Abstract
The emergence of antimicrobial resistance due to antibiotics in the environment presents significant public health, economic, and societal risks. This study addresses the need for effective strategies to reduce antibiotic residues, focusing on ciprofloxacin degradation. Magnetic iron oxide nanoparticles (IO NPs), approximately 13 nm in size, were synthesized and functionalized with branched polyethyleneimine (bPEI) to obtain a positive charge. These IO-bPEI NPs were combined with negatively charged titanium dioxide NPs (TiO2@CA) to form magnetically photocatalytic IO-TiO2 nanocomposites. Characterization techniques, including X-ray diffraction (XRD), transmission electron microscopy (TEM), scanning electron microscopy (SEM), infrared spectroscopy (IR), X-ray photoelectron spectroscopy (XPS), thermogravimetric analysis (TGA), electrokinetic measurements, and a vibrating sample magnetometer (VSM), confirmed the successful formation and properties of the nanocomposites. The nanocomposites exhibited a high specific surface area, reduced mobility of photogenerated charge carriers, and enhanced photocatalytic properties. Testing the photocatalytic potential of IO-TiO2 with ciprofloxacin in water under UV-B light achieved up to 70% degradation in 150 min, with a degradation rate of 0.0063 min−1. The nanocomposite was magnetically removed after photocatalysis and successfully regenerated for reuse. These findings highlight the potential of IO-TiO2 nanocomposites for reducing ciprofloxacin levels in wastewater, helping curb antibiotic resistance.
1. Introduction
Antibiotics are among the crucial drugs essential for improving human health. However, the rise of bacterial resistance to antibiotics and their rapid adaptation have become a global issue [1]. Despite a record low in antibiotic usage recorded in 2020, it remains one of the top three health threats, according to the European Health Emergency Preparedness and Response Authority (HERA). Infections caused by antibiotic-resistant bacteria can lead to additional healthcare and productivity costs, potentially amounting to as much as 1.5 billion euros [2]. Between 2017 and 2021, infections caused by Klebsiella pneumoniae, a rod-shaped bacterium commonly found in the intestines that can infect the lungs, increased. These infections are often treated with ciprofloxacin [3].
Ciprofloxacin, a synthetic broad-spectrum antibiotic, is utilized for its bactericidal and bacteriostatic effects against a wide range of Gram-positive and Gram-negative bacteria [4,5]. This has resulted in its increased use in clinical treatments. However, due to its structural stability, ciprofloxacin cannot be completely metabolized in the human body. Studies have reported that about 40–60% of ciprofloxacin is excreted by humans in unmetabolized form [6]. Residues from both metabolized, unused, or expired ciprofloxacin-based drugs are released into the environment. For example, a study found that hospital wastewater in France contained ciprofloxacin concentrations ranging from 0.97 to 3.39 μg/L [7]. These residues bind to minerals and organic compounds, which accumulate in soil or disperse rapidly in water [8], leading to ecotoxicological effects [5]. Ciprofloxacin ingestion through water has been associated with unhealthy symptoms such as diarrhea, headaches, vomiting, and tremors [9,10]. Urgent action is required to remove the antibiotic from wastewater to safeguard underground water supplies.
Various investigations have focused on this issue, considering the physicochemical properties of water. Common procedures employed to eliminate antibiotics include coagulation [11], flocculation [12,13], sedimentation [14], filtration [15], and disinfection [16]. However, due to the formation of stable wastewater mixtures, those classical methods proved to be either ineffective or insufficient [17], having several shortcomings. Additional investigations using catalytic oxidation [18], electro-Fenton photocatalytic oxidation [19], and adsorption proved to be more efficient, especially adsorption using nanotechnology in combination with photocatalysis [17,20]. Photodegradation offers numerous appealing advantages over traditional methods, including being environmentally friendly, cost-effective, efficient, reusable, and scalable [21,22]. In comparison, for example, to the Fenton oxidation process, where large volumes of iron slurry are obtained without the possibility of reusability, photodegradation is the preferred method [23]. Photocatalysis utilizes sunlight or artificial light, and photocatalysts degrade persistent pollutants under light irradiation and contribute to a comprehensive and sustainable wastewater treatment solution. Advanced oxidation processes (AOPs) are particularly important and attractive for the degradation of pollutants in contaminated water. These processes generate strongly oxidizing radicals under UV light, especially hydroxyl radicals (OH·). These radicals are able to break down complex compounds into simpler, non-toxic substances, making AOPs an effective method for water purification [24].
Commonly used materials for photodegradation include metal oxides [25] such as titanium dioxide (TiO2) [26], zinc oxide (ZnO), graphene oxide [27], iron oxide [28], and metal-organic frameworks [9]. Due to its cost-effectiveness and high efficiency, TiO2 is the most commonly used material [29]. TiO2, a semiconductor photocatalyst, is often used in heterogeneous processes to degrade pollutants in aqueous environments. TiO2 NPs have remarkable properties, including a high surface-to-volume ratio, strong oxidizing power, and low toxicity. As a result, TiO2 NPs are gaining ground in the field of photocatalysis and are being extensively studied for various photocatalytic applications. Importantly, a deeper understanding of TiO2 photocatalysis has facilitated the development of new photocatalytic materials with improved functionalities, such as integration with magnetic materials [24,30]. However, the effectiveness of TiO2 is hindered by its wide bandgap, which limits its efficiency in visible light and has reduced commercial interest in recent years. In fact, the controlled application and removal of TiO2 nanocomposites is essential. A major challenge is the difficulty in removing the photocatalyst from the solution medium after use. Although various technologies, primarily filtration, have been used to remove TiO2 after photocatalysis, they often result in loss or incomplete removal of TiO2 [31,32]. Consequently, further research has focused on the immobilization of TiO2 on supports such as glass plates [33], waste polystyrene [34], or magnetic nanomaterials [31,32,35,36,37,38,39,40] to address these challenges. In the field of TiO2-based photocatalysts, considerable efforts have been made to incorporate magnetic nanomaterials into TiO2-based nanocomposite structures. This integration aims to enable the rapid and efficient removal of photocatalytic materials from aqueous systems using an external magnetic field. The multifaceted approach here in this study emphasizes the potential of magnetic nanocomposites as advanced, viable, and versatile tools in addressing the complex challenges associated with wastewater remediation [41].
In particular, combining TiO2 with other metal iron oxides, such as hematite (α-Fe2O3) [29,37,42,43,44], maghemite (γ-Fe2O3) [45], or magnetite (Fe3O4) [24,31,32,36,38,40,46,47,48], can improve its performance. Correspondingly, maghemite iron oxide is non-toxic and cost-effective, making it a viable option for scaling up wastewater treatment technologies [45,49,50]. The combination of TiO2 and Fe2O3 has been found to be highly effective in the photo-oxidation of water, generating photo-induced holes that can last from milliseconds to seconds [44].
The synthesis/combination of iron oxides and TiO2 nanocomposites typically involves multi-step processes that require high temperatures [36,51] or the use of toxic materials [26,52], posing challenges in terms of cost and environmental impact. Researchers are exploring alternative methods using charge complexation, sonication for activation, or incorporation of silicium dioxide [53] as a median layer to prepare TiO2-iron oxide nanoparticles. Furthermore, under mild conditions with non-toxic solvents or activators, these nanoparticles were made using poly(N-isopropylacrylamide-co-methacrylic acid) and silver [54]. These advances aim to improve the feasibility and safety of nanocomposites in wastewater remediation. In the development of nanocomposite materials from two different NPs, hetero-agglomeration is an excellent choice due to its simplicity, lack of specific additional chemicals, and environmental friendliness. It is based on attractive forces between oppositely charged nanoparticles, resulting in composites that are irreversibly bonded together and exhibit high stability [40].
Most photocatalytic materials based on magnetic materials such as Fe3O4 or α-Fe2O3, or their combination with TiO2, have primarily been utilized for degrading dyes [24,31,32,37,38,39,40,43,44,46,48,54,55,56,57] or other organic pollutants [35,36,51]. However, few studies have focused on the degradation of the antibiotic ciprofloxacin, followed by magnetic-assisted removal, as indicated by the cited research [33,34,47,58,59,60,61,62]. In this context, photocatalytic materials developed for the degradation of ciprofloxacin have mainly focused on materials such as TiO2 [61], chlorophyll-sensitized and salicylic acid-functionalized TiO2 NPs [58], Fe2O3 nanoparticles loaded on graphitic carbon nitride [59], waste polystyrene and TiO2 composites [34], defective TiO2-x nanomaterials [60], and biosynthesized silica-supported silver nanoparticles [62]. In addition, other materials, such as ZnIn2S4/CoFe2O4 p-n junction decorated biochar [63], Gd2WO6-loaded ZnO/bentonite nanocomposite [64], Fe3O4/CdS/g-C3N4 [65], CuFe2O4@methylcellulose-based magnetic nanobiocomposite [66], nano-TiO2 immobilized polyvinylidene fluoride-based sponge beads [67] and others [68,69,70] have been used for photocatalytic degradation of ciprofloxacin, but no study uses the IO-TiO2 nanocomposite for the degradation of ciprofloxacin, which highlights the unique contribution of the present work to the scientific community. In fact, the combined approach of photocatalytic degradation of ciprofloxacin and magnetic removal of the photocatalytic material after the process is relatively unexplored in the literature and requires further investigation. Currently, there are no studies addressing the hetero-agglomeration of positively modified IO-bPEI NPs with negatively charged TiO2@CA for the photocatalytic degradation of ciprofloxacin in an aqueous environment, followed by the removal of the nanocomposite with an applied magnetic field. This gap in research clearly emphasizes the novelty and significance of the present study. Additionally, the innovative use of bPEI and citric acid in this combination has not been previously reported in the literature, making this approach both novel and more environmentally friendly. This method ensures that only the precise amount of TiO2 is adsorbed onto the IO-bPEI, thus controlling the TiO2 usage.
In this study, a precisely engineered IO-TiO2 nanocomposite was developed by combining positively charged maghemite iron oxide NPs (i.e., IO-bPEI) with negatively charged TiO2@CA NPs through a simple hetero-agglomeration method. This nanocomposite was applied for the photocatalytic degradation of ciprofloxacin under UV-B irradiation. After completion of the process, the nanocomposites could be easily removed using a permanent magnet. This represents a significant advance over existing state-of-the-art photocatalysis processes for the degradation of ciprofloxacin. Although this work was studied under UV irradiation, it lays the foundation for future studies in the visible light range. The carefully prepared nanomaterials were thoroughly investigated for their structure, morphology, composition, electrophoretic behavior, and magnetic properties using various methods such as ATR-IR spectroscopy, potentiometric titrations, XRD, XPS, SEM with EDXS, TEM, TGA, VSM, and BET, among others. Several factors affecting the degradation of ciprofloxacin under UV-B irradiation were investigated, and optimal conditions for efficient photodegradation were defined. The photocatalytic potential of the nanocomposite was also explored (i.e., UV-Vis reflectance spectra (UV-Vis-DR), photoluminescence (PL) emission, band gap). In addition, the recyclability and reusability of photocatalysts are crucial for the environmentally friendly and sustainable development of photocatalytic technology, although this is often overlooked. Therefore, the stability and reusability of nanocomposite were explored. Finally, plausible degradation mechanisms for ciprofloxacin using the developed nanocomposite are exhaustively discussed.
2. Materials and Methods
2.1. Materials
All the chemicals were used as received, without any further purification. The FeSO4 × 7H2O, Fe2(SO4)3 × 7H2O, and HCl (≥37%), branched PEI with an average MW of 25,000 Da, and ciprofloxacin with a purity of ≥98% (HPLC) were received from Sigma-Aldrich. The NH4OH (25% aqueous solution) and NaOH (>98%) were purchased from Honeywell. The absolute EtOH (anhydrous) was received from CarloErba.
In this study, we used a commercially available nano-sized colloidal dispersion of TiO2@CA, namely CCA100BS—a dispersion with neutral pH 7 (~25% TiO2@CA according to the manufacturer). These NPs were industrially produced and supplied by Cinkarna, Metallurgical and Chemical Industry Celje, Inc. in Slovenia (Celje, Slovenia). The TiO2@CA NPs in this stable dispersion were synthesized in a sol-gel process using metatitanic acid, a by-product of the sulfate synthesis process. The complete synthesis process is described in patent SI 23,501 A of Cinkarna Celje. Details can be found in the Supplementary Information. The magnet used for the magnetic separation was a nickel-plated NdFeB block magnet (size 46 × 30 × 10 mm) with an adhesive force of approx. 284 N (approx. 29 kg) and a magnetization of N40, which was purchased from supermagnete.
Ultra-pure water with a resistivity > 18 MΩ cm (Millipore, Burlington, MA, USA) was used for the preparation of all the aqueous systems in this work.
2.2. Synthesis and Functionalization of Magnetic Iron Oxide Nanoparticles with Branched Polyethylene Imine (IO-bPEI)
Magnetic iron oxide nanoparticles (IO NPs) were synthesized using a slightly modified method based on [71]. Iron sulfate salts were dissolved in 500 mL of MilliQ water under ambient air conditions at a molar ratio of Fe2+ to Fe3+ of 2.4:1. A diluted ammonium solution was then added to the mixture to adjust the pH to 3. This value was maintained for 30 min by continuous addition of the ammonium solution, resulting in the precipitation of iron hydroxides. Subsequently, 250 mL of 25% NH4OH was added to form iron oxide MNPs, and the solution was magnetically stirred for another 30 min. To purify the synthesized MNPs, MilliQ water was prepared with diluted NH4OH at pH 10. The MNPs were washed three times with 200 mL of this alkaline solution, once with pure MilliQ water with a separation time of about 1 min, and finally dispersed in MilliQ water.
Functionalization of bare IO NPs with branched polyethylenimine (bPEI) was performed under the assumption that 5 bPEI molecules would bind per 1 nm2 of the MNPs’ surface. The required amount of polysaccharide was calculated based on a theoretical specific surface area of 95.4 m2/g for ~13 nm MNPs as reported by [71]. For the functionalization of 250 mg MNPs, 100 mg bPEI was required. To achieve this, a 0.4 wt% dispersion of MNPs (62.5 mL) was prepared, and the pH was adjusted to 8 with 0.1 M NaOH. The bPEI (0.2 wt%) was dissolved in MilliQ water, also adjusted to pH 8, and then slowly added to the MNP dispersion. At this pH, the amino groups of bPEI are protonated and positively charged, while the bare MNP carries a negative charge, which facilitates electrostatic interaction (as shown by the zeta potential data in the Section 3). The final pH of the dispersion was maintained at 8. The mixture was stirred for 20 h to allow complete functionalization of the IO NPs with bPEI. After functionalization, the bPEI-coated iron oxide nanoparticles (IO-bPEI) were separated with a permanent magnet (separation time 30 s), washed several times with MilliQ water, and finally dispersed again in MilliQ water.
2.3. Preparation of IO-TiO2 Photocatalytic Nanocomposites
A simple heteroagglomeration method utilizing electrostatic interactions between oppositely charged NPs was used to prepare the magnetic photocatalytic IO-TiO2 nanocomposites, similar to the study by Makovec et al. [45], but using different modification agents and experimental conditions to perform heteroagglomeration. A commercially available dispersion of TiO2 stabilized with citric acid (i.e., TiO2@CA) was adjusted to a concentration of 1 mg/mL, with the pH set to 7.5 using 0.1 M NaOH or HCl. An aqueous dispersion containing 200 mg TiO2@CA NPs in 100 mL was gradually added to the IO-bPEI NPs dispersion. A weight ratio of 1:1 between IO-bPEI and TiO2@CA NPs was maintained. Similarly, a 0.1 wt% aqueous dispersion of IO-bPEI NPs was prepared at pH 7.5 (200 mL containing 200 mg IO-bPEI). The negatively charged TiO2@CA NPs were slowly introduced into the positively charged IO-bPEI dispersion with vigorous mixing, and the pH of the mixture was maintained at 7.5 by adding 0.1 M NaOH or HCl as required. The resulting nanocomposite was stirred for 1 h at room temperature to ensure successful heteroagglomeration. The nanocomposite was then separated with a permanent magnet with a time of separation of ~30 s and washed twice with MilliQ water. The overall concept of the work presented here is shown schematically in Scheme 1.
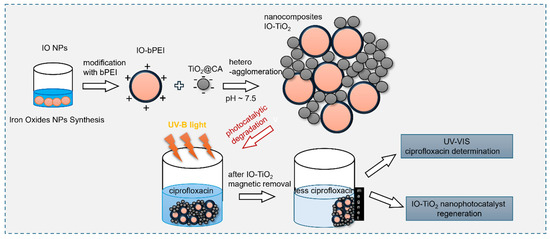
Scheme 1.
The overall concept of the work performed in the following study.
2.4. Characterization of Photocatalytic Nanocomposite
2.4.1. pH Titration Curves
Potentiometric pH titration was used to determine the amount and presumed nature of charged functional groups in aqueous solutions of bPEI and ciprofloxacin. The protonation and deprotonation behavior of the groups, which is pH-dependent, was monitored by potentiometric titrations performed in the forward direction (acidic to alkaline) over the wide pH range. The titrations were performed with HCl (0.1 mol/L) and KOH (0.1 mol/L) as titrants. A T70 multipurpose autotitrator with two burettes (Mettler Toledo, Greifensee, Switzerland) was used to add the titrants, while pH measurements were recorded using an InLab Reach combined glass electrode (Mettler Toledo, Switzerland). Titrations were performed in an inert atmosphere by bubbling nitrogen gas, and an ionic strength of 0.1 mol/L was maintained by adding 3 mol/L KCl. A detailed description of the potentiometric charge titration method can be found in the references [72,73].
2.4.2. X-ray Powder Diffraction (XRD)
The composition of the prepared materials was determined by X-ray powder diffraction (XRD) on a PANanalytical PRO MPD diffractometer with Cu Kα1 radiation (1.54056 Å). Scans were performed over a range of 10–90° in 0.034° increments. Standard reference data was obtained from the International Center for Diffraction Data (PDF).
2.4.3. Transmission Electron Microscopy (TEM)
The morphology and elemental mapping of composites were analyzed using a transmission electron microscope (TEM, JEOL JEM 2100UHR, Tokyo, Japan) operating at 200 kV, equipped with an energy dispersive X-ray spectrometer (EDXS, Oxford X-Max80, Oxfordshire, UK). For TEM observations, the particles were deposited on a carbon-coated copper grid from suspension.
2.4.4. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDXS) Mapping
The morphology of IO-bPEI, TiO2@CA, and IO-TiO2 was analyzed by scanning electron microscopy (SEM) using a JSM-7610FPlus instrument (Jeol, Tokyo, Japan). About 100 μL of the dispersion was applied to double-sided conductive carbon tape using a pipette, allowed to dry thoroughly, and then mounted on a holder. The sample was sputtered with platinum to further ensure conductivity. SEM images were taken at an accelerating voltage of 10 kV and a magnification of 5000x using a secondary electron detector. In addition, energy dispersive X-ray spectroscopy (EDXS) was performed to identify and map the distribution of certain elements, such as C, N, O, Fe, and Ti.
2.4.5. ATR-FTIR
The infrared spectra of the samples were measured using a Perkin Elmer Spectrum GX NIR FT-Raman spectrometer (Waltham, MA, USA) equipped with a diamond crystal ATR accessory and operating in transmission mode. A background spectrum was recorded first, followed by measurements of the investigated samples under identical conditions. The specific parameters used for the samples included 16 scans with a resolution of 4 cm−1, covering a wavenumber range of 4000–400 cm−1. After scanning, the spectra were processed with automatic smoothing filters, baseline corrections, and ATR corrections and normalized to 1 for comparison purposes. All measurements were performed at room temperature.
2.4.6. X-ray Photoelectron Spectroscopy (XPS)
The chemical composition of the MNP samples was analyzed using X-ray photoelectron spectroscopy (XPS). A PHI-TFA model from Physical Electronics (Chanhassen, MN, USA) was used. The main XPS chamber was maintained at an ultimate pressure of 6 × 10−8 Pa. The XPS setup included a monochromatic Al Kα1.2 radiation source with a photon energy of 1486.6 eV and a hemispherical analyzer positioned at a 45° angle. The MNP samples were mounted on double-sided copper tape and analyzed over an area with a diameter of 400 µm. An electron gun was used to neutralize the surface charge during the measurements. The C-C component in the C1s peak was calibrated to 248.8 eV. Survey scan spectra were acquired at a pass energy of 187 eV with an energy step of 0.4 eV, while high-resolution spectra were acquired at a pass energy of 27.35 eV with an energy step of 0.125 eV. Data analysis was performed using the Multipak 9.9 software supplied with the spectrometer.
2.4.7. Zeta Potential and Hydrodynamic Diameter
The zeta potential (ZP) and hydrodynamic diameter (HD) of the prepared particle dispersions, formulated as a 0.1 wt% aqueous dispersion in a 1 mM KOH electrolyte background, were determined using a Litesizer 500 analyzer (Anton Paar, Graz, Austria) at 25 °C. The ZP measurements were performed by electrophoretic light scattering (ELS), while the HD measurements were performed by dynamic light scattering (DLS). Prior to analysis, the dispersion was stirred and adjusted to the desired pH using a titration unit with 0.05 M HCl or 0.05 M KOH solutions. The dispersion was then transferred to an Omega cuvette for both ZP and size measurements and placed in the sample holder of the device. The following conditions were used for the HD measurements: pH 8 and at 25 °C, in water as solvent. The selected material was considered unknown due to the different components and complexity of the designed NPs and final nanocomposite. For ZP, the Smoluchowski approximation in water as solvent was used (refractive index 1.3303, viscosity 0.00089 Pas, relative permittivity 78.368). Data acquisition was managed using Kalliope software (version 2.22.1, Anton Paar, Graz, Austria).
2.4.8. Thermogravimetric Analysis and Magnetic Properties
The mass loss as a function of temperature was determined by thermogravimetric analysis (TGA). Approximately 5 mg of each well-dried sample was placed in 60 μL alumina crucibles for analysis using a Mettler TGA/SDTA 851 thermogravimetric analyzer (Mettler Toledo, Switzerland). The samples were heated from 25 to 600 °C at a rate of 10 K/min in a nitrogen atmosphere. The room-temperature magnetization curves of the bare IO NPs and the magnetic IO-TiO2 nanocomposites were measured using a vibrating-sample magnetometer (VSM, Lake Shore 7307, Westerville, OH, USA). The dried nanoparticle powders were placed in plastic holders and sealed for measurement. The magnetization was recorded at room temperature with an external magnetic field ranging from −10 × 103 to 10 × 103 Oe.
2.4.9. Specific Surface Area, Pore Volume, and Average Pore Size of the Materials
To determine the specific surface area, pore volume, and average pore size of the materials, N2-physisorption experiments were carried out using a Micromeritics TriStar II 3020 device equipped with the SmartPrep degasser. Degassing was performed in an N2 stream (Linde, Danbury, CT, USA, purity 6.0) at 65 °C for 24 h. Brunauer, Emmett, and Teller (BET) theory was used to calculate the specific surface area of the photocatalysts, while the theory of Barret, Joyner, and Halenda (BJH) was used to determine the pore size distribution.
2.4.10. UV-Vis Diffuse Reflectance (UV-Vis-DR) and Solid Photoluminescence (PL) Spectra
A Perkin-Elmer Lambda 35 UV-Vis spectrophotometer, equipped with an RSA-PE-19M praying mantis powder holder, was used to obtain the UV-Vis diffuse reflectance (UV-Vis-DR) spectra of the synthesized materials. Spectralon© was used for background corrections. All spectra were recorded in absorbance mode from 800 to 250 nm with a scan rate of 240 nm/min and a slit width of 2.0 nm. Kubelka-Munk theory was used to estimate the band gap values.
Solid photoluminescence (PL) spectra were recorded using a Perkin-Elmer LS-55 fluorescence spectrometer equipped with a solid plunger plate and a powder sample holder. The excitation wavelength was set to 300 nm, with a scan rate of 150 nm/min (from 340 to 560 nm) and an emission slit width of 5.5 nm.
2.5. Exploitation of Photocatalytic Performance towards Ciprofloxacin
The photocatalytic performance of the developed nanocomposite with respect to ciprofloxacin was investigated in a 250 mL glass beaker containing 50 mL of an initial 0.008 g/L ciprofloxacin solution (initial concentration in all cases before irradiation). The effect of nanocomposite dosage was investigated (i.e., for 2.5, 5, 10, 15, and 35 mg of IO-TiO2 nanophotocatalyst), and the effect of pH on the nanocomposite and ciprofloxacin was studied at pH values of 3, 5, 7, and 9. The kinetics of the degradation of ciprofloxacin were investigated for up to 2.5 h (specifically at 30, 60, 90, 120, and 150 min). For conducting the photocatalytic experiments, the photoreactor (Luzchem) was irradiated with artificial UV-B light from 6 overhead UV-B lamps (emitting light in the range of 281–315 nm, with a maximum at λ = 313 nm and an intensity of about 141 W/m2). Prior to UV-B irradiation, the IO-TiO2 and ciprofloxacin dispersion was stirred in the dark for 30 min to achieve adsorption-desorption equilibrium. Afterwards, the UV-B lamps were activated. After certain irradiation times and examining different effects, the beaker was removed from the photoreactor, and the magnetic nanocomposite was deposited with a permanent magnet. The supernatant was then additionally filtered through a 0.2 μm syringe filter to remove any residual nanocomposite that might interfere with the UV-Vis analysis. The residual ciprofloxacin concentration in the exposed dispersion was determined using a Cary 60 UV-Vis spectrophotometer, and the concentration of remained ciprofloxacin was calculated using a calibration curve (given in Supplementary Information, Figure S1). The absorbance changes of ciprofloxacin under different irradiation conditions were monitored at a maximum wavelength of 277 nm. The photocatalytic degradation was calculated using Equation (1), and the ciprofloxacin degradation efficiency was determined using Equation (2). The reusability of the nanocomposite was evaluated over four cycles, with the recovered nanocatalyst used in subsequent photocatalytic processes under optimal conditions and a constant working volume. Prior to each recycling experiment, the nanocomposite was washed with 0.1 M HCl to remove adsorbed ciprofloxacin, followed by MilliQ water. A constant stirring speed and a fixed distance between the UV-B light source and the solution were maintained throughout the photocatalytic experiments. The changes in the nanocomposite after the reusability cycles were evaluated using ATR-FTIR, thermogravimetric analysis, and surface charge measurements.
where c0 is the initial concentration of ciprofloxacin and c is the ciprofloxacin concentration during the investigation of the various influences of degradation.
Photocatalytic degradation = c/c0
Photocatalytic degradation efficiency = c0 − c/c0 (%)
The kinetics of photocatalytic degradation were investigated using a zero-order kinetic model, a pseudo-first-order kinetic model, and a second-order kinetic model. The description of the individual kinetic models used is given in Supplementary Information.
2.6. Statistical Analysis
Each set of conditions was tested in triplicate to ensure the reliability of our results, and the results are reported as means of three independent experiments, unless otherwise stated. This approach was chosen to minimize experimental variability and to obtain a robust estimate of central tendency. Data analysis was performed using Origin 2018.
3. Results and Discussion
3.1. Characterization of Designed Photocatalytic Nanocomposite
The XRD diffractogram of TiO2@CA in Figure 1 shows peaks typical of anatase TiO2 (JCPDS no. 01-075-2550), while the diffractogram of IO-bPEI shows peaks corresponding to γ-Fe2O3 (JCPDS no. 04-022-3973). The XRD diffractogram of IO-TiO2 shows peaks for both anatase TiO2 and Fe2O3. The average crystallite size of anatase in the TiO2@CA and IO-TiO2 samples was calculated from the width of the (001) diffraction peak (2θ = 25°) using the Scherrer formula. The results presented in Table S1 show that the average crystallite size of anatase in IO-TiO2 remained unchanged during the synthesis, indicating that IO-bPEI was deposited only on the surface of TiO2@CA, which is in line with the conducted hetero-agglomeration method.
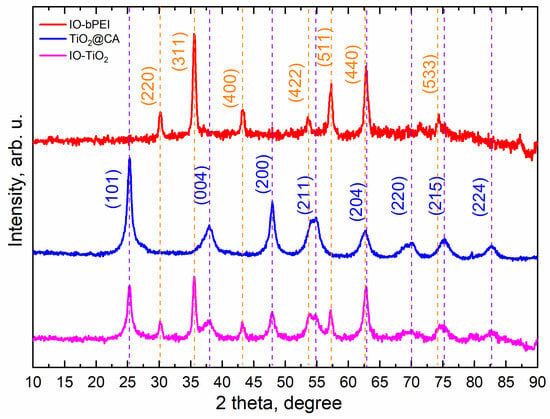
Figure 1.
X-ray diffraction (XRD) patterns for IO-bPEI, TiO2@CA, and IO-TiO2. The purple vertical lines show the peaks for anatase TiO2 together with labeled diffraction peaks (JCPDS no. 01-075-2550), and the orange vertical lines show cubic spinnel Fe2O3, marked with diffraction peaks (JCPDS no. 04-022-3973).
Transmission electron microscopy (TEM) was used to investigate the morphology and microstructure of heteroagglomerated IO-TiO2 nanocomposites (Figure 2) as well as single IO NPs (Figure S2), and TiO2@CA NPs (Figure S3). The TEM image of IO-bPEI nanoparticles (Figure S2a) shows quasi-spherical nanoparticles with a large polydispersity. The Selected Area Electron Diffraction (SAED) pattern (Figure S2b) showed the polycrystalline nature of the nanoparticles, with the diffraction rings matching well with the X-ray diffraction (XRD) data (Figure 1), suggesting a spinel crystal structure for the IO-bPEI nanoparticles. High-resolution TEM images (Figure S2c) confirmed the crystallinity of the prepared nanoparticles, which had an average size of about 13 nm. In contrast, the TiO2@CA NPs (Figure S3a) exhibited a significantly different morphology than IO-bPEI. These NPs were smaller, with a size of about 3–5 nm, and also exhibited good crystallinity (Figure S3c). The SAED pattern (Figure S3b) was consistent with the XRD results (Figure 1), indicating an anatase crystal structure for the TiO2@CA NPs. When both types of NPs were combined in a composite, the resulting nanocomposite showed integration of both nanoparticle types, with agglomerates of IO-bPEI often covered by TiO2@CA and vice versa (Figure 2). The size of the nanocomposite ranged from hundreds of nanometers to the micrometer range (Figure 2a,b), which was consistent with the measurements of the hydrodynamic diameter of the nanocomposite (results below). In agreement with the XRD pattern, the nanocomposite contained both types of NPs, as confirmed by energy dispersive X-ray spectroscopy (EDXS) in STEM mode, which showed the presence of elements characteristic of both types of nanoparticles (Figure 2c–e). Importantly, it was demonstrated that the magnetic removal of NPs is significantly more effective when the magnetic nanoparticles (MNPs) form clusters [74]. This finding underlines the crucial role of the nanocomposite structure in enhancing the efficiency of magnetic separation [45].
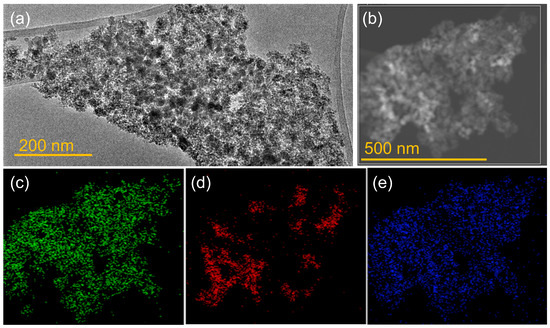
Figure 2.
(a) TEM image of IO-TiO2 nanocomposite, (b) STEM image of nanocomposite with marked area of EDS mapping. Element EDS maps of (c) Ti Kα1, (d) Fe Kα1, and (e) O Kα1.
The morphological features of the designed photocatalytic nanocomposite were investigated by scanning electron microscopy (SEM) to observe the formed structure at lower magnification (Figure 3a, Figures S4 and S5). Elemental mapping of the individual components of the nanocomposite was also performed (Figure 3b–g), together with EDXS spectra analysis (Figure 3h). The same characterization was performed for IO-bPEI (Figure S4) and TiO2@CA (Figure S5). The SEM images revealed that the formed IO-TiO2 nanocomposite has a rather homogeneous structure, which is advantageous for the improvement of the photocatalytic process. The elements present in IO-bPEI and TiO2@CA were also confirmed by elemental mapping (Figures S4 and S5). However, due to the limited magnification of the SEM images, individual NPs and the detailed structure of IO-TiO2 could not be clearly recognized, in contrast to Figure 2 and Figures S2 and S3 (Supplementary Information). The TEM was used for this purpose (Figure 2). Elemental mapping of the typical elements in the nanocomposite (C, N, O, Fe, and Ti; Figure 3b–g) showed a uniform distribution, confirming the successful formation of the nanocomposite with moderately uniformly distributed NPs. The EDXS spectrum (Figure 3h) also confirmed the presence of all constituent elements in the IO-TiO2 nanocomposite.
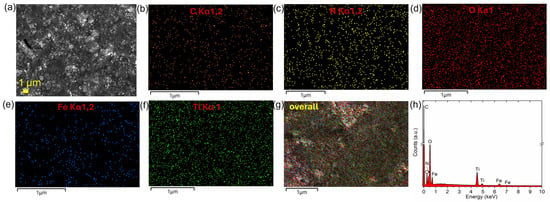
Figure 3.
SEM image of IO-TiO2 (a) together with EDXS imaging (b–g) and spectrum (h) for corresponding present elements.
The results of ATR-IR spectroscopy for all samples are shown in Figure 4. The peak at 534 cm−1 associated with Fe-O (in IO NPs), as identified in [75], is present in both the IO-bPEI and IO-TiO2 samples, indicating the successful formation of the designed nanocomposite. The peaks at 1620 cm−1 and around 3200 cm−1 correspond to the O-H bending and stretching of the water molecules in the IO NPs. The successful functionalization of IO NPs with bPEI is evidenced by new peaks. In particular, the peaks at 3400 cm−1 and 1628 cm−1 correspond to N-H stretching and bending of primary amines, while the peak at 1457 cm−1 corresponds to N-H stretching of secondary amine groups [76]. In addition, the C-N stretching peak at 1315 cm−1 confirms the presence of amine-containing molecules [77]. The presence of TiO2 is confirmed by typical peaks at 804 cm−1 and 616 cm−1, which are attributed to Ti-O stretching [78]. In addition, the presence of citric acid on TiO2 NPs was suggested by peaks characteristic of carboxylic acids. This was evident from the peaks at 1633 cm−1 and 1385 cm−1, which correspond to the symmetric and asymmetric stretching of the C=O group [49], respectively. After hetero-agglomeration of TiO2@CA with IO-bPEI, these peaks are less pronounced, which is probably due to a lower concentration. However, the broadening of the peak indicates the formation of hydrogen bonds [79] and, furthermore, an electrostatic interaction between negatively charged citric acid and positive bPEI. Indeed, the peaks present on the individual NPs are also clearly visible in the nanocomposite IO-TiO2 (Figure 4). Thus, infrared spectroscopy confirmed the successful functionalization of the IO-bPEI particles with bPEI and the hetero-agglomeration of TiO2@CA with IO-bPEI.
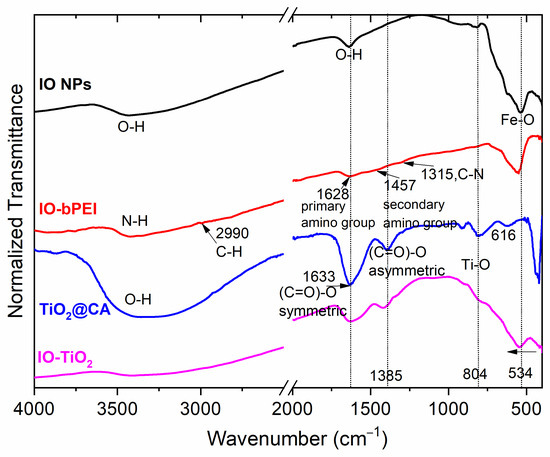
Figure 4.
Infrared spectroscopy spectra for bare IO NPs, IO-bPEI, TiO2@CA, and hetero-agglomerated nanocomposite IO-TiO2.
FTIR results are further supported by XPS results. Table 1 shows the XPS elemental composition of the NPs. Additionally, we also show in Figure 5a recorded XPS survey spectra of the samples IO-bPEI, TiO2@CA, and IO-TiO2, with characteristic peaks used for obtaining the elemental composition shown in Table 1. For the sample IO NPs, carbon, oxygen, and iron were found, which is consistent with their expected composition. The presence of a layer of bPEI (sample IO-bPEI) is confirmed by the obvious presence of nitrogen originating from amino groups in bPEI. In the case of TiO2 samples coated with CA, the presence of large amounts of oxygen and substantial amounts of titanium, as well as carbon, was found. Oxygen originates from both TiO2 and CA. The composition of the reference CA sample is also shown in Table 1, and we can see that oxygen concentration prevails over carbon, as found also for the TiO2@CA sample. For the final composite IO-TiO2 samples, we found all the elements that are characteristic of the individual components from which they are composed, i.e., iron, nitrogen, titanium, carbon, and oxygen. This result clearly demonstrates the success of the nanocomposite formation. High-resolution spectra of Fe, Ti, N, C, and O were measured as well. They are shown in Figure 5b–f. Iron peak Fe2p3/2 was found at a binding energy of 710.9 eV (Figure 5b). There is also a small satellite peak at approximately 719.5 eV, which is characteristic of Fe3+ iron [80]. The satellite peak is not as noticeable on the IO-TiO2 sample as on IO-bPEI, which could be explained by the coverage of the NPs surface with TiO2@CA particles. The titanium Ti 2p peak of TiO2@CA samples was observed at a binding energy of 458.9 eV, which is typical for TiO2 oxide (Figure 5c) [81,82]. For IO-TiO2, we observe, in addition to the peak characteristic of TiO2, another peak at a higher binding energy of approximately 460 eV. There is no data for this Ti 2p peak at such a high energy in the literature, so it can only be attributed to the presence of IO, which affects the chemical environment of titanium and thus causes changes in binding energies due to mutual interactions between TiO2@CA and IO-bPEI. In Figure 5d,e we show nitrogen and carbon peaks, which are related to the presence of organic molecules. As already mentioned, the origin of nitrogen is the amino groups of bPEI. Therefore, nitrogen is observed in the IO-bPEI sample and in IO-TiO2, where its concentration is lower (Table 1) because of the presence of TiO2@CA particles on the NPs surface and vice versa. A comparison of carbon peaks in Figure 5e and fitted carbon spectra in Figure 5f can provide information on the presence of CA because CA is characterized by carboxyl groups. In Figure 5f, we observe in the C1s spectrum of TiO2@CA the peak related to COOH groups, which clearly confirms the presence of CA on the surface. To conclude, the XPS results presented below prove the successful hetero-agglomeration of nanocomposite particles.

Table 1.
Elemental surface composition in at % for different samples.
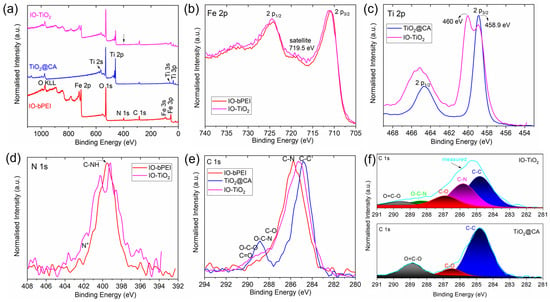
Figure 5.
Survey spectra (a) for IO-bPEI, TiO2@CA, and IO-TiO2, Fe 2p (b), Ti 2p (c), N 1s (d), (e) C 1s, and C 1s spectra of TiO2@CA and IO-TiO2 with fitted spectra (f).
At low pH (acidic conditions, e.g., 3–4), the surface of magnetic nanoparticles (IO NPs) is usually positively charged due to the adsorption of hydrogen ions (H+) and the zeta potential might be around +30 mV (Figure 6a). Above pH 5, it starts to decrease until reaching the isoelectric point (e.g., pH 5.8), where the zeta potential approaches 0 mV. At high pH (above the isoelectric point), the surface is typically negatively charged due to the adsorption of hydroxide ions (OH−). It reaches a negative zeta potential plateau value at around pH 7.5. Positive and negative zeta potential plateau levels are reached at the absolute value of zeta potential, where the stability of colloidal dispersion is guaranteed [83]. Interestingly, TiO2@CA shows very similar behavior of zeta potential as a function of pH as bare IO NPs. The zeta potential of TiO2@CA nanoparticles as a function of pH exhibits similar characteristics to IO NPs, which is reasonable since both are metal oxide nanoparticles with a tendency to be positive in acidic conditions due to the adsorption of H+ and negative at higher pH due to the adsorption of hydroxide ions (OH−) [75]. Both zeta potential plateau levels, positive and negative, are slightly higher (positive) and lower (negative) in comparison to IO NPs, which may be connected to CA, especially by the lower negative zeta potential plateau level where carboxylic acids of CA are available on the particles surface to be in protonated form [49]. IO-bPEI shows successful functionalization with bPEI, where the zeta potential is positive across the entire pH range due to the protonated amino groups [76], in line with the potentiometric titration curve of bPEI (Figure S6), where the highly positive character of the latter is revealed. There is a tendency of decreasing positive zeta potential slowly above pH 7, which may be due to less accessibility of amino groups due to their deprotonation (see potentiometric titration curve of bPEI) and thus to possible slight agglomeration. IO-TiO2 nanocomposites show the dominating character of IO-bPEI due to almost the same ZP (pH) curve as for IO-bPEI. This means that at hetero-agglomeration of IO-TiO2 nanocomposites by electrostatic interactions among IO-bPEI and TiO2@CA NPs, IO-bPEI chemistry dominates.
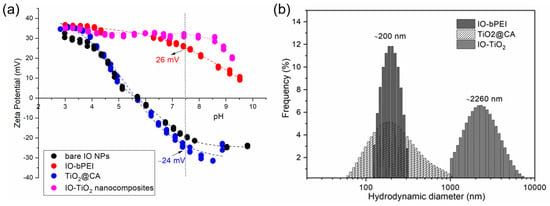
Figure 6.
Zeta potential as a function of pH for bare and differently modified IO aqueous dispersions (a), and hydrodynamic diameter as an intensity-weighted distribution at pH 8 in water medium (b).
Successful formation of nanocomposites at pH 7.5 is evident through the increase in particle size (Figure 6b) from the colloidal range of the individual systems (TiO2@CA and IO-bPEI, respectively) to the micron range when combined in the composite (IO-TiO2). In this context, the IO-bPEI nanoparticles exhibit a relatively narrow size distribution, with an average diameter of about 200 nm. This narrow distribution can be attributed to the positive repulsive forces, as shown by the zeta potential (ZP) of IO-bPEI, which is ~25 mV at pH 8. The TiO2@CA nanoparticles also have an average size of about 200 nm but exhibit a broader size distribution compared to IO-bPEI. Although both nanoparticles have similar absolute ZP values (about 25 mV at pH 8), TiO2@CA NPs tend to form larger agglomerates, which is consistent with the TEM analysis (Figure S3). As expected, the hydrodynamic diameter of the nanocomposite is larger than that of the individual components and is about 2260 nm, which is in good agreement with the TEM observations (Figure 2). Although the highest ZP value at pH 8 was measured for the IO-TiO2 nanoparticles, which typically indicates smaller particle sizes due to stronger repulsive forces, the attractive interactions between the individual nanoparticles seem to dominate. This leads to the formation of a nanocomposite with a size in the micrometer range and a moderately uniform size distribution.
Thermogravimetric analysis (TGA) was performed to determine the mass loss of the different nanomaterials, and the resulting curves were compared (Figure 7a). The bare IO NPs showed a minimum mass loss of about 3–4 wt%, probably due to the presence of physically adsorbed water. After modification with bPEI, the IO NPs showed a more significant mass loss of about 5 wt%, indicating the presence of an organic coating on the IO-bPEI. For the TiO2@CA nanoparticles, the TGA curves indicated a mass loss of 12 wt%, which is likely due to the citric acid (CA) coating and reveals distinct decomposition patterns. When TiO2@CA was combined with IO-bPEI, the nanocomposite displayed a maximum mass loss of 11 wt% at 600 °C. This indicates a successful formation of the nanocomposite. The IO-TiO2 nanocomposite exhibited a TGA curve that reflects the properties of both nanomaterials of which it is composed. The organic species within the nanocomposite began to decompose above 120 °C and decomposed almost completely up to 600 °C, likely resulting in volatile products [84]. This thermal behavior is further evidence of the successful integration of the components into the nanocomposite. The magnetic properties of the bare IO NPs and the synthesized IO-TiO2 nanocomposite were investigated by hysteresis loop measurements. As illustrated in Figure 7b, both the bare IO NPs and the nanocomposite exhibit superparamagnetic behavior characterized by negligible coercivity and remanence [85,86]. This is of crucial importance since superparamagnetic NPs do not exhibit spontaneous magnetic moments and thus prevent agglomeration due to magnetic dipole-dipole interactions, in contrast to larger particles [87,88]. The saturation magnetization of the IO NPs decreased from an initial 67 emu/g to 49 emu/g when combined TiO2@CA and IO-bPEI. This decrease is due to the presence of organic coatings and non-magnetic material within the nanocomposite. Despite this decrease, the IO-TiO2 nanocomposite retained remarkable magnetic properties that enabled effective magnetic separation of the nanocatalyst after the photocatalytic process in the presence of an external magnetic field. The strong magnetic response of the nanocomposite is significant as it enables not only enhanced photocatalytic activity but also efficient removal and reusability, minimizing additional environmental pollution. This dual functionality underlines the potential of the developed nanocomposite for practical applications in environmental remediation.
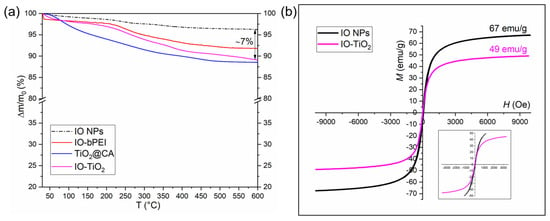
Figure 7.
TGA curves for bare IO, differently surface modified IO-bPEI, TiO2@CA, and formed nanocomposite (a). Magnetic properties of bare IO NPs and formed IO-TiO2 nanocomposite are presented in (b) with magnified part in inset.
Since the specific surface area (SBET) has a significant influence on the photocatalytic activity of a material [89,90], whereby a catalyst with a lower SBET value generally has a lower photocatalytic activity than a catalyst with a higher SBET value, nitrogen adsorption/desorption experiments were carried out to analyze the porosity of the materials investigated (Figure 8). The specific surface areas (SBET) were calculated using the Brunauer-Emmett-Teller (BET) theory. The isotherms for IO-bPEI, TiO2@CA, and IO-TiO2 (Figure 8a) correspond to the IUPAC classification of type IV with H1 hysteresis loops, indicating a mesoporous structure with cylindrical pores [91,92]. As shown in Table 2, IO-bPEI has an SBET value of 55.8 m2/g, which is significantly lower than the SBET value of TiO2@CA of 246.8 m2/g. The low SBET value of IO-bPEI could have a negative impact on its photocatalytic activity compared to TiO2@CA, as it provides less surface area for light-generated charge carriers to generate reactive oxygen species or directly degrade pollutants in water. In addition, the adsorption of degradation products on the catalyst surface during heterogeneous photocatalysis would affect the catalytic activity of IO-bPEI more than that of TiO2@CA, which has a higher SBET. When IO-bPEI nanoparticles were combined with TiO2@CA, a reduction in specific surface area (SBET) was observed compared to TiO2@CA alone. However, the specific surface area remained significant at 165.3 m2/g, which is advantageous for the adsorption and photodegradation of ciprofloxacin (Table 2). Despite the smaller pore volume (Vpore) of IO-bPEI, the Vpore of the nanocomposite increased to 0.45 cm3/g, comparable to that of TiO2@CA, indicating the presence of mesopores. A similar trend was observed for the pore diameter (dpore) for IO-TiO2, with a value of 10.1 nm, which falls between the values for IO-bPEI and TiO2@CA (Figure 8b, Table 2). The presence of large mesopores and a large effective surface area likely facilitates charge separation and increases the number of active sites within the nanocomposite structure, improving its suitability for heterogeneous photocatalytic applications.
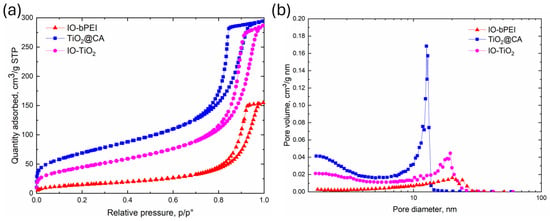
Figure 8.
(a) N2 adsorption-desorption isotherms of IO-bPEI, TiO2@CA, and IO-TiO2, and (b) corresponding BJH pore size distributions.

Table 2.
Comparison of the specific surface area (SBET), average pore diameter (dpore), and total pore volume (Vpore).
3.2. Photocatalytic Degradation of Ciprofloxacin
During photocatalytic degradation of antibiotics with TiO2-based magnetic nanocomposites, photons from the light source excite electrons from the valence band to the conduction band, creating electron-hole pairs. These electron-hole pairs initiate the degradation process by generating various radicals through reactions with water molecules. The generated radicals then degrade the antibiotic molecules adsorbed on the nanocomposite surface through oxidative and radical-driven reactions, transforming the antibiotics into less harmful by-products [20,61]. In particular, the magnetic properties of the iron oxide NPs allow easy manipulation and removal of the nanocomposite by an external magnetic force, so that it can be reused in several photodegradation cycles. Remarkably, the iron oxide NPs can also help to improve photocatalytic performance. The UV-Vis reflectance spectra (UV-Vis-DR) and the corresponding Kubelka-Munk functions for IO-bPEI, TiO2@CA, and IO-TiO2 are shown in Figure 9a and Figure S7. The band gap values of these solids determined using the Kubelka-Munk function are listed in Table 3. The TiO2@CA sample exhibits strong light absorption below 400 nm with a band gap of 3.2 eV, which is typical for anatase TiO2, [93]. This is consistent with the XRD analysis, which shows peaks in the XRD diffractogram of TiO2@CA that correspond to anatase TiO2. The UV-Vis-DR spectra of IO-bPEI show that this sample can absorb light above 400 nm with a band gap of 1.7 eV (typical for iron oxide NPs [94]), which makes IO-bPEI photocatalytically active when illuminated with visible light. In the UV-Vis-DR spectra of IO-TiO2, the contributions of TiO2@CA below 400 nm and of IO-bPEI above 400 nm are obvious. The UV-Vis-DR measurements show that the addition of IO-bPEI to TiO2@CA significantly reduces the band gap of TiO2 and that both components of the IO-TiO2 composite can generate charge carriers when illuminated with UV-B light. A key parameter that determines the catalytic activity of a photocatalyst is the recombination rate of the charge carriers generated by the light; a lower recombination rate is associated with a higher photocatalytic activity. The recombination of charge carriers generates energy in the form of photoluminescence (PL) emissions, i.e., the intensity of the PL signal of a solid material is proportional to its electron-hole recombination rate [29,95]. The PL spectra of TiO2@CA and IO-TiO2 in the solid state are shown in Figure 9b. TiO2@CA shows well-defined peaks at about 3.2, 2.9, 2.7, 2.5, and 2.3 eV. The peak at 2.9 eV corresponds to the lowest indirect transition Γ1b → X1a, while the signals around 3.1 eV and above are assigned to direct (X1b → X2b and X1b → X1a) and indirect (X1b → Γ3 and Γ1b → X2b) transitions. The peaks at 2.8, 2.7, 2.55, and 2.34 eV are associated with shallow traps in anatase [96]. The PL emission intensity of IO-TiO2 is lower than that of TiO2@CA, indicating a reduced recombination rate of electron-hole pairs. Similar findings were observed in the study of Sakineh et al. [35], who investigated a composite of TiO2 decorated on ferroferric oxides coupled with activated carbon (TFOC) under UV light for tetracycline degradation. The photocatalytic performance of the composite was enhanced by the presence of ferroferric oxides, which played a crucial role in suppressing the recombination of excited electrons and holes. In addition, another study showed that the combination of TiO2 with superparamagnetic iron oxide nanoparticles (SPIONs) significantly enhanced the degradation of dyes. Here, the inclusion of iron oxides extended the adsorption edges of the nanocomposite and improved its overall photocatalytic performance [97].
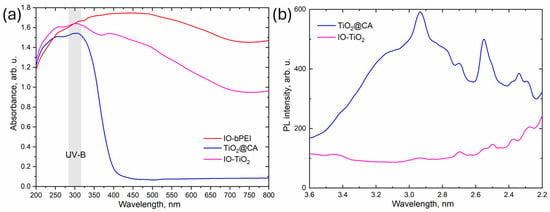
Figure 9.
(a) UV-Vis-DR spectra of IO-bPEI, TiO2@CA, and IO-TiO2, and (b) solid-state photoluminescence emission (PL) spectra of TiO2@CA and IO-TiO2.

Table 3.
Comparison of the band gap values of the investigated materials obtained with the Kubelka-Munk function (Supplementary Information Figure S7).
3.2.1. Influence of Nanocomposite Dosage
The initial parameter that significantly influences the efficiency of the photocatalytic process is the dosage of the IO-TiO2 photocatalyst. In this study, dosages from 2.5 mg to 25 mg IO-TiO2 were investigated (Figure 10a, UV-Vis spectra in Figure S8). The photocatalytic activity increased significantly with the dosage of the nanocomposite up to 10 mg. Beyond this dosage, however, no further significant improvements were observed, so that a degradation efficiency of 75–80% was achieved. The initial increase in photocatalytic degradation of ciprofloxacin can be attributed to the increased quantity of nanocomposite, which in turn increases the total effective surface area. This leads to more redox-active centers on the surface of the nanocatalyst and the formation of more radicals or active oxidants responsible for degradation [34,35]. However, a further increase in the IO-TiO2 dosage led to increased turbidity of the dispersion, which in turn reduced the penetration of UV-B light. This reduction in light penetration limits the number of photons reaching the surface of the nanocatalyst and the excitation of the water molecules, thereby reducing the formation of hydroxyl radicals [34]. Another possible explanation for the reduced photocatalytic performance at higher nanocomposite dosages is agglomeration, which reduces the effective surface area during photocatalytic degradation and thus significantly affects the overall degradation efficiency [35]. Consequently, the optimal IO-TiO2 dosage was set at 10 mg, resulting in a degradation efficiency of approximately 65%. At higher dosages, the degradation efficiency did not differ significantly and ranged between approximately 70% and 80% (Figure 10a). Therefore, 10 mg of the photocatalyst was used in the subsequent studies to investigate further effects on the photocatalytic degradation of ciprofloxacin.
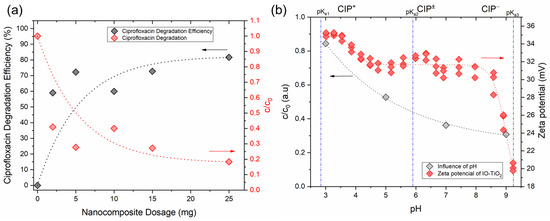
Figure 10.
Influence of the nanocomposite amount (a) and pH values (b) on ciprofloxacin photocatalytic degradation. Conditions for effect of IO-TiO2 dosage were: 50 mL of 0.008 g/L ciprofloxacin, pH 7, 2 h of irradiation with UV-B light. Conditions for effect of pH were: 10 mg of IO-TiO2, 50 mL of 0.008 g/L ciprofloxacin, 2 h of irradiation with UV-B light.
3.2.2. Influence of pH
One of the most important parameters influencing the photocatalytic degradation of ciprofloxacin is the pH of the dispersion during irradiation. The pH not only influences the surface chemistry of the nanocatalyst but also the charging behavior of the ciprofloxacin molecules. This in turn affects the interaction between the surface of the nanocatalyst, the ionized forms of ciprofloxacin, and the formation of hydroxyl radicals. Adsorption plays a decisive role in the photocatalytic degradation process. The potentiometric titration of pure ciprofloxacin (Figure S9) shows three pKa values up to a pH of 10, namely pKa1 = 2.8, pKa2 = 5.9, and pKa3 = 9.3, indicating that ciprofloxacin is in a cationic form below pH 6 (see the potentiometric curve in Supplementary Information, Figure S9) and in an anionic form above pH 9.3. Similarly, zeta potential measurements of IO-TiO2 show that the nanocomposite is positively charged, with the charge decreasing above pH 9 (Figure 10b). In our system, the low photocatalytic degradation at acidic pH (about 15% at pH 3) can be attributed to the repulsive forces between the positively charged IO-TiO2 (primary amines) and the positively charged ciprofloxacin molecules (due to the protonation of the piperazine ring nitrogen and secondary amines), which reduce the adsorption on the nanocatalyst surface (Figure 10b; UV-Vis spectra are presented in Figure S10). As the pH value increases, photocatalytic degradation also increases. At a pH of 7, there is an electrostatic attraction between the zwitterionic ciprofloxacin molecules (carboxylic acids) and the positively charged IO-TiO2 (secondary and tertiary amines of bPEI dominating at its surface), resulting in a photocatalytic degradation efficiency of 62%. A further increase in pH to 9 leads to a degradation efficiency of about 70%, which is probably due to electrostatic attractions between the deprotonated carboxylic acids of ciprofloxacin and the tertiary amines of -bPEI as dominating in IO-TiO2, as well as enhanced van der Waals interactions between the negatively charged ciprofloxacin and the positively charged IO-TiO2 nanocomposites, as reported in the literature [20,34]. Under alkaline conditions, the aqueous dispersion is more favorable for the efficient formation of OH• and O2−• radicals, leading to higher photodegradation efficiency. In particular, a higher concentration of OH- under alkaline conditions leads increasingly to oxidative OH• species, which enhance the degradation of ciprofloxacin. Since the degradation efficiency is comparable at neutral and alkaline pHs, neutral pH is preferred in view of practical applications. This minimizes the need for extensive pH adjustment and the consumption of acids and bases, making the process more feasible and cost-effective for practical applications.
3.2.3. Effect of Nanocatalyst Type and Influence of Time on Ciprofloxacin Degradation
To evaluate the effects of the individual components of IO-TiO2 on the degradation of ciprofloxacin, both IO-bPEI and TiO2@CA were examined over a period of 150 min under identical conditions (Figure 11a). IO-bPEI showed almost negligible photocatalytic degradation of ciprofloxacin under UV-B light irradiation, which can be attributed to the smaller band gap of maghemite (approx. 1.7 eV, Table 3). The degradation efficiency of the magnetic nanoparticles remained relatively constant and fluctuated between 15–30% over time. In contrast, TiO2@CA nanoparticles showed a significantly different behavior compared to IO-bPEI. Under the same conditions, TiO2@CA achieved ciprofloxacin degradation of up to 80% after 150 min of irradiation, showing a consistent degradation trend with prolonged UV-B light exposure. It should be noted that the specific surface area of IO-bPEI was about 4.5 times smaller than that of TiO2@CA (Figure 8, Table 2), which may also affect the photocatalytic activity. This high activity of TiO2 semiconductor NPs in generating reactive oxygen species is well documented [51] and leads to improved photocatalytic performance. Interestingly, when comparing the degradation efficiency of the nanocomposite with the individual nanoparticles, some synergistic effect can be observed. Yet, the degradation curve and efficiency of the nanocomposite matched those of TiO2@CA to some extent, suggesting that TiO2@CA primarily affects the overall photocatalytic degradation efficiency of ciprofloxacin in the IO-TiO2 composite. In particular, the degradation of ciprofloxacin reached about 70% after 150 min (Figure 10a). The change in the height of the peak at 277 nm with longer degradation time is shown in Figure 11b. Comparison of different kinetic models (Figure S11 in the Supplementary Information) showed moderate fitting parameters, with IO-TiO2 being the best fit to a pseudo-first order kinetic model (R2 = 0.7766). The rate constants of degradation also varied between the three types of nanoparticles (Figure 11c). The highest rate constant derived from the pseudo-first-order model was observed for TiO2@CA at 0.0083 min−1, while IO-TiO2 had a slightly lower rate constant of 0.0062 min−1.
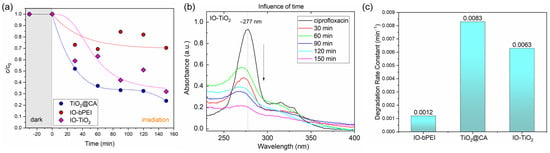
Figure 11.
Kinetic degradation of ciprofloxacin by photocatalysis using TiO2@CA, IO-bPEI, and IO-TiO2 (a), UV-Vis spectra of corresponding IO-TiO2 at different degradation times (b), and degradation rate constant for all samples (c), derived from a pseudo-first order kinetic model. The used photocatalytic conditions were pH of 7, 50 mL of 0.008 g/L ciprofloxacin, and 10 mg of nanocomposite.
3.2.4. Reusability and Stability Evaluation of the Nanocatalyst
A key factor for practical applications of photocatalysts is their reusability. Reusability minimizes costs as the nanocatalyst can be regenerated and reused in multiple photocatalytic processes, contributing to the development of environmentally friendly and sustainable photocatalysts. To investigate the reusability of IO-TiO2 for the degradation of ciprofloxacin, four cycles were performed, the results of which are shown in Figure 12. After each process, the IO-TiO2 was magnetically separated from the dispersion, regenerated with acid, and then reused in the next photocatalytic cycle. The photocatalytic performance remained stable in the first two cycles. However, in the third cycle, a decrease in efficiency of about 15% was observed after 60 min of irradiation. In the fourth cycle, the photocatalytic efficiency had dropped significantly to 40% after 60 min, which is less than half of the efficiency observed in the first cycle. This decrease can be attributed to possible leaching of Ti from the nanocomposite during the acidic wash process or to the blocking of active sites on the photocatalyst by by-products formed in previous cycles that could not be completely removed or by adsorbed ciprofloxacin residues [35]. Despite the decrease in performance after several cycles, the photocatalytic efficiency of IO-TiO2 did not decrease significantly after three cycles, indicating its potential for practical environmental and economic applications. Structural analysis by infrared spectroscopy after the photocatalytic tests revealed no significant changes in the position of the infrared bands (Figure 13a). Thermogravimetric analysis indicated a slight mass loss (Figure 13b), probably due to desorption of the organic coatings, and a shift of zeta potential towards a more acidic range (Figure 13c), possibly related to the adsorption of more acidic surface-charged compounds after photocatalysis, such as remained ciprofloxacin (Figure S9, potentiometric titration curve) or possibly adsorbed residue degradation products. In addition, Table 4 shows a comparison between the results of this study and more recent research dealing with the photocatalytic degradation of ciprofloxacin. Notably, the recyclable and magnetically controlled nanocomposite employed in this research demonstrated satisfactory performance.
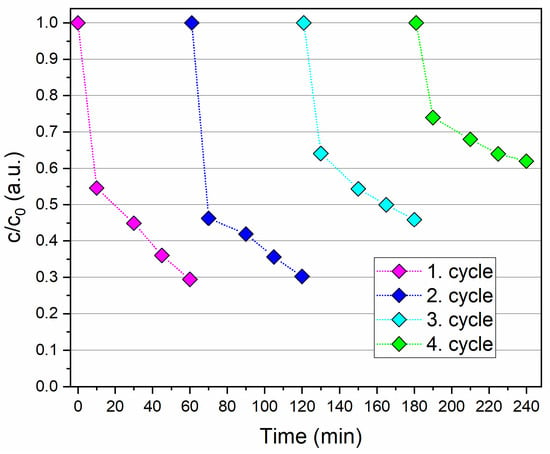
Figure 12.
Recycling experiments on the photocatalytic degradation of ciprofloxacin over IO-TiO2 photocatalyst under UV-B light irradiation. The conditions for photocatalytic degradation were pH 7, 25 mg IO-TiO2, 50 mL 0.008 mg/mL ciprofloxacin, and the time was set to 60 min for each cycle performed.
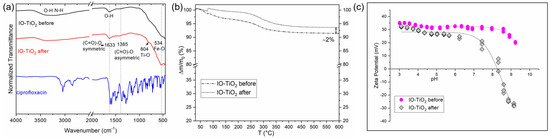
Figure 13.
Infrared spectra (a), thermogravimetric behavior (b), and electrokinetic measurements of zeta potential (c) of IO-TiO2 before and after photocatalytically processed samples.

Table 4.
Comparison of the photocatalytic performance of different materials for the degradation of ciprofloxacin in the existing literature under different experimental conditions.
3.3. Plausible Degradation Mechanism
A promising strategy to improve the photocatalytic efficiency between two different semiconductors is heterojunction coupling [30], as represented in the current system by the combination of TiO2@CA and IO-bPEI nanoparticles. This hybrid configuration offers several advantages for photocatalysis, including improved charge separation, extended carrier lifetime, increased recombination resistance, and more efficient charge transfer at the interface to the adsorbed molecules [30]. According to photoluminescence (PL) measurements in the UV-B spectral range, both the individual nanoparticles and the IO-TiO2 nanocomposite can generate charge carriers that migrate between the phases and thus reduce recombination rate (Figure 9b). Although the nanocomposite consists of a 1:1 ratio of TiO2@CA to IO-bPEI, its overall photocatalytic performance is almost identical to that of pure TiO2@CA under optimal conditions, emphasizing the significant role of IO-bPEI nanoparticles. The presence of IO-bPEI likely supports the phase separation between TiO2@CA and IO-bPEI [99], which increases the photocatalytic efficiency. XPS analysis was used to determine the positions of the valence bands (VB) of TiO2@CA and IO-bPEI by measuring their valence band maxima (VBM). The data presented in Figure S12 show that the VBM values for TiO2@CA and IO-bPEI are 2.5 eV and 0.30 eV, respectively. When we combine these VBM results with the band gap values obtained from UV-Vis diffuse reflectance (UV-Vis DR) measurements, we can create a detailed schematic representation of the energy band structure for IO-TiO2 and propose the mechanism of charge transfer under UV-B light (Figure S13). When irradiated with UV-B light, both TiO2@CA and IO-bPEI generate charge carriers. Holes in the VB of TiO2@CA can migrate into the VB of IO-bPEI, while electrons from the conduction band (CB) of IO-bPEI can migrate into the CB of TiO2@CA. This process promotes the separation of electron-hole pairs, increases their concentration on the surface of the IO-TiO2 nanocomposites, and thereby enhances the overall photocatalytic activity. This phenomenon is confirmed by PL measurements, which show a significantly lower PL intensity for the composite material compared to TiO2@CA alone, indicating a more efficient charge separation and extended carrier lifetime in the composite material. Consequently, this leads to the formation of reactive oxygen species (ROS) such as O2−• and OH•, which are highly reactive and can react effectively with ciprofloxacin to form less harmful degradation products (Figure 14) [36,45,48]. In contrast to our findings, it was observed that when the α-Fe2O3@TiO2 system was irradiated with UV light, the TiO2 was excited as expected, but the α-Fe2O3 acted as a recombination center for photoinduced charge carriers [56]. Consequently, the designed composite showed relatively poor photocatalytic activity. One possible reason for this discrepancy could be the different iron oxide used in the composite. In our study, maghemite with a spinel crystal structure was used as the magnetic nanoparticles, while [56] used hematite as the iron oxide.
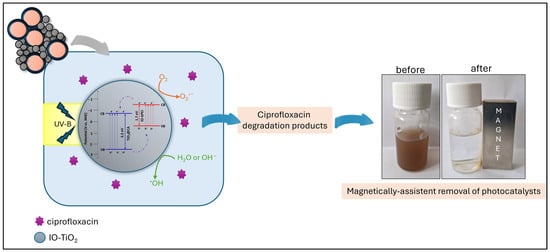
Figure 14.
Proposed photocatalytic degradation of ciprofloxacin with IO-TiO2 nanocomposite.
As already mentioned, the photocatalytic degradation of ciprofloxacin with IO-TiO2 under the influence of light and excitation by photon energy (with an energy equal to or greater than the band gap of the photocatalyst) leads to the formation of electron (e−)—hole (h+) pairs (h+ in valence band VB and e− in conductive band CB). These pairs subsequently generate highly reactive species such as superoxide anions (O2−•) and hydroxyl radicals (OH•). The proposed degradation mechanism caused by photocatalytic nanocomposite is suggested by Equations (3)–(7). Usually mentioned reactive species primarily attack the C-N bond of the piperazine ring, the fluorine atom, the carbonyl group, and the cyclopropyl group within the quinolone structure, leading to oxidation and degradation of the ciprofloxacin molecules, as reported in the current study [20]. A similar photodegradation pathway for ciprofloxacin, driven by O2−• and OH• radicals through the generation of electron-hole (e−/h+) pairs, has been observed in other photocatalytic systems. These include chlorophyll-sensitized and salicylic acid-functionalized TiO2 nanoparticles [58], Fe2O3 nanoparticles supported on graphitic carbon nitride [59], magnetic nanocomposites [47], composites of polystyrene waste with TiO2 [34], and TiO2 nanoparticles immobilized on glass substrates [33].
IO-TiO2 + hν (UV-B light) → IO-TiO2 (eCB− + hVB+)
IO-TiO2 (eCB−) + O2 → IO-TiO2 + O2−•
IO-TiO2 (hVB+) + H2O/OH− → IO-TiO2 + OH•
O2−• + ciprofloxacin molecules → ciprofloxacin degradation products
OH• + ciprofloxacin molecules → ciprofloxacin degradation products
The heterogeneous photocatalytic process described in this report comprises the following steps:
- (i)
- Transfer of ciprofloxacin from the aqueous dispersion to the surface of the IO-TiO2 nanocomposite.
- (ii)
- Adsorption of ciprofloxacin onto IO-TiO2.
- (iii)
- Photocatalytic oxidation and reduction of ciprofloxacin by reactive species as described in Equations (3)–(7).
- (iv)
- Removal of ciprofloxacin degradation products from the IO-TiO2 surface.
- (v)
- Transport of these degradation products from the IO-TiO2 surface back into the aqueous dispersion.
- (vi)
- Magnetically assisted removal of IO-TiO2 after the photocatalytic process.
- (vii)
- Reuse of IO-TiO2 for another photocatalytic process.
4. Conclusions
The world is currently struggling with significant residues of antibiotics entering aqueous systems, with ciprofloxacin being a major contributor. This is exacerbating the alarming rise in antimicrobial resistance (AMR). In this pioneering study, we successfully synthesized a magnetically controlled and photocatalytic nanocomposite IO-TiO2 for the first time by hetero-agglomerating positively charged bPEI-modified iron oxide (IO) NPs with commercially available negatively charged TiO2@CA NPs. This novel nanocomposite was tested for its ability to degrade ciprofloxacin in aqueous media under UV-B irradiation, an investigation not previously reported in the literature. The TiO2@CA exhibited an anatase crystal structure, while the IO-bPEI corresponded to a cubic spinel crystal structure. The designed nanocomposite, confirmed by XRD and SAED analyses, showed both crystal structures. The IO-bPEI NPs (individual NPs were about 13 nm in size) aggregates formed hetero-agglomerates with TiO2@CA clusters (individual NPs were 3–5 nm in size), resulting in a composite size ranging from hundreds of nanometers to micrometers. SEM and elemental mapping confirmed the formation and elemental distribution of the elements (i.e., Ti, Fe, O, N, C) in the nanocomposite. Infrared spectroscopy identified typical functional groups, while XPS provided a comprehensive understanding of the elemental states in IO-TiO2. Zeta potential measurements revealed a positive charge over a wide pH range for IO-TiO2, primarily caused by IO-bPEI, which influenced the ciprofloxacin degradation process. Hydrodynamic diameter measurements, supported by TEM results, showed a significantly larger size of the nanocomposite (~2260 nm) compared to individual NPs. TGA confirmed the presence of organic layers on both the individual NPs and the IO-TiO2 nanocomposite. Magnetic property analysis revealed a strong magnetic response with saturated magnification of 49 emu/g. BET analysis indicated a large specific surface area, which is crucial for enhanced photocatalytic processes and the formation of more reactive species. UV-Vis-DR measurements demonstrated the ability of the nanocomposite to generate charge carriers in the UV-B range, and PL results indicated reduced electron-hole recombination in the IO-TiO2 heterojunction, thereby improving photocatalytic efficiency compared to TiO2@CA alone, with twice the amount with respect to the latter in IO-TiO2. To determine the optimal parameters for enhanced photocatalytic degradation of ciprofloxacin by IO-TiO2, various effects were studied in detail, including the amount of nanocomposite, pH, time, and reusability. Optimal parameters for enhanced photocatalytic degradation of ciprofloxacin by IO-TiO2 were determined: 10 mg IO-TiO2, pH 7, and 2 h UV-B irradiation. The degradation process monitored by UV-VIS spectroscopy achieved up to 70% degradation of ciprofloxacin at a concentration of 8 mg/L after 150 min. Compared to the current state-of-the-art, the IO-TiO2 nanocomposite exhibited acceptable and satisfactory performance with remarkable magnetic response and good reusability, maintaining stability and photocatalytic behavior over three cycles. The study shows that iron oxide not only acts as a carrier for TiO2@CA NPs but also delays electron-hole recombination, which increases the photocatalytic efficiency. Overall, the successful preparation and performance of IO-TiO2 nanocomposites highlight their significant potential as magnetic photocatalytic materials for mitigating antibiotic residues in wastewater, representing a key advance in combating antibiotics in environmental systems. Despite recent advances in the use of heterogeneous TiO2-based materials for wastewater treatment, there are still technical issues that hinder its commercialization. Future studies will focus on the photocatalytic degradation of ciprofloxacin in the visible range with magnetically-based NPs and TiO2 and on the industrialization of this technology to move from laboratory scale to real applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/magnetochemistry10090066/s1 Synthesis details of TiO2@CA NPs preparation; Photocatalytic degradation kinetics equations; Figure S1: Linear correlation between ciprofloxacin concentration and absorbance in the explore range; Table S1: Comparison of the anatase TiO2 crystallite size at 25°; Figure S2: (a) TEM image of IO-bPEI nanoparticles, (b) SAED from area encircled on TEM image, (c) HR-TEM image of nanoparticles; Figure S3: (a) TEM image of TiO2@CA nanoparticles, (b) SAED from area encircled on TEM image, (c) HR-TEM image of nanoparticles; Figure S4: SEM image of IO-bPEI at smaller magnification (a) with corresponding EDXS imaging for C (b), N (c), O (d), Fe (e), overall elements (f), and EDXS spectrum (g); Figure S5: SEM image of TiO2@CA at smaller magnification (a) with corresponding EDXS imaging for C (b), O (c), Ti (d), overall elements (e), and EDXS spectrum (f); Figure S6: pH titration curve for bPEI; Figure S7: The respective Kubelka-Munk function of the UV-Vis-DR spectra of IO-bPEI, TiO2@CA, and IO-TiO2 present in Figure 8a in the main manuscript; Figure S8: Photocatalytic performance of ciprofloxacin degradation—effect of IO-TiO2 dosage; Figure S9: pH titration curve for ciprofloxacin; Figure S10: UV-Vis absorption spectra by different pH of photocatalytic degradation of ciprofloxacin; Figure S11: Kinetics modeling fitting for IO-bPEI, TiO2@CA, and IO-TiO2 utilizing zero-order kinetics (a), pseudo-first-order kinetics (b), and second-order kinetics (c); Figure S12: Valence band maximum (VBM) values for TiO2@CA and IO-bPEI; Figure S13: Schematic representation of the energy band structure for IO-TiO2 and proposed the mechanism of charge transfer under UV-B light.
Author Contributions
Conceptualization, O.P.; methodology, L.J., G.Ž., J.R., P.B., L.F.Z., A.V., A.M., M.G. and O.P.; validation, G.Ž., A.V., A.M. and O.P.; formal analysis, L.J., G.Ž., A.V., A.M., M.G. and O.P.; investigation, G.Ž., A.V., A.M. and O.P.; resources, J.R., P.B., L.F.Z. and O.P.; data curation, L.J., G.Ž., A.V., A.M. and O.P.; writing—original draft preparation, J.R., L.J., G.Ž., L.F.Z., A.V., A.M. and O.P.; writing—review and editing, L.J., G.Ž., J.R., P.B., L.F.Z., A.V., A.M., M.G. and O.P.; supervision, J.R. and O.P.; funding acquisition, J.R., L.F.Z. and O.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Slovenian Research Agency (research programs: P2-0118, P2-0150, P2-0412, P2-0082, and project J1-4416).
Data Availability Statement
Data is contained within the article or Supplementary Material.
Acknowledgments
The authors gratefully acknowledge the financial support of the Slovenian Research Agency (research programs: P2-0118, P2-0150, P2-0412, P2-0082, and project J1-4416). We would like to thank Sašo Gyergyek for kindly performing the VSM analysis and Irena Ban for the use of the TGA equipment. The authors would also like to thank Rok Valentan for help with the experimental part, Tjaša Kraševac Glaser and Simon Ekselenski for performing zeta potential analyses, and Matej Bračič for help with the potentiometric titrations.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Gupta, R.; Sharma, S. Role of alternatives to antibiotics in mitigating the antimicrobial resistance crisis. Indian J. Med. Res. 2022, 156, 517–520. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Data on Antimicrobial Resistance (AMR): Use of Antibiotics in the EU Decreases but More Needs to be Done; European Commission: Brussels, Belgium, 2022. [Google Scholar]
- Setiawan, A.; Widodo, A.D.W.; Endraswari, P.D. Comparison of ciprofloxacin, cotrimoxazole, and doxycycline on Klebsiella pneumoniae: Time-kill curve analysis. Ann. Med. Surg. 2022, 84, 104841. [Google Scholar] [CrossRef]
- Shariati, A.; Arshadi, M.; Khosrojerdi, M.A.; Abedinzadeh, M.; Ganjalishahi, M.; Maleki, A.; Heidary, M.; Khoshnood, S. The resistance mechanisms of bacteria against ciprofloxacin and new approaches for enhancing the efficacy of this antibiotic. Front. Public Health 2022, 10, 1025633. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, J.; Zeng, J.; Li, J.; Liu, Y.; Sun, X.; Xu, L.; Li, L. Complete degradation and detoxification of ciprofloxacin by a micro-/nanostructured biogenic mn oxide composite from a highly active Mn2+-oxidizing pseudomonas strain. Nanomaterials 2021, 11, 1660. [Google Scholar] [CrossRef]
- Patrolecco, L.; Rauseo, J.; Ademollo, N.; Grenni, P.; Cardoni, M.; Levantesi, C.; Luprano, M.L.; Caracciolo, A.B. Persistence of the antibiotic sulfamethoxazole in river water alone or in the co-presence of ciprofloxacin. Sci. Total Environ. 2018, 640–641, 1438–1446. [Google Scholar] [CrossRef]
- Ory, J.; Bricheux, G.; Togola, A.; Bonnet, J.L.; Donnadieu-Bernard, F.; Nakusi, L.; Forestier, C.; Traore, O. Ciprofloxacin residue and antibiotic-resistant biofilm bacteria in hospital effluent. Environ. Pollut. 2016, 214, 635–645. [Google Scholar] [CrossRef]
- Monahan, C.; Nag, R.; Morris, D.; Cummins, E. Antibiotic residues in the aquatic environment–current perspective and risk considerations. J. Environ. Sci. Health—Part A Toxic/Hazard. Subst. Environ. Eng. 2021, 56, 733–751. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Chang, H.; Wei, W.; Yu, H.; Chen, Z.; Cheng, X.; Chen, D.; Jin, Y.; Han, D.; Xu, W. Highly Effective Removal of Ciprofloxacin Antibiotic from Water by Magnetic Metal–Organic Framework. Water 2023, 15, 2531. [Google Scholar] [CrossRef]
- Mompelat, S.; Le Bot, B.; Thomas, O. Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water. Environ. Int. 2009, 35, 803–814. [Google Scholar] [CrossRef]
- de Ilurdoz, M.S.; Sadhwani, J.J.; Reboso, J.V. Antibiotic removal processes from water & wastewater for the protection of the aquatic environment—A review. J. Water Process Eng. 2022, 45, 102474. [Google Scholar]
- Saitoh, T.; Shibata, K.; Fujimori, K.; Ohtani, Y. Rapid removal of tetracycline antibiotics from water by coagulation-flotation of sodium dodecyl sulfate and poly(allylamine hydrochloride) in the presence of Al(III) ions. Sep. Purif. Technol. 2017, 187, 76–83. [Google Scholar] [CrossRef]
- Adams, C.; Wang, Y.; Loftin, K.; Meyer, M. Removal of Antibiotics from Surface and Distilled Water in Conventional Water Treatment Processes. J. Environ. Eng. 2002, 128, 253–260. [Google Scholar] [CrossRef]
- Saitoh, T.; Shibata, K.; Hiraide, M. Rapid removal and photodegradation of tetracycline in water by surfactant-assisted coagulation-sedimentation method. J. Environ. Chem. Eng. 2014, 2, 1852–1858. [Google Scholar] [CrossRef]
- Nasrollahi, N.; Vatanpour, V.; Khataee, A. Removal of antibiotics from wastewaters by membrane technology: Limitations, successes, and future improvements. Sci. Total Environ. 2022, 838, 156010. [Google Scholar] [CrossRef]
- Oh, J.; Salcedo, D.E.; Medriano, C.A.; Kim, S. Comparison of different disinfection processes in the effective removal of antibiotic-resistant bacteria and genes. J. Environ. Sci. 2014, 26, 1238–1242. [Google Scholar] [CrossRef] [PubMed]
- Apostolescu, N.; Tataru Farmus, R.E.; Harja, M.; Vizitiu, M.A.; Cernatescu, C.; Cobzaru, C.; Apostolescu, G.A. Photocatalytic Removal of Antibiotics from Wastewater Using the CeO2/ZnO Heterojunction. Materials 2023, 16, 850. [Google Scholar] [CrossRef]
- Gutiérrez-Sánchez, P.; Álvarez-Torrellas, S.; Larriba, M.; Gil, M.V.; Garrido-Zoido, J.M.; García, J. Efficient removal of antibiotic ciprofloxacin by catalytic wet air oxidation using sewage sludge-based catalysts: Degradation mechanism by DFT studies. J. Environ. Chem. Eng. 2023, 11, 109344. [Google Scholar] [CrossRef]
- Brillas, E. A review on the photoelectro-Fenton process as efficient electrochemical advanced oxidation for wastewater remediation. Treatment with UV light, sunlight, and coupling with conventional and other photo-assisted advanced technologies. Chemosphere 2020, 250, 126198. [Google Scholar] [CrossRef]
- Hu, X.; Hu, X.; Peng, Q.; Zhou, L.; Tan, X.; Jiang, L.; Tang, C.; Wang, H.; Liu, S.; Wang, Y.; et al. Mechanisms underlying the photocatalytic degradation pathway of ciprofloxacin with heterogeneous TiO2. Chem. Eng. J. 2020, 380, 122366. [Google Scholar] [CrossRef]
- Attar, R.M.S.; Alkhamis, K.M.; Alsharief, H.H.; Alaysuy, O.; Alrashdi, K.S.; Mattar, H.; Alkhatib, F.; El-Metwaly, N.M. Remarkable photodegradation breakdown cost, antimicrobial activity, photocatalytic efficiency, and recycling of SnO2 quantum dots throughout industrial hazardous pollutants treatment. Ceram. Int. 2024, 50, 36194–36209. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El-Nemr, M.A.; Elkatory, M.R.; Ragab, S.; Niculescu, V.C.; El Nemr, A. Principles of Photocatalysts and Their Different Applications: A Review; Springer International Publishing: New York, NY, USA, 2023; Volume 381, ISBN 0123456789. [Google Scholar]
- Xu, M.; Wu, C.; Zhou, Y. Advancements in the Fenton Process for Wastewater Treatment; Bustillo-Lecompte, C., Ed.; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Yee, L.Y.; Ng, Q.H.; Kartini Enche Ab Rahim, S.; Hoo, P.Y.; Chang, P.T.; Ahmad, A.L.; Low, S.C.; Shuit, S.H. A Novel Tri-Functionality pH-Magnetic-Photocatalytic Hybrid Organic-Inorganic Polyoxometalates Augmented Microspheres for Polluted Water Treatment. Membranes 2023, 13, 174. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Xu, T.; Ji, W.; Zong, X. Recent Advances on Small Band Gap Semiconductor Materials (≤2.1 eV) for Solar Water Splitting. Catalysts 2023, 13, 728. [Google Scholar] [CrossRef]
- Madrakian, T.; Afkhami, A.; Haryani, R.; Ahmadi, M. Synthesis of γ-Fe2O3/TiO2nanocomposite and its application in removal of dyes from water samples by adsorption and degradation processes. RSC Adv. 2014, 4, 44841–44847. [Google Scholar] [CrossRef]
- Ismail, N.; Imran, M.; Ramzan, M.; Anwar, A.; Alsafari, I.A.; Asgher, M.; Iqbal, H.M.N. Functionalized graphene oxide-zinc oxide hybrid material and its deployment for adsorptive removal of levofloxacin from aqueous media. Environ. Res. 2023, 217, 114958. [Google Scholar] [CrossRef]
- Bedin, K.C.; Muche, D.N.F.; Melo, M.A.; Freitas, A.L.M.; Gonçalves, R.V.; Souza, F.L. Role of Cocatalysts on Hematite Photoanodes in Photoelectrocatalytic Water Splitting: Challenges and Future Perspectives. ChemCatChem 2020, 12, 3156–3169. [Google Scholar] [CrossRef]
- Mei, Q.; Zhang, F.; Wang, N.; Yang, Y.; Wu, R.; Wang, W. TiO2/Fe2O3 heterostructures with enhanced photocatalytic reduction of Cr(vi) under visible light irradiation. RSC Adv. 2019, 9, 22764–22771. [Google Scholar] [CrossRef]
- Sakar, M.; Mithun Prakash, R.; Trong-On, D. Insights into the Tio2-based photocatalytic systems and their mechanisms. Catalysts 2019, 9, 680. [Google Scholar] [CrossRef]
- Kiziltaş, H.; Tekin, T.; Tekin, D. Preparation and characterization of recyclable investigation of the photocatalytic activity. Chem. Eng. Commun. 2021, 208, 1041–1053. [Google Scholar] [CrossRef]
- Behzadi, S.; Nonahal, B.; Royaee, S.J.; Asadi, A.A. TiO2/SiO2/Fe3O4 magnetic nanoparticles synthesis and application in methyl orange UV photocatalytic removal. Water Sci. Technol. 2020, 82, 2432–2445. [Google Scholar] [CrossRef]
- Malakootian, M.; Nasiri, A.; Amiri Gharaghani, M. Photocatalytic degradation of ciprofloxacin antibiotic by TiO2 nanoparticles immobilized on a glass plate. Chem. Eng. Commun. 2020, 207, 56–72. [Google Scholar] [CrossRef]
- Hayri-senel, T.; Kahraman, E.; Sezer, S.; Erdol-aydin, N.; Nasun-saygili, G. Photocatalytic degradation of ciprofloxacin from water with waste polystyrene and TiO2 composites. Heliyon 2024, 10, e25433. [Google Scholar] [CrossRef] [PubMed]
- Sakineh, S.; Kakavandi, B.; Noorisepehr, M.; Akbar, A. Photocatalytic oxidation of tetracycline by magnetic carbon-supported TiO2 nanoparticles catalyzed peroxydisulfate: Performance, synergy and reaction mechanism studies. Sep. Purif. Technol. 2021, 258, 117936. [Google Scholar]
- Lv, Y.; Yue, L.; Khan, I.M.; Zhou, Y.; Cao, W.; Niazi, S.; Wang, Z. Fabrication of magnetically recyclable yolk-shell Fe3O4@TiO2 nanosheet/Ag/g-C3N4 microspheres for enhanced photocatalytic degradation of organic pollutants. Nano Res. 2021, 14, 2363–2371. [Google Scholar] [CrossRef]
- Amoli, A.E.; Masoumi, M.; Sharifzadeh, M. Synthesis of TiO2-Fe2O3 nanocomposite for the photocatalytic degradation of Direct Blue 199 and Basic Yellow 28 dyes under visible light irradiation. J. Dispers. Sci. Technol. 2023, 44, 630–638. [Google Scholar] [CrossRef]
- Rani, N.; Dehiya, B.S. Magnetically recyclable copper doped core-shell Fe3O4@TiO2@Cu nanocomposites for wastewater remediation. Environ. Technol. 2022, 43, 4484–4492. [Google Scholar] [CrossRef]
- Brossault, D.F.F.; Mccoy, T.M.; Routh, A.F. Self-assembly of TiO2 Fe3O4/SiO2 microbeads: A green approach to produce magnetic photocatalysts. J. Colloid Interface Sci. 2021, 584, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Dewi, S.H.; Sutanto; Fisli, A.; Wardiyati, S. Synthesis and Characterization of Magnetized Photocatalyst Fe3O4/SiO2/TiO2 by Heteroagglomeration Method. J. Phys. Conf. Ser. 2016, 739, 012113. [Google Scholar] [CrossRef]
- Ren, G.; Han, H.; Wang, Y.; Liu, S.; Zhao, J.; Meng, X.; Li, Z. Recent Advances of Photocatalytic Application in Water Treatment: A Review. Nanomaterials 2021, 11, 1804. [Google Scholar] [CrossRef]
- Babar, S.; Gavade, N.; Shinde, H.; Gore, A.; Mahajan, P.; Lee, K.H.; Bhuse, V.; Garadkar, K. An innovative transformation of waste toner powder into magnetic g-C3N4-Fe2O3 photocatalyst: Sustainable e-waste management. J. Environ. Chem. Eng. 2019, 7, 103041. [Google Scholar] [CrossRef]
- Synowiec, M.; Dominika, Z.; Trenczek-Zajac, A.; Radecka, M. The impact of nanometric Fe2O3 on the magnetic, electronic, and photocatalytic behavior of TiO2@ Fe2O3 heterostructures. Appl. Surf. Sci. 2023, 608, 155186. [Google Scholar] [CrossRef]
- Qamar, U.; Jazib, S.; Zaidi, A.; Riaz, A.; Atiq, M.; Xin, C.; Abdul, M. Simple two-step development of TiO2/Fe2O3 nanocomposite for oxygen evolution reaction (OER) and photo-bio active applications. Colloids Surf. A Physicochem. Eng. Asp. 2023, 671, 131662. [Google Scholar]
- Makovec, D.; Sajko, M.; Drofenik, M. Magnetically recoverable photocatalytic nanocomposite particles for water treatment. Mater. Chem. Phys. 2011, 129, 83–89. [Google Scholar] [CrossRef]
- Zamani, W.; Rastgar, S.; Hedayati, A. Capability of TiO2 and Fe3O4 nanoparticles loaded onto Algae (Scendesmus sp.) as a novel bio-magnetic photocatalyst to degration of Red195 dye in the sonophotocatalytic treatment process under ultrasonic/UVA irradiation. Sci. Rep. 2023, 13, 18182. [Google Scholar] [CrossRef]
- Zulfiqar, N.; Nadeem, R.; Musaimi, O.A.I. Photocatalytic Degradation of Antibiotics via Exploitation of a Magnetic Nanocomposite: A Green Nanotechnology Approach toward Drug-Contaminated Wastewater Reclamation. ACS Omega 2024, 9, 7986–8004. [Google Scholar] [CrossRef] [PubMed]
- Beketova, D.; Motola, M.; Sopha, H.; Michalicka, J.; Cicmancova, V.; Dvorak, F.; Hromadko, L.; Frumarova, B.; Stoica, M.; Macak, J.M. One-Step Decoration of TiO2 Nanotubes with Fe3O4 Nanoparticles: Synthesis and Photocatalytic and Magnetic Properties. ACS Appl. Nano Mater. 2020, 3, 1553–1563. [Google Scholar] [CrossRef]
- Plohl, O.; Ajdnik, U.; Gyergyek, S.; Ban, I.; Vesel, A.; Glaser, T.K.; Zemljič, L.F. Superior stability and high biosorbent efficiency of carboxymethylchitosan covalently linked to silica-coated core-shell magnetic nanoparticles for application in copper removal. J. Environ. Chem. Eng. 2019, 7, 102913. [Google Scholar] [CrossRef]
- Makovec, D.; Sajko, M.; Selinik, A.; Drofenik, M. Low-temperature synthesis of magnetically recoverable, superparamagnetic, photocatalytic, nanocomposite particles. Mater. Chem. Phys. 2012, 136, 230–240. [Google Scholar] [CrossRef]
- Herrera, W.; Vera, J.; Hermosilla, E.; Diaz, M.; Tortella, G.R.; Albino, R.; Reis, D.; Seabra, A.B.; Rubilar, O. The Catalytic Role of Superparamagnetic Iron Oxide Nanoparticles as a Support Material for TiO2 and ZnO on Chlorpyrifos Photodegradation in an Aqueous Solution. Nanomaterials 2024, 14, 299. [Google Scholar] [CrossRef]
- Suliman, Z.A.; Mecha, A.C.; Mwasiagi, J.I. Effect of TiO2/Fe2O3 nanopowder synthesis method on visible light photocatalytic degradation of reactive blue dye. Heliyon 2024, 10, e29648. [Google Scholar] [CrossRef]
- Esfandiari, N.; Kashefi, M.; Mirjalili, M.; Afsharnezhad, S. On the adsorption kinetics and mechanism of enhanced photocatalytic activity of Fe3O4-SiO2-TiO2 core-multishell nanoparticles against E. coli. J. Biomed. Mater. Res.—Part A 2021, 109, 181–192. [Google Scholar] [CrossRef]
- Wang, J.; Sgarzi, M.; Němečková, Z.; Henych, J.; Licciardello, N.; Cuniberti, G. Reusable and Antibacterial Polymer-Based Nanocomposites for the Adsorption of Dyes and the Visible-Light-Driven Photocatalytic Degradation of Antibiotics. Glob. Chall. 2022, 6, 2200076. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.Q.; Zi, T.Q.; Zhao, X.R.; Liu, C.; Ren, Q.; Fang, J.B.; Li, W.M.; Li, A.D. Enhanced visible light photocatalytic activity of Fe2O3 modified TiO2 prepared by atomic layer deposition. Sci. Rep. 2020, 10, 13437. [Google Scholar] [CrossRef]
- Xia, Y.; Yin, L. Core-shell structured α-Fe2O3@TiO2 nanocomposites with improved photocatalytic activity in the visible light region. Phys. Chem. Chem. Phys. 2013, 15, 18627–18634. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, S.; Wu, W.; Tian, Q.; Cui, S.; Dai, Z.; Ren, F.; Xiao, X.; Jiang, C. 3D Flowerlike α-Fe2O3@TiO2 Core-Shell Nanostructures: General Synthesis and Enhanced Photocatalytic Performance. ACS Sustain. Chem. Eng. 2015, 3, 2975–2984. [Google Scholar] [CrossRef]
- Krishnan, S.; Shriwastav, A. Chlorophyll sensitized and salicylic acid functionalized TiO2 nanoparticles as a stable and efficient catalyst for the photocatalytic degradation of ciprofloxacin with visible light. Environ. Res. 2023, 216, 114568. [Google Scholar] [CrossRef] [PubMed]
- Rajiv, P.; Mengelizadeh, N.; McKay, G.; Balarak, D. Photocatalytic degradation of ciprofloxacin with Fe2O3 nanoparticles loaded on graphitic carbon nitride: Mineralisation, degradation mechanism and toxicity assessment. Int. J. Environ. Anal. Chem. 2021, 103, 2193–2207. [Google Scholar] [CrossRef]
- Bazzanella, N.; Bajpai, O.P.; Fendrich, M.; Guella, G.; Miotello, A.; Orlandi, M. Ciprofloxacin degradation with a defective TiO2-x nanomaterial under sunlight. MRS Commun. 2023, 13, 1252–1259. [Google Scholar] [CrossRef]
- Paul, T.; Dodd, M.C.; Strathmann, T.J. Photolytic and photocatalytic decomposition of aqueous ciprofloxacin: Transformation products and residual antibacterial activity. Water Res. 2010, 44, 3121–3132. [Google Scholar] [CrossRef]
- Golmohammadi, M.; Hanafi-Bojd, H.; Shiva, M. Photocatalytic degradation of ciprofloxacin antibiotic in water by biosynthesized silica supported silver nanoparticles. Ceram. Int. 2023, 49, 7717–7726. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y. ZnIn2S4/CoFe2O4 p-n junction-decorated biochar as magnetic recyclable nanocomposite for efficient photocatalytic degradation of ciprofloxacin under simulated sunlight. Sep. Purif. Technol. 2022, 303, 122156. [Google Scholar] [CrossRef]
- Karuppaiah, S.; Annamalai, R.; Muthuraj, A.; Kesavan, S.; Palani, R.; Ponnusamy, S.; Nagarajan, E.R.; Meenakshisundaram, S. Efficient photocatalytic degradation of ciprofloxacin and bisphenol A under visible light using Gd2WO6 loaded ZnO/bentonite nanocomposite. Appl. Surf. Sci. 2019, 481, 1109–1119. [Google Scholar]
- Zhang, N.; Li, X.; Wang, Y.; Zhu, B.; Yang, J. Fabrication of magnetically recoverable Fe3O4/CdS/g-C3N4 photocatalysts for effective degradation of ciprofloxacin under visible light. Ceram. Int. 2020, 46, 20974–20984. [Google Scholar] [CrossRef]
- Tamaddon, F.; Nasiri, A.; Yazdanpanah, G. Photocatalytic degradation of ciprofloxacin using CuFe2O4@methyl cellulose based magnetic nanobiocomposite. MethodsX 2020, 7, 74–81. [Google Scholar] [CrossRef]
- Raikar, L.G.; Patel, A.; Gandhi, J.; Gupta, K.V.K.; Prakash, H. Nano-TiO2 immobilized polyvinylidene fluoride based spongy-spheres for ciprofloxacin photocatalytic degradation: Antibacterial activity removal, mechanisms, UVA LED irradiation and easy recovery. Environ. Sci. Nano, 2024; Advance Article. Available online: https://pubs.rsc.org/en/content/articlehtml/2024/en/d4en00302k (accessed on 29 July 2024).
- Naga Lakshmi, C.; Irfan, M.; Sinha, R.; Singh, N. Magnetically recoverable Ni-doped iron oxide/graphitic carbon nitride nanocomposites for the improved photocatalytic degradation of ciprofloxacin: Investigation of degradation pathways. Environ. Res. 2024, 242, 117812. [Google Scholar] [CrossRef]
- Zeng, Y.; Wu, L.; Luo, J.; Lu, X.; Jia, B.; Liang, L.; Zhang, J. Photocatalytic degradation of ciprofloxacin by Gd-Co/g-C3N4 under low-power light source: Degradation pathways and mechanistic insights. J. Water Process Eng. 2024, 58, 104849. [Google Scholar] [CrossRef]
- Huang, Y.; Nengzi, L.C.; Zhang, X.; Gou, J.; Gao, Y.; Zhu, G.; Cheng, Q.; Cheng, X. Catalytic degradation of ciprofloxacin by magnetic CuS/Fe2O3/Mn2O3 nanocomposite activated peroxymonosulfate: Influence factors, degradation pathways and reaction mechanism. Chem. Eng. J. 2020, 388, 124274. [Google Scholar] [CrossRef]
- Campelj, S.; Makovec, D.; Drofenik, M. Preparation and properties of water-based magnetic fluids. J. Phys. Condens. Matter 2008, 20, 204101. [Google Scholar] [CrossRef]
- Zemljič, L.F.; Čakara, D.; Michaelis, N.; Heinze, T.; Kleinschek, K.S. Protonation behavior of 6-deoxy-6-(2-aminoethyl)amino cellulose: A potentiometric titration study. Cellulose 2011, 18, 33–43. [Google Scholar] [CrossRef]
- Bračič, M.; Mohan, T.; Kargl, R.; Grießer, T.; Heinze, T.; Stana Kleinschek, K. Protein repellent anti-coagulative mixed-charged cellulose derivative coatings. Carbohydr. Polym. 2021, 254, 117437. [Google Scholar] [CrossRef]
- Ali, A.; Shah, T.; Ullah, R.; Zhou, P.; Guo, M.; Ovais, M.; Tan, Z.; Rui, Y.K. Review on Recent Progress in Magnetic Nanoparticles: Synthesis, Characterization, and Diverse Applications. Front. Chem. 2021, 9, 629054. [Google Scholar] [CrossRef] [PubMed]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Reactions, Occurrences and Uses, 2nd ed.; WILEY-VCH: Weinheim, Baden, Germany, 2003. [Google Scholar]
- Plohl, O.; Finšgar, M.; Gyergyek, S.; Ajdnik, U.; Ban, I.; Fras Zemljič, L. Efficient Copper Removal from an Aqueous Anvironment using a Novel and Hybrid Nanoadsorbent Based on Derived-Polyethyleneimine Linked to Silica Magnetic Nanocomposites. Nanomaterials 2019, 9, 209. [Google Scholar] [CrossRef] [PubMed]
- Plohl, O.; Simonič, M.; Kolar, K.; Gyergyek, S.; Zemljič, L.F. Magnetic nanostructures functionalized with a derived lysine coating applied to simultaneously remove heavy metal pollutants from environmental systems. Sci. Technol. Adv. Mater. 2021, 22, 55–71. [Google Scholar] [CrossRef]
- Silva, C.G.; Sampaio, M.J.; Marques, R.R.N.; Ferreira, L.A.; Tavares, P.B.; Silva, A.M.T.; Faria, J.L. Photocatalytic production of hydrogen from methanol and saccharides using carbon nanotube-TiO2 catalysts. Appl. Catal. B Environ. 2015, 178, 82–90. [Google Scholar] [CrossRef]
- Novak, U.; Grdadolnik, J. Infrared spectra of hydrogen bond network in lamellar perfluorocarboxylic acid monohydrates. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2021, 253, 119551. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Pan, S.; Liu, X.; Guo, M.; Yu, S.F.; Huang, H.; Fan, H.; Li, G. Engineering the intermediate band states in amorphous Ti3+-doped TiO2 for hybrid dye-sensitized solar cell applications. J. Mater. Chem. A 2015, 3, 11437–11443. [Google Scholar] [CrossRef]
- Sikdar, S.; Pathak, S.; Ghorai, T.K. Aqueous phase photodegradation of rhodamine B and p-nitrophenol desctruction using titania based nanocomposites. Adv. Mater. Lett. 2015, 6, 867–873. [Google Scholar] [CrossRef]
- Plohl, O.; Zemljič, L.F.; Potrč, S.; Luxbacher, T. Applicability of electro-osmotic flow for the analysis of the surface zeta potential. RSC Adv. 2020, 10, 6777–6789. [Google Scholar] [CrossRef]
- Plohl, O.; Kralj, S.; Majaron, B.; Fröhlich, E.; Ponikvar-svet, M.; Makovec, D.; Lisjak, D. Amphiphilic coatings for the protection of upconverting nanoparticles against dissolution in aqueous media. Dalt. Trans. 2017, 46, 6975–6984. [Google Scholar] [CrossRef]
- Gyergyek, S.; Pahovnik, D.; Žagar, E.; Mertelj, A.; Kostanjšek, R.; Beković, M.; Jagodič, M.; Hofmann, H.; Makovec, D. Nanocomposites comprised of homogeneously dispersed magnetic iron-oxide nanoparticles and poly(methyl methacrylate). Beilstein J. Nanotechnol. 2018, 9, 1612–1622. [Google Scholar] [CrossRef]
- Plohl, O.; Fras Zemljič, L.; Vihar, B.; Vesel, A.; Gyergyek, S.; Maver, U.; Ban, I.; Bračič, M. Novel magnetic iron oxide-dextran sulphate nanocomposites as potential anticoagulants: Investigating interactions with blood components and assessing cytotoxicity. Carbohydr. Polym. 2024, 343, 122469. [Google Scholar] [CrossRef] [PubMed]
- Dušak, P.; Benčina, M.; Turk, M.; Bavčar, D.; Košmerl, T.; Berovič, M.; Makovec, D. Application of magneto-responsive Oenococcus oeni for the malolactic fermentation in wine. Biochem. Eng. J. 2016, 110, 134–142. [Google Scholar] [CrossRef]
- Kralj, S.; Makovec, D. The chemically directed assembly of nanoparticle clusters from superparamagnetic iron-oxide nanoparticles. RSC Adv. 2014, 4, 13167–13171. [Google Scholar] [CrossRef]
- Li, D.; Song, H.; Meng, X.; Shen, T.; Sun, J.; Han, W.; Wang, X. Effects of particle size on the structure and photocatalytic performance by alkali-treated TiO2. Nanomaterials 2020, 10, 546. [Google Scholar] [CrossRef]
- Amano, F.; Nogami, K.; Tanaka, M.; Ohtani, B. Correlation between surface area and photocatalytic activity for acetaldehyde decomposition over bismuth tungstate particles with a hierarchical structure. Langmuir 2010, 26, 7174–7180. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, J.; Ding, J.; Liu, T.; Shi, G.; Li, X.; Dang, W.; Cheng, Y.; Guo, R. Pore structure and fractal characteristics of different shale lithofacies in the dalong formation in the western area of the lower yangtze platform. Minerals 2020, 10, 72. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Lindgren, T.; Mwabora, J.M.; Avandaño, E.; Jonsson, J.; Hoel, A.; Granqvist, C.G.; Lindquist, S.E. Photoelectrochemical and optical properties of nitrogen doped titanium dioxide films prepared by reactive DC magnetron sputtering. J. Phys. Chem. B 2003, 107, 5709–5716. [Google Scholar] [CrossRef]
- Mallick, P.; Dash, B.N. X-ray Diffraction and UV-Visible Characterizations of α-Fe2O3 Nanoparticles Annealed at Different Temperature. Nanosci. Nanotechnol. 2013, 3, 130–134. [Google Scholar]
- Fang, X.; Lu, G.; Mahmood, A.; Tang, Z.; Liu, Z.; Zhang, L.; Wang, Y.; Sun, J. A novel ternary Mica/TiO2/Fe2O3 composite pearlescent pigment for the photocatalytic degradation of acetaldehyde. J. Photochem. Photobiol. A Chem. 2020, 400, 112617. [Google Scholar] [CrossRef]
- Abazović, N.D.; Čomor, M.I.; Dramićanin, M.D.; Jovanović, D.J.; Ahrenkiel, S.P.; Nedeljković, J.M. Photoluminescence of anatase and rutile TiO2 particles. J. Phys. Chem. B 2006, 110, 25366–25370. [Google Scholar] [CrossRef] [PubMed]
- Gnanasekaran, L.; Hemamalini, R.; Rajendran, S.; Qin, J.; Yola, M.L.; Atar, N.; Gracia, F. Nanosized Fe3O4 incorporated on a TiO2 surface for the enhanced photocatalytic degradation of organic pollutants. J. Mol. Liq. 2019, 287, 2–8. [Google Scholar] [CrossRef]
- Song, S.Y.; Chen, H.D.; Li, C.X.; Shi, D.S.; Ying, Y.; Han, Y.B.; Xu, J.C.; Hong, B.; Jin, H.X.; Jin, D.F.; et al. Magnetic Bi2WO6 nanocomposites: Synthesis, magnetic response and their visible-light-driven photocatalytic performance for ciprofloxacin. Chem. Phys. 2020, 530, 110614. [Google Scholar] [CrossRef]
- Navó, J.A.; Colón, G.; Trillas, M.; Peral, J.; Domènech, X.; Testa, J.J.; Padrón, J.; Rodŕguez, D.; Litter, M.I. Heterogeneous photocatalytic reactions of nitrite oxidation and Cr(VI) reduction on iron-doped titania prepared by the wet impregnation method. Appl. Catal. B Environ. 1998, 16, 187–196. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).