The Efficient Degradation of Oxytetracycline in Wastewater Using Fe/Mn-Modified Magnetic Oak Biochar: Pathways and Mechanistic Investigation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Characterization
2.3. Synthesis of Fe/Mn Bimetal-Modified Biochar
2.4. Experimental Procedure
3. Results and Discussion
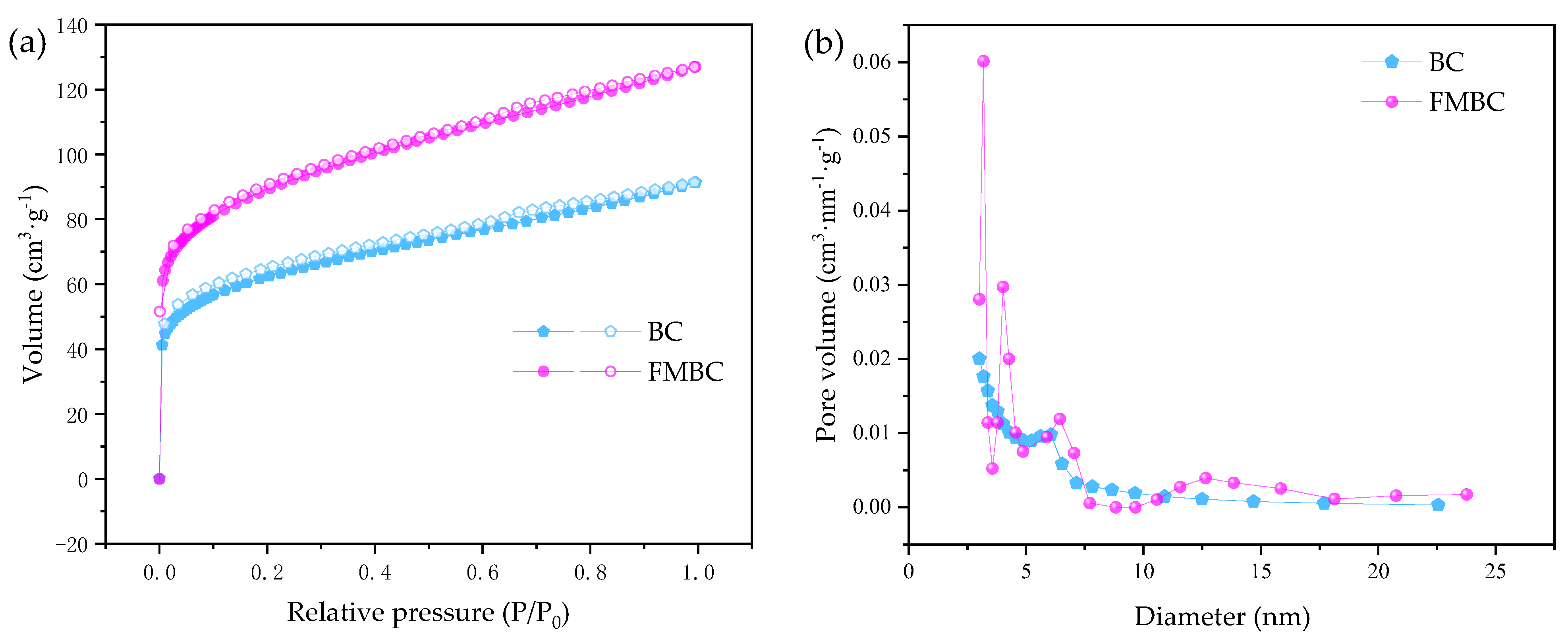
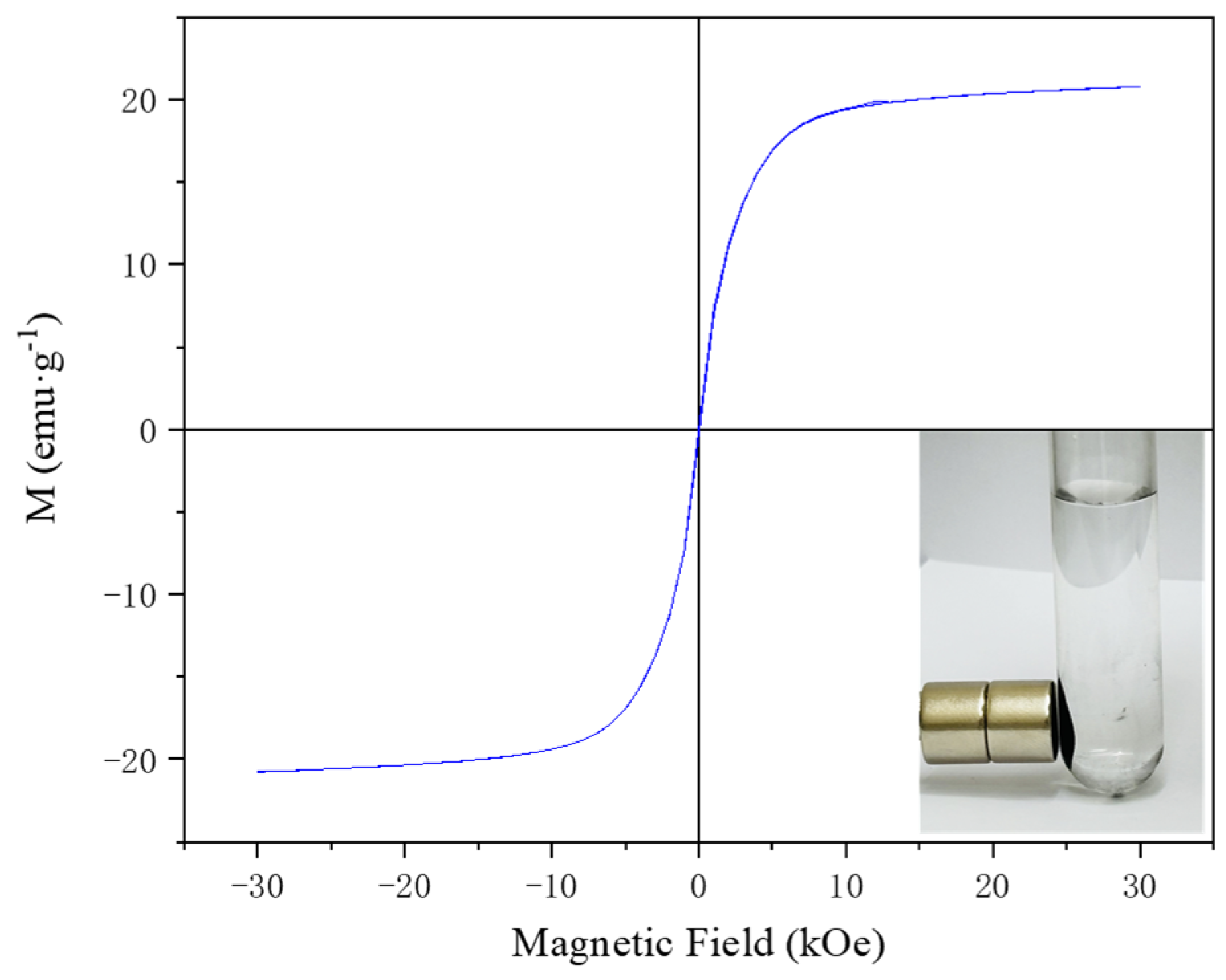
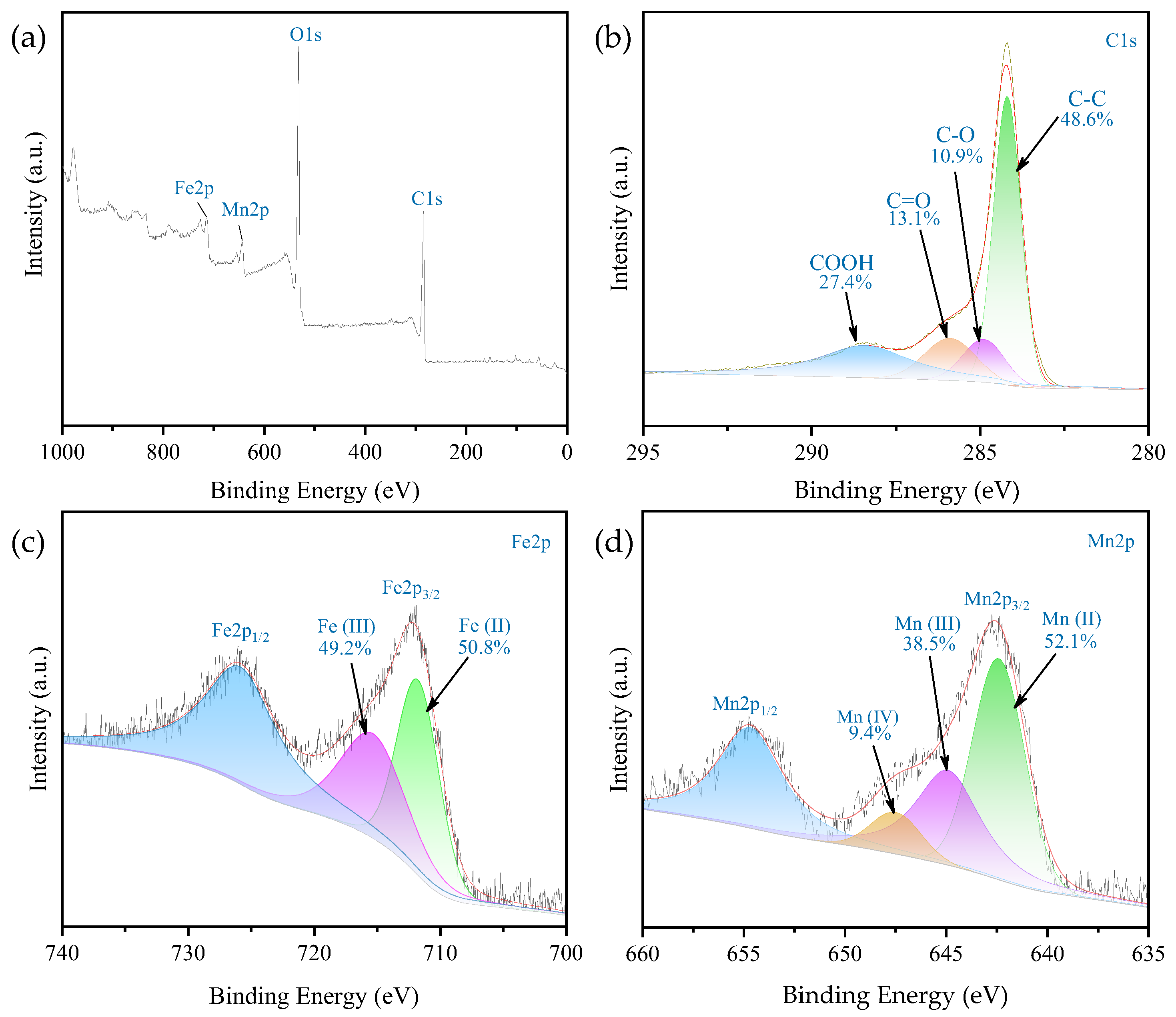
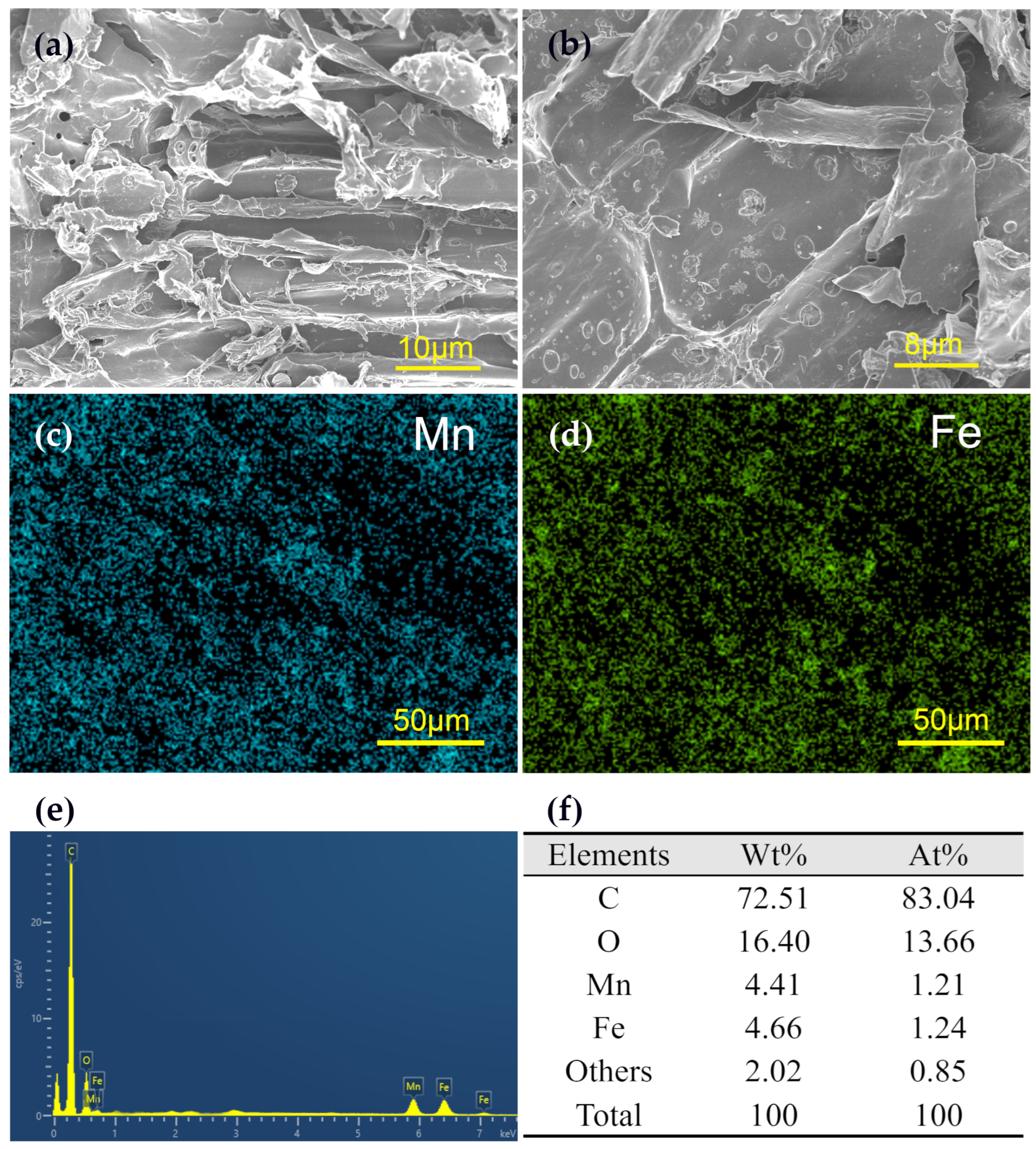
3.1. Characterization of Materials
3.2. Influence of Experimental Conditions
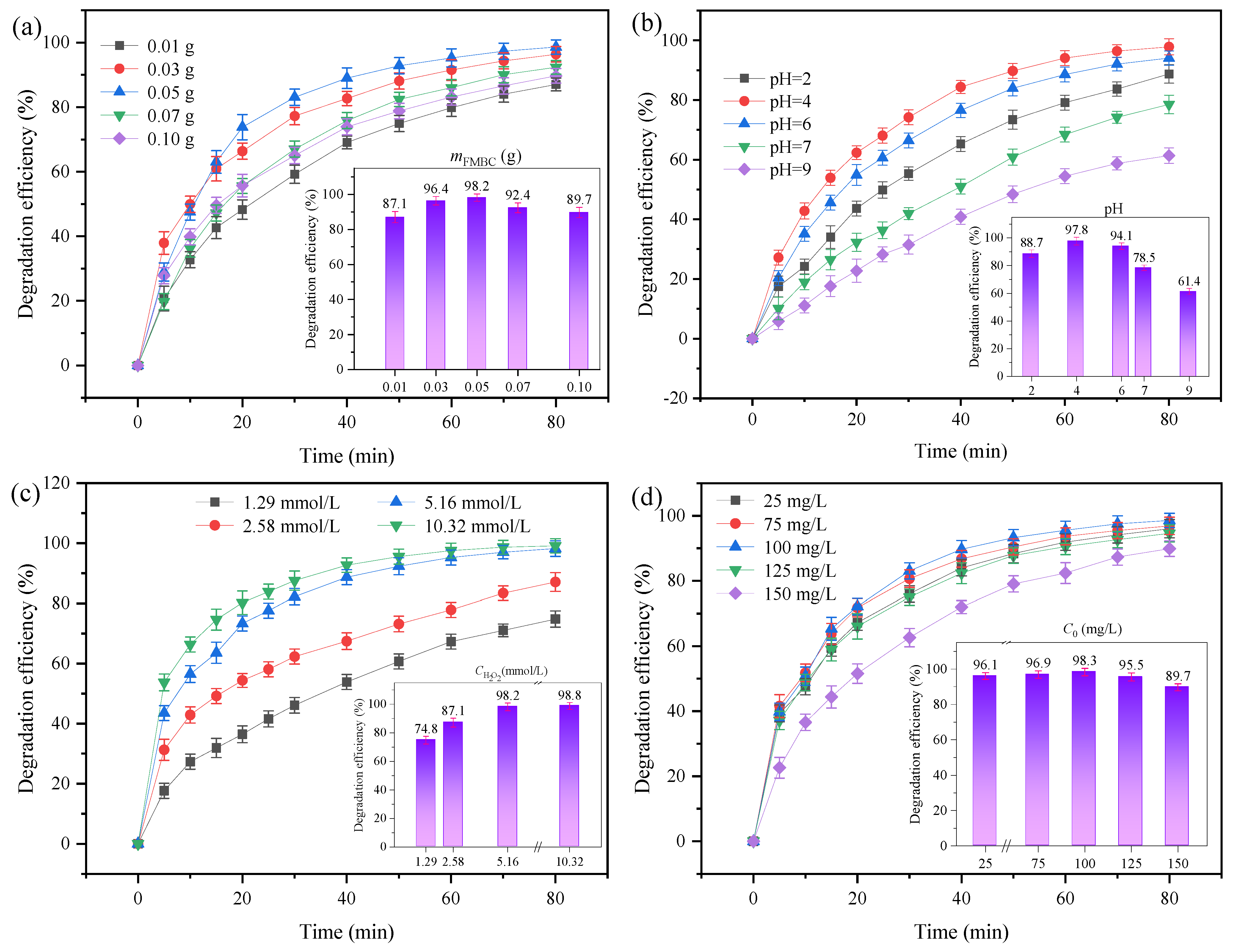
3.2.1. Influence of FMBC Dosage and Fe/Mn Ratios
3.2.2. Influence of pH on the System Performance
3.2.3. Effect of H2O2 on the System Performance
3.2.4. Effects of Different Concentrations of OTC
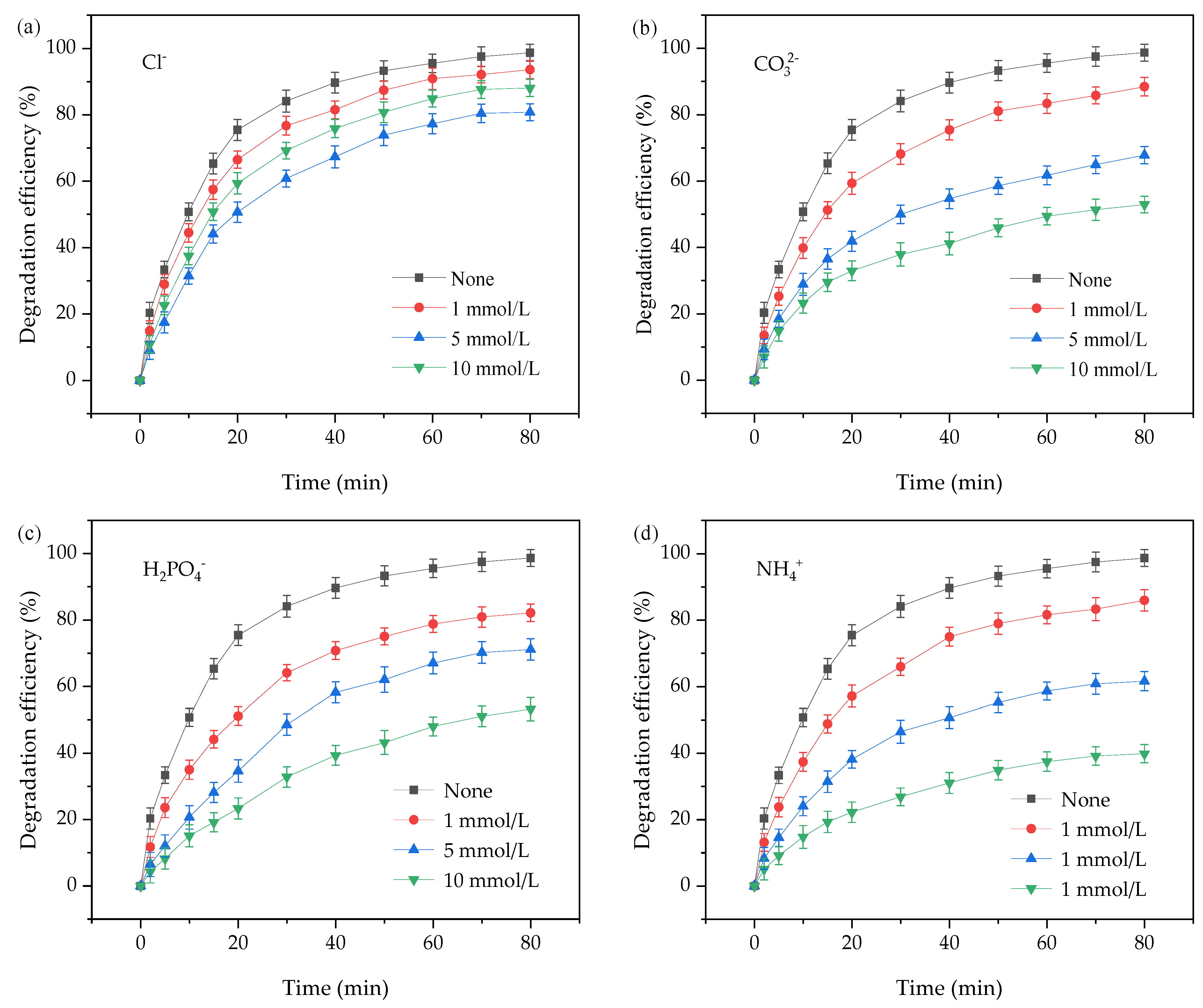
3.2.5. Effects of Various Ions on the Degradation of OTC
3.3. The KINETICS Study of the OTC Decomposition
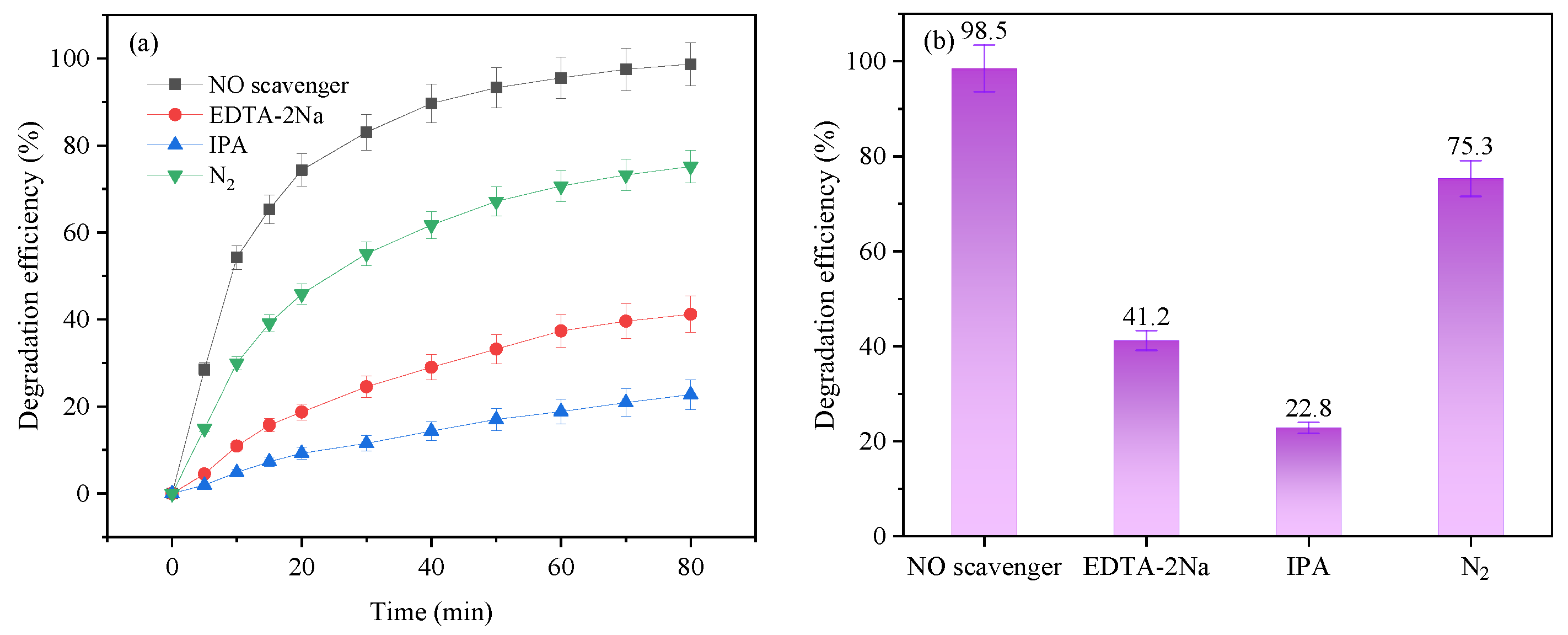
3.4. The Degradation Mechanism of Oxytetracycline (OTC)
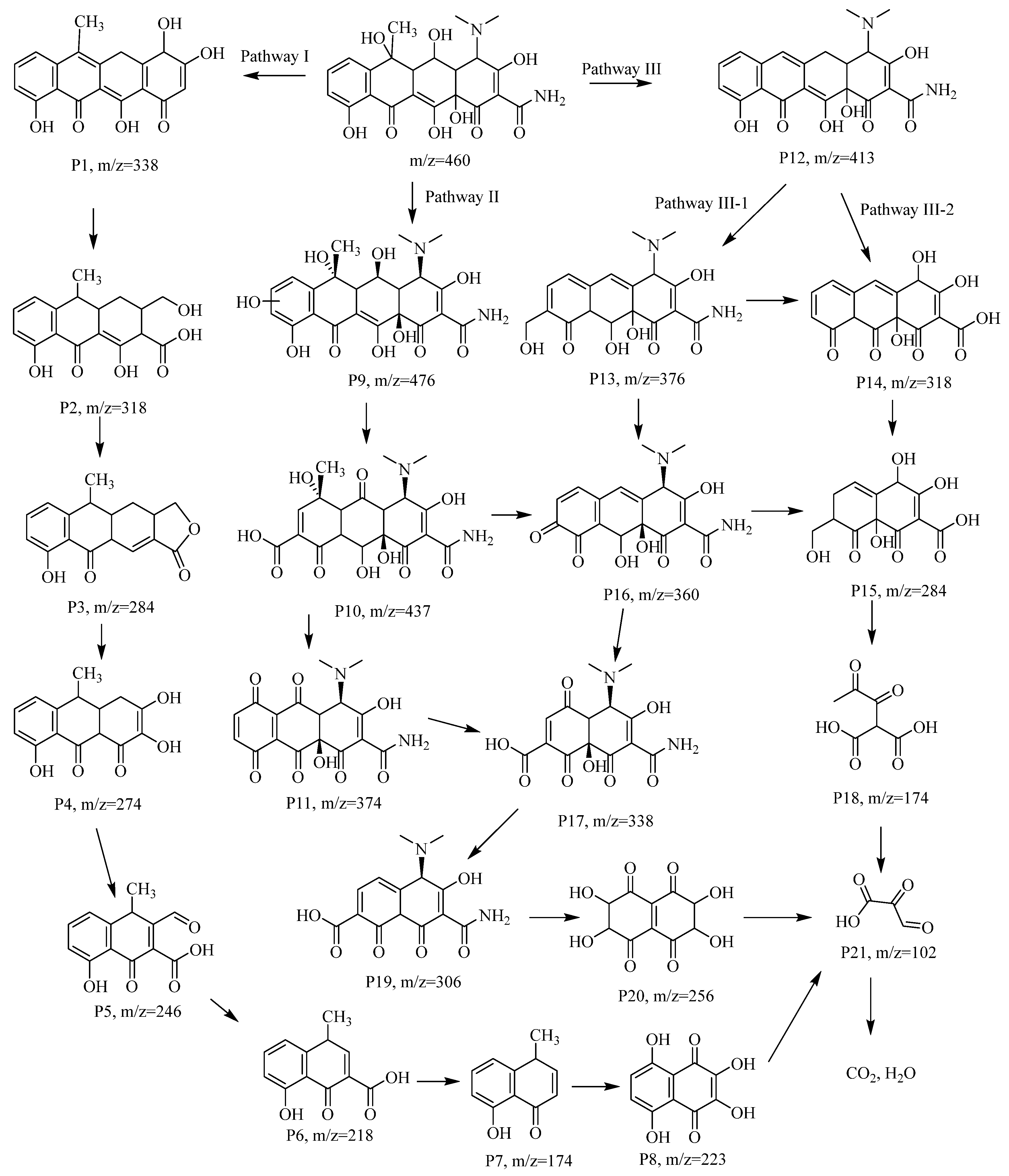
3.5. The OTC Degradation Pathways
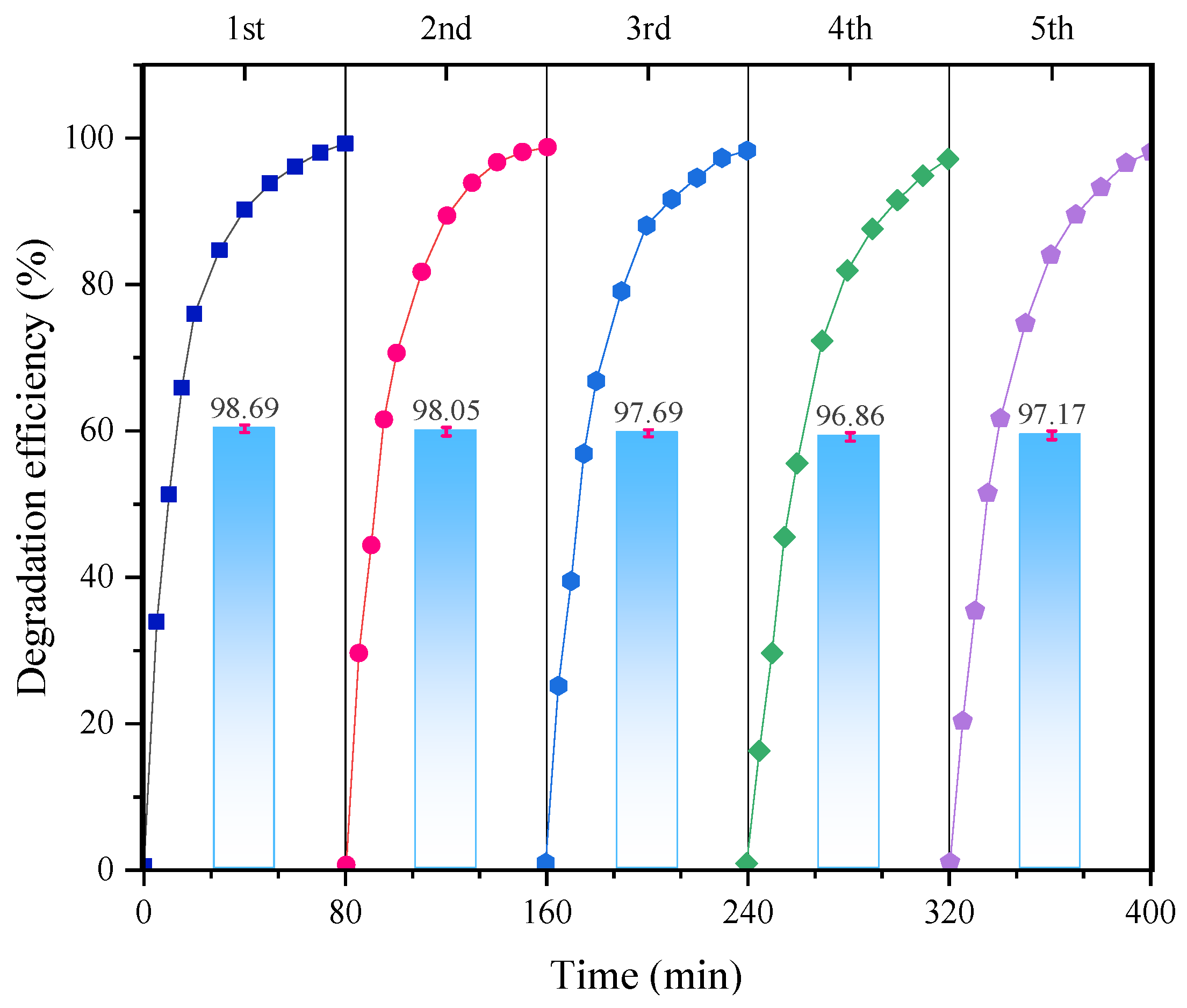
3.6. Stability and Reusability of FMBC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thangsan, P.; Wannakan, K.; Nanan, S. Biosynthesis of ZnO using Senna siamea leaf extract for photodegradation of tetracycline antibiotic and azo dye in wastewater. OpenNano 2024, 16, 100202. [Google Scholar] [CrossRef]
- Shen, L.; Shen, D.; Chen, K.; Li, M.; Ding, J.; Zhang, B.; Guo, L. Composite photocatalysts for efficient degradation of emerging contaminant tetracyclines: From material design to degradation mechanisms. Environ. Chem. 2023, 42, 2859–2875. [Google Scholar]
- Pérez-Rodríguez, M.; Pellerano, R.G.; Pezza, L.; Pezza, H.R. An overview of the main foodstuff sample preparation technologies for tetracycline residue determination. Talanta 2018, 182, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Jafari Ozumchelouei, E.; Hamidian, A.H.; Zhang, Y.; Yang, M. Physicochemical properties of antibiotics: A review with an emphasis on detection in the aquatic environment. Water Environ. Res. 2020, 92, 177–188. [Google Scholar] [CrossRef]
- Liu, X.; Lu, S.; Guo, W.; Xi, B.; Wang, W. Antibiotics in the aquatic environments: A review of lakes, China. Sci. Total Environ. 2018, 627, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Lundström, S.V.; Östman, M.; Bengtsson-Palme, J.; Rutgersson, C.; Thoudal, M.; Sircar, T.; Blanck, H.; Eriksson, K.M.; Tysklind, M.; Flach, C.-F. Minimal selective concentrations of tetracycline in complex aquatic bacterial biofilms. Sci. Total Environ. 2016, 553, 587–595. [Google Scholar] [CrossRef]
- Guo, F.; Cai, Y.; Guan, W.; Huang, H.; Liu, Y. Graphite carbon nitride/ZnIn2S4 heterojunction photocatalyst with enhanced photocatalytic performance for degradation of tetracycline under visible light irradiation. J. Phys. Chem. Solids 2017, 110, 370–378. [Google Scholar] [CrossRef]
- Wang, J.; Lei, X.; Huang, C.; Xue, L.; Cheng, W.; Wu, Q. Fabrication of a novel MoO3/Zn–Al LDHs composite photocatalyst for efficient degradation of tetracycline under visible light irradiation. J. Phys. Chem. Solids 2021, 148, 109698. [Google Scholar] [CrossRef]
- Zhou, X.; Cuasquer, G.J.P.; Li, Z.; Mang, H.P.; Lv, Y. Occurrence of typical antibiotics, representative antibiotic-resistant bacteria, and genes in fresh and stored source-separated human urine. Environ. Int. 2021, 146, 106280. [Google Scholar] [CrossRef]
- Shankar, P.R.; Balasubramanium, R. Antimicrobial resistance: Global report on surveillance 2014. Australas. Med. J. (Online) 2014, 7, 237–238. [Google Scholar]
- Liang, D.; Li, N.; An, J.; Ma, J.; Wu, Y.; Liu, H. Fenton-based technologies as efficient advanced oxidation processes for microcystin-LR degradation. Sci. Total Environ. 2021, 753, 141809. [Google Scholar] [CrossRef]
- Chen, X.; Mu, S.; Luo, Y. Degradation of petroleum pollutants in oil-based drilling cuttings using an Fe2+-based Fenton-like advanced oxidation processes. Environ. Sci. Pollut. Res. 2023, 30, 37669–37678. [Google Scholar] [CrossRef]
- de Jesus, J.H.F.; Lima, K.V.L.; Hammer, P.; Nogueira, R.F.P. Wastewater sludge recycling: An efficient catalyst for photo-Fenton degradation of antibiotics and effluent disinfection. Chem. Eng. J. 2023, 467, 143380. [Google Scholar] [CrossRef]
- Dong, Y.-D.; Shi, Y.; He, Y.-L.; Yang, S.-R.; Yu, S.-Y.; Xiong, Z.; Zhang, H.; Yao, G.; He, C.-S.; Lai, B. Synthesis of Fe–Mn-based materials and their applications in advanced oxidation processes for wastewater decontamination: A Review. Ind. Eng. Chem. Res. 2023, 62, 10828–10848. [Google Scholar] [CrossRef]
- Sharma, K.; Sudhaik, A.; Sonu; Kumar, R.; Nguyen, V.-H.; Le, Q.V.; Ahamad, T.; Thakur, S.; Kaya, S.; Nguyen, L.H.; et al. Advanced photo-Fenton assisted degradation of tetracycline antibiotics using α-Fe2O3/CdS/SiO2 based S-scheme photocatalyst. J. Water Process Eng. 2024, 59, 105011. [Google Scholar] [CrossRef]
- Wan, X.; Liu, R.; Cheng, A. Zero-valent iron-supported magnetic hydrochar derived from kitchen waste for efficient Fenton-like degradation of tetracycline hydrochloride. Sustainability 2025, 17, 1295. [Google Scholar] [CrossRef]
- Zhang, S.; Li, W.; Lv, K.; Zhu, L.; Zhang, Y.; Wang, L.; Li, Y.; Luo, J.; Huang, Z. Engineering electron transport pathways in Cobalt-Doped g-C3N4 photocatalysts: Enhanced tetracycline degradation through interlayer bridging. Catalysts 2025, 15, 366. [Google Scholar] [CrossRef]
- Pannerselvam, M.; Siva, V.; Murugan, A.; Shameem, A.S.; Bavani, T.; Jhelai, S.; Shanmugan, S.; Ali, I.H.S.; Kannan, K. Rational design of Core–Shell MoS2@ZIF-67 nanocomposites for enhanced photocatalytic degradation of tetracycline. Nanomaterials 2025, 15, 545. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, J.; Gao, J.; Liang, Z.; Li, W.; Yan, H.; Guo, R.; Wang, H. Carbon quantum dots-modified TiO2 nanoparticles for antibacterial applications. Chem. Phys. Lett. 2025, 869, 142055. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Ghadirli, M.M.; Hayati, B.; Mahmoodi, B.; Rabeie, B. Synthesis of ZIF-8 composite (g-C3N4@ZIF-8/Ag3PO4) as a catalyst for the malachite green and tetracycline degradation. Inorg. Chem. Commun. 2025, 177, 114345. [Google Scholar] [CrossRef]
- Yu, Q.; Zhao, Z.; Wei, Z.; Ding, M.; Li, Z.; Jia, J.; Zhou, M.; Yuan, L.; Bai, J.; Zhang, H. Enhanced Photo-Fenton degradation of tetracycline using MIL-101(Fe)/g-C3N4/FeOCl double Z-scheme heterojunction catalyst. Appl. Surf. Sci. 2025, 688, 162386. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Lei, Q.; Zhong, D.; Ke, Y.; Liu, W.; Yang, L. Significantly activated persulfate by novel carbon quantum dots-modified N-BiOCl for complete degradation of bisphenol-A under visible light irradiation. Sci. Total Environ. 2023, 870, 161804. [Google Scholar] [CrossRef] [PubMed]
- Ahmadijokani, F.; Molavi, H.; Tajahmadi, S.; Rezakazemi, M.; Amini, M.; Kamkar, M.; Rojas, O.J.; Arjmand, M. Coordination chemistry of metal–organic frameworks: Detection, adsorption, and photodegradation of tetracycline antibiotics and beyond. Coord. Chem. Rev. 2022, 464, 214562. [Google Scholar] [CrossRef]
- He, X.; Kai, T.; Ding, P. Heterojunction photocatalysts for degradation of the tetracycline antibiotic: A review. Environ. Chem. Lett. 2021, 19, 4563–4601. [Google Scholar] [CrossRef]
- Jin, H.; Li, L.; Luo, N.; Zhang, X.; Niu, H.; Cai, Y. Biochar supported Fe-Cu bimetal composites prepared with waste materials for removal of tetrachloropicolinic acid from high salinity wastewater. Inorg. Chem. Commun. 2023, 156, 111141. [Google Scholar] [CrossRef]
- Wen, J.; Long, Y.; Zhu, L.; Zeng, L.; Qin, X.; Song, S.; Lu, M.; Deng, L. Bimetallic CuFe2O4 photocatalytic activates persistent free radicals and accelerates electron transfer in biochar: Efficient degradation of tetracycline. Environ. Res. 2025, 275, 121413. [Google Scholar] [CrossRef]
- Li, H.; Wu, J.; Ren, A.; Qu, Y.; Zong, X.; Gong, Y.; Wang, D.; Ye, Y.; Li, Q.; Wu, Z.; et al. Shaddock peels biochar doping with Fe-Co bimetal for peroxymonosulfate activation on the degradation of tetracycline: The influence of HCO3− and PO43−. Environ. Res. 2025, 275, 121411. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, J.; Zhao, B.; Fan, X.; Xu, Y.; Liu, Y. Efficient sulfamethoxazole degradation via boosting nonradical-based peroxymonosulfate activation by biochar supported Co-Ni bimetal oxide. J. Environ. Chem. Eng. 2023, 11, 110903. [Google Scholar] [CrossRef]
- Katibi, K.K.; Shitu, I.G.; Yunos, K.F.M.; Azis, R.S.; Iwar, R.T.; Adamu, S.B.; Umar, A.M.; Adebayo, K.R. Unlocking the potential of magnetic biochar in wastewater purification: A review on the removal of bisphenol A form aqueous solution. Environ. Monit. Assess. 2024, 196, 492. [Google Scholar] [CrossRef]
- Katibi, K.K.; Yunos, K.F.; Man, H.C.; Aris, A.Z.; Mohd Nor, M.Z.; Azis, R.S. An Insight into a Sustainable Removal of Bisphenol A from Aqueous Solution by Novel Palm Kernel Shell Magnetically Induced Biochar: Synthesis, Characterization, Kinetic, and Thermodynamic Studies. Polymers 2021, 13, 3781. [Google Scholar] [CrossRef]
- Katibi, K.K.; Shitu, I.G.; Syahidah Azis, R.; Soo Kien, C.; Kean Pah, L.; Awang Kechik, M.M.; Md Yunos, K.F.; Abdulhameed Amusa, A.; Titilayo Katibi, M. Synthesis of eco-friendly bio-based coconut shell magnetic biochar for efficient bisphenol S sequestration in aqueous environment: Green technology breakthrough. Chem. Eng. Commun. 2024, 211, 1802–1827. [Google Scholar] [CrossRef]
- Abd El-Monaem, E.M.; Omer, A.M.; Heydari, A.; Ouyang, X.k.; El-Subruiti, G.M.; Xiao, Y.; Eltaweil, A.S. Harnessing the storage-release cavity of β-cyclodextrin to enhance SnFe2O4/FeCoNi-LTH catalyst efficiency in fenton-like degradation of tetracycline. Surf. Interfaces 2025, 58, 105749. [Google Scholar] [CrossRef]
- Wu, M.; Huang, J.; Xiang, Y.; Jia, M.; Xiong, W.; Yang, Z.; Peng, H.; Ye, Y. Fe/Mn modified biochar as electrode particles in electrochemical system for efficient anaerobic sludge digestion. Chem. Eng. J. 2023, 472, 144754. [Google Scholar] [CrossRef]
- Wu, Q.; Dong, C.; Chen, M.; Zhang, Y.; Cai, M.; Chen, Y.; Jin, M.; Wei, Z. Silica enhanced activation and stability of Fe/Mn decorated sludge biochar composite for tetracycline degradation. Chemosphere 2023, 328, 138614. [Google Scholar] [CrossRef]
- Qin, H.; Xiao, R.; Cheng, H.; Leng, S.; Wu, S. Heterogeneous Fenton degradation of cefotaxime sodium in water catalyzed by carboxyl-functionalized MnFe2O4 magnetic nanoparticles. Acta Sci. Circumstantiae 2022, 42, 71–80. [Google Scholar]
- Li, W.-Y.; Huo, G.; Huang, Y.; Dong, L.-J.; Lu, X.-G. Synthesis and superparamagnetism of Fe3O4 hollow nano-microspheres. Acta Phys. Sin. 2018, 67, 177501. [Google Scholar]
- Li, J.; Zhang, M.; Kong, Q.; Zeng, T.; Mao, Y.; Liu, J.; Xie, S. Effects and mechanism of uranium (VI) removal by wood biochar loaded with MnFe2O4. J. Taiwan Inst. Chem. Eng. 2025, 166, 105532. [Google Scholar] [CrossRef]
- Yang, X.; Guo, Z.; Chen, X.; Xi, S.; Cui, K.; Li, J.; Dong, D.; Wu, F.; Wu, Z. Efficient degradation of thiamethoxam pesticide in water by iron and manganese oxide composite biochar activated persulfate. Chem. Eng. J. 2023, 473, 145051. [Google Scholar] [CrossRef]
- Liu, T.; Li, C.-X.; Chen, X.; Chen, Y.; Cui, K.; Wei, Q. Magnetic MgFeO@BC derived from rice husk as peroxymonosulfate activator for sulfamethoxazole degradation: Performance and reaction mechanism. Int. J. Mol. Sci. 2024, 25, 11768. [Google Scholar] [CrossRef]
- Guo, Z.; Bai, G.; Huang, B.; Cai, N.; Guo, P.; Chen, L. Preparation and application of a novel biochar-supported red mud catalyst: Active sites and catalytic mechanism. J. Hazard. Mater. 2021, 408, 124802. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, L.; Duan, F. Effects of acid pickling and nitrogen doping on the biochar–NO reaction performance of different biochars under high O2 conditions. J. Energy Inst. 2022, 103, 128–137. [Google Scholar] [CrossRef]
- Xiang, Y.; Xu, Z.; Zhou, Y.; Wei, Y.; Long, X.; He, Y.; Zhi, D.; Yang, J.; Luo, L. A sustainable ferromanganese biochar adsorbent for effective levofloxacin removal from aqueous medium. Chemosphere 2019, 237, 124464. [Google Scholar] [CrossRef]
- Liang, Y.; Xie, J.; Liu, L.; Cui, W.; Lin, Z.; Hu, J.; Wang, S.; Li, J.; An, W. Fe (III)/rGO/Bi2MoO6 composite photocatalyst preparation and phenol degradation by photocatalytic Fenton synergy. J. Inorg. Mater. 2021, 36, 615–626. [Google Scholar]
- Li, D.; Yang, M.; Hu, J.; Ren, L.; Zhang, Y.; Li, K. Determination and fate of oxytetracycline and related compounds in oxytetracycline production wastewater and the receiving river. Environ. Toxicol. Chem. 2008, 27, 80–86. [Google Scholar] [CrossRef]
- Xu, M.; Wei, J.; Chen, X.; Pan, G.; Li, J.; Xing, L.; Zhang, Y.; Li, Y.; Wang, Z.; Li, J. Satisfactory degradation of tetracycline by a pH-universal MnFe-LDH@BC cathode in electric Fenton process: Performances, mechanisms and toxicity assessments. J. Environ. Chem. Eng. 2022, 10, 108409. [Google Scholar] [CrossRef]
- Guan, Y.-H.; Ma, J.; Liu, D.-K.; Ou, Z.-F.; Zhang, W.; Gong, X.-L.; Fu, Q.; Crittenden, J.C. Insight into chloride effect on the UV/peroxymonosulfate process. Chem. Eng. J. 2018, 352, 477–489. [Google Scholar] [CrossRef]
- Du, D.; Shi, W.; Wang, L.; Zhang, J. Yolk-shell structured Fe3O4@void@TiO2 as a photo-Fenton-like catalyst for the extremely efficient elimination of tetracycline. Appl. Catal. B Environ. 2017, 200, 484–492. [Google Scholar] [CrossRef]
- Li, L.; Lai, C.; Huang, F.; Cheng, M.; Zeng, G.; Huang, D.; Li, B.; Liu, S.; Zhang, M.; Qin, L.; et al. Degradation of naphthalene with magnetic bio-char activate hydrogen peroxide: Synergism of bio-char and Fe–Mn binary oxides. Water Res. 2019, 160, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lyu, H.; Du, Y.; Cheng, Q.; Liu, Y.; Ma, J.; Yang, S.; Lin, H. Unraveling how Fe-Mn modified biochar mitigates sulfamonomethoxine in soil water: The activated biodegradation and hydroxyl radicals’ formation. J. Hazard. Mater. 2024, 465, 133490. [Google Scholar] [CrossRef]
- Zhu, R.; Zhu, Y.; Xian, H.; Yan, L.; Fu, H.; Zhu, G.; Xi, Y.; Zhu, J.; He, H. CNTs/ferrihydrite as a highly efficient heterogeneous Fenton catalyst for the degradation of bisphenol A: The important role of CNTs in accelerating Fe(III)/Fe(II) cycling. Appl. Catal. B Environ. 2020, 270, 118891. [Google Scholar] [CrossRef]
- Lai, C.; Xu, F.; Zhang, M.; Li, B.; Liu, S.; Yi, H.; Li, L.; Qin, L.; Liu, X.; Fu, Y. Facile synthesis of CeO2/carbonate doped Bi2O2CO3 Z-scheme heterojunction for improved visible-light photocatalytic performance: Photodegradation of tetracycline and photocatalytic mechanism. J. Colloid Interf. Sci. 2021, 588, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Hu, L.; Li, X.; Deng, L.; Fan, G.; He, Y. A novel nano-sized MoS2 decorated Bi2O3 heterojunction with enhanced photocatalytic performance for methylene blue and tetracycline degradation. Ceram. Int. 2019, 45, 15824–15833. [Google Scholar] [CrossRef]
- Xia, B.; Deng, F.; Zhang, S.; Hua, L.; Luo, X.; Ao, M. Design and synthesis of robust Z-scheme ZnS-SnS2 n-n heterojunctions for highly efficient degradation of pharmaceutical pollutants: Performance, valence/conduction band offset photocatalytic mechanisms and toxicity evaluation. J. Hazard. Mater. 2020, 392, 122345. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | |||
|---|---|---|---|---|---|
| k1 (×102, min−1) | R2 | k2 (×10, min−1) | R2 | ||
| FMBC dosages (g) | 0.01 | 2.38 ± 0.03 | 0.9985 | 0.65 ± 0.03 | 0.9889 |
| 0.03 | 3.63 ± 0.05 | 0.9982 | 1.73 ± 0.08 | 0.9887 | |
| 0.05 | 4.93 ± 0.11 | 0.9961 | 3.09 ± 0.11 | 0.9918 | |
| 0.07 | 3.07 ± 0.06 | 0.9971 | 1.06 ± 0.04 | 0.9904 | |
| 0.10 | 2.48 ± 0.05 | 0.9964 | 0.78 ± 0.04 | 0.9876 | |
| H2O2 concentrations (mmol/L) | 1.29 | 1.57 ± 0.03 | 0.9979 | 0.32 ± 0.02 | 0.9778 |
| 2.58 | 2.07 ± 0.06 | 0.9932 | 0.53 ± 0.02 | 0.9878 | |
| 5.16 | 4.52 ± 0.05 | 0.9990 | 3.21 ± 0.13 | 0.9883 | |
| 10.32 | 5.26 ± 0.06 | 0.9989 | 4.69 ± 0.23 | 0.9827 | |
| pH | 2.0 | 2.61 ± 0.04 | 0.9981 | 0.63 ± 0.05 | 0.9629 |
| 4.0 | 4.66 ± 0.07 | 0.9980 | 3.19 ± 0.12 | 0.9886 | |
| 6.0 | 3.47 ± 0.03 | 0.9994 | 1.28 ± 0.10 | 0.9621 | |
| 7.0 | 1.91 ± 0.03 | 0.9977 | 0.36 ± 0.03 | 0.9616 | |
| 9.0 | 1.15 ± 0.02 | 0.9979 | 0.16 ± 0.02 | 0.9987 | |
| C0 of OTC (mg/L) | 25 | 3.59 ± 0.06 | 0.9979 | 1.84 ± 0.11 | 0.9798 |
| 75 | 3.87 ± 0.09 | 0.9963 | 2.23 ± 0.12 | 0.9823 | |
| 100 | 4.88 ± 0.07 | 0.9983 | 3.54 ± 0.20 | 0.9817 | |
| 125 | 3.25 ± 0.08 | 0.9949 | 1.39 ± 0.08 | 0.9764 | |
| 150 | 2.68 ± 0.05 | 0.9978 | 0.82 ± 0.04 | 0.9887 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Fu, Y.; Niu, X.; Wu, B.; Liu, X.; Hao, F.; Ma, Z.; Cai, H.; Liu, Y. The Efficient Degradation of Oxytetracycline in Wastewater Using Fe/Mn-Modified Magnetic Oak Biochar: Pathways and Mechanistic Investigation. Magnetochemistry 2025, 11, 49. https://doi.org/10.3390/magnetochemistry11060049
Zhou Y, Fu Y, Niu X, Wu B, Liu X, Hao F, Ma Z, Cai H, Liu Y. The Efficient Degradation of Oxytetracycline in Wastewater Using Fe/Mn-Modified Magnetic Oak Biochar: Pathways and Mechanistic Investigation. Magnetochemistry. 2025; 11(6):49. https://doi.org/10.3390/magnetochemistry11060049
Chicago/Turabian StyleZhou, Yujie, Yuzhe Fu, Xiaoxue Niu, Bohan Wu, Xinghan Liu, Fu Hao, Zichuan Ma, Hao Cai, and Yuheng Liu. 2025. "The Efficient Degradation of Oxytetracycline in Wastewater Using Fe/Mn-Modified Magnetic Oak Biochar: Pathways and Mechanistic Investigation" Magnetochemistry 11, no. 6: 49. https://doi.org/10.3390/magnetochemistry11060049
APA StyleZhou, Y., Fu, Y., Niu, X., Wu, B., Liu, X., Hao, F., Ma, Z., Cai, H., & Liu, Y. (2025). The Efficient Degradation of Oxytetracycline in Wastewater Using Fe/Mn-Modified Magnetic Oak Biochar: Pathways and Mechanistic Investigation. Magnetochemistry, 11(6), 49. https://doi.org/10.3390/magnetochemistry11060049








