Increase in Total Phenolic Content and Antioxidant Capacity in Wines with Pre- and Post-Fermentation Addition of Melissa officinalis, Salvia officinalis and Cannabis sativa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Herbs
2.3. Wine Preparation with Herbs
2.4. Total Phenolic Content Determination
2.5. Evaluation of Antioxidant Activity-DPPH Method
2.6. HPLC Analysis of Phenolic Compounds in Plant Extracts
2.7. Data Analyses
3. Results
3.1. Herbs’ Maximum Extraction Level
3.2. Total Phenolic Content
3.3. Evaluation of Antioxidant Activity
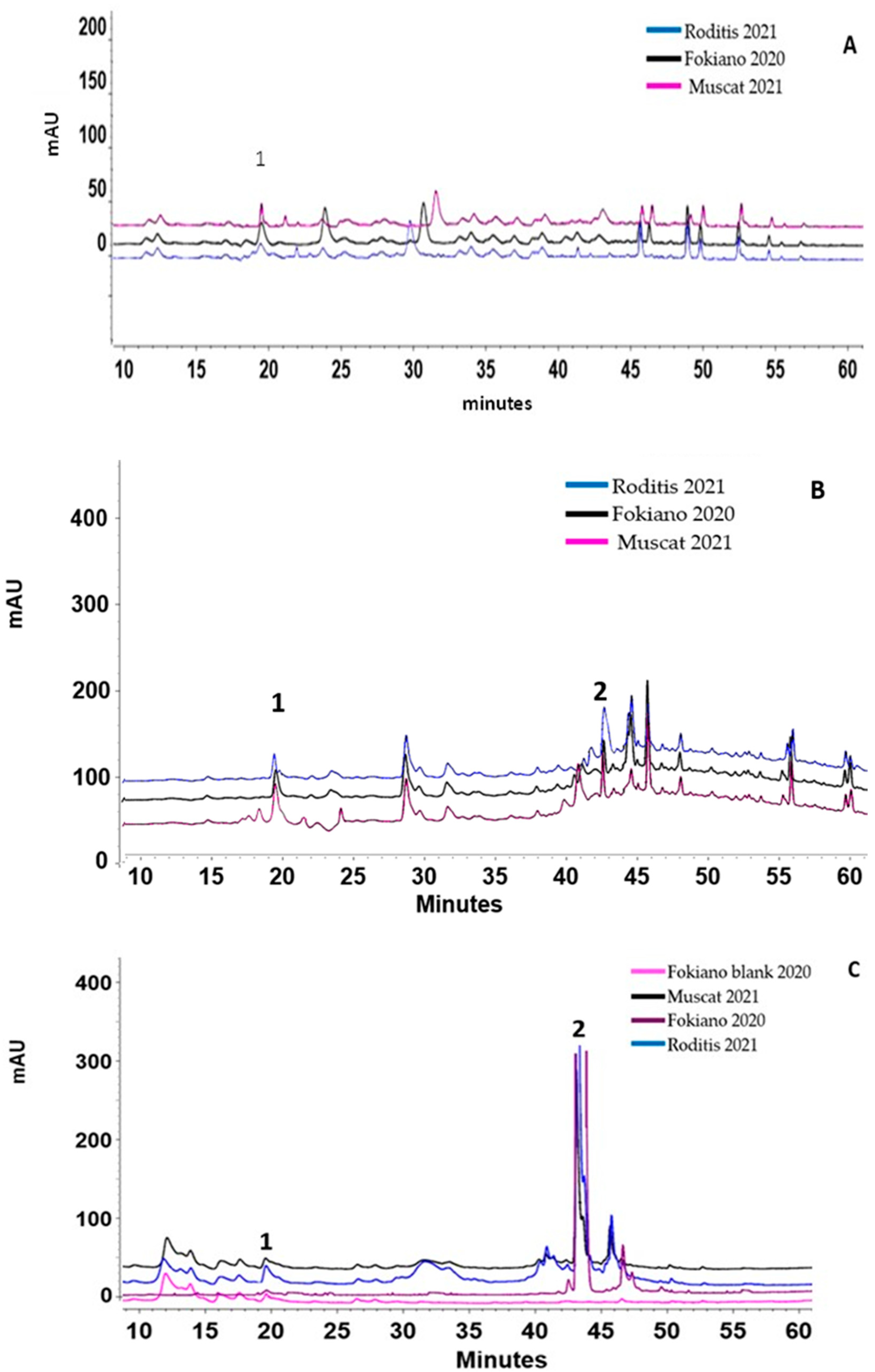
3.4. HPLC-DAD Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rasool, A.; Bhat, K.M.; Sheikh, A.A.; Jan, A.; Hassan, S. Medicinal Plants: Role, Distribution and Future. J. Pharmacogn. Phytochem. 2020, 9, 2111–2114. [Google Scholar]
- Solomou, A.D.; Martinos, K.; Skoufogianni, E.; Danalatos, N.G. Medicinal and Aromatic Plants Diversity in Greece and Their Future Prospects: A Review. Agric. Sci. 2016, 4, 9–20. [Google Scholar] [CrossRef]
- Pasias, I.N.; Ntakoulas, D.D.; Raptopoulou, K.; Gardeli, C.; Proestos, C. Chemical Composition of Essential Oils of Aromatic and Medicinal Herbs Cultivated in Greece—Benefits and Drawbacks. Foods 2021, 10, 2354. [Google Scholar] [CrossRef] [PubMed]
- Kintzios, E.S. Sage, The Genus Salvia, 1st ed.; CRC Press: London, UK, 2000; pp. 31–53. [Google Scholar] [CrossRef]
- Harutyunyan, M.; Malfeito-Ferreira, M. Historical and Heritage Sustainability for the Revival of Ancient Wine-Making Techniques and Wine Styles. Beverages 2022, 8, 10. [Google Scholar] [CrossRef]
- Tonutti, I.; Liddle, P. Aromatic Plants in Alcoholic Beverages. A Review. Flavour Fragr. J. 2010, 25, 341–350. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, P.; Zeng, X.A.; Fang, Z. The Art of Flavored Wine: Tradition and Future. Trends Food Sci. Technol. 2021, 116, 130–145. [Google Scholar] [CrossRef]
- Buglass, A.J. Handbook of Alcoholic Beverages: Technical, Analytical and Nutritional Aspects; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; Volume 1–2, ISBN 9780470512029. [Google Scholar]
- Karalija, E.; Dahija, S.; Tarkowski, P.; Zeljković, S.Ć. Influence of Climate-Related Environmental Stresses on Economically Important Essential Oils of Mediterranean salvia sp. Front. Plant Sci. 2022, 13, 864807. [Google Scholar] [CrossRef]
- Onlooker Sage against Age. Pharm. J. 1995, 255, 708.
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological Properties of Salvia officinalis and Its Components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef]
- Jakovljević, M.; Jokić, S.; Molnar, M.; Jašić, M.; Babić, J.; Jukić, H.; Banjari, I. Bioactive Profile of Various Salvia officinalis L. Preparations. Plants 2019, 8, 55. [Google Scholar] [CrossRef]
- Kennedy, T.A.; Naeem, S.; Howe, K.M.; Knops, J.M.H.; Tilman, D.; Reich, P. Biodiversity as a Barrier to Ecological Invasion. Nature 2002, 417, 636–638. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, F.; Begaa, S.; Messaoudi, M.; Benarfa, A.; Ouakouak, H.; Hassani, A.; Sawicka, B.; Simal Gandara, J. HPLC–DAD Analysis, Antimicrobial and Antioxidant Properties of Aromatic Herb Melissa officinalis L., Aerial Parts Extracts. Food Anal. Methods 2023, 16, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Petrisor, G.; Motelica, L.; Craciun, L.N.; Oprea, O.C.; Ficai, D.; Ficai, A. Melissa officinalis: Composition, Pharmacological Effects and Derived Release Systems—A Review. Int. J. Mol. Sci. 2022, 23, 3591. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant Activity and Phenolic Compounds in Selected Herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Isahq, M.S.; Afridi, M.S.; Ali, J.; Hussain, M.M.; Ahmad, S.; Kanwal, F. Proximate Composition, Phytochemical Screening, GC-MS Studies of Biologically Active Cannabinoids and Antimicrobial Activities of Cannabis indica. Asian Pac. J. Trop. Dis. 2015, 5, 897–902. [Google Scholar] [CrossRef]
- Kumar, P.; Mahato, D.K.; Kamle, M.; Borah, R.; Sharma, B.; Pandhi, S.; Tripathi, V.; Yadav, H.S.; Devi, S.; Patil, U.; et al. Pharmacological Properties, Therapeutic Potential, and Legal Status of Cannabis sativa L.: An Overview. Phytother. Res. 2021, 35, 6010–6029. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Jeena, G.; Pitakbut, T.; Vasilev, N.; Kayser, O. Cannabis sativa Research Trends, Challenges, and New-Age Perspectives. iScience 2021, 24, 103391. [Google Scholar] [CrossRef]
- Russo, E.B.; Grotenhermen, F. The Handbook of Cannabis Therapeutics, from Bench to Bedside, 1st ed.; Routledge: London, UK, 2014; ISBN 9780203820803. [Google Scholar]
- Merkyte, V.; Longo, E.; Windisch, G.; Boselli, E. Phenolic Compounds as Markers of Wine Quality and Authenticity. Foods 2020, 9, 1785. [Google Scholar] [CrossRef]
- Wurz, D.A. Wine and Health: A Review of Its Benefits to Human Health. BIO Web Conf. 2019, 12, 04001. [Google Scholar] [CrossRef]
- Kalogiouri, N.P.; Samanidou, V.F. Liquid Chromatographic Methods Coupled to Chemometrics: A Short Review to Present the Key Workflow for the Investigation of Wine Phenolic Composition as It Is Affected by Environmental Factors. Environ. Sci. Pollut. Res. 2021, 28, 59150–59164. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Kulisic, T.; Radonic, A.; Katalinic, V.; Milos, M. Use of Different Methods for Testing Antioxidative Activity of Oregano Essential Oil. Food Chem. 2004, 85, 633–640. [Google Scholar] [CrossRef]
- Kouri, G.; Tsimogiannis, D.; Bardouki, H.; Oreopoulou, V. Extraction and Analysis of Antioxidant Components from Origanum dictamnus. Innov. Food Sci. Emerg. Technol. 2007, 8, 155–162. [Google Scholar] [CrossRef]
- Karimali, D.; Kosma, I.; Badeka, A. Varietal Classification of Red Wine Samples from Four Native Greek Grape Varieties Based on Volatile Compound Analysis, Color Parameters and Phenolic Composition. Eur. Food Res. Technol. 2020, 246, 41–53. [Google Scholar] [CrossRef]
- Karagiannis, S.; Economou, A.; Lanaridis, P. Phenolic and Volatile Composition of Wines Made from Vitis vinifera Cv. Muscat Lefko Grapes from the Island of Samos. J. Agric. Food Chem. 2000, 48, 5369–5375. [Google Scholar] [CrossRef]
- Proestos, C.; Bakogiannis, A.; Komaitis, M. Determination of Phenolic Compounds in Wines. Int. J. Food Stud. 2012, 1, 33–41. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Dias, M.I.; Sousa, M.J.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic Profiles of Cultivated, in Vitro Cultured and Commercial Samples of Melissa officinalis L. Infusions. Food Chem. 2013, 136, 1–8. [Google Scholar] [CrossRef]
- Ahmad, F.; Abbas, T.; Farman, K.; Akrem, A.; Saleem, M.A.; Iqbal, M.U.; Baloch, F.S.; Mahmood, S. High-Throughput Phytochemical Characterization of Non-Cannabinoid Compounds of Cannabis Plant and Seed, from Pakistan. Pak. J. Bot. 2018, 50, 639–643. [Google Scholar]
- Fracassetti, D.; Lawrence, N.; Tredoux, A.G.J.; Tirelli, A.; Nieuwoudt, H.H.; Du Toit, W.J. Quantification of Glutathione, Catechin and Caffeic Acid in Grape Juice and Wine by a Novel Ultra-Performance Liquid Chromatography Method. Food Chem. 2011, 128, 1136–1142. [Google Scholar] [CrossRef]
- Arvaniti, O.S.; Tsolou, A.; Sakantani, E.; Milla, S.; Kallinikou, E.; Petsini, F.; Choleva, M.; Detopoulou, M.; Fragopoulou, E.; Samaras, Y. Quality Characteristics, Polyphenol Profile and Antioxidant Capacity in Red, Rosé and White Monovarietal Wines from Ionian Islands of Greece. Acta Sci. Pol. Technol. Aliment. 2022, 21, 343–357. [Google Scholar] [CrossRef]
- Panesar, P.S.; Joshi, V.K.; Panesar, R.; Abrol, G.S. Vermouth: Technology of Production and Quality Characteristics. Adv. Food Nutr. Res. 2011, 63, 251–283. [Google Scholar] [CrossRef]
- Popescu, A.; Birghila, S.; Radu, M.D.; Bratu, M.M. Evaluation of the Polyphenol Content and Antioxidant Activity of Wine Macerates (Medicinal Wines) With Sage (Salvia officinalis L. Lamiaceae) and Sea Rush (Juncus martitimus Lam. Juncaceae) Obtained Using Traditional Technology. Pol. J. Environ. Stud. 2022, 31, 3279–3285. [Google Scholar] [CrossRef] [PubMed]
- Leonard, W.; Zhang, P.; Ying, D.; Adhikari, B.; Fang, Z. Fermentation Transforms the Phenolic Profiles and Bioactivities of Plant-Based Foods. Biotechnol. Adv. 2021, 49, 107763. [Google Scholar] [CrossRef] [PubMed]
- Skotti, E.; Anastasaki, E.; Kanellou, G.; Polissiou, M.; Tarantilis, P.A. Total Phenolic Content, Antioxidant Activity and Toxicity of Aqueous Extracts from Selected Greek Medicinal and Aromatic Plants. Ind. Crops Prod. 2014, 53, 46–54. [Google Scholar] [CrossRef]
- Dastmalchi, K.; Damien Dorman, H.J.; Oinonen, P.P.; Darwis, Y.; Laakso, I.; Hiltunen, R. Chemical Composition and in Vitro Antioxidative Activity of a Lemon Balm (Melissa officinalis L.) Extract. LWT 2008, 41, 391–400. [Google Scholar] [CrossRef]
- Lee, J. Caffeic Acid Derivatives in Dried Lamiaceae and Echinacea Purpurea Products. J. Funct. Foods 2010, 2, 158–162. [Google Scholar] [CrossRef]
- Farhat, M.B.; Chaouch-Hamada, R.; Sotomayor, J.A.; Landoulsi, A.; Jordán, M.J. Antioxidant Potential of Salvia officinalis L. Residues as Affected by the Harvesting Time. Ind. Crops Prod. 2014, 54, 78–85. [Google Scholar] [CrossRef]
- Shekarchi, M.; Hajimehdipoor, H.; Saeidnia, S.; Gohari, A.R.; Hamedani, M.P. Comparative Study of Rosmarinic Acid Content in Some Plants of Labiatae Family. Pharmacogn. Mag. 2012, 8, 37–41. [Google Scholar] [CrossRef]
- Kallithraka, S.; Tsoutsouras, E.; Tzourou, E.; Lanaridis, P. Principal Phenolic Compounds in Greek Red Wines. Food Chem. 2006, 99, 784–793. [Google Scholar] [CrossRef]
- Székelyhidi, R.; Lakatos, E.; Sik, B.; Nagy, Á.; Varga, L.; Molnár, Z.; Kapcsándi, V. The Beneficial Effect of Peppermint (Mentha X piperita L.) and Lemongrass (Melissa officinalis L.) Dosage on Total Antioxidant and Polyphenol Content during Alcoholic Fermentation. Food Chem. X 2022, 13, 100226. [Google Scholar] [CrossRef]
- Lakićević, S.H.; Popović Djordjević, J.B.; Pejin, B.; Djordjević, A.S.; Matijašević, S.M.; Lazić, M.L. An Insight into Chemical Composition and Bioactivity of “Prokupac” Red Wine. Nat. Prod. Res. 2020, 34, 1542–1546. [Google Scholar] [CrossRef] [PubMed]
- Chawafambira, A. The Effect of Incorporating Herbal (Lippia javanica) Infusion on the Phenolic, Physicochemical, and Sensorial Properties of Fruit Wine. Food Sci. Nutr. 2021, 9, 4539–4549. [Google Scholar] [CrossRef] [PubMed]
- Tarapatskyy, M.; Kapusta, I.; Gumienna, A.; Puchalski, C. Assessment of the Bioactive Compounds in White and Red Wines Enriched with a Primula veris L. Molecules 2019, 24, 4074. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Zhang, P.; Ma, W.; Zeng, X.A.; Fang, Z. Pulsed Electric Field Processing of Green Tea-Infused Chardonnay Wine: Effects on Physicochemical Properties, Antioxidant Activities, Phenolic and Volatile Compounds. Food Biosci. 2023, 54, 102884. [Google Scholar] [CrossRef]


| Maximum Her Extraction in Phenolic Compounds (g herb L−1 Wine) | Maximum Herb Extraction in Antioxidant Compounds (g herb L−1 Wine) | |||
|---|---|---|---|---|
| Sample | Pre-Fermentation | Post-Fermentation | Pre-Fermentation | Post-Fermentation |
| Can/Rod 2021 | 9.31 | 10.12 | 7.66 | 9.70 |
| Can/Mus 2021 | 8.94 | 8.38 | 10.00 | 10.97 |
| Can/Fok 2020 | 10.20 | 10.89 | 11.30 | 12.60 |
| Sage/Rod 2021 | 10.01 | 10.73 | 10.86 | 11.69 |
| Sage/Mus 2021 | 7.91 | 8.34 | 9.10 | 9.65 |
| Sage/Fok 2020 | 9.12 | 9.67 | 9.65 | 9.90 |
| Mel/Rod 2021 | 9.53 | 11.38 | 9.70 | 9.49 |
| Mel/Mus 2021 | 11.29 | 11.70 | 9.38 | 8.83 |
| Mel/Fok 2020 | 9.87 | 9.03 | 7.83 | 9.10 |
| Blank Wine (no Herb) | Pre-Fermentation Herb Addition (in Must) | Post-Fermentation Herb Addition (in Stable Wine) | |||
|---|---|---|---|---|---|
| Herb/Wine Harvest Year | mg GAE L−1 | mg GAE L−1 g−1 | % Increase | mg GAE L−1 g−1 | % Increase |
| Can/Rod 2021 | 140.22 ± 1.07 a | 151.40 ± 1.33 b | 7.97 | 160.51 ± 1.47 c | 14.47 |
| Can/Mus 2021 | 192.74 ± 1.98 a | 213.51 ± 1.71 b | 10.81 | 244.33 ± 2.33 c | 26.78 |
| Can/Fok 2020 | 337.45 ± 4.79 a | 387.55 ± 3.52 b | 14.84 | 500.35 ± 4.21 c | 48.27 |
| Sage/Rod 2021 | 140.22 ± 1.73 a | 197.10 ± 2.47 b | 40.56 | 212.93 ± 3.56 c | 51.86 |
| Sage/Mus 2021 | 192.74 ± 1.22 a | 275.45 ± 2.01 b | 42.91 | 356.84 ± 2.72 c | 85.14 |
| Sage/Fok 2020 | 337.45 ± 3.56 a | 443.55 ± 3.89 b | 31.44 | 644.75 ± 4.71 c | 91.06 |
| Mel/Rod 2021 | 140.22 ± 1.85 a | 230.93 ± 2.81 b | 64.71 | 246.11 ± 2.87 c | 75.53 |
| Mel/Mus 2021 | 192.74 ± 1.51 a | 312.55 ± 2.94 b | 62.16 | 374.94 ± 3.75 c | 94.53 |
| Mel/Fok 2020 | 337.45 ± 3.44 a | 461.23 ± 4.38 b | 36.68 | 642.35 ± 4.41 c | 90.35 |
| Sample | Pre-Fermentation Herb Addition | Post-Fermentation Herb Addition | % Difference |
|---|---|---|---|
| Can/Rod 2021 | 11.33 | 20.30 | 44.2 |
| Can/Mus 2021 | 20.77 | 51.59 | 59.7 |
| Can/Fok 2020 | 50.1 | 162.9 | 69.2 |
| Sage/Rod 2021 | 56.88 | 72.71 | 22.2 |
| Sage/Mus2021 | 82.71 | 164.1 | 49.6 |
| Sage/Fok 2020 | 106.1 | 307.3 | 65.4 |
| Mel/Rod 2021 | 90.73 | 105.91 | 14.3 |
| Mel/Mus 2021 | 119.81 | 182.2 | 34.2 |
| Mel/Fok 2020 | 123.78 | 304.9 | 59.4 |
| Blank Wine (no Herb) | Pre-Fermentation Herb Addition | Post-Fermentation Herb Addition | |||
|---|---|---|---|---|---|
| Sample | mmole Trolox L−1 | mmole Trolox L−1 g−1 | % Increase | mmole Trolox L−1 g−1 | % Increase |
| Can/Rod 2021 | 1.69 ± 0.071 a | 1.79 ± 0.079 b | 5.7 | 1.84 ± 0.083 b | 9.3 |
| Can/Mus 2021 | 1.72 ± 0.069 a | 1.89 ± 0.076 b | 10.1 | 2.01 ± 0.084 b | 16.9 |
| Can/Fok 2020 | 3.14 ± 0.113 a | 3.35 ± 0.171 b | 6.8 | 3.49 ± 0.182 b | 11.3 |
| Sage/Rod 2021 | 1.69 ± 0.077 a | 1.81 ± 0.084 b | 7.1 | 1.96 ± 0.080 c | 11.1 |
| Sage/Mus 2021 | 1.72 ± 0.078 a | 1.99 ± 0.099 b | 17.1 | 2.21 ± 0.103 c | 28.4 |
| Sage/Fok 2020 | 3.14 ± 0.143 a | 3.53 ± 0.158 b | 12.8 | 3.75 ± 0.146 c | 19.4 |
| Mel/Rod 2021 | 1.69 ± 0.064 a | 2.76 ± 0.092 b | 63.0 | 2.91 ± 0.116 c | 71.6 |
| Mel/Mus 2021 | 1.72 ± 0.078 a | 2.67 ± 0.089 b | 52.7 | 3.03 ± 0.147 c | 76.6 |
| Mel/Fok 2020 | 3.14 ± 0.147 a | 3.85 ± 0.144 b | 22.6 | 4.07 ± 0.153 c | 29.7 |
| mmole Trolox L−1 g−1 | mmole Trolox L−1 g−1 | ||
|---|---|---|---|
| Sample | Pre-Fermentation Herb Addition | Post-Fermentation Herb Addition | % Difference |
| Can/Rod 2021 | 0.096 | 0.158 | 39.20 |
| Can/Mus 2021 | 0.174 | 0.292 | 40.40 |
| Can/Fok 2020 | 0.215 | 0.356 | 39.60 |
| Sage/Rod 2021 | 0.121 | 0.168 | 27.90 |
| Sage/Mus 2021 | 0.295 | 0.490 | 39.70 |
| Sage/Fok 2020 | 0.390 | 0.610 | 36.00 |
| Mel/Rod 2021 | 1.075 | 1.411 | 23.80 |
| Mel/Mus 2021 | 0.907 | 1.319 | 31.30 |
| Mel/Fok 2020 | 0.710 | 0.933 | 23.90 |
| Caffeic Acid (mg L−1) | Rosmarinic Acid (mg L−1) | |||
|---|---|---|---|---|
| Pre-Fermentation | Post-Fermentation | Pre-Fermentation | Post-Fermentation | |
| Can/Rod 2021 | n.d. * | 0.9 ± 0.10 | n.d. | nd |
| Can/Mus 2021 | n.d. | 0.9 ± 0.18 | n.d. | nd |
| Can/Fok 2020 | n.d. | 0.9 ± 0.16 | n.d. | nd |
| Sage/Rod 2021 | n.d. | 17.9 ± 0.53 | n.d. | 27.0 ± 0.33 |
| Sage/Mus2021 | n.d. | 19.1 ± 0.38 | n.d. | 27.8 ± 0.71 |
| Sage/Fok 2020 | n.d. | 20.0 ± 0.31 | n.d. | 28.7 ± 0.67 |
| Mel/Rod 2021 | n.d. | 3.1 ± 0.44 | n.d. | 39.4 ± 1.06 |
| Mel/Mus 2021 | n.d. | 3.4 ± 0.46 | n.d. | 44.8 ± 0.91 |
| Mel/Fok 2020 | n.d. | 4.8 ± 0.52 | n.d. | 53.5 ± 1.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roufa, P.; Evangelou, A.; Beris, E.; Karagianni, S.; Chatzilazarou, A.; Dourtoglou, E.; Shehadeh, A. Increase in Total Phenolic Content and Antioxidant Capacity in Wines with Pre- and Post-Fermentation Addition of Melissa officinalis, Salvia officinalis and Cannabis sativa. Horticulturae 2023, 9, 956. https://doi.org/10.3390/horticulturae9090956
Roufa P, Evangelou A, Beris E, Karagianni S, Chatzilazarou A, Dourtoglou E, Shehadeh A. Increase in Total Phenolic Content and Antioxidant Capacity in Wines with Pre- and Post-Fermentation Addition of Melissa officinalis, Salvia officinalis and Cannabis sativa. Horticulturae. 2023; 9(9):956. https://doi.org/10.3390/horticulturae9090956
Chicago/Turabian StyleRoufa, Paraskevi, Alexandra Evangelou, Evangelos Beris, Styliani Karagianni, Archontoula Chatzilazarou, Efthalia Dourtoglou, and Adnan Shehadeh. 2023. "Increase in Total Phenolic Content and Antioxidant Capacity in Wines with Pre- and Post-Fermentation Addition of Melissa officinalis, Salvia officinalis and Cannabis sativa" Horticulturae 9, no. 9: 956. https://doi.org/10.3390/horticulturae9090956
APA StyleRoufa, P., Evangelou, A., Beris, E., Karagianni, S., Chatzilazarou, A., Dourtoglou, E., & Shehadeh, A. (2023). Increase in Total Phenolic Content and Antioxidant Capacity in Wines with Pre- and Post-Fermentation Addition of Melissa officinalis, Salvia officinalis and Cannabis sativa. Horticulturae, 9(9), 956. https://doi.org/10.3390/horticulturae9090956