Abstract
Two hydrazone Schiff base-bridging ligands with different heterocycles {2-[(E)-(5-chloro-2-hydroxyphenyl)methylidene]diazanyl}(pyrazine-2-yl)methanone (H2LSchiff-1) and (E)-N′-(2-hydroxy-3-methoxybenzylidene)nicotinohydrazide (H2LSchiff-2) together with 1,3-di(2-pyridyl)-1,3-propanedione (Hdpp) were chosen to construct two new Dy2 complexes, [Dy2(LSchiff-1)2(DMF)2(dpp)2]·0.5DMF (1) and [Dy2(LSchiff-2)2(DMF)2(dpp)2]·2DMF (2). Although the [N2O6] coordination spheres are observed for the Dy3+ ions in 1 and 2, their coordination configurations have some differences (both the biaugmented trigonal prism and the Snub diphenoid J84 in 1 and only the biaugmented trigonal prism in 2). Magnetic research revealed that both 1 and 2 possess ferromagnetic interactions between two Dy3+ ions and perform as zero-field single-molecule magnets, with Ueff/k values of 49.7 K at 0 Oe for 1 and 151.8 K at 0 Oe for 2. This work suggests that the heterocycle groups (pyrazine vs. pyridine) on the hydrazone Schiff base-bridging ligands have effects on the SMM properties of 1 and 2.
1. Introduction
Single-molecule magnets (SMMs) are an important branch of molecular magnets, and their magnetic bistable properties at the nanoscale have potential applications in high-density information storage, quantum computing, and other related fields [1]. Since lanthanide ions, especially the dysprosium (III) ion, naturally have significant magnetic anisotropy and multiple unpaired electrons (corresponding to large ground-state spin values), lanthanide SMMs have become the most developed SMMs in recent years [2]. Among them, the energy barrier (Ueff) value and magnetic blocking temperature (TB) of the SMM containing a single lanthanide ion, that is, the single-ion magnet (SIM), can reach a high level [3,4,5,6,7,8], but the sensitivity of metal–organic compounds to air will affect the application of this type of SMMs; while the SMM containing multiple lanthanide ions, requires consideration of the consistency of the magnetic axis orientation of each lanthanide ion [9], its Ueff value and TB value are more difficult to make a big breakthrough. There is no doubt that the magnetic axis orientation consistency of the Ln(III) binuclear SMM is the easiest to achieve among the SMMs with multiple lanthanide (III) ions, which can be achieved through the ferromagnetic interaction between two lanthanide (III) ions [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29]. Notably, the ferromagnetic interaction can partially or completely quench quantum tunneling of magnetization (QTM), activating SMM behavior at zero dc field [11]. However, the magnetic interactions between 4f and 4f electrons are usually difficult; even if magnetic exchange occurs, it often exhibits weak antiferromagnetic coupling.
Hydrazone Schiff base-bridging ligands are commonly used to construct Ln SMMs [30,31]; they have been used to construct some ferromagnetically coupled Dy2 SMMs [10,11,12,13,14,32,33,34,35,36,37,38], the magnetic properties of which may be affected by several factors. For example, the coordination anion [32], and the substituent on the terminal ligands [33] are the most common influencing factors. From the perspective of molecular engineering, heterocycles on hydrazone Schiff base-bridging ligands are also important influencing factors. Recently, we used two hydrazone Schiff bases (Scheme 1) with different heterocycles (pyrazine vs. pyridine) as bridging ligands and both 1,3-di(2-pyridyl)-1,3-propanedione (Hdpp) (Scheme 1) and DMF as terminal ligands to construct two new Dy2 complexes: [Dy2(LSchiff-1)2(DMF)2(dpp)2]·0.5DMF {H2LSchiff-1 = {2-[(E)-(5-chloro-2-hydroxyphenyl)methylidene]diazanyl}(pyrazine-2-yl)methanone} (1) and [Dy2(LSchiff-2)2(DMF)2(dpp)2]·2DMF {H2LSchiff-2 = (E)-N’-(2-hydroxy-3-methoxybenzylidene)nicotinohydrazide} (2), which exhibit ferromagnetic interactions and perform zero-field SMM properties, with Ueff/k values of 49.7 K and 151.8 K at 0 Oe for 1 and 2, respectively, suggesting that the heterocycles on hydrazone Schiff base-bridging ligands have an effect on the magnetic properties of Dy2 SMMs.
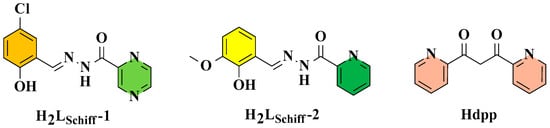
Scheme 1.
Hydrazone Schiff bases (H2LSchiff-1 and H2LSchiff-2) and 1,3-di(2-pyridyl)-1,3-propanedione (Hdpp).
2. Results
2.1. Crystal Structures
Dy2 complexes 1 and 2 are crystallized in P-1 space group and P21/c space group, respectively. As shown in Figure 1, there are two crystallographically independent molecules in 1, each consisting of two Dy3+ ions, two LSchiff-12− bridging ligands, two dpp− terminal ligands, and two coordinated DMF molecules. In addition, the lattice of 1 contains DMF solvent molecules. Notably, both independent molecules are centrosymmetric; consequently, the anisotropy axes of the two ferromagnetic Dy3+ ions in 1 have to be parallel to each other, which may be beneficial to its SMM properties. The asymmetric unit is composed of one Dy3+ ion, one LSchiff-12− bridging ligand, one dpp− terminal ligand, and one coordinated DMF molecule. In each molecule the two LSchiff-12− bridging ligands chelate two Dy3+ ions from opposite positions to form a rough plane; a dpp− terminal ligand and a DMF molecule then coordinate each dysprosium (III) ion from the upper and lower directions of the plane, and a [N2O6] coordination sphere is formed, which is coordinated by one Npyrazine atom and Oacyl atom from one LSchiff-12− ligand, one Nimine atom, one Oacyl atom and one Ophenol atom from the other LSchiff-12− ligand, two O atoms from the dpp− terminal ligand, and one ODMF atom. Further analysis with the SHAPE 2.1 software [39] revealed that the coordination configurations of Dy1 and Dy2 are the biaugmented trigonal prism and Snub diphenoid J84, respectively, with CShM values of 1.818 for the C2v symmetry and 20.565 for the D2d symmetry (Table S1). Notably, the pyridine N atom in the dpp− terminal ligand does not participate in the coordination, unlike some SMMs containing lanthanide (III) ions [40,41]. The Dy1–Dy1#1 (#1 1 − x, 2 − y, 1 − z) distance (3.942 Å) is a little longer than the Dy1-Dy1#2 (#2 −x, 1 − y, 2 − z) distance (3.932 Å). The mean distance of Dy1–O (2.324 Å) is comparable with that of Dy2–O (2.321 Å), and the average distance of Dy1–N (2.541 Å) is comparable with that of Dy2–N (2.541 Å) (Table 1). Notably, the latest CCDC retrieval revealed that 1 represents the first complex derived from the new H2LSchiff-1 ligand.
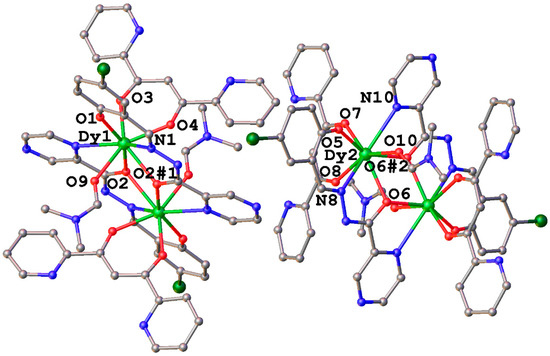
Figure 1.
Crystal structure of 1, all H atoms and solvent molecules are omitted for clarity. Symmetry code: #1 1 − x, 2 − y, 1 − z; #2 −x, 1 − y, 2 − z.

Table 1.
Selected bond lengths (Å) and angles (°) of 1.
As shown in Figure 2, the main structure of 2 is composed of two Dy3+ ions, two LSchiff-22− bridging ligands, two dpp− anions, and two coordinated DMF molecules. Additionally, 2 contains some DMF solvent molecules in its lattice as well. Different from 1, only one crystallographically independent molecule exists in 2; there is a symmetry center in this molecule as well, and the asymmetric unit is composed of one Dy3+ ion, one LSchiff-22− bridging ligand, one dpp− anion, and one coordinated DMF molecule. Similar to 1, the two LSchiff-22− bridging ligands in 2 coordinate two Dy3+ ions to form a rough plane; and a dpp− anion and a DMF molecule then finish the coordination of the Dy3+ ion, generating a [N2O6] coordination sphere. Each Dy3+ cation is coordinated by one Npydine atom and Oacyl atom of one LSchiff-22− ligand, one Nimine atom, one Oacyl atom and one Ophenol atom of the other LSchiff-22− ligand, two O atoms of the dpp− anion, and one ODMF atom. The coordination configuration of Dy1 in 2 is the biaugmented trigonal prism, with a CShM value of 2.247 for the C2v symmetry (Table S2). The Dy1–Dy1#3 (#3 1 − x, 1 − y, 1 − z) distance in 2 (3.885 Å) is slightly shorter than the Dy–Dy distance in 1 (3.942 Å and 3.932 Å) (Table 1 and Table 2). The mean distance of Dy1–O in 2 (2.315 Å) is slightly shorter than those of Dy–O in 1 (2.324 Å and 2.321 Å), and the average distance of Dy1–N in 2 (2.525 Å) is a little smaller than those of Dy–N in 1 (2.541 Å and 2.541 Å) (Table 1 and Table 2). However, the Dy1–O3–Dy1#3 bond angle in 2 {113.95(8)°} is slightly larger than the corresponding Dy–O–Dy bond angles in 1 {112.7(2)° and 112.6(2)°} (Table 1 and Table 2).
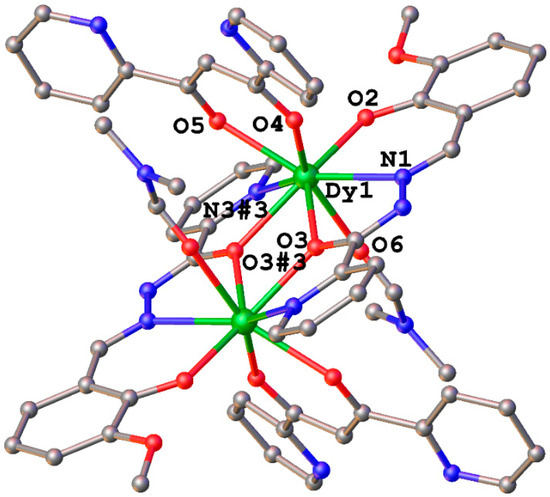
Figure 2.
Crystal structure of 2, all H atoms and solvent molecules are omitted for clarity. Symmetry code: #3 1 − x, 1 − y, 1 − z.

Table 2.
Selected bond lengths (Å) and angles (°) of 2.
2.2. Magnetic Properties
The thermal variation of χMT in the range of 2–300 K for 1 and 2 at 1000 Oe is shown in Figure 3. The χMT values at 300 K are 28.36 cm3 K mol−1 for 1 and 28.37 cm3 K mol−1 for 2, both are very close to the theoretical value of the two separated Dy3+ ions (28.34 cm3 K mol−1). Their χMT values all decrease slowly with the decrease in temperature, reaching minimums of 27.04 cm3 K mol−1 at 42 K for 1 and 28.12 cm3 K mol−1 at 55 K for 2, and then increasing sharply to 40.32 cm3 K mol−1 for 1 and 41.67 cm3 K mol−1 for 2 at 2 K. Such a trend of the χMT–T curve is caused by two factors, the depopulation of Mj levels of the Dy3+ ion and the intramolecular ferromagnetic interaction between the two Dy3+ ions [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28]. More and more ferromagnetic Dy2 SMMs based on hydrazine Schiff base ligands have been explored (Table 3), in which the Dy···Dy distance, the Dy–O bond distance and the Dy–O–Dy angle of the Dy2O2 core are important parameters that determine whether a ferromagnetic interaction is formed or not, because these three factors have a subtle regulation of the overlap between the magnetic orbitals of the two Dy3+ ions, thus affecting their magnetic interactions. As can be seen from Table 3, the ferromagnetic interaction can be formed in the range of 3.864–3.992 Å of the Dy···Dy distance, 2.317–2.380 Å of the average Dy–O bond distance, and 108.9–115.1° of the Dy–O–Dy angle for the eight-coordinate sphere. However, in the case of a nine-coordinate sphere [42], the ferromagnetic interaction may not be formed when the average Dy–O bond length is greater than 2.400 Å (Table 3). The field-variable magnetization of 1 and 2 at different low temperatures was then determined, and their M–H/T curves at 2–6 K do not coincide together (Figures S1 and S2), suggesting possible magnetic anisotropy.
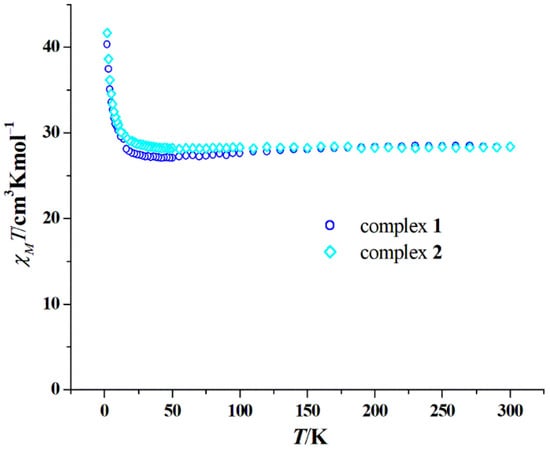
Figure 3.
Plots of χMT versus T of 1 and 2 measured under a 1000 Oe dc field.

Table 3.
Main structural parameters in the Dy2O2 core and nature of the magnetic interactions for some Dy2 SMMs with hydrazine Schiff base ligands.
The measurement of ac magnetic susceptibility revealed that both 1 and 2 are zero-field SMMs. As shown in Figure 4a, at 0 Oe the χ” versus T curves at 50–1399 Hz of 1 show clear frequency dependence, and peaks can be observed in the range of 250–1399 Hz. On the other hand, the χ” versus ν curves at 0 Oe of 1 also display frequency dependence, and peaks can be observed in the range of 2.0–6.5 K (Figure 4b). The ln(τ)–1/T plot based on these χ” versus ν curves is a curve rather than a straight line (Figure 4c), suggesting that the magnetic relaxation of 1 includes other processes such as the quantum tunneling of magnetization (QTM), the Raman process in addition to the Orbach process; therefore, this curve was fitted with τ−1 = τQTM−1 + CTn + τ0−1exp(−Ueff/kT), giving τQTM = 0.0145 s, n = 2.46, C = 1.33 s−1 K−2.46, Ueff/k = 49.7 K and τ0 = 7.9 × 10−6 s. The τ0 value of 1 (7.9 × 10−6 s) is normal for the Dy(III) SMMs (10−6 s–10−11 s) [2], and its n value of 2.46 is also within the normal range (2 < n < 9). Since the crystal structure of 1 contains two types of coordination configurations of the Dy3+ ions, its Cole–Cole curves at 0 Oe could be fitted with the sum of two modified Debye functions [43,44,45,46] (Figure 4d), giving α1 values of 0.276–0.385, and α2 values of 0.001–0.108. Moreover, no hysteresis loop can be formed at 1.9 K for 1 (Figure S3). The magnetic axes of 1 could be calculated with the electrostatic model implemented in the Magellan program [47]; as expected, the two magnetic axes in each independent molecule are arranged in perfect parallel (Figure S4).
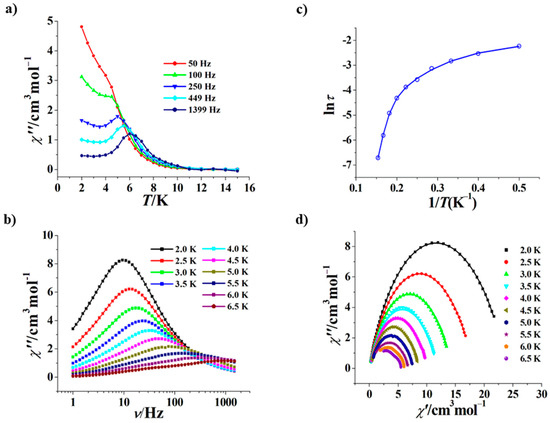
Figure 4.
χ” versus T curves for 1 (Hdc = 0 Oe) (a); χ” versus ν curves at 2.0 to 6.5 K for 1 (Hdc = 0 Oe) (b); plot of ln(τ) versus 1/T for 1 (Hdc = 0 Oe), the solid line represents the best fitting with QTM + Raman + Orbach (c); Cole–Cole curves at 2.0 to 6.5 K for 1 (Hdc = 0 Oe), the solid lines represent the best fitting with the sum of two modified Debye functions [43,44,45,46] (d).
We also investigated the effect of 1500 Oe, a commonly used dc magnetic field, on the magnetic relaxation of 1. As shown in Figure 5a, at 1500 Oe the χ” versus T curves of 1 show more clear frequency dependence, and peaks can be observed in a wider range of 10–1399 Hz with respect to 0 Oe. The χ” versus ν curves at 1500 Oe of 1 are also frequency-dependent, but peaks can be observed in a narrower range of 3.0–6.5 K with respect to 0 Oe (Figure 5b). The ln(τ)–1/T plot at 1500 Oe of 1 is still a curve (Figure S5), which was fitted using τ−1 = AT + CTn + τ0−1exp(−Ueff/kT), giving A = 0.892, n = 5.91, C = 0.00352 s−1 K−5.91, Ueff/k = 87.6 K and τ0 = 2.4 × 10−9 s. Similar to other zero-field Dy2 SMMs [30,32,33,34], the dc magnetic field (here is 1500 Oe) can partially or completely inhibit QTM, resulting in an increase in the Ueff/k value of 1 (from 49.7 K to 87.6 K) and a decrease in the τ0 value (from 7.9 × 10−6 s to 2.4 × 10−9 s).
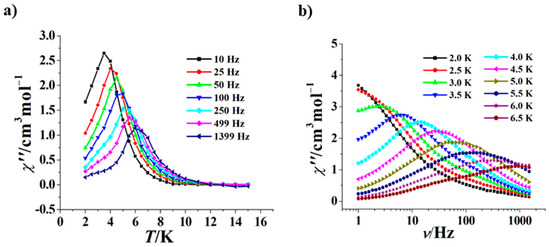
Figure 5.
χ” versus T curves for 1 (Hdc = 1500 Oe) (a); χ” versus ν curves at 2.0 to 6.5 K for 1 (Hdc = 1500 Oe) (b).
The χ” versus T curves at 0 Oe of 2 show obvious frequency dependence (Figure 6a), and peaks appear in the range of 10–1399 Hz, which is larger than that of 1 (250–1399 Hz). On the other hand, the χ” versus ν curves at 0 Oe of 2 are also frequency-dependent (Figure 6b), and peaks appear in the range of 5–15 K; the peak temperatures of 2 are obviously higher than those of 1 at 0 Oe. The ln(τ)–1/T plot derived from the χ” versus ν curves of 2 is a curve as well (Figure 6c), which could be fitted with τ−1 = CTn + τ0−1exp(−Ueff/kT), yielding n = 5.56, C = 0.00014 s−1K−5.56, Ueff/k = 151.8 K, and τ0 = 5.2 × 10−8 s. The τ0 value at 0 Oe of 2 (5.2 × 10−8 s) is smaller than that of 1 (7.9 × 10−6 s); the Ueff/k value at 0 Oe of 2 (151.8 K) is comparable with those of other ferromagnetic Dy2 SMMs based on hydrazine Schiff base ligands and achiral β-diketonate ligands [33] or homochial β-diketonate ligands [36], which is about three times that of 1 (49.7 K). The Cole–Cole curves at 0 Oe of 2 could be fitted by a generalized Debye model [48,49] (Figure 6d), giving small α values in the range of 0.00051–0.01061, suggesting narrow relaxation time distribution. In addition, at 1.9 K no hysteresis loop may be observed for 2 (Figure S6). Due to the presence of a center of symmetry in the molecule, the two magnetic axes of 2 are also perfectly parallel (Figure S7).
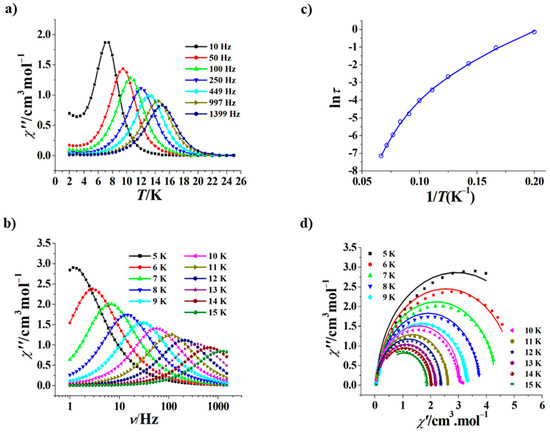
Figure 6.
χ” versus T curves for 2 (Hdc = 0 Oe) (a); χ” versus ν curves at 5 to 15 K for 2 (Hdc = 0 Oe) (b); plot of ln(τ) versus 1/T for 2 (Hdc = 0 Oe), the solid line represents the best fitting with Raman + Orbach (c); Cole–Cole curves at 5 to 15 K for 2 (Hdc = 0 Oe), the solid lines represent the best fitting with a generalized Debye model [48,49] (d).
Similarly, we also studied the effect of 1500 Oe dc field on the magnetic relaxation of 2. As shown in Figure 7a, at 1500 Oe the χ” versus T curves of 2 possess obvious frequency dependence, and peaks may be observed in the same range of 10–1399 Hz as at 0 Oe. The χ” versus ν curves at 1500 Oe of 2 are frequency-dependent as well, but peaks appear in a narrower range of 6–15 K with respect to 0 Oe (Figure 7b). The ln(τ)–1/T plot at 1500 Oe of 2 is also a curve (Figure S8), which could also be fitted with τ−1 = AT + CTn + τ0−1exp(−Ueff/kT), calculating A = 0.211, n = 5.95, C = 0.00006 s−1 K−5.95, Ueff/k = 157.9 K, and τ0 = 5.1 × 10−8 s. Similar to 1, the dc magnetic field of 1500 Oe may partially or completely inhibit QTM, which results in an increase in the Ueff/k value of 2 (from 151.8 K to 157.9 K) and a decrease in the τ0 value (from 5.2 × 10−8 s to 5.1 × 10−8 s). However, the variation amplitude of 2 is smaller than that of 1, indicating that the QTM effect of 2 is much weaker than that of 1.
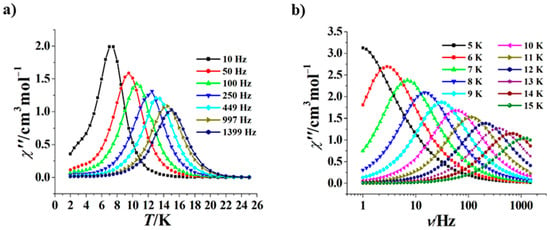
Figure 7.
χ” versus T curves for 2 (Hdc = 1500 Oe) (a); χ” versus ν curves at 5 to 15 K for 2 (Hdc = 1500 Oe) (b).
It can be seen that although both 1 and 2 have a [N2O6] coordination sphere, the change in heterocyclic groups (pyrazine vs. pyridine) on the hydrazone Schiff base-bridging ligand can lead to significant changes in the SMM properties of 1 and 2 (e.g., magnetic relaxation, QTM, energy barrier value, etc.). This is related to the difference in their coordination configurations.
3. Conclusions
In summary, two new Dy2 zero-field SMMs have been assembled using two hydrazone Schiff base-bridging ligands with different heterocycles (pyrazine vs. pyridine) as the bridging ligand and 1,3-di(2-pyridyl)-1,3-propanedione as the terminal ligand. The ferromagnetic interactions between two Dy3+ ions exist in both complexes. As expected, they have different Ueff/k values (49.7 K and 151.8 K at 0 Oe for 1 and 2, respectively). This work demonstrates that the Dy2 SMMs’ magnetic properties can be adjusted by the change in the heterocycle groups (pyrazine vs. pyridine) on the hydrazone Schiff base-bridging ligands. Such molecular engineering provides a good way to design lanthanide SMMs with better performance.
4. Materials and Methods
4.1. General Remarks
The C, H, N elemental analyses were carried out on a Thermo Flash EA1112 elemental analyzer (Thermo Fisher Scientific, Waltham, MA, USA). The infrared spectra were performed on a Bruker VERTEX 70v spectrophotometer (Bruker, Karlsruhe, Germany) with pressed KBr disk. The magnetic susceptibility measurements were measured on a Quantum Design MPMS-XL5 SQUID magnetometer (Quantum Design, San Diego, CA, USA). Diamagnetic corrections were estimated using Pascal’s constants for all constituent atoms of 1 and 2.
4.2. Preparation of H2LSchiff-1
A mixture of 5-chlorosalicylaldehyde (1565.7 mg, 10 mmol) and pyrazinoic acid hydrazide (1381.3 mg, 10 mmol) was added into 60 mL of MeOH; the mixture was refluxed for 3 h to form a light yellow precipitate, which was naturally cooled and then collected by filtration. Yield: 90%. Anal. Calcd. (%) for C12H9ClN4O2 (H2LSchiff-1): C, 52.09; H, 3.28; N, 20.25%. Found: C, 52.01; H, 3.32; N, 20.17%.
4.3. Preparation of 1
H2LSchiff-1 (66.9 mg, 0.25 mmol), 1,3-di(2-pyridyl)-1,3-propanedione (56.6 mg, 0.25 mmol), LiOH·H2O (31.5 mg, 0.75 mmol), and Dy(ClO4)3·6H2O (142.2 mg, 0.25 mmol) were added into 15 mL of DMF, this mixture was stirred for 10 h to form yellow precipitates, which were dissolved after adding 20 mL of methanol and 20 mL of CH2Cl2 to obtain a yellow solution, and yellow single crystals were grown after slowly volatilizing the solvent. Yield: 55% based on Dy. Anal. Calcd. (%) for C57.5H47.5Cl2Dy2N14.5O10.5 (1): C, 45.87; H, 3.18; N, 13.49%. Found: C, 45.81; H, 3.22; N, 13.43%. IR (KBr): ν = 3422(b,w), 3052(w), 3000(w), 2929(w), 2889(w), 1659(s), 1606(s), 1553(s), 1524(s), 1507(s), 1470(s), 1399(s), 1365(m), 1340(m), 1311(w), 1284(w), 1255(w), 1240(w), 1171(w), 1156(w), 1112(w), 1088(w), 1050(w), 1032(w), 1007(w), 994(w), 973(w), 948(w), 906(w), 875(w), 861(w), 828(w), 808(w), 774(w), 752(m), 716(m), 684(w), 653(w), 610(w), 553(w), 534(w), 506(w), 474(w), 451(w), 429(w) cm−1.
4.4. Preparation of 2
H2LSchiff-2 (64.8 mg, 0.25 mmol), 1,3-di(2-pyridyl)-1,3-propanedione (56.6 mg, 0.25 mmol), LiOH·H2O (31.5 mg, 0.75 mmol), and Dy(ClO4)3·6H2O (142.2 mg, 0.25 mmol) were added into 5 mL of DMF; this mixture was stirred for 10 h to form yellow precipitates, which were dissolved after adding 15 mL of methanol and 20 mL of CH2Cl2 to obtain a yellow solution, and yellow single crystals were grown after slowly volatilizing the solvent. Yield: 45% based on Dy. Anal. Calcd. (%) for C66H68Dy2N14O14 (2): C, 49.35; H, 4.27; N, 12.21%. Found: C, 49.25; H, 4.31; N, 12.15%. IR (KBr): ν = 3415(b,w), 3131(w), 3045(w), 2998(w), 2930(w), 2898(w), 2826(w), 1656(s), 1603(s), 1555(s), 1521(s), 1505(s), 1473(s), 1453(s), 1402(s), 1343(m), 1315(w), 1286(w), 1235(m), 1212(m), 1163(w), 1102(w), 1082(w), 1045(w), 1015(w), 993(w), 968(w), 949(w), 919(w), 861(w), 806(w), 740(m), 718(w), 694(w), 668(w), 635(w), 610(w), 587(w), 536(w), 493(w), 475(w), 456(w), 426(w) cm−1.
4.5. X-Ray Crystallography
The intensity data collection of 1 and 2 was curried on a Rigaku MM007HF diffractometer (Rigaku, Tokyo, Japan) with Mo-Kα radiation (λ = 0.71073 Å) at 170 K. The structures were solved using the olex2.solve structure solution program and refined using the ShelXL-2018 refinement package. All non-hydrogen atoms were refined anisotropically, and the hydrogen atoms were refined as riding atoms. Selected crystal data and structural refinement parameters for 1 and 2 are listed in Table 4.

Table 4.
Crystal data and structural refinement parameters for 1 and 2.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/magnetochemistry11070058/s1, Table S1, Dy (III) ion geometry analysis by SHAPE 2.1 software for 1; Table S2, Dy (III) ion geometry analysis by SHAPE 2.1 software for 2; Figure S1, M versus H/T plots at 2–6 K of 1; Figure S2, M versus H/T plots at 2–6 K of 2; Figure S3, Hysteresis loop for 1 at 1.9 K; Figure S4, Magnetic axes of the Dy3+ ions in 1 calculated using an electrostatic model; Figure S5, Plot of lnτ versus 1/T for 1 (Hdc = 1500 Oe); Figure S6, Hysteresis loop for 2 at 1.9 K; Figure S7, Magnetic axes of the Dy3+ ions in 2 calculated using an electrostatic model; Figure S8, plot of lnτ versus 1/T for 2 (Hdc = 1500 Oe). CCDC 2454030-2454031 contain the supplementary crystallographic data for 1 and 2. These data can be obtained free of charge via https://www.ccdc.cam.ac.uk/, or from the Cambridge Crystallographic Data Center, 12 Union Road, Cambridge CB2 1EZ, UK; e-mail: deposit@ccdc.cam.ac.uk.
Funding
This research was funded by the National Natural Science Foundation of China (22271289 and 21871274).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Sessoli, R.; Gatteschi, D.; Caneschi, A.; Novak, M.A. Magnetic Bistability in a Metal-Ion Cluster. Nature 1993, 365, 141–143. [Google Scholar] [CrossRef]
- Woodruff, D.N.; Winpenny, R.E.P.; Layfield, R.A. Lanthanide Single-Molecule Magnets. Chem. Rev. 2013, 113, 5110–5148. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.-D.; Wang, B.-W.; Sun, H.-L.; Wang, Z.-M.; Gao, S. An Organometallic Single-Ion Magnet. J. Am. Chem. Soc. 2011, 133, 4730–4733. [Google Scholar] [CrossRef]
- Ding, Y.-S.; Chilton, N.F.; Winpenny, R.E.P.; Zheng, Y.-Z. On Approaching the Limit of Molecular Magnetic Anisotropy: A Near-Perfect Pentagonal Bipyramidal Dysprosium(III) Single-Molecule Magnet. Angew. Chem. Int. Ed. 2016, 55, 16071–16074. [Google Scholar] [CrossRef]
- Goodwin, C.A.P.; Ortu, F.; Reta, D.; Chilton, N.F.; Mills, D.P. Molecular magnetic hysteresis at 60 kelvin in dysprosocenium. Nature 2017, 548, 439–442. [Google Scholar] [CrossRef]
- Guo, F.-S.; Day, B.M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. Magnetic hysteresis up to 80 kelvin in a dysprosium metallocene single-molecule magnet. Science 2018, 362, 1400–1403. [Google Scholar] [CrossRef]
- Canaj, A.B.; Dey, S.; Martí, E.R.; Wilson, C.; Rajaraman, G.; Murrie, M. Insight into D6h Symmetry: Targeting Strong Axiality in Stable Dysprosium(III) Hexagonal Bipyramidal Single-Ion Magnets. Angew. Chem. Int. Ed. 2019, 58, 14146–14151. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhao, C.; Feng, T.; Liu, X.; Ying, X.; Li, X.-L.; Zhang, Y.-Q.; Tang, J. Air-Stable Chiral Single-Molecule Magnets with Record Anisotropy Barrier Exceeding 1800 K. J. Am. Chem. Soc. 2021, 143, 10077–10082. [Google Scholar] [CrossRef]
- Liu, C.-M.; Zhang, D.-Q.; Hao, X.; Zhu, D.-B. Arraying Octahedral {Cr2Dy4} Units into 3D Single-Molecule-Magnet-Like Inorganic Compounds with Sulfate Bridges. Inorg. Chem. 2018, 57, 6803–6806. [Google Scholar] [CrossRef]
- Lin, P.-H.; Burchell, T.J.; Clérac, R.; Murugesu, M. Dinuclear Dysprosium(III) Single-Molecule Magnets with a Large Anisotropic Barrier. Angew. Chem. Int. Ed. 2008, 47, 8848–8851. [Google Scholar] [CrossRef]
- Guo, Y.-N.; Xu, G.-F.; Wernsdorfer, W.; Ungur, L.; Guo, Y.; Tang, J.; Zhang, H.-J.; Chibotaru, L.F.; Powell, A.K. Strong Axiality and Ising Exchange Interaction Suppress Zero-Field Tunneling of Magnetization of an Asymmetric Dy2 Single-Molecule Magnet. J. Am. Chem. Soc. 2011, 133, 11948–11951. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Zhao, L.; Chen, P.; Guo, Y.-N.; Guo, Y.; Li, Y.-H.; Tang, J. Phenoxido and alkoxido-bridged dinuclear dysprosium complexes showing single-molecule magnet behavior. Dalton Trans. 2012, 41, 2966–2971. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Li, X.-L.; Liu, Z.; Mansikkamäki, A.; Tang, J. An investigation into the magnetic interactions in a series of Dy2 single-molecule magnets. Dalton Trans. 2020, 49, 10477–10485. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jung, J.; Zhang, P.; Guo, M.; Zhao, L.; Tang, J.; Le Guennic, B. Site-Resolved Two-Step Relaxation Process in an Asymmetric Dy2 Single-Molecule Magnet. Chem. Eur. J. 2016, 22, 1392–1398. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, Y.-Q.; Sun, W.; Liu, H.; Chen, X. Syntheses, structures and magnetic properties of macrocyclic Schiff base-supported homodinuclear lanthanide complexes. Dalton Trans. 2018, 47, 11696–11704. [Google Scholar] [CrossRef]
- Ghosh, S.; Mandal, S.; Singh, M.K.; Liu, C.-M.; Rajaraman, G.; Mohanta, S. Experimental and theoretical exploration of magnetic exchange interactions and single-molecule magnetic behaviour of bis(η1:η2:μ2-carboxylate)GdIII2/DyIII2 systems. Dalton Trans. 2018, 47, 11455–11469. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Chen, Y.; Zhang, X.; Li, Y.; Liu, W.; Dong, Y. A series of dinuclear lanthanide complexes with slow magnetic relaxation for Dy2 and Ho2. Dalton Trans. 2016, 45, 16463–16470. [Google Scholar] [CrossRef]
- Liu, C.-M.; Zhang, D.-Q.; Hao, X.; Zhu, D.-B. Simultaneous assembly of mononuclear and dinuclear dysprosium(III) complexes behaving as single-molecule magnets in a one-pot hydrothermal synthesis. Sci. China Chem. 2017, 60, 358–365. [Google Scholar] [CrossRef]
- Katoh, K.; Aizawa, Y.; Morita, T.; Breedlove, B.K.; Yamashita, M. Elucidation of Dual Magnetic Relaxation Processes in Dinuclear Dysprosium(III) Phthalocyaninato Triple-Decker Single-Molecule Magnets Depending on the Octacoordination Geometry. Chem. Eur. J. 2017, 23, 15377–15386. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Han, C.-B.; Zhang, Y.-Q.; Liu, Q.-Y.; Liu, C.-M.; Yin, S.-G. Fine-Tuning Ligand to Modulate the Magnetic Anisotropy in a Carboxylate-Bridged Dy2 Single-Molecule Magnet System. Inorg. Chem. 2016, 55, 5578–5584. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, Y.-Q.; Zhu, Z.; Tang, J. Dysprosium Compounds with Hula-Hoop-like Geometries: The Influence of Magnetic Anisotropy and Magnetic Interactions on Magnetic Relaxation. Inorg. Chem. 2018, 57, 12213–12221. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Sun, W.; Yu, M.; Li, G.; Yan, P.; Murugesu, M. An unsymmetrical coordination environment leading to two slow relaxation modes in a Dy2 single-molecule magnet. Chem. Commun. 2011, 47, 10993–10995. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Bao, S.-S.; Hoshino, N.; Akutagawa, T.; Wang, B.; Ding, Y.-C.; Wei, S.; Zheng, L.-M. Solvent Responsive Magnetic Dynamics of a Dinuclear Dysprosium Single-Molecule Magnet. Chem. Eur. J. 2013, 19, 9619–9628. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-B.; Jiang, X.-M.; Zhu, S.-D.; Du, Z.-Y.; Liu, C.-M.; Xie, Y.-R.; Liu, L.-X. Anion Effects on Lanthanide(III) Tetrazole-1-acetate Dinuclear Complexes Showing Slow Magnetic Relaxation and Photofluorescent Emission. Inorg. Chem. 2016, 55, 3738–3749. [Google Scholar] [CrossRef]
- Kong, M.; Feng, X.; Wang, J.; Zhang, Y.-Q.; Song, Y. Tuning magnetic anisotropy via terminal ligands along the Dy⋯Dy orientation in novel centrosymmetric [Dy2] single molecule magnets. Dalton Trans. 2021, 50, 568–577. [Google Scholar] [CrossRef]
- Liu, C.-M.; Sun, R.; Wang, B.-W.; Wu, F.; Hao, X.; Shen, Z. Homochiral Ferromagnetic Coupling Dy2 Single-Molecule Magnets with Strong Magneto-Optical Faraday Effects at Room Temperature. Inorg. Chem. 2021, 60, 12039–12048. [Google Scholar] [CrossRef]
- Tu, H.-R.; Sun, W.-B.; Li, H.-F.; Chen, P.; Tian, Y.-M.; Zhang, W.-Y.; Zhang, Y.-Q.; Yan, P.-F. Complementation and joint contribution of appropriate intramolecular coupling and local ion symmetry to improve magnetic relaxation in a series of dinuclear Dy2 single-molecule magnets. Inorg. Chem. Front. 2017, 4, 499–508. [Google Scholar] [CrossRef]
- Zhou, Y.-Q.; Deng, W.; Du, S.-N.; Chen, Y.-C.; Ruan, Z.-Y.; Wu, S.-G.; Liu, J.-L.; Tong, M.-L. An alkoxyborate-bridging Dy2 single-molecule magnet with ferromagnetic coupling. Inorg. Chem. Front. 2024, 11, 1061–1069. [Google Scholar] [CrossRef]
- Faraonov, M.A.; Martynov, A.G.; Polovkova, M.A.; Khasanov, S.S.; Gorbunova, Y.G.; Tsivadze, A.Y.; Otsuka, A.; Yamochi, H.; Kitagawa, H.; Konarev, D.V. Single-Molecule Magnets Based on Heteroleptic Terbium(III) Trisphthalocyaninate in Solvent-Free and Solvent-Containing Forms. Magnetochemistry 2023, 9, 36. [Google Scholar] [CrossRef]
- Liu, C.-M. Assembling 4f and 3d–4f clusters as single-molecule magnets by automatic fixation of atmospheric CO2. Dalton Trans. 2025, 54, 9850–9855. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, Y.-N.; Tang, J. Recent advances in dysprosium-based single molecule magnets: Structural overview and synthetic strategies. Coord. Chem. Rev. 2013, 257, 1728–1763. [Google Scholar] [CrossRef]
- Liu, C.-M.; Hao, X.; Zhu, D.-M.; Zhang, Y.-Q. Effect of coordinated anions on ferromagnetically coupled Dy2 zero-field single-molecule magnets. Dalton Trans. 2024, 53, 6120–6127. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Wu, D.-F.; Gou, J.; Duan, Y.-Y.; Li, L.; Chen, H.-H.; Gao, H.-L.; Cui, J.-Z. Modulation of the properties of dinuclear lanthanide complexes through utilizing different β-diketonate co-ligands: Near-infrared luminescence and magnetization dynamics. Dalton Trans. 2020, 49, 2850–2861. [Google Scholar] [CrossRef]
- Liu, C.-M.; Zou, H.-H.; Zhang, Y.-Q.; Hao, X.; Ren, X.-M. Homochiral Dy2 Schiff Base Complexes Based on (R)/(S)-Chlocyphos: Single-Molecule Magnet Behaviours, Proton Conductivities and MCD Effects. Chin. J. Chem. 2025, 43, 1051–1058. [Google Scholar] [CrossRef]
- Liu, C.-M.; Hao, X.; Zhang, Y.-Q. Homochiral Dy2 zero-field single-molecule magnets derived from axial chiral ligands (R)/(S)-octahydro-1,1′-bi-2-naphthyl phosphate. Dalton Trans. 2025, 54, 4159–4166. [Google Scholar] [CrossRef]
- Liu, C.-M.; Sun, R.; Hao, X.; Li, X.-L.; Wang, B.-W. Homochiral Dy2 single-molecule magnets with strong magneto-optical Faraday effects and strong third-harmonic generation. Inorg. Chem. Front. 2024, 11, 3296–3308. [Google Scholar] [CrossRef]
- Cen, P.; Liu, X.; Zhang, Y.-Q.; Ferrando-Soria, J.; Xie, G.; Chen, S.; Pardo, E. Modulating magnetic dynamics through tailoring the terminal ligands in Dy2 single-molecule magnets. Dalton Trans. 2020, 49, 808–816. [Google Scholar] [CrossRef]
- Liu, M.-J.; Yuan, J.; Tao, J.; Zhang, Y.-Q.; Liu, C.-M.; Kou, H.-Z. Rhodamine Salicylaldehyde Hydrazone Dy(III) Complexes: Fluorescence and Magnetism. Inorg. Chem. 2018, 57, 4061–4069. [Google Scholar] [CrossRef]
- Casanova, D.; Llunell, M.; Alemany, P.; Alvarez, S. The Rich Stereochemistry of Eight-Vertex Polyhedra: A Continuous Shape Measures Study. Chem. Eur. J. 2005, 11, 1479–1494. [Google Scholar] [CrossRef]
- Caporale, C.; Sobolev, A.N.; Phonsri, W.; Murray, K.S.; Swain, A.; Rajaraman, G.; Ogden, M.I.; Massi, M.; Fuller, R.O. Lanthanoid pyridyl-β-diketonate ‘triangles’. New examples of single molecule toroics. Dalton Trans. 2020, 49, 17421–17432. [Google Scholar] [CrossRef]
- Mao, P.-D.; Yao, N.-T.; Sun, H.-Y.; Yan, F.-F.; Zhang, Y.-Q.; Meng, Y.-S.; Liu, T. Design of Heterometallic {LnIII-MV} (Ln = Dy, Er; M = W, Mo) Molecular Nanomagnets: Protonation Induced Structural Diversification. Cryst. Growth Des. 2022, 23, 450–464. [Google Scholar] [CrossRef]
- Kumar, P.; Swain, A.; Acharya, J.; Li, Y.; Kumar, V.; Rajaraman, G.; Colacio, E.; Chandrasekhar, V. Synthesis, Structure, and Zero-Field SMM Behavior of Homometallic Dy2, Dy4, and Dy6 Complexes. Inorg. Chem. 2022, 61, 11600–11621. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-N.; Xu, G.-F.; Gamez, P.; Zhao, L.; Lin, S.-Y.; Deng, R.; Tang, J.; Zhang, H.-J. Two-Step Relaxation in a Linear Tetranuclear Dysprosium(III) Aggregate Showing Single-Molecule Magnet Behavior. J. Am. Chem. Soc. 2010, 132, 8538–8539. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-M.; Zhang, D.-Q.; Zhu, D.-B. A single-molecule magnet featuring a parallelogram [Dy4(OCH2−)4] core and two magnetic relaxation processes. Dalton Trans. 2013, 42, 14813–14818. [Google Scholar] [CrossRef]
- Langley, S.K.; Chilton, N.F.; Moubaraki, B.; Murray, K.S. Single-Molecule Magnetism in Three Related {Co2IIIDy2III}-Acetylacetonate Complexes with Multiple Relaxation Mechanisms. Inorg. Chem. 2013, 52, 7183–7192. [Google Scholar] [CrossRef]
- Jin, Y.-S.; Liu, C.-M.; Zhang, Y.-Q.; Kou, H.-Z. Trinuclear Dy(III) Single- Molecule Magnets with Two-Step Relaxation. Chin. J. Chem. 2023, 41, 2641–2647. [Google Scholar] [CrossRef]
- Chilton, N.F.; Collison, D.; McInnes, E.J.L.; Winpenny, R.E.P.; Soncini, A. An electrostatic model for the determination of magnetic anisotropy in dysprosium complexes. Nat. Commun. 2013, 4, 2551. [Google Scholar] [CrossRef]
- Cole, K.S.; Cole, R.H. Dispersion and Absorption in Dielectrics I. Alternating Current Characteristics. J. Chem. Phys. 1941, 9, 341–351. [Google Scholar] [CrossRef]
- Aubin, S.M.; Sun, Z.; Pardi, L.; Krzysteck, J.; Folting, K.; Brunel, L.-J.; Rheingold, A.L.; Christou, G.; Hendrickson, D.N. Reduced Anionic Mn12 Molecules with Half-Integer Ground States as Single-Molecule Magnets. Inorg. Chem. 1999, 38, 5329–5340. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).