J.H.; Leniart, D.S.; Connor, H.D. Theory of saturation and double-resonance in ESR-spectra. 5. Average ENDOR and eldor lines. J. Chem. Phys. 1973, 58, 3089–3105. [Google Scholar] [CrossRef]
- Möbius, K.; Lubitz, W.; Plato, M. Liquid-state ENDOR and triple resonance. In Advanced EPR, Applications in Biology and Biochemistry; Hoff, A.J., Ed.; Elsevier: Amsterdam, The Netherlands, 1989; pp. 441–499. [Google Scholar]
- Biehl, R.; Lubitz, W.; Möbius, K.; Plato, M. Observation of deuterium quadrupole splittings of aromatic free-radicals in liquid-crystals by ENDOR and triple resonance. J. Chem. Phys. 1977, 66, 2074–2078. [Google Scholar] [CrossRef]
- Lubitz, W.; Dinse, K.P.; Möbius, K.; Biehl, R. Fluorine and proton ENDOR of aromatic radicals in solution. Chem. Phys. 1975, 8, 371–383. [Google Scholar] [CrossRef]
- Lubitz, W.; Plato, M.; Möbius, K.; Biehl, R. Alkali and h ENDOR on aromatic ion-pairs in solution—Indo approach. J. Phys. Chem. 1979, 83, 3402–3413. [Google Scholar] [CrossRef]
- Lubitz, W. Aufklärung der Elektronenstruktur Aromatischer Radikale Durch Ekektron-Kern-Mehrfachresonanz-Experimente an Protonen und Heterokernen. Ph.D. Thesis, Free University Berlin, Berlin, Germany, 1977. [Google Scholar]
- Lubitz, W. EPR in photosynthesis. In Electron Paramagnetic Resonance; Gilbert, B.C., Davies, M.J., Murphy, D.M., Eds.; The Royal Society of Chemistry: Cambidge, UK, 2004; Volume 19, pp. 174–242. [Google Scholar]
- Möbius, K. ENDOR and ELDOR. In Electron Spin Resonance; Ayscough, P.B., Ed.; The Royal Society of Chemistry: Cambridge, UK, 1977; Volume 4, pp. 16–29. [Google Scholar]
- Möbius, K. EPR studies of photosynthesis. In Electron Spin Resonance; Atherton, N.M., Davies, M.J., Gilbert, B.C., Eds.; The Royal Society of Chemistry: Cambridge, UK, 1994; Volume 14, pp. 203–245. [Google Scholar]
- Weber, S. Recent EPR studies on the bacterial photosynthetic reaction centre. In Electron Paramagnetic Resonance; Gilbert, B.C., Davies, M.J., McLauchlan, K.A., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2000; Volume 17, pp. 43–77. [Google Scholar]
- Murphy, D.M.; Farley, R.D. Principles and applications of ENDOR spectroscopy for structure determination in solution and disordered matrices. Chem. Soc. Rev. 2006, 35, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Bixon, M.; Fajer, J.; Feher, G.; Freed, J.H.; Gamliel, D.; Hoff, A.J.; Levanon, H.; Möbius, K.; Nechushtai, R.; Norris, J.R.; et al. Primary events in photosynthesis—Problems, speculations, controversies, and future-trends. Isr. J. Chem. 1992, 32, 369–518. [Google Scholar]
- Lendzian, F.; Lubitz, W.; Scheer, H.; Bubenzer, C.; Möbius, K. Invivo liquid solution ENDOR and triple resonance of bacterial photosynthetic reaction centers of Rhodopseudomonas sphaeroides R-26. J. Am. Chem. Soc. 1981, 103, 4635–4637. [Google Scholar] [CrossRef] [Green Version]
- Lubitz, W.; Lendzian, F.; Scheer, H.; Gottstein, J.; Plato, M.; Möbius, K. Structural studies of the primary donor cation radical P870+· in reaction centers of Rhodospirillum rubrum by electron-nuclear double resonance in solution. Proc. Natl. Acad. Sci. USA 1984, 81, 1401–1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lendzian, F.; Huber, M.; Isaacson, R.A.; Endeward, B.; Plato, M.; Bönigk, B.; Möbius, K.; Lubitz, W.; Feher, G. The electronic-structure of the primary donor cation-radical in Rhodobacter sphaeroides R-26—ENDOR and triple-resonance studies in single-crystals of reaction centers. Biochim. Biophys. Acta 1993, 1183, 139–160. [Google Scholar] [CrossRef]
- Hoff, A.J.; Deisenhofer, J. Photophysics of photosynthesis. Structure and spectroscopy of reaction centers of purple bacteria. Phys. Rep. 1997, 287, 2–247. [Google Scholar] [CrossRef]
- Rautter, J.; Lendzian, F.; Lin, X.; Williams, J.C.; Allen, J.P.; Lubitz, W. Effect of orbital asymmetry in p+ on electron transfer in reaction centers of RB. Sphaeroides. In The Reaction Center of Photosynthetic Bacteria, Structure and Dynamics; Michel-Beyerle, M.E., Ed.; Springer: Berlin, Germany, 1996; pp. 37–50. [Google Scholar]
- Rautter, J.; Lendzian, F.; Schulz, C.; Fetsch, A.; Kuhn, M.; Lin, X.; Williams, J.C.; Allen, J.P.; Lubitz, W. ENDOR studies of the primary donor cation radical in mutant reaction centers of rhodobacter sphaeroides with altered hydrogen-bond interactions. Biochemistry 1995, 34, 8130–8143. [Google Scholar] [CrossRef] [PubMed]
- Rautter, J.; Lendzian, F.; Lubitz, W.; Wang, S.; Allen, J.P. Comparative study of reaction centers from photosynthetic purple bacteria: Electron paramagnetic resonance and electron nuclear double resonance spectroscopy. Biochemistry 1994, 33, 12077–12084. [Google Scholar] [CrossRef] [PubMed]
- Rautter, J.; Gessner, C.; Lendzian, F.; Lubitz, W.; Williams, J.C.; Murchison, H.A.; Wang, S.; Woodbury, N.W.; Allen, J.P. EPR and ENDOR studies of the primary donor cation radical in native and genetically modified bacterial reaction centers. In Photosynthetic Bacterial Reaction Center II; Breton, J., Vermeglio, A., Eds.; Plenum: New York, NY, USA, 1992; Volume 237, pp. 99–108. [Google Scholar]
- Müh, F.; Lendzian, F.; Roy, M.; Williams, J.C.; Allen, J.P.; Lubitz, W. Pigment-protein interactions in bacterial reaction centers and their influence on oxidation potential and spin density distribution of the primary donor. J. Phys. Chem. B 2002, 106, 3226–3236. [Google Scholar] [CrossRef]
- Johnson, E.T.; Müh, F.; Nabedryk, E.; Williams, J.C.; Allen, J.P.; Lubitz, W.; Breton, J.; Parson, W.W. Electronic and vibronic coupling of the special pair of bacteriochlorophylls in photosynthetic reaction centers from wild-type and mutant strains of Rhodobacter sphaeroides. J. Phys. Chem. B 2002, 106, 11859–11869. [Google Scholar] [CrossRef]
- Artz, K.; Williams, J.C.; Allen, J.P.; Lendzian, F.; Rautter, J.; Lubitz, W. Relationship between the oxidation potential and electron spin density of the primary electron donor in reaction centers from Rhodobacter sphaeroides. Proc. Natl. Acad. Sci. USA 1997, 94, 13582–13587. [Google Scholar] [CrossRef] [PubMed]
- Plato, M.; Möbius, K.; Michel-Beyerle, M.E.; Bixon, M.; Jortner, J. Intermolecular electronic interactions in the primary charge separation in bacterial photosynthesis. J. Am. Chem. Soc. 1988, 110, 7279–7285. [Google Scholar] [CrossRef]
- Feher, G. The bruker lecture. Identification and characterization of the primary donor in bacterial photosynthesis: A chronological account of an EPR/ENDOR investigation. J. Chem. Soc. Perkin Trans. 2 1992, 1861–1874. [Google Scholar] [CrossRef]
- Käß, H.; Fromme, P.; Witt, H.T.; Lubitz, W. Orientation and electronic structure of the primary donor radical cation P700 in photosystem I: A single crystals EPR and ENDOR study. J. Phys. Chem. B 2001, 105, 1225–1239. [Google Scholar] [CrossRef]
- Lubitz, W. Pulse EPR and ENDOR studies of light-induced radicals and triplet states in photosystem ii of oxygenic photosynthesis. Phys. Chem. Chem. Phys. 2002, 4, 5539–5545. [Google Scholar] [CrossRef]
- Rigby, S.E.J.; Nugent, J.H.A.; O’Malley, P.J. ENDOR and special triple resonance studies of chlorophyll cation radicals in photosystem II. Biochemistry 1994, 33, 10043–10050. [Google Scholar] [CrossRef] [PubMed]
- Lubitz, W. EPR studies of the primary electron donor P700 in photosystem I. In Advances in Photosynthesis and Respiration: The Light-Driven Plastocyanin: Ferredoxin Oxidoreductase; Golbeck, J.H., Ed.; Springer: Dordrecht, The Netherlands, 2006; Volume 24, pp. 245–269. [Google Scholar]
- Daviso, E.; Prakash, S.; Alia, A.; Gast, P.; Neugebauer, J.; Jeschke, G.; Matysik, J. The electronic structure of the primary electron donor of reaction centers of purple bacteria at atomic resolution as observed by photo-cidnp C-13 NMR. Proc. Natl. Acad. Sci. USA 2009, 106, 22281–22286. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, H.; Takano, Y.; Nakamura, H. Theoretical investigation of the electronic asymmetry of the special pair cation radical in the photosynthetic type-II reaction center. J. Phys. Chem. B 2008, 112, 13923–13933. [Google Scholar] [CrossRef] [PubMed]
- Sai Sankar Gupta, K.B.; Alia, A.; de Groot, H.J.M.; Matysik, J. Symmetry break of special pair: Photochemically induced dynamic nuclear polarization NMR confirms control by nonaromatic substituents. J. Am. Chem. Soc. 2013, 135, 10382–10387. [Google Scholar] [CrossRef] [PubMed]
- Matysik, J.; Leipzig University, Leipzig, Germany. Personal Communication, 2013.
- Kurreck, H.; Huber, M. Model reactions for photosynthesis-photoinduced charge and energy-transfer between covalently-linked porphyrin and quinone units. Angew. Chem. Int. Ed. 1995, 34, 849–866. [Google Scholar] [CrossRef]
- Gust, D.; Moore, T.A. Intramolecular photoinduced electron transfer reactions of porphyrins. In The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: New York, NY, USA, 2000; Volume 8, pp. 153–190. [Google Scholar]
- Lee, W.; Kasanmascheff, M.; Huynh, M.; Quartararo, A.; Costentin, C.; Bejenke, I.; Nocera, D.G.; Bennati, M.; Tommos, C.; Stubbe, J. Properties of site-specifically incorporated 3-aminotyrosine in proteins to study redox-active tyrosines: Escherichia coli ribonucleotide reductase as a paradigm. Biochemistry 2018, 57, 3402–3415. [Google Scholar] [CrossRef] [PubMed]
- Nick, T.U.; Ravichandran, K.R.; Stubbe, J.; Kasanmascheff, M.; Bennati, M. Spectroscopic evidence for a H bond network at Y356 located at the subunit interface of active E. coli ribonucleotide reductase. Biochemistry 2017, 56, 3647–3656. [Google Scholar] [CrossRef] [PubMed]
- Halbmair, K.; Seikowski, J.; Tkach, I.; Hobartner, C.; Sezer, D.; Bennati, M. High-resolution measurement of long-range distances in RNA: Pulse EPR spectroscopy with tempo-labeled nucleotides. Chem. Sci. 2016, 7, 3172–3180. [Google Scholar] [CrossRef] [PubMed]
- Goetzl, S.; Teutloff, C.; Werther, T.; Hennig, S.E.; Jeoung, J.H.; Bittl, R.; Dobbek, H. Protein dynamics in the reductive activation of a B12-containing enzyme. Biochemistry 2017, 56, 5496–5502. [Google Scholar] [CrossRef] [PubMed]
- Saeidpour, S.; Lohan, S.B.; Anske, M.; Unbehauen, M.; Fleige, E.; Haag, R.; Meinke, M.C.; Bittl, R.; Teutloff, C. Localization of dexamethasone within dendritic core-multishell (CMS) nanoparticles and skin penetration properties studied by multi-frequency electron paramagnetic resonance (EPR) spectroscopy. Eur. J. Pharm. Biopharm. 2017, 116, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Löwenstein, J.; Lauterbach, L.; Teutloff, C.; Lenz, O.; Bittl, R. Active site of the NAD(+)-reducing hydrogenase from Ralstonia eutropha studied by EPR spectroscopy. J. Phys. Chem. B 2015, 119, 13834–13841. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.Z.; Stich, T.A.; Liou, S.H.; Soldatova, A.V.; Delgadillo, D.A.; Romano, C.A.; Spiro, T.G.; Goodin, D.B.; Tebo, B.M.; Casey, W.H.; et al. Copper binding sites in the manganese-oxidizing MNX protein complex investigated by electron paramagnetic resonance spectroscopy. J. Am. Chem. Soc. 2017, 139, 8868–8877. [Google Scholar] [CrossRef] [PubMed]
- Stich, T.A.; McAlpin, J.G.; Wall, R.M.; Rigsby, M.L.; Britt, R.D. Electron paramagnetic resonance characterization of dioxygen-bridged cobalt dimers with relevance to water oxidation. Inorg. Chem. 2016, 55, 12728–12736. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.; Britt, R.D. Electronic structure of two catalytic states of the [FeFe] hydrogenase H-cluster as probed by pulse electron paramagnetic resonance spectroscopy. Inorg. Chem. 2018, 57, 10935–10944. [Google Scholar] [CrossRef] [PubMed]
- Selmke, B.; Borbat, P.P.; Nickolaus, C.; Varadarajan, R.; Freed, J.H.; Trommer, W.E. Open and closed form of maltose binding protein in its native and molten globule state as studied by EPR spectroscopy. Biochemistry 2018, 57, 5507–5512. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.; Freed, J.H. Singular value decomposition method to determine distance distributions in pulsed dipolar electron spin resonance. J. Phys. Chem. Lett. 2017, 8, 5648–5655. [Google Scholar] [CrossRef] [PubMed]
- Franck, J.M.; Chandrasekaran, S.; Dzikovski, B.; Dunnam, C.R.; Freed, J.H. Focus: Two-dimensional electron-electron double resonance and molecular motions: The challenge of higher frequencies. J. Chem. Phys. 2015, 142, 212302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prokopiou, G.; Lee, M.D.; Collauto, A.; Abdelkader, E.H.; Bahrenberg, T.; Feintuch, A.; Ramirez-Cohen, M.; Clayton, J.; Swarbrick, J.D.; Graham, B.; et al. Small Gd(III) tags for Gd(III)-Gd(III) distance measurements in proteins by EPR spectroscopy. Inorg. Chem. 2018, 57, 5048–5059. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Feintuch, A.; Collauto, A.; Adams, L.A.; Aurelio, L.; Graham, B.; Otting, G.; Goldfarb, D. Selective distance measurements using triple spin labeling with Gd3+, Mn2+, and a nitroxide. J. Phys. Chem. Lett. 2017, 8, 5277–5282. [Google Scholar] [CrossRef] [PubMed]
- Manukovsky, N.; Feintuch, A.; Kuprov, I.; Goldfarb, D. Time domain simulation of Gd3+-Gd3+ distance measurements by EPR. J. Chem. Phys. 2017, 147, 044201. [Google Scholar] [CrossRef] [PubMed]
- Horitani, M.; Offenbacher, A.R.; Carr, C.A.M.; Yu, T.; Hoeke, V.; Cutsail, G.E.; Hammes-Schiffer, S.; Klinman, J.P.; Hoffman, B.M. C-13 ENDOR spectroscopy of lipoxygenase-substrate complexes reveals the structural basis for C-H activation by tunneling. J. Am. Chem. Soc. 2017, 139, 1984–1997. [Google Scholar] [CrossRef] [PubMed]
- Davydov, R.; Khadka, N.; Yang, Z.Y.; Fielding, A.J.; Lukoyanov, D.; Dean, D.R.; Seefeldt, L.C.; Hoffman, B.M. Exploring electron/proton transfer and conformational changes in the nitrogenase mofe protein and femo-cofactor through cryoreduction/EPR measurements. Isr. J. Chem. 2016, 56, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Cutsail, G.E.; Telser, J.; Hoffman, B.M. Advanced paramagnetic resonance spectroscopies of iron-sulfur proteins: Electron nuclear double resonance (ENDOR) and electron spin echo envelope modulation (ESEEM). Biochim. Biophys. Acta 2015, 1853, 1370–1394. [Google Scholar] [CrossRef] [PubMed]
- Kuzhelev, A.A.; Krumkacheva, O.A.; Shevelev, G.Y.; Yulikov, M.; Fedin, M.V.; Bagryanskaya, E.G. Room-temperature distance measurements using RIDME and the orthogonal spin labels trityl/nitroxide. Phys. Chem. Chem. Phys. 2018, 20, 10224–10230. [Google Scholar] [CrossRef] [PubMed]
- Wili, N.; Jeschke, G. Chirp echo fourier transform EPR-detected NMR. J. Magn. Reson. 2018, 289, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Gmeiner, C.; Dorn, G.; Allain, F.H.T.; Jeschke, G.; Yulikov, M. Spin labelling for integrative structure modelling: A case study of the polypyrimidine-tract binding protein 1 domains in complexes with short rnas. Phys. Chem. Chem. Phys. 2017, 19, 28360–28380. [Google Scholar] [CrossRef] [PubMed]
- Salvadori, E.; Luke, N.; Shaikh, J.; Leventis, A.; Bronstein, H.; Kay, C.W.M.; Clarke, T.M. Ultra-fast spin-mixing in a diketopyrrolopyrrole monomer/fullerene blend charge transfer state. J. Mater. Chem. A 2017, 5, 24335–24343. [Google Scholar] [CrossRef]
- Salvadori, E.; Fung, M.W.; Hoffmann, M.; Anderson, H.L.; Kay, C.W.M. Exploiting the symmetry of the resonator mode to enhance PELDOR sensitivity. Appl. Magn. Reson. 2015, 46, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Ema, F.; Tanabe, M.; Saito, S.; Yoneda, T.; Sugisaki, K.; Tachikawa, T.; Akimoto, S.; Yamauchi, S.; Sato, K.; Osuka, A.; et al. Charge-transfer character drives möbius antiaromaticity in the excited triplet state of twisted [28] hexaphyrin. J. Phys. Chem. Lett. 2018, 9, 2685–2690. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Nagashima, H.; Minobe, R.; Tachikawa, T.; Mino, H.; Kobori, Y. Regulated electron tunneling of photoinduced primary charge-separated state in the photosystem II reaction center. J. Phys. Chem. Lett. 2017, 8, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Tao, R.; Shibata, S.; Umeyama, T.; Tachikawa, T.; Imahori, H.; Kobori, Y. Geometries, electronic couplings, and hole dissociation dynamics of photoinduced electron–hole pairs in polyhexylthiophene–fullerene dyads rigidly linked by oligophenylenes. J. Am. Chem. Soc. 2016, 138, 5879–5885. [Google Scholar] [CrossRef] [PubMed]
- Agostini, A.; Niklas, J.; Schulte, T.; Di Valentin, M.; Bortolus, M.; Hofmann, E.; Lubitz, W.; Carbonera, D. Changing the site energy of per-614 in the peridinin-chlorophyll a-protein does not alter its capability of chlorophyll triplet quenching. Biochim. Biophys. Acta 2018, 1859, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Lohmiller, T.; Vibhute Mahesh, A.; Lubitz, W.; Savitsky, A. Multifrequency multiresonance EPR investigation of halogen-bonded complexes involving neutral nitroxide radicals. Z. Phys. Chem. 2017, 231, 867. [Google Scholar] [CrossRef]
- Krewald, V.; Retegan, M.; Neese, F.; Lubitz, W.; Pantazis, D.A.; Cox, N. Spin state as a marker for the structural evolution of nature’s water-splitting catalyst. Inorg. Chem. 2016, 55, 488–501. [Google Scholar] [CrossRef] [PubMed]
- Vallotto, C.; Williams, H.E.; Murphy, D.M.; Ayres, Z.J.; Edge, R.; Newton, M.E.; Wedge, C.J. An electron paramagnetic resonance (EPR) spectroscopy study on the γ-irradiation sterilization of the pharmaceutical excipient l-histidine: Regeneration of the radicals in solution. Int. J. Pharm. 2017, 533, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Ritterskamp, N.; Sharples, K.; Richards, E.; Folli, A.; Chiesa, M.; Platts, J.A.; Murphy, D.M. Understanding the coordination modes of [Cu(Acac)2(Imidazole)n=1,2] adducts by EPR, ENDOR, HYSCORE, and DFT analysis. Inorg. Chem. 2017, 56, 11862–11875. [Google Scholar] [CrossRef] [PubMed]
- Pelties, S.; Carter, E.; Folli, A.; Mahon, M.F.; Murphy, D.M.; Whittlesey, M.K.; Wolf, R. Influence of ring-expanded n-heterocyclic carbenes on the structures of half-sandwich Ni(I) complexes: An X-ray, electron paramagnetic resonance (EPR), and electron nuclear double resonance (ENDOR) study. Inorg. Chem. 2016, 55, 11006–11017. [Google Scholar] [CrossRef] [PubMed]
- Gränz, M.; Erlenbach, N.; Spindler, P.; Gophane, D.B.; Stelzl, L.S.; Sigurdsson, S.T.; Prisner, T.F. Dynamics of nucleic acids at room temperature revealed by pulsed EPR spectroscopy. Angew. Chem. Int. Ed. 2018, 57, 10540–10543. [Google Scholar] [CrossRef] [PubMed]
- Grytz, C.M.; Kazemi, S.; Marko, A.; Cekan, P.; Güntert, P.; Sigurdsson, S.T.; Prisner, T.F. Determination of helix orientations in a flexible DNA by multi-frequency EPR spectroscopy. Phys. Chem. Chem. Phys. 2017, 19, 29801–29811. [Google Scholar] [CrossRef] [PubMed]
- Dastvan, R.; Brouwer, E.-M.; Schuetz, D.; Mirus, O.; Schleiff, E.; Prisner, T.F. Relative orientation of potra domains from cyanobacterial OMP85 studied by pulsed EPR spectroscopy. Biophys. J. 2016, 110, 2195–2206. [Google Scholar] [CrossRef] [PubMed]
- Richert, S.; Tait, C.E.; Timmel, C.R. Delocalisation of photoexcited triplet states probed by transient EPR and hyperfine spectroscopy. J. Magn. Reson. 2017, 280, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Bowen, A.M.; Jones, M.W.; Lovett, J.E.; Gaule, T.G.; McPherson, M.J.; Dilworth, J.R.; Timmel, C.R.; Harmer, J.R. Exploiting orientation-selective deer: Determining molecular structure in systems containing Cu(II) centres. Phys. Chem. Chem. Phys. 2016, 18, 5981–5994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tait, C.E.; Neuhaus, P.; Anderson, H.L.; Timmel, C.R. Triplet state delocalization in a conjugated porphyrin dimer probed by transient electron paramagnetic resonance techniques. J. Am. Chem. Soc. 2015, 137, 6670–6679. [Google Scholar] [CrossRef] [PubMed]
- Van Doorslaer, S.; Cuypers, B. Electron paramagnetic resonance of globin proteins—A successful match between spectroscopic development and protein research. Mol. Phys. 2018, 116, 287–309. [Google Scholar] [CrossRef]
- Van Doorslaer, S. Hyperfine spectroscopy: ESEEM. eMagRes 2017, 51–69. [Google Scholar] [CrossRef]
- Motion, C.L.; Lovett, J.E.; Bell, S.; Cassidy, S.L.; Cruickshank, P.A.S.; Bolton, D.R.; Hunter, R.I.; El Mkami, H.; Van Doorslaer, S.; Smith, G.M. Deer sensitivity between iron centers and nitroxides in heme-containing proteins improves dramatically using broadband, high-field EPR. J. Phys. Chem. Lett. 2016, 7, 1411–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, S. Transient EPR. eMagRes 2017, 6, 255–269. [Google Scholar]
- Nohr, D.; Paulus, B.; Rodriguez, R.; Okafuji, A.; Bittl, R.; Schleicher, E.; Weber, S. Determination of radical–radical distances in light-active proteins and their implication for biological magnetoreception. Angew. Chem. Int. Ed. 2017, 56, 8550–8554. [Google Scholar] [CrossRef] [PubMed]
- Biskup, T.; Sommer, M.; Rein, S.; Meyer, D.L.; Kohlstädt, M.; Würfel, U.; Weber, S. Ordering of pcdtbt revealed by time-resolved electron paramagnetic resonance spectroscopy of its triplet excitons. Angew. Chem. Int. Ed. 2015, 54, 7707–7710. [Google Scholar] [CrossRef] [PubMed]
- Mims, W.B. Pulsed ENDOR experiments. Proc. R. Soc. Lond. Ser. A 1965, 283, 452–457. [Google Scholar] [CrossRef]
- Davies, E.R. New pulse ENDOR technique. Phys. Lett. A 1974, 47, 1–2. [Google Scholar] [CrossRef]
- Doan, P.E.; Lees, N.S.; Shanmugam, M.; Hoffman, B.M. Simulating suppression effects in pulsed ENDOR, and the ‘hole in the middle’ of mims and davies ENDOR spectra. Appl. Magn. Reson. 2010, 37, 763–779. [Google Scholar] [CrossRef] [PubMed]
- Van Gastel, M.; Lubitz, W. EPR investigation of [NiFe] hydrogenases. In High Resolution EPR; Berliner, L., Hanson, G., Eds.; Springer: New York, NY, USA, 2009; Volume 28, pp. 441–470. [Google Scholar]
- Mims, W.B. Envelope modulation in spin-echo experiments. Phys. Rev. B 1972, 5, 2409–2418. [Google Scholar] [CrossRef]
- Flanagan, H.L.; Singel, D.J. Analysis of n-14 eseem patterns of randomly oriented solids. J. Chem. Phys. 1987, 87, 5606–5616. [Google Scholar] [CrossRef]
- Flanagan, H.L.; Gerfen, G.J.; Lai, A.; Singel, D.J. Orientation-selective N-14 electron-spin echo envelope modulation (ESEEM)—The determination of N-14 quadrupole coupling tensor principal axis orientations in orientationally disordered solids. J. Chem. Phys. 1988, 88, 2162–2168. [Google Scholar] [CrossRef]
- Käß, H.; Bitters-Mannweidlich, E.; Andreasson, L.E.; Bönigk, B.; Lubitz, W. ENDOR and eseem of the 15N labeled labeled radical cations of chlorophyll a and the primary donor P700 in photosystem I. Chem. Phys. 1995, 194, 419–432. [Google Scholar]
- Käß, H.; Fromme, P.; Lubitz, W. Quadrupole parameters of nitrogen nuclei in the cation radical P700·+ determined by eseem of single crystals of photosystem I. Chem. Phys. Lett. 1996, 257, 197–206. [Google Scholar]
- Käß, H.; Lubitz, W. Evaluation of 2D-ESEEM data of N-15-labeled radical cations of the primary donor P-700 in photosystem I and chlorophyll a. Chem. Phys. Lett. 1996, 251, 193–203. [Google Scholar]
- Bloess, A.; Möbius, K.; Prisner, T.F. High-frequency/high-field electron spin echo envelope modulation study of nitrogen hyperfine and quadrupole interactions on a disordered powder sample. J. Magn. Reson. 1998, 134, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Savitsky, A.; Dubinskii, A.A.; Plato, M.; Grishin, Y.A.; Zimmermann, H.; Möbius, K. High-field EPR and eseem investigation of the nitrogen quadrupole interaction of nitroxide spin labels in disordered solids: Towards differentiation between polarity and proticity matrix effects on protein function. J. Phys. Chem. B 2008, 112, 9079–9090. [Google Scholar] [CrossRef] [PubMed]
- Höfer, P.; Grupp, A.; Nebenfuhr, H.; Mehring, M. Hyperfine sublevel correlation (HYSCORE) spectroscopy—A 2D electron-spin-resonance investigation of the squaric acid radical. Chem. Phys. Lett. 1986, 132, 279–282. [Google Scholar] [CrossRef]
- Silakov, A.; Reijerse, E.J.; Albracht, S.P.J.; Hatchikian, E.C.; Lubitz, W. The electronic structure of the H-cluster in the [FeFe]-hydrogenase from Desulfovibrio desulfuricans: A Q-band Fe-57-ENDOR and HYSCORE study. J. Am. Chem. Soc. 2007, 129, 11447–11458. [Google Scholar] [CrossRef] [PubMed]
- Gromov, I.; Shane, J.; Forrer, J.; Rakhmatoullin, R.; Rozentzwaig, Y.; Schweiger, A. A q-band pulse EPR/ENDOR spectrometer and the implementation of advanced one- and two-dimensional pulse EPR methodology. J. Magn. Reson. 2001, 149, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Astashkin, A.V.; Enemark, J.H.; Raitsimring, A. 26.5–40 GHz Ka-band pulsed EPR spectrometer. Concept. Magn. Reson. B 2006, 29B, 125–136. [Google Scholar] [CrossRef]
- Goldfarb, D.; Lipkin, Y.; Potapov, A.; Gorodetsky, Y.; Epel, B.; Raitsimring, A.M.; Radoul, M.; Kaminker, I. Hyscore and deer with an upgraded 95 GHz pulse EPR spectrometer. J. Magn. Reson. 2008, 194, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, D.; Kofman, V.; Libman, J.; Shanzer, A.; Rahmatouline, R.; Van Doorslaer, S.; Schweiger, A. Double nuclear coherence transfer (DONUT)-HYSCORE: A new tool for the assignment of nuclear frequencies in pulsed EPR experiments. J. Am. Chem. Soc. 1998, 120, 7020–7029. [Google Scholar] [CrossRef]
- Schosseler, P.; Wacker, T.; Schweiger, A. Pulsed eldor-detected NMR. Chem. Phys. Lett. 1994, 224, 319–324. [Google Scholar] [CrossRef]
- Kulik, L.; Epel, B.; Messinger, J.; Lubitz, W. Pulse EPR, Mn-55-ENDOR and eldor-detected NMR of the S-2-state of the oxygen evolving complex in photosystem II. Photosynth. Res. 2005, 84, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Rapatskiy, L.; Cox, N.; Savitsky, A.; Ames, W.M.; Sander, J.; Nowaczyk, M.M.; Roegner, M.; Boussac, A.; Neese, F.; Messinger, J.; et al. Detection of the water-binding sites of the oxygen-evolving complex of photosystem II using W-band O-17 electron-electron double resonance-detected NMR spectroscopy. J. Am. Chem. Soc. 2012, 134, 16619–16634. [Google Scholar] [CrossRef] [PubMed]
- Cox, N.; Nalepa, A.; Pandelia, M.-E.; Lubitz, W.; Savitsky, A. Chapter nine—Pulse double-resonance EPR techniques for the study of metallobiomolecules. In Methods in Enzymology; Qin Peter, Z., Kurt, W., Eds.; Academic Press: Cambridge, MA, USA, 2015; Volume 563, pp. 211–249. [Google Scholar]
- Potapov, A.; Epel, B.; Goldfarb, D. A triple resonance hyperfine sublevel correlation experiment for assignment of electron-nuclear double resonance lines. J. Chem. Phys. 2008, 128, 052320. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.; Agrawal, A.G.; van Gastel, M.; Gaertner, W.; Lubitz, W. Electron-electron double resonance-detected NMR to measure metal hyperfine interactions: Ni-61 in the Ni-B state of the [NiFe] hydrogenase of Desulfovibrio vulgaris miyazaki F. J. Am. Chem. Soc. 2008, 130, 2402–2403. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, G.; Spiess, H.W. Nmr-correlated high-field electron paramagnetic resonance spectroscopy. Chem. Phys. Lett. 1998, 293, 9–18. [Google Scholar] [CrossRef]
- Potapov, A.; Pecht, I.; Goldfarb, D. Resolving ligand hyperfine couplings of type 1 and 2 Cu(II) in ascorbate oxidase by high field pulse EPR correlation spectroscopy. Phys. Chem. Chem. Phys. 2010, 12, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Baute, D.; Goldfarb, D. The 17O hyperfine interaction in V17O(H217O)52+ and Mn(H217O)62+ determined by high field ENDOR aided by DFT calculations. J. Phys. Chem. A 2005, 109, 7865–7871. [Google Scholar] [CrossRef] [PubMed]
- Mehring, M.; Höfer, P.; Grupp, A. Pulsed electron nuclear double and triple resonance schemes. Ber. Bunsen-Ges. Phys. Chem. 1987, 91, 1132–1137. [Google Scholar] [CrossRef]
- Epel, B.; Goldfarb, D. Two-dimensional pulsed triple at 95 GHz. J. Magn. Reson. 2000, 146, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, D.; Epel, B.; Zimmermann, H.; Jeschke, G. 2D triple in orientationally disordered samples—A means to resolve and determine relative orientation of hyperfine tensors. J. Magn. Reson. 2004, 168, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Denysenkov, V.P.; Biglino, D.; Lubitz, W.; Prisner, T.F.; Bennati, M. Structure of the tyrosyl biradical in mouse R2 ribonucleotide reductase from high-field PELDOR. Angew. Chem. Int. Ed. 2008, 47, 1224–1227. [Google Scholar] [CrossRef] [PubMed]
- Denysenkov, V.P.; Prisner, T.F.; Stubbe, J.; Bennati, M. High-field pulsed electron-electron double resonance spectroscopy to determine the orientation of the tyrosyl radicals in ribonucleotide reductase. Proc. Natl. Acad. Sci. USA 2006, 103, 13386–13390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savitsky, A.; Dubinskii, A.A.; Flores, M.; Lubitz, W.; Möbius, K. Orientation-resolving pulsed electron dipolar high-field EPR spectroscopy on disordered solids: I. Structure of spin-correlated radical pairs in bacterial photosynthetic reaction centers. J. Phys. Chem. B 2007, 111, 6245–6262. [Google Scholar] [CrossRef] [PubMed]
- Polyhach, Y.; Godt, A.; Bauer, C.; Jeschke, G. Spin pair geometry revealed by high-field deer in the presence of conformational distributions. J. Magn. Reson. 2007, 185, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, G. Pro and contra of pulse eldor. EPR Newsletter 2005, 14, 14–16. [Google Scholar]
- Klare, J.P.; Steinhoff, H.-J. Site-directed spin labeling and pulse dipolar electron paramagnetic resonance. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley: Chichester, UK, 2010. [Google Scholar]
- Borbat, P.P.; Freed, J.H. Measuring distances by pulsed dipolar ESR spectroscopy: Spin-labeled histidine kinases. In Methods in Enzymology; Simon, M.I., Crane, B.R., Crane, A., Eds.; Elsevier: New York, NY, USA, 2007; Volume 423, pp. 52–116. [Google Scholar]
- Böhme, S.; Steinhoff, H.-J.; Klare, J.P. Accessing the distance range of interest in biomolecules; site-directed spin labeling and deer spectroscopy. Spectroscopy 2010, 24, 283–288. [Google Scholar] [CrossRef]
- Kurshev, V.V.; Raitsimring, A.M.; Tsvetkov, Y.D. Selection of dipolar interaction by the 2+1 pulse train ese. J. Magn. Reson. 1989, 81, 441–454. [Google Scholar] [CrossRef]
- Milov, A.D.; Maryasov, A.G.; Tsvetkov, Y.D. Pulsed electron double resonance (PELDOR) and its applications in free-radicals research. Appl. Magn. Reson. 1998, 15, 107–143. [Google Scholar] [CrossRef]
- Pfannebecker, V.; Klos, H.; Hubrich, M.; Volkmer, T.; Heuer, A.; Wiesner, U.; Spiess, H.W. Determination of end-to-end distances in oligomers by pulsed EPR. J. Phys. Chem. 1996, 100, 13428–13432. [Google Scholar] [CrossRef]
- Martin, R.E.; Pannier, M.; Diederich, F.; Gramlich, V.; Hubrich, M.; Spiess, H.W. Determination of end-to-end distances in a series of tempo diradicals of up to 2.8 nm length with a new four-pulse double electron electron resonance experiment. Angew. Chem. Int. Ed. 1998, 37, 2834–2837. [Google Scholar] [CrossRef]
- Larsen, R.G.; Singel, D.J. Double electron-electron resonance spin-echo modulation—Spectroscopic measurement of electron-spin pair separations in orientationally disordered solids. J. Chem. Phys. 1993, 98, 5134–5146. [Google Scholar] [CrossRef]
- Milov, A.D.; Ponomarev, A.B.; Tsvetkov, Y.D. Electron-electron double resonance in electron spin echo: Model biradical systems and the sensitized photolysis of decalin. Chem. Phys. Lett. 1984, 110, 67–72. [Google Scholar] [CrossRef]
- Pannier, M.; Veit, S.; Godt, A.; Jeschke, G.; Spiess, H.W. Dead-time free measurement of dipole-dipole interactions between electron spins. J. Magn. Reson. 2000, 142, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Freed, J.H. Two dimensional electron spin resonance and slow motions. J. Phys. Chem. A 1997, 101, 7998–8008. [Google Scholar] [CrossRef]
- Kulik, L.V.; Dzuba, S.A.; Grigoryev, I.A.; Tsvetkov, Y.D. Electron dipole-dipole interaction in eseem of nitroxide biradicals. Chem. Phys. Lett. 2001, 343, 315–324. [Google Scholar] [CrossRef]
- Bennati, M.; Prisner, T.F. New developments in high field electron paramagnetic resonance with applications in structural biology. Rep. Prog. Phys. 2005, 68, 411–448. [Google Scholar] [CrossRef]
- Denysenkov, V.P.; Prisner, T.F.; Stubbe, J.; Bennati, M. High-frequency 180 GHz PELDOR. Appl. Magn. Reson. 2005, 29, 375–384. [Google Scholar] [CrossRef]
- Holldack, K.; Schnegg, A. Thz electron paramagnetic resonance/THz spectroscopy at bessy II. JLSRF 2016, 2, A51. [Google Scholar] [CrossRef]
- Schnegg, A. Very-high-frequency EPR. eMagRes 2017, 6, 115–131. [Google Scholar]
- Ohta, H.; Okubo, S.; Ohmichi, E.; Sakurai, T.; Zhang, W.-M.; Shimokawa, T. Developments of multi-extreme high field ESR in Kobe. J. Low Temp. Phys. 2013, 170, 511–519. [Google Scholar] [CrossRef]
- Krzystek, J.; Ozarowski, A.; van Tol, J.; Liu, J.; Hill, S. High-frequency and high-field EPR/ESR in tallahassee. EPR Newsl. 2007, 22, 12–14. [Google Scholar]
- Hubbell, W.L.; Altenbach, C. Investigation of structure and dynamics in membrane-proteins using site-directed spin-labeling. Curr. Opin. Struct. Biol. 1994, 4, 566–573. [Google Scholar] [CrossRef]
- Davydov, D.R.; Yang, Z.; Davydova, N.; Halpert, J.R.; Hubbell, W.L. Conformational mobility in cytochrome p450 3a4 explored by pressure-perturbation EPR spectroscopy. Biophys. J. 2016, 110, 1485–1498. [Google Scholar] [CrossRef] [PubMed]
- Lerch, M.T.; Yang, Z.; Brooks, E.K.; Hubbell, W.L. Mapping protein conformational heterogeneity under pressure with site-directed spin labeling and double electron–electron resonance. Proc. Natl. Acad. Sci. USA 2014, 111, E1201–E1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Eps, N.; Altenbach, C.; Caro, L.N.; Latorraca, N.R.; Hollingsworth, S.A.; Dror, R.O.; Ernst, O.P.; Hubbell, W.L. G(i)- and G(s)-coupled gpcrs show different modes of g-protein binding. Proc. Natl. Acad. Sci. USA 2018, 115, 2383–2388. [Google Scholar] [CrossRef] [PubMed]
- Van Eps, N.; Caro, L.N.; Morizumi, T.; Kusnetzow, A.K.; Szczepek, M.; Hofmann, K.P.; Bayburt, T.H.; Sligar, S.G.; Ernst, O.P.; Hubbell, W.L. Conformational equilibria of light-activated rhodopsin in nanodiscs. Proc. Natl. Acad. Sci. USA 2017, 114, E3268–E3275. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Y.; Bridges, M.D.; Lopez, C.J.; Rogozhnikova, O.Y.; Trukhin, D.V.; Brooks, E.K.; Tormyshev, V.; Halpern, H.J.; Hubbell, W.L. A triarylmethyl spin label for long-range distance measurement at physiological temperatures using t−1 relaxation enhancement. J. Magn. Reson. 2016, 269, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Altenbach, C.; López, C.J.; Hideg, K.; Hubbell, W.L. Chapter three—Exploring structure, dynamics, and topology of nitroxide spin-labeled proteins using continuous-wave electron paramagnetic resonance spectroscopy. In Methods in Enzymology; Qin, P.Z., Warncke, K., Eds.; Academic Press: Cambridge, MA, USA, 2015; Volume 564, pp. 59–100. [Google Scholar]
- Lerch, M.T.; Yang, Z.; Altenbach, C.; Hubbell, W.L. Chapter two—High-pressure EPR and site-directed spin labeling for mapping molecular flexibility in proteins. In Methods in Enzymology; Qin, P.Z., Warncke, K., Eds.; Academic Press: Cambridge, MA, USA, 2015; Volume 564, pp. 29–57. [Google Scholar]
- Yang, Z.; Bridges, M.; Lerch, M.T.; Altenbach, C.; Hubbell, W.L. Chapter one—Saturation recovery EPR and nitroxide spin labeling for exploring structure and dynamics in proteins. In Methods in Enzymology; Qin, P.Z., Warncke, K., Eds.; Academic Press: Cambridge, MA, USA, 2015; Volume 564, pp. 3–27. [Google Scholar]
- López, C.J.; Fleissner, M.R.; Brooks, E.K.; Hubbell, W.L. Stationary-phase EPR for exploring protein structure, conformation, and dynamics in spin-labeled proteins. Biochemistry 2014, 53, 7067–7075. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Jiménez-Osés, G.; López, C.J.; Bridges, M.D.; Houk, K.N.; Hubbell, W.L. Long-range distance measurements in proteins at physiological temperatures using saturation recovery EPR spectroscopy. J. Am. Chem. Soc. 2014, 136, 15356–15365. [Google Scholar] [CrossRef] [PubMed]
- Schredelseker, J.; Paz, A.; López, C.J.; Altenbach, C.; Leung, C.S.; Drexler, M.K.; Chen, J.-N.; Hubbell, W.L.; Abramson, J. High resolution structure and double electron-electron resonance of the zebrafish voltage-dependent anion channel 2 reveal an oligomeric population. J. Biol. Chem. 2014, 289, 12566–12577. [Google Scholar] [CrossRef] [PubMed]
- Hubbell, W.L.; López, C.J.; Altenbach, C.; Yang, Z. Technological advances in site-directed spin labeling of proteins. Curr. Opin. Struct. Biol. 2013, 23, 725–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toledo Warshaviak, D.; Khramtsov, V.V.; Cascio, D.; Altenbach, C.; Hubbell, W.L. Structure and dynamics of an imidazoline nitroxide side chain with strongly hindered internal motion in proteins. J. Magn. Reson. 2013, 232, 53–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López, C.J.; Oga, S.; Hubbell, W.L. Mapping molecular flexibility of proteins with site-directed spin labeling: A case study of myoglobin. Biochemistry 2012, 51, 6568–6583. [Google Scholar] [CrossRef] [PubMed]
- Borovykh, I.V.; Ceola, S.; Gajula, P.; Gast, P.; Steinhoff, H.J.; Huber, M. Distance between a native cofactor and a spin label in the reaction centre of rhodobacter sphaeroides by a two-frequency pulsed electron paramagnetic resonance method and molecular dynamics simulations. J. Magn. Reson. 2006, 180, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Bordignon, E.; Steinhoff, H.-J. Membrane protein structure and dynamics studied by site-directed spin-labeling ESR. In ESR Spectroscopy in Membrane Biophysics, Biological Magnetic Resonance; Hemminga, M.A., Berliner, L.J., Eds.; Elsevier: New York, NY, USA, 2007; Volume 27, pp. 129–164. [Google Scholar]
- Gajula, P.; Borovykh, I.V.; Beier, C.; Shkuropatova, T.; Gast, P.; Steinhoff, H.J. Spin-labeled photosynthetic reaction centers from Rhodobacter sphaeroides studied by electron paramagnetic resonance spectroscopy and molecular dynamics simulations. Appl. Magn. Reson. 2007, 31, 167–178. [Google Scholar] [CrossRef]
- Grote, M.; Bordignon, E.; Polyhach, Y.; Jeschke, G.; Steinhoff, H.-J.; Schneider, E. A comparative electron paramagnetic resonance study of the nucleotide-binding domains’ catalytic cycle in the assembled maltose atp-binding cassette importer. Biophys. J. 2008, 95, 2924–2938. [Google Scholar] [CrossRef] [PubMed]
- Padmavathi, P.V.L.; Steinhoff, H.-J. Conformation of the closed channel state of colicin a in proteoliposomes: An umbrella model. J. Mol. Biol. 2008, 378, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Böhme, S.; Padmavathi, P.V.L.; Holterhues, J.; Ouchni, F.; Klare, J.P.; Steinhoff, H.-J. Topology of the amphipathic helices of the colicin a pore-forming domain in E. coli lipid membranes studied by pulse EPR. Phys. Chem. Chem. Phys. 2009, 11, 6770–6777. [Google Scholar] [CrossRef] [PubMed]
- Klare, J.P.; Steinhoff, H.-J. Spin labeling EPR. Photosynth. Res. 2009, 102, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Bordignon, E.; Brutlach, H.; Urban, L.; Hideg, K.; Savitsky, A.; Schnegg, A.; Gast, P.; Engelhard, M.; Groenen, E.J.J.; Möbius, K.; et al. Heterogeneity in the nitroxide micro-environment: Polarity and proticity effects in spin-labeled proteins studied by multi-frequency EPR. Appl. Magn. Reson. 2010, 37, 391–403. [Google Scholar] [CrossRef]
- Böhme, S.; Meyer, S.; Krueger, A.; Steinhoff, H.-J.; Wittinghofer, A.; Klare, J.P. Stabilization of g domain conformations in the trna-modifying mnme-gida complex observed with double electron electron resonance spectroscopy. J. Biol. Chem. 2010, 285, 16991–17000. [Google Scholar] [CrossRef] [PubMed]
- Grohmann, D.; Klose, D.; Klare, J.P.; Kay, C.W.M.; Steinhoff, H.-J.; Werner, F. Rna-binding to archaeal rna polymerase subunits F/E: A deer and fret study. J. Am. Chem. Soc. 2010, 132, 5954–5955. [Google Scholar] [CrossRef] [PubMed]
- Abe, C.; Klose, D.; Dietrich, F.; Ziegler, W.H.; Polyhach, Y.; Jeschke, G.; Steinhoff, H.-J. Orientation selective deer measurements on vinculin tail at X-band frequencies reveal spin label orientations. J. Magn. Reson. 2012, 216, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Pulagam, L.P.; Steinhoff, H.-J. Acidic PH-induced membrane insertion of colicin a into E. coli natural lipids probed by site-directed spin labeling. J. Mol. Biol. 2013, 425, 1782–1794. [Google Scholar] [CrossRef] [PubMed]
- Dunkel, S.; Pulagam, L.P.; Steinhoff, H.J.; Klare, J.P. In vivo EPR on spin labeled colicin a reveals an oligomeric assembly of the pore-forming domain in E. coli membranes. Phys. Chem. Chem. Phys. 2015, 17, 4875–4878. [Google Scholar] [CrossRef] [PubMed]
- Klare, J.P.; Steinhoff, H.-J. Spin labeling studies of transmembrane signaling and transport: Applications to phototaxis, abc transporters and symporters. In Methods in Enzymology; Qin, P.Z., Warncke, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 564, pp. 315–347. [Google Scholar]
- Voskoboynikova, N.; Mosslehy, W.; Colbasevici, A.; Ismagulova, T.T.; Bagrov, D.V.; Akovantseva, A.A.; Timashev, P.S.; Mulkidjanian, A.Y.; Bagratashvili, V.N.; Shaitan, K.V.; et al. Characterization of an archaeal photoreceptor/transducer complex from natronomonas pharaonis assembled within styrene-maleic acid lipid particles. RSC Adv. 2017, 7, 51324–51334. [Google Scholar] [CrossRef]
- Savitsky, A.; Möbius, K. High-field EPR. Photosynth. Res. 2009, 102, 311–333. [Google Scholar] [CrossRef] [PubMed]
- Bagryanskaya, E.G.; Bardelang, D.; Chenesseau, S.; Finet, J.P.; Jicsinszky, L.; Karoui, H.; Marque, S.R.A.; Möbius, K.; Polovyanenko, D.; Savitsky, A.; et al. EPR, NMR, and thermodynamic evidences for forced nuclear spin-electron spin interactions in the case of 1-phenyl-2-methylpropyl-1,1-dimethyl-2-nitroxide (TIPNO) attached to permethylated beta-cyclodextrin. Appl. Magn. Reson. 2009, 36, 181–194. [Google Scholar] [CrossRef]
- Savitsky, A.; Plato, M.; Möbius, K. The temperature dependence of nitroxide spin-label interaction parameters: A high-field EPR study of intramolecular motional contributions. Appl. Magn. Reson. 2010, 37, 415–434. [Google Scholar] [CrossRef]
- Savitsky, A.; Dubinskii, A.A.; Zimmermann, H.; Lubitz, W.; Möbius, K. High-field dipolar electron paramagnetic resonance (EPR) spectroscopy of nitroxide biradicals for determining three-dimensional structures of biomacromolecules in disordered solids. J. Phys. Chem. B 2011, 115, 11950–11963. [Google Scholar] [CrossRef] [PubMed]
- Bode, B.E.; Plackmeyer, J.; Prisner, T.F.; Schiemann, O. PELDOR measurements on a nitroxide-labeled Cu(II) porphyrin: Orientation selection, spin-density distribution, and conformational flexibility. J. Phys. Chem. A 2008, 112, 5064–5073. [Google Scholar] [CrossRef] [PubMed]
- Bode, B.E.; Dastvan, R.; Prisner, T.F. Pulsed electron-electron double resonance (PELDOR) distance measurements in detergent micelles. J. Magn. Reson. 2011, 211, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Dastvan, R.; Bode, B.E.; Karuppiah, M.P.R.; Marko, A.; Lyubenova, S.; Schwalbe, H.; Prisner, T.F. Optimization of transversal relaxation of nitroxides for pulsed electron-electron double resonance spectroscopy in phospholipid membranes. J. Phys. Chem. B 2010, 114, 13507–13516. [Google Scholar] [CrossRef] [PubMed]
- Endeward, B.; Butterwick, J.A.; MacKinnon, R.; Prisner, T.F. Pulsed electron-electron double-resonance determination of spin-label distances and orientations on the tetrameric potassium ion channel kcsa. J. Am. Chem. Soc. 2009, 131, 15246–15250. [Google Scholar] [CrossRef] [PubMed]
- Marko, A.; Prisner, T.F. An algorithm to analyze PELDOR data of rigid spin label pairs. Phys. Chem. Chem. Phys. 2013, 15, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Sikora, A.; Bordignon, E.; Jeschke, G.; Cafiso, D.S.; Prisner, T.F. Distance measurement on an endogenous membrane transporter in e-coli cells and native membranes using EPR spectroscopy. Angew. Chem. Int. Ed. 2015, 54, 6196–6199. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Tormyshev, V.M.; Rogozhnikova, O.Y.; Akhmetzyanov, D.; Bagryanskaya, E.G.; Prisner, T.F. Selective high-resolution detection of membrane protein-ligand interaction in native membranes using trityl-nitroxide PELDOR. Angew. Chem. Int. Ed. 2016, 55, 11538–11542. [Google Scholar] [CrossRef] [PubMed]
- Pliotas, C.; Ward, R.; Branigan, E.; Rasmussen, A.; Hagelueken, G.; Huang, H.; Black, S.S.; Booth, I.R.; Schiemann, O.; Naismith, J.H. Conformational state of the mscs mechanosensitive channel in solution revealed by pulsed electron-electron double resonance (PELDOR) spectroscopy. Proc. Natl. Acad. Sci. USA 2012, 109, E2675–E2682. [Google Scholar] [CrossRef] [PubMed]
- Reginsson, G.W.; Schiemann, O. Pulsed electron-electron double resonance: Beyond nanometre distance measurements on biomacromolecules. Biochem. J. 2011, 434, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Reginsson, G.W.; Shelke, S.A.; Rouillon, C.; White, M.F.; Sigurdsson, S.T.; Schiemann, O. Protein-induced changes in DNA structure and dynamics observed with noncovalent site-directed spin labeling and PELDOR. Nucl. Acids Res. 2013, 41, e11. [Google Scholar] [CrossRef] [PubMed]
- Schiemann, O. Studying protein DNA binding with PELDOR. Biophys. J. 2011, 100, 2. [Google Scholar] [CrossRef]
- Bode, B.E.; Plackmeyer, J.; Bolte, M.; Prisner, T.F.; Schiemann, O. PELDOR on an exchange coupled nitroxide copper(II) spin pair. J. Organomet. Chem. 2009, 694, 1172–1179. [Google Scholar] [CrossRef]
- Hagelueken, G.; Ingledew, W.J.; Huang, H.; Petrovic-Stojanovska, B.; Whitfield, C.; ElMkami, H.; Schiemann, O.; Naismith, J.H. PELDOR spectroscopy distance fingerprinting of the octameric outer-membrane protein wza from Escherichia coli. Angew. Chem. Int. Ed. 2009, 48, 2904–2906. [Google Scholar] [CrossRef] [PubMed]
- Schiemann, O.; Cekan, P.; Margraf, D.; Prisner, T.F.; Sigurdsson, S.T. Relative orientation of rigid nitroxides by PELDOR: Beyond distance measurements in nucleic acids. Angew. Chem. Int. Ed. 2009, 48, 3292–3295. [Google Scholar] [CrossRef] [PubMed]
- Frolow, O.; Endeward, B.; Schiemann, O.; Prisner, T.F.; Engels, J.W. Tpa labelled oligonucleotides for long range distance measurements by EPR. In Proceedings of the 14th Symposium on Chemistry of Nucleic Acid Components, Cesky Krumlov, Czech Republic, 8–13 June 2008; Hocek, M., Ed.; 2008; Volume 10, pp. 343–344. [Google Scholar]
- Frolow, O.; Endeward, B.; Schiemann, O.; Prisner, T.F.; Engels, J.W. Nitroxide spin labeled rna for long range distance measurements by EPR-PELDOR. Nucl. Acids Symp. Ser. 2008, 153–154. [Google Scholar] [CrossRef] [PubMed]
- Abdullin, D.; Florin, N.; Hagelueken, G.; Schiemann, O. EPR-based approach for the localization of paramagnetic metal ions in biomolecules. Angew. Chem. Int. Ed. 2015, 54, 1827–1831. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.M.; Hannam, J.S.; Schmitz, A.; Schiemann, O.; Hagelueken, G.; Famulok, M. Studying the conformation of a receptor tyrosine kinase in solution by inhibitor-based spin labeling. Angew. Chem. Int. Ed. 2017, 56, 8417–8421. [Google Scholar] [CrossRef] [PubMed]
- Hagelueken, G.; Hoffmann, J.; Schubert, E.; Duthie, F.G.; Florin, N.; Konrad, L.; Imhof, D.; Behrmann, E.; Morgner, N.; Schiemann, O. Studies on the X-ray and solution structure of feob from Escherichia coli BL21. Biophys. J. 2016, 110, 2642–2650. [Google Scholar] [CrossRef] [PubMed]
- Polyhach, Y.; Bordignon, E.; Tschaggelar, R.; Gandra, S.; Godt, A.; Jeschke, G. High sensitivity and versatility of the deer experiment on nitroxide radical pairs at Q-band frequencies. Phys. Chem. Chem. Phys. 2012, 14, 10762–10773. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, G. Deer distance measurements on proteins. Annu. Rev. Phys. Chem. 2012, 63, 419–446. [Google Scholar] [CrossRef] [PubMed]
- Gmeiner, C.; Klose, D.; Mileo, E.; Belle, V.; Marque, S.R.A.; Dorn, G.; Allain, F.H.T.; Guigliarelli, B.; Jeschke, G.; Yulikov, M. Orthogonal tyrosine and cysteine site-directed spin labeling for dipolar pulse EPR spectroscopy on proteins. J. Phys. Chem. Lett. 2017, 8, 4852–4857. [Google Scholar] [CrossRef] [PubMed]
- Gobbo, M.; Biondi, B.; De Zotti, M.; Milov, A.D.; Tsvetkov, Y.D.; Maryasov, A.G.; Formaggio, F.; Toniolo, C. Conformational properties of the spin-labeled tylopeptin b and heptaibin peptaibiotics based on PELDOR spectroscopy data. J. Pept. Sci. 2012, 18, S59–S60. [Google Scholar]
- Kuznetsov, N.A.; Milov, A.D.; Isaev, N.P.; Vorobjev, Y.N.; Koval, V.V.; Dzuba, S.A.; Fedorova, O.S.; Tsvetkov, Y.D. PELDOR analysis of enzyme-induced structural changes in damaged DNA duplexes. Mol. Biosyst. 2011, 7, 2670–2680. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, N.A.; Milov, A.D.; Koval, V.V.; Samoilova, R.I.; Grishin, Y.A.; Knorre, D.G.; Tsvetkov, Y.D.; Fedorova, O.S.; Dzuba, S.A. PELDOR study of conformations of double-spin-labeled single- and double-stranded DNA with non-nucleotide inserts. Phys. Chem. Chem. Phys. 2009, 11, 6826–6832. [Google Scholar] [CrossRef] [PubMed]
- Milov, A.D.; Samoilova, R.I.; Tsvetkov, Y.D.; De Zotti, M.; Formaggio, F.; Toniolo, C.; Handgraaf, J.-W.; Raap, J. Structure of self-aggregated alamethicin in epc membranes detected by pulsed electron-electron double resonance and electron spin echo envelope modulation spectroscopies. Biophys. J. 2009, 96, 3197–3209. [Google Scholar] [CrossRef] [PubMed]
- Milov, A.D.; Samoilova, R.I.; Tsvetkov, Y.D.; De Zotti, M.; Toniolo, C.; Raap, J. PELDOR conformational analysis of bis-labeled alamethicin aggregated in phospholipid vesicles. J. Phys. Chem. B 2008, 112, 13469–13472. [Google Scholar] [CrossRef] [PubMed]
- Milov, A.D.; Samoilova, R.I.; Tsvetkov, Y.D.; Formaggio, F.; Toniolo, C.; Raap, J. Self-aggregation of spin-labeled alamethicin in epc vesicles studied by pulsed electron-electron double resonance. J. Am. Chem. Soc. 2007, 129, 9260–9261. [Google Scholar] [CrossRef] [PubMed]
- Milov, A.D.; Tsvetkov, Y.D.; Gorbunova, E.Y.; Mustaeva, L.G.; Ovchinnikova, T.V.; Handgraaf, J.-W.; Raap, J. Solvent effects on the secondary structure of the membrane-active zervamicin determined by PELDOR spectroscopy. Chem. Biodivers. 2007, 4, 1243–1255. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.K.; Freed, J.H. Dynamics and ordering of lipid spin-labels along the coexistence curve of two membrane phases: An ESR study. Chem. Phys. Lipids 2012, 165, 348–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dzikovski, B.; Tipikin, D.; Freed, J. Conformational distributions and hydrogen bonding in gel and frozen lipid bilayers: A high frequency spin-label ESR study. J. Phys. Chem. B 2012, 116, 6694–6706. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, E.R.; Roy, A.S.; Grigoryants, V.M.; Borbat, P.P.; Earle, K.A.; Scholes, C.P.; Freed, J.H. Effect of freezing conditions on distances and their distributions derived from double electron electron resonance (DEER): A study of doubly-spin-labeled T4 lysozyme. J. Magn. Reson. 2012, 216, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Dzikovski, B.G.; Borbat, P.P.; Freed, J.H. Channel and nonchannel forms of spin-labeled gramicidin in membranes and their equilibria. J. Phys. Chem. B 2011, 115, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Fleissner, M.R.; Tipikin, D.S.; Liang, Z.; Moscicki, J.K.; Earle, K.A.; Hubbell, W.L.; Freed, J.H. Multifrequency electron spin resonance study of the dynamics of spin labeled T4 lysozyme. J. Phys. Chem. B 2010, 114, 5503–5521. [Google Scholar] [CrossRef] [PubMed]
- Orlando, B.J.; Borbat, P.P.; Georgieva, E.R.; Freed, J.H.; Malkowski, M.G. Pulsed dipolar spectroscopy reveals that tyrosyl radicals are generated in both monomers of the cyclooxygenase-2 dimer. Biochemistry 2015, 54, 7309–7312. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Borbat, P.P.; Grigoryants, V.M.; Myers, W.K.; Freed, J.H.; Soholes, C.P. Pulse dipolar ESR of doubly labeled mini tar DNA and its annealing to mini tar RNA. Biophys. J. 2015, 108, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Lai, A.L.; Clerico, E.M.; Blackburn, M.E.; Patel, N.A.; Robinson, C.V.; Borbat, P.P.; Freed, J.H.; Gierasch, L.M. Key features of an Hsp70 chaperone allosteric landscape revealed by ion-mobility native mass spectrometry and double electron-electron resonance. J. Biol. Chem. 2017, 292, 8773–8785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eschmann, N.A.; Georgieva, E.R.; Ganguly, P.; Borbat, P.P.; Rappaport, M.D.; Akdogan, Y.; Freed, J.H.; Shea, J.-E.; Han, S. Signature of an aggregation-prone conformation of tau. Sci. Rep. 2017, 7, 44739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahrenberg, T.; Rosenski, Y.; Carmieli, R.; Zibzener, K.; Qi, M.; Frydrnan, V.; Godt, A.; Goldfarb, D.; Feintuch, A. Improved sensitivity for W-band Gd(III)-Gd(III) and nitroxide-nitroxide deer measurements with shaped pulses. J. Magn. Reson. 2017, 283, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Feintuch, A.; Otting, G.; Goldfarb, D. Gd3+ spin labeling for measuring distances in biomacromolecules: Why and how? In Methods in Enzymology; Qin, P.Z., Warncke, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 563, pp. 415–457. [Google Scholar]
- Tkach, I.; Halbmair, K.; Hoebartner, C.; Bennati, M. High-frequency 263 GHz PELDOR. Appl. Magn. Reson. 2014, 45, 969–979. [Google Scholar] [CrossRef]
- Halbmair, K.; Wegner, J.; Diederichsen, U.; Bennati, M. Pulse EPR measurements of intramolecular distances in a topp-labeled transmembrane peptide in lipids. Biophys. J. 2016, 111, 2345–2348. [Google Scholar] [CrossRef] [PubMed]
- Clayton, J.A.; Qi, M.; Godt, A.; Goldfarb, D.; Han, S.G.; Sherwin, M.S. Gd3+-Gd3+ distances exceeding 3 nm determined by very high frequency continuous wave electron paramagnetic resonance. Phys. Chem. Chem. Phys. 2017, 19, 5127–5136. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.R.; Frydman, V.; Milko, P.; Iron, M.A.; Abdelkader, E.H.; Lee, M.D.; Swarbrick, J.D.; Raitsimring, A.; Otting, G.; Graham, B.; et al. Overcoming artificial broadening in Gd3+-Gd3+ distance distributions arising from dipolar pseudo-secular terms in deer experiments. Phys. Chem. Chem. Phys. 2016, 18, 12847–12859. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, E.H.; Lee, M.D.; Feintuch, A.; Cohen, M.R.; Swarbrick, J.D.; Otting, G.; Graham, B.; Goldfarb, D. A new Gd3+ spin label for Gd3+-Gd3+ distance measurements in proteins produces narrow distance distributions. J. Phys. Chem. Lett. 2015, 6, 5016–5021. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, E.H.; Yao, X.; Feintuch, A.; Adams, L.A.; Aurelio, L.; Graham, B.; Goldfarb, D.; Otting, G. Pulse EPR-enabled interpretation of scarce pseudocontact shifts induced by lanthanide binding tags. J. Biomol. NMR 2016, 64, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Gophane, D.B.; Endeward, B.; Prisner, T.F.; Sigurdsson, S.T. A semi-rigid isoindoline-derived nitroxide spin label for RNA. Org. Biomol. Chem. 2018, 16, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Stelzl, L.S.; Erlenbach, N.; Heinz, M.; Prisner, T.F.; Hummer, G. Resolving the conformational dynamics of DNA with angstrom resolution by pulsed electron-electron double resonance and molecular dynamics. J. Am. Chem. Soc. 2017, 139, 11674–11677. [Google Scholar] [CrossRef] [PubMed]
- Schnorr, K.A.; Gophane, D.B.; Helmling, C.; Cetiner, E.; Pasemann, K.; Furtig, B.; Wacker, A.; Qureshi, N.S.; Granz, M.; Barthelmes, D.; et al. Impact of spin label rigidity on extent and accuracy of distance information from pre data. J. Biomol. NMR 2017, 68, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Weinrich, T.; Granz, M.; Grunewald, C.; Prisner, T.F.; Gobel, M.W. Synthesis of a cytidine phosphoramidite with protected nitroxide spin label for EPR experiments with RNA. Eur. J. Org. Chem. 2017, 491–496. [Google Scholar] [CrossRef]
- Kamble, N.R.; Granz, M.; Prisner, T.F.; Sigurdsson, S.T. Noncovalent and site-directed spin labeling of duplex RNA. Chem. Comm. 2016, 52, 14442–14445. [Google Scholar] [CrossRef] [PubMed]
- Akhmetzyanov, D.; Ching, H.Y.V.; Denysenkov, V.; Demay-Drouhard, P.; Bertrand, H.C.; Tabares, L.C.; Policar, C.; Prisner, T.F.; Un, S. Ridme spectroscopy on high-spin Mn2+ centers. Phys. Chem. Chem. Phys. 2016, 18, 30857–30866. [Google Scholar] [CrossRef] [PubMed]
- Schöps, P.; Plackmeyer, J.; Marko, A. Separation of intra- and intermolecular contributions to the PELDOR signal. J. Magn. Reson. 2016, 269, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Erlenbach, N.; Endeward, B.; Schöps, P.; Gophane, D.B.; Sigurdsson, S.T.; Prisner, T.F. Flexibilities of isoindoline-derived spin labels for nucleic acids by orientation selective PELDOR. Phys. Chem. Chem. Phys. 2016, 18, 16196–16201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grytz, C.M.; Marko, A.; Cekan, P.; Sigurdsson, S.T.; Prisner, T.F. Flexibility and conformation of the cocaine aptamer studied by PELDOR. Phys. Chem. Chem. Phys. 2016, 18, 2993–3002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endeward, B.; Marko, A.; Denysenkov, V.P.; Sigurdsson, S.T.; Prisner, T.F. Advanced EPR methods for studying conformational dynamics of nucleic acids. In Methods in Enzymology; Qin, P.Z., Warncke, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 564, pp. 403–425. [Google Scholar]
- Barthelmes, D.; Granz, M.; Barthelmes, K.; Allen, K.N.; Imperiali, B.; Prisner, T.; Schwalbe, H. Encoded loop-lanthanide-binding tags for long-range distance measurements in proteins by NMR and EPR spectroscopy. J. Biomol. NMR 2015, 63, 275–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhmetzyanov, D.; Schops, P.; Marko, A.; Kunjir, N.C.; Sigurdsson, S.T.; Prisner, T.F. Pulsed EPR dipolar spectroscopy at q- and g-band on a trityl biradical. Phys. Chem. Chem. Phys. 2015, 17, 24446–24451. [Google Scholar] [CrossRef] [PubMed]
- Prisner, T.F.; Marko, A.; Sigurdsson, S.T. Conformational dynamics of nucleic acid molecules studied by PELDOR spectroscopy with rigid spin labels. J. Magn. Reson. 2015, 252, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Borbat, P.P.; Freed, J.H. Dipolar spectroscopy—Single-resonance methods. eMagRes 2017, 6, 465–493. [Google Scholar]
- Srivastava, M.; Georgieva, E.R.; Freed, J.H. A new wavelet denoising method for experimental time-domain signals: Pulsed dipolar electron spin resonance. J. Phys. Chem. A 2017, 121, 2452–2465. [Google Scholar] [CrossRef] [PubMed]
- Dzikovski, B.; Livshits, V.; Freed, J. Interaction of spin-labeled lipid membranes with transition metal ions. J. Phys. Chem. B 2015, 119, 13330–13346. [Google Scholar] [CrossRef] [PubMed]
- Wylie, B.J.; Dzikovski, B.G.; Pawsey, S.; Caporini, M.; Rosay, M.; Freed, J.H.; McDermott, A.E. Dynamic nuclear polarization of membrane proteins: Covalently bound spin-labels at protein-protein interfaces. J. Biomol. NMR 2015, 61, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Samanta, D.; Borbat, P.P.; Dzikovski, B.; Freed, J.H.; Crane, B.R. Bacterial chemoreceptor dynamics correlate with activity state and are coupled over long distances. Proc. Natl. Acad. Sci. USA 2015, 112, 2455–2460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeschke, G. The contribution of modern EPR to structural biology. Emerg. Top. Life Sci. 2018. [Google Scholar] [CrossRef]
- Jeschke, G. Mmm: A toolbox for integrative structure modeling. Protein Sci. 2018, 27, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Doll, A.; Jeschke, G. Wideband frequency-swept excitation in pulsed EPR spectroscopy. J. Magn. Reson. 2017, 280, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, G. The making and breaking of a substrate trap. Biophys. J. 2017, 112, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, G. Dipolar spectroscopy—Double-resonance methods. eMagRes 2016, 5, 1459–1475. [Google Scholar]
- Duss, O.; Yulikov, M.; Allain, F.H.T.; Jeschke, G. Chapter ten—Combining NMR and EPR to determine structures of large rnas and protein–rna complexes in solution. In Methods in Enzymology; Woodson, S.A., Allain, F.H.T., Eds.; Academic Press: Cambridge, MA, USA, 2015; Volume 558, pp. 279–331. [Google Scholar]
- Soetbeer, J.; Hülsmann, M.; Godt, A.; Polyhach, Y.; Jeschke, G. Dynamical decoupling of nitroxides in o-terphenyl: A study of temperature, deuteration and concentration effects. Phys. Chem. Chem. Phys. 2018, 20, 1615–1628. [Google Scholar] [CrossRef] [PubMed]
- Vercellino, I.; Rezabkova, L.; Olieric, V.; Polyhach, Y.; Weinert, T.; Kammerer, R.A.; Jeschke, G.; Korkhov, V.M. Role of the nucleotidyl cyclase helical domain in catalytically active dimer formation. Proc. Natl. Acad. Sci. USA 2017, 114, E9821–E9828. [Google Scholar] [CrossRef] [PubMed]
- Pribitzer, S.; Sajid, M.; Hülsmann, M.; Godt, A.; Jeschke, G. Pulsed triple electron resonance (TRIER) for dipolar correlation spectroscopy. J. Magn. Reson. 2017, 282, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.; Mertens, V.; Qi, M.; Nalepa, A.I.; Godt, A.; Savitsky, A.; Jeschke, G.; Yulikov, M. Computing distance distributions from dipolar evolution data with overtones: Ridme spectroscopy with Gd(III)-based spin labels. Phys. Chem. Chem. Phys. 2017, 19, 17856–17876. [Google Scholar] [CrossRef] [PubMed]
- Kerzhner, M.; Matsuoka, H.; Wuebben, C.; Famulok, M.; Schiemann, O. High-yield spin labeling of long rnas for electron paramagnetic resonance spectroscopy. Biochemistry 2018, 57, 2923–2931. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Jassoy, J.J.; Spicher, S.; Berndhäuser, A.; Schiemann, O. Performance of PELDOR, RIDME, SIFTER, and DQC in measuring distances in trityl based bi- and triradicals: Exchange coupling, pseudosecular coupling and multi-spin effects. Phys. Chem. Chem. Phys. 2018, 20, 13858–13869. [Google Scholar] [CrossRef] [PubMed]
- Jassoy, J.; Meyer, A.; Spicher, S.; Wuebben, C.; Schiemann, O. Synthesis of nanometer sized bis- and tris-trityl model compounds with different extent of spin–spin coupling. Molecules 2018, 23, 682. [Google Scholar] [CrossRef] [PubMed]
- Jassoy, J.J.; Berndhäuser, A.; Duthie, F.; Kühn, S.P.; Hagelueken, G.; Schiemann, O. Versatile trityl spin labels for nanometer distance measurements on biomolecules in vitro and within cells. Angew. Chem. Int. Ed. 2017, 56, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Kerzhner, M.; Abdullin, D.; Więcek, J.; Matsuoka, H.; Hagelueken, G.; Schiemann, O.; Famulok, M. Post-synthetic spin-labeling of rna through click chemistry for PELDOR measurements. Chem. Eur. J. 2016, 22, 12113–12121. [Google Scholar] [CrossRef] [PubMed]
- Gölz, J.P.; NejatyJahromy, Y.; Bauer, M.; Muhammad, A.; Schnakenburg, G.; Grimme, S.; Schiemann, O.; Menche, D. Design, synthesis, EPR-studies and conformational bias of novel spin-labeled DCC-analogues for the highly regioselective labeling of aliphatic and aromatic carboxylic acids. Chem. Eur. J. 2016, 22, 9591–9598. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Schiemann, O. PELDOR and RIDME measurements on a high-spin manganese(II) bisnitroxide model complex. J. Phys. Chem. A 2016, 120, 3463–3472. [Google Scholar] [CrossRef] [PubMed]
- Abdullin, D.; Hagelueken, G.; Schiemann, O. Determination of nitroxide spin label conformations via PELDOR and X-ray crystallography. Phys. Chem. Chem. Phys. 2016, 18, 10428–10437. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Abdullin, D.; Schnakenburg, G.; Schiemann, O. Single and double nitroxide labeled bis(terpyridine)-copper(II): Influence of orientation selectivity and multispin effects on PELDOR and RIDME. Phys. Chem. Chem. Phys. 2016, 18, 9262–9271. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu-Aruxandei, D.; Petrovic-Stojanovska, B.; Schiemann, O.; Naismith, J.H.; White, M.F. Taking a molecular motor for a spin: Helicase mechanism studied by spin labeling and PELDOR. Nucl. Acids Res. 2016, 44, 954–968. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, H.; Schiemann, O. Molecular spins in biological systems. In Electron Spin Resonance (ESR) Based Quantum Computing; Takui, T., Berliner, L., Hanson, G., Eds.; Springer: New York, NY, USA, 2016; pp. 51–77. [Google Scholar]
- Hagelueken, G.; Abdullin, D.; Schiemann, O. Chapter twenty-two—Mtsslsuite: Probing biomolecular conformation by spin-labeling studies. In Methods in Enzymology; Qin, P.Z., Warncke, K., Eds.; Academic Press: Cambridge, MA, USA, 2015; Volume 563, pp. 595–622. [Google Scholar]
- Abdullin, D.; Duthie, F.; Meyer, A.; Müller, E.S.; Hagelueken, G.; Schiemann, O. Comparison of PELDOR and RIDME for distance measurements between nitroxides and low-spin Fe(III) ions. J. Phys. Chem. B 2015, 119, 13534–13542. [Google Scholar] [CrossRef] [PubMed]
- Hagelueken, G.; Duthie, F.G.; Florin, N.; Schubert, E.; Schiemann, O. Expression, purification and spin labelling of the ferrous iron transporter feob from Escherichia coli BL21 for EPR studies. Protein Expr. Purif. 2015, 114, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Abdullin, D.; Hagelueken, G.; Hunter, R.I.; Smith, G.M.; Schiemann, O. Geometric model-based fitting algorithm for orientation-selective PELDOR data. Mol. Phys. 2015, 113, 544–560. [Google Scholar] [CrossRef]
- Timachi, M.H.; Hutter, C.A.J.; Hohl, M.; Assafa, T.; Böhm, S.; Mittal, A.; Seeger, M.A.; Bordignon, E. Exploring conformational equilibria of a heterodimeric abc transporter. eLife 2017, 6, e20236. [Google Scholar] [CrossRef] [PubMed]
- Celia, H.; Noinaj, N.; Zakharov, S.D.; Bordignon, E.; Botos, I.; Santamaria, M.; Barnard, T.J.; Cramer, W.A.; Lloubes, R.; Buchanan, S.K. Structural insight into the role of the ton complex in energy transduction. Nature 2016, 538, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Bleicken, S.; Jeschke, G.; Stegmueller, C.; Salvador-Gallego, R.; García-Sáez, A.J.; Bordignon, E. Structural model of active bax at the membrane. Mol. Cell 2014, 56, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Hohl, M.; Hürlimann, L.M.; Böhm, S.; Schöppe, J.; Grütter, M.G.; Bordignon, E.; Seeger, M.A. Structural basis for allosteric cross-talk between the asymmetric nucleotide binding sites of a heterodimeric ABC exporter. Proc. Natl. Acad. Sci. USA 2014, 111, 11025–11030. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Liou, S.-H.; Khadka, N.; Jenney, F.E.; Goodin, D.B.; Seefeldt, L.C.; Adams, M.W.W.; Cramer, S.P.; Larsen, D.S. Cluster-dependent charge-transfer dynamics in iron–sulfur proteins. Biochemistry 2018, 57, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Liou, S.-H.; Enkin, N.; Tkach, I.; Bennati, M. Photo-induced radical polarization and liquid-state dynamic nuclear polarization using fullerene nitroxide derivatives. Phys. Chem. Chem. Phys. 2017, 19, 31823–31829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liou, S.-H.; Myers, W.K.; Oswald, J.D.; Britt, R.D.; Goodin, D.B. Putidaredoxin binds to the same site on cytochrome P450cam in the open and closed conformation. Biochemistry 2017, 56, 4371–4378. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Levien, M.; Karschin, N.; Parigi, G.; Luchinat, C.; Bennati, M. One-thousand-fold enhancement of high field liquid nuclear magnetic resonance signals at room temperature. Nat. Chem. 2017, 9, 676–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzato, R.; Bennati, M. Cross-polarization electron-nuclear double resonance spectroscopy. ChemPhysChem 2015, 16, 3769–3773. [Google Scholar] [CrossRef] [PubMed]
- Kasanmascheff, M.; Lee, W.; Nick, T.U.; Stubbe, J.; Bennati, M. Radical transfer in E. coli ribonucleotide reductase: A NH2Y731/R411A-α mutant unmasks a new conformation of the pathway residue 731. Chem. Sci. 2016, 7, 2170–2178. [Google Scholar] [CrossRef] [PubMed]
- Bährle, C.; Nick, T.U.; Bennati, M.; Jeschke, G.; Vogel, F. High-field electron paramagnetic resonance and density functional theory study of stable organic radicals in lignin: Influence of the extraction process, botanical origin, and protonation reactions on the radical G tensor. J. Phys. Chem. A 2015, 119, 6475–6482. [Google Scholar] [CrossRef] [PubMed]
- Nick, T.U.; Lee, W.; Koßmann, S.; Neese, F.; Stubbe, J.; Bennati, M. Hydrogen bond network between amino acid radical intermediates on the proton-coupled electron transfer pathway of E. coli α2 ribonucleotide reductase. J. Am. Chem. Soc. 2015, 137, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Schlee, S.; Klein, T.; Schumacher, M.; Nazet, J.; Merkl, R.; Steinhoff, H.-J.; Sterner, R. Relationship of catalysis and active site loop dynamics in the (βα)8-barrel enzyme indole-3-glycerol phosphate synthase. Biochemistry 2018, 57, 3265–3277. [Google Scholar] [CrossRef] [PubMed]
- Meiners, A.; Meyer-Ács, M.; Bondarenko, E.; Steinhoff, H.-J.; Mittmann, K. Conjugation of spin labeled proteins to fluorescent quantum dots∗. Mater. Today 2017, 4, S180–S187. [Google Scholar] [CrossRef]
- Kathiresan, M.; Steinhoff, H.-J.; Walder, L. Tempo-labeled viologen dendrimers: Synthesis, characterization, and preliminary distance measurements. Macromol. Chem. Phys. 2017, 218, 1700142. [Google Scholar] [CrossRef]
- Diskowski, M.; Mehdipour, A.R.; Wunnicke, D.; Mills, D.J.; Mikusevic, V.; Bärland, N.; Hoffmann, J.; Morgner, N.; Steinhoff, H.-J.; Hummer, G.; et al. Helical jackknives control the gates of the double-pore K(+) uptake system ktrab. eLife 2017, 6, e24303. [Google Scholar] [CrossRef] [PubMed]
- Orekhov, P.; Bothe, A.; Steinhoff, H.-J.; Shaitan, K.V.; Raunser, S.; Fotiadis, D.; Schlesinger, R.; Klare, J.P.; Engelhard, M. Sensory rhodopsin I and sensory rhodopsin ii form trimers of dimers in complex with their cognate transducers. Photochem. Photobiol. 2017, 93, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Bagrov, D.V.; Voskoboynikova, N.; Armeev, G.A.; Mosslehy, W.; Gluhov, G.S.; Ismagulova, T.T.; Mulkidjanian, A.Y.; Kirpichnikov, M.P.; Steinhoff, H.-J.; Shaitan, K.V. Characterization of lipodisc nanoparticles containing sensory rhodopsin ii and its cognate transducer from natronomonas pharaonis. Biophysics 2016, 61, 942–949. [Google Scholar] [CrossRef]
- Gruian, C.M.; Rickert, C.; Nicklisch, S.C.T.; Vanea, E.; Steinhoff, H.-J.; Simon, S. Conformational changes and competitive adsorption between serum albumin and hemoglobin on bioceramic substrates. ChemPhysChem 2017, 18, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Kucher, S.; Korneev, S.; Tyagi, S.; Apfelbaum, R.; Grohmann, D.; Lemke, E.A.; Klare, J.P.; Steinhoff, H.-J.; Klose, D. Orthogonal spin labeling using click chemistry for in vitro and in vivo applications. J. Magn. Reson. 2017, 275, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Matthies, M.; Glinka, K.; Theiling, M.; Hideg, K.; Steinhoff, H.-J. Kinetics of rapid covalent bond formation of aniline with humic acid: Esr investigations with nitroxide spin labels. Appl. Magn. Reson. 2016, 47, 627–641. [Google Scholar] [CrossRef]
- Gölz, J.P.; Bockelmann, S.; Mayer, K.; Steinhoff, H.-J.; Wieczorek, H.; Huss, M.; Klare, J.P.; Menche, D. EPR studies of V-ATPase with spin-labeled inhibitors DCC and archazolid: Interaction dynamics with proton translocating subunit C. ChemMedChem 2016, 11, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Wunnicke, D.; Ding, P.; Yang, H.; Seela, F.; Steinhoff, H.-J. DNA with parallel strand orientation: A nanometer distance study with spin labels in the watson–crick and the reverse watson–crick double helix. J. Phys. Chem. B 2015, 119, 13593–13599. [Google Scholar] [CrossRef] [PubMed]
- Ernst, F.G.M.; Rickert, C.; Bluschke, A.; Betat, H.; Steinhoff, H.-J.; Mörl, M. Domain movements during CCA-addition: A new function for motif C in the catalytic core of the human trna nucleotidyltransferases. RNA Biol. 2015, 12, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Gast, P.; Mance, D.; Zurlo, E.; Ivanov, K.L.; Baldus, M.; Huber, M. A tailored multi-frequency EPR approach to accurately determine the magnetic resonance parameters of dynamic nuclear polarization agents: Application to amupol. Phys. Chem. Chem. Phys. 2017, 19, 3777–3781. [Google Scholar] [CrossRef] [PubMed]
- Gerolin, M.; Zerbetto, M.; Moretto, A.; Formaggio, F.; Toniolo, C.; van Son, M.; Shabestari, M.H.; Huber, M.; Calligari, P.; Polimeno, A. Integrated computational approach to the electron paramagnetic resonance characterization of rigid 310-helical peptides with toac nitroxide spin labels. J. Phys. Chem. B 2017, 121, 4379–4387. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Schilderink, N.; Subramaniam, V.; Huber, M. Membrane binding of parkinson’s protein α-synuclein: Effect of phosphorylation at positions 87 and 129 by the s to d mutation approach. Isr. J. Chem. 2017, 57, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Zurlo, E.; Meeuwenoord, N.J.; Filippov, D.V.; Huber, M. Rigid spin labels for improved distance and dynamics in intrinsically disordered proteins and peptides. Biophys. J. 2017, 112, 316a. [Google Scholar] [CrossRef]
- Shabestari, M.H.; Meeuwenoord, N.J.; Filippov, D.V.; Huber, M. Interaction of the amyloid β peptide with sodium dodecyl sulfate as a membrane-mimicking detergent. J. Biol. Phys. 2016, 42, 299–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schilder, J.; Liu, W.-M.; Kumar, P.; Overhand, M.; Huber, M.; Ubbink, M. Protein docking using an ensemble of spin labels optimized by intra-molecular paramagnetic relaxation enhancement. Phys. Chem. Chem. Phys. 2016, 18, 5729–5742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarpelli, F.; Arrieta, A.L.; Gast, P.; Groenen, E.J.J.; Milikisyants, S.; Murphy, M.E.P.; Huber, M. A single-crystal EPR study at 95 GHz of the type-2 copper site of nitrite reductase from alcaligenes faecalis. Appl. Magn. Reson. 2015, 46, 411–420. [Google Scholar] [CrossRef]
- van Son, M.; Lindhoud, S.; van der Wild, M.; van Mierlo, C.P.M.; Huber, M. Double electron–electron spin resonance tracks flavodoxin folding. J. Phys. Chem. B 2015, 119, 13507–13514. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Segers-Nolten, I.M.J.; Schilderink, N.; Subramaniam, V.; Huber, M. Parkinson’s protein α-synuclein binds efficiently and with a novel conformation to two natural membrane mimics. PLoS ONE 2015, 10, e0142795. [Google Scholar] [CrossRef] [PubMed]
- The magnetic field dependence of cross-effect dynamic nuclear polarization under magic angle spinning. J. Chem. Phys. 2015, 142, 234201. [CrossRef] [PubMed]
- Hashemi Shabestari, M.; Kumar, P.; Segers-Nolten, I.M.J.; Claessens, M.M.A.E.; van Rooijen, B.D.; Subramaniam, V.; Huber, M. Three long-range distance constraints and an approach towards a model for the α-synuclein-fibril fold. Appl. Magn. Reson. 2015, 46, 369–388. [Google Scholar] [CrossRef]
- Milov, A.D.; Tsvetkov, Y.D.; Raap, J.; De Zotti, M.; Formaggio, F.; Toniolo, C. Review conformation, self-aggregation, and membrane interaction of peptaibols as studied by pulsed electron double resonance spectroscopy. Pept. Sci. 2015, 106, 6–24. [Google Scholar] [CrossRef] [PubMed]
- Syryamina, V.N.; Samoilova, R.I.; Tsvetkov, Y.D.; Ischenko, A.V.; De Zotti, M.; Gobbo, M.; Toniolo, C.; Formaggio, F.; Dzuba, S.A. Peptides on the surface: Spin-label EPR and PELDOR study of adsorption of the antimicrobial peptides trichogin GA IV and ampullosporin a on the silica nanoparticles. Appl. Magn. Reson. 2016, 47, 309–320. [Google Scholar] [CrossRef]
- Milov, A.D.; Samoilova, R.I.; Tsvetkov, Y.D.; Peggion, C.; Formaggio, F.; Toniolo, C. Peptides on the surface. PELDOR data for spin-labeled alamethicin F50/5 analogues on organic sorbent. J. Phys. Chem. B 2014, 118, 7085–7090. [Google Scholar] [CrossRef] [PubMed]
- Milov, A.D.; Tsvetkov, Y.D.; Bortolus, M.; Maniero, A.L.; Gobbo, M.; Toniolo, C.; Formaggio, F. Synthesis and conformational properties of a toac doubly spin-labeled analog of the medium-length, membrane active peptaibiotic ampullosporin a as revealed by cd, fluorescence, and EPR spectroscopies. Pept. Sci. 2014, 102, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Lohan, S.B.; Saeidpour, S.; Solik, A.; Schanzer, S.; Richter, H.; Dong, P.; Darvin, M.E.; Bodmeier, R.; Patzelt, A.; Zoubari, G.; et al. Investigation of the cutaneous penetration behavior of dexamethasone loaded to nano-sized lipid particles by EPR spectroscopy, and confocal raman and laser scanning microscopy. Eur. J. Pharm. Biopharm. 2017, 116, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Engelhard, C.; Diensthuber, R.P.; Möglich, A.; Bittl, R. Blue-light reception through quaternary transitions. Sci. Rep. 2017, 7, 1385. [Google Scholar] [CrossRef] [PubMed]
- Consentius, P.; Loll, B.; Gohlke, U.; Alings, C.; Müller, C.; Müller, R.; Teutloff, C.; Heinemann, U.; Kaupp, M.; Wahl, M.C.; et al. Internal dynamics of the 3-pyrroline-n-oxide ring in spin-labeled proteins. J. Phys. Chem. Lett. 2017, 8, 1113–1117. [Google Scholar] [CrossRef] [PubMed]
- Saeidpour, S.; Lohan, S.B.; Solik, A.; Paul, V.; Bodmeier, R.; Zoubari, G.; Unbehauen, M.; Haag, R.; Bittl, R.; Meinke, M.C.; et al. Drug distribution in nanostructured lipid particles. Eur. J. Pharm. Biopharm. 2017, 110, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Yee, E.F.; Diensthuber, R.P.; Vaidya, A.T.; Borbat, P.P.; Engelhard, C.; Freed, J.H.; Bittl, R.; Möglich, A.; Crane, B.R. Signal transduction in light–oxygen–voltage receptors lacking the adduct-forming cysteine residue. Nat. Commun. 2015, 6, 10079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hahn, A.; Engelhard, C.; Reschke, S.; Teutloff, C.; Bittl, R.; Leimkühler, S.; Risse, T. Structural insights into the incorporation of the mo cofactor into sulfite oxidase from site-directed spin labeling. Angew. Chem. Int. Ed. 2015, 54, 11865–11869. [Google Scholar] [CrossRef] [PubMed]
- Freed, J.H. New technologies in electron spin resonance. Annu. Rev. Phys. Chem. 2000, 51, 655–689. [Google Scholar] [CrossRef] [PubMed]
- Polnaszek, C.F.; Freed, J.H. Electron-spin resonance studies of anisotropic ordering, spin relaxation, and slow tumbling in liquid-crystalline solvents. J. Phys. Chem. 1975, 79, 2283–2306. [Google Scholar] [CrossRef]
- Freed, J.H. Stochastic-molecular theory of spin-relaxation for liquid-crystals. J. Chem. Phys. 1977, 66, 4183–4199. [Google Scholar] [CrossRef]
- Polimeno, A.; Freed, J.H. Slow motional ESR in complex fluids—The slowly relaxing local-structure model of solvent cage effects. J. Phys. Chem. 1995, 99, 10995–11006. [Google Scholar] [CrossRef]
- Liang, Z.C.; Freed, J.H. An assessment of the applicability of multifrequency ESR to study the complex dynamics of biomolecules. J. Phys. Chem. B 1999, 103, 6384–6396. [Google Scholar] [CrossRef]
- Tugarinov, V.; Liang, Z.C.; Shapiro, Y.E.; Freed, J.H.; Meirovitch, E. A structural mode-coupling approach to n-15 NMR relaxation in proteins. J. Am. Chem. Soc. 2001, 123, 3055–3063. [Google Scholar] [CrossRef] [PubMed]
- Meirovitch, E.; Shapiro, Y.E.; Polimeno, A.; Freed, J.H. Protein dynamics from NMR: The slowly relaxing local structure analysis compared with model-free analysis. J. Phys. Chem. A 2006, 110, 8366–8396. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.K.; Freed, J.H. Molecular motions. In Multifrequency Electron Paramagnetic Resonance; Misra, S.K., Ed.; Wiley-VCH: Berlin, Germany, 2011; pp. 497–544. [Google Scholar]
- Polimeno, A.; Freed, J.H. A many-body stochastic approach to rotational motions in liquids. In Advances in Chemical Physics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 89–206. [Google Scholar]
- Sezer, D.; Freed, J.H.; Roux, B. Multifrequency electron spin resonance spectra of a spin-labeled protein calculated from molecular dynamics simulations. J. Am. Chem. Soc. 2009, 131, 2597–2605. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.C.; Freed, J.H.; Keyes, R.S.; Bobst, A.M. An electron spin resonance study of DNA dynamics using the slowly relaxing local structure model. J. Phys. Chem. B 2000, 104, 5372–5381. [Google Scholar] [CrossRef]
- Liang, Z.C.; Lou, Y.; Freed, J.H.; Columbus, L.; Hubbell, W.L. A multifrequency electron spin resonance study of t4 lysozyme dynamics using the slowly relaxing local structure model. J. Phys. Chem. B 2004, 108, 17649–17659. [Google Scholar] [CrossRef]
- Cornish, V.W.; Benson, D.R.; Altenbach, C.A.; Hideg, K.; Hubbell, W.L.; Schultz, P.G. Site-specific incorporation of biophysical probes into proteins. Proc. Natl. Acad. Sci. USA 1994, 91, 2910–2914. [Google Scholar] [CrossRef] [PubMed]
- Shafer, A.M.; Kalai, T.; Liu, S.Q.B.; Hideg, K.; Voss, J.C. Site-specific insertion of spin-labeled l-amino acids in xenopus oocytes. Biochemistry 2004, 43, 8470–8482. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, H.J. Methods for study of protein dynamics and protein-protein interaction in protein-ubiquitination by electron paramagnetic resonance spectroscopy. Front. Biosci. 2002, 7, C97–C110. [Google Scholar] [CrossRef] [PubMed]
- Hubbell, W.L.; Cafiso, D.S.; Altenbach, C. Identifying conformational changes with site-directed spin labeling. Nat. Struct. Mol. Biol. 2000, 7, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Hubbell, W.L.; Gross, A.; Langen, R.; Lietzow, M.A. Recent advances in site-directed spin labeling of proteins. Curr. Opin. Struct. Biol. 1998, 8, 649–656. [Google Scholar] [CrossRef]
- Hubbell, W.L.; McHaourab, H.S.; Altenbach, C.; Lietzow, M.A. Watching proteins move using site-directed spin labeling. Structure 1996, 4, 779–783. [Google Scholar] [CrossRef] [Green Version]
- Möbius, K.; Savitsky, A.; Wegener, C.; Plato, M.; Fuchs, M.; Schnegg, A.; Dubinskii, A.A.; Grishin, Y.A.; Grigor’ev, I.A.; Kuhn, M.; et al. Combining high-field EPR with site-directed spin labeling reveals unique information on proteins in action. Magn. Reson. Chem. 2005, 43, S4–S19. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, H.-J.; Savitsky, A.; Wegener, C.; Pfeiffer, M.; Plato, M.; Möbius, K. High-field EPR studies of the structure and conformational changes of site directed spin labeled bacteriorhodopsin. Biochim. Biophys. Acta 2000, 1457, 253–262. [Google Scholar] [CrossRef]
- Wegener, C.; Savitsky, A.; Pfeiffer, M.; Möbius, K.; Steinhoff, H.-J. High-field EPR-detected shifts of magnetic tensor components of spin label side chains reveal protein conformational changes: The proton entrance channel of bacteriorhodopsin. Appl. Magn. Reson. 2001, 21, 441–450. [Google Scholar] [CrossRef]
- Plato, M.; Steinhoff, H.-J.; Wegener, C.; Törring, J.T.; Savitsky, A.; Möbius, K. Molecular orbital study of polarity and hydrogen bonding effects on the g and hyperfine tensors of site directed no spin labeled bacteriorhodopsin. Mol. Phys. 2002, 100, 3711–3721. [Google Scholar] [CrossRef]
- Earle, K.A.; Smirnov, A.I. High field ESR: Applications to protein structure and dynamics, biological magnetic resonance. In Very High Frequency (VHF) ESR/EPR; Grinberg, O., Berliner, L.J., Eds.; Kluwer/Plenum Publishers: New York, NY, USA, 2004; Volume 22, pp. 96–144. [Google Scholar]
- Livshits, V.A.; Marsh, D. Hf-EPR spectra of spin labels in membranes, biological magnetic resonance. In Very High Frequency (VHF) ESR/EPR; Grinberg, O., Berliner, L.J., Eds.; Kluwer/Plenum Publishers: New York, NY, USA, 2004; Volume 22, pp. 431–465. [Google Scholar]
- Barnes, J.P.; Liang, Z.C.; McHaourab, H.S.; Freed, J.H.; Hubbell, W.L. A multifrequency electron spin resonance study of t4 lysozyme dynamics. Biophys. J. 1999, 76, 3298–3306. [Google Scholar] [CrossRef]
- Banham, J.E.; Timmel, C.R.; Abbott, R.J.M.; Lea, S.M.; Jeschke, G. The characterization of weak protein-protein interactions: Evidence from deer for the trimerization of a von willebrand factor a domain in solution. Angew. Chem. Int. Ed. 2006, 45, 1058–1061. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.W.; Borbat, P.P.; Freed, J.H. Maximum entropy: A complement to tikhonov regularization for determination of pair distance distributions by pulsed ESR. J. Magn. Reson. 2005, 177, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Bennati, M.; Robblee, J.H.; Mugnaini, V.; Stubbe, J.; Freed, J.H.; Borbat, P. EPR distance measurements support a model for long-range radical initiation in e.Coli ribonucleotide reductase. J. Am. Chem. Soc. 2005, 127, 15014–15015. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Borbat, P.P.; Gonzalez-Bonet, G.; Bhatnagar, J.; Pollard, A.M.; Freed, J.H.; Bilwes, A.M.; Crane, B.R. Reconstruction of the chemotaxis receptor-kinase assembly. Nat. Struct. Mol. Biol. 2006, 13, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Daldrop, J.O.; Saita, M.; Heyden, M.; Lorenz-Fonfria, V.A.; Heberle, J.; Netz, R.R. Orientation of non-spherical protonated water clusters revealed by infrared absorption dichroism. Nat. Commun. 2018, 9, 311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luecke, H.; Schobert, B.; Richter, H.-T.; Cartailler, J.-P.; Lanyi, J.K. Structure of bacteriorhodopsin at 1.55 resolution. J. Mol. Biol. 1999, 291, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Váró, G.; Lanyi, J.K. Kinetic and spectroscopic evidence for an irreversible step between dEPRotonation and rEPRotonation of the schiff base in the bacteriorhodopsin photocycle. Biochemistry 1991, 30, 5008–5015. [Google Scholar] [CrossRef] [PubMed]
- Druckmann, S.; Friedmann, N.; Lanyi, J.K.; Needleman, R.; Ottolenghi, M.; Shewes, M. The back photoreaction of the m intermediate in the photocycle of bacteriorhodopsin: Mechanism and evidence for two m species. Photochem. Photobiol. 1992, 56, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Hessling, B.; Herbst, J.; Rammelsberg, R.; Gerwert, K. Fourier transform infrared double-flash experiments resolve bacteriorhodopsin’s M1 to M2 transition. Biophys. J. 1997, 73, 2071–2080. [Google Scholar] [CrossRef]
- Haupts, U.; Tittor, J.; Oesterhelt, D. Closing in on bacteriorhodopsin: Progress in understanding the molecule. Annu. Rev. Biophys. Biomol. Struct. 1999, 28, 367–399. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Henderson, R. Molecular mechanism of vectorial proton translocation by bacteriorhodopsin. Nature 2000, 406, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, M.; Rink, T.; Gerwert, K.; Oesterhelt, D.; Steinhoff, H.-J. Site-directed spin labeling reveals the orientation of the amino acid side chains in the e-f loop of bacteriorhodopsin. J. Mol. Biol. 1999, 287, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Radzwill, N.; Gerwert, K.; Steinhoff, H.-J. Time-resolved detection of transient movement of helices f and g in doubly spin-labeled bacteriorhodopsin. Biophys. J. 2001, 80, 2856–2866. [Google Scholar] [CrossRef]
- Stoll, S. High-field EPR of bioorganic radicals. In Electron Paramagnetic Resonance; The Royal Society of Chemistry: London, UK, 2011; Volume 22, pp. 107–154. [Google Scholar]
- Möbius, K. Primary processes in photosynthesis: What do we learn from high-field EPR spectroscopy? Chem. Soc. Rev. 2000, 29, 129–139. [Google Scholar] [CrossRef]
- Riedi, P.C.; Smith, G.M. Progress in high-field EPR. In Electron Paramagnetic Resonance; Gilbert, B.C., Davies, M.J., Murphy, D.M., Eds.; Royal Society of Chemistry: Cambridge, UK, 2002; Volume 18, pp. 254–303. [Google Scholar]
- Smirnov, A.I. Spin-labeling in high-field EPR. In Electron Paramagnetic Resonance; Gilbert, B.C., Davies, M.J., Murphy, D.M., Eds.; Royal Society of Chemistry: Cambridge, UK, 2002; Volume 18, pp. 109–136. [Google Scholar]
- Smith, G.M.; Riedi, P.C. Progress in high-field EPR. In Electron Paramagnetic Resonance; Gilbert, B.C., Davies, M.J., McLauchlan, K.A., Eds.; Royal Society of Chemistry: Cambridge, UK, 2000; Volume 17, pp. 164–204. [Google Scholar]
- Freed, J.H. The development of high-field/high-frequency ESR, biological magnetic resonance. In Very High Frequency (VHF) ESR/EPR; Grinberg, O., Berliner, L.J., Eds.; Kluwer/Plenum Publishers: New York, NY, USA, 2004; Volume 22, pp. 19–43. [Google Scholar]
- Gulla, A.F.; Budil, D.E. Engineering and design concepts for quasioptical high-field electron paramagnetic resonance. Concept. Magn. Reson. B 2004, 22B, 15–36. [Google Scholar] [CrossRef]
- Marsh, D.; Kurad, D.; Livshits, V.A. High-field electron spin resonance of spin labels in membranes. Chem. Phys. Lipids 2002, 116, 93–114. [Google Scholar] [CrossRef]
- Möbius, K.; Savitsky, A.; Schnegg, A.; Plato, M.; Fuchs, M. High-field EPR spectroscopy applied to biological systems: Characterization of molecular switches for electron and ion transfer. Phys. Chem. Chem. Phys. 2005, 7, 19–42. [Google Scholar] [CrossRef] [PubMed]
- Prisner, T. Pulsed high-frequency EPR. In Very High Frequency (VHF) ESR/EPR, Biological Magnetic Resonance; Grinberg, O., Berliner, L.J., Eds.; Kluwer/Plenum Publishers: New York, NY, USA, 2004; Volume 22, pp. 45–93. [Google Scholar]
- Chapman, H.N.; Caleman, C.; Timneanu, N. Diffraction before destruction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369. [Google Scholar] [CrossRef] [PubMed]
- Nogly, P.; Weinert, T.; James, D.; Carbajo, S.; Ozerov, D.; Furrer, A.; Gashi, D.; Borin, V.; Skopintsev, P.; Jaeger, K.; et al. Retinal isomerization in bacteriorhodopsin captured by a femtosecond X-ray laser. Science 2018, 361, eaat0094. [Google Scholar] [CrossRef] [PubMed]
- Johansson, L.C.; Arnlund, D.; Katona, G.; White, T.A.; Barty, A.; DePonte, D.P.; Shoeman, R.L.; Wickstrand, C.; Sharma, A.; Williams, G.J.; et al. Structure of a photosynthetic reaction centre determined by serial femtosecond crystallography. Nat. Commun. 2013, 4, 2911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kern, J.; Alonso-Mori, R.; Hellmich, J.; Tran, R.; Hattne, J.; Laksmono, H.; Glöckner, C.; Echols, N.; Sierra, R.G.; Sellberg, J.; et al. Room temperature femtosecond X-ray diffraction of photosystem II microcrystals. Proc. Natl. Acad. Sci. USA 2012, 109, 9721–9726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kern, J.; Alonso-Mori, R.; Tran, R.; Hattne, J.; Gildea, R.J.; Echols, N.; Glöckner, C.; Hellmich, J.; Laksmono, H.; Sierra, R.G.; et al. Simultaneous femtosecond X-ray spectroscopy and diffraction of photosystem II at room temperature. Science 2013, 340, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Kern, J.; Tran, R.; Alonso-Mori, R.; Koroidov, S.; Echols, N.; Hattne, J.; Ibrahim, M.; Gul, S.; Laksmono, H.; Sierra, R.G.; et al. Taking snapshots of photosynthetic water oxidation using femtosecond X-ray diffraction and spectroscopy. Nat. Commun. 2014, 5, 4371. [Google Scholar] [CrossRef] [PubMed]
- Fuller, F.D.; Gul, S.; Chatterjee, R.; Burgie, E.S.; Young, I.D.; Lebrette, H.; Srinivas, V.; Brewster, A.S.; Michels-Clark, T.; Clinger, J.A.; et al. Drop-on-demand sample delivery for studying biocatalysts in action at X-ray free-electron lasers. Nat. Methods 2017, 14, 443–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kupitz, C.; Basu, S.; Grotjohann, I.; Fromme, R.; Zatsepin, N.A.; Rendek, K.N.; Hunter, M.S.; Shoeman, R.L.; White, T.A.; Wang, D.; et al. Serial time-resolved crystallography of photosystem II using a femtosecond X-ray laser. Nature 2014, 513, 261–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suga, M.; Akita, F.; Hirata, K.; Ueno, G.; Murakami, H.; Nakajima, Y.; Shimizu, T.; Yamashita, K.; Yamamoto, M.; Ago, H.; et al. Native structure of photosystem II at 1.95 Å resolution viewed by femtosecond X-ray pulses. Nature 2014, 517, 99–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suga, M.; Akita, F.; Sugahara, M.; Kubo, M.; Nakajima, Y.; Nakane, T.; Yamashita, K.; Umena, Y.; Nakabayashi, M.; Yamane, T.; et al. Light-induced structural changes and the site of O=O bond formation in PSII caught by XFEL. Nature 2017, 543, 131–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, D.T.; Ma, Z.D.; Meade, T.J.; Goldfarb, D.; Han, S.G.; Sherwin, M.S. Extending the distance range accessed with continuous wave EPR with gd3+ spin probes at high magnetic fields. Phys. Chem. Chem. Phys. 2013, 15, 11313–11326. [Google Scholar] [CrossRef] [PubMed]
- Dalaloyan, A.; Qi, M.; Ruthstein, S.; Vega, S.; Godt, A.; Feintuch, A.; Goldfarb, D. Gd(III)-Gd(III) EPR distance measurements—The range of accessible distances and the impact of zero field splitting (vol. 17, pg 18464, 2015). Phys. Chem. Chem. Phys. 2016, 18, 18614. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Gaidamakova, E.K.; Grichenko, O.; Matrosova, V.Y.; Hoeke, V.; Klimenkova, P.; Conze, I.H.; Volpe, R.P.; Tkavc, R.; Gostincar, C.; et al. Across the tree of life, radiation resistance is governed by antioxidant Mn2+, gauged by paramagnetic resonance. Proc. Natl. Acad. Sci. USA 2017, 114, E9253–E9260. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.F.; Crowe, L.M.; Crowe, J.H. Stabilization of phosphofructokinase with sugars during freeze-drying—Characterization of enhanced protection in the presence of divalent-cations. Biochim. Biophys. Acta 1987, 923, 109–115. [Google Scholar] [CrossRef]
- Lopez-Diez, E.C.; Bone, S. The interaction of trypsin with trehalose: An investigation of protein preservation mechanisms. Biochim. Biophys. Acta 2004, 1673, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.K.; Roy, I. Effect of trehalose on protein structure. Prot. Sci. 2009, 18, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Ohtake, S.; Wang, Y.J. Trehalose: Current use and future applications. J. Pharm. Sci. 2011, 100, 2020–2053. [Google Scholar] [CrossRef] [PubMed]
- Crowe, J.H.; Carpenter, J.F.; Crowe, L.M. The role of vitrification in anhydrobiosis. Ann. Rev. Physiol. 1998, 60, 73–103. [Google Scholar] [CrossRef] [PubMed]
- Crowe, L.M. Lessons from nature: The role of sugars in anhydrobiosis. Comp. Biochem. Physiol. 2002, 131, 505–513. [Google Scholar] [CrossRef]
- Simperler, A.; Kornherr, A.; Chopra, R.; Bonnet, P.A.; Jones, W.; Motherwell, W.D.S.; Zifferer, G. Glass transition temperature of glucose, sucrose, and trehalose: An experimental and in silico study. J. Phys. Chem. B 2006, 110, 19678–19684. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Ambastha, V.; Levine, A.; Sopory, S.K.; Yadava, P.K.; Tripathy, B.C.; Tiwari, B.S. Anhydrobiosis and programmed cell death in plants: Commonalities and differences. Curr. Plant Biol. 2015, 2, 12–20. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Zafar, S.A.; Hameed, A.; Nawaz, M.A.; Wei, M.A.; Noor, M.A.; Hussain, M.; Mehboob ur, R. Mechanisms and molecular approaches for heat tolerance in rice (Oryza sativa L.) under climate change scenario. J. Integr. Agric. 2018, 17, 726–738. [Google Scholar] [CrossRef]
- Nalepa, A.; Malferrari, M.; Lubitz, W.; Venturoli, G.; Möbius, K.; Savitsky, A. Local water sensing: Water exchange in bacterial photosynthetic reaction centers embedded in a trehalose glass studied using multiresonance EPR. Phys. Chem. Chem. Phys. 2017, 19, 28388–28400. [Google Scholar] [CrossRef] [PubMed]
- Shelaev, I.; Gorka, M.; Savitsky, A.; Kurashov, V.; Mamedov, M.; Gostev, F.; Möbius, K.; Nadtochenko, V.; Golbeck, J.; Semenov, A. Effect of dehydrated trehalose matrix on the kinetics of forward electron transfer reactions in photosystem I. Z. Phys. Chem. 2017, 231, 325–345. [Google Scholar] [CrossRef]
- Malferrari, M.; Savitsky, A.; Mamedov, M.D.; Milanovsky, G.E.; Lubitz, W.; Möbius, K.; Semenov, A.Y.; Venturoli, G. Trehalose matrix effects on charge-recombination kinetics in photosystem I of oxygenic photosynthesis at different dehydration levels. Biochim. Biophys. Acta 2016, 1857, 1440–1454. [Google Scholar] [CrossRef] [PubMed]
- Amesz, J.; Hoff, A.J. Biophysical Techniques in Photosynthesis; Springer: Dordrecht, The Netherlands, 1996. [Google Scholar]
- Wydrzynski, T.J.; Satoh, K. Photosystem II, the light-driven water: Plastoquinone oxidoreductase. In Advances in Photosynthesis and Respiration; Wydrzynski, T.J., Satoh, K., Eds.; Springer: Dordrecht, The Netherlands, 2005. [Google Scholar]
- Ort, D.; Yocum, C.; Heichel, I. Oxygenic Photosynthesis: The Light Reactions; Kluwer: Dordrecht, The Netherlands, 2004; Volume 4, pp. 1–9. [Google Scholar]
- Lubitz, W.; Reijerse, E.J.; Messinger, J. Solar water-splitting into h2 and o2: Design principles of photosystem ii and hydrogenases. Energy Environ. Sci. 2008, 1, 15–31. [Google Scholar] [CrossRef]
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thapper, A.; Styring, S.; Saraccao, G.; Rutherford, A.W.; Robert, B.; Magnuson, A.; Lubitz, W.; Llobet, A.; Kurz, P.; Holzwarth, A.R.; et al. Artificial photosynthesis for solar fuels—An evolving research field within ampea, a joint programme of the european energy research alliance. Green 2013, 3, 43–57. [Google Scholar] [CrossRef]
- Schlögl, R. Chemical Energy Storage; De Gruyter: Berlin, Germany, 2013; p. 479. [Google Scholar]
- Cox, N.; Pantazis, D.A.; Neese, F.; Lubitz, W. Artificial photosynthesis: Understanding water splitting in nature. Interface Focus 2015, 5, 20150009. [Google Scholar] [CrossRef] [PubMed]
- Barber, J. Photosynthetic water splitting provides a blueprint for artificial leaf technology. Joule 2017, 1, 5–9. [Google Scholar] [CrossRef]
- Florent, M.; Kaminker, I.; Nagarajan, V.; Goldfarb, D. Determination of the 14N quadrupole coupling constant of nitroxide spin probes by W-band eldor-detected NMR. J. Magn. Reson. 2011, 210, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Nalepa, A.; Möbius, K.; Lubitz, W.; Savitsky, A. High-field eldor-detected NMR study of a nitroxide radical in disordered solids: Towards characterization of heterogeneity of microenvironments in spin-labeled systems. J. Magn. Reson. 2014, 242, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Nalepa, A.; Möbius, K.; Plato, M.; Lubitz, W.; Savitsky, A. Nitroxide spin-labels—Magnetic parameters and hydrogen bond formation. A high-field EPR and EDNMR study. Appl. Magn. Reson. 2018, in press. [Google Scholar] [CrossRef]
- Kaminker, I.; Wilson, T.D.; Savelieff, M.G.; Hovav, Y.; Zimmermann, H.; Lu, Y.; Goldfarb, D. Correlating nuclear frequencies by two-dimensional eldor-detected NMR spectroscopy. J. Am. Chem. Soc. 2014, 240, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Van Landeghem, M.; Maes, W.; Goovaerts, E.; Van Doorslaer, S. Disentangling overlapping high-field EPR spectra of organic radicals: Identification of light-induced polarons in the record fullerene-free solar cell blend pbdb-t:Itic. J. Magn. Reson. 2018, 288, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gast, P.; Herbonnet, R.T.L.; Klare, J.; Nalepa, A.; Rickert, C.; Stellinga, D.; Urban, L.; Möbius, K.; Savitsky, A.; Steinhoff, H.J.; et al. Hydrogen bonding of nitroxide spin labels in membrane proteins. Phys. Chem. Chem. Phys. 2014, 16, 15910–15916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bordignon, E.; Nalepa, A.I.; Savitsky, A.; Braun, L.; Jeschke, G. Changes in the microenvironment of nitroxide radicals around the glass transition temperature. J. Phys. Chem. B 2015, 119, 13797–13806. [Google Scholar] [CrossRef] [PubMed]
- Cox, N.; Nalepa, A.; Lubitz, W.; Savitsky, A. Eldor-detected NMR: A general and robust method for electron-nuclear hyperfine spectroscopy? J. Magn. Reson. 2017, 280, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Potapov, A.; Lancaster, K.M.; Richards, J.H.; Gray, H.B.; Goldfarb, D. Spin delocalization over type zero copper. Inorg. Chem. 2012, 51, 4066–4075. [Google Scholar] [CrossRef] [PubMed]
- Nagy, N.V.; Doorslaer, S.V.; Szabó-Plánka, T.; Rompaey, S.V.; Hamza, A.; Fülöp, F.; Tóth, G.K.; Rockenbauer, A. Copper(II)-binding ability of stereoisomeric cis- and trans-2-aminocyclohexanecarboxylic acid–l-phenylalanine dipeptides. A combined cw/pulsed EPR and DFT study. Inorg. Chem. 2012, 51, 1386–1399. [Google Scholar] [CrossRef] [PubMed]
- Bruch, E.M.; Warner, M.T.; Thomine, S.; Tabares, L.C.; Un, S. Pulse electron double resonance detected multinuclear NMR spectra of distant and low sensitivity nuclei and its application to the structure of Mn(II) centers in organisms. J. Phys. Chem. B 2015, 119, 13515–13523. [Google Scholar] [CrossRef] [PubMed]
- Bruch, E.M.; Thomine, S.; Tabares, L.C.; Un, S. Variations in Mn(II) speciation among organisms: What makes d. Radiodurans different. Metallomics 2015, 7, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Fittipaldi, M.; García-Rubio, I.; Trandafir, F.; Gromov, I.; Schweiger, A.; Bouwen, A.; Van Doorslaer, S. A multi-frequency pulse EPR and ENDOR approach to study strongly coupled nuclei in frozen solutions of high-spin ferric heme proteins. J. Phys. Chem. B 2008, 112, 3859–3870. [Google Scholar] [CrossRef] [PubMed]
- Kaminker, I.; Goldberg, H.; Neumann, R.; Goldfarb, D. High-field pulsed EPR spectroscopy for the speciation of the reduced [PV2MO10O40]6− polyoxometalate catalyst used in electron-transfer oxidations. Chem. Eur. J. 2010, 16, 10014–10020. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.L.; Raitsimring, A.M.; Astashkin, A.V.; Rajapakshe, A.; Johnson-Winters, K.; Arnold, A.R.; Potapov, A.; Goldfarb, D.; Enemark, J.H. Identity of the exchangeable sulfur-containing ligand at the Mo(V) center of R160Q human sulfite oxidase. Inorg. Chem. 2012, 51, 1408–1418. [Google Scholar] [CrossRef] [PubMed]
- Cox, N.; Lubitz, W.; Savitsky, A. W-band eldor-detected NMR (EDNMR) spectroscopy as a versatile technique for the characterization of transition metal-ligand interactions. Mol. Phys. 2013, 111, 2788–2808. [Google Scholar] [CrossRef]
- Zamani, S.; Meynen, V.; Hanu, A.-M.; Mertens, M.; Popovici, E.; Van Doorslaer, S.; Cool, P. Direct spectroscopic detection of framework-incorporated vanadium in mesoporous silica materials. Phys. Chem. Chem. Phys. 2009, 11, 5823–5832. [Google Scholar] [CrossRef] [PubMed]
- Mino, H.; Ono, T. Applications of pulsed eldor-detected NMR measurements to studies of photosystem II: Magnetic characterization of YD tyrosine radical and Mn2+ bound to the high-affinity site. Appl. Magn. Reson. 2003, 23, 571. [Google Scholar] [CrossRef]
- Litvinov, A.; Feintuch, A.; Un, S.; Goldfarb, D. Triple resonance EPR spectroscopy determines the mn2+ coordination to ATP. J. Magn. Reson. 2018, 294, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Marchiori, D.A.; Oyala, P.H.; Debus, R.J.; Stich, T.A.; Britt, R.D. Structural effects of ammonia binding to the Mn4CaO5 cluster of photosystem II. J. Phys. Chem. B 2018, 122, 1588–1599. [Google Scholar] [CrossRef] [PubMed]
- Barwinska-Sendra, A.; Basle, A.; Waldron, K.J.; Un, S. A charge polarization model for the metal-specific activity of superoxide dismutases. Phys. Chem. Chem. Phys. 2018, 20, 2363–2372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, M.R.; Mendelman, N.; Radoul, M.; Wilson, T.D.; Savelieff, M.G.; Zimmermann, H.; Kaminker, I.; Feintuch, A.; Lu, Y.; Goldfarb, D. Thiolate spin population of type I copper in azurin derived from S-33 hyperfine coupling. Inorg. Chem. 2017, 56, 6163–6174. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gong, Y.J.; Litvinov, A.; Liu, H.K.; Yang, F.; Su, X.C.; Goldfarb, D. Generic tags for Mn(II) and Gd(III) spin labels for distance measurements in proteins. Phys. Chem. Chem. Phys. 2017, 19, 26944–26956. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.I.; Suess, D.L.M.; Darago, L.E.; Oyala, P.H.; Levine, D.S.; Ziegler, M.S.; Britt, R.D.; Tilley, T.D. Manganese-cobalt oxido cubanes relevant to manganese-doped water oxidation catalysts. J. Am. Chem. Soc. 2017, 139, 5579–5587. [Google Scholar] [CrossRef] [PubMed]
- Sarma, B.B.; Carmieli, R.; Collauto, A.; Efremenko, I.; Martin, J.M.L.; Neumann, R. Electron transfer oxidation of benzene and aerobic oxidation to phenol. ACS Catal. 2016, 6, 6403–6407. [Google Scholar] [CrossRef]
- Aliabadi, A.; Zaripov, R.; Salikhov, K.; Voronkova, V.; Vavilova, E.; Abdulmalic, M.A.; Ruffer, T.; Buchner, B.; Kataev, V. Electron spin density on the n-donor atoms of Cu(II)-(bis)oxamidato complexes as probed by a pulse eldor detected NMR. J. Phys. Chem. B 2015, 119, 13762–13770. [Google Scholar] [CrossRef] [PubMed]
- Rapatskiy, L.; Ames, W.M.; Perez-Navarro, M.; Savitsky, A.; Griese, J.J.; Weyhermueller, T.; Shafaat, H.S.; Hogbom, M.; Neese, F.; Pantazis, D.A.; et al. Characterization of oxygen bridged manganese model complexes using multifrequency 17O-hyperfine EPR spectroscopies and density functional theory. J. Phys. Chem. B 2015, 119, 13904–13921. [Google Scholar] [CrossRef] [PubMed]
- Hetzke, T.; Bowen, A.M.; Prisner, T.F. Eldor-detected NMR at Q-band. Appl. Magn. Reson. 2017, 48, 1375–1397. [Google Scholar] [CrossRef]
- Goldfarb, D. Eldor-detected NMR. eMagRes 2017, 101–114. [Google Scholar] [CrossRef]
- Bordignon, E. EPR spectroscopy of nitroxide spin probes. eMagRes 2017, 6, 235–253. [Google Scholar]
- Clegg, J.S. Cryptobiosis—A peculiar state of biological organization. Comp. Biochem. Phys. B 2001, 128, 613–624. [Google Scholar] [CrossRef]
- Olsson, C.; Jansson, H.; Swenson, J. The role of trehalose for the stabilization of proteins. J. Phys. Chem. B 2016, 120, 4723–4731. [Google Scholar] [CrossRef] [PubMed]
- Shukla, N.; Pomarico, E.; Chen, L.; Chergui, M.; Othon, C.M. Retardation of bulk water dynamics by disaccharide osmolytes. J. Phys. Chem. B 2016, 120, 9477–9483. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.F.; Crowe, J.H. An infrared spectroscopic study of the interactions of carbohydrates with dried proteins. Biochemistry 1989, 28, 3916–3922. [Google Scholar] [CrossRef] [PubMed]
- Belton, P.S.; Gil, A.M. Ir and raman-spectroscopic studies of the interaction of trehalose with hen egg-white lysozyme. Biopolymers 1994, 34, 957–961. [Google Scholar] [CrossRef] [PubMed]
- Sampedro, J.G.; Uribe, S. Trehalose-enzyme interactions result in structure stabilization and activity inhibition. The role of viscosity. Mol. Cell. Biochem. 2004, 256, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Cordone, L.; Cottone, G.; Giuffrida, S.; Palazzo, G.; Venturoli, G.; Viappiani, C. Internal dynamics and protein-matrix coupling in trehalose-coated proteins. Biochim. Biophys. Acta 2005, 1749, 252–281. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, G.; Mallardi, A.; Hochkoeppler, A.; Cordone, L.; Venturoli, G. Electron transfer kinetics in photosynthetic reaction centers embedded in trehalose glasses: Trapping of conformational substates at room temperature. Biophys. J. 2002, 82, 558–568. [Google Scholar] [CrossRef]
- Francia, F.; Dezi, M.; Mallardi, A.; Palazzo, G.; Cordone, L.; Venturoli, G. Protein-matrix coupling/uncoupling in “dry” systems of photosynthetic reaction center embedded in trehalose/sucrose: The origin of trehalose peculiarity. J. Am. Chem. Soc. 2008, 130, 10240–10246. [Google Scholar] [CrossRef] [PubMed]
- Francia, F.; Palazzo, G.; Mallardi, A.; Cordone, L.; Venturoli, G. Residual water modulates Qa−-to-Qb− electron transfer in bacterial reaction centers embedded in trehalose amorphous matrices. Biophys. J. 2003, 85, 2760–2775. [Google Scholar] [CrossRef]
- Malferrari, M.; Francia, F.; Venturoli, G. Retardation of protein dynamics by trehalose in dehydrated systems of photosynthetic reaction centers. Insights from electron transfer and thermal denaturation kinetics. J. Phys. Chem. B 2015, 119, 13600–13618. [Google Scholar] [CrossRef] [PubMed]
- Savitsky, A.; Malferrari, M.; Francia, F.; Venturoli, G.; Möbius, K. Bacterial photosynthetic reaction centers in trehalose glasses: Coupling between protein conformational dynamics and electron-transfer kinetics as studied by laser-flash and high-field EPR spectroscopies. J. Phys. Chem. B 2010, 114, 12729–12743. [Google Scholar] [CrossRef] [PubMed]
- Francia, F.; Palazzo, G.; Mallardi, A.; Cordone, L.; Venturoli, G. Probing light-induced conformational transitions in bacterial photosynthetic reaction centers embedded in trehalose-water amorphous matrices. Biochim. Biophys. Acta 2004, 1658, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Kleinfeld, D.; Okamura, M.Y.; Feher, G. Electron-transfer kinetics in photosynthetic reaction centers cooled to cryogenic temperatures in the charge-separated state: Evidence for light-induced structural changes. Biochemistry 1984, 23, 5780–5786. [Google Scholar] [CrossRef] [PubMed]
- McMahon, B.H.; Muller, J.D.; Wraight, C.A.; Nienhaus, G.U. Electron transfer and protein dynamics in the photosynthetic reaction center. Biophys. J. 1998, 74, 2567–2587. [Google Scholar] [CrossRef]
- Malferrari, M.; Nalepa, A.; Venturoli, G.; Francia, F.; Lubitz, W.; Möbius, K.; Savitsky, A. Structural and dynamical characteristics of trehalose and sucrose matrices at different hydration levels as probed by ftir and high-field EPR. Phys. Chem. Chem. Phys. 2014, 16, 9831–9848. [Google Scholar] [CrossRef] [PubMed]
- Koepke, J.; Krammer, E.M.; Klingen, A.R.; Sebban, P.; Ullmann, G.M.; Fritzsch, G. Ph modulates the quinone position in the photosynthetic reaction center from rhodobacter sphaeroides in the neutral and charge separated states. J. Mol. Biol. 2007, 371, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Joliot, P.; Barbieri, G.; Chabaud, R. Un nouveau modele des centres photochimiques du systeme II. Photochem. Photobiol. 1969, 10, 309–329. [Google Scholar] [CrossRef]
- Blankenship, R.E. Molecular Mechanisms of Photosynthesis; Wiley: Oxford, UK, 2014. [Google Scholar]
- Cardona, T.; Sedoud, A.; Cox, N.; Rutherford, A.W. Charge separation in photosystem ii: A comparative and evolutionary overview. Biochim. Biophys. Acta 2012, 1817, 26–43. [Google Scholar] [CrossRef] [PubMed]
- Rappaport, F.; Guergova-Kuras, M.; Nixon, P.J.; Diner, B.A.; Lavergne, J. Kinetics and pathways of charge recombination in photosystem II. Biochemistry 2002, 41, 8518–8527. [Google Scholar] [CrossRef] [PubMed]
- Umena, Y.; Kawakami, K.; Shen, J.R.; Kamiya, N. Crystal structure of oxygen-evolving photosystem ii at a resolution of 1.9. Nature 2011, 473, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Zouni, A.; Witt, H.-T.; Kern, J.; Fromme, P.; Krauss, N.; Saenger, W.; Orth, P. Crystal structure of photosystem II from Synechococcus elongatus at 3.8 Å resolution. Nature 2001, 409, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, K.N.; Iverson, T.M.; Maghlaoui, K.; Barber, J.; Iwata, S. Architecture of the photosynthetic oxygen-evolving center. Science 2004, 303, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.; Kern, J.; Irrgang, K.-D.; Latimer, M.J.; Bergmann, U.; Glatzel, P.; Pushkar, Y.; Biesiadka, J.; Loll, B.; Sauer, K.; et al. X-ray damage to the Mn4Ca complex in single crystals of photosystem II: A case study for metalloprotein crystallography. Proc. Natl. Acad. Sci. USA 2005, 102, 12047–12052. [Google Scholar] [CrossRef] [PubMed]
- Ogata, H.; Lubitz, W. Protein crystallography using free-electron lasers: Water oxidation in photosynthesis. Angew. Chem. Int. Ed. 2014, 53, 13007–13008. [Google Scholar] [CrossRef] [PubMed]
- Chapman, H.N.; Fromme, P.; Barty, A.; White, T.A.; Kirian, R.A.; Aquila, A.; Hunter, M.S.; Schulz, J.; DePonte, D.P.; Weierstall, U.; et al. Femtosecond X-ray protein nanocrystallography. Nature 2011, 470, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Messinger, J.; Renger, G. Chapter 17 photosynthetic water splitting. In Primary Processes of Photosynthesis, Part 2: Principles and Apparatus; The Royal Society of Chemistry: London, UK, 2008; Volume 9, pp. 291–349. [Google Scholar]
- Kok, B.; Forbush, B.; McGloin, M. Cooperation of charges in photosynthetic O2 evolution. Photochem. Photobiol. 1970, 11, 457–476. [Google Scholar] [CrossRef] [PubMed]
- Hillier, W.; Wydrzynski, T.J. 18O-water exchange in photosystem II: Substrate binding and intermediates of the water splitting cycle. Coord. Chem. Rev. 2008, 252, 306–317. [Google Scholar] [CrossRef]
- Dau, H.; Haumann, M. The manganese complex of photosystem II in its reaction cycle-basic framework and possible realization at the atomic level. Coord. Chem. Rev. 2008, 252, 273–295. [Google Scholar] [CrossRef]
- Klauss, A.; Haumann, M.; Dau, H. Alternating electron and proton transfer steps in photosynthetic water oxidation. Proc. Natl. Acad. Sci. USA 2012, 109, 16035–16040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, N.; Pantazis, D.A.; Neese, F.; Lubitz, W. Biological water oxidation. Acc. Chem. Res. 2013, 46, 1588–1596. [Google Scholar] [CrossRef] [PubMed]
- Lubitz, W.; Lohmiller, T.; Cox, N. Water oxidation and oxygen evolution in photosynthesis. An enzyme that changed the world. Bunsen-Mag. 2016, 101, 216–223. [Google Scholar]
- Haddy, A. EPR spectroscopy of the manganese cluster of photosystem II. Photosynth. Res. 2007, 92, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Dismukes, G.C.; Siderer, Y. Intermediates of a polynuclear manganese center involved in photosynthetic oxidation of water. Proc. Natl. Acad. Sci. USA 1981, 78, 274–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messinger, J.; Robblee, J.H.; Yu, W.O.; Sauer, K.; Yachandra, V.K.; Klein, M.P. The S0 state of the oxygen-evolving complex in photosystem ii is paramagnetic: Detection of an EPR multiline signal. J. Am. Chem. Soc. 1997, 119, 11349–11350. [Google Scholar] [CrossRef] [PubMed]
- Kulik, L.V.; Epel, B.; Lubitz, W.; Messinger, J. Electronic structure of the Mn4OxCa cluster in the S0 and S2 states of the oxygen-evolving complex of photosystem II based on pulse 55Mn ENDOR and EPR spectroscopy. J. Am. Chem. Soc. 2007, 129, 13421–13435. [Google Scholar] [CrossRef] [PubMed]
- Randall, D.W.; Sturgeon, B.E.; Ball, J.A.; Lorigan, G.A.; Chan, M.K.; Klein, M.P.; Armstrong, W.H.; Britt, R.D. 55Mn ese-ENDOR of a mixed valence Mn(III)Mn(IV) complex: Comparison with the mn cluster of the photosynthetic oxygen-evolving complex. J. Am. Chem. Soc. 1995, 117, 11780–11789. [Google Scholar] [CrossRef]
- Peloquin, J.M.; Campbell, K.A.; Randall, D.W.; Evanchik, M.A.; Pecoraro, V.L.; Armstrong, W.H.; Britt, R.D. 55Mn ENDOR of the S2-state multiline EPR signal of photosystem II: Implications on the structure of the tetranuclear mn cluster. J. Am. Chem. Soc. 2000, 122, 10926–10942. [Google Scholar] [CrossRef]
- Kulik, L.V.; Epel, B.; Lubitz, W.; Messinger, J. 55Mn pulse ENDOR at 34 GHz of the S0 and S2 states of the oxygen-evolving complex in photosystem II. J. Am. Chem. Soc. 2005, 127, 2392–2393. [Google Scholar] [CrossRef] [PubMed]
- Cox, N.; Ames, W.; Epel, B.; Kulik, L.V.; Rapatskiy, L.; Neese, F.; Messinger, J.; Wieghardt, K.; Lubitz, W. Electronic structure of a weakly antiferromagnetically coupled MnIIMnIII model relevant to manganese proteins: A combined EPR, 55Mn-ENDOR, and DFT study. Inorg. Chem. 2011, 50, 8238–8251. [Google Scholar] [CrossRef] [PubMed]
- Stich, T.A.; Yeagle, G.J.; Service, R.F.; Debus, R.J.; Britt, R.D. Ligation of D1-His332 and D1-Asp170 to the manganese cluster of photosystem II from synechocystis assessed by multifrequency pulse EPR spectroscopy. Biochemistry 2011, 50, 7390–7404. [Google Scholar] [CrossRef] [PubMed]
- Schinzel, S.; Schraut, J.; Arbuznikov, A.; Siegbahn, P.; Kaupp, M. Density functional calculations of 55Mn, 14N and 13C electron paramagnetic resonance parameters support an energetically feasible model system for the S2 state of the oxygen-evolving complex of photosystem II. Chem. Eur. J. 2010, 16, 10424–10438. [Google Scholar] [CrossRef] [PubMed]
- Cox, N.; Rapatskiy, L.; Su, J.H.; Pantazis, D.A.; Sugiura, M.; Kulik, L.V.; Dorlet, P.; Rutherford, A.W.; Neese, F.; Boussac, A.; et al. Effect of Ca2+/Sr2+ substitution on the electronic structure of the oxygen-evolving complex of photosystem II: A combined multifrequency EPR, 55Mn-ENDOR, and DFT study of the S2 state. J. Am. Chem. Soc. 2011, 133, 3635–3648. [Google Scholar] [CrossRef] [PubMed]
- Lohmiller, T.; Cox, N.; Su, J.H.; Messinger, J.; Lubitz, W. The basic properties of the electronic structure of the oxygen-evolving complex of photosystem ii are not perturbed by Ca2+ removal. J. Biol. Chem. 2012, 287, 24721–24733. [Google Scholar] [CrossRef] [PubMed]
- Krewald, V.; Retegan, M.; Cox, N.; Messinger, J.; Lubitz, W.; DeBeer, S.; Neese, F.; Pantazis, D.A. Metal oxidation states in biological water splitting. Chem. Sci. 2015, 6, 1676–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boussac, A.; Sugiura, M.; Rutherford, A.W.; Dorlet, P. Complete EPR spectrum of the S3-state of the oxygen-evolving photosystem II. J. Am. Chem. Soc. 2009, 131, 5050–5051. [Google Scholar] [CrossRef] [PubMed]
- Cox, N.; Retegan, M.; Neese, F.; Pantazis, D.A.; Boussac, A.; Lubitz, W. Electronic structure of the oxygen-evolving complex in photosystem ii prior to o-o bond formation. Science 2014, 345, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T. Ftir detection of water reactions in the oxygen-evolving centre of photosystem II. Phil. Trans. R. Soc. B 2008, 363, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- McConnell, I.L.; Grigoryants, V.M.; Scholes, C.P.; Myers, W.K.; Chen, P.Y.; Whittaker, J.W.; Brudvig, G.W. EPR-ENDOR characterization of (17O, 1H, 2 H) water in manganese catalase and its relevance to the oxygen-evolving complex of photosystem II. J. Am. Chem. Soc. 2012, 134, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.-A.; Sackett, H.; Babcock, G.T. Identification of a Mn-O-Mn cluster vibrational mode of the oxygen-evolving complex in photosystem II by low-frequency ftir spectroscopy. Biochemistry 2000, 39, 14371–14376. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.P.; Ames, W.M.; Nilsson, H.; Lohmiller, T.; Pantazis, D.A.; Rapatskiy, L.; Nowacyzk, M.M.; Neese, F.; Boussac, A.; Messinger, J.; et al. Ammonia binding to the oxygen-evolving complex of photosystem ii identiefies the solvent-exchangeable water. Proc. Natl. Acad. Sci. USA 2013, 110, 15561–15566. [Google Scholar] [CrossRef] [PubMed]
- Pantazis, D.A.; Ames, W.; Cox, N.; Lubitz, W.; Neese, F. Two interconvertible structures that explain the spectroscopic properties of the oxygen-evolving complex of photosystem II in the S2 state. Angew. Chem. Int. Ed. 2012, 51, 9935–9940. [Google Scholar] [CrossRef] [PubMed]
- Lohmiller, T.; Krewald, V.; Sedoud, A.; Rutherford, A.W.; Neese, F.; Lubitz, W.; Pantazis, D.A.; Cox, N. The first state in the catalytic cycle of the water-oxidizing enzyme: Identification of a water-derived μ-hydroxo bridge. J. Am. Chem. Soc. 2017, 139, 14412–14424. [Google Scholar] [CrossRef] [PubMed]
- Kern, J.; Chatterjee, R.; Young, I.D.; Fuller, F.D.; Lassalle, L.; Ibrahim, M.; Gul, S.; Fransson, T.; Brewster, A.S.; Alonso-Mori, R.; et al. Structures of the intermediates of kok’s photosynthetic water oxidation clock. Nature 2018, in press. [Google Scholar]
- Retegan, M.; Krewald, V.; Mamedov, F.; Neese, F.; Lubitz, W.; Cox, N.; Pantazis, D.A. A five-coordinate Mn(IV) intermediate in biological water oxidation: Spectroscopic signature and a pivot mechanism for water binding. Chem. Sci. 2016, 7, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Siegbahn, P.E.M. Structures and energetics for O2 eormation in photosystem II. Acc. Chem. Res. 2009, 42, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Komenda, J.; Sobotka, R.; Nixon, P.J. Assembling and maintaining the photosystem ii complex in chloroplasts and cyanobacteria. Curr. Opin. Plant Biol. 2012, 15, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Hore, P.J. Animal detected magnetic resonance. EPR Newsl. 2011, 21, 2–3. [Google Scholar]
- Prisner, T. Dynamic nuclear polarization at high magnetic fields. EPR Newsl. 2018, 28, 7–8. [Google Scholar]
- Moser, E.; Laistler, E.; Schmitt, F.; Kontaxis, G. Ultra-high field NMR and MRI—The role of magnet technology to increase sensitivity and specificity. Front. Phys. 2017, 5, 33. [Google Scholar] [CrossRef]
- Jeschke, G. Lecture Notes: Studying Macromolecules by EPR; ETH Zurich: Zurich, Switzerland, 2017; p. 72. [Google Scholar]
- Dzikovski, B. Sample preparation for quasioptical high-field ESR. EPR Newsl. 2007, 22, 14–15. [Google Scholar]
- Murray, P.R.; Collison, D.; Daff, S.; Austin, N.; Edge, R.; Flynn, B.W.; Jack, L.; Leroux, F.; McInnes, E.J.L.; Murray, A.F.; et al. An in situ electrochemical cell for q- and w-band EPR spectroscopy. J. Magn. Reson. 2011, 213, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, T.I.; Smirnov, A.I. High-field ESR spectroscopy in membrane and protein biophysics. In ESR Spectroscopy in Membrane Biophysics; Springer: Boston, MA, USA, 2007; pp. 165–251. [Google Scholar]
- Hoff, A.J. (Ed.) Optically-detected magnetic resonance of triplet states. In Advanced EPR, Applications in Biology and Biochemistry; Elsevier: Amsterdam, The Netherlands, 1989; pp. 633–684. [Google Scholar]
- Dinse, K.P.; Winscom, C.J. Determination of n-14 quadrupole tensor of phenazine in its lowest excited triplet-state by odnqr. J. Chem. Phys. 1978, 68, 1337–1343. [Google Scholar] [CrossRef]
- Von Borczyskowski, C.V.; Plato, M.; Dinse, K.P.; Möbius, K. Optically detected X-band ENDOR of excited triplet-state of quinoxaline and p-dichlorobenzene. Semicond. Insul. 1978, 4, 376. [Google Scholar]
- Atkins, P.W.; McLauchlan, K.A. Electron spin polarization. In Chemically Induced Magnetic Polarization; Lepley, A.R., Closs, G.L., Eds.; Wiley: New York, NY, USA, 1973; pp. 41–93. [Google Scholar]
- Freed, J.H.; Pedersen, J.B. The theory of chemically induced dynamic spin polarization. In Advances in Magnetic Resonance; Waugh, J.S., Ed.; Academic Press: New York, NY, USA, 1976; Volume 8, pp. 1–80. [Google Scholar]
- Salikhov, K.M.; Molin, Y.N.; Sagdeev, R.Z.; Buchachenko, A.L. Spin Polarization and Magnetic Effects in Radical Reactions; Elsevier: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Steiner, U.E.; Ulrich, T. Magnetic-field effects in chemical-kinetics and related phenomena. Chem. Rev. 1989, 89, 51–147. [Google Scholar] [CrossRef]
- Forbes, M.D.E.; Jarocha, L.E.; Sim, S.; Tarasov, V.F. Chapter one—Time-resolved electron paramagnetic resonance spectroscopy: History, technique, and application to supramolecular and macromolecular chemistry. In Advances in Physical Organic Chemistry; Williams, I.H., Williams, N.H., Eds.; Academic Press: Cambridge, MA, USA, 2013; Volume 47, pp. 1–83. [Google Scholar]
- Zarea, M.; Ratner, M.A.; Wasielewski, M.R. Spin polarization transfer by the radical pair mechanism. J. Chem. Phys. 2015, 143, 054101. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.; Schiemann, O.; Bode, B.; Prisner, T.F. PELDOR at S- and X-band frequencies and the separation of exchange coupling from dipolar coupling. J. Magn. Reson. 2002, 157, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.B.; Halpern, H.J. In vivo EPR imaging. In Biomedical EPR, Part A: Free Radicals, Metals, Medicine, and Physiology, Biological Magnetic Resonance Vol. 23; Eaton, S.R., Eaton, G.R., Berliner, L.J., Eds.; Springer: Boston, MA, USA, 2005; pp. 283–319. [Google Scholar]
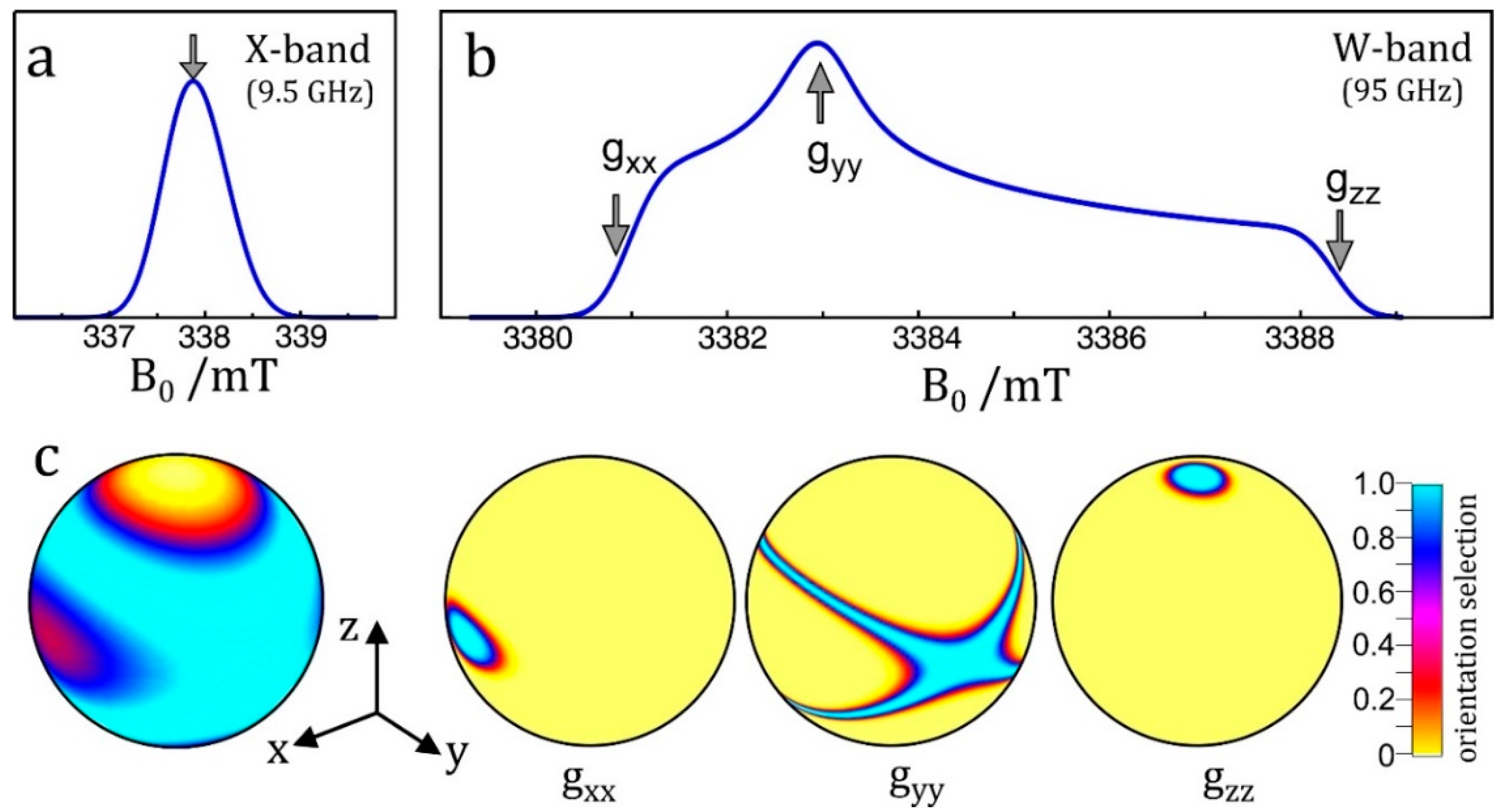
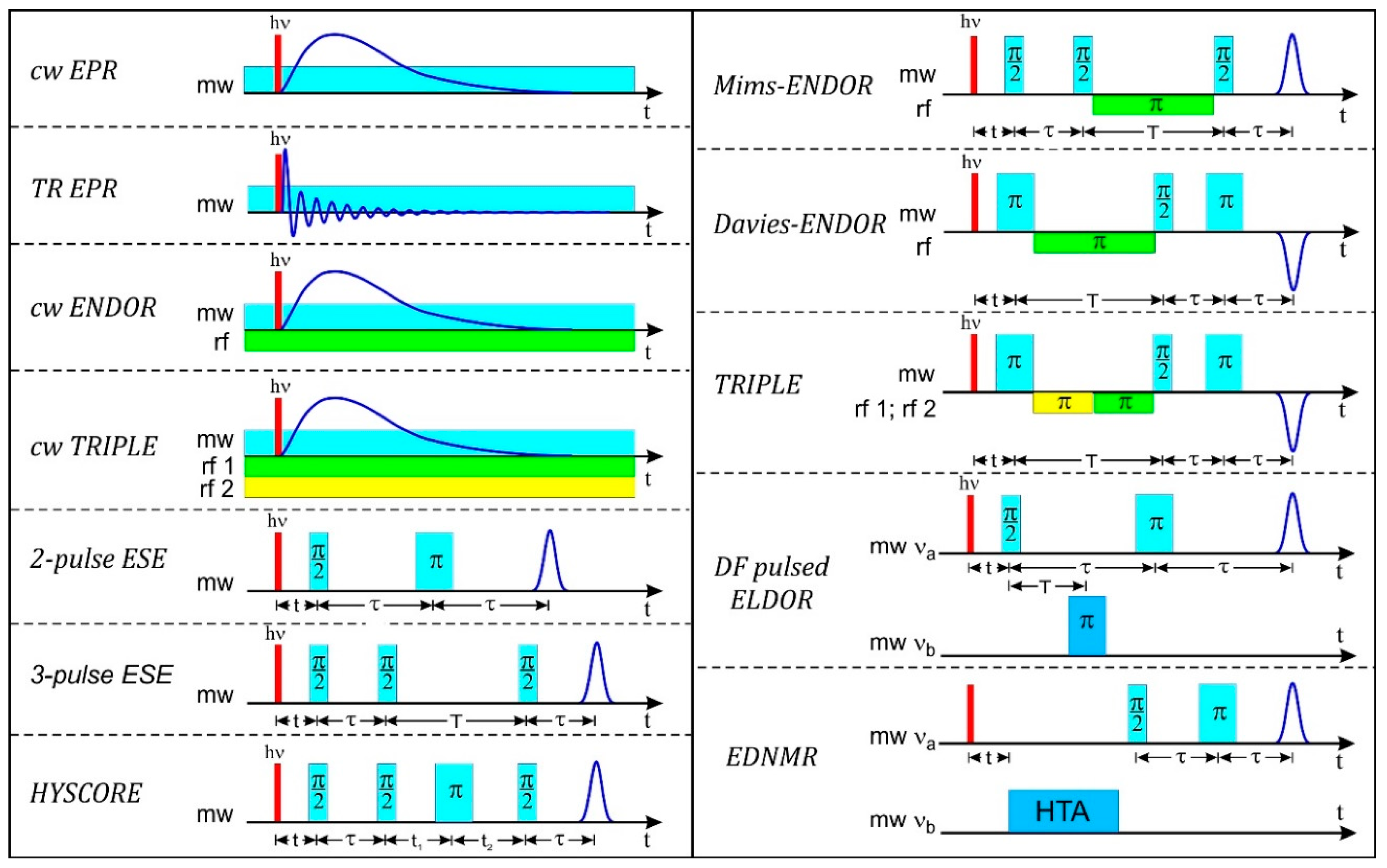
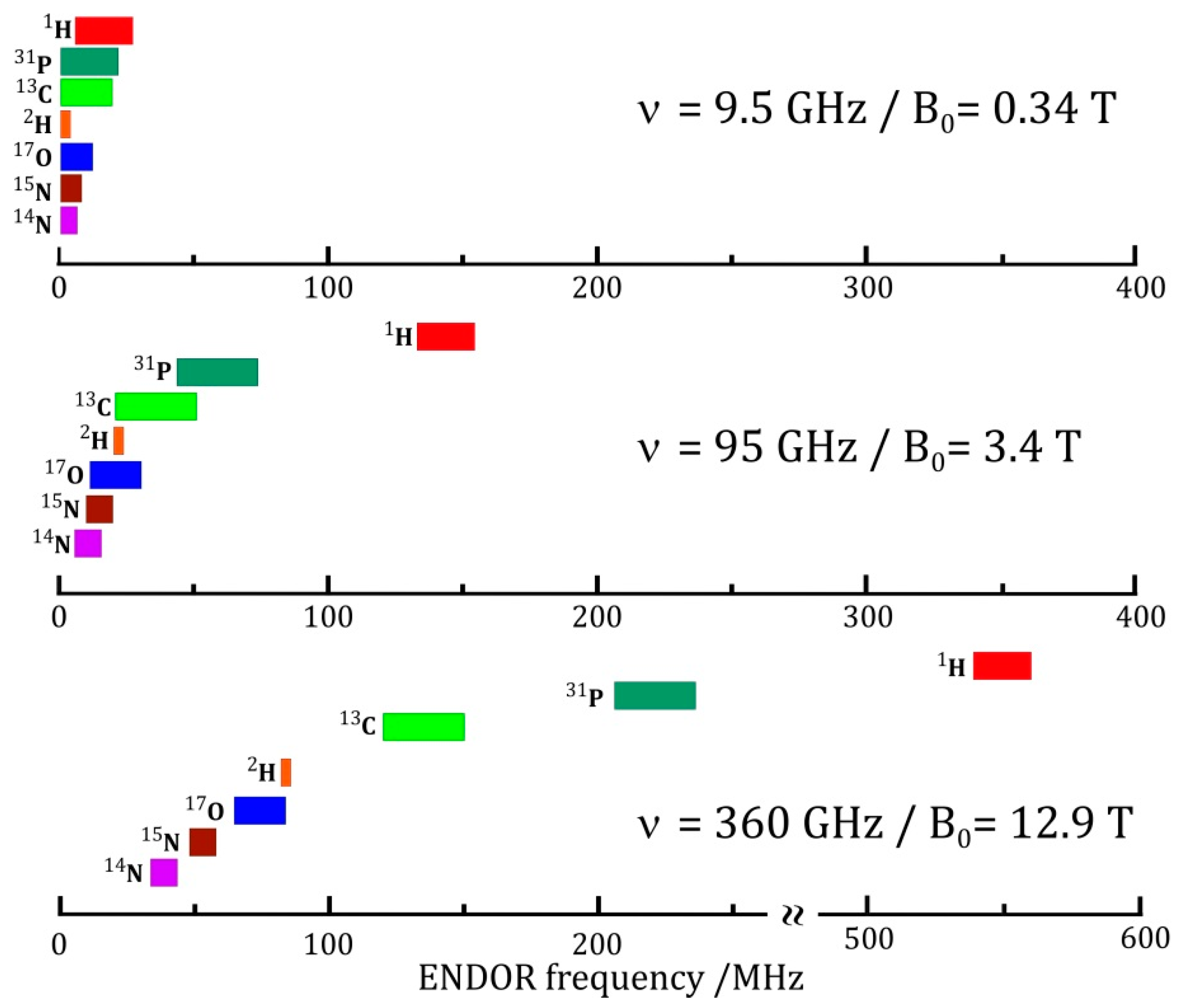
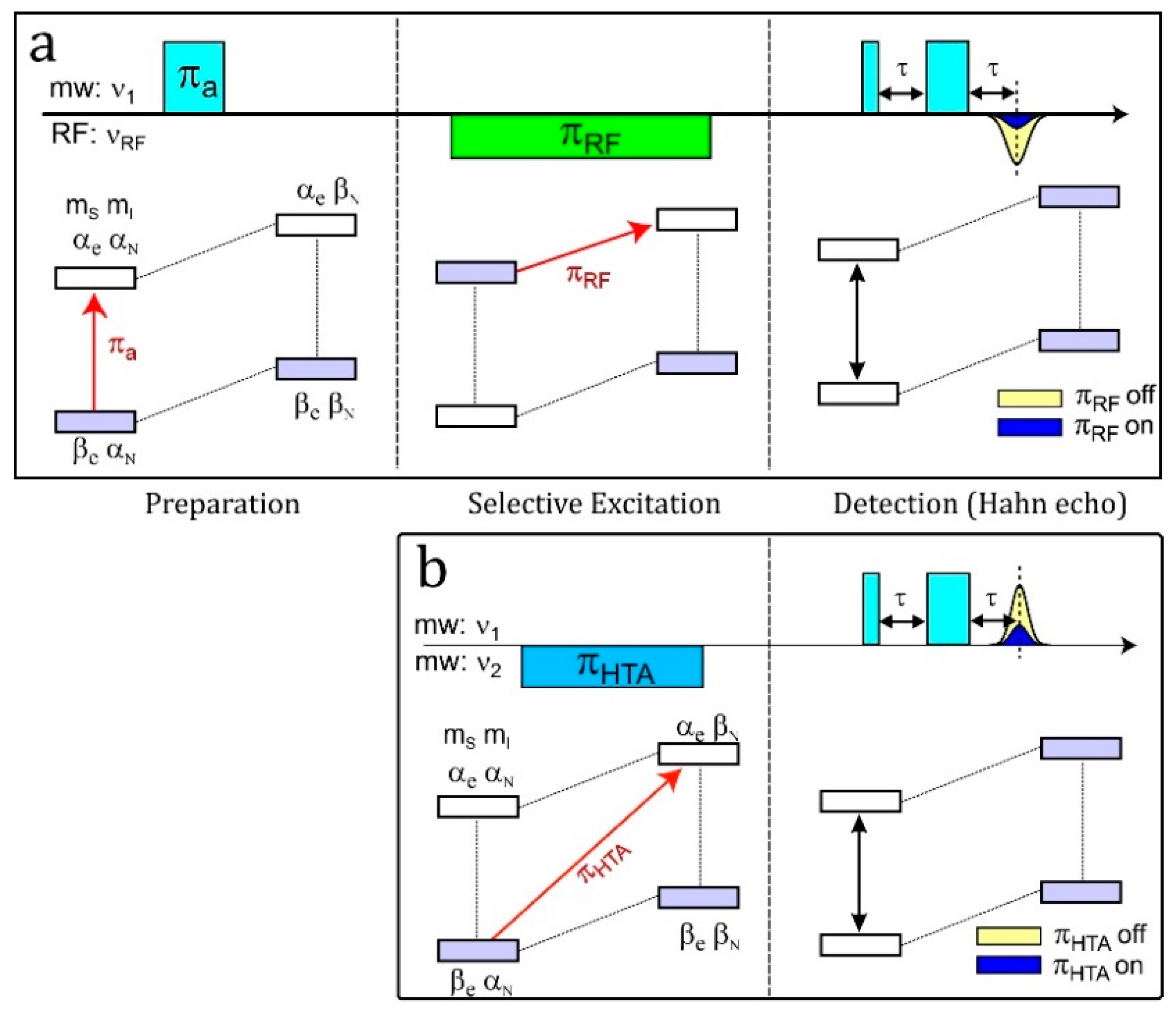
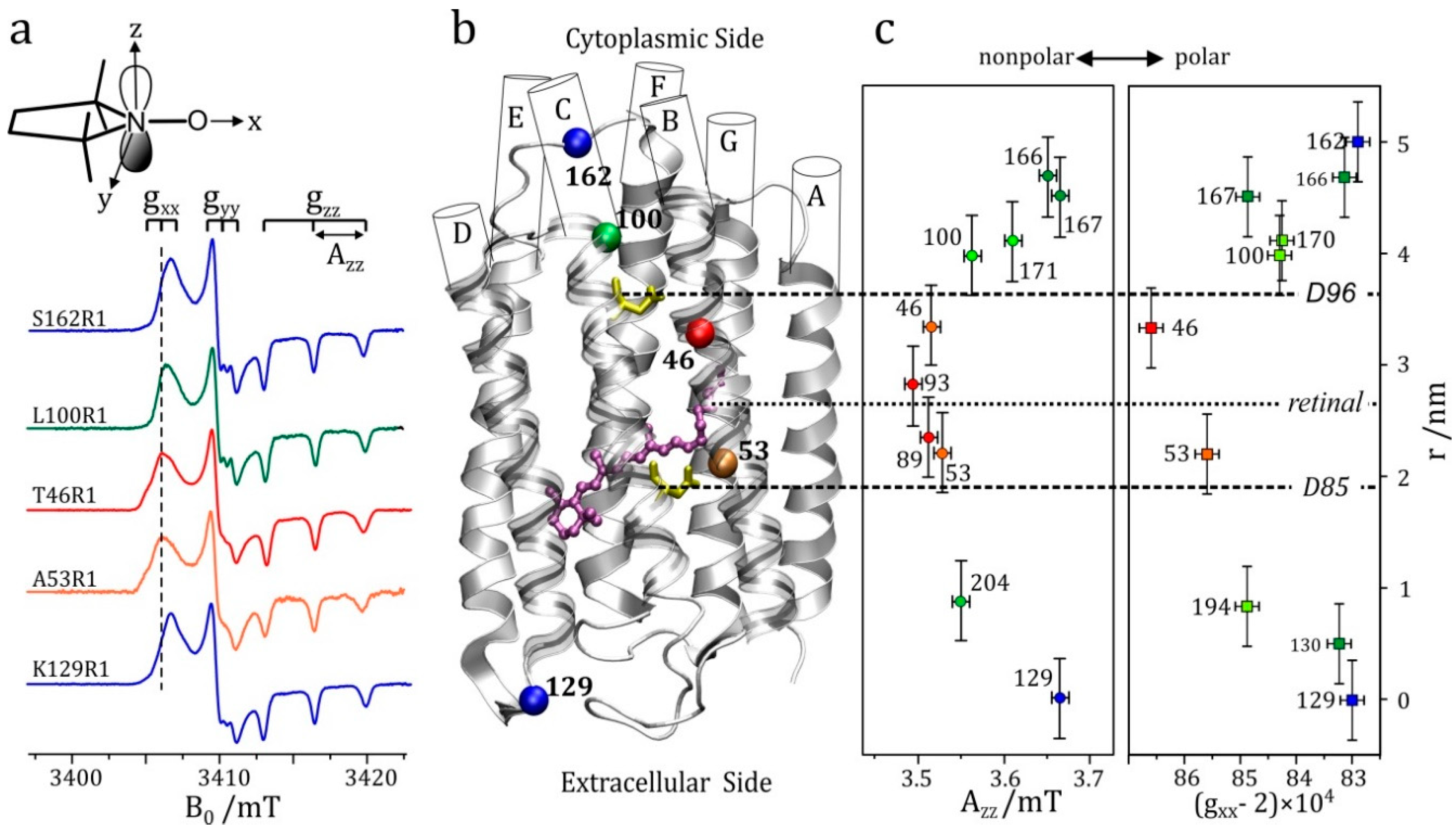
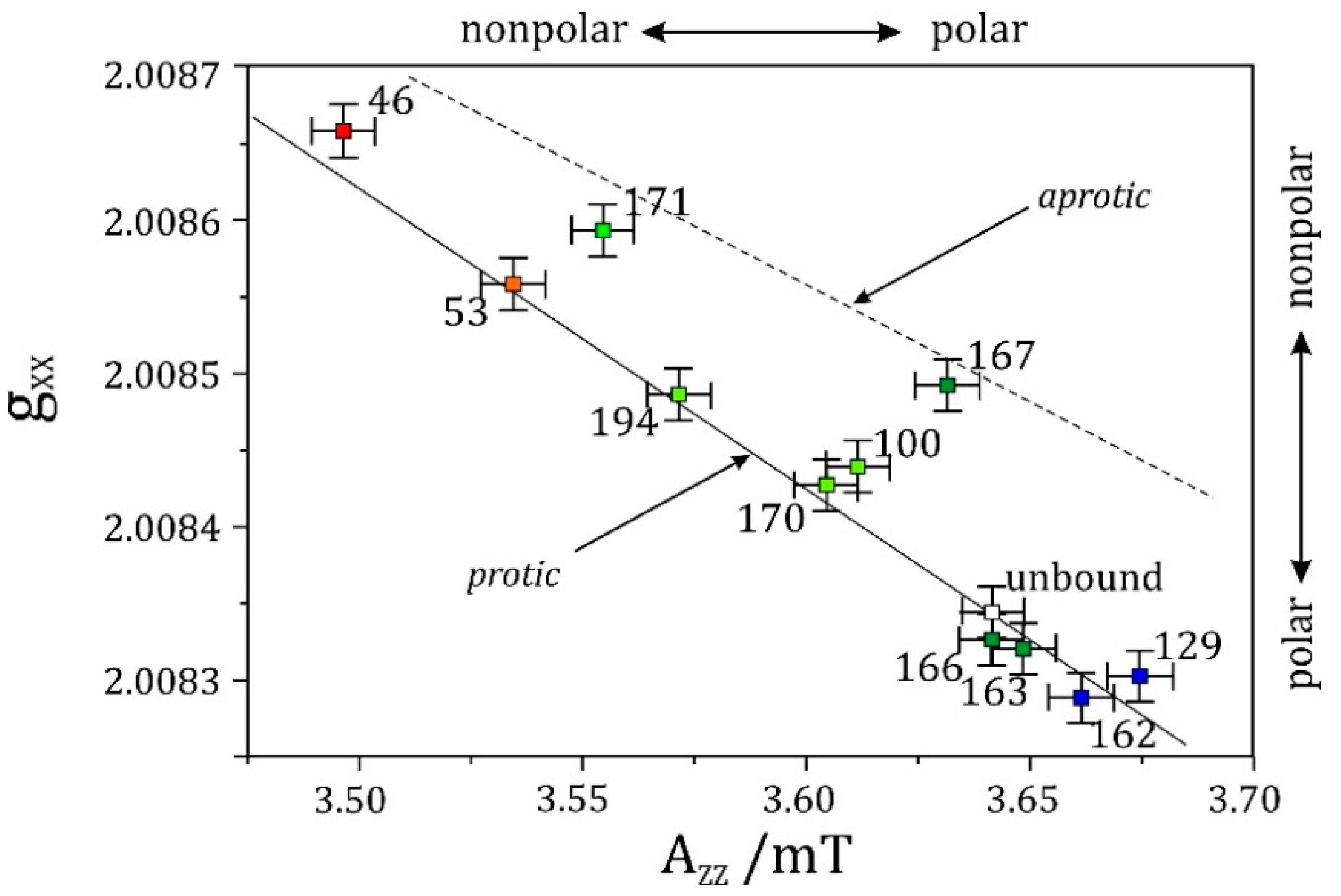
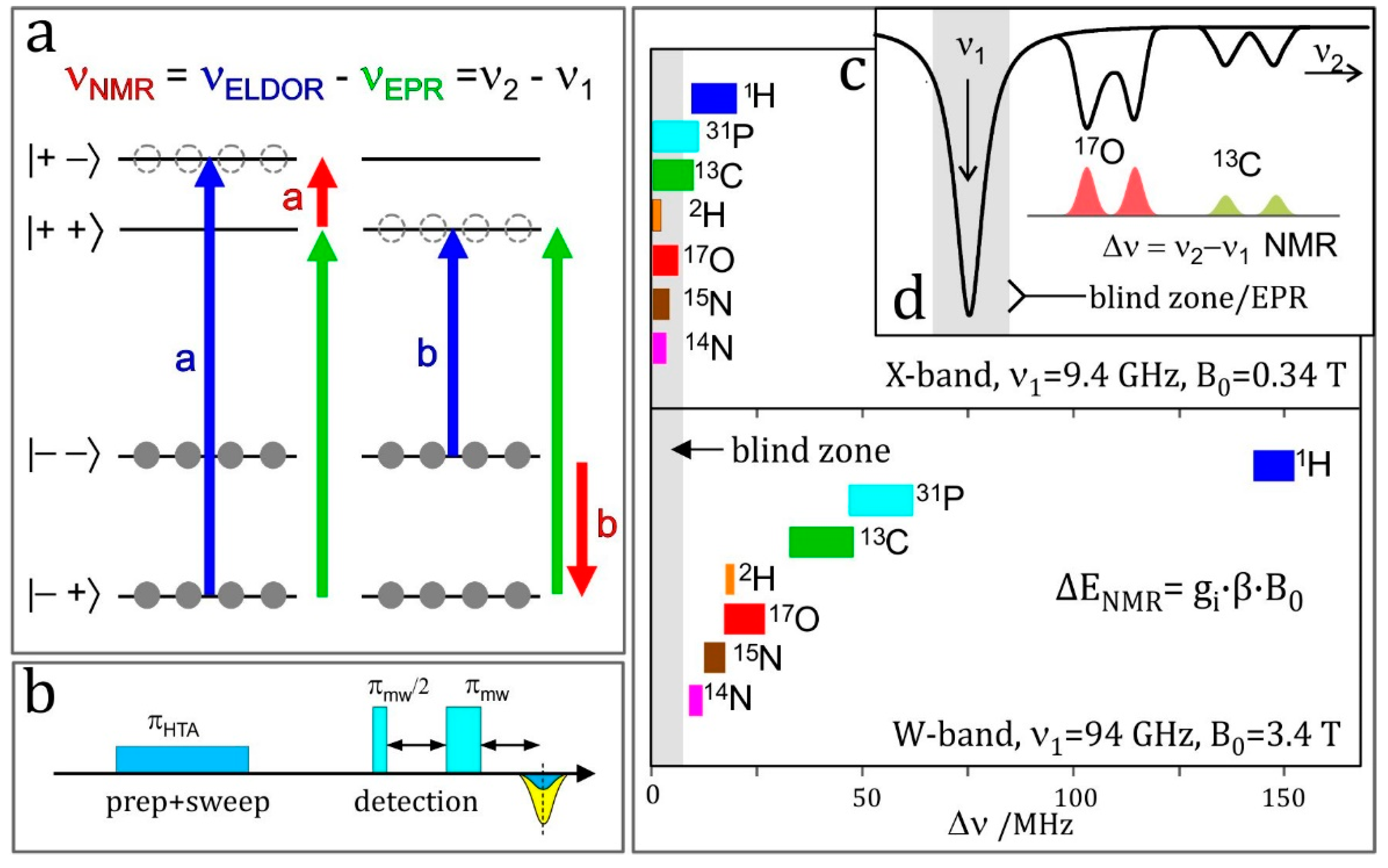
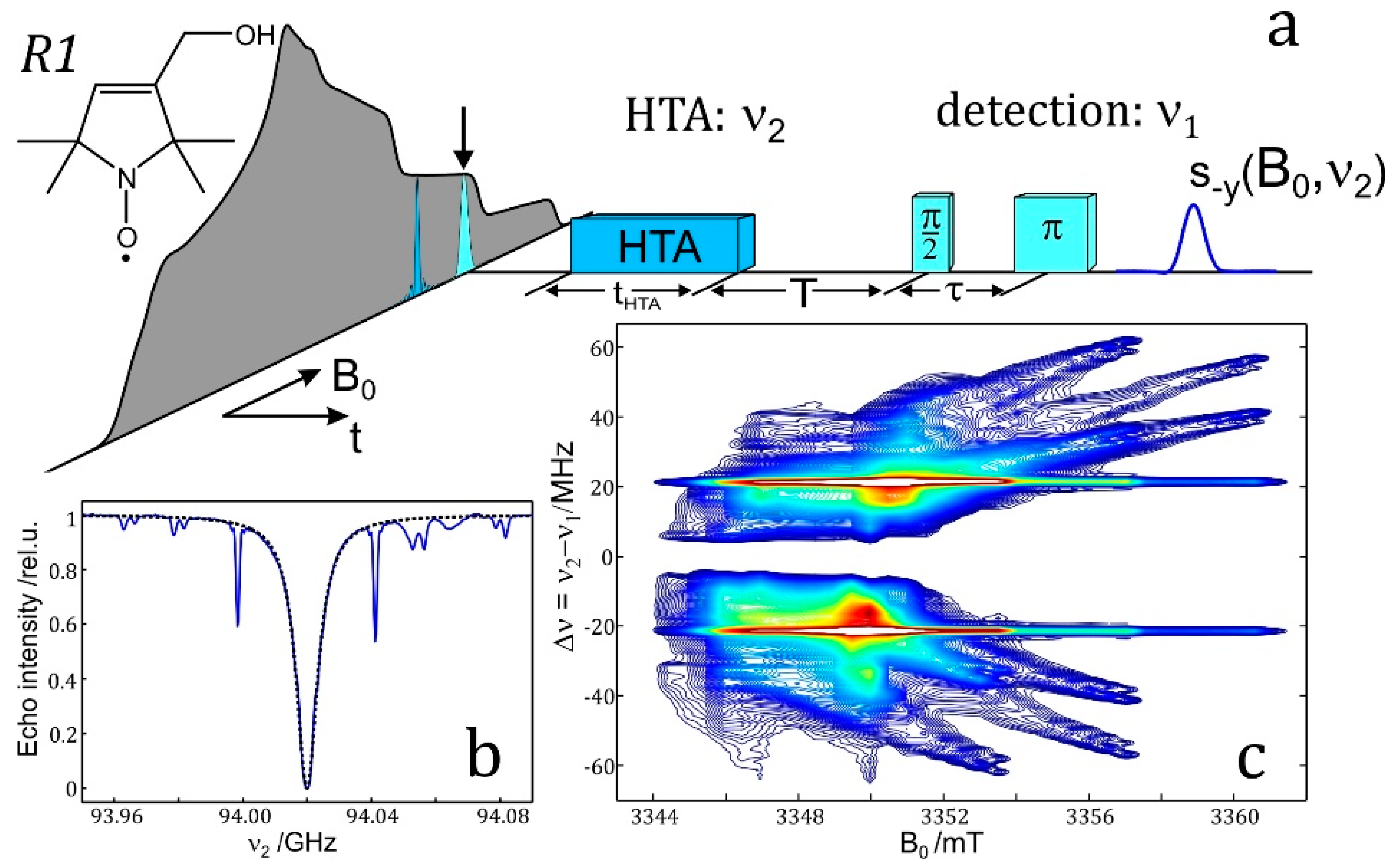
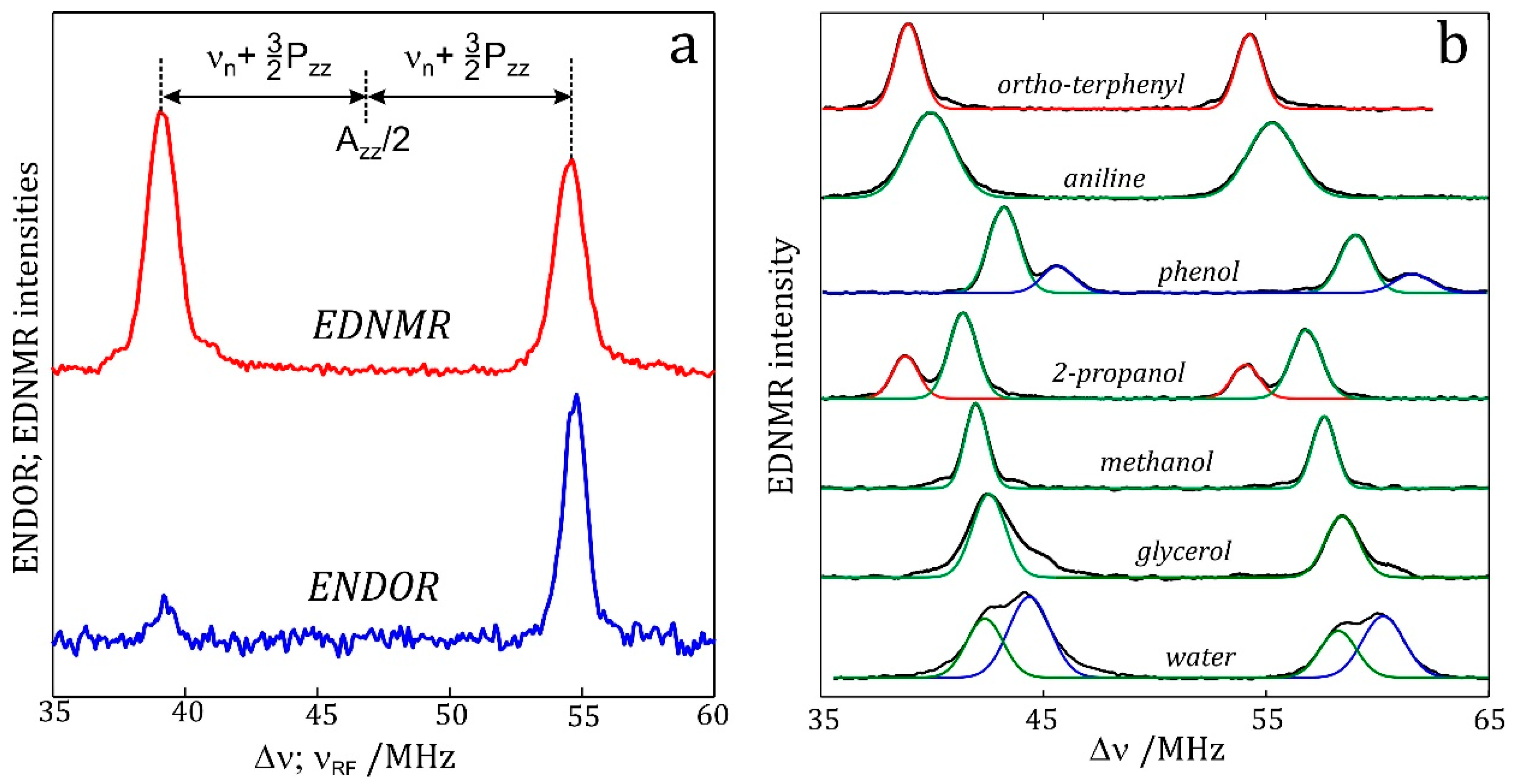
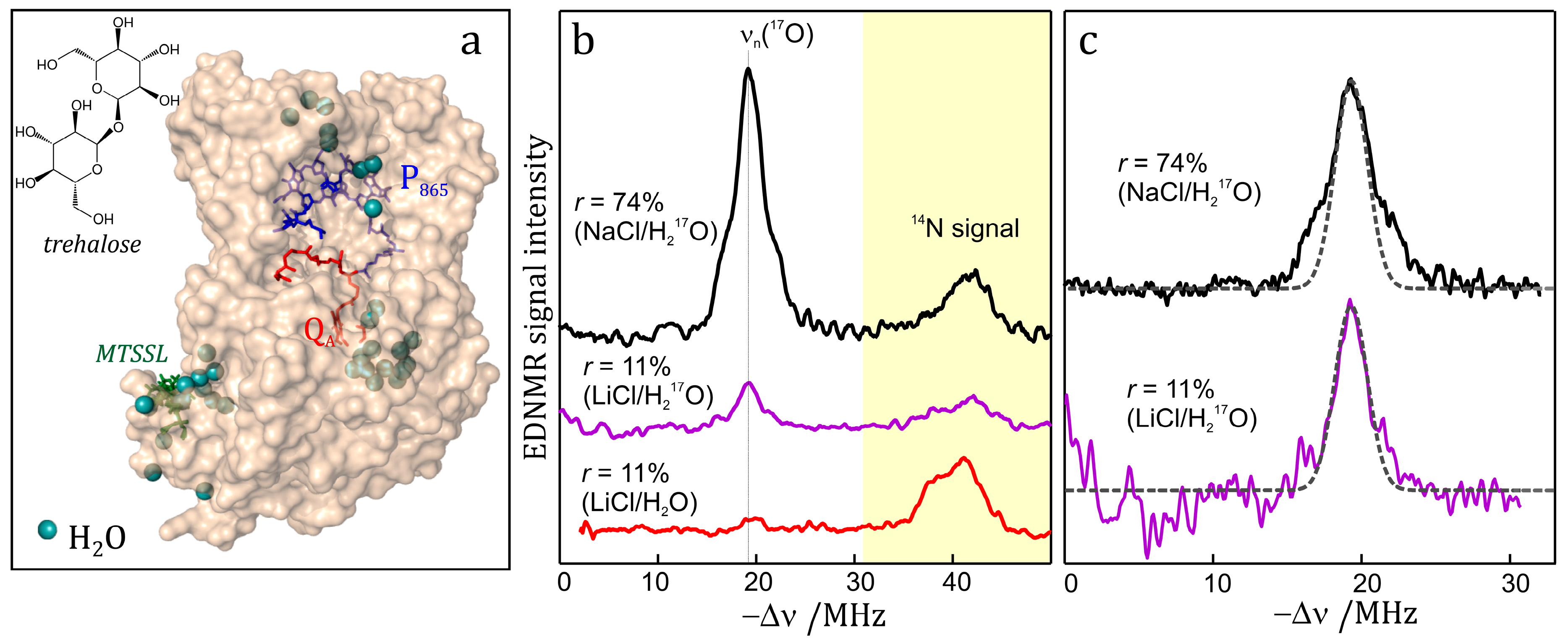
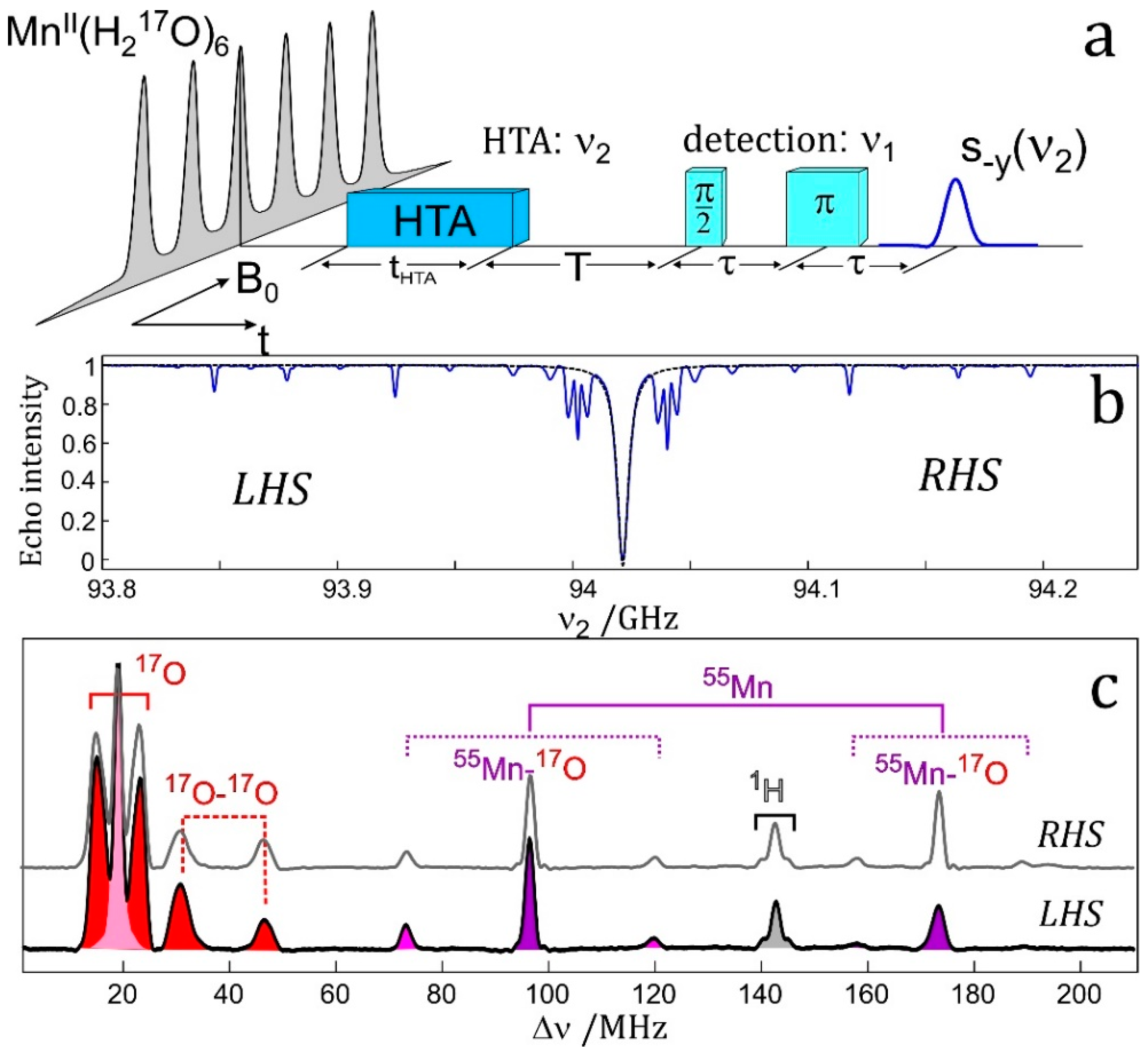
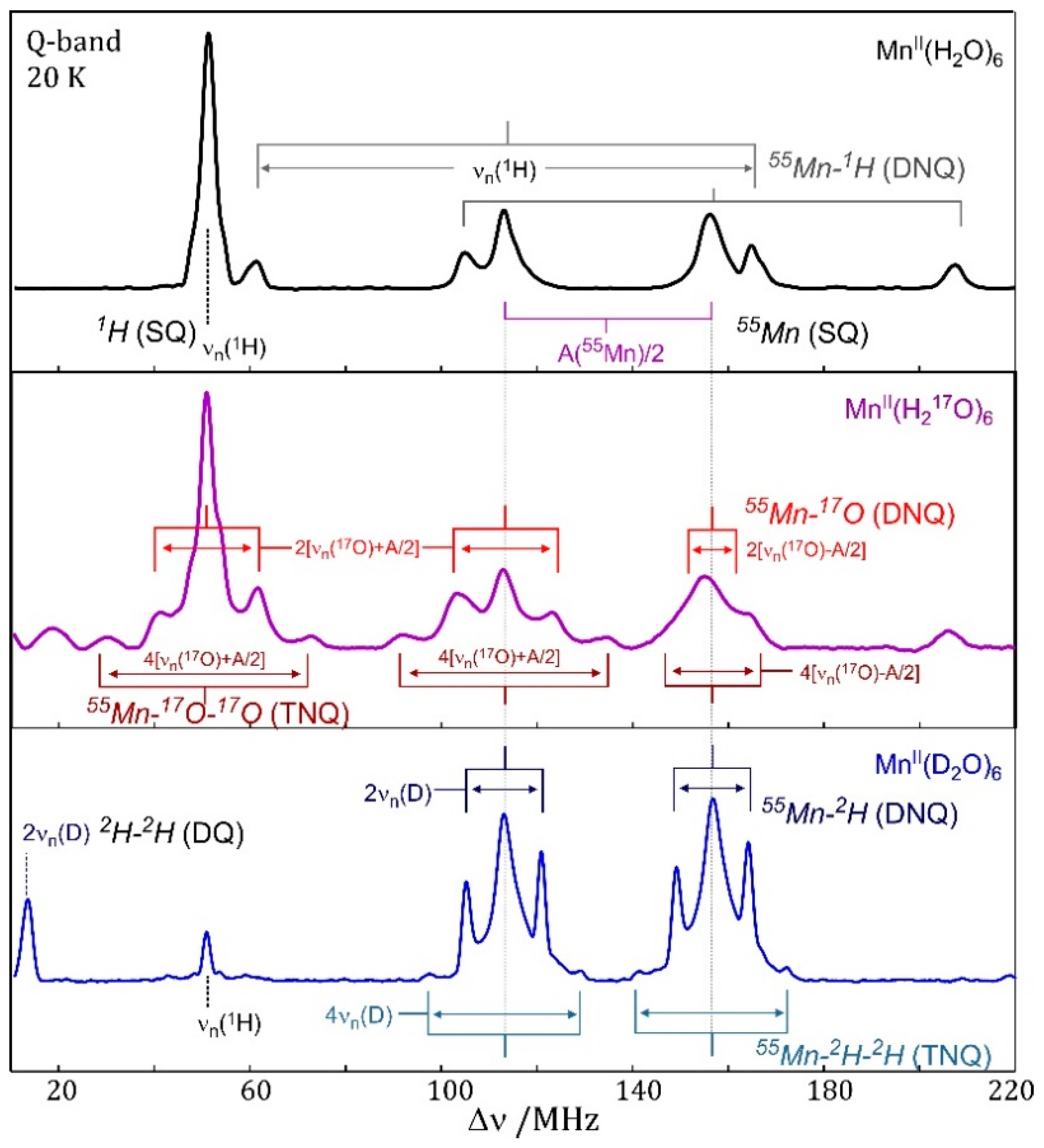
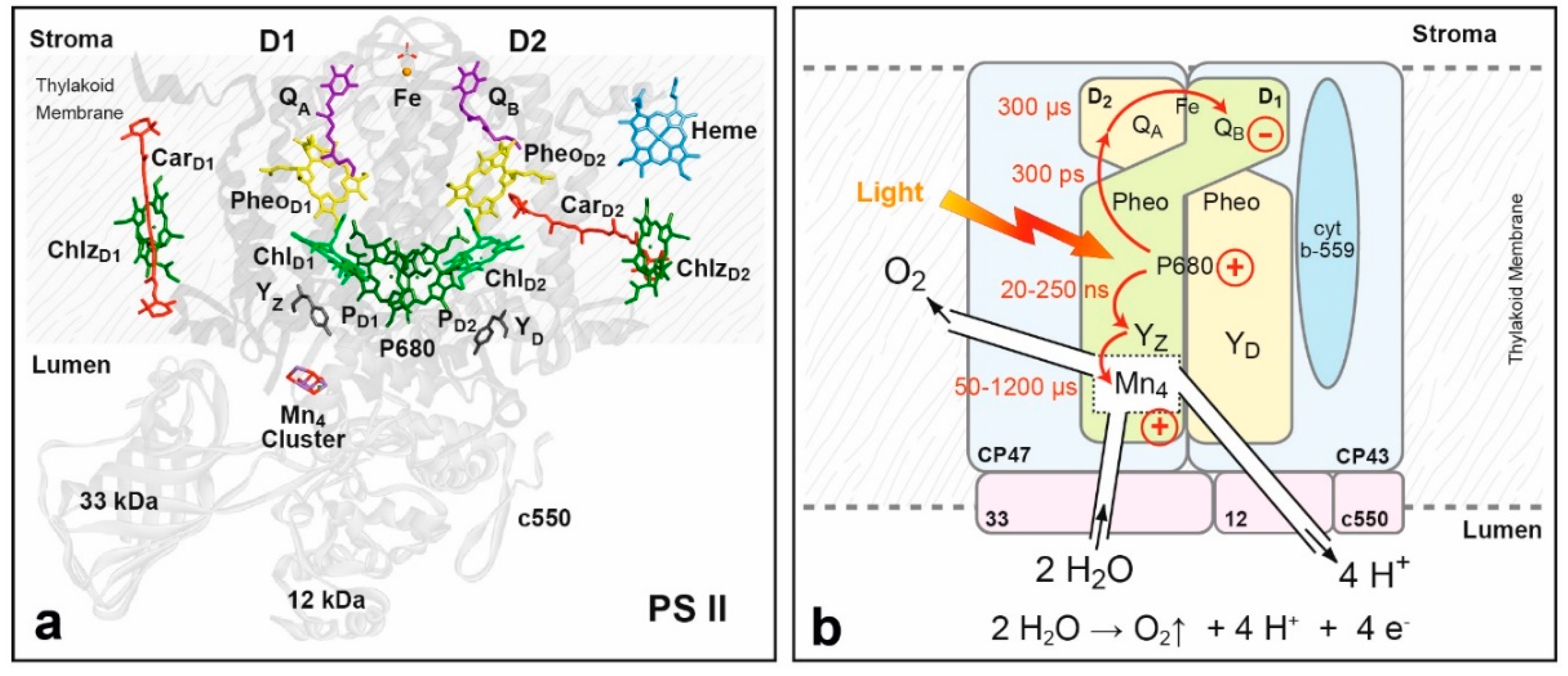
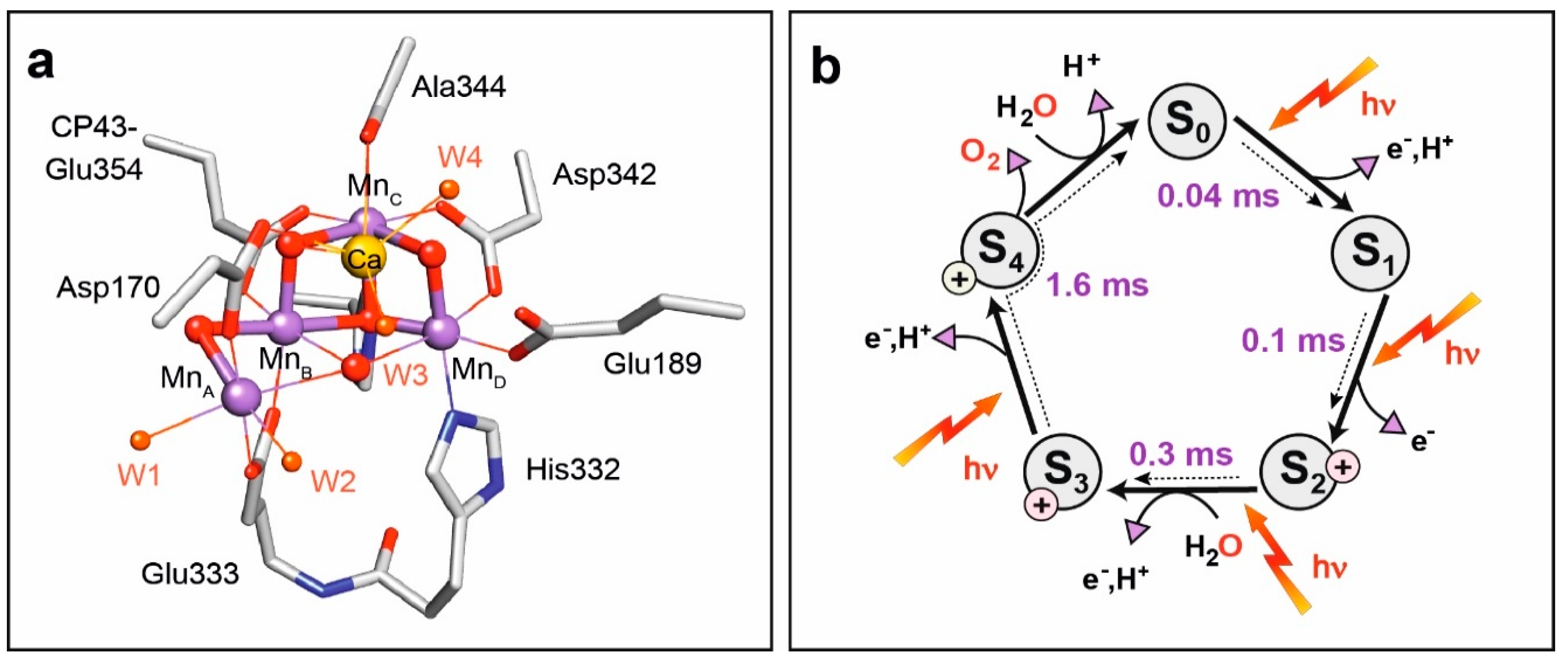
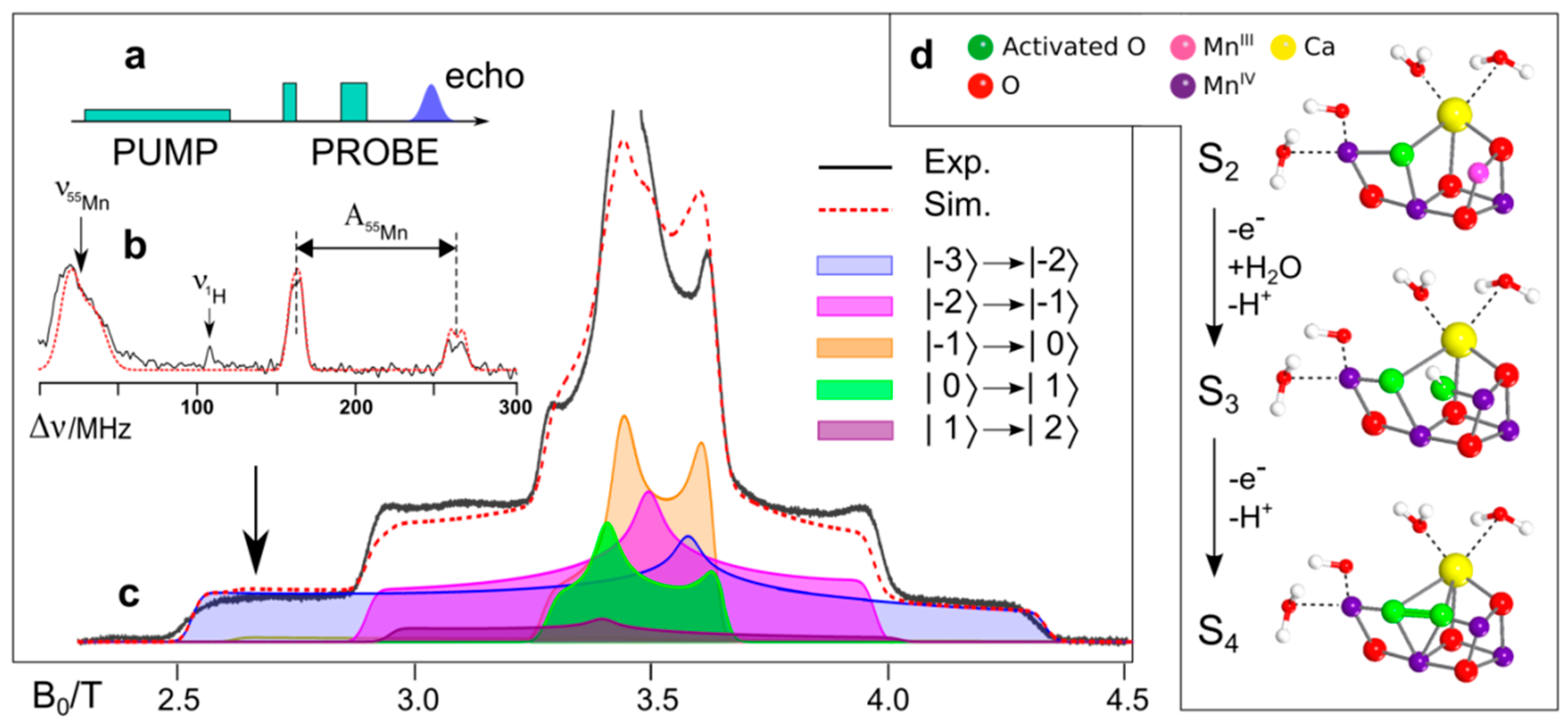

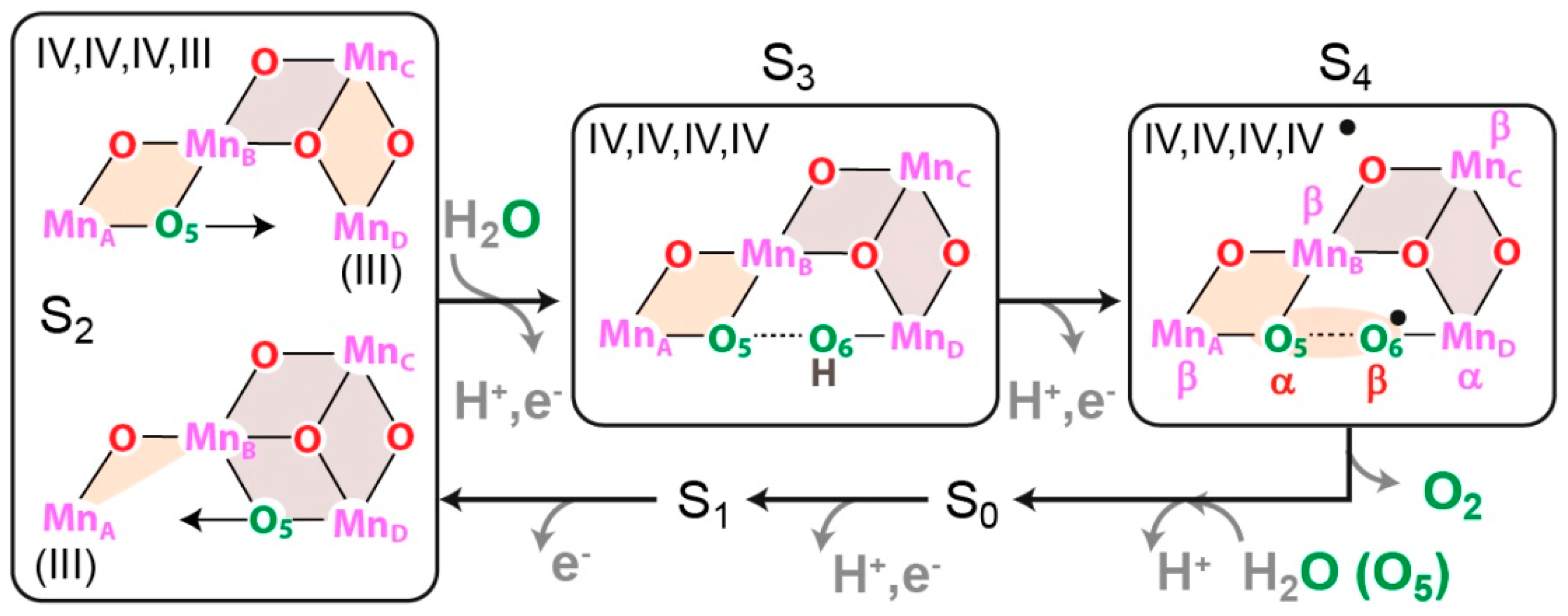
| Resonance frequency | 103 larger in EPR (GHz versus MHz) |
| Spin interactions | 106 stronger in EPR (MHz versus Hz) |
| Relaxation times | 106 shorter in EPR (ns versus ms) |
| Time resolution | 106 shorter in EPR (ns versus ms) |
| Detection sensitivity | 106 higher in EPR (nM versus mM) |
| Radical Type | Method | Anisotropic Spin Interaction | Distance Range |
|---|---|---|---|
| Monoradical | EPR | electron–nuclear | 1 Å ≤ r ≤ 3 Å |
| Monoradical | ENDOR | electron–nuclear | 1 Å ≤ r ≤ 10 Å |
| Radical pair | EPR/ESE | electron–electron | 5 Å ≤ r ≤ 30 Å |
| Radical pair | PELDOR | electron–electron | 15 Å ≤ r < 100 Å |
| Frequency GHz | EPR Mode | Detection Scheme | MW Bridge Scheme | Transmiss. Line | Resonator | MW Source | Year | Ref. |
|---|---|---|---|---|---|---|---|---|
| 150 | cw, pulse | HO | WG | OWR | TE011, FP | K, SS, BWO, IMPATT | 1977 | [41,42,43,73,74] |
| 95 | cw, pulse | HE | WG | OWR | TE011, FP | K, SS | 1985 | [44,45,48,49] |
| 95 | cw, pulse | HE | WG | OWR, OWC | FP | IMPATT | 1989 | [46,50,75] |
| 95 | cw, pulse | HE | WG | OWR | TE011 | G | 1992 | [76] |
| 95 | cw | HO | WG | OWR, DWG | TE013 | G | 1994 | [77,78] |
| 95, 140 | cw, pulse | HE | WG | OWR | TE011 | SS | 1999 | [79] |
| 95 | cw, pulse | HE | OQ | CWG | FP | SS, EIKA | 2004 | [80] |
| 95 | cw, pulse | HE | WG | OWR | TE011 | SS | 2006 | [81] |
| 95 | cw | HE | WG | OWR | TE011, LGR | G | 2007 | [82] |
| 30–120 | cw | HO | WG | WR | TE011 | MVNA | 1999 | [83] |
| 130 | cw | HO | WG | OWR | TE011 | G | 1998 | [84] |
| 130 | cw | HE | WG | OWR | TE011 | G | 2002 | [85] |
| 130 | cw | HE | WG | OWR | TE011 | G | 2005 | [86,87] |
| 95–475 | cw | HE | QO | CWG | FP | G | 1998 | [84] |
| 90, 180 | cw | HE | OQ | CWG | FP | G | 1998 | [54] |
| 140 | cw, pulse | HE | WG | OWR | FP | G, EIK | 1992 | [51,88] |
| 140 | cw, pulse | HE | WG | OWR | TE011, FP | G, GTR | 1995 | [89] |
| 180 | cw, pulse | HE | QO | CWG | TE011 | G | 2001 | [90] |
| 220 | cw | HO | QO | LS | FP | G | 1999 | [91] |
| 120, 240 | cw, pulse | HE | QO | CWG | TE011 | G | 2007 | [58] |
| 120, 240 | cw | HE | QO | CWG | FP, NRS | G | 2005 | [92] |
| 275 | cw, pulse | HE | QO | CWG | TE011 | G | 2003 | [93,94] |
| 285 | cw | HO | WG | OWC | NRS | G, SS | 1999 | [95,96] |
| 360 | cw, pulse | HE | QO | CWG | FP | G, O | 1999 | [56,63] |
| 160–525 | cw | HO | QO | CWG | NRS | FIR | 1989 | [57] |
| 240, 316 | cw | HO | QO | OWC | WGD | FIR | 2000 | [97] |
| 95–3000 | cw | HO | QO | OWC, | NRS, FP | G, FIR | 1999 | [98,99,100] |
| 604 | pulse | HE | QO | OWC, DWG | FP | FIR | 1999 | [101] |
| 170, 250 | cw | HO | QO | LS, CWG | FP | G | 1988 | [55,102,103] |
| 250–7000 | cw | HO | QO | OWC | NRS | G, BWO, FIR | 2003 | [104] |
| 38–889 | cw | HO | QO | OWC | NRS | G, GTR | 1998 | [105,106,107] |
| 110, 220, 330 | pulse | HE | QO | CWG | FP | SS | 2008 | [108] |
| 130 | cw | HO | WG | OWR | FP | G | 2018 | [109] |
| 120, 240 | cw, pulse | HE | QO | CWG | NRS | SS | 2014 | [110,111] |
| 240 | cw, pulse | HE | QO | CWG | NRS | FEL, SS | 2008 | [112,113] |
| 82.5–1100 | cw | HO | QO | CWG | NRS | SS | 2018 | [114] |
| 210, 315, 420 | cw | HO | QO | CWG | NRS | SS | 2018 | [115] |
| 150–1200 | cw | HO | QO | CWG | NRS | SIN | 2009 | [116,117] |
| 300–3000 | cw | HO | QO | CWG | NRS | FEL | 2017 | [118] |
| 300–700 | cw | HO | QO | CWG | NRS | BWO | 2015 | [119] |
| 200 | cw, pulse | HE | QO | CWG | NRS | SS | 2016 | [120] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Möbius, K.; Lubitz, W.; Cox, N.; Savitsky, A. Biomolecular EPR Meets NMR at High Magnetic Fields. Magnetochemistry 2018, 4, 50. https://doi.org/10.3390/magnetochemistry4040050
Möbius K, Lubitz W, Cox N, Savitsky A. Biomolecular EPR Meets NMR at High Magnetic Fields. Magnetochemistry. 2018; 4(4):50. https://doi.org/10.3390/magnetochemistry4040050
Chicago/Turabian StyleMöbius, Klaus, Wolfgang Lubitz, Nicholas Cox, and Anton Savitsky. 2018. "Biomolecular EPR Meets NMR at High Magnetic Fields" Magnetochemistry 4, no. 4: 50. https://doi.org/10.3390/magnetochemistry4040050
APA StyleMöbius, K., Lubitz, W., Cox, N., & Savitsky, A. (2018). Biomolecular EPR Meets NMR at High Magnetic Fields. Magnetochemistry, 4(4), 50. https://doi.org/10.3390/magnetochemistry4040050





