A Heptacobalt(II/III) Dicubane Cluster with Polyoxometalate and Acetato Ligands: Synthesis, Crystal Structure, and Magnetic Properties †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Physical Techniques
2.3. Single-Crystal X-Ray Diffraction
2.4. Powder X-Ray Diffraction (PXRD)
2.5. Synthesis of Na13[Co7(OH)6(H2O)2(CH3COO)4(PW9O34)2]·37H2O (Na13-1·37H2O)
2.6. Magnetic Measurements
3. Results
3.1. Synthesis
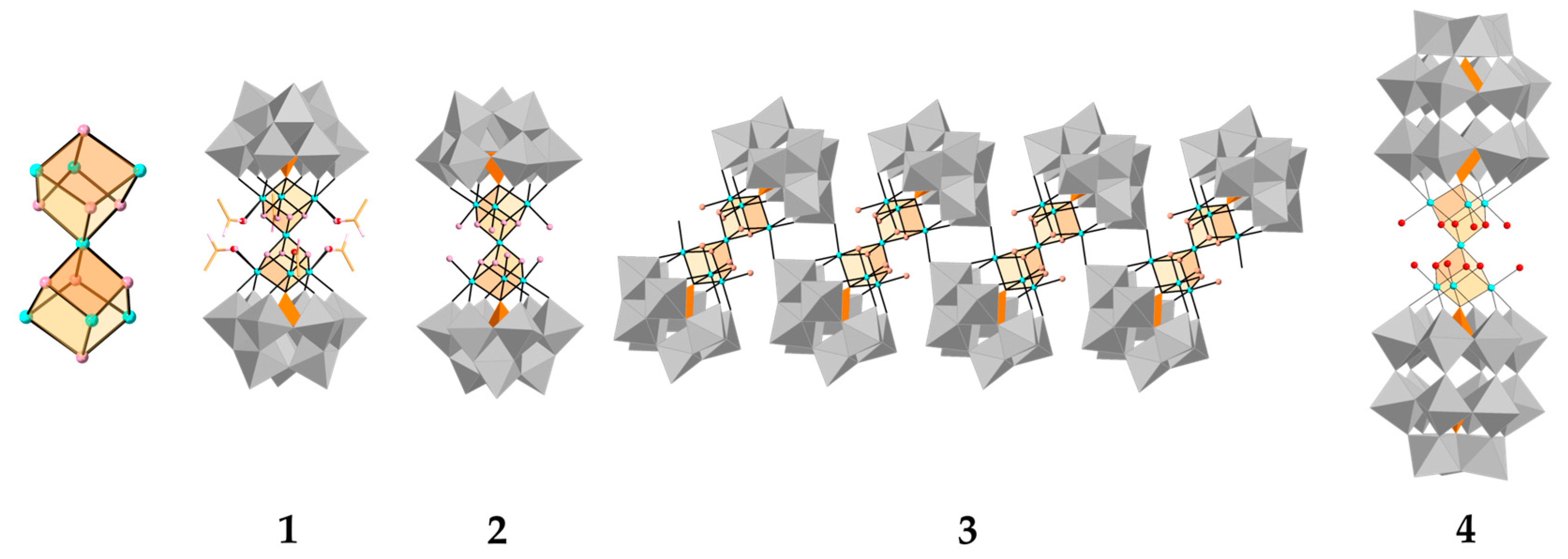
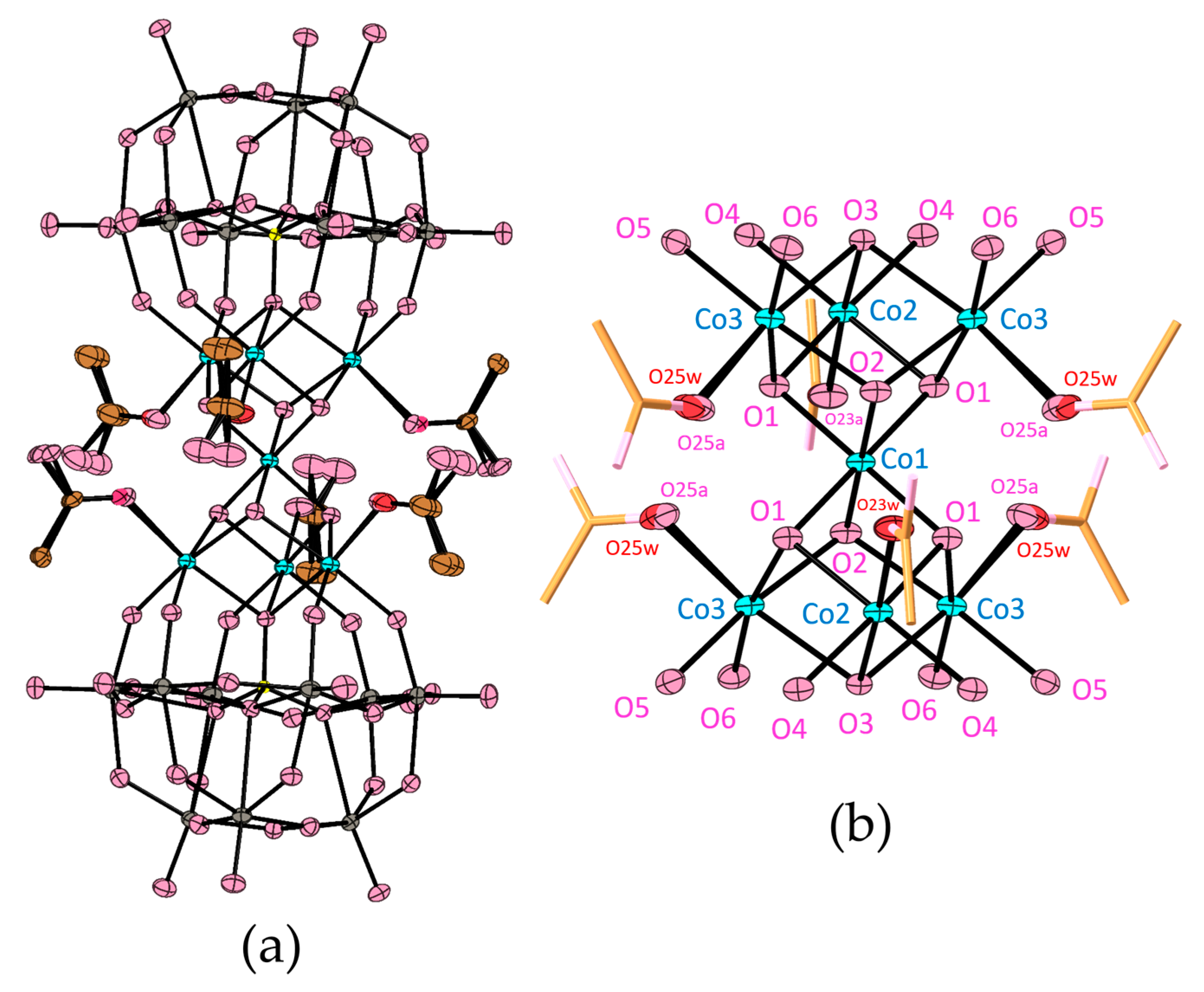
3.2. Crystal Structure
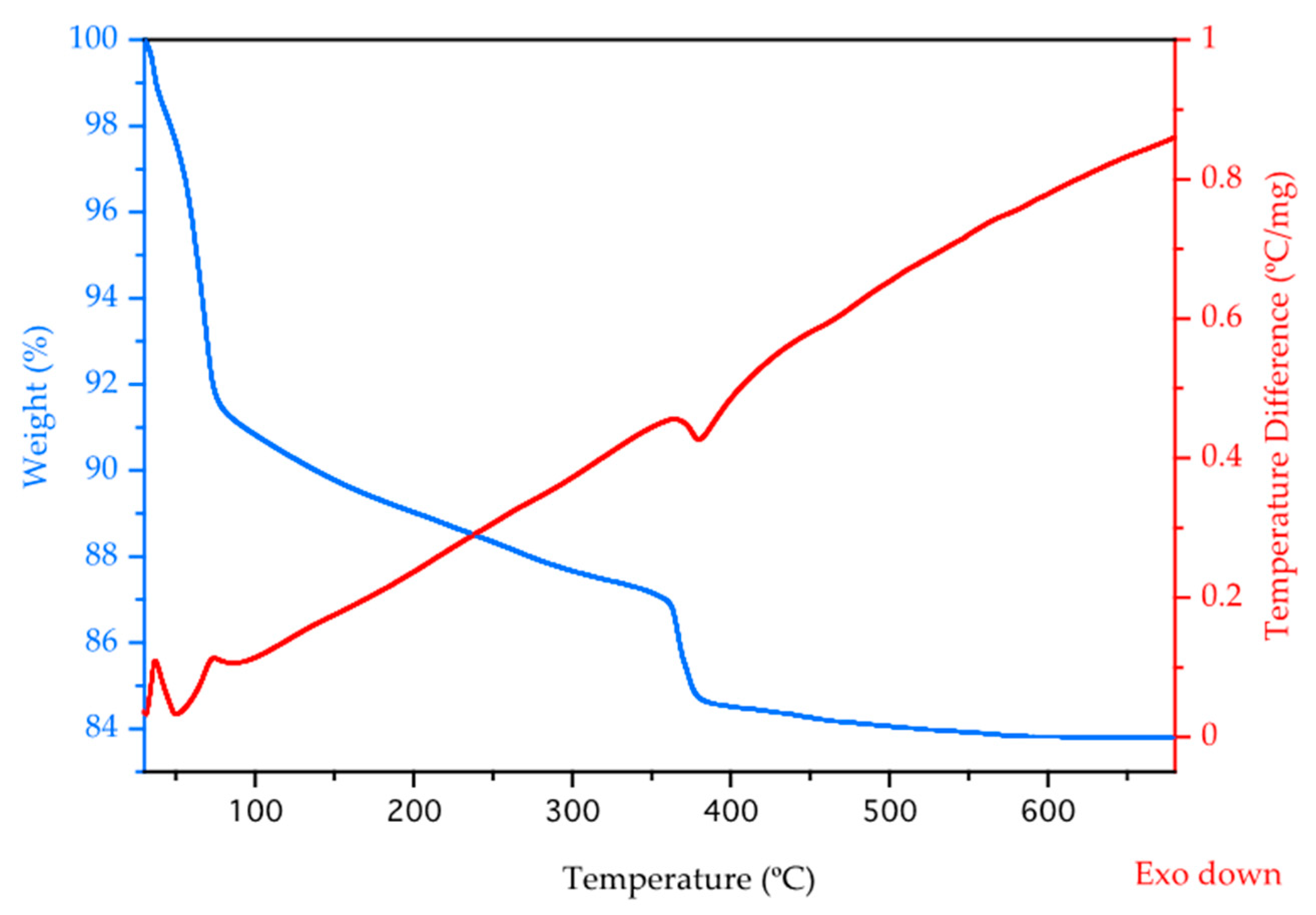
3.3. IR Spectroscopy, TGA-DTA, UV-Vis-NIR Diffuse Reflectance Spectroscopy, and PXRD
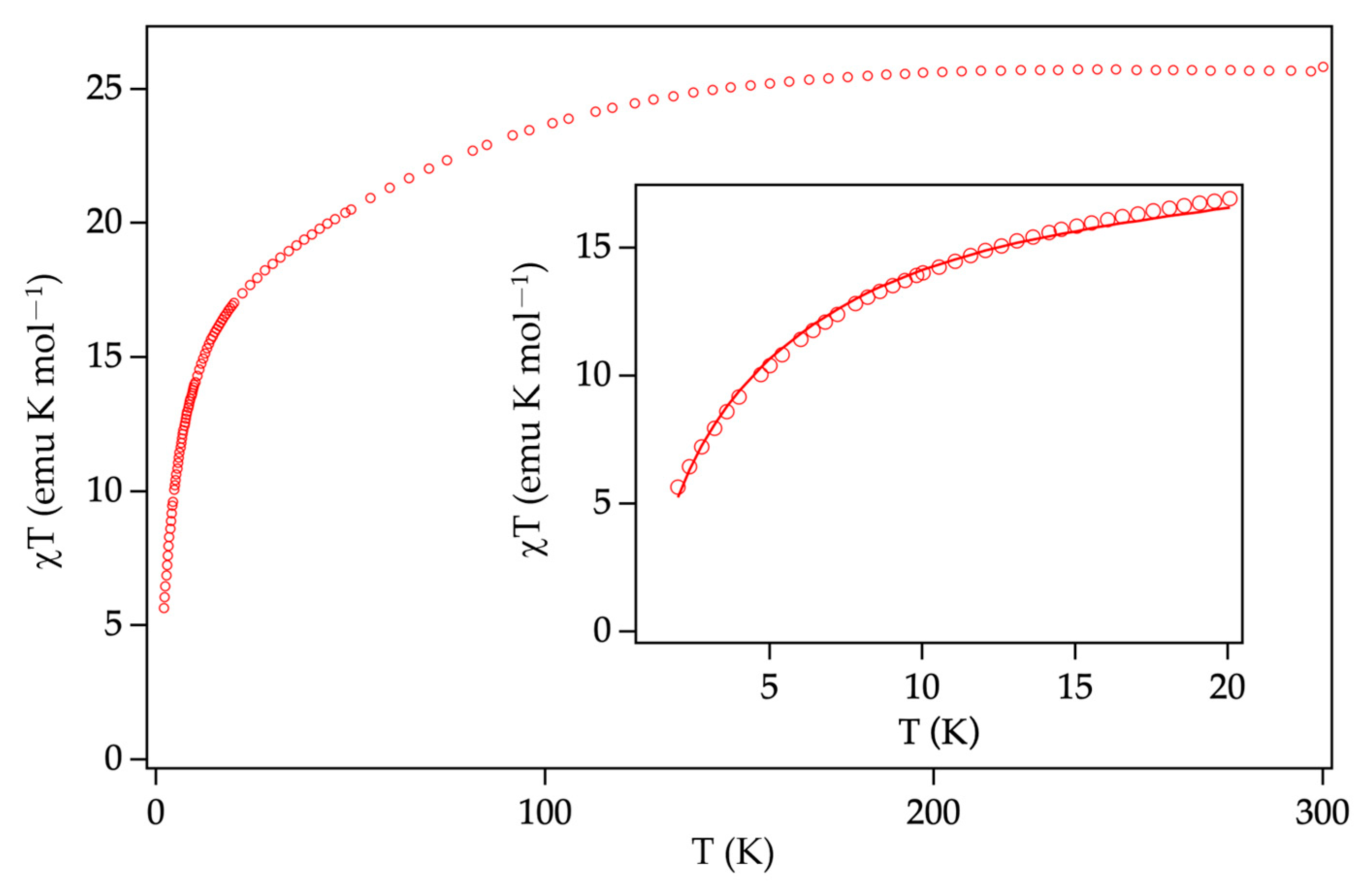
3.4. Magnetic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATR | Attenuated total reflectance |
| BVS | Bond valence sum |
| CCD | Charge-coupled device |
| DTA | Differential thermal analysis |
| EDX | Energy-dispersive X-ray spectroscopy |
| FTIR | Fourier transform infrared spectroscopy |
| IR | Infrared |
| POM | Polyoxometalate |
| PXRD | Powder X-ray diffraction |
| TGA | Thermogravimetric analysis |
| UV-Vis-NIR | Ultraviolet–visible–near infrared |
References
- Pope, M.T. Heteropoly and Isopoly Oxometalates; Springer: New York, NY, USA; Berlin/Heidelberg, Germany; Tokyo, Japan, 1983; ISBN 0387118896. [Google Scholar]
- Pope, M.T.; Müller, A. Polyoxometalate Chemistry: An Old Field with New Dimensions in Several Disciplines. Angew. Chem. Int. Ed. Engl. 1991, 30, 34–48. [Google Scholar] [CrossRef]
- Iftikhar, T.; Rosnes, M.H. Covalent Organic-Inorganic Polyoxometalate Hybrids in Catalysis. Front. Chem. 2024, 12, 1447623. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, R.; Kozhevnikov, I.V. Versatile POMOF-Based Materials: Synthesis, Mechanism, Topology and Catalytic Applications. Coord. Chem. Rev. 2025, 524, 216304. [Google Scholar] [CrossRef]
- Gumerova, N.I.; Rompel, A. Synthesis, Structures and Applications of Electron-Rich Polyoxometalates. Nat. Rev. Chem. 2018, 2, 0112. [Google Scholar] [CrossRef]
- Absalan, Y.; Gholizadeh, M.; Kim, E.-B.; Ameen, S.; Wang, Y.; Wang, Y.; He, H. Recent Progress on Organic Metal Compound/MOF Hybrids: From Controllable Synthesis to Potential Catalytic Applications. Coord. Chem. Rev. 2024, 515, 215972. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, M.; Chen, X.; Yue, B.; He, H. Heterogeneous Catalysis of Polyoxometalate Based Organic-Inorganic Hybrids. Materials 2015, 8, 1545–1567. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Chen, J.; Dong, L.; Su, Y.; Tian, S.; Zhou, Y.; Li, M. Polyoxometalates and Their Composites for Antimicrobial Applications: Advances, Mechanisms and Future Prospects. J. Inorg. Biochem. 2025, 262, 112739. [Google Scholar] [CrossRef]
- Liu, J.; Huang, M.; Zhang, X.; Hua, Z.; Feng, Z.; Dong, Y.; Sun, T.; Sun, X.; Chen, C. Polyoxometalate Nanomaterials for Enhanced Reactive Oxygen Species Theranostics. Coord. Chem. Rev. 2022, 472, 214785. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Y.; Zhao, J.; Chen, L. Recent Advances of Polyoxometalate-Based Materials Applied for Electron-Related Devices. Coord. Chem. Rev. 2024, 506, 215724. [Google Scholar] [CrossRef]
- Kruse, J.-H.; Langer, M.; Romanenko, I.; Trentin, I.; Hernandez-Castillo, D.; Gonzalez, L.; Schacher, F.H.; Streb, C. Polyoxometalate-Soft Matter Composite Materials: Design Strategies, Applications, and Future Directions. Adv. Funct. Mater. 2022, 32, 2208428. [Google Scholar] [CrossRef]
- Verissimo, M.I.S.; Evtuguin, D.V.; Gomes, M.T.S.R. Polyoxometalate Functionalized Sensors: A Review. Front. Chem. 2022, 10, 840657. [Google Scholar] [CrossRef] [PubMed]
- Housaindokht, M.R.; Jamshidi, A.; Janati-Fard, F. Recent Advances in Polyoxometalates for Spectroscopic Sensors: A Review. J. Mater. Sci. 2022, 57, 13871–13902. [Google Scholar] [CrossRef]
- Clemente-Juan, J.M.; Coronado, E.; Gaita-Arino, A. Magnetic Polyoxometalates: From Molecular Magnetism to Molecular Spintronics and Quantum Computing. Chem. Soc. Rev. 2012, 41, 7464–7478. [Google Scholar] [CrossRef]
- Yang, Z.-X.; Gong, F.; Lin, D.; Huo, Y. Recent Advances in Polyoxometalate-Based Single-Molecule Magnets. Coord. Chem. Rev. 2023, 492, 215205. [Google Scholar] [CrossRef]
- Baldoví, J.J.; Cardona-Serra, S.; Gaita-Ariño, A.; Coronado, E. Design of Magnetic Polyoxometalates for Molecular Spintronics and as Spin Qubits. In Advances in Inorganic Chemistry, Vol 69: Polyoxometalate Chemistry; VanEldik, R., Cronin, L., Eds.; Univ Valencia, Inst Ciencia Mol ICMol: Paterna, Spain, 2017; Volume 69, pp. 213–249. ISBN 0898-8838. [Google Scholar]
- Tsukerblat, B.; Palii, A.; Clemente-Juan, J.M.; Gaita-Arino, A.; Coronado, E. A Symmetry Adapted Approach to the Dynamic Jahn-Teller Problem: Application to Mixed-Valence Polyoxometalate Clusters with Keggin Structure. Int. J. Quantum Chem. 2012, 112, 2957–2964. [Google Scholar] [CrossRef]
- Palii, A.; Tsukerblat, B.; Klokishner, S.; Dunbar, K.R.; Clemente-Juan, J.M.; Coronado, E. Beyond the Spin Model: Exchange Coupling in Molecular Magnets with Unquenched Orbital Angular Momenta. Chem. Soc. Rev. 2011, 40, 3130–3156. [Google Scholar] [CrossRef]
- Clemente-Juan, J.M.; Coronado, E.; Gaita-Ariño, A.; Giménez-Saiz, C.; Güdel, H.-U.; Sieber, A.; Bircher, R.; Mutka, H. Magnetic Polyoxometalates: Anisotropic Exchange Interactions in the Co3 II Moiety of [(NaOH2)Co3(H2O)(P2W15O56)2]17−. Inorg. Chem. 2005, 44, 3389–3395. [Google Scholar] [CrossRef]
- Galán-Mascarós, J.R.; Gómez-Garcia, C.J.; Borrás-Almenar, J.J.; Coronado, E. High Nuclearity Magnetic Clusters: Magnetic Properties of a Nine Cobalt Cluster Encapsulated in a Polyoxometalate, [Co9(OH)3(H2O)6(HPO4)2(PW9O34)3]16−. Adv. Mater. 1994, 6, 221–223. [Google Scholar] [CrossRef]
- Duan, Y.; Clemente-Juan, J.M.; Giménez-Saiz, C.; Coronado, E. Cobalt Clusters with Cubane-Type Topologies Based on Trivacant Polyoxometalate Ligands. Inorg. Chem. 2016, 55, 925–938. [Google Scholar] [CrossRef]
- Bassil, B.S.; Xiang, Y.; Haider, A.; Hurtado, J.; Novitchi, G.; Powell, A.K.; Bossoh, A.M.; Mbomekallé, I.M.; de Oliveira, P.; Kortz, U. Heptanickel(II) Double-Cubane Core in Wells-Dawson Heteropolytungstate, [Ni7(OH)6(H2O)6(P2W15O56)2]16−. Chem. Commun. 2016, 52, 2601–2604. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, E.; Qi, Y.; Li, Y.; Mao, B.; Su, Z. Synthesis, Characterization, and Crystal Structures of Double-Cubane-Substituted and Asymmetric Penta-Ni-Substituted Dimeric Polyoxometalates. Cryst. Growth Des. 2007, 7, 1305–1311. [Google Scholar] [CrossRef]
- Fang, X.; Kögerler, P.; Speldrich, M.; Schilder, H.; Luban, M. A Polyoxometalate-Based Single-Molecule Magnet with an S = 21/2 Ground State. Chem. Commun. 2012, 48, 1218–1220. [Google Scholar] [CrossRef] [PubMed]
- APEX3, version v2017.3-0; Bruker AXS Apex3 Inc.: Madison, WI, USA, 2017.
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Domaille, P.J.; Hervé, G.; Tézé, A. Vanadium(V) Substituted Dodecatungstophosphates. In Inorganic Syntheses, Volume 27; Ginsberg, A.P., Ed.; Wiley: Hoboken, NJ, USA, 1990; pp. 96–104. [Google Scholar]
- Brown, I.D.; Altermatt, D. Bond-Valence Parameters Obtained from a Systematic Analysis of the Inorganic Crystal Structure Database. Acta Crystallogr. Sect. B Struct. Sci. 1985, 41, 244–247. [Google Scholar] [CrossRef]
- Wood, R.M.; Palenik, G.J. Bond Valence Sums in Coordination Chemistry. A Simple Method for Calculating the Oxidation State of Cobalt in Complexes Containing Only Co−O Bonds. Inorg. Chem. 1998, 37, 4149–4151. [Google Scholar] [CrossRef]
- Brese, N.E.; O’Keeffe, M. Bond-Valence Parameters for Solids. Acta Crystallogr. Sect. B Struct. Sci. 1991, 47, 192–197. [Google Scholar] [CrossRef]
- Finke, R.G.; Droege, M.W.; Domaille, P.J. Trivacant Heteropolytungstate Derivatives. 3. Rational Syntheses, Characterization, Two-Dimensional Tungsten-183 NMR, and Properties of Tungstometallophosphates P2W18M4(H2O)2O6810− and P4W30M4(H2O)2O11216− (M = Cobalt, Copper, Zinc). Inorg. Chem. 1987, 26, 3886–3896. [Google Scholar] [CrossRef]
- Nickolov, Z.; Georgiev, G.; Stoilova, D.; Ivanov, I. Raman and IR Study of Cobalt Acetate Dihydrate. J. Mol. Struct. 1995, 354, 119–125. [Google Scholar] [CrossRef]
- Zhang, Z.; Muhammed, M. Thermochemical Decomposition of Cobalt Doped Ammonium Paratungstate Precursor. Thermochim. Acta 2003, 400, 235–245. [Google Scholar] [CrossRef]
- Yamase, T. Photo- and Electrochromism of Polyoxometalates and Related Materials. Chem. Rev. 1998, 98, 307–326. [Google Scholar] [CrossRef]
- Weakley, T.J.R.; Malik, S.A. Heteropolyanions Containing Two Different Heteroatoms—I. J. Inorg. Nucl. Chem. 1967, 29, 2935–2944. [Google Scholar] [CrossRef]


| Formula | C8H96Co7Na13O121P2W18 |
|---|---|
| Mr (g/mol) | 6211.46 |
| Crystal system | Orthorhombic |
| Space group | Cmca (No. 64) |
| T (K) | 150 (2) |
| λ (Å) | 0.71073 |
| a (Å) | 22.2275 (8) |
| b (Å) | 20.0193 (8) |
| c (Å) | 29.0406 (11) |
| α (deg) | 90 |
| β (deg) | 90 |
| γ (deg) | 90 |
| V (Å3) | 12,922.5 (9) |
| Z | 4 |
| ρ (mg/m3) | 3.193 |
| μ (Mo Kα) (mm−1) | 17.002 |
| F(000) | 11,224 |
| Crystal size (mm) | 0.063 × 0.179 × 0.199 |
| θ range (deg) | 2.308–39.681 |
| Index ranges for h, k, l | −38/31, −35/34, −52/51 |
| Reflns. with I > 2σ(I) | 16,291 |
| Independent reflns. | 18,871 |
| Rint | 0.0527 |
| GOF on F2 | 1.074 |
| Reflns. collected | 244,378 |
| Data/restraints/parameters | 18,871/328/551 |
| R1, wR2 [I > 2σ(I)] a | 0.0384, 0.0481 |
| R1, wR2 (all data) a | 0.0977, 0.1040 |
| Largest diff. peak/hole (e Å−3) | 5.216/−6.043 |
| Type of Distance | 1 1 | 2 | 3 |
|---|---|---|---|
| Co(III)–OH | Co1–O1 1.927(3) × 4 Co1–O2 1.919(5) × 2 | 1.91(3)–1.93(3) | 1.912(11)–1.917(12) |
| Co(II)–OH | Co2–O1 2.091(3) × 4 Co3–O1 2.092(3) × 4 Co3–O2 2.090(3) × 4 | 2.06(3)–2.11(3) | 2.069(10)–2.096(11) |
| Co(II)–OP | Co2–O3 2.282(4) × 2 Co3–O3 2.273(3) × 4 | 2.24(2)–2.29(2) | 2.232(10)–2.263(10) |
| Co(II)–OW | Co2–O4 2.038(3) × 4 Co3–O5 2.026(4) × 4 Co3–O6 2.034(3) × 4 | 1.97(3)–2.09(3) | 1.984(11)–2.030(11) |
| Co(II)–O2H | Co2–O23water 2.17(4) × 2 Co3–O25water 2.16(5) × 4 | 2.13(3)–2.21(3) | 2.100(10)–2.163(11) |
| Co(II)–OAc | Co2–O23a 2.043(18) × 2 Co3–O25a 2.07(3) × 4 | — | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abellán-Dumont, G.; Clemente-Juan, J.M.; Giménez-Saiz, C. A Heptacobalt(II/III) Dicubane Cluster with Polyoxometalate and Acetato Ligands: Synthesis, Crystal Structure, and Magnetic Properties. Magnetochemistry 2025, 11, 48. https://doi.org/10.3390/magnetochemistry11060048
Abellán-Dumont G, Clemente-Juan JM, Giménez-Saiz C. A Heptacobalt(II/III) Dicubane Cluster with Polyoxometalate and Acetato Ligands: Synthesis, Crystal Structure, and Magnetic Properties. Magnetochemistry. 2025; 11(6):48. https://doi.org/10.3390/magnetochemistry11060048
Chicago/Turabian StyleAbellán-Dumont, Gonzalo, Juan Modesto Clemente-Juan, and Carlos Giménez-Saiz. 2025. "A Heptacobalt(II/III) Dicubane Cluster with Polyoxometalate and Acetato Ligands: Synthesis, Crystal Structure, and Magnetic Properties" Magnetochemistry 11, no. 6: 48. https://doi.org/10.3390/magnetochemistry11060048
APA StyleAbellán-Dumont, G., Clemente-Juan, J. M., & Giménez-Saiz, C. (2025). A Heptacobalt(II/III) Dicubane Cluster with Polyoxometalate and Acetato Ligands: Synthesis, Crystal Structure, and Magnetic Properties. Magnetochemistry, 11(6), 48. https://doi.org/10.3390/magnetochemistry11060048







