Inhibitory Activity and Mechanism of Action with Thymol against the Blueberry Pathogenic Fungi Caused by Neopestalotiopsis clavispora
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strain and Culture Conditions
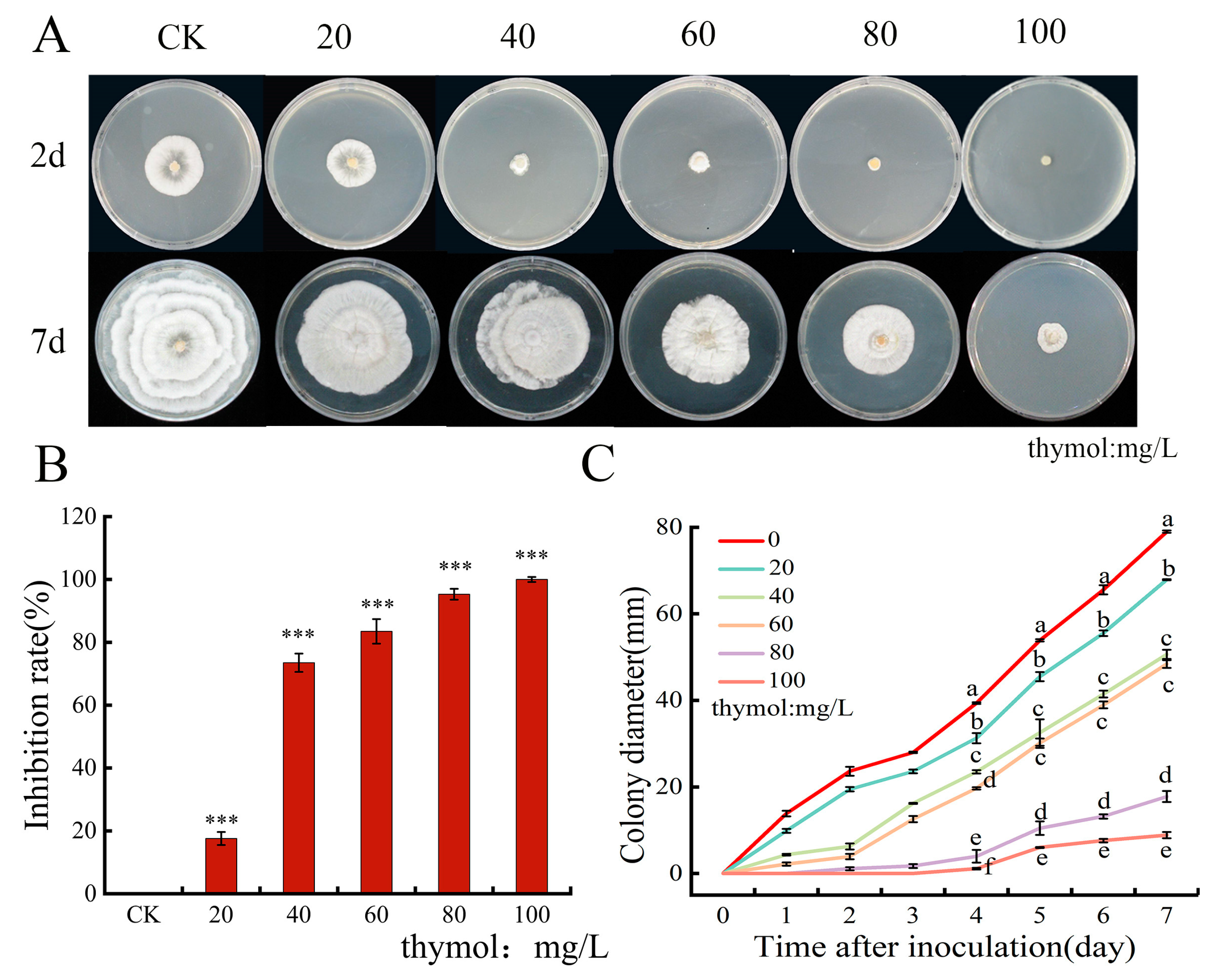
2.2. Effect of Thymol on Colony Growth
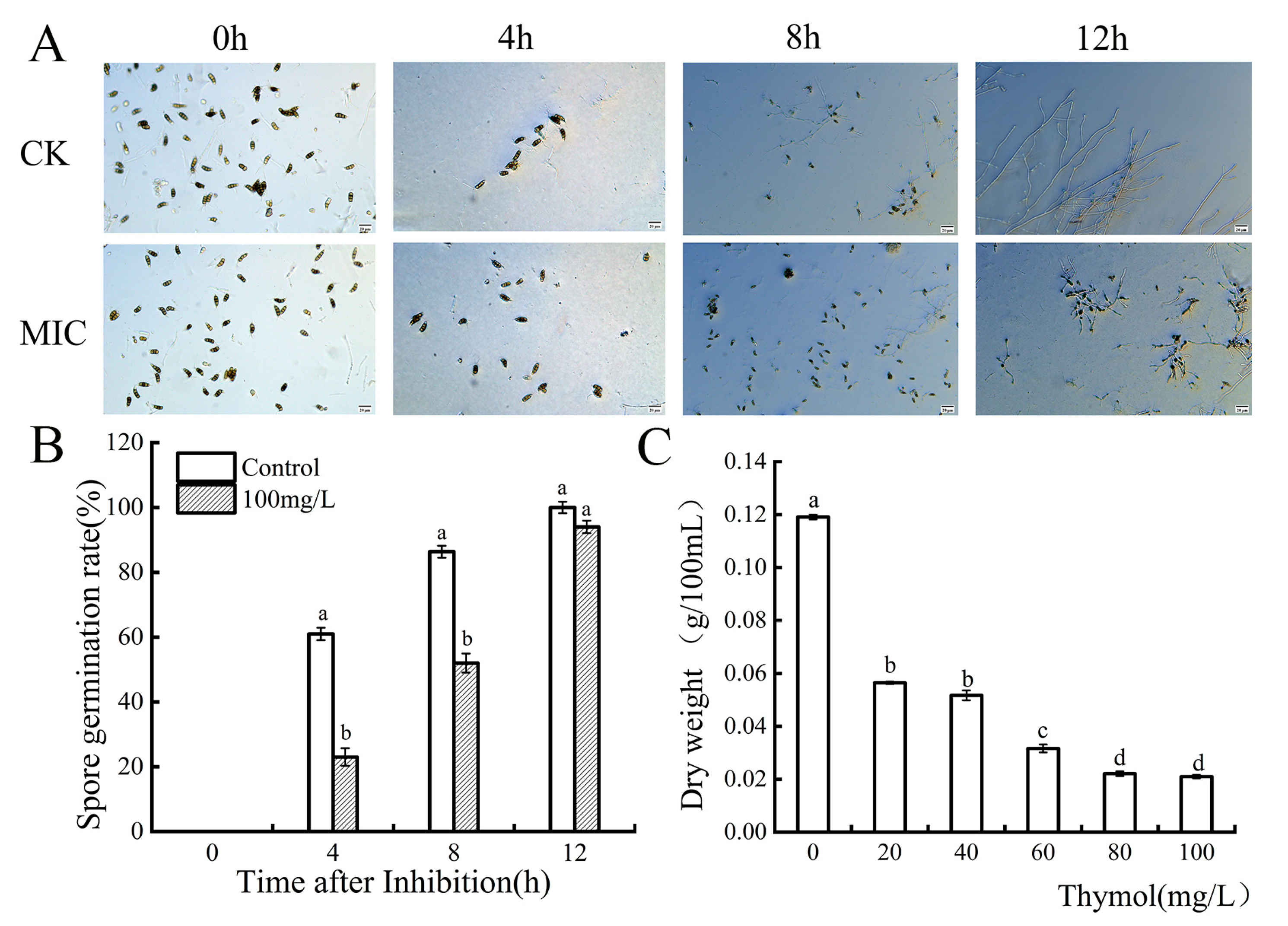
2.3. Determination of Thymol on N. clavispora Spore Germination
2.4. Effect of Thymol on the Dry Weight of N. clavispora Mycelium
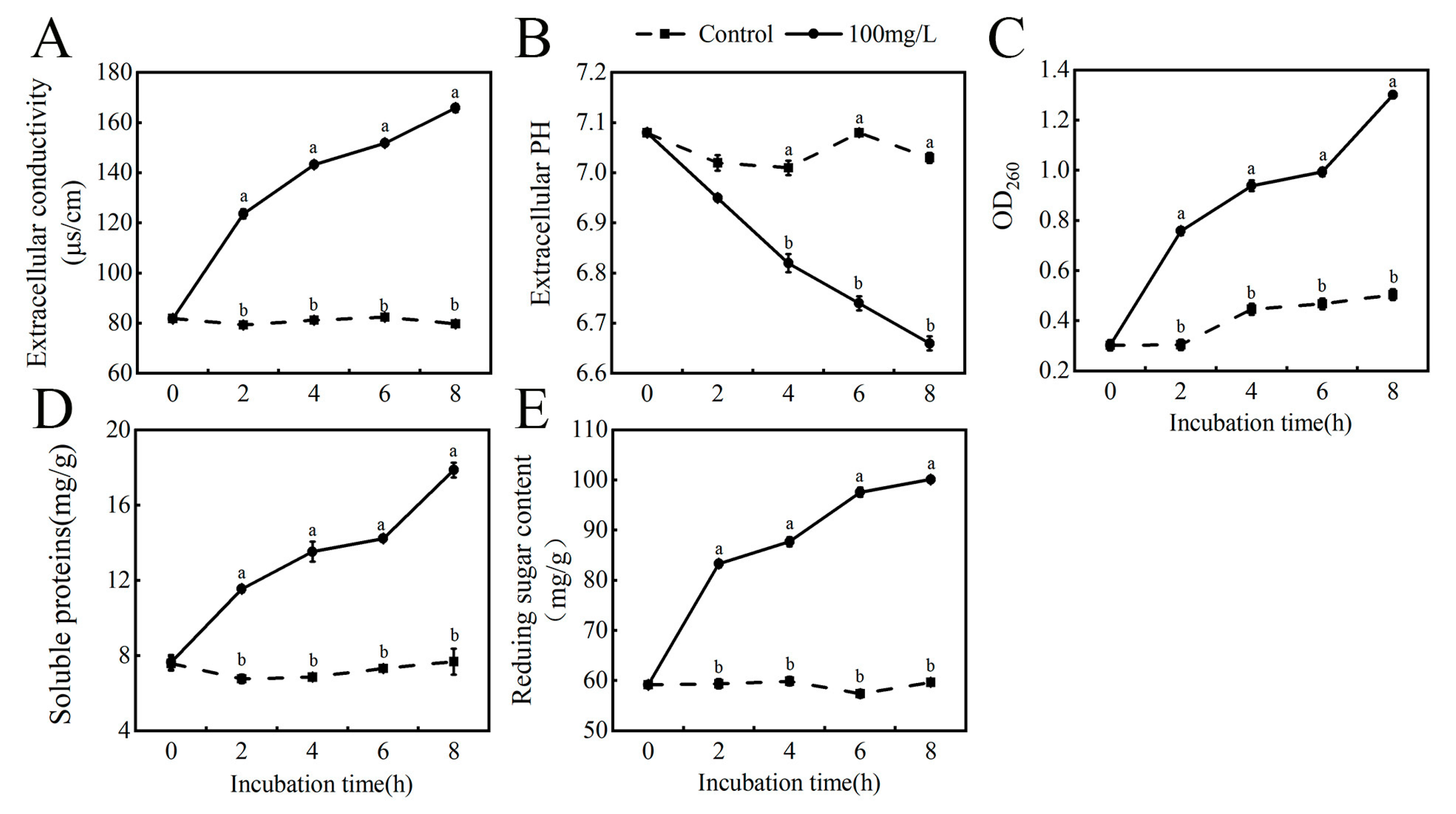
2.5. Effect of Thymol on the Relative Conductivity, Nucleic Acid Release, and pH of N. clavispora
2.6. Determination of Thymol on Extracellular Soluble Protein and Reducing Sugar Content of N. clavispora
2.7. Effect of Thymol on the Integrity of N. clavispora Cell Membrane
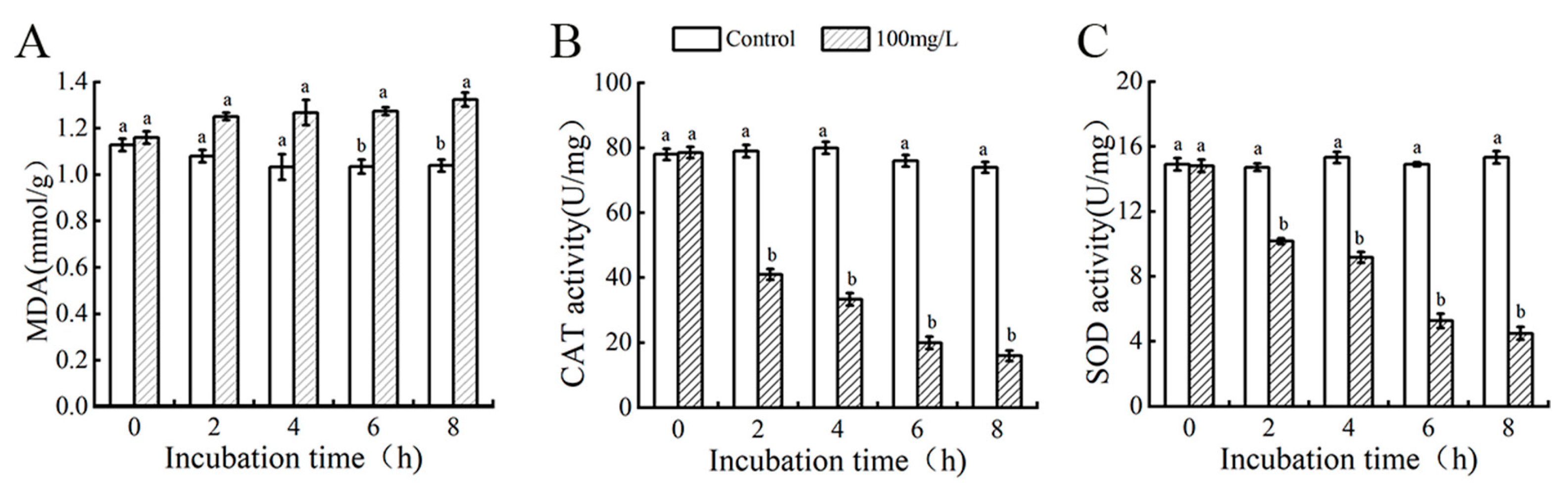
2.8. Determination of Intracellular Malondialdehyde (MDA) Content
2.9. Determination of Superoxide Dismutase and Catalase Enzyme Activities of N. clavispora Mycelium
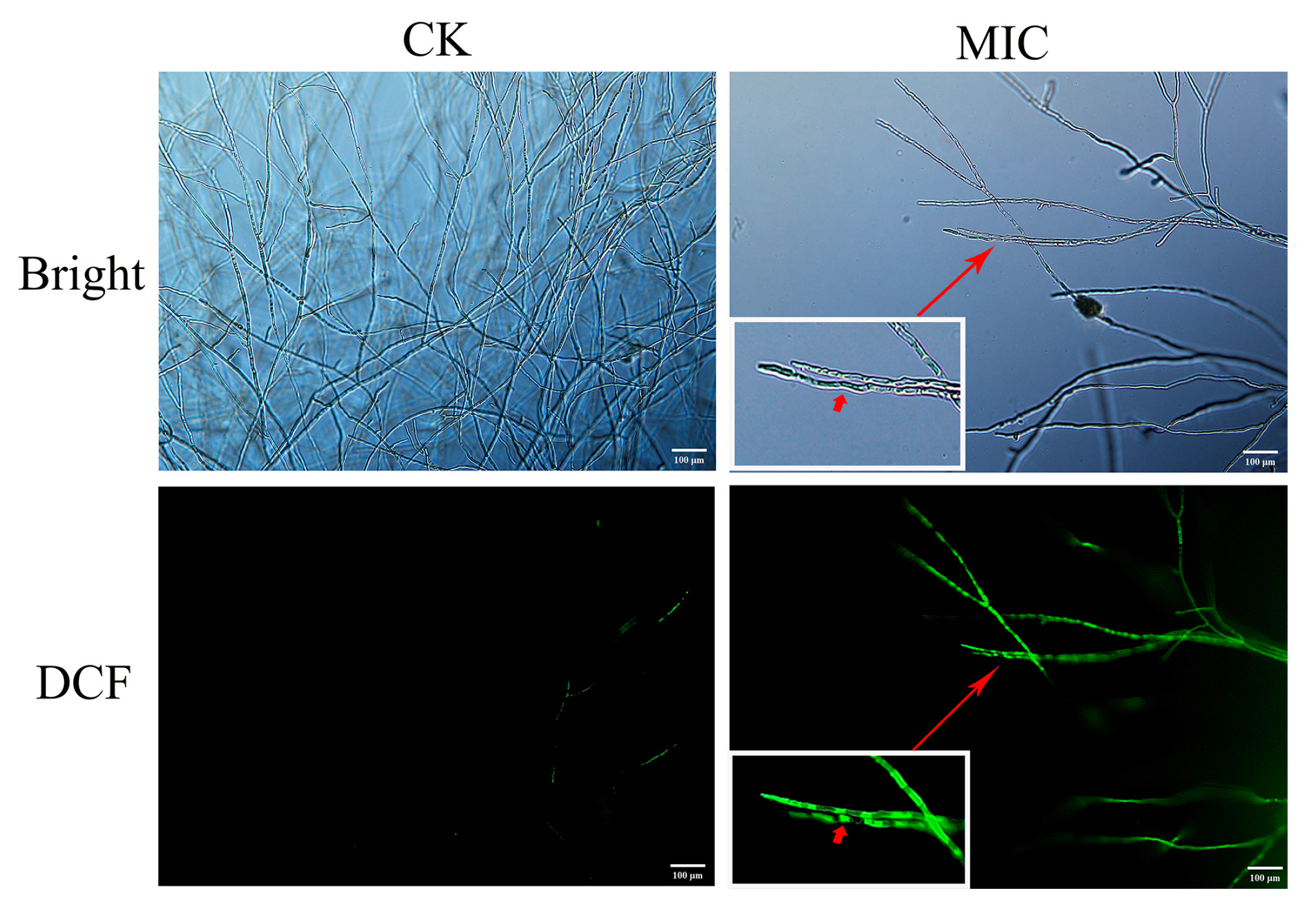
2.10. Detection of Intracellular Reactive Oxygen Species (ROS) Levels in N. clavispora
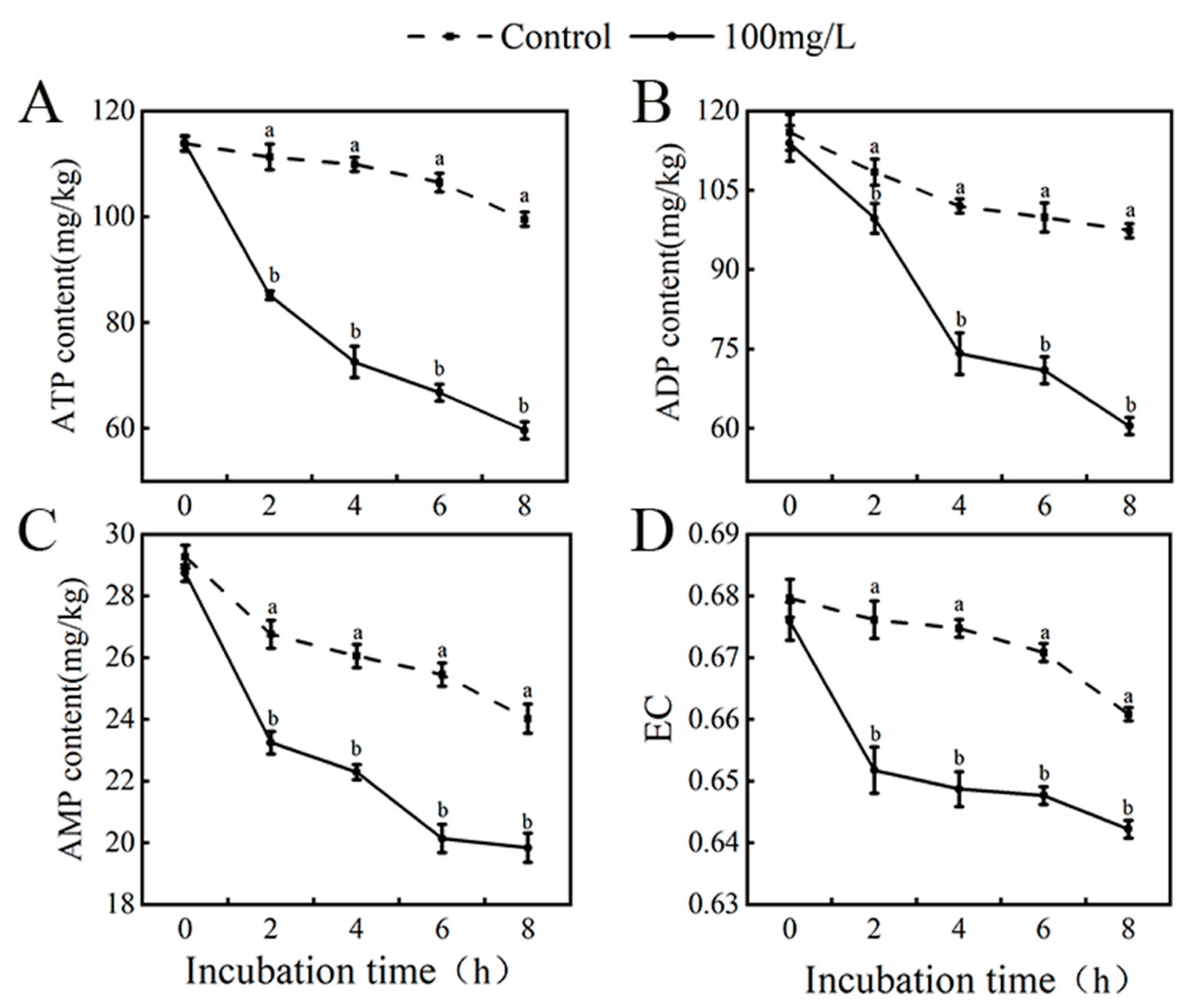
2.11. N. clavispora Energy Metabolism Analysis
2.12. Statistical Analysis
3. Results
3.1. Effect of Thymol on Colony Growth
3.2. Effect of Thymol on the Germination of N. clavispora Spores
3.3. Effect of Thymol on the Dry Weight of N. clavispora Mycelium
3.4. Effect of Thymol on the Relative Conductivity, Nucleic Acid Release, and pH of N. clavispora
3.5. Effect of Thymol on the Extracellular Soluble Protein and Reducing Sugar Content of N. clavispora
3.6. Effect of Thymol on N. clavispora Cell Membrane Integrity
3.7. Changes in Intracellular Malondialdehyde (MDA) Content
3.8. Changes in Superoxide Dismutase and Catalase Enzyme Activities of N. clavispora Mycelium
3.9. Intracellular Reactive Oxygen Species (ROS) Levels in N. clavispora Cells
3.10. Effect of Thymol Treatment on Energy of Pathogenic Mycelium
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Silva, S.; Costa, E.M.; Veiga, M.; Morais, R.M.; Calhau, C.; Pintado, M. Health promoting properties of blueberries: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ceja, A.; Loeza-Lara, P.D.; Espinosa-García, F.J.; García-Rodríguez, Y.M.; Medina-Medrano, J.R.; Gutiérrez-Hernández, G.F.; Ceja-Torres, L.F. In Vitro Antifungal Activity of Plant Extracts on Pathogenic Fungi of Blueberry (Vaccinium sp.). Plants 2021, 10, 852. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.R.; Montiel, L.G.H.; Estrada, R.R.G.; Martínez, P.G. Main diseases in postharvest blueberries, conventional and eco-friendly control methods: A review. LWT 2021, 149, 112046. [Google Scholar] [CrossRef]
- Abeli, P.J.; Fanning, P.D.; Issaacs, R.; Randolph, M.B. Blueberry fruit quality and control of blueberry maggot (Rhagoletis mendax Curran) larvae after fumigation with sulfur dioxide. Postharvest Biol. Tec. 2021, 179, 11568. [Google Scholar] [CrossRef]
- Jiang, T.; Guo, F.; Fang, T.; Hwang, C.A.; Huang, L. Efficacy of gaseous chlorine dioxide generated by sodium chlorite-carbon dioxide reaction on safety and quality of blueberries, cherry tomatoes, and grapes. Food Control. 2023, 143, 109288. [Google Scholar] [CrossRef]
- Dukare, A.S.; Paul, S.; Nambi, V.E.; Gupta, R.K.; Vishwakarma, R.K. Exploitation of microbial antagonists for the control of postharvest diseases of fruits: A review. Crit. Rev. Food Sci. Nutr. 2018, 59, 1–16. [Google Scholar] [CrossRef]
- Radi, M.; Ahmadi, H.; Amiri, S. Effect of Cinnamon Essential Oil-Loaded Nanostructured Lipid Carriers (NLC) Against Penicillium Citrinum and Penicillium Expansum Involved in Tangerine Decay. Food Biobroc. Tech. 2022, 15, 306–318. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef]
- Cruz, J.N.; Silva, S.G.; Pereira, D.S.; Filho, A.P.D.S.S.; Oliveira, M.S.D.; Lima, R.R.; Andrade, E.H.A. In Silico Evaluation of the Antimicrobial Activity of Thymol-Major Compounds in the Essential Oil of Lippia thymoides Mart. & Schauer (Verbenaceae). Molecules 2022, 27, 4768. [Google Scholar]
- Bagy, H.M.M.K.; Abo-Elyousr, K.A.M. Antibacterial activity of some essential oils on bacterial spot disease of tomato plant caused by xanthomonas axonopodis pv. vesicatoria. Int. J. Phytopathol. 2019, 8, 53–61. [Google Scholar] [CrossRef]
- Prakash, B.; Kediak, K.; Misra, P.K.; Dubey, N.K. Plant essential oils as food preservatives to control moulds, mycotoxin contamination and oxidative deterioration of agri-food commodities - potentials and challenges. Food Control. 2015, 47, 381–391. [Google Scholar] [CrossRef]
- Tomazelli Júnior, O.; Kuhn, F.; Padilha, P.J.M.; Vicente, L.R.M.; Costa, S.W.; Boligon, A.A.; Scapinello, J.; Nesi, C.N.; Dal Magro, J.; Castellví, S.L. Microencapsulation of essential thyme oil by spray drying and its antimicrobial evaluation against vibrio alginolyticus and vibrio parahaemolyticus. Braz. J. Biol. 2017, 78, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Guedes, J.; Medeiros, J.; Silva, R.; Sousa, J.; Lúcia da Conceio, M..; Souza, E. The efficacy of Mentha arvensis L. and M. piperita L. essential oils in reducing pathogenic bacteria and maintaining quality characteristics in cashew, guava, mango, and pineapple juices. Int. J. Food Microbiol. 2016, 238, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, A.; Ramezanian, A. Antifungal Activity of Thymol against the Main Fungi Causing Fruit Rot in In Vitro Conditions. Chem. Proc. 2022, 10, 79. [Google Scholar]
- Abdel-Rahim, I.R.; Abo-Elyousr, K.A.M. Using of endophytic Saccharomycopsis fibuligera and thyme oil for management of gray mold rot of guava fruits. Biol. Control. 2017, 110, 124–131. [Google Scholar] [CrossRef]
- Venturini, T.P.; Rossato, L.; Chassot, F.; Azevedo, M.I.D.; Alves, S.H. Activity of cinnamaldehyde, carvacrol and thymol combined with antifungal agents against Fusarium spp. J. Essent. Oil Res. 2021, 33, 502–508. [Google Scholar] [CrossRef]
- Lvarez-Hernández, M.H.; Martínez-Hernández, G.B.; Castillejo, N.; Martínez, J.A.; Artés-Hernández, F. Development of an antifungal active packaging containing thymol and an ethylene scavenger. Validation during storage of cherry tomatoes. Food Packag. Shelf Life. 2021, 29, 100734. [Google Scholar] [CrossRef]
- Sallam, N.M.A.; Ali, E.F.; Abo-Elyousr, K.A.M.; Bereika, M.F.F.; Seleim, M.A.A. Thyme oil treatment controls bacterial wilt disease symptoms by inducing antioxidant enzyme activity in solanum tuberosum. J. Plant Patho. 2021, 103, 563–572. [Google Scholar] [CrossRef]
- Li, Q.; Huang, K.X.; Pan, S.; Su, C.; Bi, J.; Lu, X. Thymol Disrupts Cell Homeostasis and Inhibits the Growth of Staphylococcus aureus. Contrast Media Mol. Imaging. 2022, 192, 1–12. [Google Scholar] [CrossRef]
- Shumin, Z.; Xianzhe, Z.; Reiter, R.J.; Shun, F.; Ying, W.; Sen, L. Melatonin Attenuates Potato Late Blight by Disrupting Cell Growth, Stress Tolerance, Fungicide Susceptibility and Homeostasis of Gene Expression in Phytophthora infestans. Front. Plant Sci. 2017, 8, 1993. [Google Scholar]
- Xu, Y.; Chen, L.; Zhang, Y.; Huang, Y.; Cao, J.; Jiang, W. Antimicrobial and controlled release properties of nanocomposite film containing thymol and carvacrol loaded UiO-66-NH2 for active food packaging. Food Chem. 2022, 404, 134427. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Tong, J.; Li, P.; Huang, X.; Ren, M. Chitosan is an effective inhibitor against potato dry rot caused by Fusarium oxysporum. Physiol. Mol. Plant Pathol. 2021, 113, 101601. [Google Scholar]
- Wan, C.; Shen, Y.; Nisar, M.F.; Qi, W.; Chen, J. The Antifungal Potential of Carvacrol against Penicillium Digitatum through 1H-NMR Based Metabolomics Approach. Appl. Sci. 2019, 9, 2240. [Google Scholar] [CrossRef]
- Shao, X.; Cheng, S.; Wang, H.; Yu, D.; Mungai, C. The possible mechanism of antifungal action of tea tree oil on Botrytis cinerea. J. Appl. Microbiol. 2013, 114, 1642–1649. [Google Scholar] [CrossRef]
- Shi, Y.; Zhu, Y.J.; Shao, S.Y.; Zhang, R.R.; Wu, Y.; Zhu, C.M.; Liang, X.R.; Cai, W.Q. Alkyl Ferulate Esters as Multi-functional Food Additives: Antibacterial Activity and Mode of Action against Escherichia coli in Vitro. J. Agric. Food Chem. 2018, 66, 12088–12101. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Qi, W.; Peng, X.; Chen, J.; Wan, C. Inhibitory Effect of 7-Demethoxytylophorine on Penicillium italicum and its Possible Mechanism. Microorganisms 2019, 7, 36. [Google Scholar] [CrossRef]
- Bo, T.; Liu, M.; Zhong, C.; Zhang, Q.; Jia, S.R. Metabolomic Analysis of Antimicrobial Mechanisms of ε-Poly-l-lysine on Saccharomyces cerevisiae. J. Agric. Food Chem. 2014, 62, 4454–4465. [Google Scholar] [CrossRef]
- Pasquariello, M.S.; Patre, D.D.; Mastrobuoni, F.; Zampella, L.; Scortichini, M.; Petriccione, M. Influence of postharvest chitosan treatment on enzymatic browning and antioxidant enzyme activity in sweet cherry fruit. Postharvest Biol. Tec. 2015, 109, 45–56. [Google Scholar]
- Lai, T.; Li, B.; Qin, G.; Tian, S. Oxidative damage involves in the inhibitory effect of nitric oxide on spore germination of Penicillium expansum. Curr. Microbiol. 2010, 62, 229–234. [Google Scholar] [CrossRef]
- Pang, J.; Zhang, F.; Wang, Z.; Wu, Q.; Liu, B.; Meng, X. Inhibitory effect and mechanism of curcumin-based photodynamic inactivation on patulin secretion by Penicillium expansum. Innov. Food Sci. Emerg. Technol. 2022, 80, 103078. [Google Scholar] [CrossRef]
- Peng, L.; Yang, S.; Cheng, Y.J.; Chen, F.; Pan, S.; Fan, G. Antifungal activity and action mode of pinocembrin from propolis against Penicillium italicum. Food Sci. Biotech. 2012, 21, 1533–1539. [Google Scholar]
- Pesavento, G.; Calonico, C.; Bilia, A.R.; Barnabei, M.; Calesini, F.; Addona, R.; Mencarelli, L.; Carmagnini, L.; Di Martino, M.C.; Lo Nostro, A. Antibacterial activity of Oregano, Rosmarinus and Thymus essential oils against Staphylococcus aureus and Listeria monocytogenes in beef meatballs. Food Control. 2015, 54, 188–199. [Google Scholar] [CrossRef]
- Tenn, C.C.; Wang, Y. VX-induced cell death involves activation of caspase-3 in cultured rat cortical neurons. Neurosci. Lett. 2005, 417, 155–159. [Google Scholar]
- Li, K.; Zhang, C.; Wang, W.; Chen, C.; Liu, Q.; Yin, H. First Report of Neopestalotiopsis clavispora Causing Postharvest Fruit Rot on Actinidia arguta in Liaoning Province, China. Plant Dis. 2022, 107, 217. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, S.; Banerjee, J.; Kumar, K.; Pramanik, S.; Pramanik, K.; Islam, S. Leaf spot and fruit rot of strawberry caused by Neopestalotiopsis clavispora in Indo-gangetic plains of india. Indian Phytopathol. 2018, 71, 1–5. [Google Scholar] [CrossRef]
- Abbas, M.F.; Batool, S.; Khan, T.; Rashid, M. First report of Neopestalotiopsis clavispora causing postharvest fruit rot of loquat in Pakistan. J. Plant Pathol. 2022, 104, 459. [Google Scholar] [CrossRef]
- Shi, T.; Pan, T.; Guo, M. First Isolation and Identification of Neopestalotiopsis clavispora Causing Postharvest Rot of Rosa sterilis and Its Control with Methyl Jasmonate and Calcium Chloride. Horticulturae 2013, 8, 190. [Google Scholar]
- Liu, Y.; Liu, S.; Luo, X.; Wu, X.; Ren, J.; Huang, X.; Dong, P. Antifungal activity and mechanism of thymol against Fusarium oxysporum, a pathogen of potato dry rot, and its potential application. Postharvest Biol. Tec. 2022, 192, 112025. [Google Scholar] [CrossRef]
- Gunduz, G.T.; Tuncel, G. Biofilm formation in an ice cream plant. Antonie Van Leeuwenhoek 2006, 89, 329–336. [Google Scholar] [CrossRef]
- Fadli, M.; Saad, A.; Sayadi, S.; Chevalier, J.; Hassani, L. Antibacterial activity of Thymus maroccanus and Thymus broussonetii essential oils against nosocomial infection -bacteria and their synergistic potential with antibiotics. Phytomedicine 2021, 19, 464–471. [Google Scholar] [CrossRef]
- Martinière, A.; Gibrat, R.; Sentenac, H.; Dumont, X.; Gaillard, I.; Paris, N. Uncovering pH at both sides of the root plasma membrane interface using noninvasive imaging. Proc. Natl. Acad. Sci. USA 2018, 115, 6488–6493. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, L.; Li, D.; Xia, H.; Su, X.; Peng, L. Use of active extracts of poplar buds against Penicillium italicum and possible modes of action. Food Chem. 2016, 196, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, S.; Du, S.; Sun, H. Antifungal activity of thymol and carvacrol against postharvest pathogens Botrytis cinerea. J. Food Sci. Technol. 2019, 56, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Pallavi, S.; Bhushan, J.A.; Shanker, D.R.; Mohammad, P. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Botany. 2012, 2012, 1–26. [Google Scholar]
- Yang, F.; Mi, J.; Huang, F.; Pienpinijtham, P.; Guo, Y.; Cheng, Y. Trans-cinnamaldehyde inhibits Penicillium italicum by damaging mitochondria and inducing apoptosis mechanisms. Food Sci. Hum. Well. 2022, 11, 975–981. [Google Scholar]
- Xin, Z.; Ouyang, Q.; Wan, C.; Che, J.; Tao, N. Isolation of antofine from Cynanchum atratum BUNGE (Asclepiadaceae) and its antifungal activity against Penicillium digitatum. Postharvest Biol. Tec. 2019, 157, 110961. [Google Scholar] [CrossRef]
- Zhang, M.; Ge, J.; Yu, X. Transcriptome Analysis Reveals the Mechanism of Fungicidal of Thymol Against Fusarium oxysporum f. sp. niveum. Curr. Microbiol. 2017, 75, 410–419. [Google Scholar] [CrossRef]
- Zou, X.; Wei, Y.; Jiang, S.; Xu, F.; Wang, H.; Zhan, P.; Shao, X. ROS Stress and Cell Membrane Disruption are the Main Antifungal Mechanisms of 2-Phenylethanol against Botrytis cinerea. J. Agric. Food Chem. 2022, 70, 14468–14479. [Google Scholar] [CrossRef]
- Pan, C.; Yang, K.; Famous, E.; Li, Y.X.; Liu, M.; Pan, S.; Yang, S.; Lu, G.; Ma, D.; Tian, J. Inhibitory effect of cinnamaldehyde on Fusarium solani and its application in postharvest preservation of sweet potato. Food Chem. 2023, 408, 135213. [Google Scholar] [CrossRef]
- Burt, S.A. Essential oils: Their antibacterial properties and potential applications in foods-A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar]
- Helmerhorst, E.J.; Troxler, R.F.; Oppenheim, F.G. The human salivary peptide histatin 5 exerts its antifungal activity through the formation of reactive oxygen species. Proc. Natl. Acad. Sci. USA 2022, 98, 14637–14642. [Google Scholar] [CrossRef] [PubMed]
- Okayama, S.; Kopelovich, L.; Balmus, G.; Weiss, R.S.; Subbaramaiah, K. P53 protein regulates Hsp90 ATPase activity and thereby wnt signaling by modulating aha1 expression. J. Biol. Chem. 2014, 289, 6513–6525. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sun, L.; Zh, J.; Ji, X.; Huang, R.; Fan, Y.; Ge, Y. Trehalose Regulates Starch, Sorbitol, and Energy Metabolism to Enhance Tolerance to Blue Mold of “Golden Delicious” Apple Fruit. J. Agric. Food Chem. 2022, 70, 5658–5667. [Google Scholar] [CrossRef]
- Saquet, A.A.; Streif, J.; Bangerth, F. Energy metabolism and membrane lipid alterations in relation to brown heart development in ‘conference’ pears during delayed controlled atmosphere storage. Postharvest Biol. Tec. 2003, 30, 123–132. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, S.; Shuai, L.; Luo, D.; Ba, L. Inhibitory Activity and Mechanism of Action with Thymol against the Blueberry Pathogenic Fungi Caused by Neopestalotiopsis clavispora. Horticulturae 2023, 9, 983. https://doi.org/10.3390/horticulturae9090983
Ye S, Shuai L, Luo D, Ba L. Inhibitory Activity and Mechanism of Action with Thymol against the Blueberry Pathogenic Fungi Caused by Neopestalotiopsis clavispora. Horticulturae. 2023; 9(9):983. https://doi.org/10.3390/horticulturae9090983
Chicago/Turabian StyleYe, Shengjie, Liang Shuai, Donglan Luo, and Liangjie Ba. 2023. "Inhibitory Activity and Mechanism of Action with Thymol against the Blueberry Pathogenic Fungi Caused by Neopestalotiopsis clavispora" Horticulturae 9, no. 9: 983. https://doi.org/10.3390/horticulturae9090983
APA StyleYe, S., Shuai, L., Luo, D., & Ba, L. (2023). Inhibitory Activity and Mechanism of Action with Thymol against the Blueberry Pathogenic Fungi Caused by Neopestalotiopsis clavispora. Horticulturae, 9(9), 983. https://doi.org/10.3390/horticulturae9090983





