Correction: Grewal et al. Structural and Photocatalytic Studies on Oxygen Hyperstoichiometric Titanium-Substituted Strontium Ferrite Nanoparticles. Magnetochemistry 2022, 8, 120
Reference
- Grewal, J.K.; Kaur, M.; Sharma, R.K.; Oliveira, A.C.; Garg, V.K.; Sharma, V.K. Structural and Photocatalytic Studies on Oxygen Hyperstoichiometric Titanium-Substituted Strontium Ferrite Nanoparticles. Magnetochemistry 2022, 8, 120. [Google Scholar] [CrossRef]
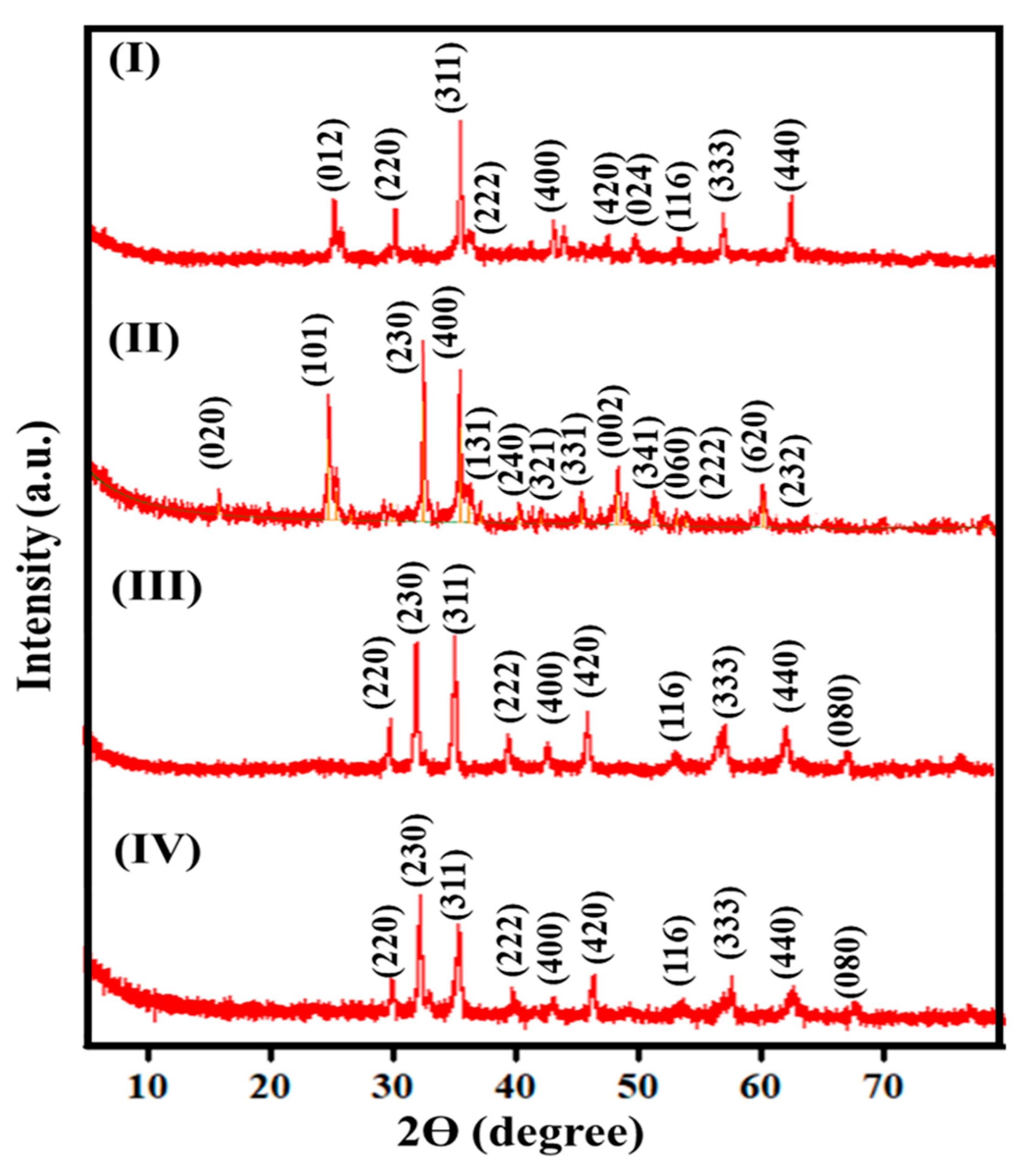
| Nanomaterial | Lattice Constant (Å) | Experimental Density (ρexp in g/cm3) | Crystallite Size (nm) | XRD Density (ρXRD in g/cm3) | % Porosity | ξ-Potential (mV) | DLS Hydrodynamic Particle Size (nm) |
|---|---|---|---|---|---|---|---|
| SrFe2O4 | 8.343 | 3.22 | 39.8 | 7.835 | 58.90 | 13.54 | 123.92 |
| TiFe2O5 | 8.730 | 2.19 | 31.7 | 3.450 | 45.52 | 14.78 | 105.52 |
| Sr0.7Ti0.3Fe2O4.3 | 8.345 | 2.87 | 25.7 | 5.641 | 49.12 | 23.54 | 101.79 |
| Sr0.4Ti0.6Fe2O4.6 | 8.386 | 2.43 | 18.0 | 5.034 | 51.73 | 32.41 | 95.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grewal, J.K.; Kaur, M.; Sharma, R.K.; Oliveira, A.C.; Garg, V.K.; Sharma, V.K. Correction: Grewal et al. Structural and Photocatalytic Studies on Oxygen Hyperstoichiometric Titanium-Substituted Strontium Ferrite Nanoparticles. Magnetochemistry 2022, 8, 120. Magnetochemistry 2024, 10, 65. https://doi.org/10.3390/magnetochemistry10090065
Grewal JK, Kaur M, Sharma RK, Oliveira AC, Garg VK, Sharma VK. Correction: Grewal et al. Structural and Photocatalytic Studies on Oxygen Hyperstoichiometric Titanium-Substituted Strontium Ferrite Nanoparticles. Magnetochemistry 2022, 8, 120. Magnetochemistry. 2024; 10(9):65. https://doi.org/10.3390/magnetochemistry10090065
Chicago/Turabian StyleGrewal, Jaspreet Kaur, Manpreet Kaur, Rajeev K. Sharma, Aderbal C. Oliveira, Vijayendra Kumar Garg, and Virender K. Sharma. 2024. "Correction: Grewal et al. Structural and Photocatalytic Studies on Oxygen Hyperstoichiometric Titanium-Substituted Strontium Ferrite Nanoparticles. Magnetochemistry 2022, 8, 120" Magnetochemistry 10, no. 9: 65. https://doi.org/10.3390/magnetochemistry10090065
APA StyleGrewal, J. K., Kaur, M., Sharma, R. K., Oliveira, A. C., Garg, V. K., & Sharma, V. K. (2024). Correction: Grewal et al. Structural and Photocatalytic Studies on Oxygen Hyperstoichiometric Titanium-Substituted Strontium Ferrite Nanoparticles. Magnetochemistry 2022, 8, 120. Magnetochemistry, 10(9), 65. https://doi.org/10.3390/magnetochemistry10090065






