Sustainable Phosphate Remediation via Hierarchical Mg-Fe Layered Double Hydroxides on Magnetic Biochar from Agricultural Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Characterization
2.3. Adsorption Experiments
2.4. Regeneration Studies
2.5. Statistical Analysis
3. Results
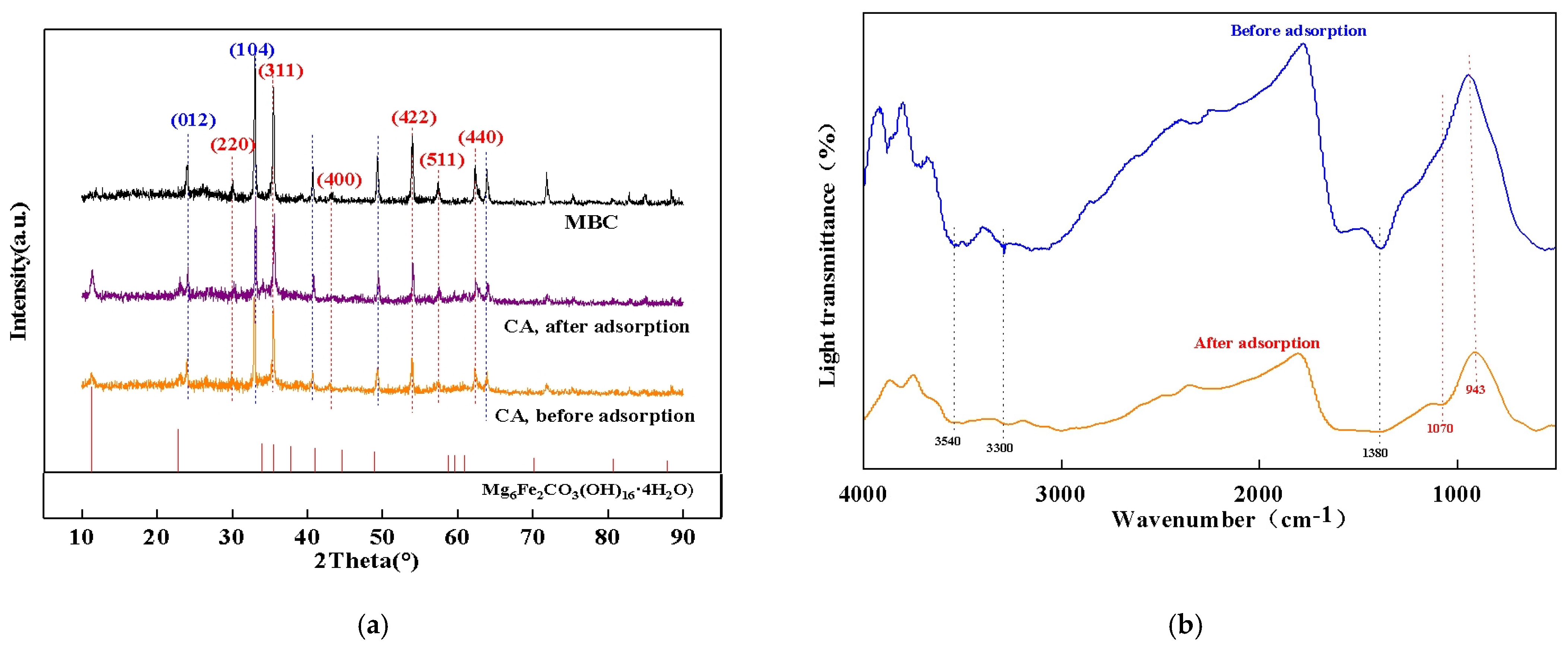
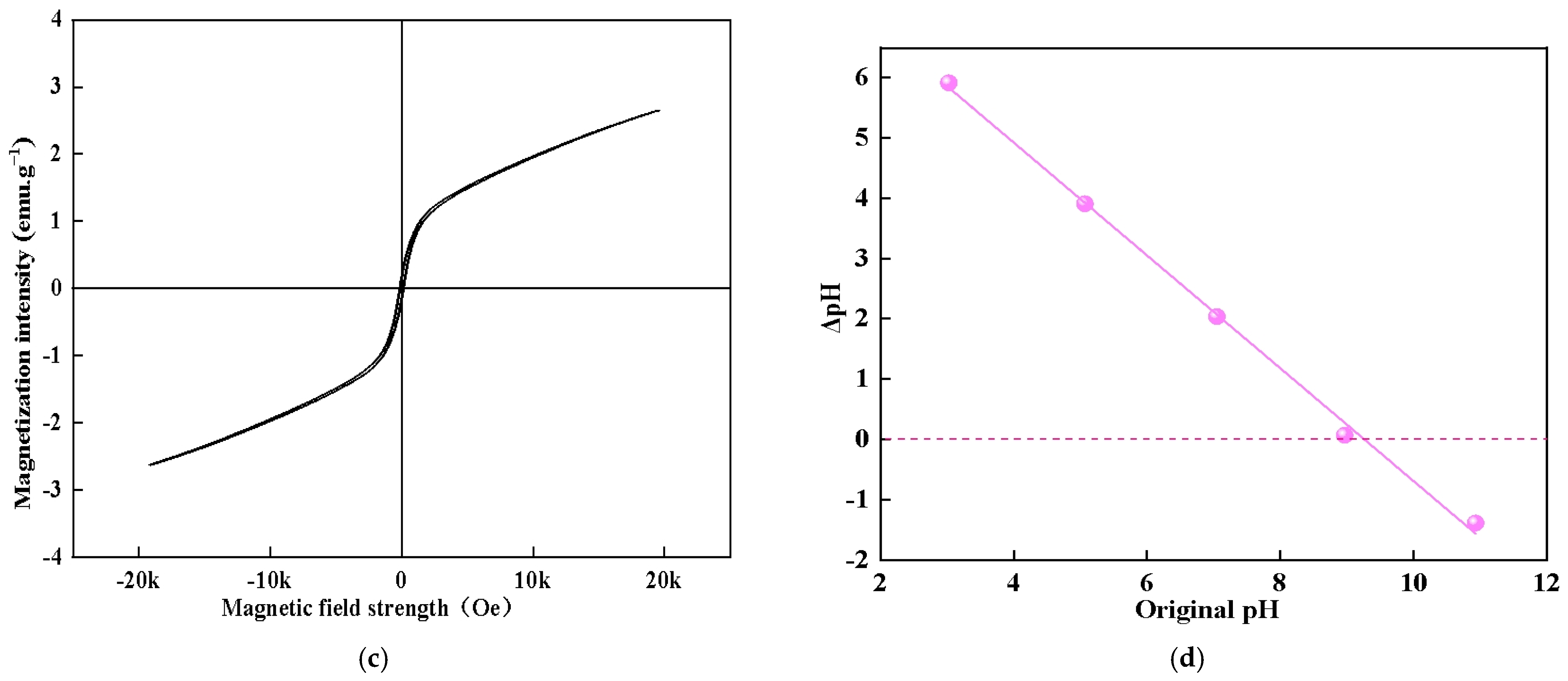
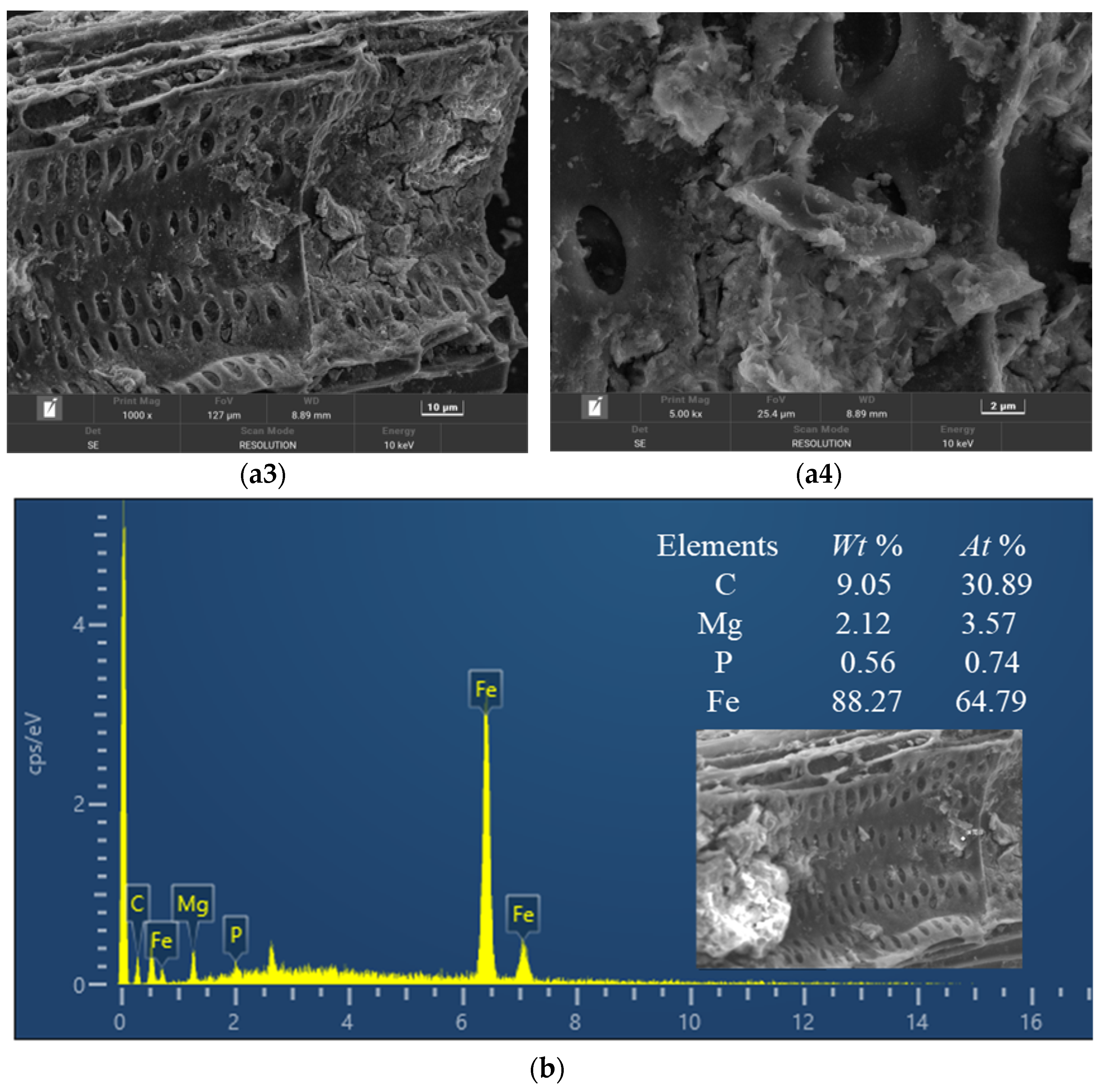
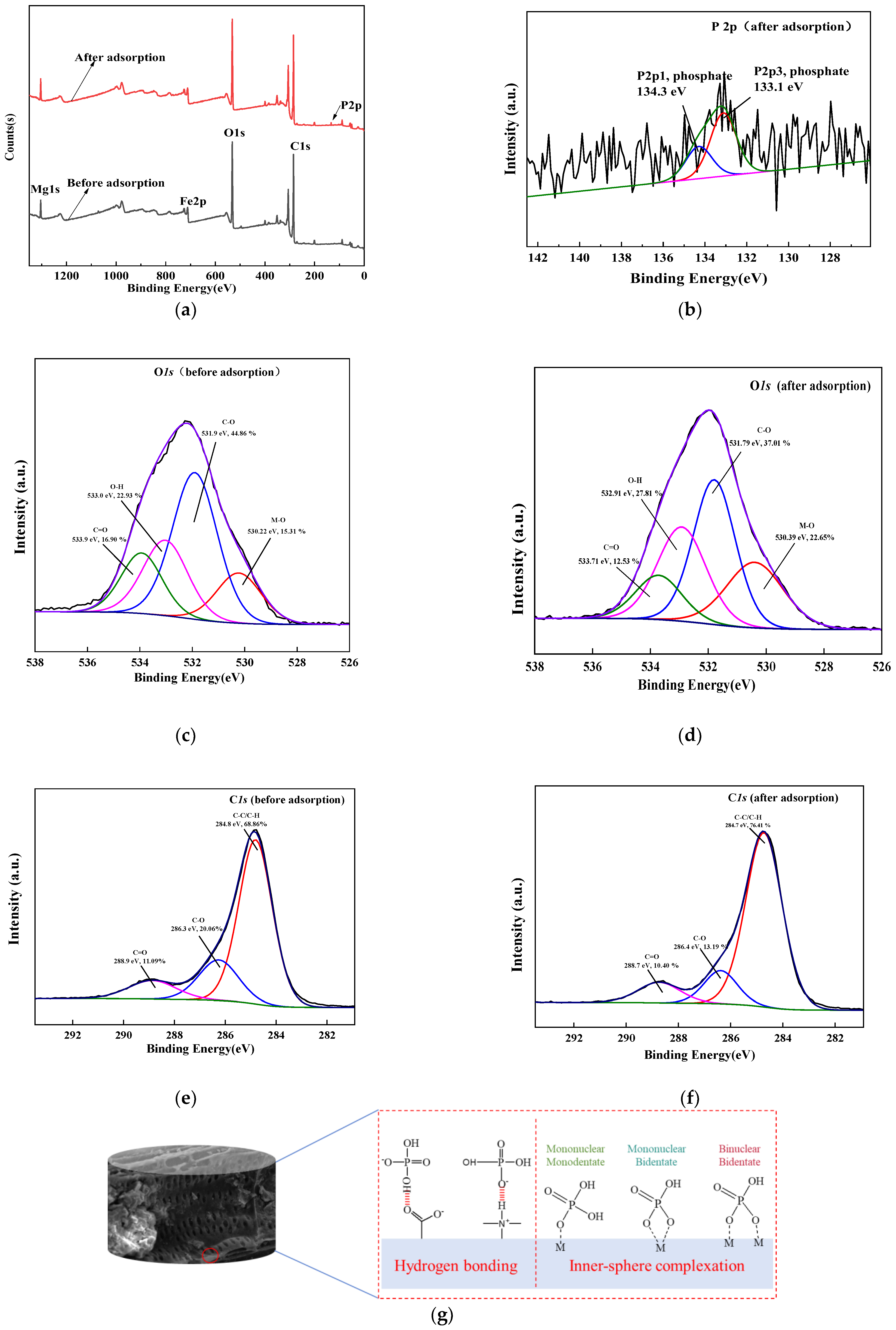
3.1. Characterization of the Composite Adsorbent
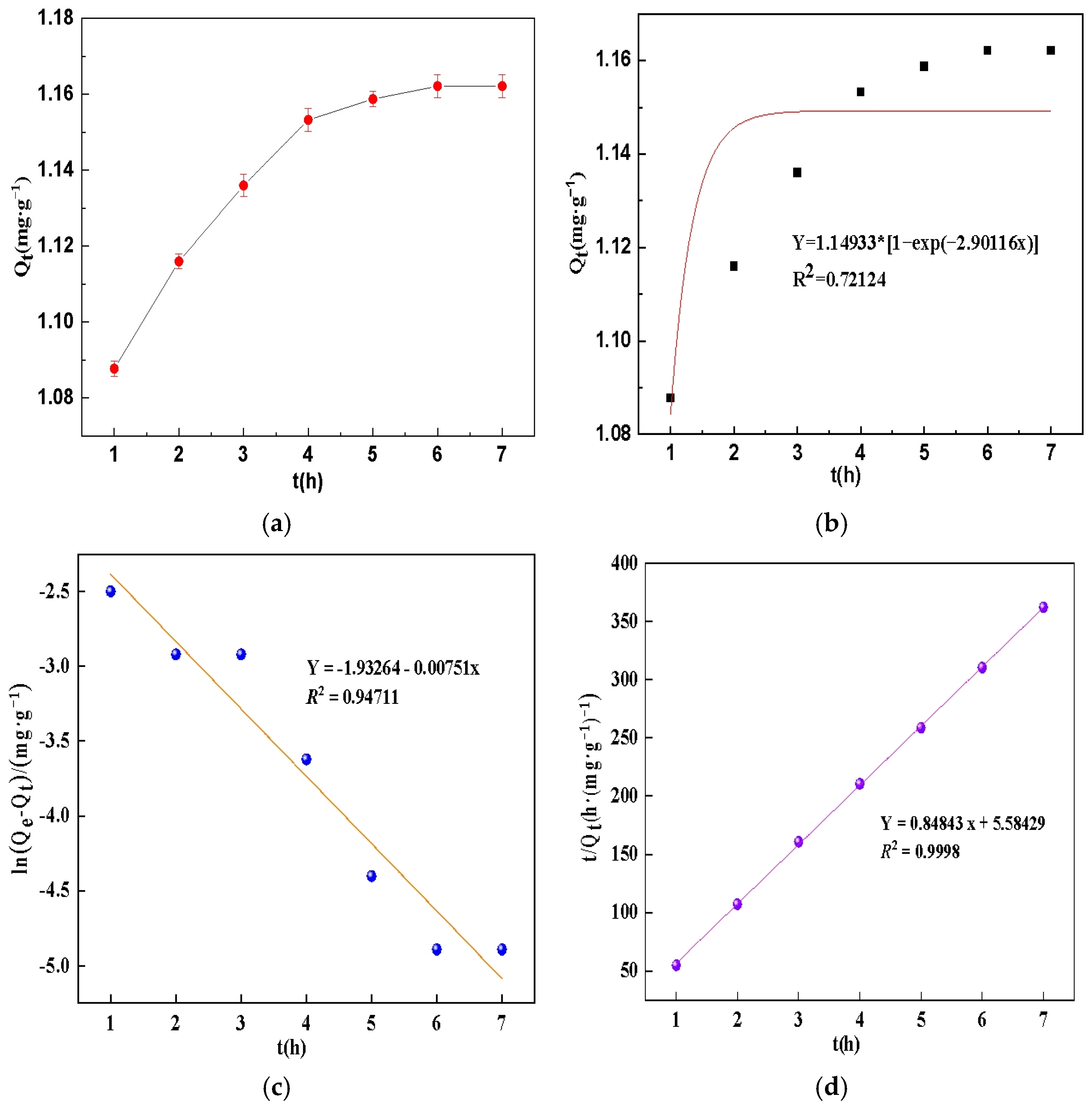
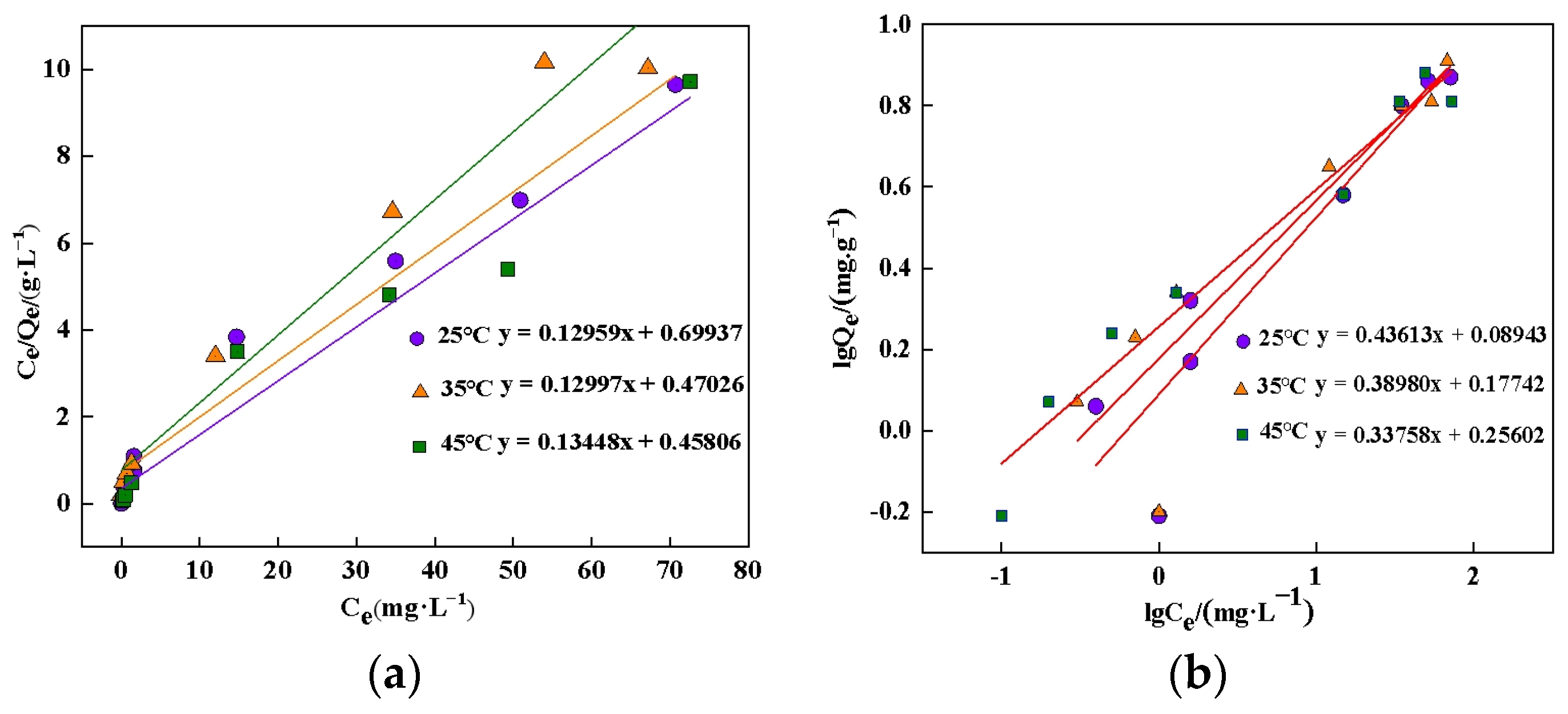
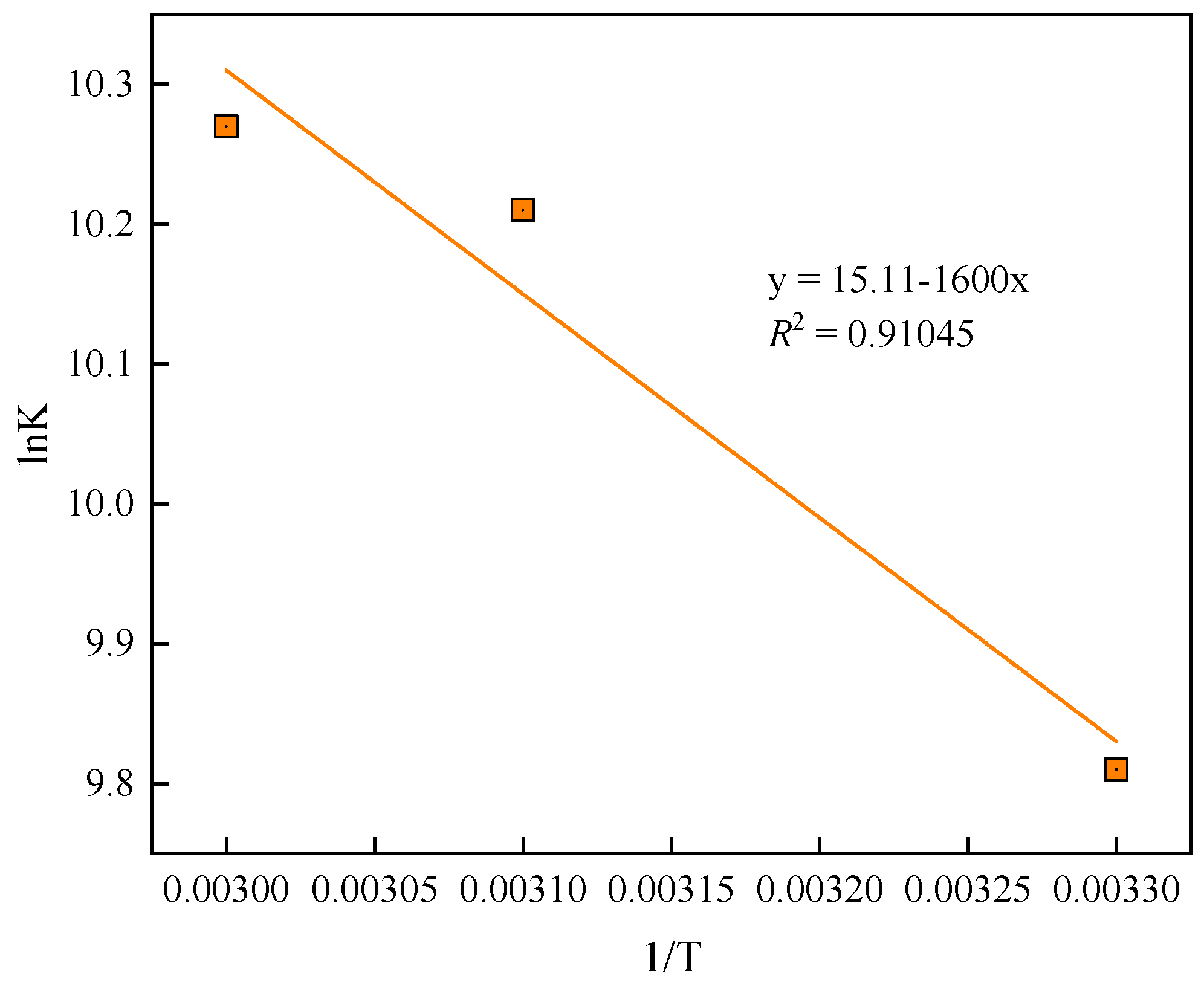
3.2. Phosphate Adsorption Kinetics, Isotherms and Thermodynamics
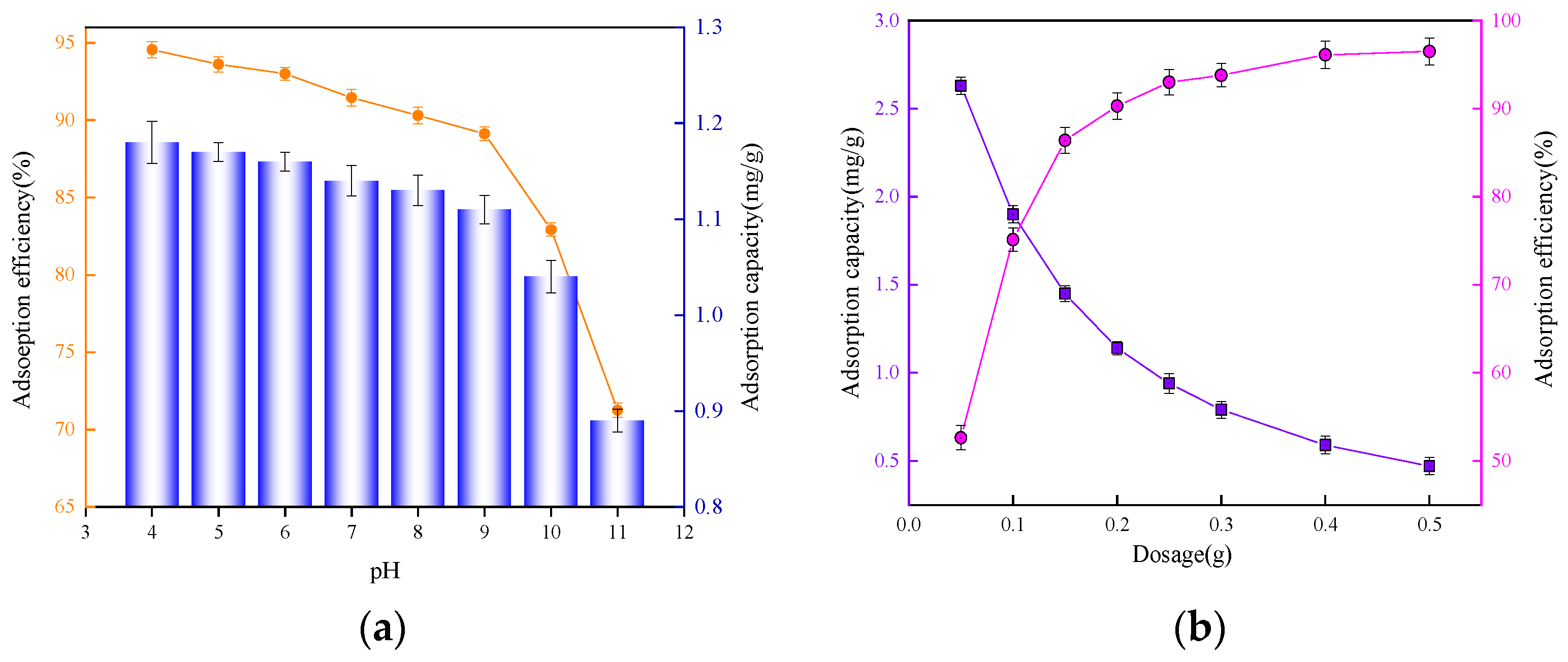
3.3. pH and Adsorbent Dosage Effects on Phosphate Removal
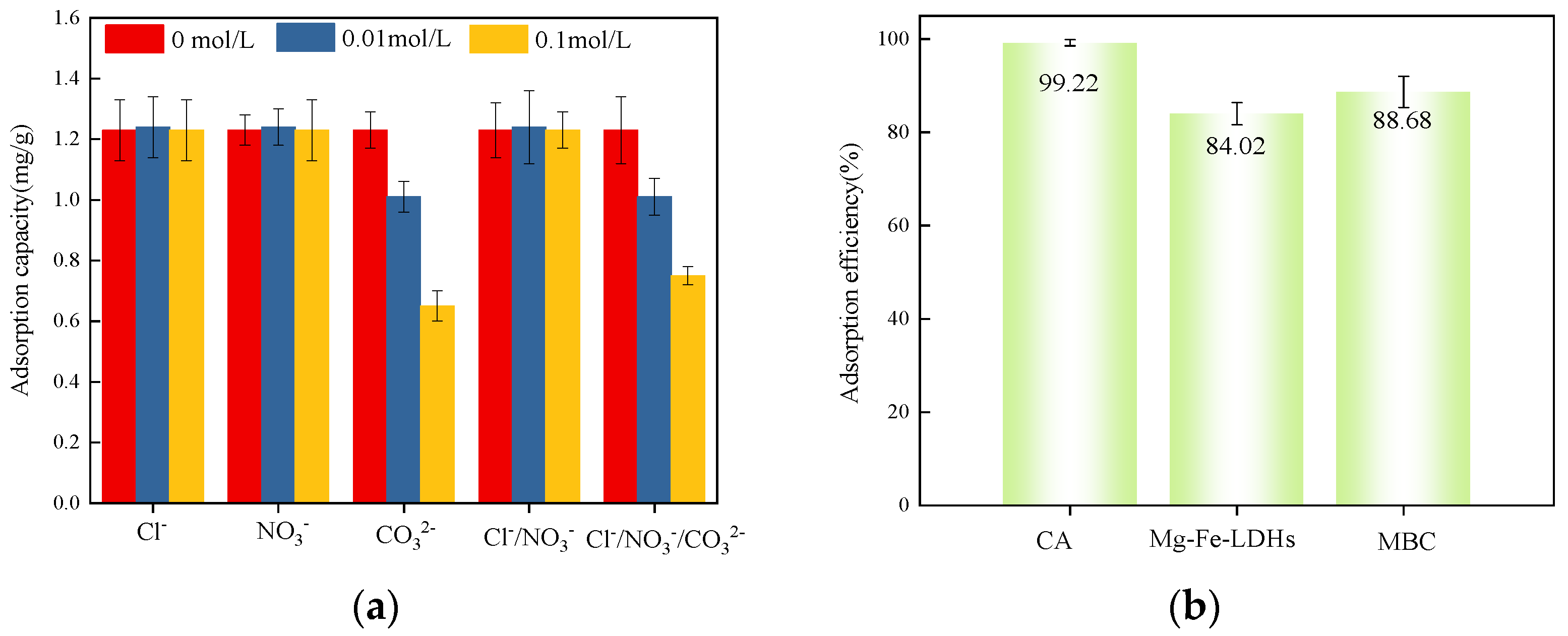
3.4. Selective Phosphate Adsorption in Multi-Anion Systems
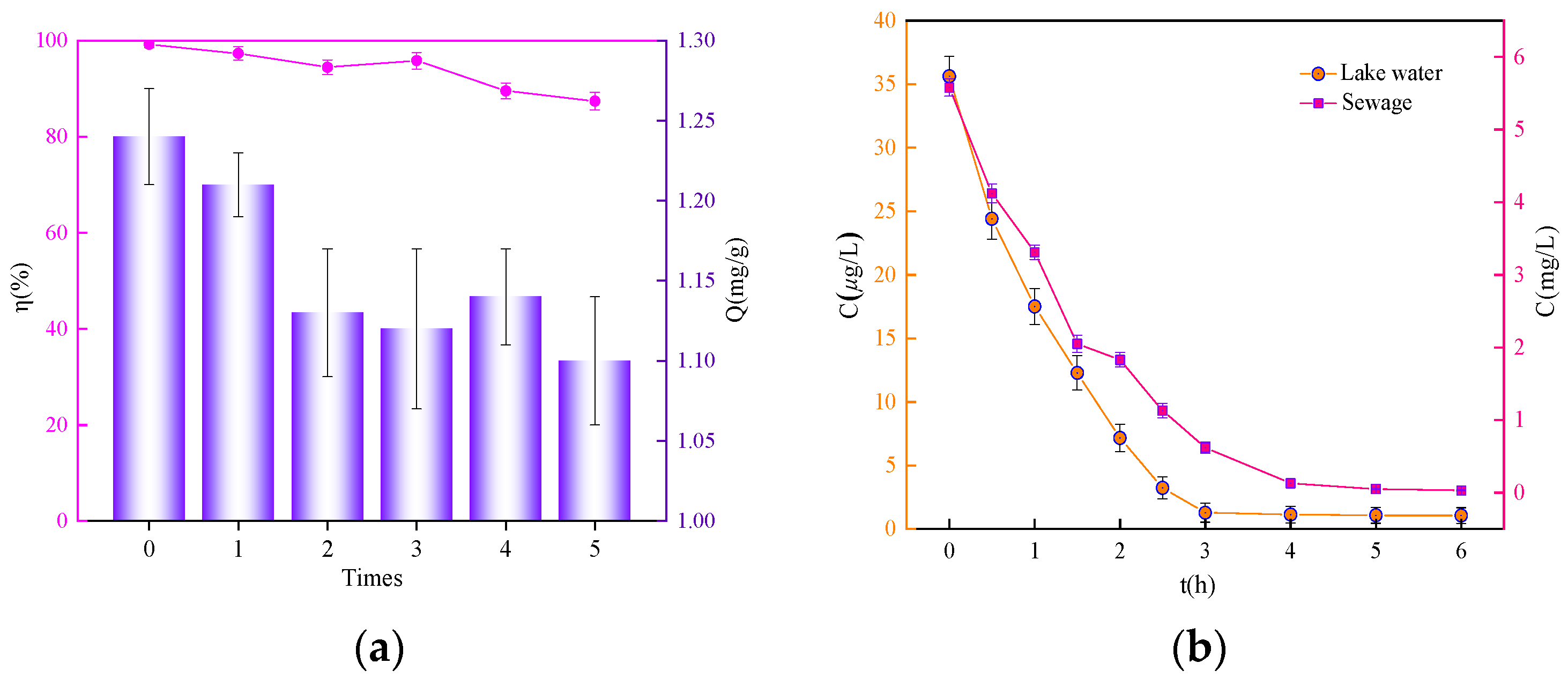
3.5. Regeneration Performance and Environmental Application
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schindler, D.W. The dilemma of controlling cultural eutrophication of lakes. Proc. Biol. Sci. 2012, 279, 4322–4333. [Google Scholar]
- Zeng, Q.; Qin, L.; Bao, L.; Li, Y.; Li, X. Critical nutrient thresholds needed to control eutrophication and synergistic interactions between phosphorus and different nitrogen sources. Environ. Sci. Pollut. Res. Int. 2016, 23, 21008–21019. [Google Scholar]
- Yue, Y.; Han, L.; Ding, B.; Yang, Y.; Yue, X.; Wang, S.; Song, Q.; Du, C. Straw-Derived Activated Carbon Decorated with Ag(3)PO(4) for Organic Pollutant Removal by a Circular Degradation Mechanism: Adsorption and Photocatalysis. ACS Omega 2024, 9, 23584–23596. [Google Scholar] [PubMed]
- Li, Y.; Barati, B.; Li, J.; Verhoestraete, E.; Rousseau, D.P.L.; Van Hulle, S.W.H. Lab-scale evaluation of Microalgal-Bacterial granular sludge as a sustainable alternative for brewery wastewater treatment. Bioresour. Technol. 2024, 411, 131331. [Google Scholar]
- Sun, F.F.; Guan, X.; Huang, Z.H.; Han, X.; Li, H.; Ma, T. Fluoride-based hydrogen bond chemistry in a layered double hydroxide cathode toward high-performance aqueous NH(4)(+) storage. Proc. Natl. Acad. Sci. USA 2025, 122, e2414112122. [Google Scholar] [PubMed]
- Shi, D.; Mao, X.; Fei, M.; Liang, C.; Luo, Y.; Xu, Z.; Hu, L. Unveiling the efficacy and mechanism of chlortetracycline degradation by MnFeCu-LDH/GO activating of peroxymonosulfate. RSC Adv. 2025, 15, 5277–5285. [Google Scholar] [PubMed]
- Tisler, S.; Mrkajic, N.S.; Reinhardt, L.M.; Jensen, C.M.; Clausen, L.; Thomsen, A.H.; Albrechtsen, H.J.; Christensen, J.H. A non-target evaluation of drinking water contaminants in pilot scale activated carbon and anion exchange resin treatments. Water Res. 2025, 271, 122871. [Google Scholar]
- Lai, Z.; Zhou, Y.; Bai, S.; Sun, Q. Opportunity and Challenge of Advanced Porous Sorbents for PFAS Removal. ChemSusChem 2025, 18, e202401229. [Google Scholar]
- Satheesan, A.K.; Madhu, R.; Nagappan, S.; Dhandapani, H.N.; De, A.; Singha Roy, S.; Mazumder, P.; Kundu, S. Current progress in layered double hydroxide-based electrocatalysts for urea oxidation: Insights into strategies and mechanisms. Chem. Commun. 2025, 61, 4092–4109. [Google Scholar]
- Alqahtani, H.A.; AlGhamdi, J.M.; Mu’azu, N.D. Synergistic Effects of Zn-Rich Layered Double Hydroxides on the Corrosion Resistance of PVDF-Based Coatings in Marine Environments. Polymers 2025, 17, 331. [Google Scholar] [CrossRef]
- Ariga, K. Layer-by-Layer Nanoarchitectonics: A Method for Everything in Layered Structures. Materials 2025, 18, 654. [Google Scholar] [CrossRef]
- Zheng, A.L.T.; Lih, E.T.Y.; Hung, Y.P.; Boonyuen, S.; Al Edrus, S.S.O.; Chung, E.L.T.; Andou, Y. Biochar-based electrochemical sensors: A tailored approach to environmental monitoring. Anal. Sci. Int. J. Jpn. Soc. Anal. Chem. 2025; online ahead of print. [Google Scholar]
- Sheikh, L.; Naz, N.; Oranab, S.; Younis, U.; Alarfaj, A.A.; Alharbi, S.A.; Ansari, M.J. Minimization of cadmium toxicity and improvement in growth and biochemical attributes of spinach by using acidified biochar. Sci. Rep. 2025, 15, 5880. [Google Scholar]
- Zhou, L.; Chen, J.; Qian, Y.; Zhang, Y.; Batjargal, E.; Tuulaikhuu, B.A.; Zhou, X. Unlocking phosphorus recovery from microalgae biomass: The enhanced transformation and release of phosphorus species. Water Res. 2025, 275, 123196. [Google Scholar] [PubMed]
- Gubitosa, J.; Rizzi, V.; Cignolo, D.; Fini, P.; Barisano, D.; Freda, C.; Petrella, A.; Cosma, P. Regenerable chitosan-biochar-TiO(2) composite sponges for hazardous pollutants removal from water: The case of carbamazepine. Int. J. Biol. Macromol. 2025, 300, 140315. [Google Scholar]
- Liu, Z.; Yan, Z.; Liu, G.; Wang, X.; Fang, J. Impacts of adding FeSO(4) and biochar on nitrogen loss, bacterial community and related functional genes during cattle manure composting. Bioresour. Technol. 2023, 379, 129029. [Google Scholar]
- Li, X.; Zhang, G.; Jia, Y.; Zou, W.; Zhang, G.; Pan, Y.; Zhou, M. Removal of bisphenol A in a heterogeneous Fenton system via biochar synthesized using different Fe precursors: Properties, effects, and mechanisms. Sci. Total Environ. 2024, 912, 168855. [Google Scholar]
- Polyakov, V.; Bauer, T.; Kirichkov, M.; Butova, V.; Gritsai, M.; Minkina, T.; Soldatov, A.; Kravchenko, E. MOF-biochar nanocomposite for sustainable remediation of contaminated soil. Environ. Sci. Pollut. Res. Int. 2025, 32, 5533–5550. [Google Scholar]
- Wu, X.; Li, R.; Lin, J. Contrasting effects of MgAl- and MgFe-based layered double hydroxides on phosphorus mobilization and microbial communities in sediment. Chemosphere 2024, 346, 140643. [Google Scholar]
- Song, J.; Cha, L.; Sillanpää, M.; Sainio, T. Removal of phosphate with a polyacrylonitrile composite functionalized by a metal organic framework-enhanced layered double hydroxide. Water Sci. Technol. 2023, 87, 1672–1685. [Google Scholar]
- Wang, H.; Zhao, W.; Chen, Y.; Li, Y. Nickel aluminum layered double oxides modified magnetic biochar from waste corncob for efficient removal of acridine orange. Bioresour. Technol. 2020, 315, 123834. [Google Scholar] [CrossRef]
- Fang, Q.; Ye, S.; Yang, H.; Yang, K.; Zhou, J.; Gao, Y.; Lin, Q.; Tan, X.; Yang, Z. Application of layered double hydroxide-biochar composites in wastewater treatment: Recent trends, modification strategies, and outlook. J. Hazard. Mater. 2021, 420, 126569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tang, L.; Luo, J.; Tan, J.; Jiang, X. Comparative study of Mg/Al-LDH and Mg/Fe-LDH on adsorption and loss control of 2,4-dichlorophenoxyacetic acid. Adv. Biotechnol. 2025, 3, 4. [Google Scholar] [CrossRef]
- Wang, X.; Shi, C.; Hao, X.; Wu, Y. Phosphate recovery from sludge-incinerated ash by adsorption with hydrotalcite synthesized by metals in the ash. Sci. Total Environ. 2023, 905, 167263. [Google Scholar] [CrossRef]
- Yu, W.; Du, N.; Gu, Y.; Yan, J.; Hou, W. Specific Ion Effects on the Colloidal Stability of Layered Double Hydroxide Single-layer Nanosheets. Langmuir ACS J. Surf. Colloids 2020, 36, 6557–6568. [Google Scholar] [CrossRef] [PubMed]
- Sala, M.; Makuc, D.; Kolar, J.; Plavec, J.; Pihlar, B. Potentiometric and ³¹P NMR studies on inositol phosphates and their interaction with iron(III) ions. Carbohydr. Res. 2011, 346, 488–494. [Google Scholar] [PubMed]
- Ren, S.; Wang, Y.; Han, Z.; Zhang, Q.; Cui, C. Synthesis of polydopamine modified MgAl-LDH for high efficient Cr(VI) removal from wastewater. Environ. Res. 2022, 215 Pt 1, 114191. [Google Scholar]
- Wang, L.; Song, J.; Yu, C. MgAl-LDH nanoflowers as a novel sensing material for high-performance humidity sensing. RSC Adv 2024, 14, 21991–21998. [Google Scholar] [CrossRef]
- Bai, B.; Wang, Q.; Sun, Y.; Zhou, R.; Chen, G.; Tang, Y. Synthesis of Porous MgAl-LDH on a Micelle Template and Its Application for Efficient Treatment of Oilfield Wastewater. Molecules 2023, 28, 6638. [Google Scholar] [CrossRef]
- Wu, K.; Xu, J.; Jiang, Y.; Jiang, Y.; Yurekli, Y.; Yue, X.; Dai, Y.; Zhang, T.; Yang, D.; Qiu, F. ZnAl-LDH/wood-based antifouling membranes for high-flux and efficient oil/water separation. J. Hazard. Mater. 2025, 490, 137739. [Google Scholar] [CrossRef]
- Song, S.; Xia, M.; Feng, Y.; Zhang, X. Synergistic Coupling Effect and Anionic Modulation of CoFe LDH@MXene for Triggered and Sustained Alkaline Water/Seawater Electrolysis. Chem. Asian J. 2025, 20, e202401295. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Mo, H.; Liu, Y.; Zeng, C.; Peng, S.; Zhan, H. As(III) removal by a recyclable granular adsorbent through dopping Fe-Mn binary oxides into graphene oxide chitosan. Int. J. Biol. Macromol. 2023, 237, 124184. [Google Scholar] [CrossRef] [PubMed]
- Charles, C.; Barrat, J.A.; Pelleter, E. Trace element determinations in Fe-Mn oxides by high resolution ICP-MS after Tm addition. Talanta 2021, 233, 122446. [Google Scholar] [CrossRef] [PubMed]
- Lartey-Young, G.; Ma, L. Optimization, equilibrium, adsorption behaviour of Cu/Zn/Fe LDH and LDHBC composites towards atrazine reclamation in an aqueous environment. Chemosphere 2022, 293, 133526. [Google Scholar] [CrossRef]
- Tian, S.Q.; Wang, L.; Liu, Y.L.; Ma, J. Degradation of organic pollutants by ferrate/biochar: Enhanced formation of strong intermediate oxidative iron species. Water Res. 2020, 183, 116054. [Google Scholar] [CrossRef]
- Radwan, I.T.; Khater, H.F.; Mohammed, S.H.; Khalil, A.; Farghali, M.A.; Mahmoud, M.G.; Selim, A.; Manaa, E.A.; Bagato, N.; Baz, M.M. Synthesis of eco-friendly layered double hydroxide and nanoemulsion for jasmine and peppermint oils and their larvicidal activities against Culex pipiens Linnaeus. Sci. Rep. 2024, 14, 6884. [Google Scholar] [CrossRef]
- Baker, A.; Iram, S.; Syed, A.; Elgorban, A.M.; Al-Falih, A.M.; Bahkali, A.H.; Khan, M.S.; Kim, J. Potentially Bioactive Fungus Mediated Silver Nanoparticles. Nanomaterials 2021, 11, 3227. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, S.; Sun, Y.; Tsang, D.C.W.; Cheng, K.; Ok, Y.S. Assembling biochar with various layered double hydroxides for enhancement of phosphorus recovery. J. Hazard. Mater. 2019, 365, 665–673. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Xin, L.; Peng, Y.; Zhang, S.; Guan, D.; Song, J. Sustainable Phosphate Remediation via Hierarchical Mg-Fe Layered Double Hydroxides on Magnetic Biochar from Agricultural Waste. Magnetochemistry 2025, 11, 27. https://doi.org/10.3390/magnetochemistry11040027
Li X, Xin L, Peng Y, Zhang S, Guan D, Song J. Sustainable Phosphate Remediation via Hierarchical Mg-Fe Layered Double Hydroxides on Magnetic Biochar from Agricultural Waste. Magnetochemistry. 2025; 11(4):27. https://doi.org/10.3390/magnetochemistry11040027
Chicago/Turabian StyleLi, Xiuling, Lei Xin, Yuhan Peng, Shihao Zhang, Delong Guan, and Jing Song. 2025. "Sustainable Phosphate Remediation via Hierarchical Mg-Fe Layered Double Hydroxides on Magnetic Biochar from Agricultural Waste" Magnetochemistry 11, no. 4: 27. https://doi.org/10.3390/magnetochemistry11040027
APA StyleLi, X., Xin, L., Peng, Y., Zhang, S., Guan, D., & Song, J. (2025). Sustainable Phosphate Remediation via Hierarchical Mg-Fe Layered Double Hydroxides on Magnetic Biochar from Agricultural Waste. Magnetochemistry, 11(4), 27. https://doi.org/10.3390/magnetochemistry11040027






